Abstract
Broadly neutralizing antibodies (bnAbs) are potential therapeutic molecules and valuable tools for studying conserved viral targets for vaccine and drug design. Interestingly, antibody responses to conserved epitopes can be highly convergent at the molecular level. Human antibodies targeting a number of viral antigens have often been found to utilize a restricted set of immunoglobulin germline genes in different individuals. Here we review recent knowledge on VH1–69-encoded antibodies in antiviral responses to influenza virus, HCV, and HIV-1. These antibodies share common genetic and structural features, and often develop neutralizing activity against a broad spectrum of viral strains. Understanding the genetic and structural characteristics of such antibodies and the target epitopes should help advance novel strategies to elicit bnAbs through vaccination.
Graphical abstract

Introduction
Most vaccines to viral pathogens rely on the induction of neutralizing antibody (nAb) responses for host protection. For highly variable viruses such as influenza virus, HCV, and HIV-1, a broadly effective or universal vaccine that can cross-protect against the diverse spectrum of known viral strains and subtypes remains elusive. This failure so far may be exacerbated by various mechanisms that these viruses use for immune evasion such as high genetic diversity, fast mutation rates facilitated by the error-prone RNA polymerase, glycan shielding of the viral envelope glycoproteins, conformational flexibility of the glycoproteins, and limited accessibility of conserved epitopes (reviewed in [1–4]). However, broadly nAbs (bnAbs) to such hypervariable RNA viruses have in fact been isolated from infected or vaccinated individuals, demonstrating the ability of the human immune system to overcome some of the viral escape mechanisms and recognize conserved epitopes on the viruses. Thus, in principle, it is possible for a broad vaccine to be designed against these viruses.
In addition to viral escape, host genetic factors such as interleukin genotypes and human leukocyte antigen (HLA) alleles are known to modulate antiviral immune responses, resulting in individual differences in surviving infection [5–7]. Single-nucleotide polymorphisms (SNPs) in HLA, cytokine and cytokine receptor genes have been reported to influence antibody responses during infection and vaccination [8,9]. The recent interest in understanding antiviral bnAbs and the conditions to elicit them in vaccination have led to the study of antibody responses at the genetic level. It is found that some antibodies against a particular epitope share a restricted set of immunoglobulin heavy chain variable region (VH) genes and are often quite similar in overall structure [10]. For example, influenza antibodies against the hemagglutinin (HA) stem region have so far been found to predominantly utilize VH1–69, VH1–18 and VH6–1 [11–14]; HCV antibodies targeting the E2 antigenic region 3 (AR3) mainly derive from VH1–69 [15–17]; HIV-1 antibodies against the gp120 CD4 binding site (CD4bs) tend to be encoded by VH1–2 and VH1–46 [18,19], and by VH1–69 when against the coreceptor-binding site on gp120 [also known as CD4-induced (CD4i) binding site] or the conserved heptad repeat 1 (HR1) region and membrane proximal external region (MPER) of gp41 [20,21]; rotavirus antibodies against virus protein 6 (VP6) preferentially use VH1–46 [22–24]. Such antibodies can be produced by multiple individuals and often exhibit broadly neutralizing activity, and thus, represent immunological solutions to induce reproducible bnAbs in the general human population by vaccination. Here we focus on the current knowledge on VH1–69-encoded bnAbs against influenza, HCV and HIV-1, and discuss how their genetic and structural information can inform rational vaccine design.
Conserved epitopes targeted by VH1–69 bnAbs
The VH1–69 gene encodes two hydrophobic residues at the tip of the heavy-chain complementarity-determining region 2 (CDRH2) loop that provide a structural basis for epitope recognition. It is, therefore, preferentially used by antibodies that target conserved hydrophobic regions of viral envelope glycoproteins.
HA is a homotrimeric glycoprotein (trimer of HA1-HA2 dimers) on the envelope of influenza A and B viruses that is responsible for viral receptor attachment and membrane fusion activities. It can be functionally and structurally separated into two domains: a hypervariable membrane-distal head and a conserved membrane-proximal stem [2] (Figure 1a). The head domain is composed only from HA1 and contains the binding site for its sialic acid receptor, while the stem consists of both HA1 and HA2 and houses the fusion machinery. Current seasonal influenza viruses and annual vaccines normally generate strain-specific antibody responses to the HA head. In the last decade, however, the discovery and isolation of human bnAbs to influenza virus have identified an increasing number of antibodies that are able to neutralize a broad spectrum of heterosubtypic influenza viruses (reviewed in [25•]). Surprisingly, these antibodies (e.g. CR6261, A6, F10, 27F3 and CR9114) exhibit restricted usage of VH1–69 and mainly target epitopes that correspond to hydrophobic pockets in the HA stem [11,26–28,29••] (Table 1 and Figure 1a). A small proportion of anitbodies (e.g. 2G1, 8M2, and F045–092) that also originate from VH1–69 are directed at or near the receptor-binding site (RBS) on the HA head (Table 1 and Figure 1b) [30–32].
Figure 1. Recognition of influenza, HCV and HIV-1 envelope glycoproteins by representative antiviral VH1–69 bnAbs.

(a+b) Binding of the influenza HA stem-specific CR9114 (a, PDB 4FQI) and RBS-specific F045–092 (b, PDB 4O58) bnAbs to the influenza HA-trimer. One HA protomer is colored in light pink and light purple for the HA1 and HA2 subunits, respectively. For clarity only one mAb is shown in each illustration. (c+d) Binding of AR3C to HCV E2 antigen region 3 (c, PDB 4MWF) and HC84–1 to E2434–446 peptide (d, PDB 4JZN) on the neutralizing face. The E2 front layer (FL) is colored in yellow and CD81 binding loop (CD81bl) in green. (e+f) Binding of 4E10 to HIV-1 membrane-proximal-external region (MPER) on gp41 (e, PDB 4XCF), and VRC13 to CD4 binding site (CD4bs) on HIV-1 gp120 that does not use a hydrophobic motif at the tip of CDRH2. (f, PDB 4YDJ). In each complex, the side chains of the hydrophobic residues at the tip of CDRH2 are shown in sticks and colored in red. The heavy chain (HC) and light chain (LC) CDRs that contribute to the binding are shown in a narrow tube representation.
Table 1.
Properties of representative VH1–69 antiviral antibodies.
| Virus | Antibody | VH1–69 allele | Genetic features | CDRH3 length1 | Epitope | Recognition properties | Neutralization breadth | PDB | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Influenza | CR6261 | F | Hydrophobic Ile-Phe (IF) sequence at the tip of CDRH2 [Met-Phe (MF) for F10] with a conserved Tyr (Y) in CDRH3 to give an IFY motif | 12 | HA stem | Heavy chain only binding; CDRH2 dominant | Influenza A group 1 | 3GBM | [27] |
| F10 | F | 15 | 3FKU | [11] | |||||
| CR9114 | F | 12 | Heavy chain dominant; CDRH2 dominant | Influenza A group 1 and 2 viruses | 4FQI | [28] | |||
| 27F3 | F | 14 | 5WKO | [29••] | |||||
| 2G1 | F | Hydrophobic IF (FF for F045–092) at the tip of CDRH2 | 11 | HA head RBS | Heavy chain dominant; CDRH2 and H3 mediated | Influenza A group 1 | 4HG4 | [32] | |
| 8M2 | F | 14 | 4HFU | [32] | |||||
| F045–092 | F | 23 | Heavy chain only binding; CDRH3 dominant | Influenza A (group 1 and 2) | 4O58; 4O5I | [30,70] | |||
| HCV | AR3A | F | Hydrophobic I/V-P-X-F motif at the tip of CDRH2 for AR3- and HC84-class antibodies; T/S-P-I-F/S motif for HEPC3/74-class antibodies | 18 | E2 AR3 | Heavy chain only binding; CDRH2 and CDRH3 mediated | Broadly neutralizing | 6BKB | [15,60••] |
| AR3B | F | 19 | 6BKC | [15,60••] | |||||
| AR3C | F | 18 | 4MWF | [15,44,60••] | |||||
| AR3D | F | 22 | 6BKD | [15,60••] | |||||
| HEPC3 | F | 17 | 6MEI; 6MEJ; 6MEK | [47••] | |||||
| HEPC74 | F | 18 | 6MEH | [47••] | |||||
| HC84–1 | F | 10 | E2 domain D | Heavy chain dominant; CDRH2 dominant | Broadly neutralizing | 4JZN | [45] | ||
| HC84–27 | F | 10 | 4JZO | [45] | |||||
| HC84–26 | F | 10 | 5ERW | [46] | |||||
| HIV-1 | 4E10 | L | Hydrophobic LL at the tip of CDRH2 | 18 | gp41 MPER and membrane lipid | Heavy chain dominant; CDRH2 dominant | Broadly neutralizing | 2FX7, 4XCF | [51,53,75,75] |
| HK20 | F | Hydrophobic IF at the tip of CDRH2 | 13 | gp41 HR1 | Heavy chain dominant; CDRH2 dominant | Less breadth and potency | 2XRA | [56] | |
| D5 | F | 10 | 2CMR | [55] | |||||
| 17b | L | Hydrophobic IL at the tip of CDRH2 | 19 | gp120 coreceptor-binding site | Heavy chain dominant; CDRH2 and H3 mediated | Limited neutralizing | 1RZ8 1GC1, 1G9N, 1G9M | [20,77,78] | |
| VRC13 | F | Polar ST at the tip of CDRH2 | 21 | gp120 CD4bs | CDRH3 dominant | Broadly neutralizing | 4YDJ | [19] |
Based on Kabat numbering, in which the CDRH3 length is 2 amino acids shorter than in the IMGT definition.
HCV envelope glycoproteins E1 and E2 form heterodimers on the viral surface and play essential roles in viral entry, assembly, fusion and budding [33,34]. E2 is the receptor binding protein that directly interacts with the host receptors tetraspanin CD81 and the scavenger receptor class B member 1 (SR-B1) [35,36•], while E1 is suggested to help modulate the E2-receptor interactions and carry out fusion with the host cell membrane [37,38]. HCV bnAbs have been mapped onto the E2 neutralizing face, a predominantly hydrophobic surface formed by the front layer and the tip of the CD81 binding loop [36•] (Figure 1c), as well as the E1E2 heterodimer (reviewed in [39•]). Biased use of VH1–69 has been reported in anti-E2 antibody responses in multiple studies [15–17,40–43]. Most of these antibodies (e.g. AR3-class antibodies, HC84-class antibodies, HEPC3/74-class, HC-1-class and AT12–009) recognize the overlapping neutralizing sites on the E2 neutralizing face (Table 1, Figure 1c and d), and neutralize diverse HCV genotypes by blocking E1E2 binding to CD81 (Table 1) [44–46,47••].
HIV-1 envelope glycoprotein (Env) consists of two non-covalently associated subunits that form trimers of the receptor binding glycoprotein gp120 and the membrane-anchored fusion protein gp41. The fusion and entry of HIV-1 to host cells is initiated by binding of gp120 to the CD4 receptor and then to CCR5 or CXCR4 co-receptor, which promotes conformational changes and leads to exposure of the gp41 hydrophobic N-terminal fusion peptide [2,48]. bnAbs against HIV-1 recognize all major exposed surfaces of the prefusion-closed Env trimer (reviewed in [49,50••]) (Figure 1e). VH1–69 has been found to be over-represented in HIV-1 CD4i antibodies [20,21]. These antibodies, exemplified by 17b and 412d, however, have no or limited neutralization by themselves (Table 1). Some VH1–69-encoded nAbs have been mapped to gp41: 4E10 and CAP206-CH12 to linear epitopes in the MPER [51–53], while HK20 and D5 to the highly conserved HR1 region [54–56] (Table 1 and Figure 1e). Recently, a CD4bs-directed antibody VRC13 originating from VH1–69 was isolated and demonstrated potent neutralizing activity [19] (Table 1 and Figure 1f).
Polymorphism of VH1–69 gene and its effect on bnAb expression
VH1–69 is one of the most polymorphic loci within the human VH gene cluster (14q32.33), exhibiting both allelic and copy number variation [57,58]. There are 17 alleles known to be associated with this gene: Ten have a phenylalanine at amino acid position 54 (Kabat numbering) in CDRH2 (F alleles) and the remaining seven have a leucine (L alleles) (Figure 2a). The VH1–69 bnAbs often originate from F alleles, mainly VH1–69*01 and VH1–69*06 (Table 1 and Figure 2), whereas weak or non-nAbs (e.g. HIV-1 antibodies 17b and CAP206-CH12 and HCV antibody AR1A) tend to be encoded by L alleles [15,20,52]. One exception is 4E10, a bnAb against HIV-1, which is encoded by the L allele VH1–69*10 [52]. The VH1–69 F/L polymorphism strongly affects serum bnAb titers to the influenza HA stem [14,59•], and the copy number also impacts the IgM VH1–69 bnAb response and overall bnAb memory B cell pool [59•].
Figure 2. VH1–69 allelic polymorphism and heavy-chain CDR sequences of representative VH1–69 antibodies.
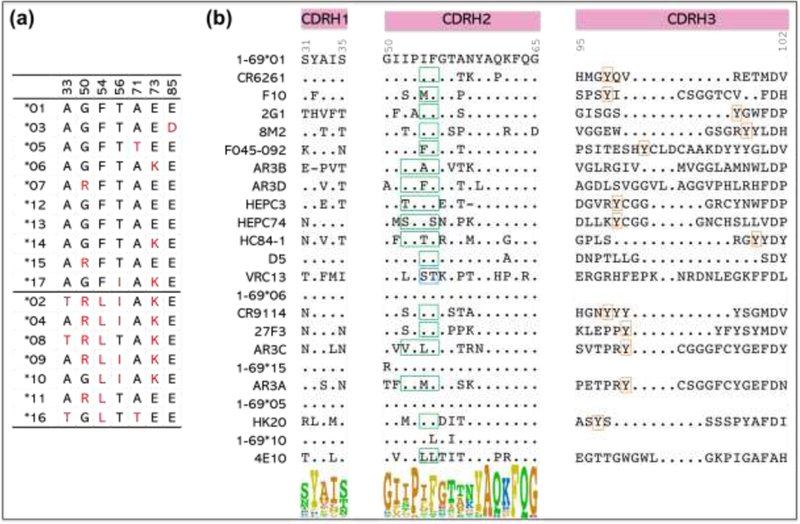
(a) Polymorphic amino-acid residues encoded by VH1–69 alleles. (b) Alignment of amino-acid sequences of the heavy-chain CDRs of representative VH1–69 antibodies to viral antigens and their germline gene alleles. The hydrophobic motif in CDRH2 tip for each antibody is boxed in green (except for VRC13, which is polar and boxed in blue), the conserved tyrosine in CDRH3 is boxed in orange. Kabat numbering is indicated. The sequence logo shows the amino-acid composition at each position in CDR H1 and H2.
Interestingly, in the general population, around 33% of individuals are homozygous for F alleles (F/F), 56% are heterozygous (F/L), and 11% are homozygous for L alleles (L/L) [12]. Such VH1–69 polymorphism appears to play a role in shaping global VH germline gene utilization [59•] and might explain in part the difference in an individual’s capacity to mount an bnAb response to viruses during infection and vaccination.
Genetic features of VH1–69 bnAbs
As noted above, one unique feature of VH1–69 bnAbs is an unusually hydrophobic CDRH2 loop that serves as a crucial anchor for interaction with conserved hydrophobic epitopes. Typically, CDRH2 contains a canonical hydrophobic residue at position 53 (isoleucine for most influenza antibodies and somatically mutated hydrophobic residues for others) and a critical phenylalanine at position 54 (Figure 2b). Some influenza VH1–69 antibodies also have a conserved tyrosine in CDRH3, which, together with the IF residues in CDRH2, represents the IFY motif that is important for binding to the HA stem (Figure 2b).
A hallmark of HIV-1 bnAbs is their extraordinary levels of somatic hypermutation (SHM) (13–32% nucleotide mutations for VH) [49]. Strikingly, most VH1–69 bnAbs (except VRC13) carry low to moderate SHM (5–16% nucleotide mutations) [12,16,17,45,46,52,60••], although not all of the mutations are required for antibody function [12,61]. This implies functionally mature VH1–69 antibodies can be generated in fewer cell replication cycles compared to the highly mutated anti-HIV antibodies. As SHM levels over 20% (nucleotide) may be difficult to achieve by vaccination [62–66], this feature of low genetic barrier in bnAb formation indicates VH1–69 as a promising gene to target for rational vaccine design.
Another feature of many human bnAbs is a long CDRH3 loop, which helps gain access to epitopes that are partially obscured, especially by surrounding glycans, or which require an extensive interacting surface with generally flat (or undulating) epitopes, even if exposed [44,60••,67,68]. Long CDRH3s are found in VH1–69 HCV AR3-class and HEPC3/74-class antibodies, influenza RBS-specific antibody F045–092, and HIV-1 antibodies 4E10 and VRC13 (17–23 amino acids, as defined by Kabat numbering) (Table 1 and Figure 2b), compared to the normal range of 9–15-residue CDRH3s in healthy blood donors [19,60••,69]. However, this long CDRH3 feature is not observed for most influenza stem-specific and many RBS-specific VH1–69 bnAbs, which are characterized by shorter CDRH3s of 11–14 residues [12,32], and for HCV HC84-class antibodies, which have even shorter CDRH3s of only 10 residues [45] (Table 1 and Figure 2b).
Structural basis for VH1–69 bnAb recognition
The binding modes for influenza stem-specific VH1–69 antibodies, such as F10, CR6261, CR9114 and 27F3, have been structurally well defined [11,27,28,29••], and revealed common binding features for this group of antibodies: (1) the majority of the buried surface and the interactions with the stem groove are mediated by the heavy chain through CDRs H1, H2, framework 3 (FR3) and H3 (for antibodies 23F3, CR6261 and CR9114, very few to no light chain interactions were observed); and (2) a conserved IFY motif (or similar MFY motif for antibody F10) arising from CDRs H2 (IF) and H3 (Y) anchors the antibody to the hydrophobic groove in the stem and provides the basis for HA stem-specific VH1–69 antibody recognition [12] (Figures 1a and 2b). Despite the conservation of heavy-chain contacts, there are significant differences in the rotation of the antibody VH domains relative to the HA stem, which may influence the potency and breadth of these antibodies [29••]. Beside the stem-specific antibodies, three RBS-specific VH1–69 antibodies, 2G1, 8M2, and F045–092, have also been structurally characterized [32,70]. These antibodies (as for antibodies encoded by other VH genes [71••]) primarily use their heavy chain CDRs (CDRH2 or CDRH3) to insert into the RBS, mimic some of the features of the sialic acid receptor, and hence block virus entry [70,72]. Like the stem-specific antibodies, they have identical IF hydrophobic residues (FF for antibody F045–092) at the tip of CDRH2 that interact with a conserved hydrophobic pocket at the base of the RBS (Figure 1b). For 8M2 and 2G1, although the heavy- and light-chain CDRs interact with the HA RBS, the recognition of the RBS is still dominated by the heavy chain, where CDRs H2 and H3 make extensive contacts with the receptor binding pocket [32]. Unlike the stem-specific antibodies, a tyrosine residue in CDRH3, Y100 for 2G1 and Y100d for 8M2, makes no interaction with the RBS. In contrast, F045–092 binds HA RBS only through the heavy-chain CDRs and the principal contacts are via CDRH3 (the hydrophobic CDRH2 makes fewer contacts with the HA); in this case, Y100b in CDRH3 makes a hydrogen bond with the RBS (Figures 1b and 2b). The long CDRH3 loop (23 amino acids) of F045–092 with an internal disulfide reaches deeper and more extensively into the RBS and leads to enhanced receptor mimicry [70] (Figure 1b). This mode of receptor mimicry is presumably the basis for the neutralization breadth of the F045–092 and other RBS-specific bnAbs [71••].
Structural studies of HCV E2 have been very challenging [36•], nevertheless, the recognition of E2 by VH1–69 antibodies have been characterized from the structures of E2 core-AR3A-D complexes, truncated E2 ectodomain-HEPC3/74 complexes, and complexes of HC84–1, HC84–27, HC84–26 and HC84–26AM (the affinity-matured HC84–26 antibody) with a linear peptide component (E2434–446) of the corresponding epitopes [44–46,47••,60••]. Similar to influenza VH1–69 antibodies, the hydrophobic CDRH2 tip of HCV AR3-class and HC84-class antibodies contains an I/V-P-X-F motif (Figure 2b) and interacts with a highly conserved hydrophobic pocket on E2. The HC84-class antibodies interacts with the E2 front layer L441 and F442, whereas the CDRH2 of the AR3-class antibodies interacts also with front layer L427 and with W529 of the CD81 binding loop (Figure 1c and d). In the CDRH2 motif, residue 53 is hydrophobic for AR3-class antibodies, but can be a polar amino acid for HC84-class antibodies. The CDRH2 tip of HEPC3/74-class antibodies is less hydrophobic and contains the T/S-P-I-F/S motif (for HEPC74, the highly conserved F54 is mutated to serine) (Figure 2b) [47••]. In addition to the hydrophobic interactions (P53 and I54 with Y443 for HEPC3 and I53 with F442 for HEPC74), their CDRH2s form a hydrogen bond with the front layer (P53 with K446 for HEPC3 and N55 with Y443 for HEPC74). Compared to HC84-class antibodies, for which both heavy and light chains interact with the linear component of the epitopes in the E2 front layer [45,46], AR3-class and HEPC3/74 class antibodies bind to a discontinuous epitope where the interactions are mediated mainly by the heavy chain CDRs [44,47••,60••], and the long CDRH3 forms multiple main-chain to main-chain hydrogen bonds with the front layer and the CD81 binding loop. This difference in the binding mode may be a consequence of differences in the CDRH3 length (17–22 residues for AR3-class and HEPC3/74-class antibodies vs 10 residues for HC84-class antibodies).
The MPER-directed HIV-1 bnAb 4E10 also has a hydrophobic CDRH2 loop that extensively interacts with a linear epitope in the highly conserved MPER of gp41 [73] (Figure 1e). Interestingly, 4E10 exhibits some polyreactivity with cardiolipin phospholipids [74], and indeed recognizes an extended epitope that includes the MPER peptide and specific lipids in the virus membrane: it contacts the lipid head groups through CDRH1, and the hydrophobic lipid tails through its CDRH3 [75]. Such lipid binding properties have been proposed to increase the local concentration of these MPER-specific antibodies in the vicinity of the epitopes for virus neutralization [76]. VH1–69 CD4i antibodies (e.g. 17b and 412d) bind to a hydrophobic surface on gp120 surrounded by basic residues that overlap with the chemokine-receptor-binding site [20,77,78]. The binding is dominated by CDRH2, which interacts with the hydrophobic center of the co-receptor binding site, and a long acidic CDRH3 (19–25 amino acids) that complements the gp120 basic residues. Indeed, CDRH3 might contain a sulfated tyrosine that mimics the co-receptor acidic N-terminus that includes tyrosine sulfation [79]. VH1–69 HR1-specific antibodies (e.g. HK20 and D5) bind a conserved hydrophobic pocket in the gp41 fusion intermediate or post-fusion conformation [54–56]. The binding is dominated by CDRH2 and a short CDRH3 (10/13 amino acids, Table 1) with minor contribution of CDRL3. Unlike all the other VH1–69 bnAbs described in this review, the CDRH2 tip of HIV-1 bnAb VRC13 is not hydrophobic but polar (S53 and T54, Figure 2b). This antibody targets the CD4bs on gp120, with the antibody interface dominated by CDRH3 [19] (Figure 1f).
Developmental pathways for VH1–69 bnAbs
Recent longitudinal analysis by next-generation sequencing (NGS) has greatly expanded our understanding of B-cell lineage development, i.e. how bnAb lineages initially arise and then evolve via SHM to acquire specific biological functions (i.e. cross-neutralization in this case). The pathways for B cells to develop breadth and potency differ depending on the epitope targeted, but involve initial V(D)J recombination and selection of B cells with a particular VH gene and/or often a long CDRH3 to generate the “precursor” or “ancestral” B cells, followed by subsequent SHM during an active immune response to antigen stimulation [50••,80–82]. To date, only a few studies have defined the developmental pathways of VH1–69 bnAb lineages targeting influenza HA stem or HCV AR3. Remarkably, these antibodies achieved breadth through a rapid lineage development, with only few mutations [12,17,60••,61]. Indeed, Lingwood et al. revealed that affinity maturation of CR6261 requires only seven (7.4%) amino acids in CDRH1 and FR3 of the germline precursor to acquire full activity [61]. Pappas et al. reported that in Y98-bearing influenza VH1–69 antibodies, a single proline to alanine mutation at position 52a in CDRH2 is sufficient to confer high affinity binding of the antibodies to the selecting H1 HA antigen [12]. More recently, we determined that some germline-encoded precursors of HCV AR3-class bnAbs bind E2 with high affinity and show strain-specific neutralization capability, although more mutations are required for cross-reactivity [60••]. These observations strengthen the hypothesis that such VH1–69 bnAbs could be easier to induce by vaccination than highly mutated bnAbs to viral antigens, and would provide broad protection against genetically variable viruses.
Viral escape from VH1–69 bnAbs
Enveloped RNA viruses such as influenza, HCV and HIV-1 exhibit a remarkable ability to adapt and evade the host immune response. Despite the impressive breadth and potency of bnAbs in vitro, these highly mutable viruses may still escape from them in cell culture and in animal models through mutations at critical sites on the target surface glycoproteins. The escape mutations can occur directly in the neutralization epitope leading to reduction in antibody binding , or indirectly outside the targeted epitope altering epitope presentation and accessibility of bnAbs for neutralization [83–88].
For influenza VH1–69 bnAbs, antibody selection of escape virus resulted in a single indirect escape mutation for CR6261 (H111L) [89] and CR9114 (V466I or R526G) [90], and four single indirect escape mutations for F10 (three in HA and one in neuraminidase) [91]. For HCV VH1–69 bnAbs, several mutations in E2 (e.g., D431E, F442L and L438F) that conferred resistence to AR3-class antibodies [88,92] or to HC11 [93] were identified in viruses with compromised viral fitness. However, escape virus was not observed for HC84-class antibodies [45,46,93] and HC-1 [93] in vitro. For HIV-1 VH1–69 bnAbs, Env mutants carrying W672G and F673L demonstrated resistant to 4E10 in vitro, although the resistance was difficult to achieve because of impaired viral infectivity [94,95].
Although escape viruses have been identified for most bnAbs, given the high conservation of the targeted neutralizing epitopes and the fitness cost of acquiring and maintaining escape mutations in the virus, a vaccine designed to target broadly neutralizing epitopes of genetically diverse viruses will likely be superior to traditional vaccine strategies.
Relevance of VH1–69 bnAbs in rational vaccine design
The discovery of the frequent occurrence of VH1–69 bnAbs against viral antigens has led to the identification of several conserved epitopes suitable for rational vaccine designs. Structural information on antibody-epitope complexes and knowledge on the development of bnAbs at the genetic level can serve to guide vaccine design approaches based on epitope or B cell ontogeny.
Epitope-based design involves identification of bnAbs, determination of structures of the epitopes, and design and engineering structural mimics of the target epitopes for immunization [50••,96]. In the case of influenza, the HA stem presents a highly conserved region for bnAbs raising optimism of developing a universal vaccine [27,71••,97,98,99•,100–102]. Several approaches have been employed to elicit bnAb responses against this domain, including design of head-stem chimeric HA, headless HA or mini-HA, and hyperglycosylated HA [71••,103–107]. For HCV, the structural and genetic information on VH1–69 bnAb-epitope complexes is now enabling immunogen design of vaccine candidates targeting the highly conserved epitopes in the neutralizing face. For HIV-1, identification of the lipid binding sites on 4E10 may aid in a MPER-targeting immunogen design that include a lipid component for eliciting bnAbs. Notably, the VH1–69 CD4i antibodies, such as E51, 412d and 17b, can enhance the association of Env with CD4bs bnAbs and, hence, improve neutralization potency of the antibody mixtures [108]. Similar synergistic neutralizing activity has also been reported in HCV bnAb mixtures[109] These data support the development of vaccines designed to elicit multiple bnAbs of complementary specificities.
B cell ontogeny-based vaccine design is based on structural information of antibody maturation to guide design of germline-targeting immunogeons to activate a specific antibody lineage [71••,110]. The VH1–69 bnAbs have been found in different individuals and share common features in gene usage, mode of recognition and developmental pathways, suggesting that appropriately designed immunogens may elicit these antibodies reproducibly in vaccinated populations. Compared to bnAbs that require high levels of SHM for maturation, the low-to-medium SHM rate for most VH1–69 bnAbs should be more readily achievable through vaccination.
Conclusion
Outstanding progress has been made over the past decade in isolation of human bnAbs against influenza, HCV and HIV-1. Among the numerous bnAbs, the VH1–69 bnAbs are of great interest. They are dominantly found in protective antibody responses to viral pathogens, and are found repeatedly at the population level. Genetic and structural characterization of these antibodies have revealed several classes of antibodies that recognize the same region in particular antigens, employ the same mode of recognition, and develop through rapid maturation pathways with low-to-medium SHM. These features make the VH1–69 bnAbs appealing candidates for rational vaccine design. Further studies aimed at designing immunogens to engage a specific B cell lineage to a highly conserved epitope such as the HA stem or HCV neutralizing face, based on the genetic and structural information of VH1–69 bnAbs summarized here, may provide a means to achieve cross-protection for broadly effective or universal vaccines for these viruses.
Acknowledgements
This work was supported in part by NIH grants AI079031 and AI123861 (to ML), AI106005 and AI123365 (to ML and IAW), and R56 AI127371 (to IAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Haynes BF, Kelsoe G, Harrison SC, Kepler TB: B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 2012, 30:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julien JP, Lee PS, Wilson IA: Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev 2012, 250:180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lorenzo C, Angus AG, Patel AH: Hepatitis C virus evasion mechanisms from neutralizing antibodies. Viruses 2011, 3:2280–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards VC, Tarr AW, Urbanowicz RA, Ball JK: The role of neutralizing antibodies in hepatitis C virus infection. J Gen Virol 2012, 93:1–19. [DOI] [PubMed] [Google Scholar]
- 5.Janiak M, Caraballo Cortes K, Demkow U, Radkowski M: Spontaneous elimination of hepatitis C virus infection. Adv Exp Med Biol 2018, 1039:45–54. [DOI] [PubMed] [Google Scholar]
- 6.Akcay IM, Katrinli S, Ozdil K, Doganay GD, Doganay L: Host genetic factors affecting hepatitis B infection outcomes: Insights from genome-wide association studies. World J Gastroenterol 2018, 24:3347–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crux NB, Elahi S: Human leukocyte antigen (HLA) and immune regulation: how do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections? Front Immunol 2017, 8:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euler Z, van Gils MJ, Boeser-Nunnink BD, Schuitemaker H, van Manen D: Genome-wide association study on the development of cross-reactive neutralizing antibodies in HIV-1 infected individuals. PLoS One 2013, 8:e54684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linnik JE, Egli A: Impact of host genetic polymorphisms on vaccine induced antibody response. Hum Vaccin Immunother 2016, 12:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry Dunand CJ, Wilson PC: Restricted, canonical, stereotyped and convergent immunoglobulin responses. Philos Trans R Soc Lond B Biol Sci 2015, 370:20140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, et al. : Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009, 16:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, et al. : Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014, 516:418–422. [DOI] [PubMed] [Google Scholar]
- 13.Joyce MG, Wheatley AK, Thomas PV, Chuang GY, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, et al. : Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 2016, 166:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheatley AK, Whittle JR, Lingwood D, Kanekiyo M, Yassine HM, Ma SS, Narpala SR, Prabhakaran MS, Matus-Nicodemos RA, Bailer RT, et al. : H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J Immunol 2015, 195:602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M: Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 2012, 109:6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merat SJ, Molenkamp R, Wagner K, Koekkoek SM, van de Berg D, Yasuda E, Bohne M, Claassen YB, Grady BP, Prins M, et al. : Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS One 2016, 11:e0165047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey JR, Flyak AI, Cohen VJ, Li H, Wasilewski LN, Snider AE, Wang S, Learn GH, Kose N, Loerinc L, et al. : Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017, 2:e92872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. : Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 2011, 333:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou T, Lynch RM, Chen L, Acharya P, Wu X, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, et al. : Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 2015, 161:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, et al. : Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A 2004, 101:2706–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams WB, Liao HX, Moody MA, Kepler TB, Alam SM, Gao F, Wiehe K, Trama AM, Jones K, Zhang R, et al. : HIV-1 Vaccines. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science 2015, 349:aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weitkamp JH, Kallewaard NL, Bowen AL, Lafleur BJ, Greenberg HB, Crowe JE Jr.: VH1–46 is the dominant immunoglobulin heavy chain gene segment in rotavirus-specific memory B cells expressing the intestinal homing receptor α4β7. J Immunol 2005, 174:3454–3460. [DOI] [PubMed] [Google Scholar]
- 23.Tian C, Luskin GK, Dischert KM, Higginbotham JN, Shepherd BE, Crowe JE Jr.: Immunodominance of the VH1–46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J Immunol 2008, 180:3279–3288. [DOI] [PubMed] [Google Scholar]
- 24.Aiyegbo MS, Eli IM, Spiller BW, Williams DR, Kim R, Lee DE, Liu T, Li S, Stewart PL, Crowe JE Jr.: Differential accessibility of a rotavirus VP6 epitope in trimers comprising type I, II, or III channels as revealed by binding of a human rotavirus VP6-specific antibody. J Virol 2014, 88:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•.Sano K, Ainai A, Suzuki T, Hasegawa H: The road to a more effective influenza vaccine: Up to date studies and future prospects. Vaccine 2017, 35:5388–5395.A recent review on influenza bnAbs and rational immunogen design for universal vaccine development.
- 26.Kashyap AK, Steel J, Oner AF, Dillon MA, Swale RE, Wall KM, Perry KJ, Faynboym A, Ilhan M, Horowitz M, et al. : Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci U S A 2008, 105:5986–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA: Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al. : Highly conserved protective epitopes on influenza B viruses. Science 2012, 337:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.••.Lang S, Xie J, Zhu X, Wu NC, Lerner RA, Wilson IA: Antibody 27F3 broadly targets influenza A group 1 and 2 hemagglutinins through a further variation in VH1–69 antibody orientation on the HA stem. Cell Rep 2017, 20:2935–2943.This study revealed the structural characteristics of influenza VH1–69 bnAb 27F3 in complex with HA stem, and described the consensus and differences in the mode of binding of HA stem-specific VH1–69 bnAbs.
- 30.Ohshima N, Iba Y, Kubota-Koketsu R, Asano Y, Okuno Y, Kurosawa Y: Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J Virol 2011, 85:11048–11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Albrecht R, Blum DL, Ramos I, Fernandez-Sesma A, Edwards KM, Garcia-Sastre A, et al. : Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J Virol 2012, 86:6334–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R, Krause JC, McBride R, Paulson JC, Crowe JE Jr., Wilson IA: A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat Struct Mol Biol 2013, 20:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouille Y, Helle F, Delgrange D, Roingeard P, Voisset C, Blanchard E, Belouzard S, McKeating J, Patel AH, Maertens G, et al. : Subcellular localization of hepatitis C virus structural proteins in a cell culture system that efficiently replicates the virus. J Virol 2006, 80:2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindenbach BD, Rice CM: The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol 2013, 11:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feneant L, Levy S, Cocquerel L: CD81 and hepatitis C virus (HCV) infection. Viruses 2014, 6:535–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Tzarum N, Wilson IA, Law M: The neutralizing face of hepatitis C virus E2 envelope glycoprotein. Front Immunol 2018, 9:1315.A recent review on structural analysis of the neutralizing sites in HCV E2 neutralizing face, providing up-to-date information for structure-based HCV vaccine design.
- 37.Bruni R, Costantino A, Tritarelli E, Marcantonio C, Ciccozzi M, Rapicetta M, El Sawaf G, Giuliani A, Ciccaglione AR: A computational approach identifies two regions of hepatitis C virus E1 protein as interacting domains involved in viral fusion process. BMC Struct Biol 2009, 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moustafa RI, Haddad JG, Linna L, Hanoulle X, Descamps V, Mesalam AA, Baumert TF, Duverlie G, Meuleman P, Dubuisson J, et al. : Functional study of the C-terminal part of hepatitis C virus E1 ectodomain. J Virol 2018, 92:e00939–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.•.Kinchen VJ, Cox AL, Bailey JR: Can broadly neutralizing monoclonal antibodies lead to a hepatitis C virus vaccine? Trends Microbiol 2018, 26:854–864.A recent summary of HCV bnAbs and their targeted epitopes.
- 40.Chan CH, Hadlock KG, Foung SK, Levy S: VH1–69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood 2001, 97:1023–1026. [DOI] [PubMed] [Google Scholar]
- 41.Keck ZY, Li TK, Xia J, Gal-Tanamy M, Olson O, Li SH, Patel AH, Ball JK, Lemon SM, Foung SK: Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol 2008, 82:6061–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keck ZY, Xia J, Wang Y, Wang W, Krey T, Prentoe J, Carlsen T, Li AY, Patel AH, Lemon SM, et al. : Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 2012, 8:e1002653. [DOI] [PMC free article] [PubMed]
- 43.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, et al. : Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 2008, 14:25–27. [DOI] [PubMed] [Google Scholar]
- 44.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, et al. : Hepatitis C virus E2 envelope glycoprotein core structure. Science 2013, 342:1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krey T, Meola A, Keck ZY, Damier-Piolle L, Foung SK, Rey FA: Structural basis of HCV neutralization by human monoclonal antibodies resistant to viral neutralization escape. PLoS Pathog 2013, 9:e1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keck ZY, Wang Y, Lau P, Lund G, Rangarajan S, Fauvelle C, Liao GC, Holtsberg FW, Warfield KL, Aman MJ, et al. : Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice. Hepatology 2016, 64:1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.••.Flyak AI, Ruiz S, Colbert MD, Luong T, Crowe JE Jr., Bailey JR, Bjorkman PJ: HCV broadly neutralizing antibodies use a CDRH3 disulfide motif to recognize an E2 glycoprotein site that can be targeted for vaccine design. Cell Host Microbe 2018, 24:703–716 e703.This article presents structures of HCV VH1–69 bnAbs HEPC3 and HEPC74 in complex with a truncated E2 ectodomain, revealing common genetic features and a mode of antigen recognition by a germline-encoded CDRH3 disulfide motif.
- 48.Chien MP, Jiang S, Chang DK: The function of coreceptor as a basis for the kinetic dissection of HIV type 1 envelope protein-mediated cell fusion. FASEB J 2008, 22:1179–1192. [DOI] [PubMed] [Google Scholar]
- 49.Kwong PD, Mascola JR: Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 2012, 37:412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.••.Kwong PD, Mascola JR: HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 2018, 48:855–871.A comprehensive review on HIV-1 bnAb recognition mechanisms and antibody-to-vaccine design strategies.
- 51.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, et al. : Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 2001, 75:10892–10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, et al. : Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One 2011, 6:e23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardoso RM, Brunel FM, Ferguson S, Zwick M, Burton DR, Dawson PE, Wilson IA: Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. J Mol Biol 2007, 365:1533–1544. [DOI] [PubMed] [Google Scholar]
- 54.Miller MD, Geleziunas R, Bianchi E, Lennard S, Hrin R, Zhang H, Lu M, An Z, Ingallinella P, Finotto M, et al. : A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc Natl Acad Sci U S A 2005, 102:14759–14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luftig MA, Mattu M, Di Giovine P, Geleziunas R, Hrin R, Barbato G, Bianchi E, Miller MD, Pessi A, Carfi A: Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol 2006, 13:740–747. [DOI] [PubMed] [Google Scholar]
- 56.Sabin C, Corti D, Buzon V, Seaman MS, Lutje Hulsik D, Hinz A, Vanzetta F, Agatic G, Silacci C, Mainetti L, et al. : Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41. PLoS Pathog 2010, 6:e1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson CT, Steinberg KM, Huddleston J, Warren RL, Malig M, Schein J, Willsey AJ, Joy JB, Scott JK, Graves TA, et al. : Complete haplotype sequence of the human immunoglobulin heavy-chain variable, diversity, and joining genes and characterization of allelic and copy-number variation. Am J Hum Genet 2013, 92:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lefranc MP: Immunoglobulins: 25 years of immunoinformatics and IMGT-ontology. Biomolecules 2014, 4:1102–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.•.Avnir Y, Watson CT, Glanville J, Peterson EC, Tallarico AS, Bennett AS, Qin K, Fu Y, Huang CY, Beigel JH, et al. : IGHV1–69 polymorphism modulates anti-influenza antibody repertoires, correlates with IGHV utilization shifts and varies by ethnicity. Sci Rep 2016, 6:20842.A comprehensive study of the influence of VH1–69 polymorphism on anti-influenza antibody repertoires at the individual and population level.
- 60.••.Tzarum N, Giang E, Kong L, He L, Prentoe J, Augestad E, Hua Y, Castillo S, Lauer G, Bukh J, et al. : Genetic and structural insights into broad neutralization of hepatitis C virus by human VH1–69 antibodies. Sci Adv 2019, 5:eaav1882.This article investigates the molecular requirements for development of VH1–69 AR3-class anti-HCV bnAbs and suggests that these antibodies can achieve broad neutralization with rapid lineage development.
- 61.Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, Wei CJ, Nabel GJ: Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 2012, 489:566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. : H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 2011, 6:e25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. : Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012, 109:9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scherer EM, Smith RA, Simonich CA, Niyonzima N, Carter JJ, Galloway DA: Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog 2014, 10:e1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schramm CA, Douek DC: Beyond hot spots: biases in antibody somatic hypermutation and implications for vaccine design. Front Immunol 2018, 9:1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poulsen TR, Jensen A, Haurum JS, Andersen PS: Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol 2011, 187:4229–4235. [DOI] [PubMed] [Google Scholar]
- 67.Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA: Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci U S A 2010, 107:11483–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, et al. : Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol 2013, 20:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H: Autoreactivity in human IgG+ memory B cells. Immunity 2007, 26:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee PS, Ohshima N, Stanfield RL, Yu W, Iba Y, Okuno Y, Kurosawa Y, Wilson IA: Receptor mimicry by antibody F045–092 facilitates universal binding to the H3 subtype of influenza virus. Nat Commun 2014, 5:3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.••.Wu NC, Wilson IA: Structural insights into the design of novel anti-influenza therapies. Nat Struct Mol Biol 2018, 25:115–121.A timely review on structural characterization of influenza HA bnAbs, and implications for structure-based design of novel vaccines and therapeutics.
- 72.Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, Dormitzer PR, Haynes BF, Walter EB, Moody MA, et al. : Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci U S A 2011, 108:14216–14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA: Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 2005, 22:163–173. [DOI] [PubMed] [Google Scholar]
- 74.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, et al. : The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol 2007, 178:4424–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irimia A, Sarkar A, Stanfield RL, Wilson IA: Crystallographic identification of lipid as an integral component of the epitope of HIV broadly neutralizing antibody 4E10. Immunity 2016, 44:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, et al. : Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A 2009, 106:20234–20239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA: Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393:648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwong PD, Wyatt R, Majeed S, Robinson J, Sweet RW, Sodroski J, Hendrickson WA: Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure 2000, 8:1329–1339. [DOI] [PubMed] [Google Scholar]
- 79.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H: Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 1999, 96:667–676. [DOI] [PubMed] [Google Scholar]
- 80.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. : Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013, 496:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. : Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014, 509:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacLeod DT, Choi NM, Briney B, Garces F, Ver LS, Landais E, Murrell B, Wrin T, Kilembe W, Liang CH, et al. : Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high-mannose patch. Immunity 2016, 44:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. : Antibody neutralization and escape by HIV-1. Nature 2003, 422:307–312. [DOI] [PubMed] [Google Scholar]
- 84.Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, et al. : Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 2009, 5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chai N, Swem LR, Reichelt M, Chen-Harris H, Luis E, Park S, Fouts A, Lupardus P, Wu TD, Li O, et al. : Two escape mechanisms of influenza A virus to a broadly neutralizing stalk-binding antibody. PLoS Pathog 2016, 12:e1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu NC, Wilson IA: A perspective on the structural and functional constraints for immune evasion: insights from influenza virus. J Mol Biol 2017, 429:2694–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keck ML, Wrensch F, Pierce BG, Baumert TF, Foung SKH: Mapping determinants of virus neutralization and viral escape for rational design of a hepatitis C virus vaccine. Front Immunol 2018, 9:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, et al. : Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 2008, 3:e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson CS, Ortega S, Chaves FA, Clark AM, Yang H, Topham DJ, DeDiego ML: Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci Rep 2017, 7:14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prachanronarong KL, Canale AS, Liu P, Somasundaran M, Hou S, Poh YP, Han T, Zhu Q, Renzette N, Zeldovich KB, et al. : Mutations in influenza A virus neuraminidase and hemagglutinin confer resistance against a broadly neutralizing hemagglutinin stem antibody. J Virol 2019, 93: e01639–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey JR, Wasilewski LN, Snider AE, El-Diwany R, Osburn WO, Keck Z, Foung SK, Ray SC: Naturally selected hepatitis C virus polymorphisms confer broad neutralizing antibody resistance. J Clin Invest 2015, 125:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Velazquez-Moctezuma R, Galli A, Law M, Bukh J, Prentoe J: Hepatitis C virus escape studies for human antibody AR3A reveals a high barrier to resistance and novel insights on viral antibody evasion mechanisms. J Virol 2018. [DOI] [PMC free article] [PubMed]
- 93.Keck ZY, Saha A, Xia J, Wang Y, Lau P, Krey T, Rey FA, Foung SK: Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J Virol 2011, 85:10451–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakowitsch S, Quendler H, Fekete H, Kunert R, Katinger H, Stiegler G: HIV-1 mutants escaping neutralization by the human antibodies 2F5, 2G12, and 4E10: in vitro experiments versus clinical studies. AIDS 2005, 19:1957–1966. [DOI] [PubMed] [Google Scholar]
- 95.Manrique A, Rusert P, Joos B, Fischer M, Kuster H, Leemann C, Niederost B, Weber R, Stiegler G, Katinger H, et al. : In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J Virol 2007, 81:8793–8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, et al. : Proof of principle for epitope-focused vaccine design. Nature 2014, 507:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krammer F, Palese P, Steel J: Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol 2015, 386:301–321. [DOI] [PubMed] [Google Scholar]
- 98.Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS: The pathway to a universal influenza vaccine. Immunity 2017, 47:599–603. [DOI] [PubMed] [Google Scholar]
- 99.•.Coughlan L, Palese P: Overcoming barriers in the path to a universal influenza virus vaccine. Cell Host Microbe 2018, 24:18–24.A thoughtful review on challenges and strategies to universal influenza vaccine development.
- 100.Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS: A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018, 218:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fox A, Quinn KM, Subbarao K: Extending the breadth of influenza vaccines: status and prospects for a universal vaccine. Drugs 2018, 78:1297–1308. [DOI] [PubMed] [Google Scholar]
- 102.Zhao C, Xu J: Toward universal influenza virus vaccines: from natural infection to vaccination strategy. Curr Opin Immunol 2018, 53:1–6. [DOI] [PubMed] [Google Scholar]
- 103.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P: Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 2012, 86:5774–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krammer F, Pica N, Hai R, Margine I, Palese P: Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 2013, 87:6542–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, Garcia-Sastre A, et al. : Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 2013, 87:10435–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. : A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349:1301–1306. [DOI] [PubMed] [Google Scholar]
- 107.Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, et al. : Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 2015, 21:1065–1070. [DOI] [PubMed] [Google Scholar]
- 108.Gardner MR, Fellinger CH, Prasad NR, Zhou AS, Kondur HR, Joshi VR, Quinlan BD, Farzan M: CD4-induced antibodies promote association of the HIV-1 envelope glycoprotein with CD4-binding site antibodies. J Virol 2016, 90:7822–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.•.Mankowski MC, Kinchen VJ, Wasilewski LN, Flyak AI, Ray SC, Crowe JE Jr., Bailey JR: Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc Natl Acad Sci U S A 2018, 115:E82–E91.Demonstration of synergistic neutralization breadth and potency of some HCV neutralizing antibody combinations. One combination of two VH1–69 bnAbs, HEPC74/HEPC98, displayed exceptionally potent, supporting vaccine design to elicit multiple neutralizing antibodies with complementary specificities.
- 110.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, et al. : Rational HIV immunogen design to target specific germline B cell receptors. Science 2013, 340:711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]


