SUMMARY
Obesity is a major modifiable risk factor for pancreatic ductal adenocarcinoma (PDAC), yet how and when obesity contributes to PDAC progression is not well understood. Leveraging an autochthonous mouse model, we demonstrate a causal and reversible role for obesity in early PDAC progression, showing that obesity markedly enhances tumorigenesis, while genetic or dietary induction of weight loss intercepts cancer development. Molecular analyses of human and murine samples define microenvironmental consequences of obesity that foster tumorigenesis rather than new driver gene mutations, including significant pancreatic islet cell adaptation in obesity-associated tumors. Specifically, we identify aberrant beta cell expression of the peptide hormone cholecystokinin (Cck) in response to obesity and show that islet Cck promotes oncogenic Kras-driven pancreatic ductal tumorigenesis. Our studies argue that PDAC progression is driven by local obesity-associated changes in the tumor microenvironment and implicate endocrine-exocrine signaling beyond insulin in PDAC development.
Keywords: Pancreatic cancer, obesity, cholecystokinin, pancreatic islets, beta cells, tumor microenvironment, genetically engineered mouse models, leptin
Graphical Abstract

In Brief
Obesity is an intrinsic driver of PDAC in mice, leading to a remodeling of beta cells to increase CCK secretion, and playing a role early in pancreatic cancer development that can be intercepted by weight loss.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer death in the United States and is expected to become the second within the next few years (Rahib et al., 2014; Siegel et al., 2020). Despite the development of combination chemotherapy, targeted small molecules, and immunotherapies that have revolutionized cancer care, long-term survival in PDAC remains low at ~10% (Ryan et al., 2014; Siegel et al., 2020). In search of drug targets, genomic studies have identified frequent mutations in the proto-oncogene KRAS, occurring in >90% of human PDAC tumors (Bailey et al., 2016; TCGA Research Network, 2017). Unfortunately, no effective direct KRAS inhibitors are currently approved for clinical use (Papke and Der, 2017). Moreover, our recent work demonstrated that PDAC cells can survive genetic ablation of KRAS both in vitro and in vivo (Chen et al., 2018; Muzumdar et al., 2017), arguing that resistance will subvert even the very best KRAS inhibitors. Therefore, alternative paradigms beyond genetic factors need to be explored to develop novel therapeutic and preventative strategies for PDAC.
Epidemiologic studies in prospective human cohorts have shown that body-mass index (BMI), a surrogate measure of obesity, is associated with increased PDAC risk, more advanced disease at diagnosis, and worse survival (Larsson et al., 2007; Yuan et al., 2013). Yet, how obesity contributes to PDAC development remains poorly understood. Previous research showed that high-fat diet (HFD)-induced obesity promotes tumor progression in transplant and autochthonous models of PDAC (Incio et al., 2016; Khasawneh et al., 2009; Philip et al., 2013; Chang et al., 2017; Zaytouni et al., 2017; Zyromski et al., 2009), and implicated dysregulation of fatty acid and nitrogen metabolism (Khasawneh et al., 2009; Zaytouni et al., 2017), COX2 activation (Philip et al., 2013), and aberrations in the desmoplastic response (Incio et al., 2016; Khasawneh et al., 2009; Chang et al., 2017) as potential mechanisms. These studies were limited by slow obesity onset in HFD models, as well as variations in fat content, types of fat, and age of administration in HFD protocols, all of which can confound results (Speakman, 2019). Importantly, these approaches provided no information on whether – and at what point – weight loss or other anti-obesity interventions might influence PDAC progression, despite significant clinical implications (Massetti et al., 2017).
To overcome these important limitations, we developed a rapid and reversible autochthonous model of obesity-associated PDAC. We demonstrate that obesity plays a causal – and reversible – role in early PDAC progression. We further identify aberrant expression of the hormone cholecystokinin (Cck) in islet beta cells in tumors as an adaptive response to obesity and show that islet Cck overexpression itself promotes pancreatic ductal tumorigenesis. Together, these studies link obesity, changes in the local microenvironment, and tumorigenesis through a previously unappreciated endocrine-exocrine signaling axis in PDAC development.
RESULTS
Obesity drives pancreatic tumorigenesis in mice
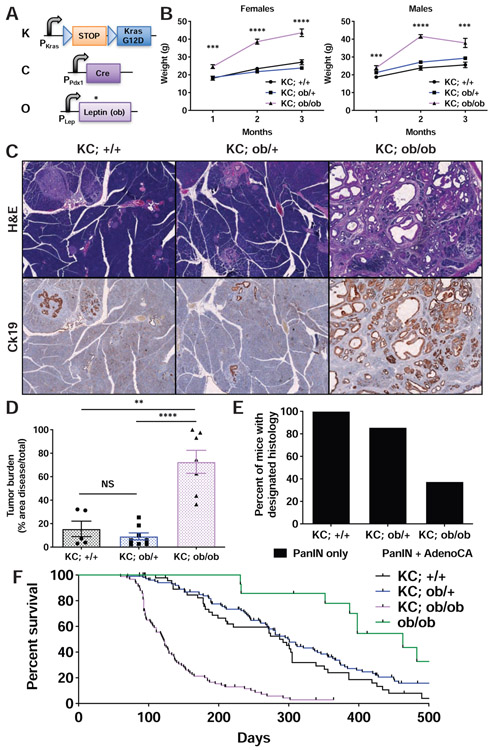
To model obesity-associated pancreatic cancer, we crossed leptin-deficient (ob/ob) mice with KC (Pdx1-Cre; KrasLSL-G12D/WT) mice predisposed to the development of precursor pancreatic intraepithelial neoplasia (PanINs) (Hingorani et al., 2003) to yield KC; ob/ob (KCO) mice (Figure 1A). Unlike HFD models, ob/ob mice exhibit early onset obesity due to impaired appetite suppression and decreased metabolism (Friedman, 2019). KCO mice were obese and developed increased primary ductal tumor burden (Figures 1B-D). They also showed enhanced progression to adenocarcinoma and markedly shortened survival compared to non-obese KC mice (Figures 1E-F). We similarly observed decreased survival using a distinct obesity model due to leptin receptor mutation (db/db) (Figures S1A-B). These phenotypes were significantly more severe than seen in HFD models (Khasawneh et al., 2009; Philip et al., 2013; Chang et al., 2017), corresponding to the degree of obesity. Consistent with human epidemiologic studies that demonstrate an association between obesity and pancreatic cancer, but not lung cancer (Lauby-Secretan et al., 2016), obesity induced by neither HFD nor ob/ob genotype shortened survival in lung cancer initiated by oncogenic Kras (Figures S1C-F). Thus, these results support a specific effect of obesity on Kras-driven PDAC progression.
Figure 1. Accelerated PDAC progression in KCO mice.
A) Schematic of transgenic/knock-in alleles in leptin-deficient KC mice (KC; ob/ob or KCO). Black arrows denote promoters. Kras and Lep promoters are endogenous. Blue triangles denote LoxP sites. A STOP cassette prevents oncogenic Kras (G12D) expression prior to Cre-mediated recombination. * denotes point mutation in ob gene causing premature stop.
B) Body weight (mean +/− s.e.m.) of KC mice of varying ob genotype over time (n=22-46 mice/group). ***p<0.001, ****p<0.0001, KC; ob/ob compared to KC; +/+ mice, two-tailed student’s t-test at each time point.
C) Representative histologic sections of pancreata from mice of designated genotypes at 3 months of age demonstrated differences in Ck19+ ductal tumor burden. Scale bar: 200 μm.
D) Tumor burden (mean +/− s.e.m.) in mice of designated genotypes at 3 months of age (n=5-8 mice/group). **p<0.01, ****p<0.0001, two-tailed student’s t-test. NS = non-significant.
E) Percentage of mice in (D) harboring PanINs and/or adenocarcinoma.
F) Kaplan-Meier survival curves for mice of designated genotypes (n=14-106 mice/group). Log-rank test: p<0.0001 KC; +/+ vs. KC; ob/ob, p<0.0001 KC; ob/+ vs. KC; ob/ob, p>0.05 KC; +/+ vs. KC; ob/+ See also Figure S1.
Weight loss intercepts early tumor progression in KCO mice
We chose the ob/ob model because it affords rapid reversal of the obesity phenotype through leptin restoration to study the effects of weight loss on PDAC development. We generated an adeno-associated viruses (AAV) that directs sustained leptin secretion (AAV-Leptin) following a single intramuscular injection (Figures 2A and S2A-B). AAV-Leptin reversed multiple phenotypes of leptin deficiency including obesity, hyperglycemia, and infertility (Figures 2B and S2C-D), as seen with leptin protein replacement (Friedman, 2019). Remarkably, early AAV-Leptin administration impeded tumor progression proportional to the degree of weight loss (Figures 2C-D). In contrast, late AAV-Leptin administration (following advanced tumor development) induced weight loss without impacting survival (Figures S2E-F). Moreover, leptin induced on-target downstream signaling in advanced murine PDAC cells in vitro but did not alter cell viability (Figures S2G-H). These data support a stage-specific effect of obesity on early PDAC development. Experiments with caloric restriction argue that it is weight loss itself that impacts tumor progression – rather than leptin signaling. Young KCO mice subject to caloric restriction exhibited significantly reduced tumor burden compared to mice fed ad libitum (Figures 2E-F), demonstrating the potential to halt or delay obesity-driven PDAC progression with weight loss.
Figure 2. Weight loss intercepts early tumor progression in KCO mice.
A) Schematic of AAV vectors administered to KCO mice. ITR = internal tandem repeat for AAV2. CAGGS = CMV enhancer chicken beta-actin promoter. GFP = green fluorescent protein.
B) Schematic of AAV treatment for tumor interception experiment. AAV-GFP-treated mice served as controls. AAV was administered at 6 weeks of age prior to development of significant tumor burden. Mice were analyzed 6 weeks later. Percent change in body weight (mean +/− s.e.m., n=8-10 mice/group) following AAV administration is shown.
C) Representative histologic sections of pancreata of mice at endpoint in (B) demonstrated a reduction in Ck19+ ductal tumors with AAV-Leptin. Quantification of tumor burden (mean +/− s.e.m., n=8-10 mice/group) is shown.
D) Greater weight loss is associated with less tumor burden in mice treated with AAV-Leptin. Each point represents one mouse (n=10). Correlation coefficient (R) and p-value from simple linear regression are shown.
E) Schematic for caloric restriction (CR) experiment. Mice began CR at 6 weeks of age as in (B). Controls remained on ad libitum diet (AL). Percent change in body weight (mean +/− s.e.m., n=6 mice/group) following intervention is shown.
F) Representative histologic sections of pancreata of mice at endpoint in (E) demonstrated a reduction in Ck19+ ductal tumors with CR. Quantification of tumor burden (mean +/− s.e.m., n=6 mice/group) is shown.
p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-tailed student’s t-test for all pairwise comparisons. Scale bars: 200 μm. See also Figure S2.
Obesity promotes PDAC progression independent of new mutations
We next sought to identify the mechanisms by which obesity contributes to pancreatic tumorigenesis in this model. We postulated that obesity might induce DNA damage in the setting of enhanced oxidative stress (Usman and Volpi, 2018) and that tumor progression in KCO mice would depend on additional driver gene mutations beyond oncogenic Kras. Precedent for this hypothesis is provided by acceleration of oncogenic Kras-induced pancreatic tumor development in mice with inactivating mutations in Trp53, Cdkn2a, or Smad4 (Bardeesy et al., 2006a; Bardeesy et al., 2006b; Hingorani et al., 2005; Muzumdar et al., 2016) – the hallmark recurrently mutated tumor suppressor genes in human PDAC (Bailey et al., 2016; TCGA Research Network, 2017)). In further support, survival of KCO mice was comparable to what we previously observed (Muzumdar et al., 2016) in KPC mice (Pdx1-Cre; KrasLSL-G12D/WT; p53flox/WTor Pdx1-Cre; KrasLSL-G12D/WT; p53LSL-R172H/WT) harboring heterozygous mutations inp53 (p=0.93, log-rank test). Surprisingly, however, immunohistochemistry (IHC) revealed persistent tumor suppressor protein expression, and exome sequencing confirmed the absence of inactivating point mutations in the associated genes (Figure 3 and Table S1). Furthermore, we did not observe recurrent pathogenic mutations in other known PDAC (Bailey et al., 2016; TCGA Research Network, 2017) or pan-cancer driver genes (Bailey et al., 2018) in KCO tumors (Tables S2-S3). Contrary to our expectations, these data argue that additional driver gene mutations are not required for obesity to promote pancreatic cancer development. To determine if a similar phenomenon is evident in human tumors, we performed targeted exome sequencing and IHC to evaluate alterations of KRAS, TP53, CDKN2A/p16, and SMAD4 in 184 human PDAC with known patient BMI at diagnosis (Table S4). Although we observed a significant increase in SMAD4 loss with elevated BMI, the total number of driver gene alterations per tumor, which we have previously shown predicts worse outcomes in patients with localized PDAC (Qian et al., 2018), did not significantly differ across categories of BMI (Table S5). Together, these data suggest that obesity may promote pancreatic tumorigenesis independent of new driver mutations.
Figure 3. Obesity promotes tumor progression independent of new driver mutations.
A) Tumor suppressor proteins frequently mutated in human PDAC were retained in 8/8 (100%) KCO tumors analyzed by IHC. Scale bar: 50 μm.
B) Exome and mRNA sequencing did not reveal new single nucleotide variants in tumor suppressor genes in KCO tumors. Average mutant allele fraction identified for each base in the coding sequence is shown. A single KPC tumor sequenced in parallel shows the presence of the expected R172H codon change. See also Tables S1-S5.
Tumors in obese mice show evidence of increased inflammation and fibrosis
To elucidate potential non-mutational mechanisms of PDAC progression, we performed bulk RNA sequencing (RNA-seq) on pancreata from obese KCO and non-obese KC and KPC mice exhibiting comparable tumor burden (Table S6). Using Independent Component Analysis (ICA), an unsupervised blind source separation technique (Hyvarinen and Oja, 2000; Muzumdar et al., 2017), we identified gene expression signatures associated with inflammation and fibrosis as upregulated in KCO compared to KC/KPC mice (Figures 4A-D and Tables S6), likely representing obesity-associated alterations in the tumor microenvironment. We confirmed abundant extracellular matrix deposition and immune cell infiltration by histologic analyses of KCO tumors (Figure 4E). To determine human relevance, we ranked primary human PDAC tumors from The Cancer Genome Atlas (TCGA) (TCGA Research Network, 2017) based on gene expression correlation to these KCO signatures. We classified tumors using previously-defined molecular subtypes (Bailey et al., 2016) and found the KCO-correlated tumors to be significantly enriched with the immunogenic subtype (Figure 4F), which is similarly associated with immune cell infiltration (Bailey et al., 2016; TCGA Research Network, 2017). These data revealed that alterations in the fibroinflammatory microenvironment are a major feature of obesity-associated pancreatic tumors and are consistent with prior work using HFD models (Incio et al., 2016; Chang et al., 2017).
Figure 4. Enhanced inflammation and fibrosis in KCO tumors.
A) Heat map of row normalized Z-scores (of mixing weights from ICA decomposition) for gene expression signatures that separate tumors from obese (KCO) and non-obese (KC and KPC) models. See Methods for details. Rows represent individual tumors. Red corresponds to positive and blue to negative Z-scores.
B) Network representation of overlapping enriched GSEA/MSigDB curated (C2) gene sets associated with the KCO signatures in (A). Cellular processes associated with related gene sets are listed.
C) GSEA using the curated gene set collection (C2 in MSigDB) revealed an enrichment of genes involved in immune cell activation/signaling and extracellular matrix/fibrosis in KCO tumors See Table S6 for complete list.
D) GSEA showed an enrichment of inflammation and fibrosis gene sets (hallmark gene set collection (H in MSigDB)) with KCO tumors.
E) Histologic analyses revealed extensive fibrosis (Sirius red staining of collagen) and Cd45+ immune cell infiltration predominantly with F4/80+ macrophages and sparse presence of Cd3+ T and B220+ B cells. Scale bars: 100 μm
F) Box and whisker plots (boxes denote 25th-75th percentile, error bars denote min/max) of standardized signature scores for TCGA tumors corresponding to each molecular subtype (Bailey et al., 2016) are shown. See Methods for details. p-values confirmed significant enrichment of TCGA tumors highly-correlated to KCO signatures with the immunogenic subtype (hypergeometric test). See also Figure S3 and Table S6.
Consequently, we tested the effects of anti-inflammatory (aspirin) and anti-fibrotic (metformin) (Chen et al., 2017; Rangarajan et al., 2018) drugs on tumor progression in KCO mice. We chose these specific agents because they have been shown to mitigate pancreatic cancer risk in selected (Li et al., 2009; Zhang et al., 2015), but not all (Khalaf et al., 2018), patient cohorts and are in widespread clinical use for other indications. Remarkably, neither aspirin nor metformin impacted tumor progression, immune cell infiltration, or fibrosis in KCO mice (Figure S3), indicating that these agents may have a limited role in preventing obesity-driven PDAC. Furthermore, these data left open the possibility that the obesity-associated changes in the fibroinflammatory microenvironment could be a consequence rather than a cause of pancreatic tumorigenesis. Therefore, we sought alternative mechanisms that may contribute to PDAC development in obesity.
Tumors in obese mice exhibit marked pancreatic islet adaptation
One of the most notable RNA-seq results was upregulation of genes associated with pancreatic islet cell function in KCO compared to KC/KPC pancreata (Figures 5A). Although expression levels of general neuroendocrine markers were mostly unchanged in KCO pancreata (Figure 5B), we observed a marked increase in the expression of genes encoding islet prohormones, proteases that process them, and secretory granule proteins (Figure 5C). We confirmed gene expression data by IHC on islets in tumor-bearing mice. While glucagon (Gcg) and its proteolytic product GLP-1 are typically expressed in peripherally-located alpha cells in non-obese rodents (Barreto et al., 2010), KCO mice displayed an expansion of Gcg and GLP-1 production throughout islets (Figure 5D). These observations are consistent with prior research demonstrating increased GLP-1 secretion from islets of obese humans and mice (Linnemann et al., 2015). Together, our results support islet adaptation to enhance hormone production, processing, and secretion in the setting of obesity.
Figure 5. Pancreatic islet adaptation in KCO tumors.
A) GSEA using the curated (C2 in MSigDB) and hallmark (H in MSigDB) gene set collections revealed an association between KCO tumors and genes expressed in pancreatic beta cells. See Table S6 for complete list.
B) Relative gene expression (mean +/− s.e.m. normalized RNA-seq expression counts with non-obese KC/KPC tumors as baseline) for general neuroendocrine markers observed in pancreatic islet cells showed mild to no significant difference between KCO (n=15) and KC/KPC (n=17) tumors. **p<0.01, two-tailed Mann-Whitney test, comparing KCO to KC/KPC. NS = non-significant.
C) Relative gene expression (mean +/− s.e.m. normalized RNA-seq expression counts with KC/KPC tumors as baseline) of islet genes in tumors from KCO mice compared to non-obese models is shown. * p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-tailed Mann-Whitney test, comparing KCO to KC/KPC expression for each gene.
D) IHC showed aberrant glucagon and GLP-1 expression throughout pancreatic islets in KCO mice compared to non-obese controls, consistent with upregulation of Gcg and Pcsk1/Pcsk2 observed by RNA-seq in (C). Scale bar: 50 μm. See also Figure S4 and Table S6.
Given these findings, we postulated that enhanced local insulin secretion may promote pancreatic tumorigenesis in obese mice. Although ob/ob mice exhibited systemic hyperinsulinemia (Figure S4A), glucose-stimulated insulin secretion by primary islets derived from ob/ob mice was reduced (Figures S4B-D), consistent with severely impaired local insulin secretion. Furthermore, analysis of insulin receptor phosphorylation suggested comparable signaling activation in tumor cells from both KC and KCO mice (Figure S4E). These data argue against local increases in insulin or insulin signaling as a primary driver of pancreatic tumorigenesis. We tested this hypothesis further by treating KCO mice with the sodium-glucose co-transporter type 2 (SGLT2) inhibitor dapagliflozin, which blocks kidney reabsorption of glucose and attenuates obesity-associated hyperglycemia. Slc5a2 (SGLT2) knockout in db/db mice improves islet function and maintenance by reducing beta cell glucotoxicity (Jurczak et al., 2011; Jurczak et al., 2018). Similarly, treatment with dapagliflozin reduced serum glucose and maintained higher circulating insulin and proinsulin (C-peptide) levels in KCO mice (Figures S4F-G), indicating preserved beta cell function. The variable effect of dapagliflozin on the maintenance of insulin production across KCO mice (Figure S4G) allowed us to quantitatively compare the relationship between insulin and tumor development in this model. We anticipated that if insulin was a main driver of tumorigenesis, tumor burden would be enhanced proportional to insulin production. Contrary to this hypothesis, C-peptide levels inversely correlated with tumor burden (Figure S4H), suggesting that alternative changes in islet hormone expression may play a greater role in tumor development.
Single cell RNA-sequencing identifies beta cell expression of Cck in obesity
To decipher islet hormone expression at higher resolution, we performed single-cell RNA sequencing (scRNA-seq) on islets isolated from congenic C57/B6 wild-type and ob/ob mice. Visualization using PHATE (Moon et al., 2019), a method for dimensionality reduction of single-cell data, displayed clusters corresponding to single hormone-expressing beta (insulin), alpha (glucagon), delta (somatostatin), and PP (Ppy) cells (Figure 6A). To characterize the differences between cells captured from ob/ob and wild-type islets, we used the newly developed MELD package (Burkhardt et al., 2019), which derives a likelihood score, called the Enhanced Experimental Signal (EES), for each cell to annotate regions of the cellular manifold that are enriched in the ob/ob or wild-type genotype. Examining the distribution of EES values within islet cell types, we observed only moderate overlap in beta cell populations from ob/ob and wild-type mice (Figure 6B), consistent with significant changes in gene expression with obesity. Moreover, beta cells exhibited higher mean EES values, indicating enrichment in ob/ob islets with a commensurate reduction in alpha, delta, and PP cells (Figure 6C). Beta cells from ob/ob mice showed increased expression of genes involved in protein translation, secretion, and endoplasmic reticulum (ER) stress. (Figure 6D and Table S7). These genes encoded hormones and secretory granule proteins also upregulated in KCO mice (Figure 6E), confirming results from bulk tumor RNA-seq.
Figure 6. scRNA-seq identifies beta cell expression of Cck in obesity.
A) PHATE visualization plots for single hormone-expressing islet clusters identified Ins1/Ins2+ beta cells, Gcg+ alpha cells, Sst+ delta cells, and Ppy+ PP cells amongst all sequenced cells.
B) PHATE plot colored by the Enhanced Experimental Signal (EES) values from MELD (Burkhardt et al., 2019) indicated the likelihood of observing each transcriptional profile in ob/ob (red) and wild-type (WT, blue).
C) The distribution of EES values in the clusters in (A) showed only moderate overlap in beta and alpha clusters between genotypes. Gray dots in each column mark the mean EES value in each cluster. Single cells color-coded by genotype showed a relative increase in beta cells in ob/ob islets (mean EES>0) and a proportional decrease in alpha, delta, and PP cells (mean EES<0).
D) Upregulated genes comparing ob/ob with WT beta cells showed significant overlap (hypergeometric test) with gene sets associated with protein translation and secretion.
E) Mean single cell expression counts (square-root transformed library size-normalized UMI/cell +/− s.d.) for beta cells showed upregulation of hormones and secretory granule genes in ob/ob islets. Each colored dot represents a single cell. Gray circles represent median expression.
F) PHATE visualization shows Cck expression exclusively in beta cells. Cck is expressed in ob/ob, but not WT, islets as shown in (E).
G) Upregulated genes comparing Cck+ versus Cck- ob/ob beta cells showed significant overlap (hypergeometric test) with gene sets associated with protein translation and secretion.
H) Gene-gene expression plots after MAGIC imputation (van Dijk et al., 2018) showed an inverse relationship between the expression of Ins1, Ins2, Slc30a8, and Insig1 with Cck in ob/ob beta cells. Spearman correlation coefficients (R) are listed for each plot. See also Figures S5-S6 and Table S7.
The top upregulated gene (by fold change) in ob/ob islets was the hormone cholecystokinin (Cck) (Figure 6E and Table S7). Cck is normally synthesized and secreted by enteroendocrine cells of the duodenum to induce pancreatic acinar cell zymogen release for digestion. It is dysregulated in the islets of obese mice (HFD and ob/ob) and has been reported to promote islet cell survival in the setting of insulin resistance, toxins, or other stresses (Lavine et al., 2015; Lavine et al., 2010). We confirmed islet Cck expression by IHC in ob/ob mice (Figures S5A). We further observed Cck expression exclusively in a subset of beta cells (Figure 6F), those of which exhibited enhanced expression of protein synthesis and secretory granule genes than their Cck- counterparts (Figure 6G and Table S7). Finally, Cck expression was inversely correlated with the expression of insulin (Ins1 and Ins2), Slc30a8 (a zinc transporter involved in insulin granule maturation), and the insulin-response gene Insig1 (Figure 6H), arguing that Cck – rather than insulin – secretion may predominate in these cells.
Local islet Cck signaling drives pancreatic ductal tumorigenesis
Since exogenous administration of cerulein, a Cck analogue, promotes Kras-driven pancreatic tumorigenesis in mice (Guerra et al., 2007), we hypothesized that, in the setting of obesity, endogenous islet-derived Cck could drive PDAC development. Systemic Cck levels were not increased in obese mice (Figure S5B), arguing that islet Cck acts locally. Cck expression was specifically observed in islets from obese models and markedly reduced by weight loss interventions (Figures 7A-B and S5C-E), consistent with an adaptive response to obesity. We further analyzed human PDAC and observed islet expression of CCK in ~60% of samples (Figures 7C-D). Although CCK expression did not correlate with BMI (Figure 7D), cancer-associated weight loss prior to diagnosis may confound this analysis. Therefore, we procured islets from human donors without known malignancy and observed a positive relationship between BMI and CCK expression (Figure 7E). Together, these data demonstrate that obesity is associated with islet Cck expression in both mice and humans.
Figure 7. Islet-derived Cck promotes pancreatic ductal cancer development.
A) Relative gene expression (mean +/− s.e.m. normalized RNA-seq expression counts with KC tumors as baseline) of Cck in obese (KCO and KCO mice treated with AAV-GFP) and non-obese models (KC, KPC, and KCO mice treated with AAV-Leptin) is shown (n=5-9 per group). **p<0.01, ***p<0.001, two-tailed Mann-Whitney test.
B) Cck was aberrantly overexpressed in pancreatic islets of KCO compared to KC and KPC mice. Scale bar: 100 μm.
C) CCK IHC on human PDAC tissues demonstrated expression specifically in islets (arrows). Scale bar: 100 μm.
D) The majority of human PDAC specimens displayed islet CCK expression by IHC at all BMI levels at diagnosis.
E) CCK expression in isolated pancreatic islets from human donors with high BMI (n=53, above median US BMI of 28.2 kg/m2) was significantly (*p<0.05, two-tailed Mann-Whitney test) greater than from low BMI donors (n=55, below median). Box and whisker plots (boxes denote 25th-75th percentile, error bars denote min/max) are shown.
F) Representative histologic sections of pancreata demonstrated an increase in Ck19+ ductal tumorigenesis in MKC (MIP-Cck; KC) mice compared to KC mice. Quantification of tumor burden (mean +/− s.e.m., n=5-6 mice/group) at 3 months of age is shown. *p<0.05, two-tailed student’s t-test. Scale bar: 200 μm.
G) Model for obesity-associated islet adaptations in PDAC development. Brown denotes Cck expression. ADM = acinar-to-ductal metaplasia. TF = transcription factor. See also Figures S3 and S7.
To test directly whether islet Cck promotes tumor progression, we utilized MIP-Cck transgenic mice, which exhibit beta cell-specific Cck expression comparable to ob/ob mice (Lavine et al., 2015). Remarkably, non-obese compound transgenic mice (MKC:MIP-Cck; Pdx1-Cre; KrasLSL-G12D/WT) displayed a significant increase in tumor burden compared to KC controls (Figure 7F), supporting the hypothesis that islet Cck overexpression can function as an independent driver of pancreatic ductal tumorigenesis. Prior work has shown that the competitive Cck receptor (Cckr) antagonist proglumide, at doses that block physiologic Cck function, impedes tumor progression in non-obese KC mice (Smith et al., 2014). Since obesity-augmented islet Cck expression appears to function as a local driver of early PDAC progression, we anticipated that proglumide treatment should be insufficient to impair progression in the setting of supraphysiologic local concentrations of Cck in KCO mice. Indeed, although proglumide induced the expected systemic pharmacodynamic effect of slowing weight gain, no significant impact on tumor development was observed (Figure S3). Thus, these data provide evidence for the importance of aberrantly-expressed pancreatic endocrine hormones beyond insulin in driving obesity-associated PDAC.
Beta cell proliferation and transcription factor signatures correlate with Cck expression
We next sought to explore the mechanisms of islet Cck induction in vivo. We hypothesized that islet stress from obesity might stimulate Cck expression to promote beta cell survival (Lavine et al., 2015). Consistent with this hypothesis, islet Cck expression was blunted in dapagliflozin-treated mice in which beta cell function was preserved (Figure S4I). We further compared the transcriptional profiles of single Cck+ and Cck− beta cells derived from ob/ob mice. Since islet expansion is a consequence of obesity (Linnemann et al., 2014) and beta cell division has been associated with polyhormone expression (Bocian-Sobkowska et al., 1999), we suspected that beta cell proliferation could act as a stimulus for Cck expression. Indeed, we observed a relative upregulation of cell cycle genes in Cck+ beta cells compared to their Cck-counterparts and an increased proportion of polyhormonal Ins2+/Gcg+ cells expressing Cck compared to monohormonal insulin-expressing (Ins1+/Ins2+) cells (Figures S6A-C). Recent work has suggested that islet resident macrophages may induce beta cell proliferation in the context of HFD-induced obesity via PDGF signaling (Ying et al., 2019). To evaluate for this in ob/ob mice, we examined Ptprc+ (Cd45+) immune cells in our islet scRNA-seq data, the majority of which expressed macrophage markers (Cd68 and Cd74) (Figures S6D-E). Islet macrophages in ob/ob mice exhibited upregulation of genes associated with proliferation and downregulation of genes associated with cytokine signaling and inflammatory responses (Figures S6F-I), mirroring HFD-induced changes (Ying et al., 2019). These macrophages also upregulated beta cell growth factors including Pdgfb (Figure S6J), implicating obesity-associated changes in islet macrophages as a potential instigator of beta cell proliferation in ob/ob mice.
To further define molecular mechanisms that contribute to islet Cck expression, we examined beta cell expression of transcription factors, given their role in regulating hormone expression during islet development. Prior work has implicated Tle3/Grg3 in the suppression of Gcg expression in beta cells as islets develop (Metzger et al., 2014). We similarly observed decreased expression of Tle3/Grg3 and another transcriptional co-repressor Ehf as the top significantly downregulated genes in Cck+ compared to Cck- cells (Figure S6K and Table S7). Furthermore, both Tle3/Grg3 and Ehf expression inversely correlated with Cck expression in ob/ob beta cells (Figure S6L). Together, these data support a role for beta cell proliferation and alterations in transcription factor expression as potential drivers of islet Cck expression in response to obesity.
Endocrine-exocrine Cck signaling in obesity
Finally, we evaluated how Cck functions in the local microenvironment to drive tumor development. While exogenous cerulein administration is commonly used as a model for experimental pancreatitis (Guerra et al., 2007), our data support a pro-tumorigenic effect of islet Cck independent of inflammation. MIP-Cck mice did not exhibit overt evidence of inflammation or fibrosis (Figure S7A), even up to one year of age (Lavine et al., 2015). Conversely, it is conceivable that local inflammation, induced by obesity, could promote islet expression of Cck. Arguing against this hypothesis, acute (cerulein- or arginine-induced) or chronic (cerulein-induced) pancreatitis did not elicit islet Cck expression in mice (Figures S7B-C). Similarly, CCK expression was not significantly observed in islets of human patients with chronic pancreatitis of varying etiologies (Figure S7D).
PDAC is commonly thought to arise from acinar or ductal cells (Kopp et al., 2012; Ray et al., 2011), suggesting that the exocrine pancreas is the target of islet Cck. Alternatively, Cck has been shown to stimulate insulin secretion from beta cells in mice (Lo et al., 2011), which in turn could modulate early tumorigenesis. Contrary to this latter hypothesis, Cck receptor (Cckar or Cckbr) transcripts were detectable in <0.05% of beta cells (compared to 12% of Prss2+/Cela1+ acinar cells) profiled from wild-type and ob/ob mice by scRNA-seq at our depth of sequencing. Furthermore, MIP-Cck mice do not exhibit increased insulin release during fasting or following glucose stimulation (Lavine et al., 2015), and ob/ob islets were impaired in glucose-stimulated insulin secretion (Figures S4B-D). Thus, we conclude that endocrine-derived Cck likely acts on acinar cells to drive tumorigenesis.
Evidence supports a direct effect of Cck on acinar cells in rodent models to induce zymogen secretion and proliferation (Kanemitsu et al., 2006; Logsdon, 1986). Although CCKR expression in human acinar cells is lower than observed in rodents, recent studies argue for a similar direct effect of CCK on intracellular signaling and zymogen release in primary human acinar cells (Liang et al., 2017; Murphy et al., 2008). Cck is thought to promote tumor formation through proliferative acinar cell regeneration and/or acinar cell reprogramming leading to acinar-to-ductal metaplasia (ADM), a prerequisite step in acinar cell-derived PDAC development (Kopp et al., 2012; Storz, 2017). Cerulein is capable of directly stimulating ADM in acinar explant cultures (Ardito et al., 2012). Furthermore, we observed an increase in Ki67+ acinar cells in MKC mice compared to KC controls (Figure S7E), consistent with enhanced islet Cck-induced acinar cell proliferation. Together, these data support a model in which obesity-induced islet Cck expression cooperates with oncogenic Kras to drive tumorigenesis via acinar cell proliferation and ductal transformation (Figure 7G).
DISCUSSION
In this study, we demonstrate that obesity plays a causal and reversible role in early PDAC progression in mice. Molecular analyses link obesity to changes in the local microenvironment and tumorigenesis via adaptive endocrine-exocrine signaling by pro-tumorigenic hormones. Hormones have long been postulated as contributing to obesity-associated cancers with principal mechanisms including dysregulation of 1) islet-derived insulin or insulin-like growth factor 1 (IGF1); 2) adipocyte secreted factors (adipokines) including leptin and adiponectin; and 3) steroid sex hormones (Murphy et al., 2018; Ulrich et al., 2018). Of these mechanisms, epidemiologic studies support a role for both insulin/IGF1 and adipokines in PDAC development, as elevated levels of insulin, proinsulin, and leptin and low levels of IGF binding protein-1 and adiponectin are associated with increased PDAC risk (Babic et al., 2016; Bao et al., 2013a; Bao et al., 2013b; Wolpin et al., 2013; Wolpin et al., 2007). Though previous studies suggested that leptin can promote PDAC proliferation (Harbuzariu et al., 2017; Mendonsa et al., 2015), our observation of accelerated tumorigenesis in obese mice deficient in leptin signaling (Figures 1 and S1A-B) supports a leptin-independent mechanism for obesity in tumor progression. Furthermore, the capacity for caloric restriction to intercept progression in KCO mice (Figures 2E-F) is consistent with obesity, rather than dysregulation of leptin signaling or the associated physiologic response to starvation (Friedman, 2019), as a driver of PDAC development.
Apart from insulin (Wang et al., 2018; Zhang et al., 2019), in vivo evidence for a pro-tumorigenic role of pancreatic islet hormones is limited. Our study identifies obesity-associated aberrant islet Cck expression as a cancer driver through a local effect in the pancreas. Ultrastructural and perfusion studies endorse the existence of an islet portal system perfusing exocrine cells (the islet-acinar axis) (Barreto et al., 2010; Williams and Goldfine, 1985). Alternatively, capillary leak or neovascularization in the setting of islet cell expansion and local tissue injury from early developing tumors may alter blood flow and permit high concentrations of islet-derived Cck to access exocrine cells. The significance of obesity-driven islet adaptations is highlighted by the observation that Cck+ beta cells show diminished expression of genes involved in insulin secretion, including insulin itself (Figure 6H), consistent with major alterations in the secretome. We further demonstrate that CCK is expressed in islets in human PDAC (Figure 7C) and that islet CCK expression correlates with BMI (Figure 7E), supporting the possibility that a similar phenomenon occurs in human PDAC development. Our findings add to the growing body of evidence that obesity may be pro-tumorigenic through its microenvironmental rather than systemic consequences (Olson et al., 2017). Although evidence supporting a role for Cck signaling in PDAC progression exists (Nadella et al., 2018; Smith et al., 2014; Smith and Solomon, 2014), prior studies focused on intestinal-derived Cck, the physiologic source of systemic Cck. On the contrary, our work highlights a local source of Cck – induced by obesity within the pancreas itself – and offers the first example of endocrine-exocrine signaling beyond insulin in PDAC development.
Our results also demonstrate that weight loss impedes the elaboration of pro-tumorigenic hormonal adaptations – including islet Cck expression – and early PDAC progression. These data are consistent with human cohort studies showing that bariatric surgery reduces cancer incidence, including for pancreatic cancer (Schauer et al., 2019; Xu et al., 2018). In addition to weight loss, we explored alternative mechanisms that may influence islet adaptation. Specifically, we identified beta cell proliferation and changes in the expression of transcription factors known to govern islet hormone expression as highly associated with islet Cck expression (Figure S6). We suspect that islet glucotoxicity (Weir, 2020) may induce Cck expression as an adaptive survival response, given the capacity of Cck to maintain islet cell survival in the setting of stress (Lavine et al., 2015). Accordingly, our data show that glucose lowering and the resultant preservation of beta cell function by SGLT2 inhibition are associated with reduced tumor progression and islet Cck expression (Figures S4F-I). Nonetheless, the precise mechanisms by which obesity induces these islet adaptations, including Cck elaboration, remain to be fully elucidated. Future studies towards this aim may offer additional strategies beyond weight loss for PDAC prevention.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Mandar Deepak Muzumdar (mandar.muzumdar@yale.edu). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals studies
Animal studies were approved by the Massachusetts Institute of Technology (MIT) and Yale University Institutional Animal Care and Use Committees (IACUC). ob (Stock #000632), db (Stock #000697), Pdx1-Cre (Stock #014647), p53fl/fl (Stock #008462), MADM11-GT (Stock #013749), MADM11-TG (Stock #013751), and C57BL/6J (Stock #000664) mice were obtained from the Jackson Laboratory. MIP-Cck mice (Lavine et al., 2015) were obtained from D. B. Davis of the University of Wisconsin. KrasLSL-G12D, p53R172H/WT, p53KO/WT, and KrasLA2 (a “hit-and-run” allele that results in spontaneous activation of oncogenic Kras) mice were generated previously by the Jacks lab (Jacks et al., 1994; Jackson et al., 2001; Johnson et al., 2001; Olive et al., 2004). Pdx1-Cre; KrasLSL-G12D/WT(KC), KC; ob/+ and KC; ob/ob (KCO), KC; db/+, KC; db/db, KrasLA2; +/+, and KrasLA2; ob/ob were produced by intercrossing mice with the appropriate alleles. KPC mice, including KC; p53R172H/WT; KC; p53fl/WT, KC; p53R172H/fl, KC; p53fl/fl, and KC; MADM11-GT,p53WT/MADM11-TG-p53KO (MADM-p53), were generated as described previously (Muzumdar et al., 2016). Mice were genotyped by PCR using template tail DNA isolated by the HotShot method and GoTaq Green Mastermix (Promega). Genotyping primers and protocols have been previously reported (Ellett et al., 2009; Lavine et al., 2015; Muzumdar et al., 2016) or are available online (http://www.jax.org).
Adult animals of both genders were used in all experiments except for islet isolation studies in which only males were used. Specific developmental age of mice used is listed in figure legends. Mice were housed in a specific-pathogen free facility, kept at room temperature with standard day-night cycles, and maintained on a mixed background unless otherwise specified. Animals were monitored at least weekly for signs of morbidity and were euthanized by CO2 asphyxiation when they met euthanasia criteria. Date of euthanasia relative to birth date was used for Kaplan-Meier survival analyses.
For diet studies, KrasLA2 mice were started on high-fat (60% kcal fat, Research Diets 12492) or low-fat (10% kcal fat, Research Diets 12240J) diets at 4 weeks of age. For caloric restriction, mice were fed 1 gram of normal chow per mouse per day, as previously described (Skowronski et al., 2017). For drug studies, KCO mice were treated with pharmaceutical-grade aspirin (2 mg/mL, Spectrum Chemical), metformin (2 mg/mL, Spectrum Chemical), proglumide (0.1 mg/mL, Sigma), or dapagliflozin (0.02 mg/mL, MedChem Express) in drinking water starting at 6 weeks of age and treated for 6 weeks continuously. Water consumption was measured weekly when treated water was replaced. KCO mice drank 7.69 +/− 0.58 (s.d.), 8.95 +/− 2.24, 8.09 +/− 3.21, 9.70 +/− 1.49, 8.97 +/− 3.98 mL drinking water daily for untreated (control), aspirin, metformin, proglumide, and dapagliflozin, respectively. Water intake was not statistically different from control for each treatment condition (two-tailed student’s t-test).
Based on water consumption, average daily doses were 17.9, 16.2, 0.97, and 0.18 mg for aspirin, metformin, proglumide, and dapagliflozin, respectively, leading to an average daily dose exposure of ~456, 436, 26 mg/kg, and 4.7 mg/kg, in-line with prior studies using these agents (Chandel et al., 2016; Saponaro et al., 2019; Smith et al., 2014). Mice were randomized following pre-stratification for gender in all treatment conditions. Weights were monitored using a standard small animal scale. Glucose was measured on a OneTouch Ultra2 glucometer on whole blood collected by tail clip. For serum studies, whole blood was collected from anesthetized mice by retro-orbital bleed or terminal cardiac puncture, allowed to clot for 30 minutes at room temperature, and spun through a serum separator tube (Sarstedt) at 10,000 rpm for 5 min prior to storage at −80°C or analysis. Serum Cck was measured by EIA (RayBiotech, Cat# EIAM-CCK). Serum insulin (RIA, Linco Cat# RI-I3K) and C-peptide (ELISA, Alpco Diagnostics Cat# 80-CPTMS-E01) were measured by the Yale Diabetes Research Core.
Cell line derivation, culture, and treatments
Murine KPC PDAC (mPDAC) cell lines (7307 and 7310) were isolated from primary autochthonous pancreatic tumors arising in female C57/B6 KC; p53R172H/WT mice (Hingorani et al., 2005). Tumors were enzymatically dissociated using a mix of collagenase IV, dispase, and DNAse I (Worthington) at 37°C for 30 minutes with further mechanical dissociation using a gentleMACS dissociator (Miltenyi Biotec). Cell suspensions were passed through a 100 μM filter, washed, and plated in complete media containing DMEM (Corning), 10% FBS (Thermo Fisher Scientific), and penicillin/streptomycin (Thermo Fisher Scientific). Cells were grown in standard culture conditions (37°C; 5% CO2) for at least five passages to separate tumor cells from tumor-associated fibroblasts. For signaling studies, cells were grown in serum-free conditions overnight, treated with 100 ng/mL recombinant murine leptin (R&D Systems, Cat# 498-OB) in serum-free media, and lysed for protein analysis after 20 minutes (peak induction of downstream signaling). Viability was assessed 72 hours after treatment using the CellTiter Glo (CTG) assay (Promega).
To generate leptin receptor knockout clones, a single guide RNA (sgRNA) sequence (5’-TGAAAGCCACCAGACCTCGA) targeting exon 8 of the murine leptin receptor (LepR) was ligated into the BsmBI site in lentiGuide-Puro (Addgene 52963) with compatible annealed oligos to generate lentiGuide-Puro-sgLepR. Lentivirus for lentiGuide-Puro-sgLepR and lentiCas9-blast (Addgene 52962) were produced by co-transfection of 293T cells (grown in the same culture media and conditions as mPDAC cells above) with plasmids containing the lentiviral backbone, packaging vector (psPAX2), and envelope (VSV-G) using TransIT-LT1 (Mirus Bio). Supernatant was collected at 48 and 72 hours and applied to target cells with 8 μg/mL polybrene (EMD Millipore) for transduction. Transduced cells were treated with 10 μg/mL blasticidin S (Thermo Fisher Scientific) and 2 μg/mL puromycin (Life Technologies) for 7 days. Cells were then sorted into single cells by limiting dilution into 96-well plates to isolate clones. Knockout clones were confirmed by genomic DNA extraction using QuickExtract DNA extraction solution (Epicentre), PCR amplification of the target locus (forward primer: 5’-GGTTCTCAGTGCACGCATTT-3 ’; reverse primer: 5 ’-ACAACGATTTTCCTGGCATCT-3’) with Q5 polymerase (NEB), and Sanger sequencing of PCR products by the Keck Biotechnology Resource Laboratory at Yale. KO1 clone harbored two frameshift mutant alleles: a 32 bp deletion and an 18 bp deletion with a 2 bp insertion (effective 16 bp deletion). KO2 clone also harbored two independent frameshift mutant alleles: a 1 bp deletion and a 13 bp deletion.
Human biospecimen acquisition
Acquisition of resected PDAC biospecimens including institutional review board approval and informed consent procedures were previously described (Qian et al., 2018). 108 human islets of northern European descent were isolated at the Oxford Consortium for Islet Transplantation (OXCIT, Oxford, UK) as previously described (Cross et al., 2012). All studies were approved by the University of Oxford’s “Oxford Tropical Research Ethics Committee” (OxTREC Reference: 2-15), or the Oxfordshire Regional Ethics Committee B (REC reference: 09/H0605/2). All organ donors provided informed consent for use of pancreatic tissue in research. Human chronic pancreatitis (etiologies included pancreatic divisum (n=5), Sphincter of Oddi dysfunction (n=2), idiopathic (n=5)) specimens were obtained from 12 adults (ages 25-60, M:F 4:8) undergoing Total Pancreatectomy and Islet Auto-Transplant (TP-IAT) program at the University of Minnesota Medical Center. Pancreatic biopsies were obtained at the time of surgery and processed for routine histologic analyses. Informed consent was obtained from all patients, and the research protocol was reviewed and approved by the University of Minnesota Institutional Review Board.
METHOD DETAILS
Mouse tissue preparation and histology
Mice were euthanized by CO2 asphyxiation and tissue was dissected, fixed in 10% neutral-buffered formalin overnight, and dehydrated in 70% ethanol prior to paraffin embedding. Adjacent 5-μm sections were cut and stained with hematoxylin and eosin or picosirius red or subject to immunohistochemistry (IHC). Primary antibodies for IHC are listed in the Key Resource Table. Mach 2 HRP-labeled micro-polymers (Biocare Medical) were used for primary antibody detection using a Thermo Scientific Autostainer 360. Slides were imaged with a modified Nikon T2R inverted microscope (MVI), 4x/10x/20x/40x objectives, and a 2.8 MP CoolSNAP Dyno CCD camera (Photometrics). Monochromatic red, green, and blue images were merged using ImageJ software (NIH).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |||
|---|---|---|---|---|---|
| Antibodies | |||||
| Rat anti-B220 | BD Biosciences | Cat# 550286; RRID:AB_393581 | |||
| Rabbit anti-beta tubulin | Cell Signaling Technology | Cat# 2128; RRID:AB_823664 | |||
| Rabbit anti-Cd3 | Abcam | Cat# ab16669; RRID:AB_443425 | |||
| Rabbit anti-Cd45 (LCA) | Abcam | Cat# ab10558; RRID:AB_442810 | |||
| Rabbit anti-Cdkn2a | Abcam | Cat# ab54210; RRID:AB_881819 | |||
| Rabbit anti-cholecystokinin | Immunostar | Cat# 20078; RRID:AB_572224 | |||
| Rabbit anti-Ck19 | Abcam | Cat# ab133496; RRID:AB_11155282 | |||
| Mouse anti-Erk1/2 | Cell Signaling Technology | Cat# 9107; RRID:AB_10695739 | |||
| Rabbit anti-F4/80 | Thermo Fisher Scientific | Cat# MA5-16363; RRID:AB_2537882 | |||
| Rabbit anti-GFP | Cell Signaling Technology | Cat# 2956; RRID:AB_1196615 | |||
| Mouse anti-Hsp90 | BD Biosciences | Cat# 610418; RRID:AB_397798 | |||
| Guinea pig anti-Insulin | Accurate Chemical | Cat# BMAT5014 | |||
| Rabbit anti-Leptin (OB) | Santa Cruz Biotechnology | Cat# sc-842; RRID:AB_2136071 | |||
| Rabbit anti-p53 | Novacastra | Cat# NCL-p53-CM5p; RRID:AB_563933 | |||
| Rabbit anti-pErk1/2 (T202/Y204) | Cell Signaling Technology | Cat# 4370; RRID:AB_2315112 | |||
| Rabbit anti-pInsR/Igf1R (Y1162/Y1163) | Thermo Fisher Scientific | Cat# 44-804G; RRID:AB_2533762 | |||
| Rabbit anti-pStat3 (Y705) | Cell Signaling Technology | Cat# 9145; RRID:AB_249100 | |||
| Rabbit anti-Smad4 | Abcam | Cat# ab40759; RRID:AB_777980 | |||
| Mouse anti-Stat3 | Cell Signaling Technology | Cat# 9139; RRID:AB_331757 | |||
| Rabbit anti-Synaptophysin | Thermo Fisher Scientific | Cat# RB-1461; RRID:AB_60081 | |||
| Anti-mouse IgG (H+L) (DyLight™ 680 Conjugate) | Cell Signaling Technology | Cat# 5470, RRID:AB_10696895 | |||
| Anti-rabbit IgG (H+L) (DyLight™ 800 4X PEG Conjugate) | Cell Signaling Technology | Cat# 5151, RRID:AB_10697505 | |||
| Peroxidase AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson Immunoresearch | Cat# 115-035-146, RRID:AB_2307392 | |||
| Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L) | Jackson Immunoresearch | Cat# 111-035-144, RRID:AB_2307391 | |||
| Mach2 Rabbit HRP-Polymer | Biocare Medical | Cat# RHRP520 | |||
| Mach2 Mouse HRP-Polymer | Biocare Medical | Cat# MHRP520 | |||
| Rabbit anti-Ki67 (SP6) | Biocare Medical | Cat# CRM 325, RRID: AB_2721189 | |||
| Rabbit anti-alpha-smooth muscle actin | Thermo Fisher Scientific | Cat# PA1-37024, RRID: AB_2223029 | |||
| Peroxidase AffiniPure Donkey anti-guinea pig | Jackson Immunoresearch | Cat# 706-035-148, RRID: AB_2340447 | |||
| Bacterial and Virus Strains | |||||
| One Shot Stbl3 chemically-competent E. coli | Thermo Fisher Scientific | Cat# C737303 | |||
| AAV2/1CAGmLeptin | Custom-made AAV vector generated by the University of Iowa Viral Vector Core | N/A | |||
| AAV2/1CAGeGFP | University of Iowa Viral Vector Core | Cat# VVC-U of Iowa-640 | |||
| Biological Samples | |||||
| Human pancreatitis biospecimens | University of Minnesota TP-IAT program | N/A | |||
| Human donor islets | Oxford Consortium for Islet Transplantation (OXCIT) | N/A | |||
| Human pancreatic cancer biospecimens | Dana-Farber Cancer Institute, University of Texas MD Anderson Cancer Center, University of Rochester, Stanford University | N/A | |||
| Chemicals, Peptides, and Recombinant Proteins | |||||
| Recombinant mouse leptin protein | R&D Systems | Cat# 498-OB | |||
| Aspirin USP | Spectrum Chemical | Cat# AS130 | |||
| Metformin Hydrochloride, BP | Spectrum Chemical | Cat# M1566 | |||
| Proglumide sodium salt | Sigma-Aldrich | Cat# M006 | |||
| High-fat diet (60% kcal fat) | Research Diets, Inc. | Cat# D12492 | |||
| Low-fat diet (10% kcal fat) | Research Diets, Inc. | Cat# 12240J | |||
| DNAse I | Worthington Biochemical | Cat# D2 | |||
| Collagenase IV | Worthington Biochemical | Cat# CLS-4 | |||
| Dispase | Worthington Biochemical | Cat# LS02100 | |||
| TransIT LT1 | Mirus Bio | Cat# MIR-2304 | |||
| QuickExtract DNA extraction solution | Epicentre | Cat# QE0905 | |||
| Puromycin dihydrochloride | Thermo Fisher Scientific | Cat# A1113803 | |||
| Blasticidin S | Thermo Fisher Scientific | Cat# A1113903 | |||
| Polybrene | EMD Millipore | Cat# TR-1003-G | |||
| Primestar HS | Takara | Cat# R040A | |||
| RIPA lysis and extraction buffer | Thermo Fisher Scientific | Cat# 89900 | |||
| Halt protease/phosphatase inhibitor cocktail | Thermo Fisher Scientific | Cat# 78440 | |||
| Cerulein | Sigma-Aldrich | Cat# C9026 | |||
| Dapagliflozin | MedChemExpress | Cat# HY-10450 | |||
| DMEM | Corning | Cat# 10-013-CV | |||
| DMEM | Sigma-Aldrich | Cat# D5030 | |||
| Fetal Bovine Serum | Gibco/Thermo Fisher Scientific | Cat# 26140-079 | |||
| Penicillin- Streptomycin | Gibco/Thermo Fisher Scientific | Cat# 15140-122 | |||
| HEPES | Gibco/Thermo Fisher Scientific | Cat# 15630-080 | |||
| L-glutamine | Gibco/Thermo Fisher Scientific | Cat# 25030-081 | |||
| Q5 High-Fidelity 2X Master Mix | New England Biolabs | Cat# M0492S | |||
| Odyssey Blocking Buffer (PBS) | LI-COR Biosciences | Cat# 927-40000 | |||
| Western Lightning Plus ECL | Perkin Elmer | Cat# NEL103001 | |||
| L-arginine monohydrochloride | Sigma-Aldrich | Cat# A5131 | |||
| GoTaq Green Master Mix | Promega | Cat# M7123 | |||
| Critical Commercial Assays | |||||
| Mouse Leptin ELISA Kit | Crystal Chem | Cat# 90030, RRID:AB_2722664 | |||
| Mouse CCK EIA Kit | RayBiotech | Cat# EIAM-CCK | |||
| Insulin RIA | Linco | Cat# RI-I3K | |||
| Mouse C-peptide ELISA Kit | Alpco Diagnostics | Cat# 80-CPTMS-E01, RRID: AB_2801468 | |||
| CellTiter-Glo Luminescent Cell Viability Assay | Promega | Cat# G7570 | |||
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific | Cat# 4368814 | |||
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat# 23227 | |||
| Allprep DNA/RNA Mini Kit | Qiagen | Cat# 80204 | |||
| Quant-iT™ PicoGreen™ dsDNA Assay Kit | Thermo Fisher Scientific | Cat# P7589 | |||
| SureSelectXT Mouse All Exon Kit | Agilent | Cat# 5190-4642 | |||
| Mouse High Range Insulin ELISA Kit | Alpco Diagnostics | Cat# 80-INSMSH-E01 | |||
| MACS Dead Cell Removal Kit | Miltenyi Biotec | Cat# 130-090-101 | |||
| Chromium Single Cell 3 ’ Reagent Kit v3 | 10X Genomics | Cat# PN-1000092 | |||
| Deposited Data | |||||
| Mouse pancreatic tumor RNA-seq data | Generated in this study and deposited in NCBI GEO | Acc# GSE131714 | |||
| Mouse islet single cell RNA-seq data | Generated in this study and deposited in NCBI GEO | Acc# GSE137236 | |||
| Mouse pancreatic tumor Exome-seq data | Generated in this study and deposited in NCBI SRA | Acc# PRJNA544740 | |||
| Human pancreatic ductal adenocarcinoma (PAAD) RNA-seq data | The Cancer Genome Atlas (https://tcga-data.nci.nih.gov/tcga); TCGA. Cancer Cell, 2017. | N/A | |||
| Experimental Models: Cell Lines | |||||
| KPC-7307 | Derived in this study from a C57/B6 KC; p53R172H/WT mice | N/A | |||
| KPC-7310 | Derived in this study from a C57/B6 KC; p53R172H/WT mice | N/A | |||
| 293T | ATCC | Cat# CRL-3216, RRID:CVCL_0063 | |||
| Experimental Models: Organisms/Strains | |||||
| Mouse: ob: B6.Cg-Lepob/J | Jackson Laboratories | IMSR Cat# JAX:000632, RRID:IMSR_JAX:000632 | |||
| Mouse: db: B6.BKS(D)-Leprdb | Jackson Laboratories | IMSR Cat# JAX:000697, RRID:IMSR_JAX:000697 | |||
| Mouse: Pdx1-Cre: B6.FVB-Tg(Pdx1-cre)6Tuv/J | Jackson Laboratories | IMSR Cat# JAX:014647, RRID:IMSR_JAX:014647 | |||
| Mouse: p53flox: B6.129P2- Trp53tm1Brn/J | Jackson Laboratories | IMSR Cat# JAX:008462, RRID:IMSR_JAX:008462 | |||
| Mouse: MADM11-GT: Igs2tm1(ACTB-EGFP,-tdTomato)Luo/J | Jackson Laboratories | IMSR Cat# JAX:013749, RRID:IMSR_JAX:013749 | |||
| Mouse: MADM11-TG: Igs2tm2(ACTB-tdTomato,-EGFP)Luo/J | Jackson Laboratories | IMSR Cat# JAX:013751, RRID:IMSR_JAX:013751 | |||
| Mouse: MIP-CCK: B6-Tg(Ins1- CCK)Davis | Gift from D.B. Davis (University of Wisconsin) | N/A | |||
| Mouse: KrasLSL-G12D: B6.129S4- Krastm4Tyj/J | Tyler Jacks lab (MIT) | IMSR Cat# JAX:008179, RRID:IMSR_JAX:008179 | |||
| Mouse: p53KO/WT:129-Trp53tm1Tyj/J | Tyler Jacks lab (MIT) | IMSR Cat# JAX:002080, RRID:IMSR_JAX:002080 | |||
| Mouse: p53R172H/WT: 129S-Trp53tm2Tyj/J | Tyler Jacks lab (MIT) | IMSR Cat# JAX:008652, RRID:IMSR_JAX:008652 | |||
| Mouse: KrasLA2:129S/Sv- Krastm3Tyj/J | Tyler Jacks lab (MIT) | IMSR Cat# JAX:008185, RRID:IMSR_JAX:008185 | |||
| Mouse: C57BL/6J: C57/B6 | Jackson Laboratories | IMSR Cat# JAX:000664, RRID:IMSR_JAX:000664 | |||
| Oligonucleotides | |||||
| Leptin cDNA Reverse | Koch Institute Swanson Biotechnology Center | TCAGCATTCAGGGCTAACATCCAACT | |||
| mLepR sgRNA Forward | Keck Biotechnology Center at Yale | CACCGTGAAAGCCACCAGACCTCGA | |||
| mLepR sgRNA Reverse | Keck Biotechnology Center at Yale | AAACTCGAGGTCTGGTGGCTTTCAC | |||
| mLepR Target Site Forward | Keck Biotechnology Center at Yale | GGTTCTCAGTGCACGCATTT | |||
| mLepR Target Site Reverse | Keck Biotechnology Center at Yale | ACAACGATTTTCCTGGCATCT | |||
| Leptin cDNA Forward | Koch Institute Swanson Biotechnology Center | ATGTGCTGGAGACCCCTGT | |||
| Recombinant DNA | |||||
| lentiGuide-puro | Addgene | Cat# 52963 | |||
| lentiCas9-blast | Addgene | Cat# 52962 | |||
| pFBAAVCAGmcsBgHpa | University of Iowa Viral Vector Core | Cat# G0345 | |||
| psPAX2 | Addgene | Cat# 12259 | |||
| pCMV-VSV-G | Addgene | Cat# 8454 | |||
| Software and Algorithms | |||||
| ImageJ | NIH | N/A | |||
| QuPath v0.1.2 | Github | N/A | |||
| Prism v8.0 | Graphpad | N/A | |||
| Image Studio Lite | LI-COR | N/A | |||
| PHATE | https://github.com/KrishnaswamyLab/PHATE | N/A | |||
| MAGIC | https://github.com/KrishnaswamyLab/MAGIC | N/A | |||
| FASTX-toolkit | Greg Hannon lab (http://hannonlab.cshl.edu/fastx_toolkit) | N/A | |||
| Burrows-Wheeler Aligner v0.5.5 | Source Forge | N/A | |||
| Picard toolkit v1.21 | Github | N/A | |||
| GATK v.1.0.5538 | Broad Institute | N/A | |||
| ANNOVAR | http://annovar.openbioinformatics.org/ | N/A | |||
| RSEM v.1.2.12 | Dewey lab/Github | N/A | |||
| Cytoscape v.3.3.0 | Cytoscape Consortium | N/A | |||
| Cell Ranger | 10X Genomics | N/A | |||
| SAS v9.4 | SAS Institute, Inc. | N/A | |||
| MELD | https://github.com/KrishnaswamyLab/MELD | N/A | |||
| diffxpy | https://github.com/theislab/diffxpy/ | N/A | |||
| R package JADE | https://www.rdocumentation.org/packages/JADE/versions/1.1-0 | N/A | |||
Adeno-associated virus generation
Total RNA was isolated from flash frozen adipose tissue derived from db/db mice using Trizol (Ambion) and reverse transcribed with a High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). Murine leptin cDNA was PCR amplified with Primestar high-fidelity PCR mix (Takara) using primers (forward 5’- ATGTGCTGGAGACCCCTGT-3’, reverse 5’-TCAGCATTCAGGGCTAACATCCAACT-3’) synthesized by the Koch Institute Swanson Biotechnology Center. Amplified leptin cDNA was cloned into the XhoI and NotI sites of the AAV2 backbone vector (pFBAAVCAGmcsBgHpa) provided by the University of Iowa Viral Vector Core (UIVVC), which generated AAV2/1CAGmLeptin (AAV-Leptin) using a baculovirus system. eGFP, cloned into the same AAV2 plasmid vector (AAV2/1CAGeGFP, AAV-GFP), was purchased from UIVVC. AAV-induced leptin expression was verified in 293T cells by western blotting 48 hours after infection. 1x1011 AAV viral particles were administered to mice via a single injection in the tibialis anterior muscle and circulating leptin levels were measured in the serum by ELISA (Crystal Chem Cat# 90030).
Immunoblotting
Cells were lysed with ice-cold RIPA buffer (Thermo Fisher Scientific), supplemented with 0.5 μM EDTA and Halt protease/phosphatase inhibitor cocktail (Thermo Fisher Scientific), rotated at 4°C for 15-30 minutes to mix, and centrifuged at maximum speed for 15 minutes to collect whole cell lysates. Protein concentration was measured with the BCA protein assay (Pierce). 30 μg of total protein per sample was loaded into 4-12% NuPAGE Tris-Bis (Thermo Fisher Scientific) or 4-20% Tris-Glycine TGX (Bio-Rad) gradient gels and separated by SDS-PAGE. Proteins were transferred to nitrocellulose (for fluorescence detection) or PVDF (for chemiluminescent detection) membranes and blocked with Odyssey Blocking Buffer (LI-COR) or 5% milk, respectively. Primary antibodies used for immunoblotting are listed in the Key Resource Table. HSP90 and beta-tubulin were used as loading controls. Primary antibodies were detected with fluorescent DyLight-conjugated (Cell Signaling Technology) or HRP-conjugated (Jackson Immunoresearch) secondary antibodies for fluorescent (LI-COR Odyssey scanner) or chemiluminescent detection (Perkin Elmer ECL), respectively. Quantification of phosphorylated protein levels was performed using Image Studio Lite (LI-COR) and normalized to total protein levels analyzed on the same membrane.
DNA and RNA isolation from mouse tumors
Mice were euthanized by cervical dislocation and pancreata were rapidly dissected, flash frozen in liquid nitrogen, and stored at −80C. Prior to DNA and RNA isolation, frozen tumors were mechanically ground in liquid nitrogen using a mortar and pestle. Genomic DNA and total RNA were extracted from ground tumor tissue using a Qiagen Allprep DNA/RNA mini kit per manufacturer’s instructions. DNA concentration was measured using Quant-iT Picogreen dsRNA Assay Kit (Thermo Fisher Scientific). RNA concentration, quality, and purity were determined using an Agilent Bioanalyzer.
Mouse tumor exome sequencing and analysis
Exome capture on mouse tumor genomic DNA was performed by Macrogen Corp. using an Agilent SureSelectXT Mouse All Exon kit. 100-nt paired-end sequencing to a raw sequence depth of 150X per sample on a HiSeq 2500 (Illumina) was subsequently performed. Variant calls were made using an approach similar to the HaJaVa platform (McFadden et al., 2016). Reads were processed to remove adapter sequences (using exact matches to 10-mer adapter start sequence: AGATCGGAAG) using the FASTX-toolkit (http://hannonlab.cshl.edu/fastx_toolkit). Surviving reads greater than or equal to 15-nt in length were retained. Reads were aligned to the mouse mm9 genome assembly (UCSC) using BWA (v0.5.5). Duplicates were removed using Picard (v1.21) MarkDuplicates utility. Local realignment of reads around indels and base quality recalibration were performed using GATK (v1.0.5538). Variant calls were made with GATK UnifiedGenotyper using the mm9 reference sequence in regions targeted by the Agilent capture kit (version G7550_20110119_mm9 from Agilent). Calls with at least 14X read coverage and dual strand support were retained and further filtered using GATK VariantFiltration (arguments - clusterWindowSize 10 -cluster 3), GATK SelectVariants (arguments "SB < −0.1", "QUAL >= 30.0", "QD >= 5.0", "HRun <= 5"), and Sanger Mouse Genomes Project Variant calls (Keane et al., 2011). Variants were annotated using Annovar (Wang et al., 2010) (version 2016Feb01) and mouse dbSNP build 142 (ftp.ncbi.nlm.nih.gov/snp/).
Mouse tumor RNA sequencing and analysis
cDNA libraries from tumor total RNA were prepared by the MIT BioMicro Center using the Neoprep library preparation system (Illumina) with indexed adaptor sequences and polyA selection. Sequencing was performed on an Illumina NextSeq to obtain paired-end 75-nt reads. All reads that passed quality metrics were mapped to UCSC mm9 mouse genome build (http://genome.ucsc.edu/) using RSEM (v1.2.12) (http://deweylab.github.io/RSEM/). All RNA-seq analyses were conducted in R. High-resolution signature analyses between tumors from obese (KCO and KCO treated with AAV-GFP) and non-obese (KC and KPC) models were performed using a blind source separation methodology based on Independent Component Analysis (ICA), as previously described (Hyvarinen and Oja, 2000; Muzumdar et al., 2017). Z-scores for each tumor for each component (signature) were calculated using ICA and reflect relative correspondence between a tumor and each signature (degree of positive or negative correlation). 3 derived signatures (Sig6, Sig12, and Sig13) showed statistically significant differences in Z-scores comparing KCO and KC/KPC tumors (p<0.01, Mann-Whitney test) and were used in subsequent analyses.
Gene Set Enrichment Analyses (GSEA) (http://software.broadinstitute.org/gsea/) were carried out using standardized signature correlation scores (for ICA signatures) with default settings. Normalized enrichment score (NES), false discovery rate (FDR), ICA signature (Sig6, Sig12, or Sig13 separating KCO and KC/KPC tumors), and MSigDB gene set rank are shown in relevant figures. Network representations of GSEA results were generated using EnrichmentMap (http://www.baderlab.org/Software/EnrichmentMap) for Cytoscape v3.3.0 (http://www.cytoscape.org) with p<0.005 and FDR<0.1. Each circle represents a gene set with circle size corresponding to gene set size and intensity corresponding to enrichment significance. Red is upregulated and blue is downregulated. Each line corresponds to minimum 50% mutual overlap with line thickness corresponding to degree of overlap. Cellular processes for gene set clusters were manually curated.
Genes with standardized signature correlation scores z > 3 (or z < −3) in Sig6, Sig12, and Sig13 were used as gene sets to stratify TCGA (https://tcga-data.nci.nih.gov/tcga/) Pancreatic Adenocarcinoma (PAAD) tumors (TCGA Research Network, 2017) using ssGSEA (Barbie et al., 2009). Associated patients within top and bottom buckets (> or < 1 s.d.) were each assessed for over-representation of previously established molecular subtypes (squamous (n=31), immunogenic (n=28), progenitor (n=53), ADEX (n=38) (Bailey et al., 2016)) using the hypergeometric test. The human tumor subtype classifications used are based on those reported by TCGA (TCGA Research Network, 2017) prior to tumor cellularity correction.
Tumor suppressor gene mutations in RNA-seq datasets were called using the GATK Best Practices workflow for SNP calling on RNA-seq data (software.broadinstitute.org/gatk/documentation/article.php?id=3891). Transcriptomic reads were trimmed to eliminate adapter sequences using an exact 10-mer match to the start of the adapter sequence (AGATCGGAAG) using Cutadapt (v1.16) and subsequently mapped to the mouse mm9 (UCSC) genome assembly using the STAR RNA-seq aligner with default parameters. Picard v2.17.0 MarkDuplicates utility was used to mark duplicates in the aligned data. GATK (v3.8.0) toolkit was used to “SplitNTrim” and reassign mapping qualities as per GATK best practices. Indel realignment and base recalibration were performed as recommended. Variants were called against the mm9 reference sequence using the GATK HaplotypeCaller and filtered using GATK VariantFiltration with recommended parameters. Variant calls were annotated using Annovar (version 2016Feb01).
Evaluation of cancer driver genes in human PDAC
Targeted exome sequencing and IHC of human PDAC biospecimens has been previously described (Qian et al., 2018). Pre-operative body-mass index (BMI) and molecular data on the main PDAC driver genes was available for 184 of 356 patients from this prior study. Of note, PDAC patients experience significant weight loss in the months preceding diagnosis, and weight at surgery may therefore not be fully reflective of weight in the prediagnostic period. In 125 patients with available weight data at 6 months before diagnosis, we compared weight at surgery with weight 6 months before diagnosis, and observed a strong correlation (Spearman’s rank correlation coefficient = 0.90, p<0.0001). This suggests that although weight may decrease in months preceding diagnosis, the rank order of 2 measurements is likely to be highly consistent. We used World Health Organization categories to classify patients as normal weight (<25 kg/m2), overweight (25-30 kg/m2), or obese (>30 kg/m2). Using SAS software (SAS Institute, Inc., Version 9.4), we performed logistic regression adjusted for age at surgery (continuous), gender (men, women), study site, grade (well/moderately differentiated, poorly differentiated/undifferentiated, unknown), perineural invasion (no, yes, unknown), lymphovascular invasion (no, yes, unknown), N stage (N0, N1, unknown), T stage (T1/T2, T3/T4, unknown), and tumor location (head/uncinate, body, tail, unknown), to evaluate the association between BMI (normal, overweight, obese) and driver alterations in human PDAC. We calculated odds ratios (ORs) and 95% confidence intervals (CIs). We used the Kruskal-Wallis test to compare the number of driver alterations across BMI categories.
Murine islet GSIS studies
Islets from C57/B6 wild-type and ob/ob male mice (n=4 mice/group) were isolated and perifused for glucose stimulated insulin secretion (GSIS) studies as previously described (Jesinkey et al., 2019) with a few minor changes, which are noted. Isolated islets were recovered overnight and were perfused the following day in DMEM (D5030, Sigma) supplemented with 24 mM NaHCO3, 10 mM HEPES, 2.5 mM glucose, 2 mM glutamine, and 0.2% BSA. The islets first equilibrated for 1 hour at 2.5 mM glucose on the perifusion instrument. After the stabilization period, islets were perfused with basal (2.5 mM) glucose for 10 minutes followed by stimulatory glucose (16.7 mM) for 45 minutes. After stimulation with glucose, the islets were exposed to basal glucose for 15 minutes followed by a final 30 mM KCl step to ensure that the insulin secreting machinery distal to mitochondrial metabolism was intact in the two groups tested. During the perifusion, eluent was collected into a 96-well plate format and both secreted and total islet insulin concentrations were determined by a Mouse High Range Insulin ELISA assay kit (Alpco Diagnostics) and normalized to islet DNA using Picogreen.
Single-cell RNA sequencing of mouse islets
Murine pancreatic islets used for single-cell analysis were isolated as described for GSIS studies, except samples were dispersed into single cells immediately after isolation using accutase (Gibco) and resuspended in 1 mL of PBS. Dead cells were removed using a MACS Dead Cell Removal Kit (Miltenyi Biotec) and cell concentration determined using a Countess II Automated Cell Counter (Thermo Scientific). Single-cell library preparation was performed using a Chromium Single Cell 3’ Reagent Kit v3 (10X Genomics) and sequenced by Illumina HiSeq. Gene counts matrices were generated using CellRanger. Genes detected in fewer than 15 cells were removed and cells with library sizes greater than 15,000 UMI/cell were removed as potential doublets. We observed a bimodal distribution of mitochondrial gene detection and removed cells in the top 12.5% of library-size normalized mitochondrial expression as apoptotic cells. We then focused our analysis on single-hormone expressing islet cells as measured by the expression of Ins1/Ins2, Gcg, Sst, and Ppy, removing dual hormone-expressing cells and cells expressing markers of acinar, ductal, endothelial, or immune cells.
Cells were visualized using PHATE (Moon et al., 2019) with default parameters and gene expression was denoised using MAGIC (van Dijk et al., 2018) with default parameters. The Enhanced Experimental Signal (EES) was calculated using MELD (Burkhardt et al., 2019) with default parameters. Overlap of upregulated/downregulated genes comparing ob/ob vs. wild-type (WT) (q<0.0001, log2FC>0.5, mean normalized expression counts >0.5) or Cck+ (expression>0) vs. Cck- (q<0.0001, mean normalized expression counts > Cck) beta cells with gene ontology (C5 biological process and cellular components) and hallmark (H) gene sets in MSigDB (http://software.broadinstitute.org/gsea/) were computed using the hypergeometric test. Statistical testing for differential expression was performed using the Mann-Whitney U test as implemented in diffxpy.
CCK evaluation in human biospecimens
IHC for detection of CCK expression in human PDAC samples was performed on 4-μm sections of formalin-fixed paraffin-embedded tissue blocks containing neoplastic and non-neoplastic pancreatic parenchyma that were obtained as part of specimens resected for PDAC treatment, as previously described (Qian et al., 2018). Antigen retrieval was performed using citrate buffer (pH 6.0, Dako) in a 2100 Antigen Retriever system (Electron Microscopy Sciences). Sections were then incubated with dual endogenous peroxidase block (Dako) for 20 minutes at room temperature, protein block (Dako) for 10 minutes at room temperature, and anti-CCK8 primary antibody for 1 hour at 4°C. Chromogenic visualization was subsequently performed using the EnVision+ System- HRP (Dako) with a 3 minute diaminobenzidine (DAB) incubation followed by counterstaining with hematoxylin. For evaluation of CCK expression, the surrounding exocrine pancreas served as an internal negative control. If present within the section, neuroendocrine cells within the epithelium of the duodenum served as internal positive controls. The immunohistochemical assessment of CCK expression was conducted without knowledge of BMI or other patient data.
For human donor islets, RNA was extracted, sequenced, and analyzed as previously described (van de Bunt et al., 2015). Reads per kilobase of CCK transcript per million mapped reads (RPKM) was log2 normalized for analysis. For a subset of donors, BMI was not available, and samples could not be used in analysis. Given limited sample size, low and high BMI groups were specified based on the median United States BMI (28.2 kg/m2) defined by the most recent 2015-2016 National Health and Nutrition Examination Survey (NHANES) (https://www.cdc.gov/nchs/nhanes/index.htm). This also closely approximated median BMI in the donor cohort of 28 kg/m2.
Induction of murine pancreatitis
Acute pancreatitis was induced in adult C57/B6 mice (~25 grams in weight) using cerulein (Sigma; six hourly 50 μg/kg intraperitoneally (IP) injections) or arginine (Sigma; three hourly 3 g/kg IP injections). Mice were euthanized and processed for histology at 12 and/or 48 hours after the first injection. Chronic pancreatitis was induced in adult C57/B6 mice with cerulein given six hourly 50 μg/kg IP injections three times a week for 2 weeks prior to analysis. Acute or chronic PBS-treated mice were used as controls. Two mice from each treatment group were analyzed.
QUANTIFICATION AND STATISTICAL ANALYSIS
Percentage of tumor burden was quantified blinded to group on scanned slides (Aperio) by measuring cross-sectional tumor area (PanIN or PDAC including stroma) relative to total pancreas area using ImageJ or QuPath v0.1.2 software. Details of statistical tests used for each experiment including n, definition of center, and precision measures are described in the figure legends. All error bars denote standard error of mean (s.e.m.) unless otherwise denoted. A p-value of <0.05 was used to denote statistical significance. In general, p-values for comparisons of two groups were determined by two-tailed student’s t-test (for normally distributed data) or Mann-Whitney U test (for non-parametric data), as noted in the figure legends, using Prism (Graphpad). The log-rank test was used for Kaplan-Meier survival analyses using Prism. All replicates were included in these analyses and represent measurements from distinct samples (biologic replicates) unless otherwise noted in the figure legends.
DATA AND CODE AVAILABILITY
The datasets generated and/or analyzed during the current study are available in the NCBI Sequencing Read Archive (SRA) under accession number PRJNA544740 and the NCBI Gene Expression Omnibus (GEO) under accession numbers GSE131714 and GSE137236. Primary murine PDAC cell lines and constructs are available upon request. Python scripts for scRNA-seq analyses are available on GitHub (https://github.com/KrishnaswamyLab). Computer code for RNA-seq signature analysis (ICA) will be made available upon request. Other software tools (including version numbers) for exome and RNA-seq analyses are listed above or referenced.
Supplementary Material
Table S4. Characteristics of PDAC patients with available body mass index (BMI) and molecular alterations data, Related to Figure 3.
Table S5. Association of BMI with PDAC driver gene alterations in human tumors, Related to Figure 3.
Number (percentage) of tumors that are either wild-type (WT) or mutant (MUT; mutation by exome sequencing or loss by IHC) for each driver gene stratified by patient BMI. Odds ratios (OR) from logistic regression models were adjusted for age at surgery (continuous), study site, gender (men, women), grade (well/moderately differentiated, poorly differentiated/undifferentiated, unknown), perineural invasion (no, yes, unknown), lymphovascular invasion (no, yes, unknown), N stage (N0, N1, unknown), T stage (T1/T2, T3/T4, unknown), and tumor location (head/uncinate, body, tail, unknown). For cumulative analysis of PDAC drivers, total number of patients (N) and mean (standard deviation) number of driver alterations per tumor were stratified by patient BMI. p-value is based on the Kruskal-Wallis test.
Figure S1. Obesity drives pancreatic but not lung tumorigenesis in mice, Related to Figure 1.
A) Body weight (mean +/− s.e.m.) of KC mice of varying db genotype over time (n=4-12 mice/group).
B) Kaplan-Meier survival curves for KC mice of varying db genotype (n=14-18 mice/group). p-value of log-rank test is shown.
C) Body weight (mean +/− s.e.m.) of KrasLA2 mice (Johnson et al., 2001) fed a low-fat (LFD) or high-fat (HFD) diet (n=18-20 mice/group).
D) Kaplan-Meier survival curves for KrasLA2 mice fed a LFD or HFD (n=40-42 mice/group). p-value of log-rank test is shown.
Body weight (mean +/− s.e.m.) of KrasLA2 mice of varying ob genotype (n=3-9 mice/group).
F) Kaplan-Meier survival curves for KrasLA2 mice of varying ob genotype (n=15-19 mice/group). p-value of log-rank test is shown.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-tailed student’s t-test for weight comparisons at each time point.
Figure S2. Effects of leptin administration in cells and mice, Related to Figure 2.
A) Western blot showed dose-dependent leptin protein expression and secretion into media by 293T cells 48 hours after infection with AAV-Leptin versus AAV-GFP control. HSP90 is cellular protein loading control. WCL = whole cell lysate.
B) Serial ELISA measurements of serum leptin concentrations (mean +/− s.e.m.) following intramuscular injection of AAV-Leptin in adult ob/ob mice (n=5) demonstrated sustained leptin expression.
C) AAV-Leptin treatment reduced non-fasting blood glucose (mean +/− s.e.m.) 6 weeks post-infection of KCO mice (n=8-10 mice/group). ****p<0.0001, two-tailed student’s t-test. Mice were infected at 6 weeks of age.
D) AAV-Leptin restored fertility in ob/ob male and female mice. Shown are early postnatal progeny born from an AAV-Leptin-treated ob/ob female mated with an AAV-Leptin-treated ob/ob male.
E) Schematic of AAV treatment of tumor-bearing mice. AAV is administered at 3 months of age once tumors have progressed (see Figures 1C-E). Percent change in body weight for each mouse (n=10 mice/group) following AAV administration is shown.
F) Kaplan-Meier survival curves for mice in (E) showed no significant difference (p>0.05, log-rank test) between AAV treatments (n=10 mice/group).
G) Recombinant murine leptin (100 ng/mL) activated MAPK (pErk1/2) and STAT3 (pStat3) signaling in murine Kras/p53 mutant PDAC (mPDAC) cells (KPC-7307 and KPC-7310), which is abolished in leptin receptor knockout clones (KPC-7307 KO1 and KO2). Quantification of pErk/Erk and pStat3/Stat3 levels (normalized to untreated) is shown. Beta-tubulin is additional protein loading control. Leptin dose was chosen to be within an order of magnitude of that observed in the serum of AAV-Leptin-treated mice in (B).
H) Recombinant murine leptin (100 ng/mL) has no effect (p>0.05, two-tailed student’s t-test) on mPDAC cell viability (mean +/− s.e.m., normalized to untreated) 72 hours are treatment (n=5 replicates/condition).
Figure S3. Treatment studies in KCO mice, Related to Figures 4 and 7.
A) Schematic shows treatment strategy in which mice are placed on drinking water containing drug at 6 weeks of age and analyzed for tumor progression 6 weeks later. Percent change in body weight (mean +/− s.e.m.) is shown for each treatment (n=7-10 mice/group). The Cck receptor antagonist proglumide showed a modest slowing of weight gain.
B) Quantification of tumor burden (mean +/− s.e.m.) is shown for each treatment and control drinking water (n=7-10 mice/group). No significant differences were observed across pairwise comparisons of treatments with control (p>0.05, two-tailed student’s t-test).
C) Representative histologic sections of pancreata of mice at endpoint demonstrate no significant decrease in tumor burden (Ck19), inflammation (Cd45+ immune cells), or fibrosis (Sirius Red staining) across treatments in (B). Scale bar: 200 μm.
Figure S4. Insulin secretion and response in obese mice, Related to Figure 5.
A) Obese leptin-deficient (ob/ob) mice exhibited increased fasting serum insulin and proinsulin (C-peptide) levels (mean +/− s.e.m.) compared to non-obese controls (ob/+) at 10 weeks (n=2-3 mice/group). *p<0.05, **p<0.01, two-tailed student’s t-test.
B) Glucose-stimulated insulin secretion (mean +/− s.e.m., n=4 replicates/group) from perifused islets derived from 12 week-old C57/B6 wild-type (WT) and ob/ob mice (n=4 pooled mice/group) is shown. *p<0.05, **p<0.01, two-tailed student’s test for each timepoint.
C) Total insulin content (mean +/− s.e.m., normalized to DNA content) of cultured islets in (B) was not significantly different between groups.
D) Area under the curve (AUC) (mean insulin ng/mL/ng DNA +/− 95% confidence intervals) showed reduced glucose-stimulated insulin secretion (combined 1st and 2nd phase) for ob/ob islets in (B). AUC for potassium chloride (KCl)-induced depolarization was not significantly different, suggesting no change in overall insulin stores.
E) IHC revealed activated insulin receptor/Igf1 receptor (phospho-InsR/Igf1R) in PanINs in non-obese (KC) and obese (KCO) mice, consistent with insulin-induced signaling. pInsR/Igf1R increased in higher-grade PanINs (arrows). Pancreatic islets were internal positive controls (asterisks). Scale bar: 50 μm.
F) Average non-fasting glucose levels (mean +/− s.e.m.) of KCO mice during treatment with dapagliflozin (n=6-9 mice/group). ****p<0.0001, two-tailed student’s t-test.
G) Non-fasting insulin and C-peptide levels (mean +/− s.e.m) of mice in (F) after 6 weeks of treatment with dapagliflozin or control drinking water. Mice were started on treatment at 6 weeks of age. **p<0.01, two-tailed-student’s t-test.
H) C-peptide levels in (G) were inversely correlated with tumor burden. Each point represents one mouse (n=15, lavender circles are control, blue squares are dapagliflozin-treated). Correlation coefficient (R) and p-value of simple linear regression are shown.
I) IHC showed an inverse relationship between islet insulin and Cck expression in the dapagliflozin-treated KCO mice with the highest and lowest C-peptide levels in (G). Scale bar: 100 μm.
Figure S5. Obesity-associated islet Cck expression in mice, Related to Figures 6 and 7.
A) Obese (ob/ob) mice exhibited progressive expression of Cck in pancreatic islets (denoted by Syn (synaptophysin) immunostaining).
B) Non-fasting serum Cck levels (mean +/− s.e.m.) did not differ between 7-9 week-old obese ob/ob and non-obese ob/+ mice (n=7 mice/group).
C) AAV-leptin treatment of ob/ob mice reduced Cck expression by RNA-seq on bulk pancreata. Normalized Cck expression counts (mean +/− s.e.m, n=2 mice/group) are shown.
D) Obese KC; db/db mice exhibited increased islet Cck expression compared to non-obese KC; db/+ mice. Representative islets from two mice of each genotype are shown.
E) Islet Cck expression is abolished following weight loss induced by AAV-Leptin treatment or caloric restriction (CR) of KCO mice. Insulin denotes beta cells. Mice were analyzed at 12 weeks of age following intervention starting at 6 weeks of age.
All scale scale bars are 100 μm.
Figure S6. Cell proliferation and transcription factor alterations are associated with beta cell Cck expression, Related to Figure 6.
A) Cck+ beta cells showed relative upregulation of cell cycle genes (Kowalczyk et al., 2015). Fold change difference in average gene expression comparing Cck+ and Cck- beta cells are shown across these genes. Genes upregulated in Cck+ cells correspond to green dots, while orange dots correspond to genes upregulated in Cck- cells. This finding was confirmed by significant overlap (hypergeometric test) between genes upregulated in Cck+ cells (q<0.05) and the Cell Cycle gene ontology (C5 GO) gene set from MSigDB (inset).
B) Mean single cell expression counts (square-root transformed library size-normalized UMI/cell +/− s.d.) for beta cells (Ins2+) showed Cck expression predominantly in Ins2+/Ins1 and Ins2+/Gcg+ cells. Each colored dot represents a single cell. Gray circles represent median expression.
C) A greater percentage of polyhormonal Ins2+/Gcg+ cells expressed Cck than monohormonal Ins2+/Ins1+ cells.
D) PHATE visualization shows distribution of Ptprc+ (Cd45+) immune cells recovered by scRNA-seq of islets from ob/ob and wild-type (WT) mice.
E) The majority of Ptprc+ (Cd45+) cells in (D) expressed macrophage markers (Cd68, Cd74).
F) Gene ontology gene sets (C5 GO sets from MSigDB) showed significant overlap (hypergeometric test) with gene expression of islet macrophages from ob/ob (green) and WT (orange) mice.
G) allmark gene sets (H sets from MSigDB) showed significant overlap (hypergeometric test) with gene expression of islet macrophages from ob/ob (green) and WT (orange) mice.
H) Islet macrophages from ob/ob mice showed upregulation of cell proliferation genes.
I) Islet macrophages from ob/ob mice showed downregulation of cytokine, inflammation, and interferon-response genes.
J) Islet macrophages from ob/ob mice showed upregulation of beta cell growth factors.
K) Ehf and Tle3 expression was absent or markedly reduced in Cck+ beta cells from ob/ob mice.
L) Gene-gene expression plots after MAGIC (van Dijk et al., 2018) showed an inverse relationship between the expression of Ehf and Tle3 with Cck in ob/ob beta cells. Spearman correlation coefficients (R) are listed for each plot.
Figures S7. Exploring mechanisms of islet Cck-driven pancreatic tumorigenesis, Related to Figure 7.
A) MIP-Cck transgenic mice exhibited islet-specific Cck expression without evidence of increased pancreatic inflammation (Cd45+ leukocyte infiltration) or fibrosis (Sirius red collagen staining or presence of smooth muscle actin (SMA)-positive fibroblasts) compared to wild-type controls.
B) Cck was not significantly expressed in pancreatic islets in murine models of acute pancreatitis (CER = cerulein-induced at 12 or 48 hours, ARG = arginine-induced at 48 hours, PBS is control).
C) Cck was rarely expressed (arrows) in pancreatic islets in a murine model of chronic pancreatitis (cerulein-treated for 2 weeks). Frequencies of islets without staining (negative) or rare Cck+ cells (arrows) are shown.
D) CCK was rarely expressed (arrows) in pancreatic islets obtained from human patients with chronic pancreatitis. Frequencies of islets without staining (negative) or rare CCK+ cells (arrows) are shown. Of a total of 127 evaluable islets, 4 harbored rare CCK+ cells.
E) MKC mice displayed an increase in proliferating (Ki67+) acinar cells (arrows) compared to KC mice. The number of Ki67+ acinar cells per 10X field (mean +/− s.e.m., 4-5 fields/mouse, n=5 mice/group) is plotted. *p<0.05, two-tailed student’s t-test.
All scale bars are 100 μm.
Table S1. Tumor suppressor gene single nucleotide variants in KCO tumors, Related to Figure 3.
Mutant allele fractions for each coding nucleotide (compared to mm9 reference database) of tumor suppressor genes (Trp53, CDKN2A/p16, and SMAD4) derived from whole exome sequencing and RNA-seq are shown for individual KCO tumors and averaged across all tumors Average base coverage across KCO tumors is as follows: Trp53 (84X exome, 127X RNA), CDKN2A/p16 (40X exome, 89X RNA), and SMAD4 (103X exome, 86X RNA). Blank cells denote lack of base coverage in sequencing, which was rare and sporadic. A single KPC tumor (7075) was used as a positive control for single nucleotide variant (SNV) detection (resulting in a R172H codon substitution as noted). No tumor suppressor gene SNVs were detected in KCO tumors.
Tables S2. Single nucleotide variants in murine tumors, Related to Figure 3.
All single nucleotide variants (SNV) called in KCO (n=13) and KPC (n=1) tumors by whole exome sequencing are listed by tumor. Variant information includes location (chromosome, cytoband, and position in mm9 genome), genic location (exonic, intergenic, UTR), nucleotide change (var vs. ref), read counts for variant (tumor_reads2) and reference (tumor_reads1), and variant frequency (tumor_var_freq). Exon SNVs were further classified as synonymous or non-synonymous with reference to transcript (RefSeq), exon number (Exon_num), and coding sequence position (AA_change).
Table S3. Compilation of non-synonymous single nucleotide variants in murine tumors, Related to Figure 3.
Compiled non-synonymous single nucleotide variants (SNVs) identified on whole exome sequencing data derived from all sequenced KCO and KPC tumors from Table S2. A total of 531 non-synonymous SNVs were called from 14 tumors (~37.7 SNVs per KCO mouse (n=13) and 41 non-synonymous SNVs in KPC mouse (n=1)). Induced Kras, Lep (ob), and Trp53 mutations were observed as expected. Of consensus pan-cancer genetic drivers (Bailey et al., 2018), Arid5b, Cacna1a, Eef2, and Kit harbored recurrent non-synonymous SNVs. These SNVs were not observed in human cancer (COSMIC) to suggest pathogenicity.
Table S6. Independent component analysis (ICA) of mouse tumor RNA-seq, Related to Figures 4 and 5.
Log (base 2) transformed normalized expression counts per gene in individual KCO, KC, and KPC tumors used in RNA-seq analyses. Mouse number, genotype, and AAV treatments are shown. Gene signatures (Sig6, Sig12, and Sig13) that distinguish obese (KCO) and non-obese (KC and KPC) tumors by ICA with p<0.05 are shown. Per-gene expression correlation Z-scores with respect to the signature are listed (positive is correlated with KCO tumors) for each gene signature and ordered by log2 fold change comparing above groups. Gene set enrichment analysis (GSEA) results correlated with KCO tumors (UP) are shown comparing ICA-derived signatures (6, 12, and 13) with curated (C2) and hallmark (H) gene sets in MSigDB.
Table S7. Beta cell comparisons from scRNA-seq data, Related to Figure 6.
Composite gene expression differences of scRNA-seq data comparing ob/ob (OB) vs. wild-type (WT) beta cells or Cck+ positive vs. Cck- OB beta cells. Shown are genes ordered by log2FC (fold change) including mean expression counts, p-values, and q-values, as measured using a Mann-Whitney U test implemented in diffxpy for each gene.
HIGHLIGHTS.
Obesity accelerates oncogenic Kras-driven pancreatic ductal tumorigenesis in mice
Genetic or dietary weight loss intercepts pancreatic cancer progression
Obesity is associated with aberrant pancreatic islet cholecystokinin expression
Islet cholecystokinin overexpression drives pancreatic ductal cancer development
ACKNOWLEDGEMENTS
We thank F. Gorelick, M. Lemmon, and F. Wilson for critical reading of the manuscript; K. Mercer for technical assistance; K. Cormier and C. Condon from the Hope Babette Tang (1983) Histology Facility of the Koch Institute Swanson Biotechnology Center and A. Brooks, P. Gaule, Y. Bai, B. Acs, and D. Rhim from Yale Pathology Tissue Services for histology assistance; M. Robert and R. Bronson for pathology assistance; S. Levine, V. Dayal, and J. Penterman from the MIT BioMicro Center for bulk RNA-seq support; J. Heitke, G. Wang, C. Castaldi, and S. Mane from the Yale Center for Genome Analysis for assisting with scRNA-seq; the University of Iowa Viral Vector core for AAV constructs and generation; M. Batsu and R. Jacobs from the Yale Diabetes Research Core for serum ELISA/RIA studies; the Oxford Human Islet Isolation facility for the provision of human islets for research; T. Kolodecik and F. Gorelick for providing murine pancreatitis specimens; F. Zheng for CRISPR constructs; and A. Berns, D. B. Davis, A. Lowy, L. Luo, and Jackson Laboratories for mice.
This work was supported in part by a Lustgarten Foundation Research Investigator Grant (C.S.F. and M.D.M.), Cancer Center Support (core) grants P30-CA016359 (C.S.F.) and P30-CA014051 (T.J.) from the National Cancer Institute, a Pilot Grant from the Yale Cancer Center (M.D.M.), a Yale Cancer Innovations Award (M.D.M.), and an NIH Director’s New Innovator Award (DP2-CA248136) (M.D.M.). The National Institute for Health Research, Oxford Biomedical Research Centre funded islet provision. C.G. is funded by the NIH/NIGMS (T32-GM007499). D.B.B. is funded by the NICHD (F31-HD097958). A.B. is funded by the NCI (K07-CA222159) and a Bob Parsons Fellowship. A.L.G. received support as a Wellcome Senior Fellow in Basic Biomedical Science with funding in Oxford by the Wellcome Trust (095101, 200837, 106130, 203141), Medical Research Council (MR/L020149/1), European Union Horizon 2020 Programme (T2D Systems), and the NIDDK (U01-DK105535, U01-DK085545). M.I.M. received support as a Wellcome Senior Investigator and an NIHR Senior Investigator. Relevant funding support for this work came from Wellcome (090532, 098381, 212259) and the NIHR (NF-SI-0617-10090). The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. R.G.K. is funded by the NIDDK (P30-DK045735, R01-DK110181) and a Yale Cancer Innovations Award. S.K. is funded by the NIH (R01-GM130847), HIPC (2U19AI089992), and the Chan Zuckerberg Initiative (CZF2019-002440). B.M.W. acknowledges support from the Hale Family Center for Pancreatic Cancer Research, Lustgarten Foundation, NIH (U01-CA210171), Stand Up to Cancer, Noble Effort Fund, Wexler Family Fund, and Promises for Purple. T.J. is a Howard Hughes Medical Institute Investigator, the David H. Koch Professor of Biology, and a Daniel K. Ludwig Scholar. M.D.M. is supported by the NCI (K08-CA2080016) and was supported by a Conquer Cancer Foundation-American Society for Clinical Oncology (CCF-ASCO) Young Investigator Award and the NIH Loan Repayment Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
K.J.D. is currently an employee of Sherlock Biosciences. M.D.B. acknowledges research support from ViaCyte and Dexcom and serves on the medical advisory boards for Novo Nordisk and ARIEL Precision Medicine. A.L.G. has received honoraria from Merck and Novo Nordisk and has received research funding from Novo Nordisk. M.I.M. has served on advisory panels for Pfizer, Novo Nordisk, Zoe Global; he has received honoraria from Merck, Pfizer, Novo Nordisk and Eli Lilly and research funding from Abbvie, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, Servier, and Takeda. As of June 2019, M.I.M. is an employee of Genentech, and a holder of Roche stock. R.G.K. is co-founder and a Scientific Advisory Board member of Elucidata, a Consultant/Advisory Board member for Agios, Janssen, BI-Lilly, and Pfizer, and a recipient of sponsored research agreements from Agios, AstraZeneca/BMS, Lilly, Pfizer, and Poxel. S.K. is a paid scientific advisor to AI Therapeutics. B.M.W. declares research funding from Celgene Inc. and Eli Lilly & Company, and consulting for BioLineRx Ltd., Celgene Inc., G1 Therapeutics Inc., and GRAIL Inc. T.J. is a Board of Directors member of Amgen and Thermo Fisher Scientific, co-Founder and Scientific Advisory Board member of Dragonfly Therapeutics, co-founder of T2 Biosystems, and Scientific Advisory Board member of SQZ Biotech with equity holding in all five companies; he receives funding from the Johnson & Johnson Lung Cancer Initiative and Calico. C.S.F. reports receiving personal fees from Eli Lilly, Entrinsic Health, Pfizer, Merck, Sanofi, Roche, Genentech, Merrimack Pharma, Dicerna, Bayer, Celgene, Agios, Gilead Sciences, Five Prime Therapeutics, Taiho, KEW, and CytomX Therapeutics and receiving support from CytomX Therapeutics. M.D.M. acknowledges research support from Genentech.
REFERENCES
- Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, et al. (2012). EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22, 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic A, Bao Y, Qian ZR, Yuan C, Giovannucci EL, Aschard H, Kraft P, Amundadottir LT, Stolzenberg-Solomon R, Morales-Oyarvide V, et al. (2016). Pancreatic Cancer Risk Associated with Prediagnostic Plasma Levels of Leptin and Leptin Receptor Genetic Polymorphisms. Cancer Res 76, 7160–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim J, Reardon B, et al. (2018). Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 173, 371–385 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52. [DOI] [PubMed] [Google Scholar]
- Bao Y, Giovannucci EL, Kraft P, Qian ZR, Wu C, Ogino S, Gaziano JM, Stampfer MJ, Ma J, Buring JE, et al. (2013a). Inflammatory plasma markers and pancreatic cancer risk: a prospective study of five U.S. cohorts. Cancer Epidemiol Biomarkers Prev 22, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, et al. (2013b). A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst 105, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, et al. (2009). Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. (2006a). Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A 103, 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. (2006b). Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20, 3130–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto SG, Carati CJ, Toouli J, and Saccone GT (2010). The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299, G10–22. [DOI] [PubMed] [Google Scholar]
- Bocian-Sobkowska J, Zabel M, Wozniak W, and Surdyk-Zasada J (1999). Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochem Cell Biol 112, 147–153. [DOI] [PubMed] [Google Scholar]
- Burkhardt DB, Stanley JS, Perdigoto AL, Gigante SA, Herold KC, Wolf G, Giraldez AJ, van Dijk D, and Krishnaswamy S (2019). Quantifying the effect of experimental perturbations in single-cell RNA-sequencing data using graph signal processing. BioRxiv 532846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, Christian S, Haegebarth A, Algire C, and Pollak M (2016). Are Metformin Doses Used in Murine Cancer Models Clinically Relevant? Cell Metab 23, 569–570. [DOI] [PubMed] [Google Scholar]
- Chang H-H, Moro A, Takakura K, Su H-Y, Mo A, Nakanishi M, Waldron RT, French SW, Dawson DW, et al. (2017). Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. Plos One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, et al. (2017). Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer 16, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Muzumdar MD, Dorans KJ, Robbins R, Bhutkar A, Del Rosario A, Mertins P, Qiao J, Schafer AC, Gertler F, et al. (2018). Adaptive and Reversible Resistance to Kras Inhibition in Pancreatic Cancer Cells. Cancer Res 78, 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SE, Hughes SJ, Clark A, Gray DW, and Johnson PR (2012). Collagenase does not persist in human islets following isolation. Cell Transplant 21, 2531–2535. [DOI] [PubMed] [Google Scholar]
- Ellett JD, Evans ZP, Zhang G, Chavin KD, and Spyropoulos DD (2009). A rapid PCR-based method for the identification of ob mutant mice. Obesity (Silver Spring) 17, 402–404. [DOI] [PubMed] [Google Scholar]
- Friedman JM (2019). Leptin and the endocrine control of energy balance. Nature Metabolism. [DOI] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, and Barbacid M (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302. [DOI] [PubMed] [Google Scholar]
- Harbuzariu A, Rampoldi A, Daley-Brown DS, Candelaria P, Harmon TL, Lipsey CC, Beech DJ, Quarshie A, Ilies GO, and Gonzalez-Perez RR (2017). Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget 8, 7740–7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, and Tuveson DA (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, and Oja E (2000). Independent component analysis: algorithms and applications. Neural Netw 13, 411–430. [DOI] [PubMed] [Google Scholar]
- Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, et al. (2016). Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discovery 6, 852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, and Weinberg RA (1994). Tumor spectrum analysis in p53-mutant mice. Curr Biol 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, and Tuveson DA (2001). Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15, 3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesinkey SR, Madiraju AK, Alves TC, Yarborough OH, Cardone RL, Zhao X, Parsaei Y, Nasiri AR, Butrico G, Liu X, et al. (2019). Mitochondrial GTP Links Nutrient Sensing to beta Cell Health, Mitochondrial Morphology, and Insulin Secretion Independent of OxPhos. Cell Rep 28, 759–772 e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, and Jacks T (2001). Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116. [DOI] [PubMed] [Google Scholar]
- Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, et al. (2011). SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczak MJ, Saini S, Ioja S, Costa DK, Udeh N, Zhao X, Whaley JM, and Kibbey RG (2018). SGLT2 knockout prevents hyperglycemia and is associated with reduced pancreatic beta-cell death in genetically obese mice. Islets 10, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu D, Sakagami J, Motoyoshi T, Nakajima T, and Kataoka K (2006). Effects of the cholecystokinin A receptor antagonist loxiglumide on the proliferation and cell cycle time of pancreatic acinar cells in rats. Pancreas 32, 190–196. [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, et al. (2011). Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf N, Yuan C, Hamada T, Cao Y, Babic A, Morales-Oyarvide V, Kraft P, Ng K, Giovannucci E, Ogino S, et al. (2018). Regular Use of Aspirin or Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs Is Not Associated With Risk of Incident Pancreatic Cancer in Two Large Cohort Studies. Gastroenterology 154, 1380–1390 e1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasawneh J, Schulz MD, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, Buck A, Schwaiger M, Saur D, Schmid RM, et al. (2009). Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A 106, 3354–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris J.P.t., Pan FC, Akiyama H, Wright CV, Jensen K, et al. (2012). Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk MS, Tirosh I, Heckl D, Rao TN, Dixit A, Haas BJ, Schneider RK, Wagers AJ, Ebert BL, and Regev A (2015). Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res 25, 1860–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, and Wolk A (2007). Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 120, 1993–1998. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, and International Agency for Research on Cancer Handbook Working, G. (2016). Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 375, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine JA, Kibbe CR, Baan M, Sirinvaravong S, Umhoefer HM, Engler KA, Meske LM, Sacotte KA, Erhardt DP, and Davis DB (2015). Cholecystokinin expression in the beta-cell leads to increased beta-cell area in aged mice and protects from streptozotocin-induced diabetes and apoptosis. Am J Physiol Endocrinol Metab 309, E819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine JA, Raess PW, Stapleton DS, Rabaglia ME, Suhonen JI, Schueler KL, Koltes JE, Dawson JA, Yandell BS, Samuelson LC, et al. (2010). Cholecystokinin is upregulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology 151, 3577–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Yeung SC, Hassan MM, Konopleva M, and Abbruzzese JL (2009). Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Dolai S, Xie L, Winter E, Orabi AI, Karimian N, Cosen-Binker LI, Huang YC, Thorn P, Cattral MS, et al. (2017). Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem 292, 5957–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann AK, Baan M, and Davis DB (2014). Pancreatic beta-cell proliferation in obesity. Adv Nutr 5, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, and Davis DB (2015). Glucagon-Like Peptide-1 Regulates Cholecystokinin Production in beta-Cells to Protect From Apoptosis. Mol Endocrinol 29, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D'Alessio D, Woods SC, and Tso P (2011). Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60, 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon CD (1986). Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol 251, G487–494. [DOI] [PubMed] [Google Scholar]
- Massetti GM, Dietz WH, and Richardson LC (2017). Excessive Weight Gain, Obesity, and Cancer: Opportunities for Clinical Intervention. JAMA 318, 1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, Santiago PM, Kim-Kiselak C, Platt JT, Lee E, et al. (2016). Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc Natl Acad Sci U S A 113, E6409–E6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonsa AM, Chalfant MC, Gorden LD, and VanSaun MN (2015). Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One 10, e0126686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DE, Liu C, Ziaie AS, Naji A, and Zaret KS (2014). Grg3/TLE3 and Grg1/TLE1 induce monohormonal pancreatic beta-cells while repressing alpha-cell functions. Diabetes 63, 1804–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KR, van Dijk D, Wang Z, Gigante S, Burkhardt DB, Chen WS, Yim K, Elzen A.v.d., Hirn MJ, Coifman RR, et al. (2019). Visualizing structure and transitions in high-dimensional biological data. Nature Biotechnology 37, 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P, et al. (2008). Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 135, 632–641. [DOI] [PubMed] [Google Scholar]
- Murphy N, Jenab M, and Gunter MJ (2018). Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol 15, 659–670. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Chen PY, Dorans KJ, Chung KM, Bhutkar A, Hong E, Noll EM, Sprick MR, Trumpp A, and Jacks T (2017). Survival of pancreatic cancer cells lacking KRAS function. Nat Commun 8, 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Dorans KJ, Chung KM, Robbins R, Tammela T, Gocheva V, Li CM, and Jacks T (2016). Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers. Nat Commun 7, 12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadella S, Burks J, Al-Sabban A, Inyang G, Wang J, Tucker RD, Zamanis ME, Bukowski W, Shivapurkar N, and Smith JP (2018). Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor. Am J Physiol Gastrointest Liver Physiol 315, G699–G712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, and Jacks T (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860. [DOI] [PubMed] [Google Scholar]
- Olson OC, Quail DF, and Joyce JA (2017). Obesity and the tumor microenvironment. Science 358, 1130–1131. [DOI] [PubMed] [Google Scholar]
- Papke B, and Der CJ (2017). Drugging RAS: Know the enemy. Science 355, 1158–1163. [DOI] [PubMed] [Google Scholar]
- Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, Ji B, Huang H, Wang H, Fleming JB, et al. (2013). A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology 145, 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, Welch MW, Brais LK, Da Silva A, Li T, et al. (2018). Association of Alterations in Main Driver Genes With Outcomes of Patients With Resected Pancreatic Ductal Adenocarcinoma. JAMA Oncol 4, e173420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, and Matrisian LM (2014). Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74, 2913–2921. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, et al. (2018). Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 24, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KC, Bell KM, Yan J, Gu G, Chung CH, Washington MK, and Means AL (2011). Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One 6, e16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Hong TS, and Bardeesy N (2014). Pancreatic Adenocarcinoma. New England Journal of Medicine 371, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Saponaro C, Gmyr V, Thevenet J, Moerman E, Delalleau N, Pasquetti G, Coddeville A, Quenon A, Daoudi M, Hubert T, et al. (2019). The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release. Cell Rep 28, 1447–1454 e1444. [DOI] [PubMed] [Google Scholar]
- Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, Powers JD, Yenumula PR, and Arterburn DE (2019). Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann Surg 269, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, and Jemal A (2020). Cancer statistics, 2020. CA Cancer J Clin 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Skowronski AA, Ravussin Y, Leibel RL, and LeDuc CA (2017). Energy homeostasis in leptin deficient Lepob/ob mice. PLoS One 12, e0189784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Cooper TK, McGovern CO, Gilius EL, Zhong Q, Liao J, Molinolo AA, Gutkind JS, and Matters GL (2014). Cholecystokinin Receptor Antagonist Halts Progression of Pancreatic Cancer Precursor Lesions and Fibrosis in Mice. Pancreas 43, 1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, and Solomon TE (2014). Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol 306, G91–G101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR (2019). Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- Storz P (2017). Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 14, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA Research Network (2017). Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32, 185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CM, Himbert C, Holowatyj AN, and Hursting SD (2018). Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol 15, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M, and Volpi EV (2018). DNA damage in obesity: Initiator, promoter and predictor of cancer. Mutat Res 778, 23–37. [DOI] [PubMed] [Google Scholar]
- van de Bunt M, Manning Fox JE, Dai X, Barrett A, Grey C, Li L, Bennett AJ, Johnson PR, Rajotte RV, Gaulton KJ, et al. (2015). Transcript Expression Data from Human Islets Links Regulatory Signals from Genome-Wide Association Studies for Type 2 Diabetes and Glycemic Traits to Their Downstream Effectors. PLoS Genet 11, e1005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk D, Sharma R, Nainys J, Yim K, Kathail P, Carr AJ, Burdziak C, Moon KR, Chaffer CL, Pattabiraman D, et al. (2018). Recovering Gene Interactions from Single-Cell Data Using Data Diffusion. Cell 174, 716–729 e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, and Hakonarson H (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nasiri AR, Damsky WE, Perry CJ, Zhang XM, Rabin-Court A, Pollak MN, Shulman GI, and Perry RJ (2018). Uncoupling Hepatic Oxidative Phosphorylation Reduces Tumor Growth in Two Murine Models of Colon Cancer. Cell Rep 24, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC (2020). Glucolipotoxicity, beta-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes 69, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, and Goldfine ID (1985). The insulin-pancreatic acinar axis. Diabetes 34, 980–986. [DOI] [PubMed] [Google Scholar]
- Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, Stampfer MJ, Sato K, Ma J, Buring JE, et al. (2013). Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst 105, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, Cochrane BB, Rohan TE, Ma J, Pollak MN, et al. (2007). Circulating insulin-like growth factor binding protein-1 and the risk of pancreatic cancer. Cancer Res 67, 7923–7928. [DOI] [PubMed] [Google Scholar]
- Xu M, Jung X, Hines OJ, Eibl G, and Chen Y (2018). Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas 47, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Lee YS, Dong Y, Seidman JS, Yang M, Isaac R, Seo JB, Yang BH, Wollam J, Riopel M, et al. (2019). Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting beta Cell Proliferation and Function in Obesity. Cell Metab 29, 457–474 e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Bao Y, Wu C, Kraft P, Ogino S, Ng K, Qian ZR, Rubinson DA, Stampfer MJ, Giovannucci EL, et al. (2013). Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol 31, 4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaytouni T, Tsai PY, Hitchcock DS, DuBois CD, Freinkman E, Lin L, Morales-Oyarvide V, Lenehan PJ, Wolpin BM, Mino-Kenudson M, et al. (2017). Critical role for arginase 2 in obesity-associated pancreatic cancer. Nat Commun 8, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AMY, Magrill J, de Winter TJJ, Hu X, Skovso S, Schaeffer DF, Kopp JL, and Johnson JD (2019). Endogenous Hyperinsulinemia Contributes to Pancreatic Cancer Development. Cell Metab 30, 403–404. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Wan YD, Sun YL, Li J, and Zhu RT (2015). Aspirin might reduce the incidence of pancreatic cancer: A meta-analysis of observational studies. Sci Rep 5, 15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, Swartz-Basile DA, and Nakshatri H (2009). Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery 146, 258–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4. Characteristics of PDAC patients with available body mass index (BMI) and molecular alterations data, Related to Figure 3.
Table S5. Association of BMI with PDAC driver gene alterations in human tumors, Related to Figure 3.
Number (percentage) of tumors that are either wild-type (WT) or mutant (MUT; mutation by exome sequencing or loss by IHC) for each driver gene stratified by patient BMI. Odds ratios (OR) from logistic regression models were adjusted for age at surgery (continuous), study site, gender (men, women), grade (well/moderately differentiated, poorly differentiated/undifferentiated, unknown), perineural invasion (no, yes, unknown), lymphovascular invasion (no, yes, unknown), N stage (N0, N1, unknown), T stage (T1/T2, T3/T4, unknown), and tumor location (head/uncinate, body, tail, unknown). For cumulative analysis of PDAC drivers, total number of patients (N) and mean (standard deviation) number of driver alterations per tumor were stratified by patient BMI. p-value is based on the Kruskal-Wallis test.
Figure S1. Obesity drives pancreatic but not lung tumorigenesis in mice, Related to Figure 1.
A) Body weight (mean +/− s.e.m.) of KC mice of varying db genotype over time (n=4-12 mice/group).
B) Kaplan-Meier survival curves for KC mice of varying db genotype (n=14-18 mice/group). p-value of log-rank test is shown.
C) Body weight (mean +/− s.e.m.) of KrasLA2 mice (Johnson et al., 2001) fed a low-fat (LFD) or high-fat (HFD) diet (n=18-20 mice/group).
D) Kaplan-Meier survival curves for KrasLA2 mice fed a LFD or HFD (n=40-42 mice/group). p-value of log-rank test is shown.
Body weight (mean +/− s.e.m.) of KrasLA2 mice of varying ob genotype (n=3-9 mice/group).
F) Kaplan-Meier survival curves for KrasLA2 mice of varying ob genotype (n=15-19 mice/group). p-value of log-rank test is shown.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-tailed student’s t-test for weight comparisons at each time point.
Figure S2. Effects of leptin administration in cells and mice, Related to Figure 2.
A) Western blot showed dose-dependent leptin protein expression and secretion into media by 293T cells 48 hours after infection with AAV-Leptin versus AAV-GFP control. HSP90 is cellular protein loading control. WCL = whole cell lysate.
B) Serial ELISA measurements of serum leptin concentrations (mean +/− s.e.m.) following intramuscular injection of AAV-Leptin in adult ob/ob mice (n=5) demonstrated sustained leptin expression.
C) AAV-Leptin treatment reduced non-fasting blood glucose (mean +/− s.e.m.) 6 weeks post-infection of KCO mice (n=8-10 mice/group). ****p<0.0001, two-tailed student’s t-test. Mice were infected at 6 weeks of age.
D) AAV-Leptin restored fertility in ob/ob male and female mice. Shown are early postnatal progeny born from an AAV-Leptin-treated ob/ob female mated with an AAV-Leptin-treated ob/ob male.
E) Schematic of AAV treatment of tumor-bearing mice. AAV is administered at 3 months of age once tumors have progressed (see Figures 1C-E). Percent change in body weight for each mouse (n=10 mice/group) following AAV administration is shown.
F) Kaplan-Meier survival curves for mice in (E) showed no significant difference (p>0.05, log-rank test) between AAV treatments (n=10 mice/group).
G) Recombinant murine leptin (100 ng/mL) activated MAPK (pErk1/2) and STAT3 (pStat3) signaling in murine Kras/p53 mutant PDAC (mPDAC) cells (KPC-7307 and KPC-7310), which is abolished in leptin receptor knockout clones (KPC-7307 KO1 and KO2). Quantification of pErk/Erk and pStat3/Stat3 levels (normalized to untreated) is shown. Beta-tubulin is additional protein loading control. Leptin dose was chosen to be within an order of magnitude of that observed in the serum of AAV-Leptin-treated mice in (B).
H) Recombinant murine leptin (100 ng/mL) has no effect (p>0.05, two-tailed student’s t-test) on mPDAC cell viability (mean +/− s.e.m., normalized to untreated) 72 hours are treatment (n=5 replicates/condition).
Figure S3. Treatment studies in KCO mice, Related to Figures 4 and 7.
A) Schematic shows treatment strategy in which mice are placed on drinking water containing drug at 6 weeks of age and analyzed for tumor progression 6 weeks later. Percent change in body weight (mean +/− s.e.m.) is shown for each treatment (n=7-10 mice/group). The Cck receptor antagonist proglumide showed a modest slowing of weight gain.
B) Quantification of tumor burden (mean +/− s.e.m.) is shown for each treatment and control drinking water (n=7-10 mice/group). No significant differences were observed across pairwise comparisons of treatments with control (p>0.05, two-tailed student’s t-test).
C) Representative histologic sections of pancreata of mice at endpoint demonstrate no significant decrease in tumor burden (Ck19), inflammation (Cd45+ immune cells), or fibrosis (Sirius Red staining) across treatments in (B). Scale bar: 200 μm.
Figure S4. Insulin secretion and response in obese mice, Related to Figure 5.
A) Obese leptin-deficient (ob/ob) mice exhibited increased fasting serum insulin and proinsulin (C-peptide) levels (mean +/− s.e.m.) compared to non-obese controls (ob/+) at 10 weeks (n=2-3 mice/group). *p<0.05, **p<0.01, two-tailed student’s t-test.
B) Glucose-stimulated insulin secretion (mean +/− s.e.m., n=4 replicates/group) from perifused islets derived from 12 week-old C57/B6 wild-type (WT) and ob/ob mice (n=4 pooled mice/group) is shown. *p<0.05, **p<0.01, two-tailed student’s test for each timepoint.
C) Total insulin content (mean +/− s.e.m., normalized to DNA content) of cultured islets in (B) was not significantly different between groups.
D) Area under the curve (AUC) (mean insulin ng/mL/ng DNA +/− 95% confidence intervals) showed reduced glucose-stimulated insulin secretion (combined 1st and 2nd phase) for ob/ob islets in (B). AUC for potassium chloride (KCl)-induced depolarization was not significantly different, suggesting no change in overall insulin stores.
E) IHC revealed activated insulin receptor/Igf1 receptor (phospho-InsR/Igf1R) in PanINs in non-obese (KC) and obese (KCO) mice, consistent with insulin-induced signaling. pInsR/Igf1R increased in higher-grade PanINs (arrows). Pancreatic islets were internal positive controls (asterisks). Scale bar: 50 μm.
F) Average non-fasting glucose levels (mean +/− s.e.m.) of KCO mice during treatment with dapagliflozin (n=6-9 mice/group). ****p<0.0001, two-tailed student’s t-test.
G) Non-fasting insulin and C-peptide levels (mean +/− s.e.m) of mice in (F) after 6 weeks of treatment with dapagliflozin or control drinking water. Mice were started on treatment at 6 weeks of age. **p<0.01, two-tailed-student’s t-test.
H) C-peptide levels in (G) were inversely correlated with tumor burden. Each point represents one mouse (n=15, lavender circles are control, blue squares are dapagliflozin-treated). Correlation coefficient (R) and p-value of simple linear regression are shown.
I) IHC showed an inverse relationship between islet insulin and Cck expression in the dapagliflozin-treated KCO mice with the highest and lowest C-peptide levels in (G). Scale bar: 100 μm.
Figure S5. Obesity-associated islet Cck expression in mice, Related to Figures 6 and 7.
A) Obese (ob/ob) mice exhibited progressive expression of Cck in pancreatic islets (denoted by Syn (synaptophysin) immunostaining).
B) Non-fasting serum Cck levels (mean +/− s.e.m.) did not differ between 7-9 week-old obese ob/ob and non-obese ob/+ mice (n=7 mice/group).
C) AAV-leptin treatment of ob/ob mice reduced Cck expression by RNA-seq on bulk pancreata. Normalized Cck expression counts (mean +/− s.e.m, n=2 mice/group) are shown.
D) Obese KC; db/db mice exhibited increased islet Cck expression compared to non-obese KC; db/+ mice. Representative islets from two mice of each genotype are shown.
E) Islet Cck expression is abolished following weight loss induced by AAV-Leptin treatment or caloric restriction (CR) of KCO mice. Insulin denotes beta cells. Mice were analyzed at 12 weeks of age following intervention starting at 6 weeks of age.
All scale scale bars are 100 μm.
Figure S6. Cell proliferation and transcription factor alterations are associated with beta cell Cck expression, Related to Figure 6.
A) Cck+ beta cells showed relative upregulation of cell cycle genes (Kowalczyk et al., 2015). Fold change difference in average gene expression comparing Cck+ and Cck- beta cells are shown across these genes. Genes upregulated in Cck+ cells correspond to green dots, while orange dots correspond to genes upregulated in Cck- cells. This finding was confirmed by significant overlap (hypergeometric test) between genes upregulated in Cck+ cells (q<0.05) and the Cell Cycle gene ontology (C5 GO) gene set from MSigDB (inset).
B) Mean single cell expression counts (square-root transformed library size-normalized UMI/cell +/− s.d.) for beta cells (Ins2+) showed Cck expression predominantly in Ins2+/Ins1 and Ins2+/Gcg+ cells. Each colored dot represents a single cell. Gray circles represent median expression.
C) A greater percentage of polyhormonal Ins2+/Gcg+ cells expressed Cck than monohormonal Ins2+/Ins1+ cells.
D) PHATE visualization shows distribution of Ptprc+ (Cd45+) immune cells recovered by scRNA-seq of islets from ob/ob and wild-type (WT) mice.
E) The majority of Ptprc+ (Cd45+) cells in (D) expressed macrophage markers (Cd68, Cd74).
F) Gene ontology gene sets (C5 GO sets from MSigDB) showed significant overlap (hypergeometric test) with gene expression of islet macrophages from ob/ob (green) and WT (orange) mice.
G) allmark gene sets (H sets from MSigDB) showed significant overlap (hypergeometric test) with gene expression of islet macrophages from ob/ob (green) and WT (orange) mice.
H) Islet macrophages from ob/ob mice showed upregulation of cell proliferation genes.
I) Islet macrophages from ob/ob mice showed downregulation of cytokine, inflammation, and interferon-response genes.
J) Islet macrophages from ob/ob mice showed upregulation of beta cell growth factors.
K) Ehf and Tle3 expression was absent or markedly reduced in Cck+ beta cells from ob/ob mice.
L) Gene-gene expression plots after MAGIC (van Dijk et al., 2018) showed an inverse relationship between the expression of Ehf and Tle3 with Cck in ob/ob beta cells. Spearman correlation coefficients (R) are listed for each plot.
Figures S7. Exploring mechanisms of islet Cck-driven pancreatic tumorigenesis, Related to Figure 7.
A) MIP-Cck transgenic mice exhibited islet-specific Cck expression without evidence of increased pancreatic inflammation (Cd45+ leukocyte infiltration) or fibrosis (Sirius red collagen staining or presence of smooth muscle actin (SMA)-positive fibroblasts) compared to wild-type controls.
B) Cck was not significantly expressed in pancreatic islets in murine models of acute pancreatitis (CER = cerulein-induced at 12 or 48 hours, ARG = arginine-induced at 48 hours, PBS is control).
C) Cck was rarely expressed (arrows) in pancreatic islets in a murine model of chronic pancreatitis (cerulein-treated for 2 weeks). Frequencies of islets without staining (negative) or rare Cck+ cells (arrows) are shown.
D) CCK was rarely expressed (arrows) in pancreatic islets obtained from human patients with chronic pancreatitis. Frequencies of islets without staining (negative) or rare CCK+ cells (arrows) are shown. Of a total of 127 evaluable islets, 4 harbored rare CCK+ cells.
E) MKC mice displayed an increase in proliferating (Ki67+) acinar cells (arrows) compared to KC mice. The number of Ki67+ acinar cells per 10X field (mean +/− s.e.m., 4-5 fields/mouse, n=5 mice/group) is plotted. *p<0.05, two-tailed student’s t-test.
All scale bars are 100 μm.
Table S1. Tumor suppressor gene single nucleotide variants in KCO tumors, Related to Figure 3.
Mutant allele fractions for each coding nucleotide (compared to mm9 reference database) of tumor suppressor genes (Trp53, CDKN2A/p16, and SMAD4) derived from whole exome sequencing and RNA-seq are shown for individual KCO tumors and averaged across all tumors Average base coverage across KCO tumors is as follows: Trp53 (84X exome, 127X RNA), CDKN2A/p16 (40X exome, 89X RNA), and SMAD4 (103X exome, 86X RNA). Blank cells denote lack of base coverage in sequencing, which was rare and sporadic. A single KPC tumor (7075) was used as a positive control for single nucleotide variant (SNV) detection (resulting in a R172H codon substitution as noted). No tumor suppressor gene SNVs were detected in KCO tumors.
Tables S2. Single nucleotide variants in murine tumors, Related to Figure 3.
All single nucleotide variants (SNV) called in KCO (n=13) and KPC (n=1) tumors by whole exome sequencing are listed by tumor. Variant information includes location (chromosome, cytoband, and position in mm9 genome), genic location (exonic, intergenic, UTR), nucleotide change (var vs. ref), read counts for variant (tumor_reads2) and reference (tumor_reads1), and variant frequency (tumor_var_freq). Exon SNVs were further classified as synonymous or non-synonymous with reference to transcript (RefSeq), exon number (Exon_num), and coding sequence position (AA_change).
Table S3. Compilation of non-synonymous single nucleotide variants in murine tumors, Related to Figure 3.
Compiled non-synonymous single nucleotide variants (SNVs) identified on whole exome sequencing data derived from all sequenced KCO and KPC tumors from Table S2. A total of 531 non-synonymous SNVs were called from 14 tumors (~37.7 SNVs per KCO mouse (n=13) and 41 non-synonymous SNVs in KPC mouse (n=1)). Induced Kras, Lep (ob), and Trp53 mutations were observed as expected. Of consensus pan-cancer genetic drivers (Bailey et al., 2018), Arid5b, Cacna1a, Eef2, and Kit harbored recurrent non-synonymous SNVs. These SNVs were not observed in human cancer (COSMIC) to suggest pathogenicity.
Table S6. Independent component analysis (ICA) of mouse tumor RNA-seq, Related to Figures 4 and 5.
Log (base 2) transformed normalized expression counts per gene in individual KCO, KC, and KPC tumors used in RNA-seq analyses. Mouse number, genotype, and AAV treatments are shown. Gene signatures (Sig6, Sig12, and Sig13) that distinguish obese (KCO) and non-obese (KC and KPC) tumors by ICA with p<0.05 are shown. Per-gene expression correlation Z-scores with respect to the signature are listed (positive is correlated with KCO tumors) for each gene signature and ordered by log2 fold change comparing above groups. Gene set enrichment analysis (GSEA) results correlated with KCO tumors (UP) are shown comparing ICA-derived signatures (6, 12, and 13) with curated (C2) and hallmark (H) gene sets in MSigDB.
Table S7. Beta cell comparisons from scRNA-seq data, Related to Figure 6.
Composite gene expression differences of scRNA-seq data comparing ob/ob (OB) vs. wild-type (WT) beta cells or Cck+ positive vs. Cck- OB beta cells. Shown are genes ordered by log2FC (fold change) including mean expression counts, p-values, and q-values, as measured using a Mann-Whitney U test implemented in diffxpy for each gene.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the NCBI Sequencing Read Archive (SRA) under accession number PRJNA544740 and the NCBI Gene Expression Omnibus (GEO) under accession numbers GSE131714 and GSE137236. Primary murine PDAC cell lines and constructs are available upon request. Python scripts for scRNA-seq analyses are available on GitHub (https://github.com/KrishnaswamyLab). Computer code for RNA-seq signature analysis (ICA) will be made available upon request. Other software tools (including version numbers) for exome and RNA-seq analyses are listed above or referenced.