Abstract
Objectives:
It has been known for decades that neurons in vitro and in vivo respond in a polarity-specific manner to changes in their electrical environment. Likewise, investigators have passed direct current (DC) across the human head for decades in attempts to alter brain function and behavior. Recent human data, however, have put this technique on a more solid empirical footing and it has re-emerged from obscurity as a“new,”noninvasive means of neuromodulation, called transcranial direct current stimulation (TDCS).
Materials and Methods:
Here, we offer a selective literature review together with our own research on the basic mechanisms and human applications of TDCS in neurophysiologic, cognitive, and behavioral research. We discuss a possible role for TDCS in enhancing normal brain function and treating neurologic and behavioral disorders.
Results:
While there are uncertainties about how TDCS produces behavioral effects and how the current is distributed in the human brain, TDCS has safely produced a variety effects on human brain function in small studies.
Conclusions:
The field is very young and many findings will require replication. Nevertheless, TDCS appears to have the potential to be a simple and safe means of neuromodulation.
Keywords: Brain stimulation, cognitive enhancement, electrical Helds, transcranial stimulation
INTRODUCTION AND BACKGROUND
Noninvasive, focal modulation of brain function became a reality with the advent of repetitive transcranial magnetic stimulation (rTMS) in the 1990s. While rTMS has the advantages of temporal precision, spatial focality, and the ability to evoke quantifiable, online responses, it has drawbacks as a neuromodulatory technique, including safety issues, high cost, and a bulky delivery system with high power requirements. Particularly, where the experimental or therapeutic goal is facilitation of brain processes, alternative means are desirable. Transcranial direct current (DC) polarization of the brain, a partially forgotten technique for modulating brain function that enjoyed periods of clinical and scientific interest as long as two centuries ago (1), is now being re-explored. This article describes what is known about the basic mechanisms and reviews recent literature on human applications of the technique in the areas of neurophysiology, cognitive neuroscience, and neuroreha-bilitation. Modern articles, illustrative of the principles discussed, were selected from a Medline search for the term“transcranial direct current stimulation.”It is not intended as an exhaustive review of the subject.
The technique is commonly known as transcranial direct current stimulation (TDCS) despite the fact that it creates a modulatory change in the electrical environment of neurons, rather than a discrete stimulus. In this sense, its action is similar to the local application of a pharmacological agent. This is in contrast to rTMS, which fires neurons with each pulse, and probably produces its neuromodulatory effects by altering synaptic effcacy through mechanisms similar or identical to long-term synaptic potentiation and depression (2). Although TDCS works by passing very weak currents through a poor conductor, the human head, a growing body of evidence shows that it can change the response of cerebral neurons and alter and, perhaps, improve cognitive functions in patients with neurobehavioral disorders, as well as healthy individuals.
The effects of static electrical fields on cortical neurons in vivo have been known almost since the advent of intracellular recording. Single-cell studies show that DC fields do not directly activate neurons, but, rather, change their excitability and firing rates by redistributing charges and changing the membrane potential along their axes (Fig. 1) (3). In mammals, weak polarizing currents applied to the brain surface can produce lasting changes in cortical-evoked potentials and the activity of individual cortical neurons (4–6). These effects are highly selective for neurons oriented longitudinally in the plane of the electric field. For instance, when the active electrode is applied to the cortical surface, the effect is selective for radially oriented cortical output (pyramidal) neurons (7). The directional selectivity of the DC effect can also be demonstrated in isolated neuronal preparations in vitro (8) and in brain areas, such as the cerebellum (9). In virtually all studies, surface-anodal polarization of the cortex (anode near the dendritic poles of radially oriented neurons) increases the firing rates of spontaneously active cells, but does not cause spontaneous firing. Surface-cathodal polarization has the opposite effect, down-modulating firing. Data indicate that these effects are mediated by changes in voltage-sensitive cation channels (10,11).
Figure 1.
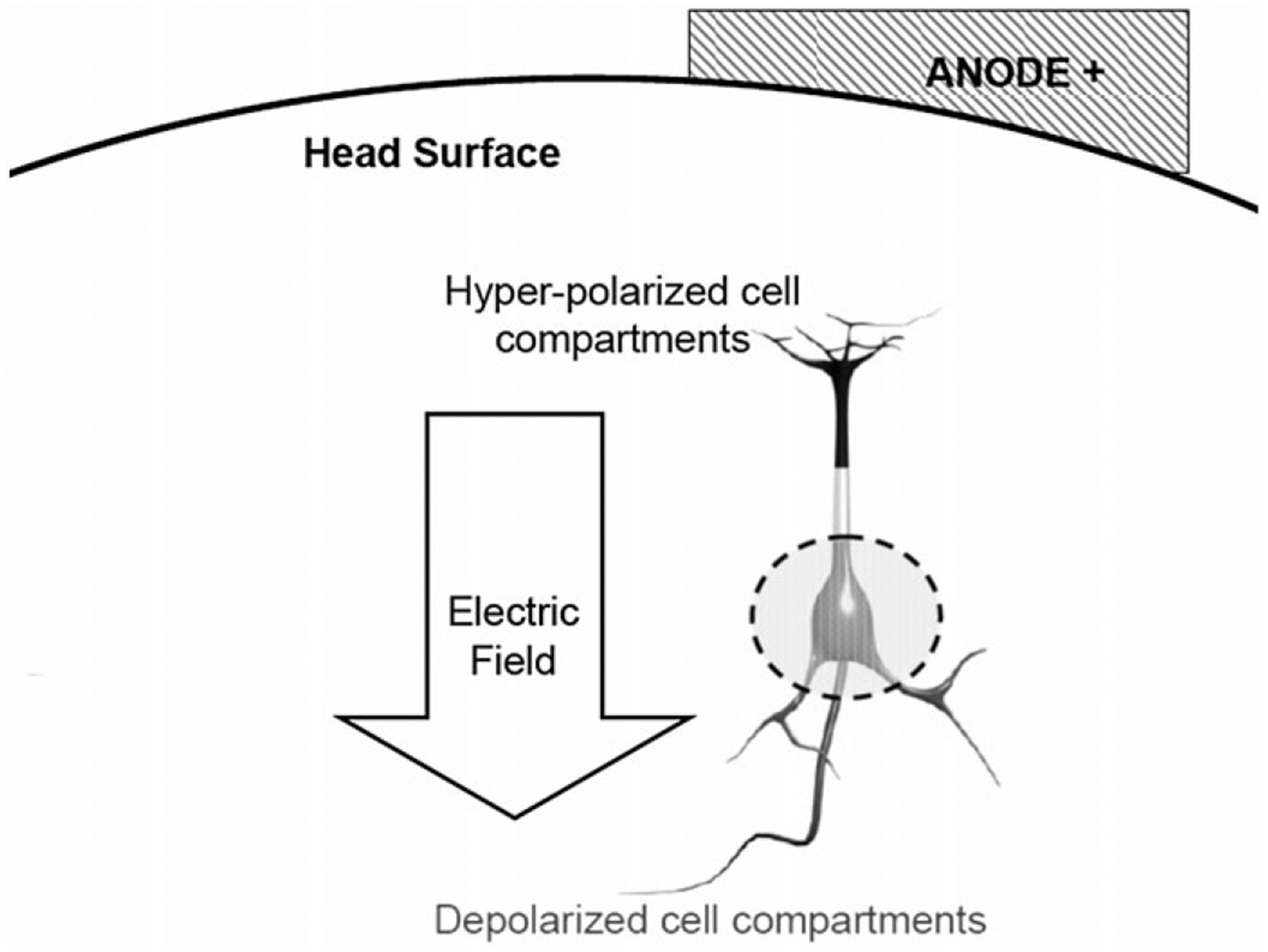
Cartoon of transcranial direct current stimulation. The anode, placed on the head surface, drives positive charge along the axis of a radially oriented neuron, hyperpolarizing nearby (dendritic regions) and depolarizing the cell body (circled). Courtesy of Dr. Marom Bikson.
Modern human experimentation with TDCS current has generally employed current-controlled apparatus to deliver currents in the 0.5–2 mA range through large (25–35 cm2) moistened sponge electrodes applied to the head. Current densities at the electrode face have generally been between 20 and 80 μA/cm2. Physical models suggest that approximately half the current is shunted through the scalp (12) and another significant fraction through the cerebrospinal fluid (13,14). With a current of 2.0 mA delivered to the scalp, current density in nearby brain tissue has been estimated at 1 μA/ cm2, yielding a field strength of 0.22 mV/mm. There are no direct measures of the focality of the technique. However, detailed, finite element models of current flow in the head, based on brain MRIs, suggest that the current diffuses rapidly away from the electrodes and concentrates in cerebrospinal fluid spaces where conductivity is high; for example, in sulci between the electrodes, rather than directly under them (15,16). Even very simple spherical head models (12) indicate that most of the current is concentrated around the edges of the electrodes. This brings into question the claims of investigators having polarized specified areas, simply because they have centered large electrodes over them. The physiologic action of the current is presumably near the surface, as the electric field diffuses rapidly in the volume of the head. Recent brain polarization experiments (see below) usually place both electrodes on the head. However, at least in theory, the reference electrode can be placed anywhere on the body, thereby ensuring that it exerts no neurophysiologic effects of its own.
The safety of brief exposure to TDCS at moderate intensities has been studied (17–19), and no adverse effects on psychomotor performance, the electroencephalogram, or other clinical measures of brain function have been reported. Intensities in the conventional range produce mild skin irritation and an easily tolerated sensation. However, skin burns are possible when high currents were delivered through small electrodes. An old report (20) described an episode of transient respiratory paralysis and longer-lasting limb incoordination experienced by a subject who received ten times the intended amperage. The reference electrode was placed on a limb, raising the possibility that current became concentrated near neural structures in the neck. No such side-effects have been reported in modern studies with extracephalic electrodes (21,22).
HUMAN MOTOR CORTEX AND MOTOR EFFECTS
Priori et al. (23) were the first to show that a weak current (0.5 mA through a 25 cm2 electrode) could modulate the excitability of the human motor cortex, as measured by the amplitude of the motorevoked potential (MEP) from TMS. The effect of TDCS on the MEP was described and explored further by Nitsche et al. (24–26) who obtained their best results with the reference electrode over the orbit contralateral to the treated motor cortex (24). This arrangement has the disadvantage of having the reference over the brain and theoretically capable of producing its own effects. In subsequent experiments (27), they showed that the acute and lasting facilitatory effects of surface-anodal polarization on MEP amplitude were blocked by the Na+ channel blockers carbamazepine and flunarizine, while the lasting effect was prevented with dextromethorphan, which antagonizes N-methyl-D-aspartate glutamate receptors, in addition to other effects. This was interpreted as involvement of “plastic” mechanisms in the cortex, presumably through changes in the effcacy of excitatory synapses. However, consistent with the in vitro and animal data, the lasting effect of motor cortex stimulation is present when the MEP is evoked with an electric pulse that stimulates the corticospinal fibers below the cortex (28), indicating that changes in neuronal excitability are involved. This mechanism also is compatible with the prevention of the lasting effect of cortical polarization by Na+ channel blockers (10,11).
TDCS of the motor cortex can improve motor function and shows promise as an adjunct to therapeutic retraining in brain-lesioned patients. Anodal polarization of the affected motor cortex in chronic stroke patients increased the effect of hand rehabilitation training (29). Cathodal polarization of the unaffected motor cortex can also improve hand function, presumably by reducing interhemispheric competition (30). In Parkinson disease, anodal polarization of the primary motor cortex can also improve motor function (31).
The majority of studies using TDCS have focused on the cerebral cortex. However, the cerebellum provides another target for noninvasive stimulation in humans. Compared with sham, anodal polarization of the right cerebellum appears to enhance inhibitory output to primary motor areas, while cathodal polarization produces the opposite effect (32).
SENSORY AND PERCEPTUAL EFFECTS
In a manner analogous to its effects in the motor cortex, TDCS can also modulate the sensitivity of sensory areas to exogenous stimulation. Surface-anodal polarization of the occipital area enhances contrast sensitivity, while cathodal current does the opposite (33). Effects on the excitability of visual cortex can also be measured as the threshold for the production of phosphenes with occipital TMS (34,35), which decreases with anodal current, or by the amplitude of the visual-evoked potential (36), which increases. Cathodal current delivered to the somatosensory cortex increased a tactile discrimination threshold (37); concordant results were found for the cortical components of the somatosensory-evoked potential (38). Conversely, tactile spatial acuity improved with anodal TDCS of the somatosensory cortex (39).
As in the motor system, TDCS can produce potentially therapeutic effects on sensory processes. Anodal polarization of the motor cortex appears to have an analgesic effect in spinal cord injury patients with neurogenic pain (40). In patients with fibromyalgia, anodal polarization of primary motor cortex can diminish pain for at least three weeks after treatment (41). These results suggest that artificially modulating cortical excitability can influence some of the maladaptive changes in the brain associated with chronic pain. The mechanism of action is poorly understood, but animal and human studies indicate that stimulation of the primary motor cortex, and not the sensory cortex, reduces the abnormal thalamic activity associated with chronic pain (42).
COGNITIVE EFFECTS
As might be expected, TDCS can enhance cognitive processes occurring in targeted brain areas. In one of the earliest demonstrations of this principle, anodal polarization of the motor cortex was used to speed the implicit acquisition of a repeated sequence of key presses, in the “serial reaction time task” (43). Interestingly, application of the same current to premotor and prefrontal areas, regions thought to participate in implicit learning, had no such effect.
In other studies, however, prefrontal cortex polarization produced an array of interesting and potentially useful effects on implicit learning. In one instance, anodal polarization of the left prefrontal cortex accelerated the acquisition of implicit knowledge about the probabilistic relationship between sets of cues and outcomes (44). Several studies have looked for effects of TDCS on various aspects of memory. Anodal current delivered to the dorsolateral prefrontal area improved response accuracy on a “3-Back,” delayed match-to-sample task in healthy subjects (45). This type of effect may increase response accuracy for up to 30 min after treatment (46). In another set of experiments (47), anodal current delivered to the left perisylvian area enhanced associative verbal learning, improving both speed and accuracy, during the acquisition of novel object names. In a particularly interesting study (48), anodal current applied to both lateral frontal areas during slow-wave sleep, but not while awake, enhanced retention of word pairs and mirror-tracing skill acquired previously. Unlike most other recent studies, the current was applied through small (8-mm diameter) electrodes, producing a relatively high-current density at the skin (26 μA/cm2).
We performed a large (N = 103), single-blind trial designed primarily to establish safety (17). Anodal polarization of the left prefrontal area at 40 μA/cm2 produced a trend-level increase in the ability to generate lists of words beginning with specified letters, relative to sham and cathodal polarization. The same effect reached statistical significance when the current was doubled. Cathodal current produced no significant changes and there were no effects of either current polarity on the electroencephalogram or a variety of other tasks, such as response inhibition and reaction time, which were included as safety tests and controls for nonspecific effects. It also was notable that the mean performance increment in word generation was about 20%. Nine of ten subjects in the anodal group showed it, the exception being a single, left-handed individual.
In single-session demonstration studies, brain polarization can improve cognitive function in patients with various impairments. For instance, anodal polarization of the left dorsolateral prefrontal cortex improved working memory, tested with a three-back delayed match task, in patients with Parkinson disease (49). Others (21) showed that anodal current applied to both temporoparietal areas with the anode off the head improved memory for words in Alzheimer disease patients. TDCS can also improve recovery from cognitive deficits after focal brain damage. In an unexpected outcome, cathodal current applied over the left frontotemporal areas in eight patients with chronic nonfluent aphasia increased picture-naming accuracy, whereas anodal and sham stimulation had no effect (50). The varied and contradictory results in this area may be explained, in part, by differences in methodology, such as electrode size and placement, current intensity, and duration. Additional systematic research is needed to clarify the effect of TDCS on cognitive processes, but there is potential for using noninvasive stimulation for cognitive enhancement.
EFFECTS ON MOOD AND BEHAVIOR
The earliest clinical application of DC brain polarization was in the field of mood disorders, and there was some mainstream clinical interest until the 1970s (51). In the most notable study (20), 32 subjects (most judged to be subclinically depressed), recruited from the staff and patients in a hospital psychiatric clinic, were treated with currents of up to 0.5 mA, delivered through two 0.5-inch diameter electrodes placed above the orbits and referenced to an off-head electrode. Current densities at the skin were comparable to those in modern studies. Treatment was continued for several hours while the subjects went about their business at the hospital. Clinician raters, blind to the polarity in each case, observed and questioned the subjects periodically. They were able to guess the polarity correctly in 26 of the 32 subjects, based on informal observation. Under anodal treatment, subjects tended to become elated and talkative; when the current was reversed, affect became withdrawn and depressed. The current had to be reduced in some subjects because the cathodal effect, in particular, caused concern among the raters. In a subsequent, randomized, double-blind trial in 24 clinically depressed patients (52), anodal current (≤250 μA) and sham were delivered for 12 days each in a cross-over design. While patients improved on both treatments, active current was associated with a significantly greater improvement in clinician ratings. Recently, Boggio et al. (53) conducted a 14-day trial in depressed patients, comparing ten daily sessions of left prefrontal anodal current with a sham and an “active” control (occipital anodal current). Anodal polarization of the dorsolateral prefrontal cortex significantly decreased clinical depression scores on two scales, compared with both controls. In a double-blind study (22), we were unable to produce emotional changes in a group of healthy subjects with bifrontal current delivered for 20 min through large (25 cm2) electrodes. However, two individuals experienced subjective and objective changes consistent with the experience of Lippold and Redfearn (20)
There is evidence that TDCS can influence other behaviors mediated by frontal lobe circuits. In healthy subjects, polarization of the lateral prefrontal cortex can modulate risk-taking behavior on gambling tasks: right anodal/left cathodal DC decreased the frequency of risky choices, while left anodal/right cathodal DC increased risky decision-making (54,55). Unilateral polarization seemed to have no effect. The effect of TDCS on decision-making during gambling tasks may generalize to other forms of risky and impulsive behavior, such as substance abuse and overeating. Bilateral polarization of the lateral prefrontal cortex reduced alcohol cravings compared with sham stimulation, but there was no apparent difference between right anodal/left cathodal and left anodal/right cathodal electrode placements (56). Bilateral anodal polarization of the dorsolateral prefrontal cortex also significantly reduced cue-provoked smoke cravings (57). Right anodal/left cathodal polarization, as well as the reverse polarity, appeared to modulate appetitive responses to images of food (58).
A study by Knoch et al. (59) applied TDCS simultaneously to both members of pairs of subjects playing the ultimatum game (60). Participants who received cathodal TDCS to the right prefrontal cortex were more likely to accept unfair money offers than those in the sham condition. These results suggest that cathodal TDCS of the right prefrontal cortex could modulate self-interest or emotiondriven decision-making and offer a parallel to the results of a rTMS study (61) from the same group showing that disrupted activity in that area increased acceptance of unfair offers during the game.
COMMENT AND CONCLUSIONS
As the foregoing illustrates, this is a field with great promise and a rapidly increasing store of data requiring replication and explanation. At present, TDCS seems to hold the greatest promise as a neuromodulatory technique for therapeutic application. However, the apparently low risk invites using it to enhance cerebral capacities in healthy people. Here, we tend to think first of cognitive abilities, but there may also be the possibility of modifying emotional processing. Ethical issues related to neural enhancement in healthy people (62), and particularly to noninvasive brain stimulation (63), have been raised. Guidelines have been issued for clinicians who are asked by their patients to provide neural enhancements and the existing regulatory apparatus is competent to address approval of treatments for this purpose (64). The ethical consensus seems to be that, while prescription of enhancements is not obligatory, it is permissible and even desirable when the social benefits outweigh the risks (64). One can imagine instances where there would be a social imperative for safe enhancement, e.g., for critical personnel in health care, transportation, and military operations.
Unlike prescription medication and, particularly, invasive techniques such as deep brain stimulation, there are currently few barriers to accessing TDCS outside of sanctioned channels. This may render regulatory, if not ethical considerations moot, and the simplicity and ease of circumventing patents on devices could prevent large-scale commercialization or even industry applications for approval. For this reason, industry-supported, pivotal trials may be unlikely. At least in the near future, small studies may provide the best opportunity to define the risks and benefits. Presently, the risks seem low, but undesirable effects, for example, temporary impairment of nonenhanced brain areas and functions is possible, if neural or metabolic resources are shifted to enhanced areas. Caution is certainly advisable, especially in long-term applications. We encourage all investigators to include safety outcomes, especially validated screens for impairment, in their studies.
If TDCS is to become a neuroscience tool in the mode of rTMS, the conventional technique of applying moistened sponges to the head needs to be improved and a better understanding of how the stimulating current is distributed in the head is essential (15,16). More precise methods, using smaller, closely spaced electrodes and spatially distributed references are on the horizon (15), but will require clinical validation. There is also an important knowledge gap between single-cell and behavioral data. Future studies will have to address how modulating neural activity in large cortical areas causes behavioral changes. Providing and testing a detailed theory is, however, hampered by the above-mentioned targeting uncertainty. Regardless of these issues, the simplicity, relative safety, and apparent promise of TDCS are likely to attract increased attention in the next few years.
COMMENTS.
The main issue that is brought by the authors of this comprehensive (and very timely) review is whether direct current (DC) polarization is a true neuromodulation. With ubiquitous use of alternating currents with transcutaneous and implanted approaches, and more recently introduced transcranial magnetic stimulation, DC stimulation has been investigated in only handful of centers and for relatively few indications. It definitely alters function of nervous system in a temporary and reversible way, and the response seems to be parameter-dependent—so it does seem to fit all criteria for neuromodulation. The only things missing are clear understanding of its mechanism and reproducibility of results as it seems that each group of researchers get somewhat different results in each subsequent study.
Lack of uniform specifications for devices, electrodes, application sites, and stimulation parameters makes it very diffcult to draw meaningful conclusions regarding the modality in general, but I agree with the authors that the potential for future applications of this modality is rather large. Effects on mood, depression, cognition, and learning will be likely targets for DC brain stimulation—and I look forward to seeing more scientific information on these and other applications in the near future.
Acknowledgments
The authors wish to thank Devera G. Schoenberg, MSc, for her skillful editing. This work was supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke (NINDS) and the Defense Advanced Research Projects Agency (DARPA).
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/bw/submit.asp?ref=1094-7159&site=1
REFERENCES
- 1.Priori A Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol 2003;114:589–595. [DOI] [PubMed] [Google Scholar]
- 2.Classen J,Stefan K.Changes in TMS measures induced by repetitive TMSIn:Wasser-mann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, eds. The Oxford handbook of transcranial stimulation. Oxford: Oxford University Press, 2008: 185–200. [Google Scholar]
- 3.Bikson M, Radman T, Datta A. Rational modulation of neuronal processing with applied electric fields. Conf Proc IEEE Eng Med Biol Soc 2006;1:1616–1619. [DOI] [PubMed] [Google Scholar]
- 4.Bindman LJ, Lippold OC, Redfearn JW. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature 1962;196:584–585. [DOI] [PubMed] [Google Scholar]
- 5.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol 1962;5:436–452. [DOI] [PubMed] [Google Scholar]
- 6.Purpura DP, McMurtry JG. Intacellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 1964;18:166–185. [DOI] [PubMed] [Google Scholar]
- 7.Hern JEC, Landgren S, Philips CG, Porter R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon’s motor cortex. J Physiol (Lond) 1962;168:890–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terzuolo CA, Bullock TH. The measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc Natl Acad Sci USA 1956;42:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol 1986;371:89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CY, Hounsgaard J, Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol 1988;402:751–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez L, Chan CY, Okada YC, Nicholson C. Multimodal characterization of population responses evoked by applied electric field in vitro:extracellular potential,magnetic evoked field, transmembrane potential, and current-source density analysis. J Neurosci 1991;11:1998–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 2006;117:1623–1629. [DOI] [PubMed] [Google Scholar]
- 13.Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV. Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr Clin Neurophysiol 1993;86:183–192. [DOI] [PubMed] [Google Scholar]
- 14.Nathan SS, Lesser RP, Gordon B, Thakor NV. Electrical stimulation of the human cerebral cortex.Theoretical approach. Adv Neurol 1993;63:61–85. [PubMed] [Google Scholar]
- 15.Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations:FEM analysis.J Neural Eng 2008;5:163–174. [DOI] [PubMed] [Google Scholar]
- 16.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial DC stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulat 2009;2:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer MB, Mattu U, Grafman J, Lomarev MP, Sato S, Wassermann EM. Safety and Cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005;64:872–876. [DOI] [PubMed] [Google Scholar]
- 18.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 2003;114:2220–2222.author reply 2222–2223. [DOI] [PubMed] [Google Scholar]
- 19.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 2007;72:208–214. [DOI] [PubMed] [Google Scholar]
- 20.Lippold OC, Redfearn JW. Mental Changes Resulting From The Passage Of Small Direct Currents Through The Human Brain. Br J Psychiatry 1964;110:768–772. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci R, Mameli F, Guidi I et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 2008;71:493–498. [DOI] [PubMed] [Google Scholar]
- 22.Koenigs M, Ukueberuwa D, Campion P, Grafman J, Wassermann E. Bilateral frontal transcranial direct current stimulation:Failure to replicate classic findings in healthy subjects. Clin Neurophysiol 2009;120:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport 1998;9:2257–2260. [DOI] [PubMed] [Google Scholar]
- 24.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 (Pt 3):633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57:1899–1901. [DOI] [PubMed] [Google Scholar]
- 26.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W.Modulation of cortical excitability by weak direct current stimulation—technical, safety and functional aspects. Suppl Clin Neurophysiol 2003;56:255–276. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche MA, Fricke K, Henschke U et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 2003;553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol 2005;568:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hummel F, Celnik P, Giraux P et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490–499. [DOI] [PubMed] [Google Scholar]
- 30.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 2007;25:123–129. [PubMed] [Google Scholar]
- 31.Fregni F, Boggio PS, Santos MC et al. Noninvasive cortical stimulation with transc- ranial direct current stimulation in Parkinson’s disease. Mov Disord 2006;21:1693–1702. [DOI] [PubMed] [Google Scholar]
- 32.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 2009;29:9115–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antal A,Nitsche MA,Paulus W.External modulation of visual perception in humans. Neuroreport 2001;12:3553–3555. [DOI] [PubMed] [Google Scholar]
- 34.Antal A, Kincses TZ, Nitsche MA, Paulus W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsycho- logia 2003;41:1802–1807. [DOI] [PubMed] [Google Scholar]
- 35.Antal A, Kincses TZ, Nitsche MA, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res 2003;150:375–378. [DOI] [PubMed] [Google Scholar]
- 36.Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 2004;45:702–707. [DOI] [PubMed] [Google Scholar]
- 37.Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. Eur J Neurosci 2004;20:313–316. [DOI] [PubMed] [Google Scholar]
- 38.Matsunaga K,Nitsche MA,Tsuji S,Rothwell JC.Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neuro- physiol 2004;115:456–460. [DOI] [PubMed] [Google Scholar]
- 39.Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol 2008;119:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fregni F, Boggio PS, Lima MC et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006;122:197–209. [DOI] [PubMed] [Google Scholar]
- 41.Fregni F, Gimenes R, Valle AC et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 2006;54:3988–3998. [DOI] [PubMed] [Google Scholar]
- 42.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 2008;15:1124–1130. [DOI] [PubMed] [Google Scholar]
- 43.Nitsche MA, Schauenburg A, Lang N et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 2003;15:619–626. [DOI] [PubMed] [Google Scholar]
- 44.Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia 2004;42:113–117. [DOI] [PubMed] [Google Scholar]
- 45.Fregni F,Boggio PS,Nitsche M et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 2005;166:23–30. [DOI] [PubMed] [Google Scholar]
- 46.Ohn SH, Park CI, Yoo WK et al. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 2008;19:43–47. [DOI] [PubMed] [Google Scholar]
- 47.Floel A, Rosser N, Michka O, Knecht S, Breitenstein C. Noninvasive brain stimulation improves language learning. J Cogn Neurosci 2008;20:1415–1422. [DOI] [PubMed] [Google Scholar]
- 48.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci 2004;24:9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boggio PS, Ferrucci R, Rigonatti SP et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci 2006;249:31–38. [DOI] [PubMed] [Google Scholar]
- 50.Monti A,Cogiamanian F,Marceglia S et al. Improved naming after transcranial direct current stimulation in aphasia.J Neurol Neurosurg Psychiatry 2008;79:451–453. [DOI] [PubMed] [Google Scholar]
- 51.Lolas F Brain polarization: behavioral and therapeutic effects. Biol Psychiatry 1977;12:37–47. [PubMed] [Google Scholar]
- 52.Costain R, Redfearn JW, Lippold OC. A controlled trial of the therapeutic effect of polarization of the brain in depressive illness. Br J Psychiatry 1964;110:786–799. [DOI] [PubMed] [Google Scholar]
- 53.Boggio PS,Rigonatti SP,Ribeiro RB et al. A randomized,double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol 2008;11:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex:a direct current stimulation study. J Neurosci 2007;27:12500–12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fecteau S, Pascual-Leone A, Zald DH et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci 2007;27:6212–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boggio PS,Sultani N,Fecteau S et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008;92:55–60. [DOI] [PubMed] [Google Scholar]
- 57.Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 2008;69:32–40. [DOI] [PubMed] [Google Scholar]
- 58.Fregni F, Orsati F, Pedrosa W et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 2008;51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knoch D,Nitsche MA,Fischbacher U,Eisenegger C,Pascual-Leone A,Fehr E.Studying the neurobiology of social interaction with transcranial direct current stimulation— the example of punishing unfairness. Cereb Cortex 2008;18:1987–1990. [DOI] [PubMed] [Google Scholar]
- 60.Güth W, Schmittberger R, Schwarze S. An experimental analysis of ultimatum bargaining. J Econ Behav Organ 1982;3:367–388. [Google Scholar]
- 61.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 2006;314:829–832. [DOI] [PubMed] [Google Scholar]
- 62.Farah MJ. Neuroethics: the practical and the philosophical. Trends Cogn Sci 2005;9:34–40. [DOI] [PubMed] [Google Scholar]
- 63.Illes J, Gallo M, Kirschen MP. An ethics perspective on transcranial magnetic stimulation (TMS) and human neuromodulation. Behav Neurol 2006;17:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larriviere D, Williams MA, Rizzo M, Bonnie RJ. Responding to requests from adult patients for neuroenhancements:guidance of the Ethics,Law and Humanities Committee. Neurology 2009;73:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]


