Abstract
BACKGROUND
Although amyloid-β and microstructural brain changes are both effective biomarkers of Alzheimer’s disease, their independent or synergistic effects on cognitive decline are unclear.
OBJECTIVE
To examine associations of amyloid-β and brain microstructure with cognitive decline in amnestic mild cognitive impairment and dementia.
METHODS
Restriction spectrum imaging (RSI), CSF amyloid-β and longitudinal cognitive data were collected on 23 healthy controls and 13 individuals with mild cognitive impairment or mild Alzheimer’s disease. Neurite density (ND) and isotropic free water diffusion (IF) were computed in fiber tracts and cortical regions of interest. We examined associations of amyloid-β with regional and whole-brain microstructure, and assessed whether microstructure mediates effects of amyloid-β on cognitive decline.
RESULTS
Lower ND in limbic and association fibers and higher medial temporal lobe IF predicted baseline impairment and longitudinal decline across multiple cognitive domains. ND and IF predicted cognitive outcomes after adjustment for amyloid-β or whole-brain microstructure. Correlations between microstructure and cognition were present for both amyloid-positive and amyloid-negative individuals. Amyloid-β correlated with whole-brain, rather than regional, ND and IF.
CONCLUSION
Amyloid-β correlates with widespread microstructural brain changes, whereas regional microstructure correlates with cognitive decline. Microstructural abnormalities predict cognitive decline regardless of amyloid, and may inform about neural injury leading to cognitive decline beyond that attributable to amyloid.
Keywords: MRI, diffusion imaging, Alzheimer’s disease, memory, aging, mild cognitive impairment, cognitive decline, amyloid, dementia
Introduction
In Alzheimer’s disease (AD), amyloid-β burden becomes abnormally elevated up to a decade prior to dementia diagnosis [1], suggesting that it is an early step in the progressive neurodegenerative cascade. On the backdrop of amyloid-β accumulation, the disease course is accompanied by cytoarchitectural changes, including neurite and synapse loss, demyelination, gliosis, and cell death [2–5]. Whereas amyloid-β levels rise before observable neurodegeneration, atrophy corresponds more closely with cognitive symptoms [6]. If white matter changes are more sensitive to early cognitive deficits than gray matter atrophy as has been suggested [7], they may better track cognitive decline than amyloid, though few studies have directly compared these biomarkers. The closer correspondence of structural changes than amyloid neuropathology to cognitive symptoms implies that, even if amyloid is an early disease trigger, the ensuing neurodegeneration may diverge along a distinct pathogenic avenue. However, the unique contributions of amyloid-β and cytoarchitectural change to cognitive decline, and any synergistic effects between them, remain unclear.
Diffusion tensor imaging (DTI) studies have identified associations between amyloid-β and degenerative microstructural changes. In cognitively normal older adults, higher amyloid-β burden correlates with reduced fractional anisotropy (FA) of the fornix and corpus callosum [8, 9], and amyloid-positive individuals demonstrate higher white matter axial diffusivity [10] and accelerated decline in parahippocampal cingulum FA [11]. Induced amyloid-β pathology in mice reduced the apparent diffusion coefficient [12] and increased white matter radial diffusivity in conjunction with axonal and myelin loss [13], suggesting that amyloid-β may directly alter cell organization. Alternatively, amyloid-β may exacerbate injury from concomitant factors that compromise cytoarchitectural integrity. Reports that amyloid-β and tau propagation are associated with genes related to dendrites and axons, respectively [14], suggest that neuropathological spread of these proteins could elicit distinct effects on neurites.
Others have reported absent or unexpected links between amyloid-β and microstructural change, and inconclusive findings regarding their effects on cognitive function. For instance, in AD tau, but not amyloid-β, is associated with FA and mean diffusivity (MD) and corresponding cognitive deficits [15], and microstructure predicts cognitive decline regardless of amyloid-β [16]. Other studies reported no difference in FA between amyloid-positive and negative controls [11], and no correlation between FA and amyloid-β [17]. Another found associations between amyloid-β and higher axial diffusivity, but no other DTI measures [10]. Paradoxically, one study reported increased FA and reduced MD, typically interpreted as preserved cell integrity, with elevated amyloid burden [18]. Incongruence across prior findings may stem from the aggregate influence of an array of cytoarchitectural properties on conventional diffusion imaging metrics. Thus, examining more refined measures of brain microstructure may clarify the nuanced relationship between neuropathological burden and neural injury.
Advanced diffusion MRI methods have improved our ability to characterize complex cell architecture beyond the resolution of DTI. Restriction spectrum imaging (RSI) uses a multi-shell, multi-direction acquisition to integrate length-scale and diffusion orientation information into the same tissue model. RSI thus enables separation of restricted, hindered, and free water diffusion, which histological studies suggest correspond respectively with intra-neurite, extra-neurite, and CSF compartments [19]. We recently reported that RSI measures of restricted and free water diffusion are sensitive to cognitive impairment [20] and predict cognitive decline [21] in prodromal AD. Further, we observed associations of amyloid-β with neurite density (ND) and isotropic free water (IF), but not with conventional DTI measures [20], suggesting that RSI may better identify subtle neuropathology-related cytoarchitectural change that is undetectable by DTI.
Here, we expanded upon our previously reported correlation between amyloid-β and brain microstructure to investigate the role of amyloid-β in the association between microstructure and cognitive decline across the AD spectrum. We hypothesized that RSI measures would correlate with amyloid-β, but that RSI would predict cognitive impairment and longitudinal cognitive change regardless of amyloid-β. We therefore also expected that associations between brain microstructure and cognitive decline would not depend upon amyloid.
Materials and methods
Participants
Participants were recruited from the UC San Diego Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) and underwent standardized clinical evaluation by the ADRC Clinical Core. Participants were given a consensus diagnosis of heathy control (HC), AD, according to INCDS-ADRDA criteria [22], or amnestic or multi-domain mild cognitive impairment (MCI), according to criteria outlined by Petersen et al. [23]. Participants were excluded if they had safety contraindications for MRI, uncorrected vision or hearing loss, significant illness, substance abuse, or major psychiatric or neurologic illness. Additional exclusion criteria for HC included taking psychotropic or cognitive enhancing medications, or a Mattis Dementia Rating Scale (DRS) <130 [24] or Clinical Dementia Rating score greater than zero. Mini-Mental State Examination (MMSE) scores were 25 or greater for HC and MCI, indicating absence of dementia, and 18 or greater for AD, indicating mild to moderate dementia.
Data from 36 participants (23 HC, 7 amnestic MCI, 6 mild AD) who completed clinical evaluation, cognitive assessment, lumbar puncture, and neuroimaging at baseline (2013–2016), and whose imaging data were free of significant artifact, were included for analysis. An average of 3.5±0.9 (range 1.0–5.3) years post-baseline, 33 participants returned for follow-up clinical and cognitive evaluation.
Study procedures were approved by the UC San Diego human subjects’ protection program and participants provided informed, written consent prior to participation. Surrogate consent was provided for participants with advanced cognitive impairment.
Cognitive assessment
At baseline and follow-up, a neuropsychological test battery [25] was administered by a trained examiner in a quiet room. The MMSE and DRS are cognitive screening tools that respectively test global cognition [26] and declining cognitive status [27]. The Trail-Making Test measures psychomotor processing speed and executive function [28]; we subtracted the Part A score from the Part B score to evaluate executive function, controlling for processing speed. Animal naming evaluates verbal semantic fluency, and requires participants to name as many unique animals as possible within one minute [29]. The WMS-R Logical Memory subtest prompts participants to report details of a passage, immediately and after delay (Wechsler, 1987). The California Verbal Learning Test (CVLT) evaluates recall from a list of categorized words; this study analyzed immediate and delayed free recall [30]. Different versions (CVLT-I and CVLT-II) were administered at baseline and follow-up, with the exception of one participant who received CVLT-II at both assessments separated by a 3.3-year interval. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test assesses word list delayed recall [31].
Imaging data acquisition and processing
MRI data were acquired on one of two 3.0 Tesla Discovery 750 scanners (GE Healthcare, Milwaukee, WI, USA) with an eight-channel phased array head coil at the UC San Diego Center for Functional MRI. The MRI sequences included a three-plane localizer; a sagittal 3D fast spoiled gradient echo T1-weighted volume optimized for maximum gray/white matter contrast (TE=3.2 ms, TR=8.1 ms, inversion time=600 ms, flip angle=8°, FOV=24 cm, frequency=256, phase=192, voxel size=1×1×1.2 mm, scan time 8:27); and an axial 2D single-shot pulsed-field gradient spin-echo echo-planar imaging sequence (45-directions, b-values=0, 500, 1500, 4000 s/mm2 and 15 gradient directions for each non-zero b-value; TE=80.6 ms, TR=8 s, frequency=96, phase=96, FOV=240, slice thickness=2.5 mm, scan time 6:34).
As previously described [20, 21], the automated processing stream integrated FreeSurfer (http://surfer.nmr.mgh.harvard.edu) with additional tools developed in-house. RSI data were corrected for motion and eddy current [32], B0 susceptibility [33], and gradient nonlinearity [34] distortions. Images were inspected and data containing uncorrectable artifacts were excluded. Images were automatically registered to T1-weighted structural images [35], and white matter tracts were labeled using a probabilistic atlas (AtlasTrack) [36]. Gray matter, white matter, and CSF boundaries were delineated and cortical regions of interest were defined according to the Desikan-Killiany atlas [37]. To minimize partial volume effects, voxels containing primarily gray matter or CSF were excluded from white matter tracts, and voxels containing primarily white matter or CSF were excluded from gray matter measures [38]. ND, a composite of all restricted diffusion (presumed intraneurite [19]), and IF (presumed in CSF) were calculated within regions of interest previously demonstrating microstructural changes in mild AD [20, 21]. ND was computed in fornix, parahippocampal cingulum, uncinate, inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and arcuate. ND and IF were computed in hippocampal gray matter and entorhinal cortex white matter. Global RSI measures were computed as the averages across all fibers, all gray matter, and all cortical white matter.
Amyloid-β quantification
Lumbar puncture was performed by a neurologist, using a Sprotte atraumatic 24-gauge needle, in the morning after the participant had fasted overnight. Approximately 15–20 ml of CSF was gently mixed, centrifuged in a polypropylene conical tube at 1500 rpm for 10 min, then aliquotted into Sarstedt 0.5-ml cryotubes, snap-frozen immediately, and stored at –80°C until assayed. Levels of amyloid-β−40 and amyloid-β−42 were measured using mass spectrometry (Quest Diagnostics). CSF samples with gross blood contamination or with red blood cell counts >10/ml were not used. Participants were classified as amyloid-positive using an amyloid-β−42/40 ratio cutoff <0.16 based on discrimination between AD and HC in a previous independent sample [39].
Data analysis
Longitudinal cognitive change was computed as the raw follow-up cognitive test score minus the raw baseline score, divided by years of follow-up, to yield a score of points of change per year. MMSE change scores were excluded from analysis due to ceiling effects.
Differences in demographics, amyloid-β and cognitive function between HC, MCI, and AD groups were assessed using Kruskal-Wallis tests for continuous variables and Fisher’s exact tests for categorical variables. Post-hoc comparisons were corrected for multiple comparisons with Bonferroni correction. Differences between amyloid-positive and amyloid-negative participants were tested using univariate ANOVA for continuous variables and chi-squared tests for categorical variables.
Partial correlations were computed between amyloid-β−42 and baseline cognitive function, annualized cognitive change, and RSI measures, across all participants. Differences in RSI measures were compared between amyloid-positive and amyloid-negative participants using univariate ANOVA.
Across all participants, partial correlations were computed between RSI and cognitive function or decline, and RSI variables that significantly correlated with cognitive measures were input as candidate predictors in stepwise linear regressions for each cognitive outcome. Regressions were repeated with adjustment for amyloid-β−42, and again with adjustment and for the respective global RSI measure for each regional regressor (i.e., global ND for fiber tract ND, global cortical gray matter ND or IF for hippocampal ND or IF, and global cortical white matter IF for entorhinal IF). Correlations between RSI measures and cognitive function or cognitive change were repeated, stratified by amyloid status. Sensitivity analyses were conducted on amyloid-stratified correlations after excluding one AD participant classified as amyloid-negative. For correlations that reached significance within either amyloid group, group differences in correlation strengths were compared using Fisher r-to-z transformation.
Cognitive measures were adjusted for age, sex, and education, and annualized change scores were additionally adjusted for baseline cognitive score. RSI measures were adjusted for scanner. Significance was set to p<0.05. P-values for ND and IF were Bonferroni corrected for multiple comparisons across eight and two regions, respectively (p<0.00625 for ND, p<0.025 for IF). Data were analyzed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA).
Results
Participant characteristics
Baseline demographics according to diagnosis and amyloid status are presented in Table 1. Participants ranged in age from 63–91 (mean±SD, 74.5±6.6) years and 64% were women. MCI and AD groups contained a greater proportion of men than HC (p=0.005). HC, MCI, and AD participants did not differ by age, education, length of cognitive follow-up, or interval between lumbar puncture and MRI.
Table 1.
Participant characteristics at baseline, by diagnosis or amyloid status.
| HC (N=23) | MCI (N=7) | AD (N=6) | Amyloid− (N=20) | Amyloid+ (N=16) | |
|---|---|---|---|---|---|
| Age [Range] |
74.5 ± 5.2 [65–82] |
72.3 ± 7.8 [63–84] |
77.0 ± 10.2 [64–91] |
75.0 ± 6.7 [65–91] |
73.8 ± 6.7 [63–84] |
| Sex (% women) a | 83 | 29 | 33 | 60 | 69 |
| Education (years) | 16.0 ± 2.0 | 17.9 ± 1.5 | 15.0 ± 2.8 | 16.1 ± 2.0 | 16.4 ± 2.4 |
| Cognitive follow-up (years) | 3.6 ± 0.8 | 3.4 ± 0.7 | 2.7 ± 1.4 | 3.5 ± 0.7 | 3.4 ± 1.2 |
| Difference between lumbar puncture and MRI (years) | 1.3 ± 1.2 | 1.3 ± 1.2 | 1.7 ± 1.4 | 1.4 ± 1.2 | 1.3 ± 1.3 |
| MMSE a,b [Range] |
29.5 ± 1.1 [26–30] |
27.3 ± 1.9 [25–30] |
22.7 ± 3.3 [18–27] |
29.2 ± 1.5 [24–30] |
26.3 ± 3.6 [18–30] |
| Fiber ND a | 0.78 ± 0.03 | 0.76 ± 0.03 | 0.72 ± 0.04 | 0.77 ± 0.04 | 0.76 ± 0.04 |
| Gray matter IF a | 0.44 ± 0.04 | 0.45 ± 0.02 | 0.50 ± 0.03 | 0.44 ± 0.04 | 0.47 ± 0.04 |
| White matter IF | 0.21 ± 0.03 | 0.24 ± 0.04 | 0.26 ± 0.03 | 0.22 ± 0.04 | 0.23 ± 0.03 |
| Amyloid-positive (%) | 30 | 57 | 83 | 0 | 100 |
| Amyloid-β-42 (ng/ml) b | 2.9 ± 1.1 | 2.0 ± 0.8 | 2.1 ± 1.1 | 3.1 ± 1.1 | 1.9 ± 0.7 |
p<0.05, difference among HC/MCI/AD (Kruskal-Wallis or Fisher’s exact test)
p<0.05, difference between amyloid- and amyloid+ (Univariate ANOVA or chi squared test)
Mean ± SD unless otherwise noted. MMSE scores are adjusted for age, sex and education. ND and IF measures are adjusted for scanner.
Seven HC (30%), four MCI (57%) and five AD (83%) were classified as amyloid-positive. Age, sex, education, cognitive follow-up period, and interval between lumbar puncture and baseline MRI did not differ by amyloid status.
Cognitive function by diagnosis and amyloid status
Participants demonstrated significant decline in CVLT and CERAD delayed recall (one-sampled t-test versus zero, ps<0.01). Baseline cognitive performance and annualized cognitive decline by diagnosis and amyloid status are shown in Supplemental Table 1. Diagnostic groups differed at baseline on all cognitive tests except Trails B-A; MCI scored worse than HC on the CVLT and CERAD tests, and AD scored worse than HC on all tests except Trails B-A and worse than MCI on the MMSE (ps<0.05). Amyloid-positive individuals scored lower on MMSE, DRS, logical memory, CVLT and CERAD, and declined more rapidly on logical memory, than amyloid-negative individuals (ps<0.05).
Amyloid-β−42 positively correlated with baseline performance on the MMSE (r=0.48, p=0.005) and DRS (r=0.42, p=0.01), and with delayed recall on logical memory (r=0.40, p=0.03), CVLT (r=0.41, p=0.02) and CERAD (r=0.44, p=0.009). Amyloid-β−42 positively correlated with annualized change on logical memory immediate recall (r=0.43, p=0.03).
Associations between amyloid-β and microstructure
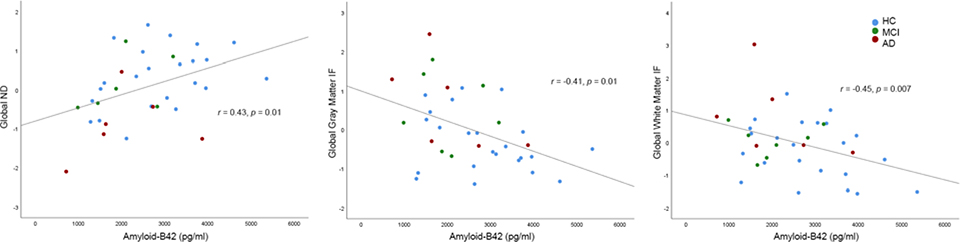
Amyloid-β−42 positively correlated with global ND (r=0.43, p=0.01), and negatively correlated with global gray (r=−0.41, p=0.01) and white (r=−0.45, p=0.007) matter IF (Table 2A, Figure 1). Regionally, amyloid-β−42 positively correlated with arcuate ND (r=0.52, p=0.001), and negatively correlated with entorhinal IF (r=−0.42, p=0.01), but these correlations were no longer significant after controlling for global ND or IF, respectively (ps>0.05). Amyloid-positive individuals had lower fiber ND and higher white matter IF than amyloid-negative individuals (p<0.05).
Table 2.
Partial correlations between global RSI measures (adjusted for scanner) and (A) amyloid-β-42, (B) baseline cognitive function (adjusted for age, sex, education), or (C) annualized cognitive change (adjusted for age, sex, education, baseline score).
| Fiber ND | Gray matter IF | White matter IF | ||
|---|---|---|---|---|
| A. | Amyloid-β-42 | 0.43 * | −0.41 * | −0.45 ** |
| B. | MMSE | 0.69 *** | −0.51 ** | −0.52 ** |
| DRS | 0.65 *** | −0.55 ** | −0.52 ** | |
| Trails B-A | −0.34 | 0.25 | 0.41 * | |
| Verbal fluency | 0.56 *** | −0.55 ** | −0.43 * | |
| LM-Immediate | 0.44 * | −0.46 ** | −0.43 * | |
| LM-Delayed | 0.47 ** | −0.41 * | −0.36 * | |
| CVLT-Immediate | 0.27 | −0.32 | −0.35 | |
| CVLT-Delayed | 0.36 | −0.34 | −0.40 * | |
| CERAD | 0.64 ** | −0.54 ** | −0.49 ** | |
| C. | DRS | −0.01 | −0.37 | −0.18 |
| Trails B-A | −0.46 * | 0.33 | 0.34 | |
| Verbal fluency | 0.43 * | 0.06 | −0.06 | |
| LM-Immediate | 0.18 | −0.36 | −0.31 | |
| LM-Delayed | 0.20 | −0.43 * | −0.36 | |
| CVLT-Immediate | 0.36 | 0.04 | −0.19 | |
| CVLT-Delayed | 0.02 | 0.12 | 0.03 | |
| CERAD | −0.04 | −0.05 | −0.23 | |
p<0.05
p<0.01
p<0.001
MMSE (Mini-Mental State Exam), DRS (Dementia Rating Scale), LM (Logical Memory), CVLT (California Verbal Learning Test), CERAD (Consortium to Establish a Registry for Alzheimer's Disease)
Figure 1.
Correlations between global RSI measures and amyloid-β−42. RSI measures are adjusted for scanner (standardized residuals).
Associations between microstructure and baseline cognitive function
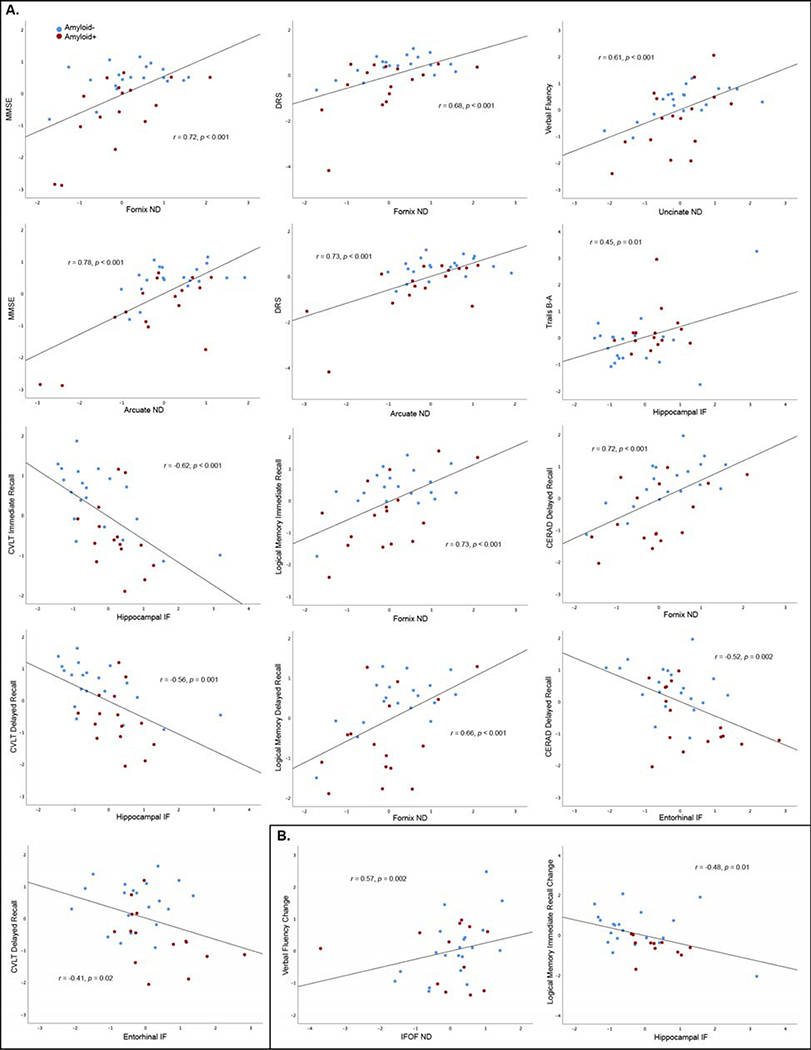
MMSE, DRS, verbal fluency, logical memory and CERAD scores correlated with all global RSI measures, and Trails B-A and CVLT delayed recall correlated with global white matter IF (Table 2B). Table 3A and Figure 2A present results from linear regression models to predict baseline cognitive scores from regional RSI measures (candidate RSI variables significantly correlated with cognitive performance are presented in Supplemental Table 2). Higher fornix ND predicted better performance on MMSE, DRS, logical memory, and CERAD tests. Higher arcuate ND predicted better MMSE and DRS scores, and higher uncinate ND predicted better verbal fluency. Lower hippocampal IF predicted better Trails B-A and CVLT immediate and delayed recall, and lower entorhinal IF predicted better CVLT and CERAD delayed recall. When amyloid-β−42 or the respective global RSI measure were included as covariates, models were essentially unchanged (Table 3A).
Table 3.
Significant predictors from stepwise linear regression models combining RSI measures to predict (A) baseline cognitive test scores (adjusted for age, sex, education, and scanner) or (B) cognitive decline (additionally adjusted for baseline cognitive function).
| A. BASELINE | RSI measure | Standardized β | Standardized β adjusted for amyloid-β-42 | Standardized β adjusted for global ND or IF |
|---|---|---|---|---|
| MMSE | Arcuate ND Fornix ND |
0.60 *** 0.43 * |
0.56 ** 0.43 * |
0.68 ** 0.48 * |
| DRS | Arcuate ND Fornix ND |
0.55 ** 0.40 * |
0.52 ** 0.40 * |
0.62 * 0.44 * |
| Trails B-A | Hippocampal IF | 0.34 * | 0.33 * | 0.34 * |
| Verbal fluency | Uncinate ND | 0.72 *** | 0.67 *** | 0.58 |
| LM-Immediate | Fornix ND | 0.87 *** | 0.84 *** | 0.99 *** |
| LM-Delayed | Fornix ND | 0.75 *** | 0.67 *** | 0.76 ** |
| CVLT-Immediate | Hippocampal IF | −0.54 *** | −0.52 *** | −0.49 *** |
| CVLT-Delayed | Hippocampal IF Entorhinal IF |
−0.43 ** −0.28 * |
−0.40 ** −0.17 |
−0.39 ** −0.26 |
| CERAD | Fornix ND Entorhinal IF |
0.71 *** −0.31 * |
0.67 *** −0.26 |
0.60 ** −0.27 |
| B. ANNUALIZED CHANGE | ||||
| Verbal fluency | IFOF ND | 0.77 ** | 0.87 ** | 1.32 * |
| LM-Immediate | Hippocampal IF | −0.46 * | −0.42 * | −0.38 * |
p<0.05
p<0.01
p<0.001
MMSE (Mini-Mental State Exam), DRS (Dementia Rating Scale), LM (Logical Memory), CVLT (California Verbal Learning Test), CERAD (Consortium to Establish a Registry for Alzheimer's Disease), IFOF (inferior fronto-occipital fasciculus)
Figure 2.
RSI measures that best predicted baseline cognitive performance (A) or annualized cognitive change (B) in linear regression models. RSI measures are adusted for scanner, baseline cognitive tests scores are adjusted for age, sex, and education, and annualized change scores are additionally adjusted for baseline test score (all variables are standardized residuals).
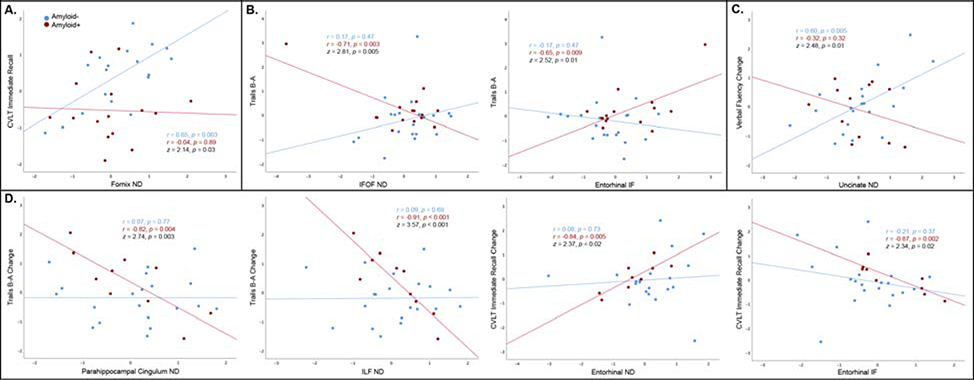
Regional microstructure significantly correlated with DRS, verbal fluency, Trails B-A, logical memory, CVLT and CERAD scores for amyloid-negative participants, and with MMSE, DRS, and Trails B-A for amyloid-positive participants (Supplemental Table 3A). Results were essentially unchanged after excluding one amyloid-negative AD participant. Fornix ND more strongly correlated with CVLT immediate recall for amyloid-negative than amyloid-positive participants (z=2.14, p=0.03; Figure 3A). In contrast, IFO ND (z=2.81, p=0.005) and entorhinal IF (z=2.52, p=0.01) more strongly correlated with Trails B-A for amyloid-positive than amyloid-negative individuals (Figure 3B).
Figure 3.
Regional RSI measures are shown for which correlations with baseline cognitive function (A, B) or annualized cognitive change (C, D) were stronger for amyloid-negative than amyloid-positive (A, C), and or amyloid-positive than amyloid-negative (B, D) participants (Fisher r-to-z, p<0.05). RSI measures are adjusted for scanner, baseline cognitive tests scores are adjusted for age, sex, and education, and annualized change scores are additionally adjusted for baseline test score (all variables are standardized residuals).
Associations between microstructure and cognitive decline
Lower global ND predicted faster verbal fluency and Trails B-A decline, and higher global gray matter IF predicted faster logical memory delayed recall decline (Table 2C). Table 3B and Figure 2B present linear regression models predicting cognitive decline from regional RSI measures. Lower IFOF ND (r=0.57, p=0.002) predicted more rapid verbal fluency decline and higher hippocampal IF predicted more rapid logical memory immediate recall decline (r=−0.48, p=0.01). All RSI predictors remained significant after adjustment for amyloid-β−42 or their respective global RSI measure (Table 3B).
Regional RSI measures significantly predicted verbal fluency decline within amyloid-negative participants, and decline in Trails B-A and CLVT immediate recall within amyloid-positive participants (Supplemental Table 3B). Lower uncinate ND predicted more rapid verbal fluency decline for amyloid-negative than amyloid-positive individuals (z=2.48, p=0.01; Figure 3C). Lower parahippocampal cingulum (z=2.74, p=0.003) and ILF (z=3.57, p<0.001) ND predicted more rapid decline on Trails B-A, and lower entorhinal ND (z=2.37, p=0.02) and higher entorhinal IF (z=2.34, p=0.02) predicted more rapid CVLT immediate recall decline, for amyloid-positive than amyloid-negative participants (Figure 3D).
Discussion
In this study of older adults across the spectrum from HC to mild dementia, we observed associations of abnormal amyloid-β−42 with reduced whole-brain neurite density and increased isotropic free water diffusion, pointing to a globally deleterious effect of amyloid pathology on cytoarchitectural integrity. However, amyloid was insufficient to explain effects of regional microarchitectural injury on cognitive performance, as anatomically-specific RSI measures predicted cognitive impairment and decline regardless of amyloid-β levels. Microstructure predicted cognitive outcomes for individuals with both high and low amyloid levels, and associations between microstructural injury and cognitive impairment did not systematically differ by pathology, further implicating amyloid-independent pathways by which cognitive decline follows microstructural compromise.
As previously reported in this sample [20, 21], lower ND and higher IF predicted poorer performance and more rapid decline across multiple cognitive domains. Here, we further report that although abnormal amyloid-β also correlated with cognitive impairment and decline, these relationships were restricted to the memory domain, expanding upon prior evidence that amyloid-β is more weakly linked with cognitive decline than neurodegeneration [6]. Critically, prediction of performance and decline on all cognitive tests by RSI was robust to adjustment for amyloid-β, aligned with previous findings that reduced FA predicts cognitive decline regardless of amyloid [16]. Thus, it appears unlikely that amyloid-β is the primary driver of cognitive decline mediated by microarchitectural damage in all cases examined here. Parallel pathophysiological processes related to concomitant risk factors and pathology, such as neurofibrillary tangles, neuroinflammation, or cerebrovascular, metabolic or immune dysfunction, may additionally mediate cytoarchitectural damage underlying cognitive decline [15, 40, 41]. Subgroup analyses revealed that brain microstructure predicted cognitive performance and rates of cognitive decline for both amyloid-positive and amyloid-negative individuals, with no systematic difference in the strength of these correlations by amyloid status. These findings offer further evidence that cytoarchitectural damage predicts risk for cognitive impairment even for individuals at low pathologically-determined risk for AD. Broadly, these observations point to separable pathways leading to cognitive dysfunction across the spectrum from healthy aging to mild AD, including some mediated by and others independent of amyloid-β.
Correlations between regional RSI measures and cognitive function were largely robust to adjustment for global microstructure. The anatomic specificity of these associations contrasts with prior findings that global, rather than tract-specific, FA, predicted cognitive decline independently of amyloid [16]. This difference may derive from the improved power of RSI over DTI to elucidate tissue microarchitecture. Regions in which ND and IF predicted cognitive performance were generally consistent with their previously reported involvement in the respective cognitive functions. For instance, lower fornix ND and higher hippocampal and entorhinal IF predicted greater dementia severity and poorer global cognitive function and memory, as well as more rapid logical memory decline, corroborating the established role of medial temporal and limbic regions in episodic memory [42]. Higher hippocampal IF also predicted worse executive function, which could stem from disrupted hippocampal-prefrontal circuits supporting working memory [43]. Lower arcuate ND predicted greater global cognitive impairment and dementia severity, consistent with prior reports of compromised arcuate integrity in MCI and AD [44]. Reduced uncinate and IFOF ND predicted verbal fluency performance and decline. Damage to these association tracts may disrupt mnemonic processes integratively supporting verbal recall and semantic assessment. This mapping of regional microstructural alterations onto established functions suggests that RSI may be sensitive to disruption of neural circuits essential to normal cognitive processing.
In contrast to the regional associations of RSI with cognitive function, amyloid-β levels correlated with whole-brain microstructure. Several prior DTI studies reporting correlations between CSF amyloid-β levels and regional microstructural compromise [8, 10, 11] did not report whether such correlations were widespread throughout the brain. Although CSF amyloid-β may be more sensitive to early pathological changes than amyloid PET [45], CSF measures are blind to deposition topography. Given that regional associations between amyloid and DTI measures have been inconsistent [9, 17, 18], further investigation integrating amyloid-PET with advanced diffusion imaging techniques will help to clarify whether foci of high neuropathological burden correspond with microstructural compromise.
A strength of this study was the availability of neuroimaging, comprehensive longitudinal cognitive evaluation, and CSF data on a cohort spanning a clinical spectrum from cognitively normal to mild dementia. Applying RSI in a sample with CSF measures and longitudinal cognitive follow-up of up to five years provided a rare opportunity to examine interactive effects of amyloid pathology and refined microstructural properties on cognitive decline, a more sensitive measure of clinical prognosis than concurrent cognitive status. However, our relatively small sample may have limited power to detect subtle effects, particularly on rates of cognitive decline, which may proceed slowly in preclinical stages. This small sample precluded comparison of associations between RSI and cognitive measures by amyloid status within cognitively normal participants; probing the relationship between microstructural change and cognitive decline in preclinical AD will be an important arena for further investigation. We note that three MCI and one AD participant did not meet criteria for amyloid-positivity. However, dichotomized AD risk has recently been questioned [46], as biomarker sensitivity may improve when assessed as a continuum, with even subthreshold amyloid levels predicting subsequent AD pathology [47]. Though we cannot rule out the presence of non-AD etiology, or subthreshold AD pathology, in our amyloid-negative group, this would not preclude our finding that microstructure predicts cognitive impairment regardless of amyloid. Finally, although our results implicate independent pathways by which amyloid-β and cytoarchitectural injury may impair cognitive function, future longitudinal studies may shed light on the temporal relationships between these factors and their respective influences on disease progression.
In summary, anatomically-specific cytoarchitectural changes estimated with RSI are more sensitive to cognitive decline than is amyloid-β. Abnormal amyloid levels did not account for the associations between microstructure and cognitive decline, implicating predominantly amyloid-independent pathways by which cell damage leads to cognitive dysfunction. Associations between microstructure and cognitive impairment did not systematically differ by amyloid status, further suggesting that RSI may be an informative marker of disease symptoms regardless of amyloid neuropathology. RSI-based metrics of microstructural compromise appear sensitive to amyloid-independent pathways leading to cognitive decline, and may be of greater prognostic value than amyloid for predicting dementia onset and disease progression.
Supplementary Material
Acknowledgments
This work was supported by American Federation for Aging Research award 20190572; donors of Alzheimer’s Disease Research, a program of BrightFocus Foundation; NIA grant P30 AG062429; and U.S. Department of Veterans Affairs CSR&D Merit Award 5I01CX000565.
Footnotes
Conflicts of Interest: LKM holds stock in CorTechs Laboratories, Inc. JBB has served on advisory boards for Elan, Bristol-Myers Squibb, Avanir, Novartis, Genentech, and Eli Lilly and holds stock in CorTechs Labs, Inc. and Human Longevity, Inc. AMD is a founder and holds equity in CorTechs Laboratories, Inc., and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. and receives funding through research agreements with General Electric Healthcare and Medtronic, Inc. This arrangement have been reviewed and approved by UCSD, in accordance with its conflict of interest policies. NSW is Chief Technology Officer and holds equity in Healthlytics, Inc. DG has served on the advisory board for vTv Pharmaceuticals and Data Monitoring Boards for Proclara and Cognition Therapeutics. No other authors declare competing financial interests.
References
- [1].Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O (2012) Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69, 98–106. [DOI] [PubMed] [Google Scholar]
- [2].Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27, 1372–1384. [DOI] [PubMed] [Google Scholar]
- [3].Brun A, Englund E (1981) Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology 5, 549–564. [DOI] [PubMed] [Google Scholar]
- [4].Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W (1995) Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 45, 883–888. [DOI] [PubMed] [Google Scholar]
- [5].Svennerholm L, Gottfries CG (1994) Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J Neurochem 62, 1039–1047. [DOI] [PubMed] [Google Scholar]
- [6].Jack CR Jr., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC, Alzheimer’s Disease Neuroimaging I (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lancaster MA, Seidenberg M, Smith JC, Nielson KA, Woodard JL, Durgerian S, Rao SM (2016) Diffusion Tensor Imaging Predictors of Episodic Memory Decline in Healthy Elders at Genetic Risk for Alzheimer’s Disease. J Int Neuropsychol Soc 22, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gold BT, Zhu Z, Brown CA, Andersen AH, LaDu MJ, Tai L, Jicha GA, Kryscio RJ, Estus S, Nelson PT, Scheff SW, Abner E, Schmitt FA, Van Eldik LJ, Smith CD (2014) White matter integrity is associated with cerebrospinal fluid markers of Alzheimer’s disease in normal adults. Neurobiol Aging 35, 2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chao LL, Decarli C, Kriger S, Truran D, Zhang Y, Laxamana J, Villeneuve S, Jagust WJ, Sanossian N, Mack WJ, Chui HC, Weiner MW (2013) Associations between white matter hyperintensities and beta amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One 8, e65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Molinuevo JL, Ripolles P, Simo M, Llado A, Olives J, Balasa M, Antonell A, Rodriguez-Fornells A, Rami L (2014) White matter changes in preclinical Alzheimer’s disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol Aging 35, 2671–2680. [DOI] [PubMed] [Google Scholar]
- [11].Rieckmann A, Van Dijk KR, Sperling RA, Johnson KA, Buckner RL, Hedden T (2016) Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging 42, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mueggler T, Meyer-Luehmann M, Rausch M, Staufenbiel M, Jucker M, Rudin M (2004) Restricted diffusion in the brain of transgenic mice with cerebral amyloidosis. Eur J Neurosci 20, 811–817. [DOI] [PubMed] [Google Scholar]
- [13].Sun SW, Liang HF, Mei J, Xu D, Shi WX (2014) In vivo diffusion tensor imaging of amyloid-beta-induced white matter damage in mice. J Alzheimers Dis 38, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sepulcre J, Grothe MJ, d’Oleire Uquillas F, Ortiz-Teran L, Diez I, Yang HS, Jacobs HIL, Hanseeuw BJ, Li Q, El-Fakhri G, Sperling RA, Johnson KA (2018) Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat Med 24, 1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kantarci K, Murray ME, Schwarz CG, Reid RI, Przybelski SA, Lesnick T, Zuk SM, Raman MR, Senjem ML, Gunter JL, Boeve BF, Knopman DS, Parisi JE, Petersen RC, Jack CR Jr., Dickson DW (2017) White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging 56, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rabin JS, Perea RD, Buckley RF, Neal TE, Buckner RL, Johnson KA, Sperling RA, Hedden T (2019) Global White Matter Diffusion Characteristics Predict Longitudinal Cognitive Change Independently of Amyloid Status in Clinically Normal Older Adults. Cereb Cortex 29, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang SJ, Adluru N, Kim WH, Johnson SC, Bendlin BB, Singh V (2019) Associations Between Positron Emission Tomography Amyloid Pathology and Diffusion Tensor Imaging Brain Connectivity in Pre-Clinical Alzheimer’s Disease. Brain Connect 9, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC (2014) Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: A multimodal imaging investigation. Neuroimage Clin 4, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM (2013) Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum Brain Mapp 34, 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reas ET, Hagler DJ Jr., White NS, Kuperman JM, Bartsch H, Cross K, Loi RQ, Balachandra AR, Meloy MJ, Wierenga CE, Galasko D, Brewer JB, Dale AM, McEvoy LK (2017) Sensitivity of restriction spectrum imaging to memory and neuropathology in Alzheimer’s disease. Alzheimers Res Ther 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reas ET, Hagler DJ, White NS, Kuperman J, Bartsch H, Wierenga CE, Galasko D, Brewer JB, Dale AM, McEvoy LK (2018) Microstructural brain changes track cognitive decline in mild cognitive impairment. Neuroimage: Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [23].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56, 303–308. [DOI] [PubMed] [Google Scholar]
- [24].Monsch AU, Bondi MW, Salmon DP, Butters N, Thal LJ, Hansen LA, Wiederholt WC, Cahn DA, Klauber MR (1995) Clinical validity of the Mattis Dementia Rating Scale in detecting Dementia of the Alzheimer type. A double cross-validation and application to a community-dwelling sample. Arch Neurol 52, 899–904. [DOI] [PubMed] [Google Scholar]
- [25].Salmon D, Butters N (1992) Neuropsychological assessment of dementia in the elderly. Principles of geriatric neurology. Philadelphia: FA Davis 144, 63. [Google Scholar]
- [26].Tombaugh TN, McIntyre NJ (1992) The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40, 922–935. [DOI] [PubMed] [Google Scholar]
- [27].Mattis S (1988) Dementia Rating Scale. Professional Manual, Psychological Assessment Resources, Florida. [Google Scholar]
- [28].Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills 8, 271–276. [Google Scholar]
- [29].Borkowski JG, Benton AL, Spreen O (1967) Word fluency and brain damage. Neuropsychologia 5, 135–140. [Google Scholar]
- [30].Delis DC, Massman PJ, Butters N, Salmon DP, Shear PK, Demadura T, Filoteo JV (1992) Spatial cognition in Alzheimer’s disease: subtypes of global-local impairment. J Clin Exp Neuropsychol 14, 463–477. [DOI] [PubMed] [Google Scholar]
- [31].Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- [32].Zhuang J, Hrabe J, Kangarlu A, Xu D, Bansal R, Branch CA, Peterson BS (2006) Correction of eddy-current distortions in diffusion tensor images using the known directions and strengths of diffusion gradients. J Magn Reson Imaging 24, 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holland D, Kuperman JM, Dale AM (2010) Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage 50, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A (2006) Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30, 436–443. [DOI] [PubMed] [Google Scholar]
- [35].Wells WM 3rd, Viola P, Atsumi H, Nakajima S, Kikinis R (1996) Multi-modal volume registration by maximization of mutual information. Med Image Anal 1, 35–51. [DOI] [PubMed] [Google Scholar]
- [36].Hagler DJ Jr., Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM (2009) Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp 30, 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- [38].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [39].Weber DM, Tran D, Goldman SM, TS W, Ginns EI, Lagier RJ, Rissman RA, Brewer JB, Clarke NJ (in press) A High-throughput Mass Spectrometry-based Clinical Assay for the Quantitation of Beta-amyloid 40 and 42 in Cerebrospinal Fluid Clinical Chemistry. [DOI] [PubMed] [Google Scholar]
- [40].Iadecola C (2016) Vascular and Metabolic Factors in Alzheimer’s Disease and Related Dementias: Introduction. Cell Mol Neurobiol 36, 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- [42].Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27, 279–306. [DOI] [PubMed] [Google Scholar]
- [43].Wall PM, Messier C (2001) The hippocampal formation--orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 127, 99–117. [DOI] [PubMed] [Google Scholar]
- [44].Zhang X, Sun Y, Li W, Liu B, Wu W, Zhao H, Liu R, Zhang Y, Yin Z, Yu T, Qing Z, Zhu B, Xu Y, Nedelska Z, Hort J, Zhang B, Alzheimer’s Disease Neuroimaging I (2019) Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer’s disease. Neuroimage Clin 22, 101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jack CR Jr., Holtzman DM (2013) Biomarker modeling of Alzheimer’s disease. Neuron 80, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McRae-McKee K, Udeh-Momoh CT, Price G, Bajaj S, de Jager CA, Scott D, Hadjichrysanthou C, McNaughton E, Bracoud L, Ahmadi-Abhari S, de Wolf F, Anderson RM, Middleton LT, Alzheimer’s Disease Neuroimaging I (2019) Perspective: Clinical relevance of the dichotomous classification of Alzheimer’s disease biomarkers: Should there be a “gray zone”? Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- [47].Leal SL, Lockhart SN, Maass A, Bell RK, Jagust WJ (2018) Subthreshold Amyloid Predicts Tau Deposition in Aging. J Neurosci 38, 4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.