Abstract
While radiosensitizing chemotherapy has improved survival for several types of cancer, current chemoradiation regimens remain ineffective for many patients and have substantial toxicities. Given the strong need for the development of novel radiosensitizers to further improve patient outcomes, the Radiation Research Program (RRP) and the Small Business Innovation Research (SBIR) in the National Cancer Institute (NCI) issued a Request for Proposals (RFP) through the NCI SBIR Development Center’s contracts pathway. We sought to determine the research outcomes for the NCI SBIR Development Center’s funded proposals for the development of radiosensitizers. We identified SBIR-funded contracts and grants for the development of radiosensitizers from 2009 to 2018 using the National Institutes of Health (NIH) Reporter database. Research outcomes of the NCI SBIR Development Center-funded proposals were determined using a comprehensive internet search. We searched PubMed, clinicaltrials.gov, company websites and google.com for research articles, abstracts and posters, clinical trials, press releases and other news, related to progress in the development of funded radiosensitizers. To protect the intellectual property of the investigators and small businesses, all information obtained and reported is publicly available. The SBIR Program has funded four contracts and 11 grants for the development of novel radiosensitizers. Two companies have received phase IIb bridge awards. Overall, 50% of companies (6/12) have successfully advanced their investigational drugs into prospective clinical trials in cancer patients, and all but one company are investigating their drug in combination with radiation therapy as described in the NCI SBIR Development Center proposal. To date, only one company has initiated a randomized trial of standard of care with or without their radiosensitizer. In conclusion, the NCI SBIR Development Center has funded the development of novel radiosensitizers leading to clinical trials of novel drugs in combination with radiation therapy. Continued follow-up is needed to determine if any of these novel radiosensitizers produce improved tumor control and/or overall survival.
INTRODUCTION
Radiation therapy is a mainstay of the curative and palliative treatment of cancer. The addition of radiosensitizing chemotherapy to definitive radiation therapy has been one of the most significant breakthroughs in the treatment of cancer over the past several decades. Radiosensitizing chemotherapy regimens have improved cure rates for patients with brain, head and neck, lung, gastrointestinal, genitourinary and gynecologic cancers. The most commonly used regimens in clinical practice involve historic drugs such as cisplatin, carboplatin, paclitaxel, mitomycin C and fluorouracil. The development of novel radiosensitizers to further improve survival and/or decrease toxicity has been lagging, especially compared to the proliferation of new drugs for patients with recurrent or metastatic disease. For instance, since 2006 there has been only one drug, cetuximab, approved for concurrent use with radiation therapy compared to more than 250 new drugs approved by the U.S. Food and Drug Administration (FDA) (1).
Given the unmet need for novel radiosensitizers to improve survival and/or decrease toxicity, the National Cancer Institute’s (NCI) Small Business Innovation Research (SBIR) Program, in collaboration with the Radiation Research Program, issued a Request for Proposals (RFP) for the development of novel radiosensitizers. The general framework for innovative drug advancement through the NCI SBIR Development Center pathway and the necessary pre-clinical data for developing radiosensitizers have been previously described elsewhere (2, 3). Briefly, the NCI SBIR Development Center provides research funding to for-profit small businesses in the early stages of drug development so that necessary investigational new drug (IND) enabling studies can be completed. In addition to the NCI SBIR Development Center phase I and phase II funding, the NCI also has an innovative funding opportunity called the NCI SBIR Development Center Phase IIb Bridge Award to support the next stage of development for companies that have completed SBIR phase II. The purpose of this award is to address the funding gap known as the “Valley of Death” between the end of the NCI SBIR Development Center Phase II award and the subsequent round of financing needed to advance a product or service toward commercialization. To achieve this goal, the Bridge Award funding opportunity is specifically designed to incentivize partnerships between federally funded SBIR Phase II awardees and third-party investors and/or strategic partners. Companies can request up to $4 million in total costs for development and are strongly encouraged to match the NCI funding from third party (non-federal) investors. The ultimate goal of the NCI SBIR Development Center Program is to facilitate the commercial success of innovative technologies and treatments that serve the public good.
We determined the research outcomes of NCI SBIR Development Center-funded grants and contracts for the development of novel radiosensitizers. Analyzing research outcomes is necessary to benchmark the effectiveness of this funding mechanism to assess the yield on taxpayer investment. Additionally, identifying recurring obstacles and/or indicators of success would be helpful for improving this funding mechanism in the future.
MATERIALS AND METHODS
Funding information for all National Institutes of Health (NIH) awarded projects is publicly available using the NIH RePORTER database, https://projectreporter.nih.gov/reporter.cfm. We used the NIH RePORTER database to search for NCI SBIR Development Center-funded grants and contracts in the field of radiosensitizers from 2009 to 2018 using the following search terms: “radiation,” “sensitizer,” “radiosensitizer,” “modulator” and “radiomodulator”. Funding information, including the phase of an award, funding years, company details, investigational agent and background information were obtained from the NIH RePORTER. We chose to begin the search for year 2009, since there was an active collaboration effort between the RRP and the newly established SBIR development Center at the NCI from that point onwards. The RRP sponsored RFP for the development of radiosensitizers was issued from 2012 to 2014.
Publicly available research outcomes were obtained using a comprehensive internet search. A PubMed search using the company name, investigational agent and principal investigator of the NCI SBIR Development Center proposal was used to identify published research findings. We searched www.clinicaltrials.gov using the company name and investigational agent to identify completed, active or withdrawn clinical trials. We also searched individual company websites for press releases or other news regarding progress in the development of the investigational agent. Finally, we performed a www.google.com search using the company name and investigational agent to capture any other information, including abstracts presented at research conferences and news articles.
RESULTS
Phase I and II Funding Milestones
NCI SBIR-funded contracts and grants for the development of novel radiosensitizers are summarized in Tables 1 and 2 (4–16). The contract mechanism funded four unique projects from 2012 to 2014 and the grants mechanism funded eleven unique projects from 2009 to 2017. Among the SBIR contracts, three companies (3/4) transitioned from phase I to phase II funding. To date, three (3/11) of the radiosensitizer grants have transitioned from phase I to phase II funding and two companies have received phase IIb bridge awards. Public commercialization was noted for four companies (Tables 1 and 2).
TABLE 1.
NCI SBIR-Funded Contracts for Radiation Sensitizers in Chronological Order
| Company | |
|---|---|
| Celldex | Suvica, Inc. |
| Drug and mechanism | |
| CDX-301 (Flt3L): Hematopoietic growth factor that increases the number of dendritic cells in blood. | SVC 112 (Bouvardin analog): Inhibits protein synthesis at the level of translation elongation. |
| Organ | |
| NSCLC | Head and neck cancer (HNC) |
| Award type and year(s) | |
| Phase I contract and phase II grant; 2012, 2015 | Phase I and II contracts; 2013, 2015 |
| Phase I aims | |
| Combine RT with two powerful immune modulators, Fms-like tyrosine kinase ligand (Flt3L) and an antibody that activates CD27 and investigate the benefits of these immune modulators when combined with RT in mouse models to provide the basis for future clinical studies. | To perform preliminary pharmacokinetic and toxicity studies and to test the efficacy of the drug on preclinical models of human HNC. |
| Phase I results | |
| No public data found | Bouvardin and radiation-enhanced clonogenic death in HNC cell lines and augmented antitumor effects in HNC tumor xenografts in mice (4). There were no clinical signs of toxicity in mice treated with bouvardin such as loss of >10% body weight, lethargy, or loss of appetite (4). |
| Phase II aims | |
| Manufacture clinical grade Fltr3L and to determine the safety, feasibility, and efficacy of combining lung stereotactic radiation therapy and Fltr3L therapy in patients with NSCLC. | Improve synthesis of SVC112, perform toxicity in rat and dogs, show differential activity of SVC112 in cancer cells versus normal cells and perform efficacy studies in human xenografts in mice. |
| Phase II results | |
| Phase II clinical trial underway for stereotactic radiotherapy and Fltr3L therapy in patients with advanced NSCLC; NCT02839265. | No public data found |
| Follow-up | |
| Publications (but unrelated): http://www.celldex.com/science/publications.php#cdx301 | No clinical trials. |
| Commercialization deal headline and year | |
| (Pre-SBR funding: Celldex Therapeutics acquires rights to immune stimulatory molecules from Amgen; 2009.) | n/a |
| Deal type and subtype | |
| Mergers and acquisitions; asset transactions | n/a |
| Drug and deal value (U.S. $M) | |
| CDX-301; 0.9 | n/a |
| Company | |
| Omm Scientific | Shuttle |
| SaliPhe (saliphenylhalamide): Inhibitor of the V-ATPase pump responsible for acidification. | IPdR (5-iodo-2pyrimidinone-2′-deoxyribose), a prod rug of the radiosensitizer IUdR (5-iodo-2′-deoxyuridine): Increased susceptibility of TdR analog-substituted DNA to the generation of highly reactive uracil free radicals by radiation. |
| NSCLC | GI cancer |
| Phase I contract; 2013 | Phase I and II contracts; 2014, 2015 |
| To prepare stable dosing solutions of the drug, perform preclinical efficacy and safety experiments using NSCLC cell lines and normal human bronchial epithelial cell lines, and to conduct genomic expression and metabolism studies on untreated and treated cells. | To produce GMP manufactured IPdR capsules, submit a letter of intent (LOI) to CTEP, and to prepare a protocol and IRB approval for a phase I clinical trial. |
| No public data found | No public data found |
| n/a | To determine the safety and feasibility of oral IPdR in the phase I dose-escalation clinical trial. |
| n/a | Phase I clinical trial is underway for patients with advanced GI cancers receiving radiotherapy; NCT02381561 |
| No clinical trials. | No public data found |
| n/a | n/a |
| n/a | Strategic alliances; licensing agreement |
| n/a | Doranidazole; 0 |
phase IIb bridge awards. phase IIb bridge awards.
TABLE 2.
NCI SBIR-Funded Grants for Radiation Sensitizers in Chronological Order
| Company | |
|---|---|
| Omniox, Inc. | NuvOx Pharma, LLC |
| Drug and mechanism | |
| OMX-4.80, a H-NOX oxygen carrier: Increases tumor oxygenation. | NVX-108, a dodecafluoropentane emulsion (DDFPe): Increases tumor oxygenation. |
| Organ | |
| None specified | Glioblastoma (GBM) |
| Award type and year(s) | |
| Phase I, II grants and Bridge; 2008, 2010, 2012 | Phase I, II grants and Bridge; 2010, 2014, 2017 |
| Phase I aims | |
| To identify leading candidates for therapeutic development, to evaluate penetration of H-NOX into tissue, to evaluate reduction in hypoxia in mice carrying human tumor xenografts treated with H-NOX, and to perform pharmacokinetic and toxicology studies. |
|
| Phase I results | |
| Phase I studies successfully identified a lead candidate that penetrates deep into tumors and raises oxygen levels in hypoxic zones. OMX-4.80 administered I.V. into mice bearing orthotopic glioblastoma penetrated into intracranial tumors and reduced tumor hypoxia in a dose-dependent manner (7). Combination of OMX-4.80 and radiation decreased tumor growth and increased survival. Toxicology studies in rodents and dogs revealed a good safety profile (7). PEGylated version of OMX-4.80 (OMX-4.80P) was developed to improve the circulation half-life, and half-life and was shown to reduce hypoxia in rodent glioblastoma models and was tell tolerated in rodents and dogs (8). | NVX-108 increased tumor pO2 up to 400% in mice with pancreatic tumor xenografts (5). The addition of NVX-108 to radiation and carbogen resulted in 2-fold reduction in tumor volume (5). |
| Phase II aims | |
| Determine the degree of RT enhancement by lead H-NOX candidates, and refine them for optimal biodistribution, tumor oxygenation, RT enhancement and safety. Phase IIB aims at continued preclinical research, safety testing, and clinical development through phase 1 trials. |
|
| Phase II results | |
| No public data found | NVX-108 increased pancreatic tumor xenograft pO2 via fiber-optic probe measurement although no differences were noted on MRI TOLD imaging (6). |
| Follow-up | |
| No clinical trials. | Phase I clinical trial of NVX-108 with radiation and temozolomide in patients with newly-diagnosed glioblastoma multiforme (NCT02189109). This trial is reportedly completed but not yet published. |
| Commercialization deal headline and year | |
| n/a | RiboMed Biotech and NuvOx Pharma enter into partnership; 2013. |
| Deal type and subtype | |
| n/a | Strategic alliances; partnerships. |
| Drug and deal value (U.S. $M) | |
| n/a | NVX-108; 0 |
| Company | ||
|---|---|---|
| MedVas Concepts, LLC | Omniox, Inc. | Medical Guidance Systems, LLC |
| Drug delivery system: Selective delivery of chemotherapy to tumor endothelial cells using cell surface proteins that are upregulated by exposure to ionizing radiation. | H-NOX variants with less immunogenicity than OMX-4.80: Increases tumor oxygenation. | Tiptuximab, an antibody that binds specifically to radiation inducible TIP1 on cancer: TIP1 is an inducible protein on cancer cells in response to radiation therapy. |
| None specified | None specified | None specified |
| Phase I grant; 2012 | Phase I grant; 2014 | Phase I grant; 2014 |
| To demonstrate proof-of-concept including selective delivery of a vascular disrupting agent to endothelial cells and effective inhibition of tumor growth in an animal model of disease. |
|
|
| No public data found | No public data found | Conjugating the anti-TIP1 antibody to radio-isotopes was effective for in vivo imaging (9, 10). Anti-TIP1 antibodies activated immune effector cells in mouse models of cancer (9, 11). |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
| No clinical trials | No clinical trials | No clinical trials |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
| Company | ||
|---|---|---|
| Biomimetix JV, LLC | Apogee Biotechnology Corp. | Medical Guidance Systems, LLC |
| BMX-001: Metalloporphyrin antioxidant compound with radioprotector and radiosensitizer properties. | ABC294640, a SK2 inhibitor: Sphingosine kinases (SK1 and SK2) are frequently overexpressed in many human cancers and inhibition of SK activity has anti-proliferative effects on tumor cells. | GIRLRG, a peptide ligand: Binds to radiation-inducible stress proteins and can specifically deliver cytotoxic agents to cancer cells. |
| Glioblastoma | Prostate cancer | None specified |
| Fast-Track I and II grants; 2015, 2016 | Phase I grant; 2015 | Phase I grant; 2016 |
|
|
Test the hypothesis that inducible cell-surface proteins on cancer can be exploited to achieve cancer specific drug delivery in patients. To conjugate the lead peptide to PEG and chelators that will serve to image the spatial and temporal distribution of peptides in planned clinical trials. |
| Phase I/II randomized clinical trial of patients with high grade glioma receiving radiation therapy and temozolomide (NCT02655601). | ABC294640 decreased castration-resistant prostate cancer cell viability in vitro and diminished the growth of TRAMP-C2 xenografts in vivo (12). The inhibition of both SK2 and dihydroceramide desaturase may account for these effects (12). ABC294640 downregulate Myc and AR expression and activity (13). | Related publications: (14, 15). |
| No public data found | n/a | n/a |
| n/a | n/a | n/a |
| Phase I clinical trials in anal cancer and with head and neck cancer (NCT03386500; NCT02990468). | No trials specific to prostate cancer. Clinical trials for various cancers (NCT03377179; NCT02939807; NCT02757326). | No clinical trials |
| (Pre-SBIR funding: BioMimetix enters into licensing agreement with Duke University, 2011). | n/a | n/a |
| Strategic alliances; licensing agreement. | n/a | n/a |
| BMX-001; 0 | n/a | n/a |
| Company | ||
|---|---|---|
| Medical Guidance Systems, LLC | DEKK-TEC, Inc. | NERx Biosciences, Inc. |
| Antibodies that are specific to the PDZ domain on TIP1: Interrupt cell viability signal transduction pathways. | 4-demethyl-4-cholesteryloxycarbonyl-penclomedine (DM-CHOC-PEN): Alkylating agent that may prevent DNA repair and sensitize cancer cells to radiation | DNA-PK inhibitor: Inhibits repair of DNA damage which potentiates cellular sensitivity to radiation. |
| None specified | Brain metastases | None specified |
| Phase I grant; 2016 | Phase I grant; 2017 | Phase I grant; 2017 |
| To determine the efficacy of anti-TIP1/PDZ IgG compared to anti-TIP1/PDZ scFv antibodies in mouse models of cancer and to determine the role of immune effector cells in the cancer response to anti-TIP1 antibodies. |
|
Expand upon identified DNA-PK inhibitors and dvance the development of these molecules for use as anti-cancer therapeutics and to increase the efficacy of radiation and chemotherapy. |
| Anti-PDZ antibodies enhanced the effects of radiotherapy in mice models of glioma and lung cancer (16). | Ongoing phase I clinical trial of DM-CHOC-PEN with WBRT in patients with brain mets: NCT03371004 | No public data found |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
| No clinical trials | Trial is ongoing in collaboration with Tulane University Medical Center. | No clinical trials |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
| n/a | n/a | n/a |
Radiosensitizers in Clinical Trials
Overall, six companies (6/12) have successfully progressed into prospective clinical trials in cancer patients to determine the safety and efficacy of their investigational drugs. Two companies awarded contracts, and four companies awarded grants, have advanced their drugs into clinical trials. Celldex Therapeutics, Inc. (Needham, MA), Shuttle Pharmaceuticals (Rockville, MD), NuvOx Pharma (Tucson, AZ) and BioMimetix JV, LLC (Greenwood Village, CO) have advanced their radiosensitizers into clinical trials for the cancers indicated in their NCI SBIR Development Center research proposals, while Apogee Biotechnology Corporation (Hummelstown, PA) has advanced its drug into trials as monotherapy (without concurrent radiation) and for cancers not specified in the SBIR radiosensitizer research proposal. Only BioMimetix has initiated a randomized trial of their investigational drug.
Celldex Therapeutics, Inc. received phase I and II funding to develop Fms-like tyrosine kinase ligand (Flt3L). Flt3L is a hematopoietic growth factor that increases the number of dendritic cells in blood and tissues and can act in conjunction with radiosurgery to stimulate an immune response against cancer cells. Celldex already completed a phase I clinical trial of their Flt3L drug (CDX-301) in 30 healthy volunteers. Aside from one case of grade 3 community-acquired pneumonia, there were no other infections, toxicities or serious adverse events (17). CDX-301 increased peripheral expansion of monocytes, hematopoietic stem cells and progenitor cells, which supports the potential for CDX-301 to augment the immune effect against cancer. A single-arm phase II clinical trial has been initiated for the combination of stereotactic radiotherapy and Fltr3L therapy in patients with advanced NSCLC that has progressed after standard systemic therapy (https://clinicaltrials.gov; NCT02839265). Fltr3L therapy (CDX-301) is administered as daily subcutaneous injections (75 μg/kg) for 5 days beginning with the first day of radiation therapy. The trial is being conducted in collaboration with the Albert Einstein College of Medicine (Bronx, NY).
Shuttle Pharmaceuticals, LLC is developing 5-iodo-2-pyrimidinone-2′-deoxyribose (IPdR) for locally advanced rectal cancer. IPdR is a prodrug of the radiosensitizer IUdR (5-iodo-2′-deoxyuridine), which is a halogenated nucleoside analog. In collaboration with Rhode Island Hospital (Providence, RI), a phase I clinical trial is underway with the goal of determining the maximum tolerated dose of oral IPdR for patients with metastatic gastrointestinal tumors receiving palliative radiation therapy (https://clinicaltrials.gov; NCT02381561). Oral IPdR is given daily for 28 consecutive days with concurrent palliative radiation therapy. On day 8, patients undergo palliative radiation therapy for 5 days a week for 3 weeks.
Apogee Biotechnology Corporation received a phase I grant to develop an oral sphingosine kinase-2 inhibitor, ABC294640, as a radiosensitizer for the treatment of prostate cancer. In a phase I clinical trial initiated in 2011, it was determined that ABC294640 was safe and well tolerated in patients with advanced solid malignancies (18). On March 31, 2015, RedHill Biopharma Ltd. (Tel Aviv, Israel/Raleigh, NC) acquired ABC294640 from Apogee for an upfront price of $1.5 million, $4 million in milestone payments and tiered royalties in the low double-digits (19). At that time, Apogee had already received cumulative funding exceeding $14 million for the support of ABC294640 from the NCI, BARDA, DoD, the FDA Office of Orphan Products Development and the Pennsylvania Department of Health. RedHill Biopharma is currently pursuing the anti-cancer properties of ABC294640 as monotherapy in clinical trials for cholangiocarcinoma, hepatocellular carcinoma and multiple myeloma (https://clinicaltrials.gov/; NCT03377179; NCT02939807; NCT02757326).
BioMimetix is developing BMX-001, a metalloporphyrin antioxidant, with both radioprotector and radiosensitizer properties. In collaboration with Duke University (Durham, NC), BioMimetix has initiated a phase I/II randomized clinical trial of radiation therapy and temozolomide with or without BMX-001 in patients with high-grade glioma, with the hope of protecting against cognitive deterioration and improving survival (https://clinicaltrials.gov/; NCT02655601). The company is also investigating BMX-001 as a radioprotectant of gastrointestinal symptoms in a phase I clinical trial of patients with anal cancer receiving definitive chemoradiation and as a radioprotectant of mucositis in a phase I clinical trial of patients with squamous cell head and neck cancer undergoing definitive chemoradiation (https://clinicaltrials.gov/; NCT03386500; NCT02990468).
DEKK-TEC, Inc. (New Orleans, LA) received phase I funding to combine 4-demethyl-4-cholesteryloxycarbonylpenclomedine (DM-CHOC-PEN), an alkylating agent with anti-cancer properties, with whole-brain radiation therapy for the treatment of brain metastases in a prospective clinical trial. DEKK-TEC had already received prior SBIR funding and completed phase I and II clinical trials in adults and adolescents and young adults that revealed a manageable toxicity profile and some objective treatment responses (20–22). The phase I trial of DM-CHOC-PEN and cranial radiation is currently ongoing in collaboration with Tulane University Medical Center, Ochsner Medical Center (both in New Orleans, LA) and recently, New York-Presbyterian Queens/Weill Cornell Medicine (New York, NY) (https://clinicaltrials.gov/; NCT03371004).
DISCUSSION
There is a definite need for the development of novel radiosensitizers to improve patient survival and/or reduce the toxicity of chemoradiation regimens. The SBIR Program has provided small businesses with valuable funding to complete pre-clinical studies needed to translate promising radiosensitizers into prospective clinical trials, with the ultimate goal of drug commercialization for public benefit. We assessed the research outcomes of SBIR-funded companies to determine the progress made in the development of novel radiosensitizers.
We found that 50% of companies (6/12) that received NCI SBIR Development Center funding have advanced their drugs into prospective clinical trials. All but one of these six companies are investigating their drug in combination with radiation therapy for the cancers described in the NCI SBIR Development Center research proposals. It is notable that these promising drugs often have multiple potential indications (e.g., anti-cancer therapy, radioprotector and radiosensitizer). Further, continued follow-up is needed to determine the proportion of these investigational drugs that enter late-phase clinical trials and ultimately, routine clinical practice.
This study provides useful benchmarks for assessing research outcomes for a cohort of SBIR-funded proposals for the development of radiosensitizers. We used a comprehensive internet search that identified all pertinent publicly available information. The NCI SBIR Development Center Program is unique in that there are clearly defined milestones for commercialization of novel therapies (IND approval, prospective clinical trials and FDA approval), which allowed us to quantify the progress of research proposals. However, there are also significant limitations to this analysis. The research proposals funded in the past several years may be too early in their development to assess their research outcomes. We also only analyzed publicly available data, which does not capture progress in drug development that remains confidential; although, substantial achievements are unlikely in the absence of prospective clinical trials, which are necessary for the commercialization of new drugs. Finally, the research outcomes for this cohort of radiosensitizer proposals should be compared to the results of other NCI SBIR Development Center contracts and grants.
It is difficult to assess the impact of federal funding in drug development, especially for companies that might have multiple products or multiple indications for the same product in their pipeline. Even if one product fails to achieve clinical success, it may be useful in another context or impact the genesis of a successful derivative product. This makes collection of metrics using publicly available data extremely challenging and is an inherent limitation of this work.
CONCLUSION
The NCI SBIR Development Center pathway is a pipeline for developing promising radiosensitizers with potential for commercial success and improved outcomes for cancer patients. NCI SBIR Development Center funding has supported the translation of promising therapies in the pre-clinical stage of development into clinical trials. Further continued follow-up, with a focus on clinical trials outcome analysis, is needed to determine the proportion of these novel radiosensitizers that are demonstrated to improve tumor control and/or survival. Possible next steps include the use of lower doses of radiation and/or chemotherapy with the use of radiosensitizers to determine if dose de-escalation is possible. Careful pre-clinical analysis of radiosensitizer use is needed to determine if their use changes the biology of radiation therapy. For example, does gene expression change and do these changes suggest new approaches to treat the tumor, given new agent availability and new biomarker capacities? Does exosome content change and point to a new response biology that can be exploited in this context? And do these agents affect the new biology of very rapid dose rate delivery or “FLASH” radiation therapy?
We should bear in mind the ongoing challenges to bringing agents into clinical use via radiation-drug trials (Fig. 1) (23). Combining radiation with novel anti-cancer drugs can increase toxicity, which may be a barrier for phase I trials. Also, radiation sensitizers are typically proposed for treatment of patients in the definitive or nonmetastatic setting, which can require larger studies and longer follow-up than for trials in the metastatic setting. To overcome these challenges, investigators should focus on more specific drug targets and accrue trials more quickly. International collaborations will be necessary for many trials (24). This is both known and supported by the NCI. Finally, these agents require testing with new agents such as hadron beams, radiopharmaceuticals (beta and alpha emitters) and immunotherapy.
FIG. 1.
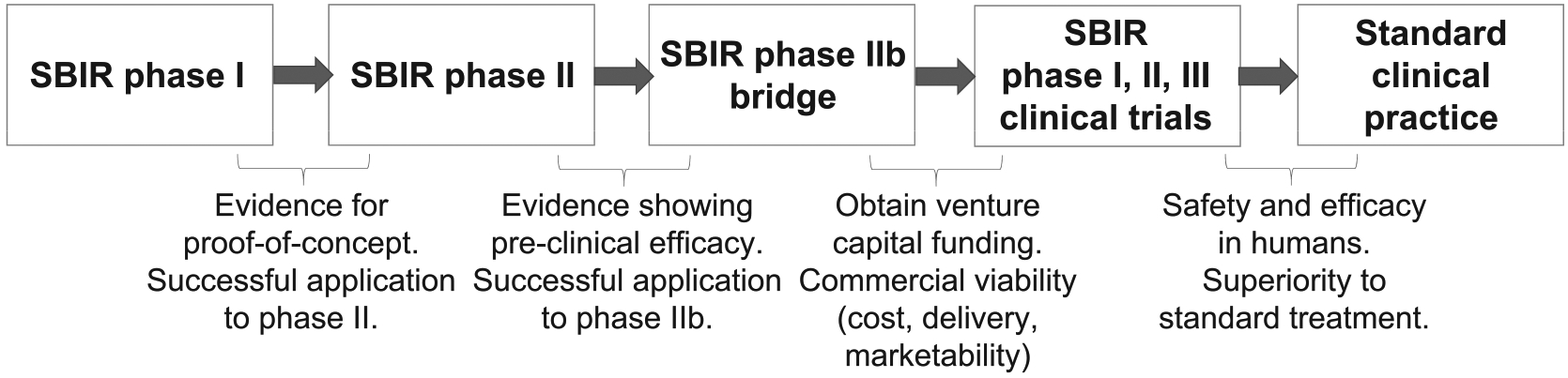
Schematic showing the challenges along the SBIR pathway for development of novel radiosensitizers.
ACKNOWLEDGMENTS
We thank the many members of the NCI and the grantees who helped collect these data. This work represents a collaboration between the RRP and the SBIR Development Center. The RRP initiated these contract solicitations and prepared this manuscript for publication. The SBIR Development Center funded the contracts under these solicitations. The views expressed are those of the authors; no endorsement by the NCI or other agencies has been given or inferred. All information presented here is publicly available.
REFERENCES
- 1.Walker AJ, Kim H, Saber H, Kluetz PG, Kim G, Pazdur R. Clinical development of cancer drugs in combination with external beam radiation therapy: US Food and Drug Administration perspective. Int J Radiat Oncol Biol Phys 2017; 98:5–7. [DOI] [PubMed] [Google Scholar]
- 2.Prasanna PG, Narayanan D, Hallett K, Bernhard EJ, Ahmed MM, Evans G, et al. Radioprotectors and radiomitigators for improving radiation therapy: The Small Business Innovation Research (SBIR) gateway for accelerating clinical translation. Radiat Res 2015; 184:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colevas AD, Brown JM, Hahn S, Mitchell J, Camphausen K, Coleman CN. Radiation Modifier Working Group of the National Cancer Institute. Development of investigational radiation modifiers. J Natl Cancer Inst 2003; 95:646–51. [DOI] [PubMed] [Google Scholar]
- 4.Stickel SA, Gomes NP, Frederick B, Raben D, Su TT. Bouvardin is a radiation modulator with a novel mechanism of action. Radiat Res 2015; 184:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JL, Leos RA, Baker AF, Unger EC. Radiosensitization of Hs-766T pancreatic tumor xenografts in mice dosed with dodecafluoropentane nano-emulsion - preliminary findings. J Biomed Nanotechnol 2015; 11:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodhead G, Costello J, Unger E. Reversal of tumor hypoxia following intravenous delivery of nano-droplet DDFP, a novel oxygen-transport agent: fiber-optic oxygen probe measurement and MRI characterization. J Vasc Interv Radiol 2016; 27:S296. [Google Scholar]
- 7.Krtolica A, Le Moan N, Serwer L, Yoshida Y, Ozawa T, Butowski N, et al. Treatment with Omx-4.80, a tumor-penetrating tunable oxygen carrier, reduces tumor hypoxia and dramatically enhances radiation therapy in intracranial models of glioblastoma. Neuro Oncol. 2014; 16:ii3–ii4. [Google Scholar]
- 8.Le Moan N, Getz J, Ng S, Davis T, Bedard C, Davis A, et al. Hypoxia reduction in intracranial glioblastoma models by Omx-4.80p, a pegylated engineered H-NOX oxygen carrier that is long-lasting in circulation and safe. Neuro Oncol 2014; 16:v86. [Google Scholar]
- 9.Yan H, Kapoor V, Nguyen K, Akers WJ, Li H, Scott J, et al. Anti-tax interacting protein-1 (TIP-1) monoclonal antibody targets human cancers. Oncotarget 2016; 7:43352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan H, Nguyen K, Kapoor V, Mnich S, Scott J, Li H, et al. Targeting of a radiation inducible tax-interaction protein 1 (Tip 1) as a novel molecule for cancer treatment. Cancer Res 2015; 75:4368. [Google Scholar]
- 11.Hallahan D, Kapoor V, Thotala D, Yan H. Activation of immune cells and enhanced efficacy of radiotherapy by anti-TIP1 antibodies in cancer. Radiat Oncol 2016; 119:S477. [Google Scholar]
- 12.Venant H, Rahmaniyan M, Jones EE, Lu P, Lilly MB, Garrett-Mayer E, et al. The sphingosine kinase 2 inhibitor ABC294640 Reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Mol Cancer Ther 2015; 14:2744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, Smith CD. Downregulation of critical oncogenes by the selective SK2 inhibitor ABC294640 hinders prostate cancer progression. Mol Cancer Res 2015; 13:1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor V, Dadey DY, Nguyen K, Wildman SA, Hoye K, Khudanyan A, et al. Tumor-specific binding of radiolabeled PEGylated GIRLRG peptide: A novel agent for targeting cancers. J Nucl Med 2016; 57:1991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadey DYA, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D, et al. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin Cancer Res 2017; 23:2556–64. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor V, Dadey D, Hoye K, Collins A, Thotala D, Hallahan D. Antibody targeting PDZ domain of TIP-1 induces proliferation arrest through AKT/mTOR signaling inhibition in lung cancer and glioblastoma. Cancer Res 2017; 77:4599. [Google Scholar]
- 17.Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P, et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant 2015; 50:924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britten CD, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2017; 23:4642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RedHill Biopharma acquires phase II first-in-class oral small molecule SK2 inhibitor from Apogee Biotech. Tel Aviv, Israel/ Raleigh, NC: RedHill Biopharma; 2015. (https://bit.ly/2wMjP88) [Google Scholar]
- 20.Weiner RS, Friedlander P, Gordon C, Saenger Y, Ware M, Mahmood T, et al. A first-in-humans phase I cancer clinical trial for 4-demethyl-4-cholesteryloxycarbonylpenclomedine (DMCHOC-PEN). Cancer Res 2013; 73:1185. [Google Scholar]
- 21.Weiner RS, Mahmood T, Gordon C, Ware ML, Morgan LR, Cosgriff TM, et al. Phase II clinical trial results for 4-demethyl-4-cholesteryloxycarbonylpenclomedine (DM-CHOC-PEN) in NSCLC involving the CNS. Cancer Res 2016; 76:CT129. [Google Scholar]
- 22.Morgan LR, Rodgers AH, Weiner RS, Mahmood T, Ware ML, Bhandari M, et al. Early clinical trial results for 4-demethyl-4-cholesteryloxycarbonylpenclomedine (DM-CHOC-PEN) in ado lescents and young adults (AYA) with cancers. Mol Cancer Ther 2018; 17:A086. [Google Scholar]
- 23.Falls KC, Sharma RA, Lawrence YR, Amos RA, Advani SJ, Ahmed MM, et al. Radiation-drug combinations to improve clinical outcomes and reduce normal tissue toxicities: current challenges and new approaches: Report of the symposium held at the 63rd Annual Meeting of the Radiation Research Society, 15–18 October 2017; Cancun, Mexico. Radiat Res 2018; 190:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol 2016; 13:627–42. [DOI] [PubMed] [Google Scholar]


