Summary
Simple decisions arise from the evaluation of sensory evidence. But decisions are determined by more than just evidence. Individuals establish internal, decision criteria that influence how they respond. Where or how decision criteria are established in the brain remains poorly understood. Here, we show that neuronal activity in the superior colliculus (SC) predicts changes in decision criteria. Using a novel ‘Yes-No’ task that isolates changes in decision criterion from changes in decision sensitivity, and computing neuronal measures of sensitivity and criterion, we find that SC neuronal activity correlates with the decision criterion regardless of the location of the choice report. We also show that electrical manipulation of activity within the SC produces changes in decisions consistent with changes in decision criteria and are largely independent of the choice report location. Our correlational and causal results together provide strong evidence that SC activity signals the position of a decision criterion.
eTOC Blurb
Crapse, et al., describe neuronal activity in the superior colliculus correlating with decision criteria, rather than a decision variable. Manipulation of SC neuronal activity with microstimulation changed the criterion predictably, demonstrating a causal role for the SC in decision criteria.
Introduction
Understanding how the brain makes even simple perceptual decisions remains an enigma in neuroscience. Work in trained animals performing two choice discrimination tasks suggests that sensory evidence is accumulated in sensorimotor regions of the brain until a threshold is reached. In dynamic decision models, the decision threshold is defined as the stopping point of evidence accumulation and describes how much sensory evidence is required to reach a decision. In static decision models, such as signal detection theory, the quantity or strength of sensory evidence required for a particular decision is determined by the position of a decision criterion and is equivalent to the starting point of evidence accumulation in dynamic models (Ratcliff et al. 2016). Much is known about where in the brain of human and non-human animals sensory signals are processed and where decision signals are accumulated (Britten et al. 1992; Shadlen et al. 1996; Heekeren et al. 2008; de Lafuente and Romo 2005), but far less is known about where or how decision criteria in static models or decision thresholds in dynamic models, are determined. Theoretical work proposes that the rapid rise of discharge in neurons of the superior colliculus (SC) around the time of an eye movement (the response modality most often used to report decisions) detects the crossing of a decision threshold that is determined by the basal ganglia (Lo and Wang 2006). Recent experimental work in humans shows that the basal ganglia play a role in threshold adjustments for decisions involving speed-accuracy trade-offs, sensory conflict, or memory (Forstmann et al. 2010; Cavanagh et al. 2011; Frank et al. 2007; Perugini et al. 2016). The SC plays a role in many processes such as target selection, planning, attention and decision-making (Horwitz et al. 2004; Kim and Basso 2008; Thevarajah et al. 2009; Kim and Basso 2010; Horwitz and Newsome 1999; Horwitz and Newsome 2001; Felsen and Mainen 2008; Ratcliff et al. 2003; Ratcliff et al. 2007) and receives input from the basal ganglia (Jiang et al. 2003; Liu and Basso 2008; Appell and Behan 1990; Kaneda et al. 2008) and much of cerebral cortex (Fries 1984; Lynch and Tian 2006; May 2006; Basso and May 2017) making it a plausible site to play a role in decision-making. Using a novel approach in monkeys that isolates changes in decision criterion from decision sensitivity and employing signal detection theory methods, we tested the hypothesis that the prelude activity of neurons in the SC signals the position of the decision criterion for simple, perceptual decisions.
The application of signal detection theory (SDT) to neurophysiological studies in monkeys has been instrumental in revealing the mechanisms of decision-making (Green and Swets 1966; Crapse and Basso 2015). Two critical behavioral measures arise from a SDT approach: sensitivity - a measure of how difficult it is to separate the signal in a sensory stimulus from noise, and criterion - a measure of how probable one decision is over another. Two choice discrimination tasks typically used in studies of decision-making, do not yield the behavioral outcomes required to isolate changes in sensitivity from changes in decision criterion. For this, stimulus detection approaches are appropriate. Taking advantage of this, we developed a ‘Yes’-’No’ task that incorporates sensorimotor priming to isolate changes in criterion from changes in sensitivity to determine whether the SC plays a role in signaling decision criteria. In our novel task, monkeys reported whether or not they observed orientation in a Glass pattern stimulus (Glass 1969; Nankoo et al. 2012). We varied the coherence of the Glass pattern to make the detection of orientation more or less difficult, a property easily parameterized with Glass patterns (Kiorpes et al. 2012). To isolate changes in criterion from changes in sensory sensitivity, we manipulated the frequency of occurrence of the patterns containing orientation information (structure) or no orientation information (no-structure) across blocks of trials. Manipulations of this sort create biases toward particular choices, a form of sensorimotor priming (Kristjansson et al. 2002). Importantly, we also dissociated the ‘Yes’ or ‘No’ decision from the choice report by requiring monkeys to report their ‘Yes’ choices to a green target and ‘No’ choices to a red target that switched positions randomly on each trial. We found that priming induced reliable shifts in criterion while leaving decision sensitivity unchanged (Green and Swets 1966; Brown and Steyvers 2005). We also recorded from SC neurons throughout the session, measuring pre- and post-priming neuronal activity and performance simultaneously. Neurophysiological recordings revealed that the activity of neurons within the SC before the onset of the choice response, reliably reflected the changes in the position of the decision criterion and measurement of the neuronal criterion correlated with monkeys’ behavioral criterion. Simulations of neuronal discharge rates generated from a decision variable model and a distance-to-criterion model showed that SC neuronal activity was best explained by a distance-to-criterion model. A causal manipulation of SC activity with electrical stimulation at sites where neurons correlating with the position of the criterion were recorded, mimicked the changes in the position of the criterion seen after sensorimotor priming. Based on our results, we conclude that the activity of SC neurons does not simply detect threshold crossings for perceptual decisions (Lo and Wang 2006) but rather, the prelude activity signals the position of the criterion for perceptual decisions.
Results
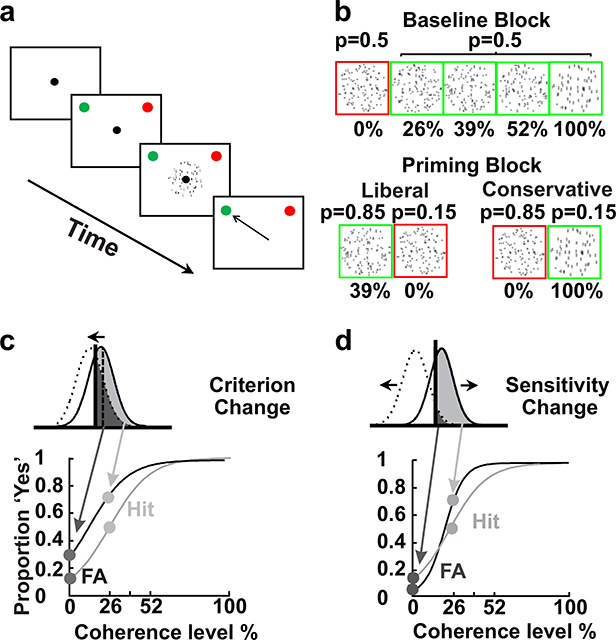
Monkeys reported the presence or absence of structure (vertical orientation) in Glass patterns (Supplemental Videos 1 and 2) by making a saccade to either a green target (‘Yes’) or a red target (‘No’) that switched positions randomly between two possible locations, one in each hemifield, ensuring a dissociation between the decision and the choice report. Reporting ‘Yes’ or ‘No’ in the different conditions of structure present or absent provided all the trial types required for an SDT analysis of the behavior (Figure 1). Following a block of equally-balanced structure and no-structure trials with an unequal block of structure and no-structure trials and then a final block of equally-balanced trials again (Figure 1b), allowed us to assess the influence of priming on monkeys’ decision sensitivity and criterion position. Figure 1c and d illustrate the possible outcomes of the priming manipulation. Changes in the criterion correlate with changes in the response bias or threshold (α) of the psychometric function and symmetric changes in both Hit and FA rates (Figure 1c) whereas, changes in sensitivity correlate with changes in the slope of the psychometric curve (β) and asymmetric changes in Hit and false alarm (FA) rates (Figure 1d).
Figure 1. Psychophysics of a ‘Yes-No’ decision task and sensorimotor priming.
a, Schematic of the spatial arrangement of the task and the sequence of the task events. The black outlined squares show the monitor and the black circle shows the fixation spot. The red and green circles indicate the choice targets and note that they changed position randomly. The small, black arrow shows an example choice. b, Illustration of the task statistics. The no-structure stimulus was a Glass pattern with 0% coherence (red) and the structure stimuli were 26%, 39%, 52%, 100% coherent Glass patterns (green). The probability of occurrence of the no-structure and structure stimuli was equal (balanced block). P indicates the probability of stimulus occurrence. In the unbalanced block, either the 39% coherent stimulus occurred more often compared to the 0% (no-structure) stimulus (85:15; Liberal priming trials) or the no-structure stimulus occurred more often than the 100% stimulus (85:15; Conservative priming trials). c. Signal detection theory predictions and psychometric functions for changes in criterion. d. Signal detection theory predictions and psychometric functions for changes in sensitivity.
Sensory priming induces changes in the position of the criterion independent of sensitivity
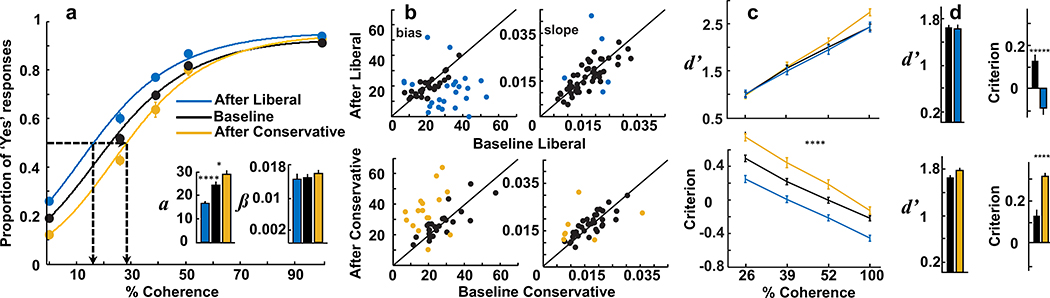
Figure 2a shows the mean task performance for 93 sessions from two monkeys (44 from Monkey B and 49 from Monkey S) plotted as the proportion of ‘Yes’ responses against Glass pattern coherence for baseline performance (Figure 2a, black, n=93), after priming with structure (Liberal, Figure 2a, blue; n=52; 28 from Monkey B and 24 from Monkey S) and after priming with no-structure (Conservative, Figure 2a, orange; n=41; 16 from Monkey B and 25 from Monkey S). Note that these data come from the second, balanced block of trials after the priming block of trials. Liberal priming caused a leftward shift in the psychometric function and a threshold reduction from 24.3% to 17.7% coherence (Figure 2a, leftmost dashed lines), indicating that the monkeys reported ‘Yes’ more often than baseline. Conservative priming resulted in the opposite pattern: a rightward shift of the psychometric function and an increase in threshold from 24.3% to 29.4% coherence (Figure 2a, rightmost dashed lines) indicating that the monkeys reported ‘No’ more often. Fitting logistic functions to the performance data showed that after liberal priming, the threshold decreased significantly ( difference: −6.6, p<0.001, Wilcoxon rank sum, z(143)=−3.13), whereas the slope remained unchanged on average ( difference: −0.0008, Wilcoxon rank sum, p=0.56, z(143)=−0.5751). On 5/52 sessions, the slope changed significantly after liberal priming, but this number differed significantly from the number of sessions resulting in changes in threshold after liberal priming (23/52; threshold vs. slope, p<0.0001; Chi-squared(1)=15.8). Following conservative priming, the threshold increased significantly ( difference: 5.1, Wilcoxon rank sum, z(132)=2.2767, p=0.02) whereas the slope remained unchanged on average ( difference: 0.002, Wilcoxon rank sum, z(132)=0.9089, p=0.3634). The incidence of significant slope changes was similarly low for conservative priming sessions (6/41) and was outnumbered by session changes in threshold (17/41; threshold vs. slope, p=0.007, Chi-squared(1)=7.3; Figure 2b). We also fit our behavioral data to cumulative Gaussian functions and analyzed changes in lapse rate (λ) which, when unaccounted for, can lead to spurious estimates of psychometric function parameters (Wichmann and Hill 2001). We found no significant change in λ across the baseline, conservative and liberal priming conditions (ANOVA, F(2,183)=2.54, p=0.08). ANOVAs conducted on the cumulative Gaussian function estimates of threshold and slope confirmed the logistic fits and indicated no significant change in slope (F(2,183)=0.58, p=0.56) but a highly significant change in the threshold parameter (F(2,183)=6.42, p=0.002). Finally, an analysis of reaction times across all coherences revealed an effect of priming condition on reaction times but no differences across coherence (2-way ANOVA, priming condition factor: F(2,930)=9.24, p=0.0001; coherence factor: F(4,930)=1.35, p=0.25; Supplemental Figure 1a).
Figure 2. Priming causes changes in decision criteria but not sensitivity.
a, Proportion of ‘Yes’ responses is plotted against % coherence. The data are fitted with logistic functions. Black points and curve show the data from the balanced block of trials before priming (Baseline; each black point is an average of 93 sessions from two monkeys). Blue data and curve are from the balanced block of trials that occurred after Liberal priming (n=52) and the orange data and curve are from the balanced block of trials occurring after the Conservative priming trials (n=41). The dotted lines highlight the shifts in the curves that occurred after priming relative to baseline. The insets show the fitted parameters from the logistic fits (see Methods). b, The threshold and slope measured from the fits of the data before priming are plotted against the threshold and slope measured during the balanced block of trials occurring after priming for all 93 sessions from two monkeys. Colored data points show statistically significant changes as determined by permutation test (p<0.05). c. d’ and criterion values (c) for all three conditions are plotted as a function stimulus coherence. Same color convention as panel a. d, d’ and c are plotted for the Liberal priming and Conservative priming trials before (baseline) and after priming. The black bars for d’ and c show the data for the baseline trials whereas the colored bars show the data for the balanced block that occurred after priming trials. Orange is for conservative priming and blue is for liberal priming. Error bars represent standard errors of the mean. * p < 0.05; ** p < 0.01; **** p < 0.001; ***** p<0.0005.
We computed d’, a measure of sensitivity, and c, a measure of criterion position, for all 4 coherence levels and for the baseline and after priming blocks across all sessions (Figure 2c). d’ increased as coherence increased but did not differ with priming condition (ANOVA, coherence: F(3,732)=199.93, p<0.00001; condition: F(2,732)=2.63, p=0.07). Criterion decreased as coherence increased, but unlike d’, differed significantly between baseline and after conservative and liberal priming conditions (ANOVA, coherence: F(3,732)=166.85, p<0.00001; condition: F(2,732)=76.47, p<0.00001; see Supplemental Table 1 for -z(FA)). Collapsing the data over all coherences and measuring the rates of the different trial types as well as calculating the overall d’ and c, confirmed our finding that priming resulted in changes to the position of the criterion primarily (Figure 2d). After liberal priming sessions, Hit and FA rates increased by 6.1% and 6.5% respectively (t(143)=3.5990, p<0.001; t(143)=2.4442, p=0.02, respectively) and no net change in d’ (t(143)=−0.4172, p = 0.68), but a significant decrease in c occurred, indicating that monkeys became more liberal after priming with structured Glass patterns ( difference: −0.22, t(143)=−3.12, p<0.002). After conservative priming, Hit rate decreased, although not significantly ( difference: −0.031, t(132)=−0.9784, p=0.33) and FA rate decreased significantly ( difference: −0.068, t(132)=−3.2101, p<0.002). As we saw with liberal priming, d’ remained unchanged after priming with a 0% coherence Glass pattern stimulus (t(132)=1.8292, p=0.07), whereas c increased significantly ( difference: 0.18, t(132)=2.5126, p=0.0132) indicating that monkeys became more conservative in responding after priming with no-structure. An analysis of the after-priming data examining early block performance (first 100 trials) vs later block performance (last 100 trials) revealed that the changes caused by priming were robust, lasting for the entire duration of the after-priming block (Supplemental Figure 1b–e). Finally, a multiple regression analysis comparing changes in threshold and slope and changes in d’ and c for the behavior, revealed no significant interactions, indicating that changes in these parameters were independent (Supplemental Table 2). Taken together, the results show that this method of sensorimotor priming successfully isolates changes in decision criterion.
SC neuronal activity before and after priming
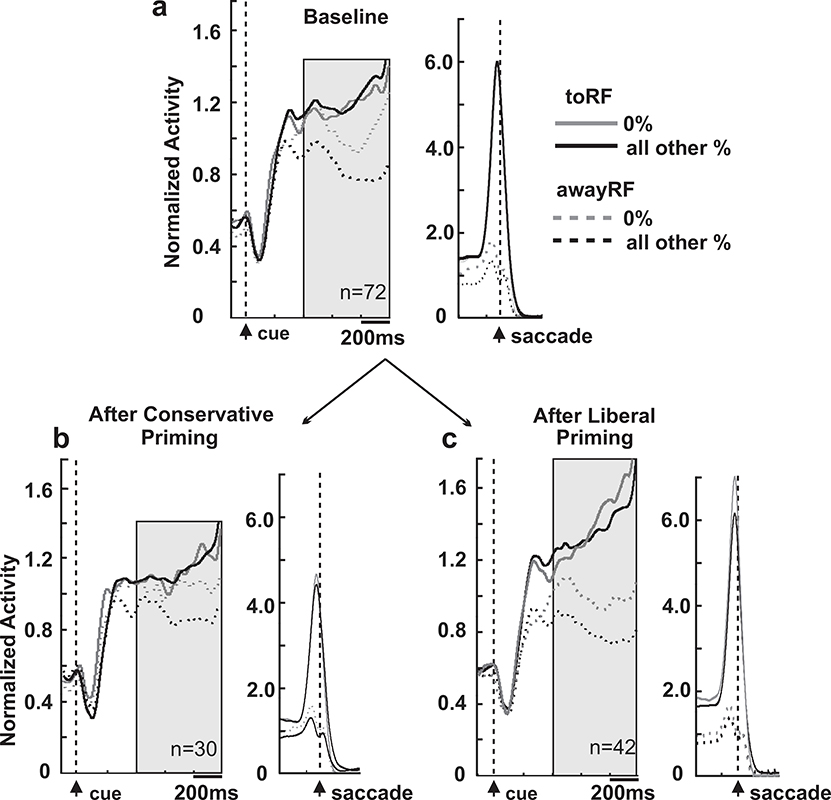
We recorded from 121 intermediate layer SC neurons from 2 trained monkeys performing the ‘Yes’-’No’ task. Sixty-seven of these neurons were recorded during the 3 blocks of liberal priming sessions and of these 42 (23 from monkey B, 19 from monkey S) showed prelude activity after cue onset and contribute to the analysis (Methods). Fifty-four neurons were recorded during the 3 blocks of conservative priming sessions and of these, 30 were analyzed (13 from monkey B, 17 from monkey S). The 49 neurons excluded showed little to no modulation during baseline performance. Figure 3 shows the averaged, normalized spike density functions for all 72 neurons recorded before priming (30 from conservative sessions + 42 from liberal sessions) for the 0% coherence trials (grey lines) and collapsed over structure coherences (26–100%; black lines). The solid traces show the activity for ‘Yes’ choices made toward the RF and the dashed traces show the activity for ‘Yes’ choices made away from the RF. Note that on ‘Yes’ choices made away from the RF, the ‘No’ target was in the neuron’s RF. Therefore, neuronal activity measured from ‘Yes’ choices reported toward the RF provide information about the neuron’s vote for a ‘Yes’ decision whereas neuronal activity measured from ‘Yes’ choices reported away from the RF, provide information about the neuron’s vote for a ‘No’ decision in analogy to the neuron-antineuron approach (Britten et al. 1992). Collicular neuronal activity is associated with decision-making in a manner similar to that seen in the lateral intraparietal cortex (LIP) (Kim and Basso 2008; Horwitz and Newsome 2001; Ratcliff et al. 2003; Ratcliff et al. 2007) and we reasoned that we would see similar activity in the SC associated with our Glass pattern task and indeed, Figure 3a shows this qualitatively. For ‘Yes’ choices made into the RF, neuronal activity in the SC was similar for the 0% coherence trials (FA trials) and the mean of all other coherence trials (Hit trials; cf., grey and black solid lines Figure 3a). For ‘Yes’ choices made away from the RF, however, neuronal activity in the SC differed for the 0% coherence and the mean of all other coherence trials. The activity for the structure stimuli (all non 0% coherence trials) was lower than that seen for the 0% coherence trials for the awayRF choices. We quantified the differences in ‘Yes-toRF’ and ‘Yes-awayRF’ activity for the 0% and structured stimuli with traditional ROC analysis, calculating areas under the ROC curve (AUC; Supplemental Figure 2). The AUC for the 0% coherence trials was 0.57 whereas that for the mean of the structure coherence trials was 0.66. These differences were significantly different (Wilcoxon rank sum, p < 0.005) and are consistent with previous reports (Kim and Basso 2008; Horwitz and Newsome 2001). Supplemental Figure 3 shows a main effect for coherence across the AUCs calculated for each coherence condition (0.57, 0.56, 0.62, 0.65 and 0.75; Kruskal-Wallis, df(4,209) Χ2=25.01, p<0.0001).
Figure 3. Superior colliculus relative neuronal activity scales with coherence and changes with priming.
a, Normalized spike density functions (sdf; σ = 10 ms) for the sample of neurons (n=72) recorded during the baseline block for each session. Black and grey traces show neuronal activity plotted across time for choices made to (solid lines) and away from (dashed lines) the RF of the recorded neurons. Left panels are aligned on Glass pattern onset (cue), and right panels are aligned to saccade onset. Transparent gray rectangles indicate analysis bins used for all analyses. b, Same as in a for the subset of neurons tested during conservative priming sessions (n=30). c, Same as in a for the subset of neurons tested during liberal priming sessions (n=42).
We next assessed whether a similar pattern appeared after priming. For the 30 conservative session neurons, the relative level of activity between ‘Yes’ choices made toward the RF (solid lines) and away from the RF (dashed lines), decreased following conservative priming (Figure 3b) whereas for the 42 liberal session neurons, the relative levels of activity increased after liberal priming (Figure 3c). Note that, as with the behavioral analysis, these data are from the second balanced block of trials so the stimulus frequency is the same as in the initial block of trials. The only difference is the intervening 200 priming trials. Quantifying this by computing the AUC for pooled ‘Yes’ report trials (0% + all structure coherences) showed that 7/30 of the neurons recorded during conservative priming sessions underwent significant reductions in AUC and 8/42 of neurons recorded during liberal priming sessions underwent significant increases in AUC. The mean AUCs calculated across all neurons belonging to each priming condition before priming was 0.64 (+/− 0.17), increased to 0.68 (+/− 0.13) after liberal priming, and decreased to 0.61 (+/− 0.18) after conservative priming, but these differences did not reach statistical significance (Supplemental Figure 3e; Kruskal-Wallis, df=(2,141), Χ2=2.06, p=0.36). Consistent with our behavioral results, sensitivity as measured by AUC, scales with coherence but changes little with sensorimotor priming.
Neuronal and behavioral measures of decision criterion co-vary
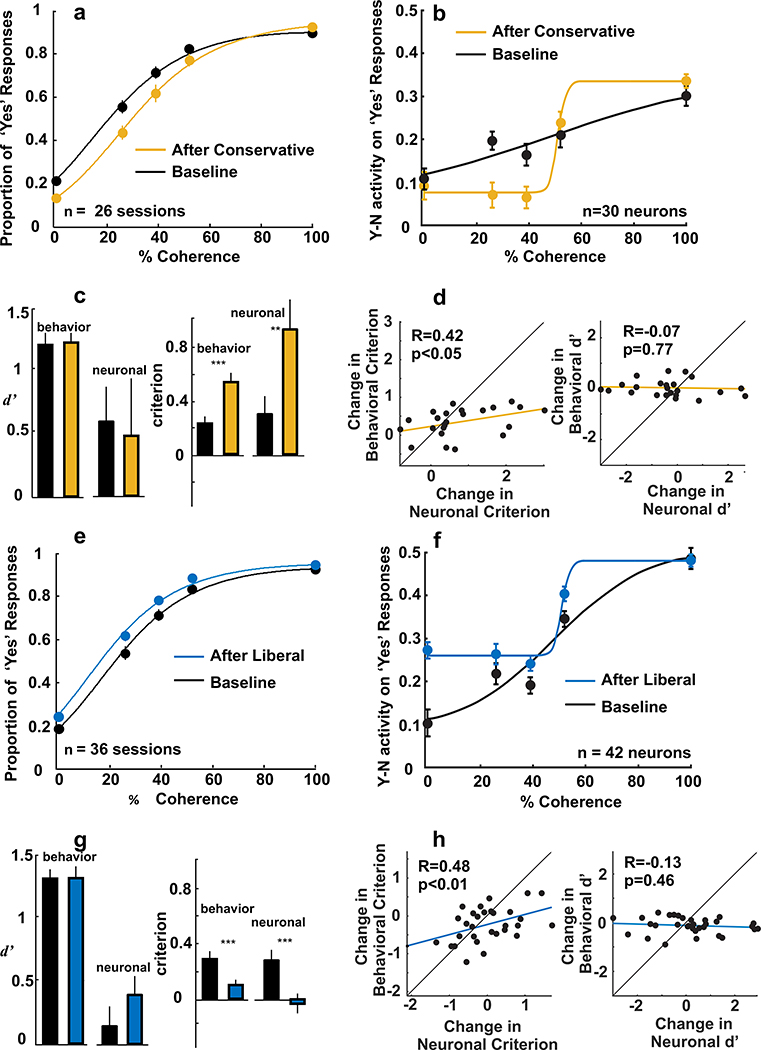
Figure 4a shows behavioral performance before and after conservative priming for all behavioral data collected during neuronal recording sessions (n=26 sessions and 30 neurons). These data are a subset of those shown in Figure 2. Figure 4b shows neuronal performance for the same dataset, before and after conservative priming. Plotted is the relative level of activity for ‘Yes’ choices made toward and away from the RF as a function of Glass pattern coherence on Hit (structure coherence trials) and FA (0% coherence) trials. To determine the relative level of activity for Hit and FA trials we calculated the mean discharge rate during the cue epoch (400–1000ms after Glass pattern onset) and defined ‘Yes’ activity as the activity measured on trials in which the monkeys made a ‘Yes’ choice into the RF for structure coherence trials (Hit) and a ‘Yes’ choice into the RF for 0% coherence trials (FA). ‘No’ activity was defined as the activity measured on trials in which the ‘No’ target was in the RF and the monkey made a ‘Yes’ choice away from the RF on structure coherence trials (Hit) and 0% coherence trials (FA). Next, we subtracted ‘No’ activity from ‘Yes’ activity (‘Y-N’) and fitted a cumulative Gaussian function. Following conservative priming, ‘Y-N’ activity decreased overall, consistent with the overall decrease in ‘Yes’ responding shown in Figure 4b (see Supplemental Figure 5a). To determine whether behavioral measures of sensitivity and criterion (d’ and c) and neuronal measures of sensitivity and criterion co-varied, we computed the traditional measures of d’ and c for the behavioral data and extended these computations to compute neuronal measures of d’ and c. The neuronal calculations were identical to those used for the behavioral measures except we used the ‘Y-N’ discharge rate rather than the behavioral choices to calculate d’ and c (see Methods). Behavioral d’ remained statistically unchanged after conservative priming (p=0.42, permutation test; Figure 4c) whereas behavioral c increased significantly ( difference: 0.31, p<0.005, permutation test; Figure 4c), indicating an overall increase in ‘No’ responding. These behavioral changes were mirrored by neuronal changes. Neuronal d’ remained constant (p=0.35, permutation test; Figure 4c) whereas neuronal c increased significantly ( difference: 0.62, p<0.001, permutation test; Figure 4c). Indeed, these changes were robust over time (Supplemental Figure 4). Finally, to determine whether behavioral and neuronal measures of d’ and c directly correlated, the left panel of Figure 4d shows change in behavioral criterion plotted as a function of change in neuronal criterion for each behavioral session-neuron pair. We found that changes in behavioral criterion induced by conservative priming correlated significantly with changes in neuronal criterion (Pearson’s r=0.42, p<0.05). In contrast, no correlation was found between changes in behavioral and neuronal d’ (Figure 4d, right; r=−0.07, p=0.77).
Figure 4. Neuronal and behavioral measures of sensitivity and criterion.
a, Proportion of ‘Yes’ responses is plotted against % coherence for 26 conservative priming sessions in which neuronal activity (n=30) was recorded simultaneously with behavior. Data and logistic fits in black show the baseline performance, before conservative priming. Data and logistic fits in orange show performance after conservative priming. Note that these data show a subset of the data shown in Figure 2 for which we recorded the behavior and the neuronal activity simultaneously. b. ‘Y-N’ neuronal activity on ‘Yes’ trials is plotted against % coherence for the 30 neurons recorded during the 26 conservative priming sessions plotted in panel a. Black shows the before priming data. Orange show the after conservative priming neuronal performance. Qualitatively, the ‘Y-N’ activity on ‘Yes’ choices shifts in directions compatible with the behavioral data, but note that direct one-to-one comparisons between the behavioral data in Figure 4a and the ‘Y-N’ activity is not possible since the behavioral data are expressed as proportions bound between 0 and 1, whereas the neuronal data are ‘Y-N’ activity on ‘Yes’ choices with no upper bound (see Supplemental Figure 5a). c. Mean and SE of d’ (left) and criterion (right) for both behavior and neuronal activity for the balanced block of trials before (black) and after (orange) conservative priming. d., Left. Change in behavioral criterion plotted against change in neuronal criterion for the same set of neurons and conservative priming sessions plotted in panels a and b. Each data point represents a single session-neuron pair. Orange line represents line of best fit. Right, Change in behavioral d’ plotted against change in neuronal d’ for the conservative priming behavioral and neuronal data depicted in panel a. e. Same as in a for 36 Liberal priming sessions in which neuronal activity (n=42) was recorded at the same time as behavior was measured. f. Same as in b for the 42 neurons recorded during the 36 Liberal priming sessions displayed in panel e. See also Supplemental Figure 5b. g. Same conventions as panel c for the liberal priming data. h. Left, Change in behavioral criterion plotted against change in neuronal criterion for the Liberal priming behavioral and neuronal data depicted in panels e and f. Right, Change in behavioral d’ plotted against change in neuronal d’ for the liberal priming data shown in panel g. Error bars show bootstrapped standard errors of the mean. ** p<0.01; *** p<0.005.
Figures 4e and 4f show the influence of liberal priming for all behavioral and neuronal data collected during all neuronal recording sessions (n=36 sessions and 42 neurons). Following liberal priming, ‘Yes’ responding increased (Figure 4e) and ‘Y-N’ activity on ‘Yes’ response trials increased (Figure 4f; see Supplemental Figure 5b for neuronal ‘Yes rate’ analysis). Overall, liberal priming caused no net change in d’ (Figure 4g, left; p=0.48, permutation test), whereas c decreased significantly (Figure 4g, right; difference: −0.2, p<0.005, permutation test) indicating a greater propensity for monkeys to report ‘Yes’. For the neuronal data, we found that the neuronal d’, remained statistically similar (p=0.11, permutation test) and that the neuronal c showed a statistically significant reduction with liberal priming ( difference: −0.32, p< 0.0001, permutation test, Figure 4g). Similar to the conservative behavioral and neuronal criterion changes, the changes in behavioral and neuronal criterion following liberal priming showed statistically significant correlations (Figure 4h, left; r=0.48, p<0.01), whereas the changes in behavioral and neuronal d’ did not (Figure 4h, right; r=−0.13, p=0.46). Performing a multiple regression analysis comparing changes in the neuronal d’ and c, revealed no significant interactions for the liberal priming data, indicating that changes in these parameters were independent for this condition. Although there were no significant changes in d’ for the conservative priming condition, there was a significant interaction between the neuronal d’ and c, indicating a relationship between these measures in this condition (Supplemental Table 2). Taken together, the results uncover a direct correlation between neuronal and behavioral measures of sensitivity and criterion and provide compelling evidence that the activity of the SC correlates with the decision criterion.
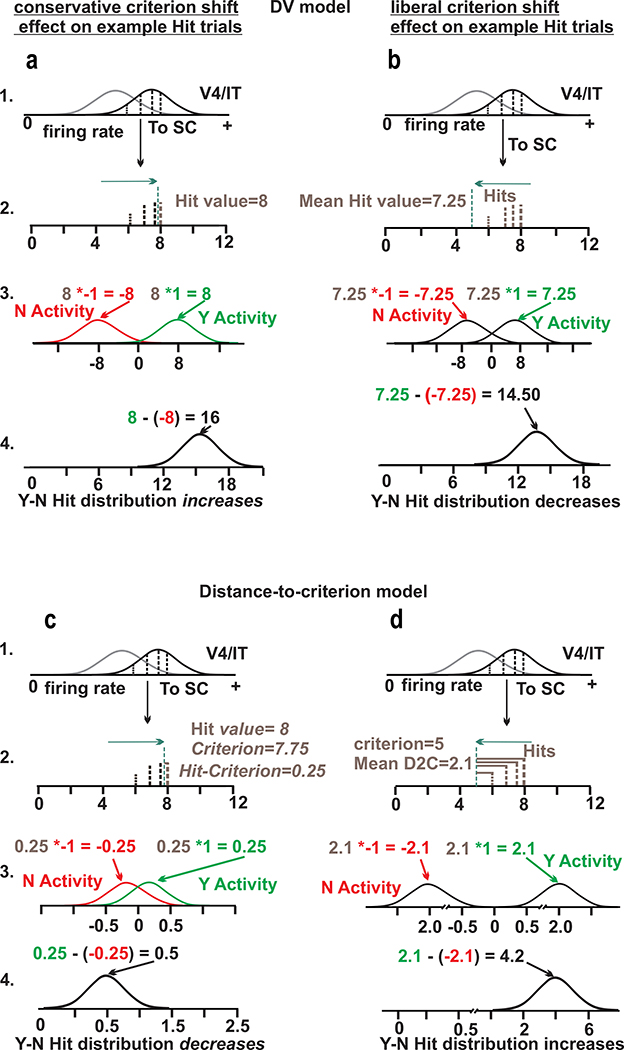
To understand the relationship between the behavior of the monkeys, changes in decision criterion and changes in SC neuronal activity, we simulated discharge rates for two possible models of what signals the SC may encode and compared the simulated predictions with the actual discharges obtained (see Methods). Supplemental Figure 6 shows the approach used to generate simulated data for example Hit trials and illustrates the two models used; a decision variable (DV) model, which assesses the balance of ‘Yes and ‘No’ evidence and a distance-to-criterion model.
Figure 5 shows how the two models make different predictions using Hit trials as an example. The DV model predicts that after conservative priming ‘Y-N’ activity measured on Hit, FA, CR and Miss trials increases, whereas after liberal priming, the ‘Y-N’ activity decreases. Figure 5a and b show an example for Hit trials before and after a conservative and liberal criterion shift, respectively, for the DV model. Following a rightward criterion shift, the means of the structure distribution trials to the right of the criterion are relatively high, with a mean value of 8 (the 3 trials of lower discharge rate fall to the left of the criterion and are therefore not Hit trials). Note that for illustrative purposes each draw represents multiple draws from the structure distribution to convey changes to the means of the simulated activity. Following the assignment of + or − based on the location of the choice report as described above, the ‘Y’ activity becomes 8 and the ‘N’ activity becomes −8. Note that for simplicity, the Hit value of 8 is used here for both the ‘No’ and ‘Yes’ activity, though during each iterated trial, two draws were made, one for the ‘Y’ activity and one for the ‘N’ activity. This results in a ‘Y-N’ Hit distribution with a mean value of 16. This contrasts to ‘Y-N’ activity on Hit trials following liberal criterion shifts (Figure 5b). Despite identical draws from the sensory area structure distributions, the liberal criterion value of 5 means that all four of the draws are now to the right of the criterion and thus exceed the criterion. This drives down the mean Hit value to 7.25, which results in a final ‘Y-N’ Hit distribution with a mean of 14.5, a value lower than that obtained on conservative criterion shifts, despite identical structure distribution draws (cf., 1; Figure 5a and b). The distance-to-criterion model predicts that after conservative priming, ‘Y-N’ activity measured on Hit, FA, CR and Miss trials decreases, whereas after liberal priming these activities increase (Figure 5c and d). During conservative criterion changes, the criterion shifts rightward to a value of 7.75. Subtracting the criterion from the mean Hit value of 8 yields a distance-to-criterion value of 0.25. Signing the value and taking the difference (Figure 5c, 3 and 4) yields a ‘Y-N’ activity distribution with a mean value of 0.5. Following a liberal criterion shift, the criterion has a value of 5 which results in all four trials exceeding the criterion and a mean distance-to-criterion value of 2.1 (Figure 5d). Following the signing operations and ‘Y-N’ subtraction, the final ‘Y-N’ Hit value is now larger, with a value of 4.2. Note that all four draws from the structure distribution are identical for all panels in Figure 5.
Figure 5. Simulations of conservative and liberal criterion shifts.
a. The DV model during conservative criterion shifts. (1) Two sensory area distributions are plotted. Four samples (black vertical lines) from the structure distribution are shown. (2) 1 of the samples is a Hit (i.e. has a value > criterion) and its value is shown in brown. (3) The value of the Hit sample is signed (multiplied by −1 or +1) depending on the target in the model RF. Colored distributions represent activity for the ‘No’ and ‘Yes’ choice targets, red and green, respectively. (4) The difference between the two distributions is taken, b. The DV model during liberal criterion shifts. Same conventions as a, but with a criterion value of 5. c. The Distance-to-criterion model during conservative criterion shifts. (1) Same 4 samples from a. used for comparison. (2) horizontal bars represent criterion distance. (3) and (4) same operations as in panels a and b. d. The Distance-to-criterion model during liberal criterion shifts. Same conventions as c. See text and Supplemental Figure 6 for further description.
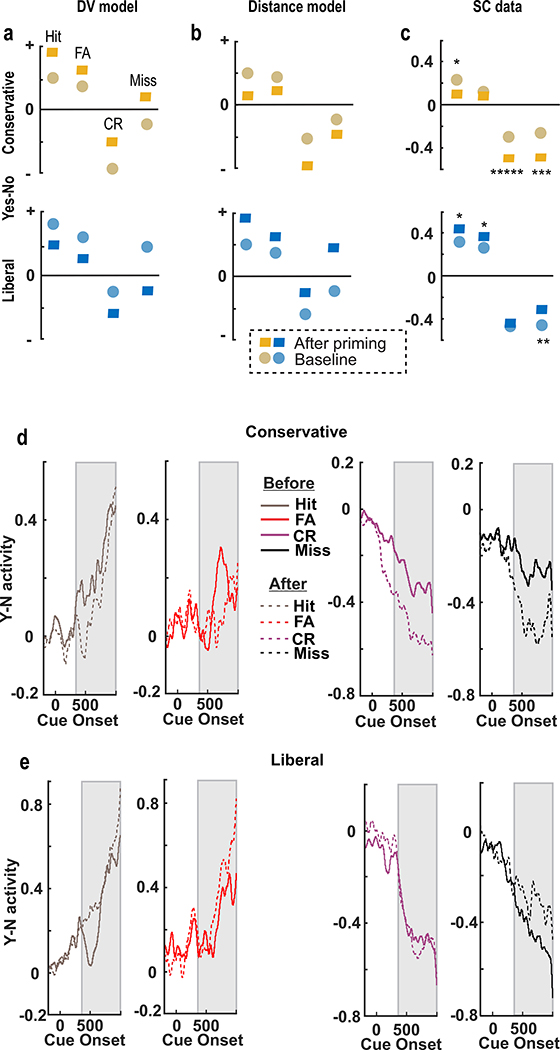
The DV and the distance models have opposite predictions for the changes in neuronal activity that occur with priming and allow us to assess which model best explains the activity of SC associated with changes in decision criterion. Figure 6a and b show the predicted results for the DV and distance-to-criterion model for direct comparison with the results from the recordings made in the SC before and after conservative and liberal priming (Figure 6c). The circles in Figure 6c show the normalized ‘Y-N’ neuronal activity before priming and the squares show the activity after priming for the different SDT trial types; Hit, FA, CR and Miss. We calculated the ‘Y-N’ activity for Hit and FA trial types as described above. To compute the ‘Y-N’ activity for CR and Miss trial types, we defined ‘Yes’ activity as the activity measured on trials in which the ‘Yes’ target was in the neuron’s RF and the monkeys made a ‘No’ choice away from the RF for 0% coherence trials (CR) and a ‘No’ choice away from the RF for structure coherence trials (Miss). ‘No’ activity was defined as the activity measured on trials in which the ‘No’ target was in the RF and the monkey made a ‘No’ choice into the RF on 0% coherence trials (CR) and structure coherence trials (Miss). We then subtracted ‘No’ activity from ‘Yes’ activity to yield ‘Y-N’ activity for these SDT trial types. Orange shows the conservative priming results and blue shows the liberal priming results and more saturated colors show the after priming results. To control for variability in neuronal discharge rates across the sample of neurons, we normalized the discharge rate for each neuron before and after priming by the average discharge rate measured over all trials (Methods). As the distance model predicts, the ‘Y-N’ activity for Hit, FA, CR and Miss trials decreased after conservative priming. The reduction from 0.25 to 0.16 for Hit trials was statistically significant (permutation test, p<0.05) as was the reduction from −0.30 to −0.51 and −0.26 to −0.49 measured in CR and Miss trials (permutation test, p<0.0005 and p<0.005). The decrease from 0.12 to 0.09 in FA trials, although in the correct direction, failed to reach statistical significance (permutation test, p=0.6). Figure 6c also shows the results from the SC neuronal recordings made before and after liberal priming (blue). Here too, the direction of change in activity after priming corresponded best to that predicted by the distance-to-criterion model. The ‘Y-N’ increase in activity was statistically significant for Hit, FA and Miss trials (0.32 to 0.44, 0.25 to 0.35 and −0.45 to −0.31; permutation tests, p<0.05, p<0.05, and p<0.01, respectively). The change in neuronal activity on CR trials after liberal priming failed to reach statistical significance (−0.45 to −0.47, permutation test, p=0.9). To appreciate the temporal dynamics of ‘Y-N’ activity, Figure 6d and e shows ‘Y-N’ activity plotted over time for the data shown in Figure 6c, before and after conservative (Figure 6d) and liberal priming (Figure 6e). Overall, these results support the hypothesis that the activity of the SC correlates best with the position of the decision criterion.
Figure 6. Superior colliculus neuronal activity signals the position of a decision criterion.
a. DV model simulated Yes-No (‘Y-N’) activity before (circles) and after (parallelograms) Conservative priming (orange, top), and Liberal priming (blue, bottom). ‘Y-N’ activity is plotted for Hit, FA, CR, and Miss trials. Arrows indicate the direction of the changes following priming. b. Distance model simulated ‘Y-N’ activity. Same conventions as in a. c. Mean ‘Y-N’ neuronal activity across all 4 SDT outcomes before (Baseline) and after Conservative (left), and Liberal (right) priming. Error bars are obscured by the symbols. d. Temporal dynamics of ‘Y-N’ activity on Hit, FA, CR and Miss trials before (solid traces) and after (dashed traces) Conservative priming for the same neurons plotted in panel c, top. Data are aligned to the onset of the Glass pattern cue. Grey window shows the analysis epoch used to calculate the data points plotted in panel c. e. Temporal dynamics of ‘Y-N’ activity before (solid traces) and after (dashed traces) Liberal priming. Same conventions as d. * p < 0.05; ** p < 0.01; *** p < 0.005; ***** p<0.0005.
Electrical manipulation of SC activity changes the decision criterion
If SC neuronal activity correlates with the position of the decision criterion, we reasoned that manipulation of the activity in the SC should change this signal and alter decisions in predictable ways. Specifically, we predicted that manipulation of SC activity on trials in which the ‘No’ choice was in the stimulation field would result in more conservative choosing and that manipulation of the SC on trials in which the ‘Yes’ choice was in the stimulation field would result in more liberal choosing. Note that the ‘Yes’ or ‘No’ choice target could occur in either hemifield, so we could assess whether stimulation simply changed the likelihood of saccades to one location or whether stimulation caused changes in the likelihood of a ‘Yes’ or ‘No’ decision regardless of the location of the choice report.
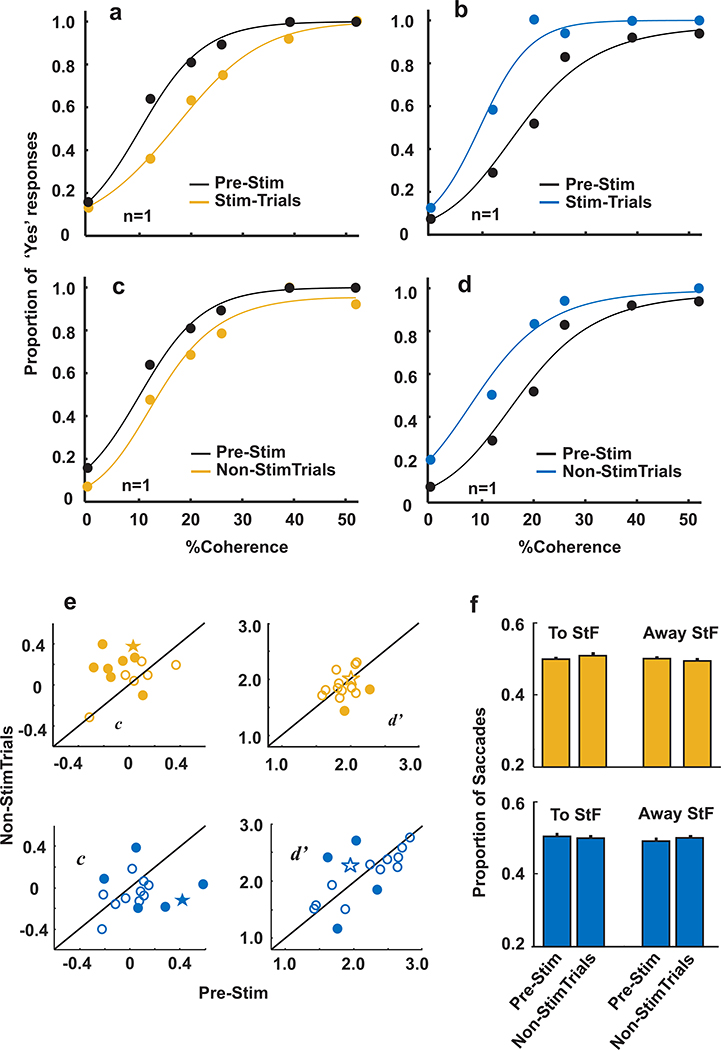
Monkeys performed three blocks of equally-balanced ‘Yes-No’ trials as before except in the second block we introduced electrical stimulation of the SC and the Glass patterns containing structure or no structure occurred with equal probability. Whenever the ‘Yes’ choice was in the stimulation field regardless of the Glass pattern stimulus, monkeys received stimulation of the SC (subthreshold to evoking a saccade) on 50% of the trials (liberal stimulation). Similarly, whenever the ‘No’ choice was in the stimulation field, regardless of the Glass pattern stimulus, monkeys received stimulation of the SC on 50% of the trials (conservative stimulation). Liberal and conservative stimulation occurred in separate sessions for both monkeys and were counterbalanced across days pseudo-randomly. Figure 7a shows an example result from a single conservative stimulation session from one monkey. In this example, the proportion of ‘Yes’ responding decreased when stimulation occurred on half of the trials in which the ‘No’ choice target was in the stimulation field. Figure 7b shows another example of a liberal stimulation session. Stimulation of the SC on 50% of the trials in which the ‘Yes’ choice target appeared in the stimulation field increased the proportion of ‘Yes’ responses. Although the stimulation occurred on 50% of the trials in which the ‘No’ choice target was in the stimulated hemifield (StF), these ‘Yes’ or ‘No’ responses changed even on the 50% of trials in which there was no stimulation (Figure 7c and d). Note also that the decision report, ‘Yes’ or ‘No’ occurred in either hemifield, a point to which we return below. These example results show that electrical stimulation of the SC changed monkeys’ proportion of choosing ‘Yes’ across the block of stimulated trials. They also show that the effects of the stimulation transferred to trials in which no stimulation occurred, suggesting that the SC stimulation produced a generalized change in the position of the decision criterion and consistent with known slow shifts in decision criteria (Brown and Steyvers 2005).
Figure 7. Stimulation of the SC induces changes in the position of the decision criterion independent of saccades.
a. Example session depicting behavioral performance before (Pre-Stim; black) and during Conservative stimulation (Stim-Trials; orange). Proportion of ‘Yes’ responses plotted as a function of coherence. b. Same as in a for an example session before (Pre-Stim) and with Liberal stimulation (Stim-Trials; blue). Note that during the stimulation block, stimulation of the SC occurred on only 50% of the trials. c. Proportion of ‘Yes’ responses plotted against coherence for the Pre-Stim block of trials from the Conservative sessions (black) and the trials from the stimulation block that did not receive stimulation (Non-Stim trials; orange). d. Same as in c for the Liberal stimulation sessions. e, Mean c and d’ without stimulation (Non-StimTrials) plotted against c and d’ for the block of trials before stimulation (Pre-stim) for the Conservative stimulation sessions (orange) and the Liberal stimulation sessions (blue). Solid circles = permutation test, p< 0.05 and open circles = p>0.05. Stars show the examples from panels a-d. f. top, Mean proportion of saccades from the Conservative stimulation sessions made to the stimulation field (ToStF) or away from the stimulation field (AwayStF) for the Pre-Stim trials and the 50% of trials in the Conservative stimulation sessions (orange) that did not receive stimulation (Non-StimTrials). Bottom, same as top but for the Liberal stimulation sessions (blue).
To quantify the influence of stimulation on monkeys’ decisions, we focused on the Non-StimTrials of the stimulation block for a few reasons. First, we wanted to assess whether the effects of stimulation transferred to Non-StimTrials, as suggested by the results shown in Figure 7a–7d and as might be expected if the stimulation produced a generalized change in monkeys’ decision criterion. Second, the change in the proportion of ‘Yes’ responding might be explained by an increased tendency to make saccades to one or the other choice target, so a change in criterion seen on Non-StimTrials would rule out an interpretation based on modulation of eye movements alone. Finally, our strategy of stimulating only when the ‘No’ target was in the StF for conservative sessions and only when the ‘Yes’ choice was in the StF for liberal sessions precluded the collection of certain Stimtrials necessary for c and d’ analyses (for example, ‘Yes’ choices to the StF cannot occur on conservative stimulated trials; see Methods).
Figure 7e shows the distributions of mean d’ and c computed only for Non-StimTrials plotted against mean d’ and c computed from the PreStimTrials for 14/15 conservative sessions and the 16/17 liberal sessions (1 session from each was removed due to insufficient number of FA trials). Points falling along the unity line indicate no differences in the average d’ or c with stimulation (on Non-StimTrials). Filled circles show individual sessions that showed significant changes in mean value and the stars show the examples from Figure 7a–d. As predicted for a generalized change in decision criterion, there was a mean positive difference in criterion of 0.14 between the Non-StimTrials and the Pre-StimTrials with conservative stimulation (permutation test, p<0.00001) whereas there was no significant difference in d’ for these trials (−0.03; permutation test p=0.6). Similarly, for the liberal stimulations trials, there was a mean negative difference in criterion between the Non-StimTrials and the Pre-Stim trials with liberal stimulation (−0.07; permutation test p<0.01) whereas the d’ values were statistically indistinguishable (0.01; permutation test p=0.79). Similar results were found for the threshold and the slope derived from fitted logistic functions to each session’s data, with and without stimulation. (Supplemental Figure 7). An analysis of lapse rates found no significant changes in λ with and without stimulation for both the conservative (t-test, t(28)=−0.64, p=0.53) and liberal stimulation sessions (t-test, t(32)=1.44, p=0.16). Supplemental Figure 8 shows the time course of the stimulation effects. Finally, linear regression analyses revealed no significant interactions between changes in d’ and c for both the conservative and liberal stimulation sessions (Supplemental Table 2).
The ability of electrical stimulation to adjust the monkeys’ ‘Yes’ response bias for interleaved non-stimulated trials is significant because it provides causal evidence that the signal introduced on stimulated trials persisted across trials and globally influenced the position of the monkeys’ decision criterion. Importantly, the ‘Yes’ and ‘No’ choice responses were indicated by a saccade into either hemifield, and since we examined only Non-StimTrials, the changes in criterion are unlikely to arise from an increase in the likelihood of making a saccade to the stimulated location. To ensure that this was true, we unpacked this result further and plotted the proportion of saccades into and away from the stimulated hemifield (ToStF and AwayStF) for the Non-StimTrials and compared these to the proportion of ToStF and AwayStF saccades for trials in the PreStim block for both conservative and liberal stimulation sessions (Figure 7f). For both the conservative and liberal stimulation session data, the proportion of saccades ToStF and AwayStF remained statistically indistinguishable from the PreStim proportions (ToStF mean= 0.5 before, 0.51 after; AwayStF mean= 0.5 before, 0.49 after; ANOVA, F(3,56)=0.27, p=0.84 and ToStF mean= 0.51 before, 0.50 after; AwayStF mean= 0.49 before, 0.5 after; ANOVA, F(3,64)=0.99, p=0.40). These results provide strong evidence that stimulation of the SC alters monkeys’ global decision criterion independently of the location of the saccade made to report the decision.
Finally, the effects of stimulation of the SC for both liberal and conservative stimulation conditions caused no significant differences in reaction time among preStim, Non-Stim, Stim and postStim trials for the conservative stimulation (ANOVA, F(3,336)=0.45, p=0.72) or liberal stimulation conditions (ANOVA, F(3,382)=1.74, p=0.16; Supplemental Figure 9). The stimulation effects cannot be explained by changes in behavioral strategies related to different reward rates on stimulation trials since reward rates did not differ across blocks of trials (Supplemental Figure 10). Taken together we conclude that the SC signals the position of a criterion for perceptual decisions.
Discussion
We provide evidence that the prelude activity of SC neurons signals the position of a criterion in a ‘Yes-No’ perceptual decision task. The ‘Yes-No’ decision task we developed allowed measurement of Hit, FA, CR and Miss trials that are critical for isolating changes in criterion from changes in sensitivity during decision-making: similar changes in Hit and FA rates indicate changes in decision criterion, whereas opposite changes in Hit and FA rates indicate changes in sensitivity. By changing the probability that a particular stimulus coherence would occur over a block of trials, we introduced a type of sensorimotor priming that selectively altered monkeys’ decision criterion independent of changes in decision sensitivity. More frequent occurrence of a Glass pattern with a clear orientation signal induced more liberal responding whereas more frequent occurrence of a Glass pattern with no orientation signals induced more conservative responding.
We recorded from SC neurons while monkeys made decisions and reported those decisions with saccades either toward or away from the recorded neurons’ RF. Each neuron’s activity served as a proxy for the activity of both neuronal populations encoding the ‘Yes’ choice and the ‘No’ choice, respectively. Similar to previous work, we computed the neuronal activity difference between the two possible choices and analyzed how the ‘Y-N’ activity changed with changes in criterion. We compared the SC activity changes to predictions generated from 2 models of SC, a DV model positing that SC activity signals a balance of evidence or decision variable, and a distance-to-criterion model positing that SC signals distance from the decision criterion. These models make opposite predictions: the DV model predicts increases in ‘Y-N’ activity with a change in decision criterion toward more conservative responding and decreases in ‘Y-N’ activity with a change in decision criterion toward more liberal responding. The distance-to-criterion model predicts the opposite: decreases in activity for a shift toward more conservative responding and increases in activity for a shift toward more liberal responding. We found that SC activity best matched the distance-to-criterion model indicating that the prelude activity in the SC signals the position of a decision criterion which can be read out through a simple subtraction operation. Such an operation could be performed by SC burst neurons which, as viewed within dynamic decision modeling frameworks, are thought to signal threshold crossing by emitting a burst of spikes driving the saccadic eye movement to the appropriate choice target (Lo and Wang 2006).
Electrical manipulation of the SC results in similar shifts in decision criteria as seen with priming, providing causal evidence for a role of SC activity in signaling the position of the decision criterion. Stimulation when the ‘Yes’ choice target was in the StF resulted in more liberal responding as if the decision criterion shifted leftward, and stimulation when the ‘No’ choice target was in the StF resulted in more conservative responding as if the decision criterion shifted rightward. We specifically analyzed the Non-StimTrials to rule out an influence of the stimulation on saccade probability and indeed, found that that for both the conservative and the liberal stimulation sessions, the proportions of saccades ToStF or AwayStF did not change, in spite of consistent changes in the proportion of ‘Yes’ responding. Thus, the stimulation altered the monkeys’ decisions to report ‘Yes’ or ‘No’, as if the stimulation created a stronger link between the Glass pattern and the color of the stimulus rather than the location of the choice target. This suggests that the stimulation of the SC modulates a global change in decision criterion. It is important to note however, that the effects of the electrical stimulation were not all or none. In some cases, electrical stimulation had little effect on monkeys’ choices. We believe that this indicates that other brain areas likely cooperate with the SC to provide a signal of decision criteria, most notably perhaps is the prefrontal cortex (Ferrera, Yanike, and Cassanello 2009).
Relationship to previous findings
Perceptual decisions are thought to evolve within sensorimotor regions of the brain including the lateral intraparietal area of cerebral cortex, the dorsolateral frontal cortex, and even the SC (Roitman and Shadlen 2002; Horwitz et al. 2004; Horwitz and Newsome 1999; Horwitz and Newsome 2001; Gold and Shadlen 2000; Kim and Basso 2008; Kim and Basso 2010; Kim and Shadlen 1999; Felsen and Mainen 2008; Ratcliff et al. 2003; Ratcliff et al. 2007). Work in humans points to the basal ganglia and frontal cortex as regions involved in determining criteria for decisions involving speed/accuracy trade-offs, conflict, or requiring memory (Frank et al. 2007; Forstmann et al. 2010; Cavanagh et al. 2011). These data are largely correlational. Evidence in monkeys shows that frontal cortical signals correlate with changes in decision criteria during visual motion speed categorization or vibrotactile detection (Ferrera et al. 2009; Carnevale et al. 2015). To our knowledge, there are no studies linking behavioral changes in decision criterion to changes in neuronal activity isolated from changes in sensitivity combined with causal manipulation of neuronal activity to induce changes in decision criterion, as we show here.
One recent study took a similar approach as we did here, albeit toward a different goal, to dissociate sensitivity from criterion to understand the different components of attention (Luo and Maunsell 2015). In an attention task in which monkeys reported a change in stimulus orientation, manipulation of reward contingencies resulted in selective changes in either the criterion or sensitivity. Luo and Maunsell recorded from neurons in area V4, an area known to be involved in attention, while monkeys performed the attention task to assess whether changes in V4 neuronal activity correlated with changes in criterion or sensitivity. The authors found little evidence of V4 activity correlating with changes in criterion. Rather, V4 activity correlated with changes in sensitivity only.
The result of Luo and Maunsell together with our result, provide insight into a puzzling finding reported by Krauzlis and colleagues in which reversible inactivation of the SC produced deficits in attentional performance, but left cortical signatures of attention unimpaired (Zenon and Krauzlis 2012). A possible explanation is that the SC plays a role in decision criteria whereas the cerebral cortex plays a role in determining sensitivity. This is consonant with a series of previous SC studies that found choice biases following SC manipulations (McPeek and Keller 2004; Lovejoy and Krauzlis 2010; Nummela and Krauzlis 2010; Carello and Krauzlis 2004; Thevarajah et al. 2009; Muller et al. 2005). Our results provide compelling evidence that the SC signals the position of a decision criterion, and this can explain how SC inactivation produces attentional deficits while leaving cerebral cortical signatures of attention intact: the SC signals the position of the decision criterion and the cerebral cortex determines decision sensitivity, so adjustments of sensitivity occurring with attention cannot be expressed when SC is inactivated (but see (Lovejoy and Krauzlis 2017)). Postulating an alternative attentional network that bypasses the cerebral cortex to explain attentional effects of SC inactivation is therefore unnecessary (Krauzlis et al. 2013).
A role for the SC in signaling the position of decision criteria
Theoretical work proposes the SC as a partner with the basal ganglia-cortex circuit controlling decision criterion (Lo and Wang 2006). In this model, informed by data from monkeys performing a motion discrimination task (Roitman and Shadlen 2002), the decision threshold is determined by the weight of cortico-striatal synapses, which determines how much drive is needed to suppress the output of the basal ganglia which in turn, releases the SC from inhibition. The disinhibition from the basal ganglia allows the SC to discharge and drive the report of the decision. In this model, the SC is a passive participant in the threshold setting process in that it detects threshold crossing and initiates the choice response. Our data update this model and support the novel idea that the ‘Y-N’ prelude activity in the SC signals the position of the decision criterion. Our working model posits a criterion signal, originating perhaps from the basal ganglia, that normalizes SC activity through subtractive inhibition. The effect of this inhibition is a criterion position signal that could be used by other structures or within the SC itself for generating the saccadic choice. Indeed, it is well-known that output neurons of the basal ganglia projecting to the SC are modulated during events that precede the onset of eye movements and therefore, may provide this modulatory input (Liu and Basso 2008; Mahamed et al. 2014; Basso and Liu 2007) (Supplemental Figure 11a). We propose that during liberal criterion shifts inhibition from the basal ganglia decreases when the ‘Yes’ choice is in a neuron’s RF and increases when the ‘No’ choice is in a neuron’s RF (Supplemental Figure 11b). This results in increased ‘Yes’ activity and decreased ‘No’ activity, translating into increased ‘Y-N’ activity. The situation is reversed during conservative criterion shifts with inhibition from the basal ganglia increasing when the ‘Yes’ choice is in a neuron’s RF and inhibition decreasing when the ‘No’ target is in the RF (Supplemental Figure 11c). This results in increased ‘No’ activity and decreased ‘Yes’ activity, translating to decreased ‘Y-N’ activity. We think therefore, that the stimulation is providing a bias signal that together with the inhibitory drive from the basal ganglia and the intrinsic inhibitory connectivity within the SC, results in a change in the position of the criterion. The role of the basal ganglia in this mechanism is a hypothesis that remains to be tested.
The SC has reciprocal connectivity with most areas of the cerebral cortex (Fries 1984; Clower et al. 2001; Lyon et al. 2010; Lynch and Tian 2006; Lynch et al. 1994). Since we used electrical stimulation, it is possible that the effects we observed result from the influence of the SC on cerebral cortical areas through antidromic activation or other mechanisms. Because of the strong correspondence we observed between behavioral and neuronal measures of decision criterion, together with the fact that cortical stimulation typically results in little change to decision criterion (Hanks et al. 2006; Salzman et al. 1990; Fetsch et al. 2014; Romo et al. 1998), we believe an explanation based on antidromic activation is unlikely. Rather, the SC is likely to participate with other areas such as the frontal cortex in criterion signaling. Like sensorimotor areas that are thought to be involved in decision-making insofar as they are involved in controlling particular effectors, it remains to be seen whether the SC signals criterion position for decisions reported by actions other than eye movements. Since our results show that manipulations of SC activity influenced the criterion largely independently of the direction of the eye movement report, and previous experiments show a role for SC in attention whether an eye or arm movement is used (Nummela and Krauzlis 2010; Lovejoy and Krauzlis 2010), we propose that SC signals the position of the criterion for perceptual decisions regardless of the effector used to report the decision. This however, remains to be tested. Finally, an important direction for future work is to determine the cellular and synaptic mechanisms within the SC and its inputs from the basal ganglia that underlie its ability to adjust decision criteria.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCES SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michele A. Basso (mbasso@mednet.ucla.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Two experimentally-naïve adult (6–7 and 10–11 years old) male rhesus monkeys (Macaca mulatta), pair-housed (with other monkeys), weighing between 9–11 Kg (monkey B and monkey S ) were implanted with eye loops for measuring eye position (Judge, Richmond, and Chu 1980), a post for immobilizing the head and a recording chamber for accessing the SC. Positioning devices and recording chambers were placed using MRI-guided surgical software (BrainSight, Rogue Research, Montreal, CA) and stereotaxic coordinates (0ML, −3AP, angled 38° posteriorly). All surgical procedures were performed under general anesthesia using aseptic procedures. All experimental protocols were approved by the UCLA Chancellor’s animal research committee and complied with and generally exceeded standards set by the Public Health Service policy on the humane care and use of laboratory animals.
METHOD DETAILS
Behavioral Procedures
We used a real-time experimental data acquisition and visual stimulus generation system, Rex and Vex developed and distributed by the Laboratory of Sensorimotor Research National Eye Institute in Bethesda MD (Hays, Richmond, and Optican 1982) to create the behavioral paradigm and acquire two channels of eye position data. Using the magnetic induction technique(Fuchs and Robinson 1966), voltage signals proportional to horizontal and vertical components of eye position were filtered (8 pole Bessel −3dB, 180 Hz), digitized at 16-bit resolution and sampled at 1 kHz (National Instruments; Austin, TX; PCI-6036E). The data were saved to disk for off-line analysis. We used an automated procedure to define saccadic eye movements by applying velocity and acceleration criteria of 20°/s and 8000°/s2, respectively. The adequacy of the algorithm was verified and adjusted as necessary on a trial-by-trial basis by the experimenter.
We designed a ‘Yes-No’ dynamic Glass pattern perceptual judgement task to assess perceptual decisions before and after manipulation of stimulus presentation statistics (Supplemental Videos 1 and 2). Head restrained monkeys sat in a custom-sized chair facing a CRT monitor (1024 × 768 pixel resolution, 85 Hz refresh rate) at a distance of 37 cm. A photocell secured to the monitor sent a transistor-transistor logic pulse to the experimental PC providing an accurate measure of the timing of stimulus events. A trial began when the monkey looked at a white dot appearing at the center of the monitor. After a brief delay of ~500 ms, randomized from an exponential distribution to prevent prediction, a red and green isoluminant (1.4°, 166 cd/m2) target appeared, one in each hemifield. The hemifield in which the red or the green choice target appeared varied randomly from trial-to-trial (Ferrera, Yanike, and Cassanello 2009; Bennur and Gold 2011). After a second randomized delay (600–1050 ms), a dynamic Glass pattern stimulus (Nankoo et al. 2012; Glass 1969) consisting of vertically-oriented dot pairs (pattern diameter = 6°, 26 cd/m2; dot size=0.1°; dot separation=0.182°; total density=5 dots/deg.2) appeared at the center of the monitor together with the white fixation point and remained on the screen for a random duration between 800–1500 ms at which point it disappeared, instructing the monkey to report its choice with an eye movement. The coherence of the Glass pattern varied randomly on each trial from among 26%, 39%, 52%, 100% or 0% to calculate psychometric functions and extract relevant parameters from logistic functions fitted to the data. The monkeys indicated their decisions by making a saccade to the green choice target if it perceived structure in the Glass pattern or the red target if it perceived no structure. Monkeys remained fixating at the choice target for ~300ms to receive a sip of juice as a reward if correct or a 2400 ms time-out if incorrect.
Each session consisted of three, 200 correct trial ‘Yes-No’ decision blocks. For the first and third blocks of trials, the stimulus presentation statistics were balanced between 0% (‘No’) and four structure coherence levels (26%, 39%, 52%, 100%; ‘Yes’). The middle block varied from session to session pseudo-randomly as either a conservative priming block or a liberal priming block. For the conservative priming block, 0% and 100% were the two possible stimuli shown with presentation probabilities of 0.85 for 0% and 0.15 for 100%. The 100% coherence stimulus served as a catch stimulus to ensure the animal was engaged in the task and performing accurate ‘Yes-No’ decisions. For the liberal priming block, 0% stimulus occurred with a 0.15 probability and the 39% stimulus occurred with a 0.85 probability (Figure 1b). The 39% coherence stimulus was chosen because, similar to 0% coherence, it was one stimulus level removed from the near threshold coherence level of 26%. For liberal priming, 0% served as the catch stimulus.
Electrophysiological Procedures
We recorded neurons in the intermediate layers of the SC with tungsten microelectrodes (Frederick Haer, Bowdoin, ME, ~1.3–2 MOhm measured at 1 kHz), inserted through a guide tube positioned by a grid system (Crist et al. 1988). The electrodes were moved in depth by an electronic microdrive system controlled by a graphical user interface on a PC running Windows (Nan Instruments, Israel). Action potential waveforms were bandpass filtered (300 Hz-5.5 kHz; 6 pole Butterworth) and amplified by a differential amplifier and then sampled and digitized at 30 kHz using the BlackRock NSP hardware system controlled by the Cerebus software suite (BlackRock Microsystems, Utah). Neurons were isolated online using time and amplitude windowing criteria and times of occurrence of action potentials were digitized at 16 bit resolution and sampled at 1 kHz and saved to disk. Neuronal waveform data were digitized at 16 bit resolution and sampled at 30 kHz and saved to disk. Sorting was confirmed with offline waveform inspection and principal component analysis using the Plexon Off-line Sorter software x64 V3 (Plexon, Texas).
Response fields (RF) of SC neurons were mapped online. Mapping was done by moving a spot around the monitor and having monkeys make delayed saccades to the different spots. We listened for maximal discharge and also monitored X-Y spatial plots of the discharge rate for each saccade on-line. We considered the center of the RF of a neuron to be the location at which a saccade was associated with maximal discharge (audibly and visually). Preliminary recordings indicated that SC build-up neurons were the neuronal class most modulated by the task so we preferentially isolated and studied neurons that contained visual responses followed by ramping activity that terminated with a saccadic burst. Only neurons with RF eccentricities greater than 8° were studied to ensure no overlap of the RF with the centrally-placed Glass pattern stimulus. For performance of the Glass pattern decision task, one choice target was placed in the center of the RF and the other choice target was placed in the opposite hemifield at an angle of 90 degrees relative to the fixation point and these positions were randomly interleaved on a trial-by-trial basis to dissociate the choice report from the direction of the eye movement.
Electrical Stimulation Procedures
After completion of all behavioral and recording experiments in both monkeys, we introduced electrical stimulation to the SC while the same monkeys performed the ‘Yes-No’ decision task. The goal of these experiments was to determine whether manipulation of SC neuronal activity would preferentially alter monkeys’ decision criterion. For the stimulation experiments, we introduced two new Glass pattern coherence levels (13% and 20%) and removed the 100% coherence level so that we could measure changes at a finer level. We also used a VR5 schedule of reward to ensure the animals responded as accurately as possible without expecting a reward on each trial (Ferster and Skinner 1957). We reasoned that on a FR1 schedule, if the stimulation altered the animals’ percept, behavior would habituate, or worse, monkeys would develop a strategy to counteract the influence of the stimulation, giving a false read-out of the effects of stimulation (Cicmil et al. 2015; Basso and Liu 2007; Liu and Basso 2008; Gattass and Desimone 2014; Murphey and Maunsell 2007; Murphey et al. 2009). Similarly, we observed that performing stimulation experiments every day resulted in adaptation, so we switched to performing electrical stimulation experiments randomly 2–3 days a week. In total, we performed 36 stimulation experiments (19 liberal stimulation, 17 conservative stimulation) in two monkeys. We excluded 4 of the stimulation experiments since one of the monkeys seemed to detect the stimulation and adopt adaptive strategies. For these sessions, the monkeys would either abort mid-trial when stimulated by making a saccade to a random location, or have unusually long reaction times on stimulated trials when cued to make its saccadic report. These behaviors led us to conclude that the monkeys had some awareness of the stimulation, so we excluded these sessions. This resulted in a final analysis set composed of 17 liberal stimulation (14 from monkey B and 3 from monkey S) and 15 conservative stimulation sessions (9 from monkey B and 6 from monkey S). Stimulation experiments occurred in 3 blocks as we did for the behavioral and recording experiments. In the behavioral and recording experiments we report the data from the baseline condition and the after priming conditions. We did this to ensure that all the visual stimulation and the stimulus statistics remained the same to ensure changes in neuronal activity could be attributed to bona fide changes in decisions. For the stimulation experiments, we used the same three block strategy: 400 total trials (correct + incorrect) of baseline no stimulation, 400 total trials of liberal or conservative stimulation and then 400 total trials of no stimulation. The liberal or conservative stimulation sessions were randomized pseudo-randomly across experimental days. Because we expected to see changes only on the sessions with stimulation, for these data we report results for the first baseline condition (No stim) and the stimulation session (With stim). For liberal stimulation sessions, trains of electrical pulses (200 Hz, 150 μs biphasic, current-balanced pulses) subthreshold to evoking saccades, were delivered randomly (p=0.5) for the entire period of Glass pattern presentation (800–1200 ms) on trials in which the green (‘Yes’) target was in the stimulation field (StF) as determined by the endpoint of electrically-evoked saccades tested before beginning each stimulation experiment, regardless of the Glass pattern shown. For conservative stimulation sessions, electrical stimulation occurred randomly (p=0.5) on trials in which the red (‘No’) target was in the stimulation field regardless of the Glass pattern shown. Current level varied from session to session but was always set well below the threshold for evoking saccades (16–40 μA) at values < 3 μA.
Decision variable and distance-to-criterion models
To ascertain whether the ‘Y-N’ neuronal activity in the SC signals a decision variable (DV) or a distance-to-criterion signal we simulated neuronal discharge rates constrained by the behavioral data. For each recording session, we calculated behavioral d’ and criterion across all coherences, and used these measures to construct artificial V4/IT No-structure and Structure discharge rate distributions. Each discharge rate distribution was modeled as a normal distribution N(μ,σ2), with means and variances of the normal distribution estimated from each session’s before and after priming d’ value, respectively, according to the following equation:
| (1) |
For each session, and were estimated by linear fits to Hit and FA rates plotted in zROC space (see above). The numerator values were then computed by multiplying the denominator terms by the session’s d’ value, followed by division by 2. This resultant value represents the mean difference between the two distributions, the numerator term.
To simulate SC discharge rates, we then randomly sampled from these V4/IT distributions under conditions in which a ‘Yes’ choice target was in the modeled SC neuron’s RF and conditions in which the ‘No’ choice target was the modeled SC RF, respectively. On each iteration we drew two samples, one representing a trial when the ‘Yes’ choice target was in the RF and one representing a trial when the ‘No’ choice target was in the RF and compared the values of the drawn samples to the value of the behavioral criterion. Structure and No-structure distribution draws that were greater than the criterion were classified as Hits and FAs, respectively. Structure and No-structure distribution draws less than the criterion were classified as Misses and CRs, respectively. Since SC neurons have spatial RFs, Hit trials and FA trials occurring when the ‘No’ target was in the RF, and Miss and CR trials occurring when the ‘Yes’ target was in the RF were multiplied by −1. This multiplicative factor signs the activity to simulate the behavior of real SC neurons, i.e. increasing discharge rates for saccades made to the RF and decreasing discharge rates for saccades made away from the RF. For the DV model, on each iteration we subtracted the ‘No’ activity from the ‘Yes’ activity for each SDT outcome yielding a modeled ‘Y-N’ activity value. For the distance-to-criterion model, we used the following equation to define criterion distance:
| (2) |
Where || denotes the absolute value. On each iteration the distance-to-criterion was calculated for each of the two samples followed by the same ‘Yes-No’ subtraction operation to yield a simulated distance-to-criterion signal. For each behavioral session’s data, we repeated the above procedure 1000 times to yield distributions of modeled DV and distance-to-criterion signals. To simulate predicted SC activity following criterion shifts, the above procedure was repeated using the behavioral criterion values calculated from the second set of equally balanced trials (After Conservative, After Liberal).
Supplemental Figure 6 shows the approach used to generate simulated data for example Hit trials. Supplemental Figure 6a illustrates the procedure for the decision variable (DV) model, which assesses the balance of ‘Yes and ‘No’ evidence. Supplemental Figure 6b shows the same for a distance-to-criterion model. In Supplemental Figure 6, we walk through an example for each of the models.
QUANTIFICATION AND STATISTICAL ANALYSIS
Behavioral Data Analysis
In signal detection theory, (STD) a ‘Yes-No’ task yields four possible trial outcomes: ‘Hit’, ‘Miss’, ‘Correct Rejection’ (CR) and ‘False Alarm’ (FA). In our task, Hit and Miss outcomes occurred when the structure stimuli appeared and the participant reported ‘Yes’ and ‘No’, respectively. CR and FA outcomes occurred when the no-structure stimulus appeared and the participant reported ‘No’ and ‘Yes’ respectively. We used these trial outcomes to quantify behavioral performance. Hit rate was calculated for each block and session as
| (3) |
False alarm rate was calculated as:
| (4) |
Sensitivity, or d’, is the ratio of the differences between the means of two distributions to the sum of the standard deviations of the distributions and can be approximated as:
| (5) |
where invcdf signifies the inverse cumulative density function of the normal distribution. d’ quantifies how well an observer can determine from which distribution a random sample was drawn. A d’ of zero indicates complete overlap of the two distributions and therefore an inability to determine from which distribution a random sample was drawn. We calculated d’ from Hit rates and FA rates associated with each stimulus coherence level shown and also for Hit rates and FA rates averaged across all coherences in order to enhance statistical power and enable direct comparisons with psychometric function thresholds and slopes which summarize performance across all coherences. Problems associated with pooling across coherences are minimal since the criterion changes we observed were relatively constant across coherences, in spite of changes in d’ with coherence.
An assumption inherent in calculating d’ across coherences is equal variance of the noise and signal distributions across all coherences. We assessed this assumption for each session by plotting transformed Hit and FA rates in zROC space and calculating the slope of the resulting best fit line, using a method adapted from (Macmillan and Creelman 2004). This process consisted of several steps. First, we associated each of the 4 Hit rates (26%, 39%, 52%, 100% coherence) with 4 replicates of the FA rate. This is necessary because there is only 1 FA rate per session. We reasoned that the FA rate is relatively constant within each block and thus can be paired with each Hit rate. We then took the cumulative sum of the Hit rates and divided the result by the sum of the Hit rates to generate Hit rates normalized to ROC space. This process was repeated for the FA rate replicates, resulting in 4 ROC space Hit rates and 4 ROC space FA rates. Finally, we converted the ROC space rates to zROC space by calculating the z-score for each of these rates. These were then co-plotted and a best fitting line determined by linear regression. The slope of this line was then used for correction of unequal variance. Equal variance is indicated by a slope of 1. We found that most sessions had slopes within the range of 0.8–1.0, indicating nearly equal variance. To confirm the validity of this method, and explicitly assess the effect of differing sensitivities across the coherences (see Figure 2), for each coherence we co-plotted in ROC space, the mean Hit rate and corresponding mean FA rate for the Before priming, after Conservative priming, and Liberal priming data, respectively. This resulted in 4 plots, 1 for each structured coherence, comprised of 3 pairs of Hit Rates and FA rates, each corresponding to the before, and after Conservative and after Liberal priming data, respectively. We then converted the data to zROC space and computed the slope of the best fitting line. We found that the mean slope values were within the range of 0.98–0.99, indicating almost equal variance. To assess the variability of the mean slope values, we generated bootstrapped estimates of the confidence intervals for each coherence and found they had ranges of 0.97–0.999. To correct for unequal variance (Macmillan and Creelman 2004), each slope value was then used to correct the original d’ value to yield d’a. The d’ adjustment equation is written as:
| (6) |
Criterion, or c, is calculated as:
| (7) |
which quantifies the position of the decision criterion relative to the two distributions and indicates whether the participant is biased towards ‘Yes’ choices (c<0) or ‘No’ choices (c>0), or has no bias (c=0; Green and Swets 1966). For completeness we also computed k, a measure of the criterion used for responding as:
| (8) |
We also quantified behavioral performance using psychometric functions by plotting the proportion of ‘Yes’ responses as a function of coherence and fitting these data with a logistic function of the form:
| (9) |
where p(Y) is the proportion of ‘Yes’ responding and C is dot pair coherence. α and β are free parameters fit using maximum likelihood methods, that determine the slope or sensitivity of the psychometric function (β) and the threshold or response bias (α). Slope values (β) were calculated as the derivative of the psychometric curve at 50% behavioral performance. Threshold values (α) were calculated by finding the x-axis position of the psychometric function at 50% behavioral performance. Lapses in behavioral performance can bias estimates of psychometric function parameters if not properly controlled (Wichmann and Hill 2001). To confirm the reliability of our logistic function fits, we also fit our data to cumulative Gaussian functions with lapses, guesses, thresholds and slopes as free parameters. The cumulative Gaussian function was of the form:
| (10) |
where γ represents the guess rate, λ represents the lapse rate and v represents the variance, a parameter setting the function’s slope. We assessed the statistical significance for differences in α, β, λ, d’ and c parameters among conditions (baseline, after liberal priming and after conservative priming) using either 2-way ANOVA followed by multiple comparisons tests with the Tukey-Kramer method, or individual comparisons using t-tests with Bonferroni corrected p values. Paired or unpaired t-tests were used as appropriate. Before and after priming differences between α and β for each individual session were assessed by permutation tests.
To test for interactions between threshold and slope and changes in d’ and c, we created multiple linear regression models with linear and pairwise interaction terms. The regression was of the form:
| (11) |
where y is the model response (e.g., the after priming criterion value) and x1 and x2 are the predictor terms (e.g., before priming criterion value and the before and after priming d’ difference respectively). After fitting the interaction coefficients were assessed for significance with an F-test.
All analyses and curve fitting were performed using Matlab v8.5 (R2015a; Mathworks) and the Matlab psychophysics toolbox, Palamedes (Prins and Kingdom 2009).
Neuronal Data Analysis
Only SC neurons that exhibited significant task modulation were used for analysis. We measured mean cue activity (300–500 after cue onset) and baseline activity (100 ms before cue onset) and defined neurons as task modulated if these two epochs of activity differed significantly (t-test, p< 0.05). These neurons were defined as buildup neurons, those having prelude activity that precedes the robust saccade-related discharge (Li and Basso 2005). Neurons without significant prelude activity were excluded from analysis, including phasic visual and burst neurons. For each trial, we convolved the times of occurrence of action potentials with a Gaussian (σ = 10 ms) (MacPherson and Aldridge 1979) to create a spike density function (SDF). The mean discharge rate for all analyses was then computed across trials for a 600 ms epoch beginning 400 ms after the onset of the Glass pattern cue. We selected the epoch’s start time and length was chosen to ensure exclusion of any transients associated with the onset of foveal stimuli (note large dip in activity (Li, Kim, and Basso 2006)) and to minimize variability associated with differences in timing across trials and difficulty levels. For each neuron, all trials were normalized relative to a mean 200 ms epoch (200–400 ms after Glass pattern onset) obtained by averaging across all baseline block trials. We tried normalizing activity to a more conventional pre-target onset epoch, but found that due to relatively low baseline discharge rates of many of the recorded SC neurons, normalization by this epoch often produced exaggerated rates and even infinite values for cases in which activity during the pre-target-onset epoch was 0 sp/s. Having done this, we noted that the baseline activity appeared different between the liberal and conservative priming conditions. We believe the reason for this is because neurons recorded in the conservative priming condition tended to have higher discharge rates compared to those recorded in the liberal condition. The reason for this is likely because during the monkeys were over-trained during the initial set of experiments on the conservative priming condition. This may also explain in part, the monkeys’ tendency to report ‘No’ more readily (see Figure 2) and for the stimulation to have a larger effect on these trials (see Figure 7).
To determine whether overall SC activity changed as a function of liberal or conservative priming, for each neuron we performed ROC analyses between activity recorded when the animal made saccades to the RF (toRF) and activity when the animal made saccades away from the RF (awayRF). All coherences were pooled, and binned data (see above) computed from each neuron’s toRF and awayRF responses, were used to calculate areas under the ROC curves for individual neurons (AUC). This procedure was performed separately for the Baseline data, After Liberal priming data, and After Conservative priming data across all coherences. To further assess the relationship of SC activity and decisions, we computed the difference in activity between trials in which the ‘Yes’ target was in the RF and trials in which the ‘No’ target was in the RF (here referred to as the ‘Y-N’ activity). For these data, we estimated the means and standard errors of the ‘Y-N’ activity using a bootstrapping procedure. Starting from the raw data, 1000 samples were drawn with replacement from the ‘Yes’ distribution and the ‘No’ distribution for Hit, FA, CR and Miss trials. The ‘Y-N’ activity was computed yielding a distribution of 1000 ‘Y-N’ discharge rates for each trial type: Hit, Miss, CR and FA, before and after priming. Means and standard errors were then calculated. To assess significance between the before and after priming ‘Y-N’ activity values, we performed permutation tests using standard procedures from resampling theory. First, for each trial type, Hit, Miss, FA and CR, the ‘Yes’ before-priming data were pooled with the ‘Yes’ after-priming data and randomly reassigned to two new before-priming and after-priming ‘Yes’ distributions, each consisting of 1000 samples. An identical procedure was performed on the ‘No’ before-priming and after-priming data yielding 4, new, statistically indistinguishable distributions. Subtracting the ‘No’ values from the ‘Yes’ values for each of the 2 sets of distributions yielded 2, chance ‘Y-N’ distributions. Finally, to assess whether the ‘Y-N’ activity changed with priming, we subtracted the before priming data from the after priming data to yield a final, chance ‘difference of ‘Y-N’ activities’ distribution. This final distribution is the difference one would expect by chance if priming exerted no influence on the ‘Y-N’ activity. To extract a p value, the number of ‘difference of ‘Y-N’ activity’ values that exceeded the actual change in ‘Y-N’ activity seen with priming was then divided by 1000, the total number of permutations. The resulting number is the p value. If this number was below 0.05, the change in ‘Y-N’ activity caused by priming was statistically significant. The ‘Y-N’ activity values before and after priming are reported in the results.
Neurometric functions were fitted to the neuronal ‘Y-N’ activity on ‘Yes’ choice trials across all coherences with cumulative Gaussian functions of the form described above (Equation 10). To enable more direct comparisons between behavioral performance and neuronal activity we calculated neuronal d’, c, and neuronal ‘Yes’ rates. For neuronal d’ and c, the computations were identical to that described above for the behavior except that the data used to compute the neuronal d’ and c were the ‘Y-N’ values for the neuronal activity calculated between the ‘Yes’ responses and ‘No’ responses for the different trial conditions: Hit, Miss, FA and CR, resulting in four ‘Y-N’ conditions, one for each SDT trial type. In more detail, for Hit trials (‘Yes’ choices on structure coherence presentation) and FA trials (‘Yes’ choices on 0% coherence presentation), ‘Yes’ activity is the activity measured on trials in which the monkeys made a ‘Yes’ choice into the RF for structure coherence trials (Hit) and a ‘Yes’ choice into the RF for 0% coherence trials (FA). ‘No’ activity is the activity measured on trials in which the ‘No’ target was in the RF and the monkey made a ‘Yes’ choice away from the RF on structure coherence trials (Hit) and 0% coherence trials (FA). For CR (‘No’ choices on 0% coherence presentation) and Miss trials (‘No’ choices on structure coherence presentation), ‘Yes’ activity is the activity measured on trials in which the ‘Yes’ target was in the neuron’s RF and the monkeys made a ‘No’ choice away from the RF for 0% coherence trials (CR) and a ‘No’ choice away from the RF for structure coherence trials (Miss). For CRs and Misses, ‘No’ activity was defined as the activity measured on trials in which the ‘No’ target was in the RF and the monkey made a ‘No’ choice into the RF on 0% coherence trials (CR) and structure coherence trials (Miss). We then used the ‘Y-N’ activity across the 4 SDT trial types to compute neuronal Hit rates and neuronal FA rates using equations 3 and 4. We then plugged these values into equations 5–7 to generate neuronal d’ and neuronal c. Neuronal ‘Yes’ rates for each coherence were computed as:
| (12) |
We only calculated neuronal ‘Yes’ rates for coherences ranging from 0% to 39% coherence, since the monkeys made insufficient numbers of ‘No’ choices on 52% coherence and 100% coherence trials. Neuronal ‘Yes’ rates and neuronal d’ and c are useful because they permit direct comparisons to the corresponding behavioral ‘Yes’ rates (as plotted in psychometric functions) and behavioral d’ and c. It should be noted that discharge rate data are not typically normally distributed at lower discharge rates, violating an assumption of d’ and c. However, we found similar results using A’ and B”, non-parametric versions of d’ and c, respectively, so for simplicity and consistency with previous literature we only report parametric d’ and criterion.
Behavioral-neuronal correlations were assessed by computing each session’s behavioral and neuronal d’ and criterion values, before and after priming, and performing a Pearson correlation analysis on the changes in behavioral d’ and criterion vs the changes in neuronal d’ and criterion, respectively. To ensure robustness, we only analyzed sessions in which at least 3 trials occurred for each SDT outcome. This resulted in the exclusion of 9 neuron-session pairs for the significant liberal priming data set and 7 neuron-session pairs from the significant conservative priming data set.
To test for interactions, we performed the same multiple regression analyses as described above for the d’ and criterion measures computed from the neuronal data.
Electrical Stimulation Analysis
We fitted logistic functions to the plots of proportion of ‘Yes’ responses as a function of coherence and determined the baseline block (No stimulation) and with-stimulation block threshold and slope. Statistical analyses used were also the same as described above. To test whether electrical stimulation effects generalized across hemifields, for each session we calculated the proportion of saccades (for all correct and error trials) directed to the stimulation field (StF) and the remaining fraction directed away from the StF. These proportions were then compared to the respective proportions observed during the baseline block of trials preceding the with-stimulation block.
To assess the temporal dynamics of c and d’ we computed mean criterion and mean d’ as a function of session trial number. Data were sorted into 2 sets: 1: all non-stimulated trials and 2: all stimulated trials. Mean criterion and d’ values were computed for each of the 2 trial sets in a moving 30 trial bin that was incremented in steps of one trial across the stimulation and after-stimulation blocks.
To rule out reward-related influences on the stimulation results (Cicmil et al. 2015), for each of the two stimulation conditions we computed mean reward rate as a function of within session trial number across all three blocks and assessed significance by performing a 1-way ANOVA on the mean values of the three blocks across all sessions.
Statistical Procedures
Most statistical procedures are described in detail in the appropriate subsections of the Methods. Briefly, normality was assessed prior to all statistical comparisons with the Shapiro-Wilk test. Those samples that failed normality were then tested using non-parametric methods. All hypothesis tests between groups were assessed with paired t-tests for matched samples, 2-sample t-tests for unmatched samples and Wilcoxon rank sum tests if one or both samples failed normality. Multiple comparisons were corrected by the Bonferroni method. For behavioral and stimulation data, one-way and 2-way ANOVAs were used to test for differences among 3 or more groups. If the ANOVA yielded significant main effects for at least one factor we followed, where appropriate, with post-hoc multiple comparison tests corrected with the Tukey-Kramer method or Bonferroni. Tests for interactions between behavioral and neuronal measures before and after priming, with and without stimulation, were performed by multiple linear regression as described above. All bootstrapping and permutation tests used 1000 iterations of the sample data to generate robust estimates of the population. We tried higher numbers of iterations (e.g., 2500, 5000) but found that the estimates stabilized reliably by 1000 iterations. Categorical data were analyzed with the chi-squared (Χ2) test. Effect sizes were assessed with the Hedges g method. Means plus standard errors of the mean are used in the figures to describe the data and effect sizes. The threshold for significance for all hypothesis tests was 0.05, two-tailed. All data analysis and statistical testing were performed in Matlab using custom scripts.
DATA AND SOFTWARE AVAILABILITY
All data and Matlab scripts are available upon request to the Lead Contact.
Supplementary Material
KEY RESOURCES TABLE.
| REAGANT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Model: Organisms Strain | ||
| Rhesus Macaque (Macaca mulatta) | Primate Center | N/A |
| Software and Algorithms | ||
| MATLAB | Mathworks | http://www.mathworks.com |
| REX/VEX | LSR/NEI/NIH | https://nei.nih.gov/intramural/lsr |
Highlights.
Priming changes monkey’s decision criteria without changing decision sensitivity.
SC neuronal activity correlates with decision criteria.
Stimulation of the SC produces reliable shifts in monkeys’ decision criteria.
Stimulation induced shifts are largely independent of saccades.
Acknowledgements
We are grateful to our colleagues Drs. Greg DeAngelis, James Bisley, Koorosh Mirpour, Marc Sommer and Hrishikesh Rao for comments on previous versions of the manuscript. We thank Dr. Xueqi Cheng for expert programming support and Dr. Tony Movshon for sharing the code to generate Glass patterns. We are grateful to Adam Meyers and Renee Hlavka for excellent animal care. This work was supported by NIH EY13962 and EY24516 (MAB) and a post-doctoral fellowship from the Neural Microcircuits Training Program at UCLA T32NS05820-07 (TBC).
Footnotes
Declaration of Financial Interests: The authors have no financial or competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appell PP, and Behan M (1990). Sources of subcortical GABAergic projections to the superior colliculus of the cat. The Journal of Comparative Neurology, 302: 143–58. [DOI] [PubMed] [Google Scholar]
- Basso MA, and Liu P (2007). Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol, 97: 4129–42. [DOI] [PubMed] [Google Scholar]
- Basso MA, and May PJ (2017). Circuits for action and cognition: a view from the superior colliculus. Annual Review of Vision Science, 3:197–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, and Gold JI (2011). Distinct Representations of a Perceptual Decision and the Associated Oculomotor Plan in the Monkey Lateral Intraparietal Area. J. Neurosci, 31: 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, and Movshon JA (1992). The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience, 12: 4745–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, and Steyvers M (2005). The dynamics of experimentally induced criterion shifts. J Exp Psychol Learn Mem Cogn, 31: 587–99. [DOI] [PubMed] [Google Scholar]
- Carello CD, and Krauzlis RJ (2004). Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron, 43: 575–83. [DOI] [PubMed] [Google Scholar]
- Carnevale F, de Lafuente W, Romo R, Barak O, and Parga N (2015). Dynamic Control of Response Criterion in Premotor Cortex during Perceptual Detection under Temporal Uncertainty. Neuron, 86: 1067–77. [DOI] [PubMed] [Google Scholar]
- Cavanagh J,F, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, and Frank MJ (2011). Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci, 14: 1462–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicmil N, Cumming BG, Parker AJ, and Krug K (2015). Reward modulates the effect of visual cortical microstimulation on perceptual decisions. eLife, 4: e07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC and Strick PL (2001). The Inferior Parietal Lobule Is the Target of Output from the Superior Colliculus, Hippocampus, and Cerebellum. J. Neurosci, 21: 6283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, and Basso MA, (2015). Insights into decision making using choice probability. J Neurophysiol, 114: 3039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DDG,. Komatsu H, and Wurtz RH (1988). A grid system and a microsyringe for single cell recording. Journal of Neuroscience Methods 26: 117–22. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, and Romo R (2005). Neuronal correlates of subjective sensory experience. Nat Neurosci, 8: 1698–703. [DOI] [PubMed] [Google Scholar]
- Felsen G, and Mainen ZF (2008). Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron, 60: 137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Yanike M, and Cassanello C (2009). Frontal eye field neurons signal changes in decision criteria. Nat Neurosci, 12: 1458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster CB, and Skinner BF (1957). Schedules of reinforcement (Prentice-Hall; ). [Google Scholar]
- Fetsch CR, Kiani R, Newsome WT, and Shadlen MN (2014). Effects of cortical microstimulation on confidence in a perceptual decision. Neuron, 83: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers E, Bogacz R, and Turner R (2010). Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proceedings of the National Academy of Sciences, 107: 15916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, and Sherman SJ (2007). Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science, 318: 1309–12. [DOI] [PubMed] [Google Scholar]
- Fries W (1984). Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol, 230: 55–76. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, and Robinson DS (1966). A method for measuring horizontal and vertical eye movement chronically in the monkey. Journal of Applied Physiology, 21: 1068–70. [DOI] [PubMed] [Google Scholar]
- Gattass R, and Desimone R (2014). Effect of microstimulation of the superior colliculus on visual space attention. J Cogn Neurosci, 26: 1208–19. [DOI] [PubMed] [Google Scholar]
- Glass L (1969). The Moire effect from random dots. Nature, 223: 578–80. [DOI] [PubMed] [Google Scholar]
- Gold JI, and Shadlen MN (2000). Representation of a perceptual decision in developing oculomotor commands, Nature, 404: 390–94. [DOI] [PubMed] [Google Scholar]
- Green DM, and Swets JA (1966). Signal Detection Theory and Psychophysics (Wiley: New York: ). [Google Scholar]
- Hanks TD, Ditterich J, and Shadlen MN (2006). Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci, 9: 682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, and Optican LM (1982). A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf. Proc, 2: 1–10. [Google Scholar]
- Heekeren HR, Marrett S, and Ungerleider S (2008). The neural systems that mediate human perceptual decision making, Nat Rev Neurosci, 9: 467–79. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, and Newsome WT (2004). Representation of an abstract perceptual decision in macaque superior colliculus. J. Neurophysiol, 91: 2281. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, and Newsome WT (1999). Separate signals for target selection and movement specification in the superior colliculus. Science, 284. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, and Newsome WT (2001). Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol, 86: 2543–58. [DOI] [PubMed] [Google Scholar]
- Jiang H, Stein BE, and McHaffie JG (2003). Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature, 423: 982. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, and Chu FC (1980). Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Research, 20: 535–38. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, and Isa T (2008). Nigral inhibition of GABAergic neurons in mouse superior colliculus. J. Neurosci, 28: 11071–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, and Basso MA (2010). A probabilistic strategy for understanding action selection. J Neurosci, 30: 2340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, and Basso MA (2008). Saccade target selection in the superior colliculus: a signal detection theory approach. J. Neurosci, 28: 2991–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, and Shadlen MN (1999). Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nature Neurosci, 2: 176–85. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Price T, Hall-Haro C, and Movshon JA (2012). Development of sensitivity to global form and motion in macaque monkeys (Macaca nemestrina). Vision Res, 63: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, and Zenon A (2013). Superior colliculus and visual spatial attention. Annu Rev Neurosci, 36: 165–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson A, Wang D, and Nakayama K (2002). The role of priming in conjunctive visual search. Cognition, 85: 37–52. [DOI] [PubMed] [Google Scholar]
- Li X, and Basso MA (2005). Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci, 25: 11357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kim B, and Basso MA (2006). Transient pauses in delay-period activity of superior colliculus neurons. J Neurophysiol, 95: 2252–64. [DOI] [PubMed] [Google Scholar]
- Liu P, and Basso MA (2008). Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol, 100: 1098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, and Wang XJ (2006). Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci, 9: 956. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, and Krauzlis RJ (2010). Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci, 13: 261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy LP, and Krauzlis RJ (2017). Changes in perceptual sensitivity related to spatial cues depends on subcortical activity. Proc Natl Acad Sci U S A, 114: 6122–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, and Maunsell JHR (2015). Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron, 86: 1182–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC, Hoover JE, and Strick PL (1994). Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res, 100: 181–6. [DOI] [PubMed] [Google Scholar]
- Lynch JC, and Tian RJ (2006). Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog Brain Res, 151: 461–501. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, and Callaway EM (2010). A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron, 65: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, and Creelman CD (2004). Detection Theory: A user’s guide (Psychology Press; ). [Google Scholar]
- MacPherson JM, and Aldridge JW (1979). A quantitative method of computer analysis of spike train data collected from behaving animals. Brain Res, 175: 183–87. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Garrison TJ, Shires J, and Basso MA (2014). Stimulation of the substantia nigra influences the specification of memory-guided saccades. J Neurophysiol, 111: 804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ (2006). The mammalian superior colliculus: laminar structure and connections. Prog Brain Res, 151: 321–78. [DOI] [PubMed] [Google Scholar]
- McPeek RM, and Keller EL (2004). Deficits in saccade target selection after inactivation of superior colliculus. Nature Neuroscience, 7 757–63. [DOI] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG and Newsome WT (2005). Inaugural Article: Microstimulation of the superior colliculus focuses attention without moving the eyes. PNAS, 102: 524–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JHR (2007). Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol, 17: 862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JHR, Beauchamp MS, and Yoshor D (2009). Perceiving electrical stimulation of identified human visual areas. Proc Natl Acad Sci U S A, 106: 5389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankoo J, Madan CR, Spetch ML, and Wylie DR (2012). Perception of dynamic Glass patterns. Vision Research, 72: 55–62. [DOI] [PubMed] [Google Scholar]
- Nummela SU, and Krauzlis RJ (2010). Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol, 104: 1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini A, Ditterich J, and Basso MA (2016). Patients with Parkinson’s Disease Show Impaired Use of Priors in Conditions of Sensory Uncertainty. Curr Biol, 26: 1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins N, and Kingdom FAA (2009). Palamedes: Matlab routines for analyzing psychophysical data http://www.palamedestoolbox.org.
- Ratcliff R, Cherian A, and Segraves MA (2003). A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J. Neurophysiol, 90: 1392–407. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith RL, and Segraves MA (2007). Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J. Neurophysiol, 97: 1756–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, and McKoon G (2016). Diffusion Decision Model: Current Issues and History. Trends Cogn Sci, 20: 260–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, and Shadlen MN (2002). Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci, 22: 9475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, and Salinas E (1998). Somatosensory discrimination based on cortical microstimulation. Nature, 392: 387–90. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, and Newsome WT (1990). Cortical microstimulation influences perceptual judgments of motion direction. Nature, 346: 174–77. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, and Movshon JA (1996). A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J. Neurosci, 16: 1486–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajah D, Mikulic A, and Dorris MC (2009). Role of the Superior Colliculus in Choosing Mixed-Strategy Saccades. J. Neurosci, 29: 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, and Hill NJ (2001). The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys, 63: 1293–313. [DOI] [PubMed] [Google Scholar]
- Zenon A, and Krauzlis RJ (2012). Attention deficits without cortical neuronal deficits.Nature, 489: 434–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and Matlab scripts are available upon request to the Lead Contact.