Abstract
The current study presents evidence on metallic and metal oxide engineered nanomaterial (ENM) emissions into the environment and an analytic perspective of the outcomes of evaluated studies with respect to different individual end points along the lifecycle trajectory. The key findings suggest that 1) the published literature on emissions of metallic ENMs is limited in both the number and information available on the characteristics of emitted ENMs; 2) the studies are classified as experimental and computational studies focused on predicting ENM emissions; 3) the majority of studies investigated ENM emissions during nanomaterial use and waste management, followed by raw material manufacturing, and finally, nano-enabled product manufacturing; 4) the studies primarily reported the concentration/quantity of emitted ENMs, whereas the physical–chemical characteristics of emitted ENMs were rarely measured or reported; and 5) the published literature primarily focused on emissions of silver and titanium dioxide ENMs and lacked similar information on other surging metallic and metal oxide ENMs such as nano-zero valent iron (nZVI), aluminum (Al), and aluminum oxide (Al2O3) ENMs. The evidence suggests that emitted nanoparticles into the air cover a wide range of concentrations below and above the allowable occupational exposure limits. The concentrations of nanoparticles in water systems are considered in the toxic to very toxic range for a variety of biological species. Given the critical gaps in knowledge, one cannot read across different sources of emissions for metallic and metal oxide ENMs hampering efforts with respect to understanding realistic scenarios for transformations in the natural environment and biological media.
Keywords: Emission, Engineered nanomaterials, Physical-chemical properties, Environment
1. Introduction
Over the past two decades, the use of engineered nanomaterials and nano-enabled products has been abundantly on the rise in a multitude of industries including healthcare, energy, electronics, and consumer products (Gottschalk et al., 2013, Ju-Nam and Lead, 2008). This increased use has raised concerns over the potential increased emissions of ENMs into the environment with respect to their health, safety and negative effects on biological species (i.e., human and non-human) and natural environment (Bystrzejewska-Piotrowska et al., 2009, Keller et al., 2013, Klaine et al., 2008, Kühnel and Nickel, 2014). In this respect, it is important to characterize not only nanomaterial emissions, but also, the physical-chemical properties of the emitted particles in order to adequately understand engineered nanomaterials’ (ENMs’) potential chemical, physical and biological transformations in interaction with key environmental conditions (Handy et al., 2008, Hartmann et al., 2014).
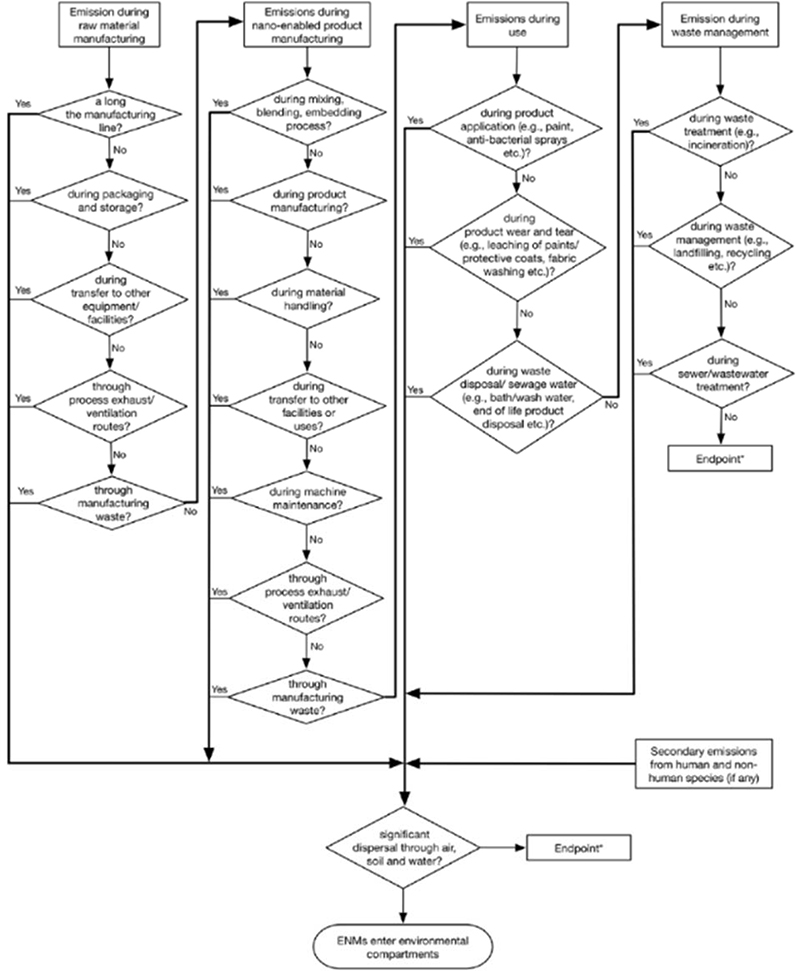
ENMs are emitted into the environment from four sources, namely, raw material manufacturing, nano-product manufacturing, product use, and waste management. As shown in Fig. 1, each source of ENM emissions and releases may consist of several avenues. Prior reviews on the subject have evaluated production data for the prediction of ENM concentration levels during emissions and releases into the environment (Table S1, supplementary information) with little information documented on the physical-chemical properties of the emitted ENMs. In addition, the body of knowledge is built on individual ENMs. Currently, there are a large number of individual ENMs that are synthesized in research labs and manufacturing settings, and it is expected that they will continue to increase into the future. Rather than evaluating individual nanomaterials, scientists and regulators often categorize ENMs in groups that share some physical and chemical properties. While there is no standard ENM classification to date, various classification schemes have been proposed (Farré et al., 2011, Ju-Nam and Lead, 2008, Klaine et al., 2008, Peralta-Videa et al., 2011). For example, under the Canada-United States Regulatory Cooperation Council Nanotechnology Initiative, a draft classification scheme for industrial ENMs was developed in 2014 proposing seven classes: (1) carbon nanotubes, (2) inorganic carbon, (3) metal, metal salts and metalloids, (4) metal oxides and metalloid oxides, (5) semi-conductor quantum dots, (6) organics, and (7) other classes (e.g., metal alloys, and nanoclays) (RCC, 2014). This classification scheme was developed with the intent to, among other things, select appropriate analogue/read-across information within a class of nanomaterials.
Fig 1.
Sources of emissions and releases fut ENMs.
The objective of this paper is to analyze state-of-the-art research on metallic and metal oxide ENM emissions and releases into the environment and to assess the gaps in knowledge in order to advance the body of knowledge for regulatory purposes. The specific aims of this research are three-fold: (1) to document the available knowledge in the peer-reviewed scientific literatures with respect to the emissions and releases of metallic and metal oxide nanoparticles into environmental compartments, (2) to outline the data gaps in released ENM, and (c) to discuss the positive and negative implications of emission data availability and gaps with respect to environmental compartments and biological species. To our knowledge, there is no integrated study on the analysis of peer-reviewed literature on metallic and metal oxide ENM emissions and releases into the environment. This study is intended to fill in this gap. It should be noted that it is well documented that silver and titanium dioxide ENMs are the earliest and most widely used metallic and metal oxide ENMs particularly in consumer products (Benn et al., 2010, Ji et al., 2010, Shukla et al., 2011). In this study we were also interested in assessing exposure data for other types of surging metallic and metal oxide ENMs such as nano-zero valent iron (nZVI), copper (Cu), aluminum (Al), gold (Au), aluminum oxide (Al2O3), and copper oxide ENMs which have been in increased use in the past few years (Aitken et al., 2006, Fu et al., 2014, Keller et al., 2013, Khot et al., 2012).
2. Methods
The methodology utilized in this research is adopted from the standard steps employed in evidence-based medicine (Akobeng, 2005, Luckmann, 2001, Rosenberg and Donald, 1995, Sackett, 1997, Sackett and Rosenberg, 1995, Straus and Sackett, 1998) and consists of five steps, namely, (1) formulating answerable environmental questions, (2) finding the evidence, (3) appraising the evidence, (4) applying the evidence, and (5) evaluating performance. Although this is becoming standard practice in the emerging nanotechnology research, evidence-based methodologies have its roots in evidence-based medicine.
The fundamental question in this research is “based on the current state of knowledge, what are the quantity and characteristics of metallic and metal oxide ENMs such as nano silver (Ag) and titanium dioxide (TiO2) nanoparticles currently emitted into the environment from raw material manufacturing, nano-enabled product manufacturing, product use and waste management?”. To evaluate the evidence, a benchmark is needed. Air emission data are often presented as the number of particles per unit volume. In aqueous systems, concentration data are reported as mass per unit volume. Based on the assumption that the evaluated nanoparticles are spherical, the volume of these particles (VNP) can be calculated as a sphere. Assuming further that the bulk material density does not change at the nanoscale, the mass based emissions can be calculated as Em=En∗VNP∗ρ,w here Em is the mass-based emission, En is the particle number-based emission, VNP is the volume of one nanoparticle, and ρ is the bulk density of the chemical constituting the nanoparticle (e.g., silver density was used for Ag ENMs). One would thus be able to compare and integrate air emission data with those of aqueous solutions.
Another benchmark that is needed for ENM emission evaluation is a consistent list of nanoparticle characteristics deemed essential to understand the physical–chemical properties of the emitted particles. In this respect, the minimum principle of information as suggested by the Organization for Economic Co-operation and Development (OECD) and others was adopted for the purpose of this study (OECD, 2007). The characterization data examined includes: physical characteristics of material or material morphology (e.g., particle size, size, distribution, shape), core material make-up (e.g., elemental composition, purity level, crystallinity) of ENM, and surface characteristics governing ENM interactions with the surroundings (e.g., surface area, surface charge, and surface coating) (Lubick, 2009, Nativo et al., 2008, Tolaymat et al., 2015b).
The next step in the process, that is, finding the evidence, requires a search methodology that includes the selection of electronic databases and keywords, the manipulation of keywords using Boolean operators, and the application of inclusion and exclusion criteria to filter out evidence relevant to the research question. Three databases, namely, American Chemical Society (ACS) Publications®, Sciencedirect®, and Academic Search Complete® were accessed in the electronic search using the following keywords: emission, release, disperse, engineered nanoparticle or nanomaterial, environment, air, water, soil, metal, gold, silver, iron, copper, and aluminum. Keywords related to fate and transport of metallic and metal oxide ENMs were represented in this search by the keyword “release.” In general, fate and transport studies investigate the mobility among environmental compartments for already emitted ENMs. The major determinant that was used in this search to distinguish an emission study from a transport study was the presence of a source of emission that falls within the emission source categories presented herein. For example, the release of ENMs in the leachate from municipal solid waste is included because waste includes nano-enabled products and thus, represents a source of emission of ENMs. On the other hand, transport studies of commercially obtained or in house synthesized ENMs in soil are not considered emission studies because the emission sources of ENMs do not belong to any of the previously discussed emission source categories.
The Boolean operators were logically formulated as follows for the keyword manipulation: (metal OR silver OR gold OR iron OR copper OR aluminum OR metal oxide OR titanium dioxide OR zinc dioxide OR cerium oxide OR aluminum oxide OR copper oxide) AND (nanomaterial OR nanoparticle) AND (emission OR release OR disperse AND (environment OR air OR water OR soil). This logical manipulation of keywords was designed to increase the pool of target articles to produce the first output of the electronic search in terms of article titles and abstracts. The development of appropriate inclusion criteria is needed to select the articles that are specifically relevant to answering the research question and to ensure the accuracy of the conclusions reached by this evaluation. The inclusion criteria for the target articles involved peer-reviewed papers that have data on emissions of metallic and metal oxide ENMs from the sources presented in Table S1 (i.e., raw material manufacturing, nano-enabled product manufacturing, use, and waste management).
Following the electronic search process, the physical evaluation of extracted evidence was conducted by applying the aforementioned inclusion criteria in several sequential steps: (1) evaluating evidence’s titles relevance to metallic and metal oxide ENMs or nanomaterial emissions; (2) analysis of evidence’s abstracts for environmental emissions of metallic and metal oxide ENMs; and (3) full retrieval of evidence for more detailed inclusion/exclusion evaluation. Bibliographies of retrieved articles were also evaluated for pertinent evidence unavailable from the electronic search. The electronic search was concluded on 05/30/2016 and only studies published in English were used.
The third step in the methodology – appraising the evidence - selected articles were closely examined to extract the following information: (a) emission sources, (b) characteristics (i.e., physical characteristics such as size, size distribution, shape; core material make-up such as elemental composition, speciation, impurity, and crystal structure; and surface characteristics such as surface area, surface coating, and surface chemistry) of ENMs prior to emission (i.e., beginning state) and after being emitted (i.e., end state) into the environment, (c) concentration and quantity of ENMs at the beginning and end states, and (d) factors influencing ENM beginning and end states.
In addition to appraising the emission data, an assessment of nanomaterial detection and characterization in the reviewed evidence was documented as current methods are in their infancy (von der Kammer et al., 2011). Three levels of certainty (low, medium, and high) were established in the current study to classify the obtained data and were based on emission media, characteristics of measured particle, and analytical measurement methods and techniques (Tolaymat et al., 2015a). In simple environmental media (e.g., deionized water and synthetic aqueous suspensions), data on ENM emission quantity and characteristics were considered of high certainty. Those related to characterizing ENM speciation using ion selective electrodes and the consequent quantification of the amount of emitted ENMs were of medium certainty. Certain sample preparation techniques for conducting characterization experiments such as burning the samples prior to usage for size analysis can reduce the measurement certainty and was considered of medium certainty. In complex environmental samples such as landfill leachate or wastewater samples, data were considered of low certainty.
Applying the evidence is another step in the methodology in which the data synthesized from the evidence base is discussed with respect to the positive and negative implications of metallic and metal oxide nanomaterials and nano-enabled products on the natural and engineered environments as well as biological species (e.g., bacteria, microbes, humans, animals, plants). In the final step, the performance of the research reported herein is discussed with respect to several elements such as study limitations in terms of the devised methodology, data gaps in the evidence base, and implications of existing knowledge with respect to the natural/engineered environments and biological species.
It should be noted that the evidence-based methodology was applied separately for each class of ENMs. That is, the methodology was applied first for metallic ENMs; thereafter, it was deployed for metal oxide ENMs. The results are reported for each class separately.
3. Results and discussion
3.1. Overall appraisal and evidence analysis by emission routes for experimental studies on metallic ENMs
The electronic search yielded as expected a large number of citations (>6000). Upon applying the inclusion criteria, a smaller number of these manuscripts emerged for further examination. The majority of the excluded manuscripts were either not specific to ENM emissions or to metallic ENMs. After a detailed evaluation of the fully retrieved articles, only a small number was included in the evidence appraisal (43) for the emission routes examined in this study. This finding clearly indicates the need for more data in this research area.
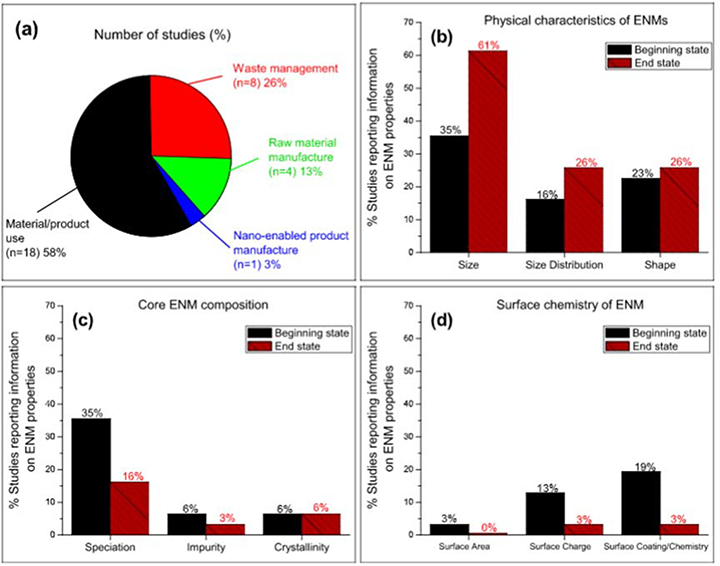
The majority of studies investigated metallic ENM emissions during material/product use (18), followed by waste management (8), then raw material manufacturing (4) (Fig. 2a). Additionally, there was only one study on the emissions from nano-enabled product manufacturing process (Lee et al., 2013). In general, there was a narrow focus within each category of metallic ENM source emission as discussed in the next section. Therefore, research needs to be conducted more broadly to allow for the evaluation of emissions of these nanoparticles along the products’ lifecycle. Size was the dominant physical characteristic reported for metallic ENMs and to a much lesser extent size distribution and shape (Fig. 2b). With respect to core make-up composition, speciation was the most reported element and impurity and crystallinity were rarely documented (Fig. 2c). Furthermore, surface characteristics of ENMs were poorly detailed (Fig. 2d). The overall evidence further suggests that nanosilver is the most widely metallic ENM studied, with very few exceptions (Kaegi et al., 2013, Kiser et al., 2012, Moreno et al., 2015, Yang et al., 2016). This is may be attributed to the fact that nano silver has been one of the very early applications in nano-enabled products. This further highlights another limitation in which the assessment of emissions of other metallic nanoparticles is lacking.
Fig. 2.
Analysis of evidence base for metallic ENM experimental studies: a) source emissions, b) physical characteristics (Size BS “n = 11”, ES “n = 19”; Size Distribution BS “n = 5”, ES “n = 8”; Shape BS “n = 7”, ES “n = 8”), c) core material make-up (Speciation BS “n = 11”, ES “n = 5”; Impurity BS “n = 2”, ES “n = 1”; crystallinity BS “n = 2”, ES “n = 2”), and d) surface characteristics (Surface Area BS “n = 1”, ES “n = 0; Surface Chare BS “n = 4”, ES “n = 1”; Surface Coating/Chemistry BS “n = 6”, ES “n = 1”). Fottnote: n = number of studies.
An assessment of the environmental conditions examined in the experimental studies suggests that emissions were mostly conducted at room temperature, with the exception of six studies that were conducted at temperatures up to 100 °C (Echegoyen and Nerín, 2013, Hedberg et al., 2014, Lee et al., 2012b, Mitrano et al., 2014, Quadros et al., 2013, Ramos et al., 2016). Environmental temperature can indeed influence the physical–chemical characteristics such as inducing agglomeration of emitted ENMs (El Badawy et al., 2011). The pH of aqueous emission media varied widely from 4.1 to 10.6. Changes in pH can cause marked dissolution to metallic ENMs and can alter, among other things, the surface charge (and hence, stability; i.e., resistant to aggregation) and the size of emitted ENMs (Bian et al., 2011, El Badawy et al., 2010). Studies in aqueous solutions utilized liquid media with varying ionic composition and strength. Examples include Milli-Q water and 10.8 g L−1 of sodium chloride (NaCl) in water, as well as synthetic urine, sweat, and saliva containing various types of compounds (e.g., ammonium chloride “NH4Cl”, potassium thiocyanate “KSCN”, potassium dihydrogen phosphate “KH2PO4”, sodium sulfate “Na2SO4”, sodium nitrate “NaNO3”, calcium carbonate “Ca(NO3)2”, and surfactants) (Quadros et al., 2013). The concentration and cation/anion valence of the experimental media can influence the stability of emitted ENMs. For example, divalent cations (i.e., calcium ion “Ca2+”) at similar concentrations have much stronger ability to cause ENM aggregation relative to monovalent cations (e.g., sodium ion “Na+”) (El Badawy et al., 2010). Certain emission studies utilized media containing organic molecules, such as media containing 15 mg L−1 of dissolved organic matter. The presence of these molecules in leaching media can influence the extent and stability of emitted ENMs (Loosli et al., 2013). A summary of the experimental studies on ENM emissions is provided in Table S2 (supplementary information).
With respect to evidence appraisal by emission routes for metallic ENMs, four studies examined the emissions of metallic ENMs in raw manufacturing. Three studies investigated nano silver and one study inquired about the emissions of Al, Ag and Cu ENMs. Park et al. gathered data about worker exposure to Ag ENMs during liquid phase reactions and grinding (Park et al., 2009). Lee et al. collected exposure data on Ag ENMs in the injection room as well other locations in the manufacturing setting (Lee et al., 2012a). Ham et al. gathered information on Al, Ag and Cu ENMs from two manufacturing facilities and two welding workplaces containing incidental nanoparticles (Ham et al., 2012). Lastly, Miller et al. characterized exposures to airborne silver nanoparticle emissions in a refinery (Miller et al., 2010). The physical chemical characteristics and the concentration data are summarized in Table S3a (supplementary information). One should note that only size was reported for the emitted state from among the nine particle characteristics. Indeed, one should document such important data.
Lee et al. investigated exposure to silver nanoparticles in two printed electronics workplaces (Lee et al., 2013). All the printed electronics operations were conducted in a clean room. Similar to their study in raw manufacturing (Lee et al., 2012a), the researchers only reported the nanoparticle size during the emitted state (Table S3b, supplementary information). The concentration levels were also documented.
The evidence base for sources of emissions during product use was derived only for silver nanoparticles and is demonstrated in Table S3c (supplementary information). Quadros and Marr and Hagendorfer et al. examined silver nanoparticles during consumer spray applications, and Tsai et al. studied silver nanoparticles during manual handling operations in fume foods (Hagendorfer et al., 2009, Quadros and Marr, 2011, Tsai et al., 2008). Silver nanoparticles were also examined in various experiments containing textile product use (Benn and Westerhoff, 2008, Lombi et al., 2014, Mitrano et al., 2014, Quadros et al., 2013), aqueous media stimulating sweat/laundry detergent solutions/surface water (Hedberg et al., 2014), ceramic surface (Bielefeldt et al., 2013), point of use disinfection (Loo et al., 2013), antimicrobial food containers (Echegoyen and Nerín, 2013), and coated wooden facades (Künniger et al., 2014). In general, size was poorly documented for the aforementioned studies and most physical chemical characteristics were not reported. Concentration levels were reported in almost all studies.
In another series of emission studies in 2015 and 2016, the trend continued with nano silver as the dominantly studied metallic ENMs with little emphasis on a complete profile of the ENM physical-chemical properties as shown in Table S3c (supplementary information). Mackevica et al. examined the release of Ag nanoparticles from commercial toothbrushes (Mackevica et al., 2016). In food product applications, Metak et al. studied the migration of Ag ENMs from nano silver impregnated polymer containers into orange juice packing and found no significant traces relative to the controls (Metak et al., 2015). Similarly, Ramos et al. found that the released silver ENMs from plastic containers was dependent on the temperature and the exposure time (Ramos et al., 2016). In another food application, Verleysen et al. demonstrated that a simple treatment with water of silver pearls meant for decoration of pastry lead to the release of a subfraction of Ag ENMs (Verleysen et al., 2015). Moreno et al. demonstrated that most particles breathed on rail subway platforms are highly ferruginous and in the nano range (Moreno et al., 2015). They further stated that flakes released from brakes are very chemically distinctive with trace elements present in nanominerals in the crystalline form. Finally, Tavares et al. found that both Ag and Au ENMs added to soils were retained almost completely within 24 h (Tavares et al., 2015). Moreover, under aerated soil conditions, the actual availability of nano silver and gold are low in soils.
Only one study was reported on the emissions of silver nanoparticles during landfill leachate (Bolyard et al., 2013). Six investigations analyzed the emissions of silver nanoparticles in wastewater (Chao et al., 2011, Kaegi et al., 2013, Kim et al., 2010, Kiser et al., 2012, Li et al., 2013, Wang et al., 2012). Lastly, one study examined both Ag and Au ENMs in wastewater and natural water (Yang et al., 2016). Similar to the aforementioned studies, researchers did not pay attention to the details of the physical chemical properties. For example, only about half the studies documented the nanoparticle size (Table S3d, supplementary information).
3.2. Critical gaps in knowledge in metallic ENM experimental data
OECD’s minimum principle of information was used as a guideline for evaluating the physical-chemical properties of emitted metallic ENMs (OECD, 2007). All examined experimental studies did not present the full characterization data needed for proper evaluation as suggested by the OECD. This further complicates our ability not only to read across the emissions of different types of metallic nanomaterials, but also, to generalize emission observations for a particular class of ENMs such as metallic ENMs.
Although size is the major characteristic granting ENMs their unique properties, and therefore a major determinant of particle fate, transport, and toxicity (Albanese and Chan, 2011, Dehner et al., 2011), 61% of the studies reported the size of emitted ENMs at the end state (i.e., state after emission), and only 35% of the studies documented size at the beginning state (i.e., state before emission) (Fig. 2b). This is an important piece of information to make the connection between the pristine and emitted forms of nanoparticles. In general, metallic ENM size was below 100 nm for both the beginning and emitted states. Only six studies were found emitting metallic ENMs in excess of 100 nm (i.e., 150–10,000 nm) (Benn and Westerhoff, 2008, Hagendorfer et al., 2009, Lee et al., 2013, Metak et al., 2015, Quadros and Marr, 2011, Tavares et al., 2015). Thus, the data suggests that the emitted metallic ENMs remain in the typical nanoscale where they exhibit their unique size-dependent properties (Jiang et al., 2008).
Unlike the particle size, data regarding metallic ENM size distribution at both the beginning and end states have been limited in the published literature. That is, 16% of the studies reported size distribution at the beginning state and 26% investigated size distribution at the end state (Fig. 2b). The reported size distributions included both mono-dispersed and poly-dispersed metallic ENMs depending on several factors, such as emission media and other experimental conditions (e.g., pH and ionic strength).
Shape is an important characteristic that plays a significant role in the implications of metallic ENMs. For example, research suggests that triangular-shaped silver ENMs are more toxic relative to spherical-shaped nanoparticles (Pal et al., 2007). A small percentage of the literature (45% of studies) presented information on ENM shape (Fig. 2b) which was predominantly spherical (11 studies), with only three studies reporting other shapes such as irregular, ellipsoidal and octahedral shapes (Benn and Westerhoff, 2008, Kim et al., 2010, Moreno et al., 2015).
Albeit they are of critical importance for accurate assessment of ENM implications, the speciation and oxidation states of emitted ENMs were largely not examined in the studies (35% for the beginning state, and 16% at the end state) (Fig. 2c). Since silver nanomaterial was the most dominant metallic ENM examined, total silver was only reported from an emission standpoint with additional details on speciation and oxidation states such as the presence of silver sulfide, silver chloride, and ionic silver forms. Depending on the oxidation state of silver (zero-valent silver “Ag0” vs silver ion “Ag+”) and its elemental phase (e.g., Ag0, Ag2S or silver sulfide, AgCl or silver chloride), the potential risks of emissions can markedly vary. For example, Ag+ is frequently reported as having significantly higher toxicological impacts relative to Ag0 (Ivask et al., 2014). Another experimental work limited the toxic effects of Ag nanoparticles to its dissolution to Ag+ (Xiu et al., 2012). Also, silver sulfide form is less bioavailable and less toxic to living organisms (Reinsch et al., 2012).
The impurities and crystallinity of emitted ENMs were often overlooked in the published literature (Fig. 2c). Two studies by (Bielefeldt et al. (2013) and Wang et al. (2012) reported information on ENM impurities and indicated the presence of (Na, Fe, B and P) and (8–10% Ag+) respectively, at the beginning state, while Moreno et al. (2015) revealed the existence of (Barium or Ba, zinc or Zn and Cu) impurities at the end state (Fig. 2c). Three studies indicated that metallic ENMs had a crystal structure, without providing further details on the type of crystal structure (Loo et al., 2013, Moreno et al., 2015, Wang et al., 2012). Indeed, information on metallic ENM impurities is needed because sizable levels of impurities might lead to quantitative differences in toxicological effects (OECD, 2007). Also, the crystal structure of metallic ENMs (although not applicable to all ENMs) may influence the interaction of emitted ENMs with the surrounding environment (OECD, 2007).
The surface area, charge, and coating of emitted metallic ENMs are also rarely reported in the published literature (Fig. 2d). Only one study investigated the surface area of ENMs (i.e., 3.32 m2 g−1), and only at the beginning state (Gottschalk et al., 2009) (Fig. 2c). It is well documented that ENMs with a large surface area indicate a large ratio of surface atoms to bulk atoms, and thus, have higher reactivity and potential risks (Lowry et al., 2012). The reported surface charge of ENMs was mainly negative and ranged from −6 mV to −53.3 mV, depending on the type of coating and the chemistry of the environmental media. The surface charge (i.e., sign and magnitude) can be an indicator of the extent of stability of electrostatically stabilized ENMs, and thus can serve as a marker of their mobility, and potential toxicity (El Badawy et al., 2010). The impacts of negatively charged ENMs can vary in the environment relative to positively charged ENMs (El Badawy et al., 2011). Few studies gathered information on ENM surface coating, especially at the end state such as the use of uncoated ENMs, citrate-, polyvinylpyrrolidone (PVP)-, casein-, gum Arabic- and tanning acid-coated ENMs (Fig. 2d). It is to be noted that the type of coating determines the surface chemistry, and accordingly, the surface charge and stabilization mechanism of ENMs (El Badawy et al., 2010). Thus, it becomes an important determinant of emitted ENM behavior in the environment (El Badawy et al., 2010).
The majority of experimental studies reported information on emission quantities which varied depending on the experimental conditions and media. For example, some studies reported mass concentrations and showed a wide range of emissions from 1 ng L−1 to 160 mg L−1 and from 0.00006 to 0.426 mg m−3 (Tables S3a–d, supplementary information). Air emission studies mainly reported concentrations and ranged from 3260 to > 106 particles per cm3 (Tables S3a–d, supplementary information).
The data provided by experimental studies were of high certainty levels except in few cases. For example, one study reported the use of an ion selective electrode (Zuliani and Diamond, 2012), having several limitations for determining the concentrations of silver ions, and consequently, the concentration of silver ENMs released into the emission media (Benn and Westerhoff, 2008).
3.3. Appraisal of evidence for metallic ENM computational studies and critical gaps
Overall, thirteen computational studies attempted to predict the concentration of emitted ENM based on modeling and lifecycle assessment tools. The majority (∼56%) of these studies focused on predicting ENM emissions through mass flow analyses starting with product use to emission during waste management. Approximately 33% of the studies predicted ENM emissions during use only. Unlike experimental studies, computational-based studies presented variability among the study’s boundaries where in some cases results were reported for a region, others as a country. The models’ input data were obtained from various sources such as the published literature, extrapolation from proxy parameters, and surveys sent out to producers of ENMs, as well as other entities in the supply chain (e.g., manufacturers, downstream, users, others) (Piccinno et al., 2012) This contributed to a large degree of variability observed among the results of computational studies.
Some computational studies reported the quantity of emissions based on continent or country. For example, the predicted emissions of Ag nanoparticles ranged from 2.8 to 80 tons year−1 in the United States (Hendren et al., 2011), 3.1 tons year−1 in Switzerland (Schmid and Riediker, 2008), and a median of 5.5 tons year−1 for Europe. Additionally, some predictions were regional. One study predicted compartmental concentration (distribution) of released Ag nanoparticles in the Los Angeles region at the end of a 1-year simulation: air (∼10−2 ng m−3); water (10−4 ng L−1); soil (∼10−2 μg kg−1 soil); sediment (∼10−1 μg kg−1 sediment) (Liu and Cohen, 2014). Other studies estimated emissions for various engineered environmental systems (Keller et al., 2013), predicting worldwide emissions of ENMs in landfills in the approximate range of 200 metric tons per year. Furthermore, some studies predicted emissions at a company level such as volume estimates of Ag NPs production in large U.S. companies (1000 kg year−1 as lower bound, and 6000 kg year−1 as upper bound) and small companies (100 kg year−1 as lower bound, and 1000 kg year−1 as upper bound) (Hendren et al., 2011).
3.4. Analysis of experimental studies for metal oxide ENMs and critical gaps
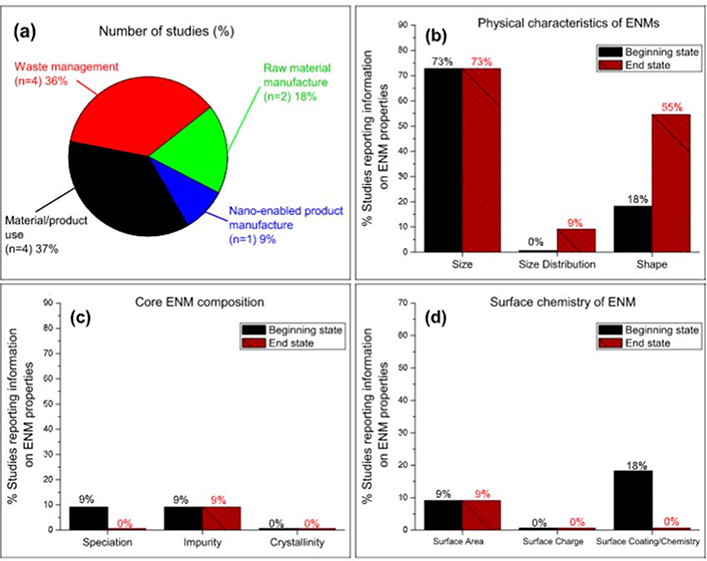
Similar to metallic ENM emission studies, the experimental investigations for metal oxide ENMs were limited in numbers. As shown in Fig. 3a, the studies were equally distributed between material product use (n = 4) and waste management (n = 4), followed by raw material manufacture (n = 2), then only one study for nano-enabled product manufacturing.
Fig. 3.
Analysis of evidence base for metal oxide ENM experimental studies: a) source emission (Size BS “n =8”, ES “n = 8”; Size Distribution BS “n = 0”, ES “n = 1”; Shape BS “n = 2”, ES “n = 6”), b) physical characteristics (Speciation BS “n =1”, ES “n = 0”; Impurity BS “n = 1”, ES “n = 1”; Crystallinity BS “n = 0”, ES “n = 0”), c) core material makeup, and d) surface characteristics (Surface Area BS “n = 1”, ES “n = 1”; Surface Charge BS “n = 0”, ES “n = 0”; Surface Coating/Chemistry BS “n = 2”, ES “n = 0”). Footnote: n = number of studies.
Most emission studies on metallic oxide ENMs were mostly conducted for titanium oxide (TiO2). Kaegi et al. assessed the release of TiO2 from exterior facades into surface waters (Kaegi et al., 2008). It was estimated that there was a significant release in quantities in the amount of 3.5 × 107 particles L–1. Windler et al., in an investigation examining the release of TiO2 from consumer textiles, determined the presence of about 0.01–0.06% of the titanium present in the textiles (Windler et al., 2012). A concentration of 1.5–15 μg L–1 of titanium was found in wastewater. Ham et al. characterized and compared task-based nanoparticle exposure profiles for titanium oxide ENMs in the workplace (Ham et al., 2012). A total of 1,032,889 particles cm–3 was found with 5th–95th percentile values spanning from 464,959 to 2,468,792 particles cm–3. Lee et al. monitored the possible exposure to nano TiO2 in the workplace (Lee et al., 2011). The concentrations ranged from 0.10 to 4.99 mg m–3, a value that is lower than the occupational exposure limit of 10 mg m–3 set by the Korean Ministry of Labor and American Conference of Governmental Industrial Hygienests. Hsu and Chein investigated the emission of TiO2 nano-powder coating materials under simulated conditions (Hsu and Chein, 2007). Among the three selected substrates (wood, polymer, tile), it was found that tile coated with TiO2 nano-powder was found to have the highest particle emission. Exposure to UV light showed an increase in the release of particles below 200 nm from TiO2 coating products. Bolyard et al. examined the behavior of TiO2 ENMs in landfill leachate (Bolyard et al., 2013). Coated nanoparticles did not affect biological processes when added to leachate. Similar findings were obtained in the same study for zinc oxide (ZnO) nanoparticles. Kiser et al. reported the presence of 100 to nearly 3000 μg L–1 of Ti in raw sewage in wastewater treatment plant (Kiser et al., 2009). Ti concentrations in effluents ranged from <5 to 15 μg L–1.
The release of aluminum oxide ENMs (or nano-alumina) was examined by Tsai et al. during the manual handling of nanoparticles (Tsai et al., 2008). The obtained measurements demonstrated the significant release of airborne nanoparticles from the fume hood into the laboratory environment and the investigator’s breathing area. In another study examining aluminum oxide nanoparticles, Pakrashi et al. demonstrated the bioavailability of nano-alumina in a freshwater system (Pakrashi et al., 2013). In addition to the aforementioned study of Bolyard et al. (2013) on ZnO nanoparticles, Lombi et al. researched the release of zinc oxide nanoparticles during the anaerobic digestion of wastewater and post-treatment processing of sewage sludge (Lombi et al., 2012). The results indicated that Zn when added as a soluble salt or as nanoparticles was rapidly converted to sulfides in all treatments. Lastly, Lu et al. studied the emission of cerium dioxide ENMs or ceria into the aquatic environment (Lu et al., 2010). In general, the distribution and accumulation characteristics of ceria nanoparticles in various aquatic organisms were different.
Fig. 3b–3d provide a detailed analysis of the physico-chemical characteristics of metal oxide ENMs in emission studies. It appears that, as found for metallic ENMs, size was the most studied physical property for metal oxide ENMs, followed by shape, then size distribution (Fig. 3b). The reporting of chemical properties was lacking in the examined studies (Fig. 3c and 3d). Certainly, this is a clear gap in knowledge as previously reported for metallic ENMs. Table S4 provides an examination of the physic-chemical properties for each of the experimental studies on metal oxide ENMs.
3.5. Appraisal of computational studies for metal oxide ENMs and gaps in knowledge
Computational studies on the emission of metal oxide ENMs are larger in number than those for experimental studies. Keller and co-workers examined the production of metal oxide ENMs in various geographical areas around the globe together with a tracking of these production estimates along their lifecycle trajectory. Based on global market information and material flow modeling, it was found by Keller et al. that titania, alumina, and iron and zinc oxides dominate the ENM market in terms of mass flow as evidenced from their use in various applications such as coatings, paints, pigments, electronics and cosmetics (Keller et al., 2013). An estimated 63–91% of over 260,000–309,00 tons of global ENM production ended up in landfills, with the remainder in soils (8–28%), water systems (0.4–7%), and atmospheric compartment (0.1–1.5%). Titanium dioxide resulted in the highest amount of releases, followed by zinc oxide, iron oxide, aluminum oxide, then copper oxide. Keller and Lazareva examined the releases in various regions around the globe along the ENM lifecycle trajectory (Keller and Lazareva, 2013). Keller et al. analyzed the release of metal oxide ENMs from personal care products (Keller et al., 2014). Using a consumer survey, estimates show that in the US zinc oxide with 1800–2100 tonns year–1 and titanium dioxide with 870–1000 tonns year–1 represented 94% of ENMs released into the environments or landfills from the use of personal care products. ENMs in sunscreen represent around 81–82% of total release from titanium and zinc dioxides, followed by facial moisturizer (7.5%), foundation (5.7%), and hair coloring products (3.1%). Lastly, Lazareva and Keller modeled the releases of TiO2, ZnO, Al2O3, and CeO2 from wastewater treatment plants (Lazareva and Keller, 2014). A comparative assessment was demonstrated for three mega metropolitan areas around the globe (New York, London, Shanghai). A significant uncertainty was found in the model parameters.
Nowack and coworkers estimated the releases of metal oxide ENMs into the environment. Mueller and Nowack reported that the predicted environmental concentrations of nano-titania in water are 0.7–16 μg L–1 and close to or higher than the predicted no effect concentrations for titanium dioxide ENMs (<1 μg L–1) (Mueller and Nowack, 2008). Similar to the work of Keller and others, Piccinno et al. examined industrial production quantities and uses of various metal oxide ENMs in Europe and around the world (Piccinno et al., 2012). The most produced ENM is TiO2 with up to 10,000 tons of worldwide production. CeO2, FeOx, AlOx, and ZnO are produced between 100 and 1000 tonns year–1. In another study, Gottschalk et al. modeled environmental concentrations of TiO2 and ZnO in different regions (Gottschalk et al., 2009). It was concluded based on the results that the risk to aquatic organisms was indeed existent from nano-TiO2 and nano-ZnO in sewage effluents for all considered regions. In a subsequent study, Gottschalk et al. (2013) examined the risk of metal oxide ENMs in water and soils (Gottschalk et al., 2013). They concluded that there was a marginal risk for the studied ENMs in surface water and some risk in sewage treatment plant effluents echoing some of the results of Keller and co-workers.
Hendren et al. and Liu and Cohen followed similar approaches to those reported by Keller, Nowack and others for a number of metal oxide ENMs (Hendren et al., 2011, Liu and Cohen, 2014). Markus et al. predicts that nanoparticles are capable of being transported over long distances, in much the same way as suspended particulate matter (Markus et al., 2016). Babaizadeh and Hassan and Shandilya et al. examined the emissions of nano-titanium oxide coating from residential windows and TiO2 nanoparticles from building materials, respectively confirming the dominating use of titanium dioxide in various applications (Babaizadeh and Hassan, 2013, Shandilya et al., 2015). O’Brien and Cummins provided a ranking order of environmental and health risk for a number of nanomaterials (O’Brien and Cummins, 2010).
4. Discussion
The evidence for emissions of metallic and metal oxide ENMs into the environment is produced from four sources, namely, manufacturing of raw materials, nano-enabled products, product use, and waste management services and has been covered in the published literature with varying emphasis. For example, four studies assessed nanosilver emissions in raw material production facilities along the manufacturing line. Assuming spherical shapes and average particles for the purpose of comparisons across the studies, the following concentrations for air emissions were obtained: (a) 9.5 × 10−5 – 1.1 × 10−4 mg Lair−1 (Park et al., 2009) (b) 6.0 × 10−8 – 4.3 × 10−4 mg Lair−1 (Lee et al., 2012a) (c) 2.2 × 10−6 mg Lair−1 (Ham et al., 2012) and (d) > 6.9 × 10−4 mg Lair−1 (Miller et al., 2010). These levels of concentrations provide a wide range below and above current occupational exposure limits of 2 × 10−6 mg Lair−1 (Lee et al., 2012a). As for nano-enabled product manufacturing, Lee et al. obtained similar values in the range of 2.0 × 10−8 – 2.4 × 10−7 mg Lair−1 (Lee et al., 2013).
In general, the concentration level depends on several factors including, among others, the type of source emission as well as its proximity to the source. For example, the findings of Lee et al. showed the highest concentrations in the injection room (Lee et al., 2012a). Other areas demonstrated much lower concentrations. Park et al. found that the increase in particle number concentration during the liquid phase process was higher than that during processes that involve the handling of dry power (Park et al., 2009). The aforementioned study concluded that since risk in liquid-phase process is undervalued compared to the gas or vapor-phase processes, the impact of exposure to aerosolized nanoparticles in liquid-phase process should be investigated in greater details. Miller et al. determined that all of the workers were exposed to levels of silver above the OSHA permissible exposure limit (Miller et al., 2010). Furthermore, the researchers found that measurements obtained near the furnace increased up to 100-fold above the baseline during the pouring of molten metal. In light of the above, engineering and administrative technologies should be instituted to reduce exposure levels by workers in nano-manufacturing facilities.
Another important source of emissions emanates from consumer product use such as during product application as well as wear and tear. Based on the same assumptions of spherical shapes and average particle sizes, the emission data reported in Hagendorfer et al. (2009) and Tsai et al. (2008) were converted to the following range of concentrations of silver nanoparticles emitted into the air: 7.9 × 10−6 – 5.5 × 10−5 mg Lair−1 and 4.4 × 10−4 mg Lair−1, respectively. These values appear to be well above the allowable levels in the occupational environment (Miller et al., 2010). Other studies examined emissions of silver nanoparticles during waste management activities with similar findings. For example, Wang et al. (2012)., Kaegi et al. (2013) and Kiser et al. (2012) found nanosilver in wastewater effluents in the range of 0.1–1.3 mg L−1. A study by Bolyard et al. (2013) documented a 3.8 ± 0.7 mg L−1 range of concentration during landfill activities. If released into the environment, these concentrations are in the toxic range reported for a number of species such as crustaceans, algae, fish, nematode, bacteria, yeast/fungi, mammalian cells in vitro, Vibrio fischeri, and protozoa (Bondarenko et al., 2013). It is to be noted that the toxic ranges reported in the literature for ENMs are typically obtained from laboratory studies under controlled conditions and these toxic ranges may be drastically lower or higher in real environments as result of the potential transformations taking place to ENMs.
Although metallic and metal oxide nanoparticle emissions into the environment have been documented for the four aforementioned sources, the evidence gathered in this research was generated from a limited number of studies that were classified as experimental and computational. These studies are too few to cover the vast area of source emissions at a multitude of individual endpoints along the lifecycle trajectory of ENMs. Certainly, a significant amount of future research is in order to cover the critical gaps in knowledge. All experimental studies were conducted mainly on silver and titanium dioxide nanoparticles. Although silver and titanium dioxide nanomaterials have the largest number of reported applications in consumer products (Xiu et al., 2012), the emissions of other metallic and metal oxide nanoparticles such as nZVI, Al and Al2O3 ENMs into the environment deserve attention as well. Other metallic and metal oxide ENMs have enjoyed a great surge in applications over the past few years (Buzea et al., 2007, Salata, 2004, Zaleska-Medynska et al., 2016). As such, one cannot generalize about the emission outcomes to other types of metallic and metal oxide ENMs. Additionally, aqueous solutions were the most investigated media, with lesser emphasis on emissions in air and soil.
In general, source emissions during product use captured the bulk of work with a narrow scope on certain processes (i.e., leaching). There is a need to broaden the scope of future research to all sources of emissions, including raw material, product manufacturing, and waste management. Studies on emissions did not report the minimum principle of information as recommended by OECD, thereby leaving critical gaps in knowledge for assessing the negative implications of ENMs. This will further hamper research efforts to understand the fate and transport of nanomaterials, as well as their impacts with respect to biological species (i.e., humans and non-humans).
The failure to report many of the ENM physical–chemical characteristics is largely attributed to the lack of standardized methodologies for analyzing ENMs in complex environmental samples. Thus, the method development for ENM characterization should be an area of priority for future research. Furthermore, nano-manufacturing industries may need to establish integrated and comprehensive continuous monitoring programs for ENM emissions in the workplace. This is particularly important because the available data points to the size of emitted ENMs, being mostly below 100 nm, a nanoscale range at which ENMs exhibit the highest potential risk.
In general, the published literature lacks details with respect to the impact of experimental conditions on the obtained characteristics of emitted ENMs. These characteristics are sensitive to the surrounding environmental and experimental conditions. Accordingly, various transformations can take place and result in increasing or decreasing the levels of potential risk (if any). Therefore, the interpretation and use of emission results should be bound by the experimental test conditions used in generating the emission data.
There is a significant shortage in the number of computational studies reported in the published literature. The outcomes of these studies were highly variable, which may be attributed to, for example, the assumptions made for analytic purposes and the lack of realistic estimates and data for use in modeling efforts. It is to be noted that the analytics involved in computational studies should be based on sound principles to enrich the field of nano-informatics along the lifecycle trajectory, from the source to the receptor. This area warrants significant advances in future research in order to develop relevant databases to assist the different stakeholders in their multitudes of endeavors.
In light of the appraisal, there are lessons to be learned in dealing with emerging technologies. All experimental studies did not take into account the further use of generated data by other stakeholders in the larger society-environment-economy systems. To allow for better utility of the data, future research may be needed to adequately parameterize the ENM physical–chemical characteristics, together with a thorough investigation of the influence of experimental conditions on the emission data.
In the absence of critical information on the emitted nanoparticles into the environment, we are left with fewer options in terms of how to deal with the potential implications of such releases. Sense-making methodologies, in addition to experimental investigations, should be established on the basis of historical data to deal with the issues at hand.
Furthermore, emissions into aqueous solutions were the most investigated relative to other media (e.g., air and soil). This is rather significant because it hampers our ability to read across the potential environmental emissions scenarios of ENMs.
5. Concluding remarks
The evidence gathered in this research suggests that the scientific literature primarily focused on emissions of silver and titanium dioxide ENMs into the environment and was limited in terms of the number of studies. Within this context, releases of nanoparticles originated from four sources, namely, raw manufacturing, nano-enabled product manufacturing, product use, and waste management services. The majority of studies investigated ENM emissions during nanomaterial use and waste management, followed by raw material manufacturing, and finally, nano-enabled product manufacturing. The reviewed studies did not adequately characterize the physical-chemical properties in pristine and emitted forms of ENMs according to the OECD minimum principle of information. Preliminary results suggest that the concentrations of emitted nanoparticles into the air cover a wide range below and above the allowable occupational exposure limits. Furthermore, the concentrations of nanoparticles in water systems, if left uncontrolled, could also pose a concern for a variety of biological species.
Supplementary Material
Acknowledgement
This research was funded by the U.S EPA Office of Research and Development. The manuscript has been subjected to the Agency’s review process and approved for publication. The opinions expressed in this paper are those of the author(s) and do not reflect the official positions and policies of the USEPA. Any mention of products or trade names does not constitute recommendation for use by the USEPA.
References
- Aitken R, Chaudhry M, Boxall A, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup. Med, 56 (2006), pp. 300–306. [DOI] [PubMed] [Google Scholar]
- Akobeng AK. Principles of evidence based medicine. Archives Dis. Child, 90 (2005), pp. 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano, 5 (2011), pp. 5478–5489. [DOI] [PubMed] [Google Scholar]
- Babaizadeh H, Hassan M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater, 40 (2013), pp. 314–321. [Google Scholar]
- Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. J. Environ. Qual, 39 (2010), pp. 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol, 42 (2008), pp. 4133–4139. [DOI] [PubMed] [Google Scholar]
- Bian S-W, Mudunkotuwa IA, Rupasinghe T, Grassian VH. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir, 27 (2011), pp. 6059–6068. [DOI] [PubMed] [Google Scholar]
- Bielefeldt AR, Stewart MW, Mansfield E, Scott Summers R, Ryan JN. Effects of chlorine and other water quality parameters on the release of silver nanoparticles from a ceramic surface. Water Res, 47 (2013), pp. 4032–4039. [DOI] [PubMed] [Google Scholar]
- Bolyard SC, Reinhart DR, Santra S. Behavior of engineered nanoparticles in landfill leachate. Environ. Sci. Technol. (2013), 130710152553007. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Archives Toxicol., 87 (2013), pp. 1181–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases, 2 (2007), pp. MR17–MR71. [DOI] [PubMed] [Google Scholar]
- Bystrzejewska-Piotrowska G, Golimowski J, Urban PL. Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag, 29 (2009), pp. 2587–2595. [DOI] [PubMed] [Google Scholar]
- Chao J.-b., Liu J.-f., Yu S.-j., Feng Y.-d., Tan Z.-q., Liu R, Yin Y.-g.. Speciation analysis of silver nanoparticles and silver ions in antibacterial products and environmental waters via cloud point extraction-based separation. Anal. Chem, 83 (2011), pp. 6875–6882. [DOI] [PubMed] [Google Scholar]
- Dehner CA, Barton L, Maurice PA, DuBois JL. Size-dependent bioavailability of hematite (α-Fe2O3) nanoparticles to a common aerobic bacterium. Environ. Sci. Technol, 45 (2011), pp. 977–983. [DOI] [PubMed] [Google Scholar]
- Echegoyen Y, Nerín C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol, 62 (2013), pp. 16–22 [DOI] [PubMed] [Google Scholar]
- El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol, 44 (2010), pp. 1260–1266. [DOI] [PubMed] [Google Scholar]
- El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol, 45 (2011), pp. 283–287. [DOI] [PubMed] [Google Scholar]
- Farré M, Sanchís J, Barceló D. Analysis and assessment of the occurrence, the fate and the behavior of nanomaterials in the environment. TrAC Trends Anal. Chem, 30 (2011), pp. 517–527. [Google Scholar]
- Fu F, Dionysiou DD, Liu H. The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J. Hazard. Mater, 267 (2014), pp. 194–205. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO 2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol, 43 (2009), pp. 9216–9222. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ. Pollut, 181 (2013), pp. 287–300. [DOI] [PubMed] [Google Scholar]
- Hagendorfer H, Lorenz C, Kaegi R, Sinnet B, Gehrig R, Goetz NV, Scheringer M, Ludwig C, Ul rich A. Size-fractionated characterization and quantification of nanoparticle release rates from a consumer spray product containing engineered nanoparticles. J. Nanoparticle Res, 12 (2009), pp. 2481–2494. [Google Scholar]
- Ham S, Yoon C, Lee E, Lee K, Park D, Chung E, Kim P, Lee B. Task-based exposure assessment of nanoparticles in the workplace. J. Nanoparticle Res, 14 (2012). [Google Scholar]
- Handy RD, Von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology, 17 (2008), pp. 287–314. [DOI] [PubMed] [Google Scholar]
- Hartmann NIB, Skjolding LM, Hansen SF, Baun A, Kjølholt J, Gottschalk F. Environmental Fate and Behavior of Nanomaterials: New Knowledge on Important Transfomation Processes. Danish Environmental Protection Agency; (2014). [Google Scholar]
- Hedberg J, Skoglund S, Karlsson M-E, Wold S, Odnevall Wallinder I, Hedberg Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Environ. Sci. Technol, 48 (2014), pp. 7314–7322. [DOI] [PubMed] [Google Scholar]
- Hendren CO, Mesnard X, Dröge J, Wiesner MR. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ. Sci. Technol, 45 (2011), pp. 2562–2569. [DOI] [PubMed] [Google Scholar]
- Hsu L-Y, Chein H-M. Evaluation of Nanoparticle Emission for TiO2 Nanopowder Coating Materials, Nanotechnology and Occupational Health. Springer; (2007), pp. 157–163. [Google Scholar]
- Ivask A, ElBadawy A, Kaweeteerawat C, Boren D, Fischer H, Ji Z, Chang CH, Liu R, Tolaymat T, Telesca D, Zink JI, Cohen Y, Holden PA, Godwin HA. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano, 8 (2014), pp. 374–386. [DOI] [PubMed] [Google Scholar]
- Ji Z, Jin X, George S, Xia T, Meng H, Wang X, Suarez E, Zhang H, Hoek EM, Godwin H. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol, 44 (2010), pp. 7309–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res, 11 (2008), pp. 77–89. [Google Scholar]
- Ju-Nam Y, Lead JR. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ, 400 (2008), pp. 396–414. [DOI] [PubMed] [Google Scholar]
- Kaegi R, Ulrich A, Sinnet B, Vonbank R, Wichser A, Zuleeg S, Simmler H, Brunner S, Vonmont H, Burkhardt M. Synthetic TiO 2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut, 156 (2008), pp. 233–239. [DOI] [PubMed] [Google Scholar]
- Kaegi R, Voegelin A, Ort C, Sinnet B, Thalmann B, Krismer J, Hagendorfer H, Elumelu M, Mueller E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res, 47 (2013), pp. 3866–3877. [DOI] [PubMed] [Google Scholar]
- Keller AA, Lazareva A. Predicted releases of engineered nanomaterials: from global to regional to local. Environ. Sci. Technol. Lett, 1 (2013), pp. 65–70. [Google Scholar]
- Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res, 15 (2013). [Google Scholar]
- Keller AA, Vosti W, Wang H, Lazareva A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanoparticle Res, 16 (2014), pp. 1–10. [Google Scholar]
- Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot, 35 (2012), pp. 64–70. [Google Scholar]
- Kim B, Park C-S, Murayama M, Hochella MF. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ. Sci. Technol, 44 (2010), pp. 7509–7514. [DOI] [PubMed] [Google Scholar]
- Kiser M, Westerhoff P, Benn T, Wang Y, Perez-Rivera J, Hristovski K. Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol, 43 (2009), pp. 6757–6763. [DOI] [PubMed] [Google Scholar]
- Kiser MA, Ladner DA, Hristovski KD, Westerhoff PK. Nanomaterial transformation and association with fresh and freeze-dried wastewater activated sludge: implications for testing protocol and environmental fate. Environ. Sci. Technol, 46 (2012), pp. 7046–7053. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem, 27 (2008), p. 1825. [DOI] [PubMed] [Google Scholar]
- Kühnel D, Nickel C. The OECD expert meeting on ecotoxicology and environmental fate — towards the development of improved OECD guidelines for the testing of nanomaterials. Sci. Total Environ, 472 (2014), pp. 347–353. [DOI] [PubMed] [Google Scholar]
- Künniger T, Gerecke AC, Ulrich A, Huch A, Vonbank R, Heeb M, Wichser A, Haag R, Kunz P, Faller M. Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environ. Pollut, 184 (2014), pp. 464–471. [DOI] [PubMed] [Google Scholar]
- Lazareva A, Keller AA. Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustain. Chem. Eng, 2 (2014), pp. 1656–1665. [Google Scholar]
- Lee JH, Ahn K, Kim SM, Jeon KS, Lee JS, Yu IJ. Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. J. Nanoparticle Res, 14 (2012). [Google Scholar]
- Lee JH, Kwon M, Ji JH, Kang CS, Ahn KH, Han JH, Yu IJ. Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal. Toxicol, 23 (2011), pp. 226–236. [DOI] [PubMed] [Google Scholar]
- Lee JH, Sohn EK, Ahn JS, Ahn K, Kim KS, Lee JH, Lee TM, Yu IJ. Exposure assessment of workers in printed electronics workplace. Inhal. Toxicol, 25 (2013), pp. 426–434. [DOI] [PubMed] [Google Scholar]
- Lee SJ, An HH, Han WB, Kim H-S, Yoon CS. Effect of temperature and humidity on coarsening behavior of Au nanoparticles embedded in liquid crystalline lipid membrane. Langmuir, 28 (2012), pp. 10980–10987. [DOI] [PubMed] [Google Scholar]
- Li L, Hartmann G, Döblinger M, Schuster M. Quantification of nanoscale silver particles removal and release from municipal wastewater treatment plants in Germany. Environ. Sci. Technol (2013), 130620163339004. [DOI] [PubMed] [Google Scholar]
- Liu HH, Cohen Y. Multimedia environmental distribution of engineered nanomaterials. Environ. Sci. Technol, 48 (2014), pp. 3281–3292. [DOI] [PubMed] [Google Scholar]
- Lombi E, Donner E, Scheckel KG, Sekine R, Lorenz C, Goetz NV, Nowack B. Silver speciation and release in commercial antimicrobial textiles as influenced by washing. Chemosphere, 111 (2014), pp. 352–358. [DOI] [PubMed] [Google Scholar]
- Lombi E, Donner E, Tavakkoli E, Turney TW, Naidu R, Miller BW, Scheckel KG. Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol, 46 (2012), pp. 9089–9096. [DOI] [PubMed] [Google Scholar]
- Loo S-L, Fane AG, Lim T-T, Krantz WB, Liang Y-N, Liu X, Hu X. Superabsorbent cryogels decorated with silver nanoparticles as a novel water technology for point-of-use disinfection. Environ. Sci. Technol, 47 (2013), pp. 9363–9371. [DOI] [PubMed] [Google Scholar]
- Loosli F, Le Coustumer P, Stoll S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids, pH and concentration effects on nanoparticle stability. Water Res, 47 (2013), pp. 6052–6063. [DOI] [PubMed] [Google Scholar]
- Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environ. Sci. Technol, 46 (2012), pp. 6893–6899. [DOI] [PubMed] [Google Scholar]
- Lu K, Zhang Z, He X, Ma Y, Zhou K, Zhang H, Bai W, Ding Y, Wu Z, Zhao Y. Bioavailability and distribution and of ceria nanoparticles in simulated aquatic ecosystems, quantification with a radiotracer technique. J. Nanosci. Nanotechnol, 10 (2010), pp. 8658–8662. [DOI] [PubMed] [Google Scholar]
- Lubick N. Silica nanoparticles flow in (and out of) waste. Environ. Sci. Technol, 43 (2009), pp. 8708–8709. [DOI] [PubMed] [Google Scholar]
- Luckmann R, Sackett David L., Straus Sharon E., Richardson W. Scott, Rosenberg William, Haynes Brain (Eds.), Evidence-based Medicine: How to Practice and Teach EBM (second ed.) (2001) Churchill Livingstone, 2000. J Intensive Care Med 16, 155–156. [Google Scholar]
- Mackevica A, Olsson ME, Hansen SF. The release of silver nanoparticles from commercial toothbrushes. J. Hazard. Mater. (2016). [DOI] [PubMed] [Google Scholar]
- Markus A, Parsons J, Roex E, De Voogt P, Laane R. Modelling the transport of engineered metallic nanoparticles in the river Rhine. Water Res, 91 (2016), pp. 214–224. [DOI] [PubMed] [Google Scholar]
- Metak AM, Nabhani F, Connolly SN. Migration of engineered nanoparticles from packaging into food products. LWT-Food Sci. Technol, 64 (2015), pp. 781–787. [Google Scholar]
- Miller A, Drake PL, Hintz P, Habjan M. Characterizing exposures to airborne metals and nanoparticle emissions in a refinery. Ann. Occup. Hyg, 54 (2010), pp. 504–513. [DOI] [PubMed] [Google Scholar]
- Mitrano DM, Rimmele E, Wichser A, Erni R, Height M, Nowack B. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano, 8 (2014), pp. 7208–7219. [DOI] [PubMed] [Google Scholar]
- Moreno T, Martins V, Querol X, Jones T, BéruBé K, Minguillón MC, Amato F, Capdevila M, de Miguel E, Centelles S. A new look at inhalable metalliferous airborne particles on rail subway platforms. Sci. Total Environ, 505 (2015), pp. 367–375. [DOI] [PubMed] [Google Scholar]
- Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol, 42 (2008), pp. 4447–4453. [DOI] [PubMed] [Google Scholar]
- Nativo P, Prior IA, Brust M. Uptake and Intracellular fate of surface-modified gold nanoparticles. ACS Nano, 2 (2008), pp. 1639–1644. [DOI] [PubMed] [Google Scholar]
- O’Brien N, Cummins E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J. Environ. Sci. Health Part A, 45 (2010), pp. 992–1007. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Important Issues on Risk Assessment of Manufactured Nanomaterials, OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials (2007). Report No. 33, ENV/JM/MONO (2012) 8, Paris: March, 28 2012 (Accessed on 28 February 2015).
- Pakrashi S, Dalai S, Humayun A, Chakravarty S, Chandrasekaran N, Mukherjee A. Ceriodaphnia dubia as a potential bio-indicator for assessing acute aluminum oxide nanoparticle toxicity in freshwater environment. PloS one, 8 (2013), p. e74003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the Nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol, 73 (2007), pp. 1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kwak BK, Bae E, Lee J, Kim Y, Choi K, Yi J. Characterization of exposure to silver nanoparticles in a manufacturing facility. J. Nanoparticle Res, 11 (2009), pp. 1705–1712. [Google Scholar]
- Peralta-Videa JR, Zhao L, Lopez-Moreno ML, de la Rosa G, Hong J, Gardea-Torresdey JL. Nanomaterials and the environment: a review for the biennium 2008–2010. J. Hazard. Mater, 186 (2011), pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res, 14 (2012). [Google Scholar]
- Quadros ME, Marr LC. Silver nanoparticles and total aerosols emitted by nanotechnology-related consumer spray products. Environ. Sci. Technol, 45 (2011), pp. 10713–10719. [DOI] [PubMed] [Google Scholar]
- Quadros ME, Pierson R, Tulve NS, Willis R, Rogers K, Thomas TA, Marr LC. Release of silver from nanotechnology-based consumer products for children. Environ. Sci. Technol, 47 (2013), pp. 8894–8901. [DOI] [PubMed] [Google Scholar]
- Ramos K, Gómez-Gómez M, Cámara C, Ramos L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS. Talanta, 151 (2016), pp. 83–90. [DOI] [PubMed] [Google Scholar]
- RCC. Government of Canada NanoPortal. Regulatory Cooperation Council Nanotechnology Initiative Final Report – Work Element 2, Priority Setting: Development of a Joint Nanomaterials Classification Scheme. (2014), http://nanoportal.gc.ca/666215CB-929F-4F75-B78C-4D9DD0F1093B/FINAL%20DRAFT%20RCC%20Nanotechnology%20Initiative%20Work%20Element%202.pdf (Accessed on 20 June 2016).
- Reinsch BC, Levard C, Li Z, Ma R, Wise A, Gregory KB, Brown GE, Lowry GV. Sulfidation of silver nanoparticles decreases Escherichia coli growth Inhibition. Environ. Sci. Technol, 46 (2012), pp. 6992–7000. [DOI] [PubMed] [Google Scholar]
- Rosenberg W, Donald A. Evidence based medicine: an approach to clinical problem-solving. BMJ Br. Med. J, 310 (1995), p. 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL. Evidence-based Medicine, Seminars in Perinatology. Elsevier; (1997), pp. 3–5. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Rosenberg WMC. On the need for evidence-based medicine. Health Econ., 4 (1995), pp. 249–254. [DOI] [PubMed] [Google Scholar]
- Salata O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol, 2 (2004), pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Riediker M. Use of nanoparticles in swiss Industry: a targeted survey. Environ. Sci. Technol, 42 (2008), pp. 2253–2260. [DOI] [PubMed] [Google Scholar]
- Shandilya N, Le Bihan O, Bressot C, Morgeneyer M. Emission of titanium dioxide nanoparticles from building materials to the environment by wear and weather. Environ. Sci. Technol, 49 (2015), pp. 2163–2170. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. Vitro, 25 (2011), pp. 231–241. [DOI] [PubMed] [Google Scholar]
- Straus SE, Sackett DL. Getting research findings into practice: using research findings in clinical practice. Br. Med. J, 317 (1998), p. 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares D, Rodrigues S, Cruz N, Carvalho C, Teixeira T, Carvalho L, Duarte A, Trindade T, Pereira E, Römkens P. Soil–pore water distribution of silver and gold engineered nanoparticles in undisturbed soils under unsaturated conditions. Chemosphere, 136 (2015), pp. 86–94. [DOI] [PubMed] [Google Scholar]
- Tolaymat T, El Badawy A, Sequeira R, Genaidy A. An integrated science-based methodology to assess potential risks and implications of engineered nanomaterials. J. Hazard. Mater, 298 (2015), pp. 270–281. [DOI] [PubMed] [Google Scholar]
- Tolaymat T, El Badawy A, Sequeira R, Genaidy A. A system-of-systems approach as a broad and integrated paradigm for sustainable engineered nanomaterials. Sci. Total Environ, 511 (2015), pp. 595–607. [DOI] [PubMed] [Google Scholar]
- Tsai S-J, Ada E, Isaacs JA, Ellenbecker MJ. Airborne nanoparticle exposures associated with the manual handling of nanoalumina and nanosilver in fume hoods. J. Nanoparticle Res, 11 (2008), pp. 147–161. [Google Scholar]
- Verleysen E, Van Doren E, Waegeneers N, De Temmerman P-J, Abi Daoud Francisco M, Mast J. TEM and SP-ICP-MS analysis of the release of silver nanoparticles from decoration of pastry. J. Agric. Food Chem, 63 (2015), pp. 3570–3578. [DOI] [PubMed] [Google Scholar]
- von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ. Toxicol. Chem, 31 (2011), pp. 32–49. [DOI] [PubMed] [Google Scholar]
- Wang Y, Westerhoff P, Hristovski KD. Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J. Hazard. Mater, 201–202 (2012), pp. 16–22. [DOI] [PubMed] [Google Scholar]
- Windler L, Lorenz C, Von Goetz N, Hungerbuhler K, Amberg M, Heuberger M, Nowack B. Release of titanium dioxide from textiles during washing. Environ. Sci. Technol, 46 (2012), pp. 8181–8188. [DOI] [PubMed] [Google Scholar]
- Xiu Z.-m., Zhang Q.-b., Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett, 12 (2012), pp. 4271–4275. [DOI] [PubMed] [Google Scholar]
- Yang Y, Long C-L, Li H-P, Wang Q, Yang Z-G. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci. Total Environ, 563 (2016), pp. 996–1007. [DOI] [PubMed] [Google Scholar]
- Zaleska-Medynska A, Marchelek M, Diak M, Grabowska E. Noble metal-based bimetallic nanoparticles: the effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci, 229 (2016), pp. 80–107. [DOI] [PubMed] [Google Scholar]
- Zuliani C, Diamond D. Opportunities and challenges of using ion-selective electrodes in environmental monitoring and wearable sensors. Electrochim. Acta, 84 (2012), pp. 29–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.