Abstract
Pigments are an essential part of everyday life on Earth with rapidly growing industrial and biomedical applications. Synthetic pigments account for a major portion of these pigments that in turn have deleterious effects on public health and environment. Such drawbacks of synthetic pigments have shifted the trend to use natural pigments that are considered as the best alternative to synthetic pigments due to their significant properties. Natural pigments from microorganisms are of great interest due to their broader applications in the pharmaceutical, food, and textile industry with increasing demand among the consumers opting for natural pigments. To fulfill the market demand of natural pigments new sources should be explored. Cold-adapted bacteria and fungi in the cryosphere produce a variety of pigments as a protective strategy against ecological stresses such as low temperature, oxidative stresses, and ultraviolet radiation making them a potential source for natural pigment production. This review highlights the protective strategies and pigment production by cold-adapted bacteria and fungi, their industrial and biomedical applications, condition optimization for maximum pigment extraction as well as the challenges facing in the exploitation of cryospheric microorganisms for pigment extraction that hopefully will provide valuable information, direction, and progress in forthcoming studies.
Keywords: Cryosphere, Microbial pigments, Cold-adapted microbes, Carotenoid, Melanin
Introduction
Think for a while, without hemoglobin how oxygen molecules can be carried within a living body or without chlorophyll and other necessary pigments; how plants and other primary producers could prepare their food? Surely impossible. It shows life on this planet depends on pigments. Pigments have been used as coloring agents since the prehistoric era. Sir William Henry Perkin prepared the earliest synthetic dye in 1856 named mauvine. The development of mauvine initiated a historical revolution of synthetic dyes (Walford 1980). Initially, the synthetic dyes gained much attraction due to several characteristics such as easy to develop, economical, no undesirable flavors, excellent coloring properties, and needed in a small amount to use. However, most of the synthetic dyes used had never been tested for their toxic nature for health and adverse effects on the environment (Downham and Collins 2000). In general, synthetic dyes are made up of chemical compounds composed of lead, mercury, copper, chromium, sodium chloride, benzene, and toluene that are harmful to human health. Several synthetic colorants previously permitted by the Food and Drug Administration (FDA) to use in medicines, food, and cosmetics development were later found carcinogenic (Rao et al. 2017). Some of these synthetically-derived colorants banned by the FDA include ethyl acrylate, benzophenone, eugenyl methyl ether (methyl eugenol), pulegone, myrcene, and pyridine. Similarly, several synthetic dyes like cochineal red, tartrazine, and sunset yellow initiate allergies directly or indirectly by combining with other colorants. The Center for Science in the Public Interest in Washington appealed to the FDA in 2008 to disallow synthetic food colorants since of their association with behavioral harms among children (Potera 2010). Most synthetic dyes previously used for various purposes are now quit due to apparent hazards such as carbon black (extensively used as printing ink pigment) a potential carcinogen and benzidine a causative agent of bowel cancer (Rao et al. 2017). Moreover, the unethical release of untreated dye effluents from industries persists for a longer time due to higher stability. Due to the aforementioned drawbacks of synthetic dyes, global demand for natural pigments has been increased (Manikprabhu and Lingappa 2013). Being aware of the adverse effects causing by synthetic dyes, now consumers prefer natural ingredients in their food instead of artificial (Downham and Collins 2000). This has raised the demand for naturally derived pigments.
The use of natural colorants is believed to be relatively safe, since their nature is biodegradable, harmless, and non-carcinogenic (Cristea and Vilarem 2006). Globally, the trend is shifting towards the consumption of biodegradable and environmentally friendly commodities; therefore, the demand for natural colorants is increasing in pharmaceutical, dyestuff, foodstuff, and cosmetics. Waste materials that are environmental pollutants have been used for microbial pigment production, which made this process more sustainable (Joshi et al. 2003; Kamla et al. 2012). Natural biocolorants that are an alternative to synthetic pigments could be obtained from plants and microorganisms. Natural pigments obtained from microbes are more ideal over plants due to the solubility, and stability of pigments and the easy availability of microbes for culturing (Rao et al. 2017). The fast growth of microorganisms in inexpensive medium, easy downstream processing, and their constant cultivation independent of the seasonal variations are advantages of microbes over plants as a source of pigment production (Manikprabhu and Lingappa 2013). Moreover, the use of plants on a large scale can cause damage to the precious species; therefore, the practice is not sustainable (Downham and Collins 2000). Additionally, the yearly growth rate of natural dyes has been projected 5–10% compared to synthetically derived dyes having a lower growth rate of 3–5% (Parmar and Phutela 2015). Pigments produced by microorganisms are not merely colors but consist of diverse chemical components having multidimensional biological activities (Kim 2013). Among microorganisms, bacteria, fungi, and microalgae offer an alternate source for natural pigments (Joshi et al. 2003; Choi et al. 2015; Rao et al. 2017; Pandey et al. 2018; Ramesh et al. 2019).
Earth’s cryosphere has been thought too hostile to harbor life for a long time. However, being extreme ecological conditions, diverse microbial communities exist in the glacial ecosystem (Anesio et al. 2017; Hassan et al. 2018; Rafiq et al. 2019) that maintain active biochemical routes (Anesio and Laybourn-Parry 2012). The microbial communities inhabiting the cryosphere are constantly exposed to several stress conditions such as extremely low temperatures, oligotrophic conditions, freeze–thaw cycles, ultraviolet (UV) radiations, and higher salinity. To cope with these hostile conditions, microorganisms adapt several protective strategies including morphological alteration and production of various metabolites. Most of these metabolites have important biotechnological and industrial applications (Cavicchioli et al. 2011). Among these metabolites, pigments are charismatic traits of these cold-adapted microorganisms that are studied to exploit for several industrial applications. Pigments production mostly occurs within cytoplasm as a responsive agent to hostile ecological conditions presenting several ecological functions (Pagano and Dhar 2015). Physiochemically extreme environments such as the cryosphere provide a suitable setup to microorganisms for pigment production with unique qualities and applications. In this review, pigments produced by cold-adapted bacteria and fungi in the cryosphere and their potential applications in various industries are discussed. Moreover, condition optimization for maximum pigment yield as well as the challenges facing in the exploitation of cryospheric microorganisms for pigment extraction are discussed.
Challenges in cryosphere and microbial adaptation mechanisms
Cryosphere has been dominated and colonized by diverse microorganisms collectively termed as psychrophiles or cold-adapted microorganisms. Life spin and activity of several microorganisms significantly reduce below 0 °C (Tribelli and López 2018). However, psychrophilic and psychrotrophic microorganisms withstand the hostile environments of cryosphere and adapt to grow below 5 °C (close to zero). Low temperature reduces thermal energy with increased viscosity of solvents and solubility of gasses such as oxygen molecules and reactive oxygen species (ROS), reduced solubility of nutrients and solutes, decreased diffusion as well as amplify desiccation, osmotic stress, and ice development. Moreover, life in the cryosphere experience several environmental stresses including high salinity, low availability of nutrients, oxidative stress, low water activity, and freeze–thaw cycles. High-pressure stress is subjected to microbial population inhabiting in deep-sea and sub-glacial habitats. Additionally, psychrophilic microbes are exposed to extremes of light, exposure to UV radiations, and bright light at high elevation and low light in frosted lakes, permafrost, and deeper ice sheets. To cope with these life-endanger challenges microorganisms adapt and develop certain sophisticated strategies (Margesin and Collins 2019; Collins and Margesin 2019). Previously, several microbiological, physiological, biochemical, biophysical, and molecular-based approaches have been adapted to identify biogeographical distribution, physiological adaptation, diversity, and ecological role of psychrophiles. However, recent advances in ‘omics’ technologies such as genomics, metagenomics, transcriptomics, and proteomics have uncovered novel adaptation mechanisms and environmental functions of microorganisms in the cryosphere (Singh et al. 2014; Barauna et al. 2017; Tribelli and Lopez 2018). Several mechanisms adapted by microorganisms to cope with the hostile environment of cryosphere are shown in Fig. 1.
Fig. 1.
Common strategies adapted by psychrophiles to cope with low temperatures and other stresses in the cryosphere
At low temperatures, psychrophiles alter their cellular envelope and components to provide shape, support, and protection to cells; regulate nutrient uptake and solute transport; and participate in cell division, adhesion, signaling, and sensing. This alteration is against the low temperature that reduces membrane permeability, fluidity, diffusion rates, and cause cell rupturing due to freeze–thaw cycles and ice formation. Psychrophiles modify the composition of fatty acid in lipid bilayer present in the cell membranes and become homeoviscous (D’Amico et al. 2001; Siddiqui et al. 2013). Contents of unsaturated fatty acids, short-chain, methyl branched, and/or cis-isomeric can manage the low temperature stress (Hassan et al. 2020) by lowering the liquid phase phospholipid bilayer to gel phase and sustain their function. For this adaptation, upregulation and over-representation of genes occur that encode proteins involved in membrane biogenesis and biosynthesis and desaturation of fatty acids (desaturases that also guard against ROS), biosynthesis of branched-chain fatty acids (KAS-II, KAS-III) and cis-isomerization (fatty acid cis/trans isomerases) (Medigue et al. 2005; De Maayer et al. 2014; He et al. 2015; Goordial et al. 2016). Furthermore, psychrophiles upregulate the membrane transport proteins that could counter the reduced diffusion rates at lower temperatures (Bakermans et al. 2007; De Maayer et al. 2014). Long-chain polyunsaturated fatty acids (LC-PUFAs) (Yoshida et al. 2016) also shield the membrane by generating hydrophobic edges between the lipid bilayers, which prevents the entrance of ROS augmented at low temperatures. Polyunsaturated fatty acids (PUFAs) can act as chaperons for proteins in the membrane, which could be functional in cell division and the efflux process (Okuyama et al. 2008; Yoshida et al. 2016). Turk et al. (2011) reported higher membrane fluidity in psychrotolerant yeast Rhodosporidium diobovatum suggesting that LC-PUFAs maintain the integrity and functionality of the plasma membrane. Similarly, psychrophiles upregulate certain genes encoding peptidoglycan biosynthesis and thicken their peptidoglycan (Mykytczuk et al. 2013), which can protect psychrophiles against freeze–thaw cycle, ice formation, and osmotic pressure. The lipopolysaccharides (LPS) layer in psychrophiles are shortened in length lacking the O-chain component (Corsaro et al. 2017) that may enhance the flexibility and stability of the outer membrane. Genes encoding for the synthesis of outer membrane components, proteins, and LPS (largely glycosyl transferases) was reported upregulated at lower temperatures (Frank et al. 2011; De Maayer et al. 2014). Benforte et al. (2018) reported that how a mutation in the glycosyl transferase gene (wapH) reduced the growth pattern of the Antarctic bacterium at lower temperatures. Several psychrophiles have higher genome contents encoding for compatible solute biosynthesis and uptake genes that could accumulate compatible solutes in molar concentrations, with glycine betaine, glycerol, mannitol, trehalose, sarcosine, sucrose, and sorbitol (Mykytczuk et al. 2013; Ghobakhlou et al. 2015; Goordial et al. 2016) that could restore osmotic balance and cell shrinkage. Fungi synthesize certain compatible solutes to cope with osmotic pressure and dehydration in the cryosphere such as mannitol that acts as a cryoprotective (Feofilova et al. 1994). Psychrophiles produce antifreeze proteins (AFPs) that bind to ice and inhibit ice development and recrystallization. AFPs preserve the liquid present in the close vicinity of cells (Raymond et al. 2008). These AFPs also stabilize cell membranes, structural integrity, and position the cell for increase contact towards nutrients and oxygen in the phototrophic region (Lorv et al. 2014; Bar Dolev et al. 2016; Voets 2017). Another protein called ice-nucleating proteins (INPs) reported in psychrophiles prevent cryoinjury of the cell by inhibiting intracellular ice formation (Lorv et al. 2014). Cold-adapted bacteria produce a high concentration of extracellular polymeric substances (EPS) at low temperatures (Mykytczuk et al. 2013; Caruso et al. 2018) which could form a protective shell around the cells. EPS produced by psychrophilic microorganisms could act as osmoprotective, ROS scavenging, and cryoprotective (Ewert and Deming 2013; Deming and Young 2017). Along with low temperature adaptation, biosurfactant (glycolipid) reported from Antarctic yeast showed ice recrystallization inhibition activity (Kitamoto et al. 2001), also such biosurfactants act as osmolytes (Perfumo et al. 2018). The main adverse effect of low temperature is on the cellular reaction rate of mesophilic microorganisms that either halts their cellular growth or cause death due to cellular lysis. However, psychrophiles produce enzymes that perform high catalytic activities in cold environments, therefore, optimally grow at low temperatures (Santiago et al. 2016; Collins and Gerday 2017). Psychrophiles overcome the obstacle of reduced enzyme activity by developing a changed system for the transportation of nutrients and waste products; altered cellular processes of transcription and translation, and cell division cycle, and decrease the fluidity of membrane to grow in low temperature (D'Amico et al. 2006). Cold-adapted organisms produce chaperones that efficiently fold proteins and DNA/RNA and stabilize the secondary structures of these molecules at low temperatures. Psychrophiles upregulate the production of protein and DNA/RNA chaperones as cold acclimation proteins (Lim et al. 2000). Recently by adapting omics approaches have discovered various traits that are common to psychrophiles (Tribelli and Lopez 2018). One such trait is the metabolic adjustment at sub-zero temperature that takes place in psychrophiles such as the downregulation of primary metabolic pathways and replacement with alternate secondary pathways and accumulations and digestion of spare compounds. Cellular metabolic routes like glycolysis, tricarboxylic acid cycle, electron transport chain, and pentose phosphate pathway get downregulated in cold-adapted microorganisms (Medigue et al. 2005; Piette et al. 2011; Tribelli et al. 2015). Such metabolic reprogramming could protect oxidative stress and conserve energy for long-term survival. Another important protective mechanism adapted by psychrophilic microorganisms is the production of pigments. Psychrophiles reported from ice-cores and glaciers were capable to produce pigments (Shen et al. 2018). Melanin pigment reported in fungi protects stresses such as ionizing radiation, desiccation, oxidizing agents, and UV radiations (Butler and Day 1998; Gorbushina 2003). Antarctic fungal species having melanin were reported, which were resistant to UV radiation (Hughes et al. 2003). Several fungal strains produce mycosporines that absorb UV radiations (Sommaruga et al. 2004). Similarly, psychrophilic bacteria produce carotenoid as a protection mechanism in cryospheric environments (Vila et al. 2019). Moreover, these pigments also play role in photoprotection (in combination with other molecules like mycosporine and scytonemin like amino acids, and act as a protector against intense light range and UV irradiation), act as cryoprotectants, antioxidants, and antimicrobials (Dieser et al. 2010; Pandey et al. 2018).
Role of pigments in cold-adapted microbes
In general, pigments are secondary metabolites produced by microbes and are not necessarily produced by all kinds of microorganisms. Cold-adapted bacteria and fungi inhabiting cryospheric environments produce a diverse range of pigments (Table 1). The pigment production by microbes in extremely low temperature environments is a mechanism to survive ecological stresses. They consume pigment molecules as an energy source (Madigan et al. 2012), for the process of photosynthesis (Siefirmann-Harms 1987), as a confrontation tool against stress (Martin-Cerezo et al. 2015), oxidants, extreme temperature and desiccation (Wada et al. 2013), and for safety against UV irradiation (Becker-Hapak et al. 1997). Moreover, pigments also act as an antimicrobial agent against other bacteria (Suresh et al. 2015) and sometimes act as a shield to protect the cell against natural antimicrobial compounds secreted by other bacteria (van Duin et al. 2002). High diversity of pigment molecules are reported from microorganisms inhabiting in glaciers (Foght et al. 2004), ice cores (Zhang et al. 2008), and marine surface waters (Agogué et al. 2005) which shows that this pigment production is essential to cope with the environmental stresses in the cryosphere. Such as carotenoid modulate the cellular membrane fluidity of bacteria as an adaptive strategy in the cryosphere. Polar microbial communities are concurrently exposed to UV radiations, highly active photosynthetic radiations, and extremely low temperatures which make them more prone to photo-damage (Roos and Vincent 1998). Therefore, the adaptation of protective strategies by microorganisms is crucial in these habitats. In such harsh environmental conditions, pigment production efficiently decreases photo-damage and could provide resources for osmoregulation, thus provide tolerance against salinity, desiccation, and freeze–thaw cycles and prevent serious damages such as inhibition of metabolic processes and cellular repair mechanisms (Mueller et al. 2005). Depend upon the composition and concentration of pigments, the production could visibly provide coloration to snow such as green due to chlorophylls, various yellow shades due to xantophylls, and orange to red due to carotenoids (Anesio et al. 2017).
Table 1.
Pigments produced by cold-adapted bacteria and fungi, characterization, and biological activities
| Type of microorganism | Name of organism | Habitat | Pigment | Color | Biological function | References |
|---|---|---|---|---|---|---|
| Bacteria | Micrococcus roseus | Antarctic soil | Carotenoid | Yellow | Regulation of membrane fluidity | Chattopadhyay et al. (1997) |
| Arthrobacter agilis | Sea ice, Antarctica | Carotenoid | Yellow | Membrane stabilization at low temperature | Fong et al. (2001) | |
| Microbial mat; multiple bacteria isolated | Ward Hunt Ice Shelf, Nunavut, Canada | Carotenoid, Scytonemins | Yellow | Protection against reactive oxygen species, coping radiations, and other stresses | Mueller et al. (2005) | |
| Multiple bacteria isolated | Antarctic sites: Pony Lake and Cotton Glacier | Carotenoid | Yellow, orange, and dark rose red | Prevent against freeze–thaw cycles. Protection against UV radiation | Dieser et al. 2010 | |
| Sanguibacter suarezii KK6, Kocuria turfanensis KK7, Kocuria rosea KK12, and Planococcus maritimus KK21 | Leh and Ladakh soil, Himalayas | Carotenoid (Lycopene) | Yellow and orange | Survival strategy in a cold environment | Kushwaha et al. (2014) | |
| Arthrobacter isolates | Scott Base, Granite Harbour, Minna Bluff and Marble Point (Antarctic) | Carotenoid | Genes reported | Cold adaptation by stabilizing cell membrane, resistance to oxidative stress | Dsouza et al. (2015) | |
| 30 different heterotrophic pigmented bacteria were isolates | Fildes Peninsula, King George Island, Antarctica | Carotenoid | Yellow and orange | Survival strategy in a cold environment | Vila et al. (2019) | |
| Novel species very close to Janthinobacterium lividum | Organic residue of a water tank keeping rainbow trout | Mixture of violacein and deoxyviolacein | Violet | Antibacterial activity and enhance competitive ability | Nakamura et al. (2002) | |
| Corynebacterium glutamicum ATCC | ATCC |
Decaprenoxanthin (Carotenoid); Lycopene (Carotenoid) |
Yellow and red | Photoprotection and light-harvesting | Heider et al. (2012) | |
| Sphingomonas faeni and other | Anthropogenic cold environments | Astaxanthin (Carotenoid) | Yellow | Antagonistic effect against competitors | Mageswari et al. (2015) | |
| Janthinobacterium sp. HHS7 | Xinjiang glacier, China | Violacein | Violet | Adaptation to extremely low temperature | Lu et al. (2009) | |
| 11 psychrophilic Flavobacterium strains | Laigu, Zepu, Renlongba, and Gawalong glaciers in Bome County, Tibetan Autonomous Region, P.R. China | Flexirubin-type pigment | Yellow | Adaptation to extremely low temperature | Liu et al. (2019) | |
| Pseudomonas. Cryobacterium, Psychrobacter, Flavobacterium, and many others | Yuzhufeng Glacier, Tibetan Plateau, China | carotenoids such as α- and β-carotene, 19′-butanoyloxy-fucoxanthin, fucoxanthin, diatoxanthin, peridinin and zea/lutein, with α-carotene as the dominant carotenoid | (orange, reddish-orange, yellow, pink, brown, and white | Survival strategy in a cold environment | Shen et al. (2018) | |
| Genus Arthrobacter; Arthrobacter psychrophenolicus sp. nov | Alpine ice cave, Austria | Unidentified | Yellow | Survival strategy in a cold environment | Margesin et al. (2004) | |
| Thirty-seven heterotrophic bacterial isolates |
Franz Josef and Fox Glaciers, New Zealand |
Unidentified | Yellow and orange | Survival strategy in a cold environment | Foght et al. (2004) | |
| Multiple bacteria isolated | Prydz Bay, Eastern, Antarctica | Unidentified | Yellow, red, orange, pink | Survival strategy in a cold environment | Bowman et al. (1997) | |
| Multiple bacterial isolates are reported | Gulkana Glacier, Alaska | Unidentified | Pink, orange, white, cream, and yellow | Resistance and adaptation to cold temperatures | Segawa et al. (2011) | |
| Multiple bacterial isolates are reported | Siachen Glacier, Pakistan | Unidentified | Several colors pigments | Adaptation against cold temperature | Rafiq et al. (2017) | |
|
Proteobacteria, Firmicutes Actinobacteria Bacteroidetes |
Himalayan Glaciers in Uttarakhand, India | Unidentified | Lemon yellow, orange, brown, violet, pinkish-red and pale yellow | Adaptation against cold temperature | Panwar et al. (2019) | |
| Janthinobacterium sp. strain Ant5-2 | Lake Podprudnoye, Schirmacher Oasis, East Antarctica | Violacein | Purple violet | photoprotective role. UV and cold tolerance | Mojib et al. (2013) | |
| total of 31 g-positive bacteria was reported | King George Island, Antarctica Peninsula) | Unidentified | Yellow, red, orange, and amber | inhibit potential surface competitors | Leiva et al. (2015) | |
| Lysobacter oligotrophicus | Antarctica | (Lo-melanin) melanin | Dark-brown | Protection against UV-B irradiation. Scavenge ROS | Kimura et al. (2015) | |
| Hymenobacter actinosclerus and Chryseobacterium chaponense | King George Island, Antarctica | Carotenoids | Yellow and red | tolerate excessive UV irradiation | Órdenes-Aenishanslins et al. (2016) | |
| Pedobacter terrae | Doumer Island, Antarctica | Different pigments belong to carotenoids group | Red | Antioxidant activity, protection against UV radiation | Correa-Llantén et al. (2012) | |
| Multiple isolates were reported | coastal Antarctic Station Dumont d’Urville, European alpine snow pits, Nevado Illimani, Bolivia, and Antarctic Station Artigas, King George Island | Unidentified | Multiple colors | Uv radiation protection | González-Toril et al. (2008) | |
| Leeuwenhoekiella aequorea, Pseudomonas pelagia, Halomonas boliviensis, Rhodococcus yunnanensis, Algoriphagus ratkowskyi | Kongsfjorden, a glacial fjord on the west coast of Svalbard, | (Zeaxanthin) carotenoid | Yellow and red | cryoprotective agent in regulating membrane fluidity | Singh et al. (2017) | |
| Kocuria polaris sp. nov | McMurdo Dry Valley, Antarctica | Carotenoid | Orange | Reddy et al. (2003) | ||
| Arthrobacter sp. G20 | Caspian Sea | Carotenoid | Red | Afra et al. (2017) | ||
| Cyanobacterial mats | Antarctic stromatolitic and endolithic cyanobacterial communities | Scytonemin | Photoprotection | Wynn-Williams et al. (1999) | ||
| Lichen and cyanobacterial samples | Antarctic desert habitats, | β-carotene, Scytonemin, phycocyanin, etc | UV protection | Wynn-Williams and Edwards (2002) | ||
| Cyanobacteria-dominated microbial mats (several isolates) | McMurdo Sound region, Antarctica | Scytonemin, carotenoids, | Multiple colors | Vincent et al. (1993) | ||
| Fungi | Several yeasts were isolated | Antarctica (Admiralty Bay, King George Island, and Port Foster Bay and Deception Island) |
Carotenoids and Other pigments |
Several colors | Protection against UV radiation | Vaz et al. (2011) |
|
Dioszegia sp., Rhodotorula mucilaginosa, Rhodotorula laryngis |
Italian alpine glacier (Tonale Pass, TN, Italy) | Carotenoids | Orange to salmon-colored | Protection against UV radiation | Amaretti et al. (2014) | |
| Thelebolus microsporus | McLeod Island, Larsemann Hills, in the Prydz Bay area of East Antarctica |
β-carotene (Carotenoid) |
Bright orange to yellow-orange | Stress tolerance of UV radiation and extreme cold | Singh et al. (2014) | |
|
Xanthophyllomyces dendrorhous |
King George Island, Antarctic Peninsula | Astaxanthin, phoenicoxanthin, β-carotene, (Carotenoid) | red, pale-yellow and yellow | photoprotective role | Contreras et al. (2015) | |
| Penicillium sp. (GBPI_P155) | Himalayan region of India |
Carotenoid (Derivatives) |
Dark orange pigment | Survival of the cold temperature | Pandey et al. (2018) | |
| Multiple fungal isolates were reported | Batura Glacier, Karakoram, Pakistan | Unidentified | Multiple colors | Adaptation against cold temperature | Hassan et al. (2018) | |
| Multiple endophytic fungal isolates were reported | Admiralty Bay, King George Island, South Shetland Islands, Antarctica | Melanin | Dark blackish | Protection against environmental stresses in cold habitats | Rosa et al. (2009) | |
| Antarctomyces pellizariae sp. nov., | Coppermine Peninsula, Robert Island, in the South Shetland Islands, Antarctica | Unidentified | Blue | Cryo-protection of fungus | de Menezes et al. (2017) | |
| Cystofilobasidium capitatum and Sporobolomyces ruberrimus | Zooplankton (Antarctica) | β-Carotenoid | Resistance to ultraviolet B (UVB | Moliné et al. (2009) | ||
| Sporobolomyces salmonicolor, Cryptococcus albidus, and Cryptococcus laurentii | Livingston Island, Antarctica | β-carotene | Resistance to ultraviolet A (UVA) | Dimitrova et al. (2010) | ||
| Sporobolomyces salmonicolor | Livingston Island, Antarctica | β-Carotene, torularhodin, and torulene | Antioxidant activity | Dimitrova et al. (2013) | ||
| Rhodotorula mucilaginosa, Rhodotorula larynges, Dioszegia sp. | (King George Island, Antarctica | 2-γ-Carotene, Torulene, γ-carotene, and lycopene, Torulene, and lycopene, OHK torulene | Pink, pale red, red, orange | UV-C radiation tolerance | Villarreal et al. 2016 | |
| Nadsoniella nigra | Antarctica | Melanin | Black | Increase resistance in piglets and reduction of morbidity and mortality | Chyizhanska and Beregova (2009) | |
| Several yeasts strains | Antarctic biotopes | Melanin | Coal-black | Tashirev et al. (2010) | ||
| Cryptococcus sp. and Torrubiella sp | Sedimentary rocks (Union Glacier) Antarctic | Mycosporine | Pink and cream | Protection against UV radiations | Barahona et al. (2016) | |
| Dioszegia patagonica | (King George Island | Carotenoid | Orange | Trochine et al. (2017) | ||
| Arthrobotrys ferox | Moss samples (Wood Bay, Victoria Land Antarctica | Carotenoid and mycosporines | Resistance to ultraviolet-B (UVB | Arcangeli et al. (1997, 2000); Arcangeli and Cannistraro (2000) | ||
| 6 yeast strains and 11 bacterial strains | Galindez Island in the Argentine Islands in the north-western Antarctic Peninsula | Carotenoids and other pigments | Multiple colors | UV resistance | Vasileva-Tonkova et al. (2014) |
Pigments produced by cold-adapted bacteria and fungi
Carotenoid
Carotenoids are the main natural pigments widely produced by plants and microorganisms and initially were reported by H.W.F Wackenroder (Wackenroder 1831). Currently, carotenoids signify the largest and highly diverse known group of natural pigments, and 1183 carotenoid structures are compiled from 702 source organisms by Carotenoid Database Japan (https://carotenoiddb.jp). Carotenoid is the family of tetraterpenoids consists of an extensively conjugated polyene, which absorbs blue light and appears from yellow to red in shade (Fraser and Bramley 2004). The structure of carotenoids acquires the form of a polyene hydrocarbon chain that is occasionally terminated by rings with or without extra oxygen atoms. Carotenoids are classified into carotenes, which are hydrocarbons (torulene, lycopene, and α- to ε-carotenes) or xanthophylls that possess keto, hydroxyl, and/or carboxyl groups (lutein, zeaxanthin, torularhodin, and astaxanthin,) (Table 2). Naturally, carotenoids are extensively produced by plants and microorganisms as a photo-protectants. The color of carotenoids ranging from yellow to deep red depending on the structure (Alija et al. 2005; Rao and Rao 2007). The maximum absorption capacity of carotenoids is ranged from 440 to 520 nm, having a stronger molar absorption coefficient (ca. 105 L mol−1 cm−1) (Marizcurrena et al. 2019). Cryospheric habitats especially glacial environment provide suitable conditions for pigment production by microorganisms. These pigments also protect the bacterial cells either screening or absorbing UV radiation (Dieser et al. 2010). Carotenoids safeguard microbial cells from photo-oxidative injury and other environmental stresses at low temperatures either by inhibiting the ROS generation through thermal degeneracy of the extra energy or satisfying the excited states of singlet oxygen and chlorophyll (Tian and Hua 2010). Moreover, these pigments play a crucial role in the physiological plasticity of Antarctic microorganisms, efficiently respond to a lower temperature and freeze–thaw cycles (Singh et al. 2017). Additionally, carotenoids play a role in cell differentiation and regulation during cell cycles.
Table 2.
Types of pigments from cold-adapted microorganisms, their applications, and chemical structures
| Pigments | Applications | Structures |
|---|---|---|
|
Lycopene ((all-E)-Lycopene) |
Food additives, Antioxidant activities, Antimicrobial activities, and as Sun protector |
C40H56 (psi,psi-Carotene) |
|
Torulene (Torulin) |
Antioxidant activity, Cosmetics additives, Anti-cancerous activities, and Antimicrobial activities |
C40H54 (3′,4′-Didehydro-beta,psi-carotene) |
|
Beta-carotene (β-Carotene) |
Food colorant, Antioxidant activity, and Precursor of vitamin A in food |
C40H56 (beta-Carotene) |
|
Xanthophylls (lutein) |
Feed additives, Protection against free radicals, and use in the pharmaceutical industry |
C40H56O ((3R,3′R,6′R)-beta,epsilon-Carotene-3,3′-diol) |
| Prodigiosin | Dyeing agent in the textile industry, Coloring agents in the food industry, Antibacterial antiviral, and anticancer activities |
C20H25N3O (4-Methoxy-5-[(Z)-(5-methyl-4-pentyl-2H-pyrrol-2-yliden)methyl]-1H,1′H-2,2′-bipyrrol) |
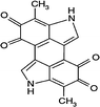
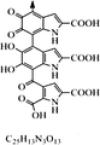
| Melanin | Antibacterial activity against antibiotic-resistant pathogens, Antioxidant activity, and cytotoxic activity |
C18H10N2O4 (6,14-dimethyl-4,12-diazapentacyclo[8.6.1.12,5.013,17.09,18]octadeca-1(17),2,5,9(18),10,13-hexaene-7,8,15,16-tetrone) |
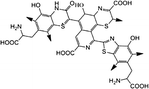
| Pheomelanin | Markers in fossils, Antioxidant activity, Photoprotective effects |
C34H29N6O12S3 (Pheomelanin) |
| Eumelanin | Antioxidant activity, Biomedical activities |
C25H13N3O13 (Eumelanin) |
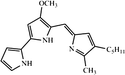
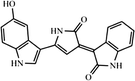
| Violacein | Dyeing agent in the textile industry, Food industry, Antibacterial, fungicidal and antiviral activities, Antioxidant and cytotoxic activities, Use in cosmetics and medicine |
C20H13N3O3 (3-[2-hydroxy-5-(5-hydroxy-1H-indol-3-yl)-1H-pyrrol-3-yl]indol-2-one) |
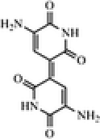
| Indigoidine | As bioindicator, Antimicrobial activities, and the Textile industry as a dyeing agent |
C10H8N4O4 (3-(5-amino-2-hydroxy-6-oxo-1H-pyridin-3-yl)-5-iminopyridine-2,6-dione) |
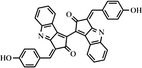
| Scytonemin | Anti-inflammatory and Anti-proliferative drugs, Antioxidant agents, Use in cosmetics |
C36H20N2O4 (3E,3′E)-3,3′-Bis(4-hydroxybenzyliden)-1,1′-bicyclopenta[b]indol-2,2′(3H,3′H)-dion) |
Several studies reported carotenoid producing bacteria and fungi from low temperature environments (Table 1). Vila et al. (2019) reported 30 bacterial strains capable of carotenoids production from Fildes Peninsula, King George Island, Antarctica. Kim et al. (2015) isolated carotenoid producing Planococcus faecalis from stools of Antarctic penguins. Carotenoid production was reported in heterotrophic bacteria from Antarctica as a strategic tool against ecological stresses (Dieser et al. 2010). The accumulation of C-50 carotenoid (Bacterioruberin and its glycosylated derivatives) observed in psychrotrophic Arthrobacter agilis from an Antarctic sea ice sample. This accumulation of pigments might be responsible for the regulation of cellular membrane fluidity at low temperatures (Fong et al. 2001). Moreover, several polar and non-polar carotenoid pigments produced by Antarctic strains of Sphingobacterium antarcticus and Micrococcus roseus (Chattopadhyay and Jagannadham 2001). Silva et al. (2019) reported carotenoid producing bacterial strains isolated from the Antarctic continent. Three different carotenoids such as zeaxanthin, β-cryptoxanthin, and β-carotene were reported from Antarctic bacterium Sphingobacterium antarcticus. Similarly, carotenoid producing cold-adapted Penicillium sp was reported in the Himalaya region of India (Pandey et al. 2018). Several carotenoid-producing yeasts were reported in Italian alpine glaciers (Amaretti et al. 2014). Vaz et al. (2011) reported multiple yeasts isolates from Antarctica capable of carotenoid production.
Prodigiosin
Prodigiosin is a red linear tripyrrole pigment primarily reported from Serratia marcescens (Boger and Patel 1987). Prodigiosin was named after its extraction from Bacillus prodigious later given the name of S. marcescens (Gerber 1975). Prodigiosin produces only in later growth stages of bacteria (Harris et al. 2004). Biosynthesis of prodigiosin is controlled by quorum sensing (Thomson et al. 2000). Certain proposed eco-physiological roles of prodigiosin are; air diaspora of bacteria (Burger and Bennett 1985), metabolic precursor for NAD(P)H or proline (Hood et al. 1992), light energy storage (Ryazantseva et al. 1995), ion exchange (Seganish and Davis 2005), energy spilling function in S. marcescens (Haddix et al. 2008) and acts as an antimicrobial agent to provide a competitive advantage within communities (Starič et al. 2010). Borić et al. (2011) studied that prodigiosin is protective against UV radiation in Vibrio sp. DSM 14379. Prodigiosin was extracted from psychrotrophic bacterial strain Janthinobacterium lividum isolated from Alaskan soil (Schloss et al. 2010). Shen et al. (2018) reported several red pigment-producing bacterial strains inside deep ice core collected from the Yuzhufeng Glacier, Tibetan Plateau. Centurion et al. (2019) reported genes in Antarctic volcanic island sediments responsible for the biosynthesis of 3-oxoacyl-[acyl-carrying protein] reductase (K00059) enzyme that belongs to the fatty acid synthesis pathway type II associated to the production of prodigiosin.
Melanin
Melanin is dark in color (brown to dark green, or fully black) and higher molecular weight biological pigment found in hair, feather, skin, eyes, scales, and some interior membranes. Melanin is chemically a polymerized product of phenolic and/or indolic compounds (Tarangini and Mishra 2014). Melanin is further classified into three groups based on structure and color (i) pheomelanins (red or yellow), (ii) eumelanins (black-brown), and (iii) allomelanins (black to dark brown) (Table 2). Due to variations in the occurrence and structure of melanin, its biosynthesis is not from a single route (Solano 2014). Melanin synthesis has been associated in providing resistance against UV- and visible light-irradiations, confrontation the attack of cell wall enzymes, fortification against oxidizing and reducing agents, and acts as an antiviral agent to enhanced the competitive and survival abilities in environmental stresses (Castro-Sowinski et al. 2007; Solano 2014). Melanin is a strong absorber of UV radiations and provides strong protective functions to microbes that’s why extreme low temperature ecosystems are suitable habitats for microbial biosynthesis of melanin (Gessler et al. 2014).
In general, melanin pigment is insoluble both in organic and aqueous solvents, however, Kimura et al. (2015) isolated bacteria strain Lysobacter oligotrophicus from the Antarctic environment that produced water-soluble heteropolymer (Lo-melanin). Lo-melanin protects against UV radiation and scavenges ROS. Melanin is commonly present in polar dark septate hyphae, and guards hyphae against low temperatures and play a crucial role in their persistence (Robinson 2001). Chyizhanska and Beregova (2009) isolated melanin from Antarctic yeasts. An Antarctic fungus, Friedmanniomyces endolithicus produces highly melanized thick-walled cells that protected cells from UV radiation (Onofri et al. 2004). Similarly, Rosa et al. (2009) isolated melanin-producing endophytic fungal strains from the leaves of Deschampsia antarctica and about 80% were black molds. In the Antarctic rocky deserts, rock-inhabiting fungi produce melanin pigments that protect the cells from extreme cold and heat, polychromatic UV radiations, extreme pH and osmotic conditions, and provides tolerance towards potentially toxic metals (Selbmann et al. 2015). Melanin was purified from cold-tolerant and oligotrophic bacterium L. oligotrophicus isolated from the Skarvsnes region, East Antarctica (Fukuda et al. 2013). UV-B radiations induced melanogenesis in L. oligotrophicus cells, that protected the suspensions of E. coli DH5α cells from UV radiations by melanin solutions. In short, melanin biosynthesis by microorganisms can play crucial roles in low temperature environments such as to cope with low temperature, protection against UV radiations, freeze–thaw cycling and desiccation, scavenging ROS. These microbes could be a microbial model for exobiological/astrobiological studies.
Violacein
Violacein is violet or purple bisindole water-insoluble pigment and first reported from Chromobacterium violaceum from the Amazon River in Brazil (Durán et al. 1953). In nature, violacein protects cells from UV radiations (Füller et al. 2016). Several studies have reported the antibacterial, antiviral, anticancer, antiulcerogenic, antileishmanial, and enzymatic modulation activities of violacein pigments (Duran et al. 2007; Soliev et al. 2011). The maximum UV absorption capacity of violacein is λ = 260 nm, which suggests its crucial role in the protection of cells from visible and UV radiations (Marizcurrena et al. 2019). In nature, violacein is associated with biofilm production (Pantanella et al. 2007) and its production is regulated by quorum sensing thus acts as a marker of quorum sensing molecules (Burt et al. 2014). Violacein plays a vital role in protecting bacterial cells from predation (Choi et al. 2015). Apart from several other habitats, violacein has been reported from several low temperature environments. Lu et al. (2009) reported a novel psychrophilic bacterium from Xinjiang glaciers, China, capable of violacein production. Violacein producing psychrophilic Janthinobacterium svalbardensis bacterium was isolated from Glacier, Spirsbergen, Arctic region (Ambrožič et al. 2013). Kim et al. (2012) reported violacein producing psychrophilic bacterium Janthinobacterium sp. from alpine glacier cryoconite. Hakvag et al. (2009) reported violacein producing Collimonas sp. from Arctic coastal water in Trøndelag, Norway. Similarly, Nakamura et al. (2002) reported violet pigment-producing psychrotrophic bacterium from the water tank keeping rainbow and exploited its pigment for antibacterial potential. Shivaji et al. (1991) isolated the bacterium of Janthinobacterium genus that produced violacein and further Durán et al. (2007) evaluated the pigments for several therapeutic activities. Mojib et al. (2010) extracted violacein-like purple violet pigment from Janthinobacterium sp. reported from Proglacial Lake Podprudnoye, Schirmacher Oasis, Antarctica that inhibited the growth of Mycobacterium smegmatis and Mycobacterium tuberculosis. At low doses, the same pigment showed activity against methicillin-resistant and multiple drug-resistant clinical strains of Staphylococcus aureus (Huang et al. 2012).
Indigoidine
Indigoidine is a brilliant blue, water-soluble pigment synthesized by very few microorganisms (Sutthiwong et al. 2014) namely Erwinia chrysanthemi (Reverchon et al. 2002), Phaeobacter sp. (Cude et al. 2012), Streptomyces chromofuscus (Yu et al. 2013) and Vogesella indigofera (Day et al. 2017), and the biological and environmental role of indigoidine is unclear, however, it has been described that this pigment could protect against oxidative stress (Reverchon et al. 2002). Indigoidine also possesses antimicrobial activities as stated in Leisingera isolates (Gromek et al. 2016). Consequently, microbes producing indigoidine could have advantage of competition in the environment due to the antibiotic and antioxidant properties of indigoidine. Furthermore, indigoidine acts as intracellular signaling molecules related to motility (Reverchon et al. 2002; Cude et al. 2012). Additionally, indigoidine provides adaptability to microbial cells such as Vogesella sp. reported from Andean Patagonia in iron-rich environments (Day et al. 2017). Several isolates of Antarctic genus Arthrobacter were reported to produce indigoidine (Sutthiwong et al. 2014). Arthrobacter genus (family Micrococcaceae) are more common in Antarctic surroundings such as sediments and soils (Reddy et al. 2003; Dsouza et al. 2015). Bacteria belong to this genus produce different pigments including blue indigoidine (Sutthiwong et al. 2014). Kobayashi et al. (2007) reported indigoidine production from Shewanella violacea DSS12 a piezophilic and psychrophilic bacterial strain isolated from deep-sea sediments of the Ryukyu Trench. Liao et al. (2019) studied the multipartite genomes and sRNome of Arctic Pseudoalteromonas fuliginea BSW20308 and reported upregulated genes encoding indigoidine biosynthetic.
Scytonemin
Scytonemin is a secondary metabolite of small hydrophobic alkaloids having yellowish-brown color (Table 2). Cyanobacteria when get exposed to UVA-blue wavelengths, scytonemin was produced (Fleming and Castenholz 2007). Scytonemin and its methoxylated and methylated derivatives are mostly reported in the upper portion of the microbial mats; that could protect the cells from extreme environments, which makes this pigment a potential biomarker molecule for studying the presumed exobiology habitats. Exposure of cyanobacteria to UV radiation produces scytonemin along with several other protective metabolites (Sinha et al. 1998). Therefore, these molecules might have performed a crucial role in Antarctica during the initial life stages on this planet (Dillon and Castenholz 1999). The palaeolimnological investigation that was carried out in 62 east Antarctic lakes revealed that the composition of pigments was different in different depths of lakes and higher scytonemin was reported in shallow lakes. Since cyanobacteria regulate their pigment production, this difference of pigment contents could be due to the light-harvesting ability of pigments at different depths (Sabbe et al. 2004). Several Antarctic cyanobacterial genera that are capable to produce scytonemin are reported in Table 1.
Applications of microbial pigments
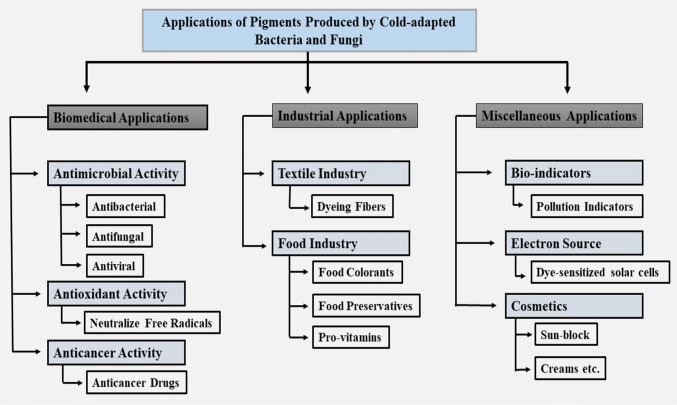
Versatile pigments produced by microorganisms are more focused during present research due to their extensively observed applications in textile industries, food industries, and biomedical purposes. Global trade of naturally derived pigments has been increased by 29% and reached 600 million USD from 2007 to 2011 (Tuli et al. 2015). This indicates that the pigments produced by microorganisms would dominate the organic market and pigment industries very soon (Ramesh et al. 2019). Such economic growth and vast applications of pigments rationalize the exploration of new sources of natural pigments. Cryospheric environments might provide the best source for the exploration of novel pigments producing microorganisms. Biotechnological applications of psychrophilic microorganisms remained a dream for decades. However, in the early 1990s researchers started focusing their possible usefulness in genetic bioengineering, food and mining industries (Gounot 1991). Currently, several of these expectations are being confirmed, since psychrophilic microorganisms and their secondary metabolites are now acting as sustainable and priceless resources for biotechnological development. Among other invaluable products, pigments produced by these psychrophilic microorganisms are the center of focus in modern research. Table 1 illustrates the microorganisms isolated from cryospheric environments producing pigments of potential applications in industries (Fig. 2). General applications of microbial pigments are as following.
Fig. 2.
Schematic representation of the pigments applications obtained from cold-adapted microbes
Industrial applications
Textile industry
Textile industries use 1.3 million tons of synthetic dyes and precursors (worth of 23 billion USD) 15% of which leak as effluents during their use (Venil et al. 2013; Chequer et al. 2013). Unluckily, enough portion of these dyes escape the conventional processes of wastewater treatment and exist as a potentially toxic environmental pollutant with adversities on public health and environment (Ogugbue and Sawidis 2011). Consequently, a great concern exists to replace the synthetic dyes in the textile industry with environmentally friendly dyes. Pigments obtained from microorganisms are environmentally friendly that is considered appropriate for the textile industry (Chadni et al. 2017). Violacein, a multipurpose pigment extracted from C. violaceum is capable of dyeing both natural and synthetic fibers and has gained increasing importance in textiles (Wan et al. 2014; Venil et al. 2016). Prodigiosin a bright red pigment from Vibrio sp. is suitable for dyeing a range of fibers including nylon, acrylics, wool, and silk (Alihosseini et al. 2008). Similarly, prodigiosin reported from Serratia sp. BTW J8 was demonstrated to color various fabric types such as chiffon, cotton, pure silk, poplene, century cotton, organdi, dupoil silk, terrycotton, polyester, and nylon (Krishna 2008). The blue pigment indigoidine produced by fungal host Rhodosporidium toruloides possesses potential applications in the dye industry (Wehrs et al. 2019). Pigment extracted from J. lividum shows a bluish-purple color tone on cotton, silk, and wool, while with vinylon and nylon it appears dark blue (Shirata et al. 2000). Likewise, the yellow pigment extracted from Thermomyces exhibited a higher dyeing affinity for silk fabric compared to other fabrics (Poorniammal et al. 2013). Similarly, red and deep blue pigments reported from Streptomyces strains NP2 and NP4 exhibited substantial variations in dyeing ability depending upon fabric materials. For instance, acrylic and polyamide fibers were vibrantly stained, however, cellulose and cotton fibers were weakly stained (Kramar et al. 2014). In the view of such extensive use of microbial pigments in the textile industry could increase their market value; therefore, new microbial sources are crucial to explore for pigment production and cryosphere inhabit broad microbial diversity capable of pigment production.
Food industry
One important goal in the food industry is to develop foods having an attractive appearance that has been achieved with synthetic dyes. Progressively, food industries are now using natural food colorants, since certain synthetic food colorants have demonstrated adverse health problems after consumption. Due to the shortage of natural food additives resources, their demand is increasing particularly in the food industry (Aberoumand 2011). This demand can be fulfilled by providing a more natural and healthy means of food colors offering a clean and safe tag declaration. Scientists have isolated food-grade pigments from bacterial strains that might provide a natural food colorant having tremendous stability, health safety profile and also act as antioxidants and preservatives (Nigam and Luke 2016). Pigment approval as food color and nutritional supplements greatly depend upon the health safety of consumers and product freshness. Thus, the use of pigmented molecules in food, cosmetics, medicine, and other medical devices is under the control of the Federal Food, Drug, and Cosmetic Act (Chapter VII, Sect. 721) and authorization should be taken before use for the specific time (https://www.fda.gov/).
Several microbial pigments like astaxanthin from Xanthophyllomyces dendrorhous, a red pigment obtained from Monascus sp., β-carotene produced by Blakeslea trispora, lycopene from Fusarium sporotrichioides and Erwinia uredovora, riboflavin reported from Ashbya gossypii, and Arpink red™ from Penicillium oxalicum were supplemented in different food items to enhance its appeal (Dharmaraj et al. 2009). For instance, carotenoids obtained from bacteria are used in feed as pro-vitamins and coloring agent in food industries (Nelis and De Leenheer 1991). Carotenoids are studied to be used as coloring agents for soft drinks, cooked sausages, and baked items (Konuray and Erginkaya 2015). It can also protect food from intense light and maintain the food quality by acting as a sunscreen (Chattopadhyay et al. 2008). Spray-dried prodigiosin from S. marcescens has been applied effectively as a coloring agent in milk, yogurt, and carbonated drinks (Namazkar and Ahmed 2013). Violacein obtained from bacterial sources has been successfully used in food industries (Dufosse 2018). Canthaxanthin is used in food items such as fish, candy, cheese, fruits, meat, snacks, beverages, wine, and beer. Currently, several pigments obtained from microorganisms are approved and used in foods for several purposes (Nigam and Luke 2016).
Biomedical applications
Antimicrobial activities
Infectious diseases are the second major reason for global human deaths and third in developed countries after non-contagious diseases (WHO 2018). In the recent few decades, new drug entry in the market has been decreased and microbial resistance against antibiotics is increasing. This increasing trend of microbial antibiotic resistance amplified the demand for novel antimicrobial agents. As an alternative to antibiotics, several microbial pigments have been successfully evaluated for antimicrobial activities. Umadevi and Krishnaveni (2013) studied pigments extracted from Micrococcus luteus that exhibited inhibitory potential against wound pathogens such as Pseudomonas sp. Klebsiella sp. and Staphylococcus sp. Carotenoids extracted from Holomonas sp. exhibited antimicrobial potential against antibiotic-resistant S. aureus, Klebsiella sp., and Pseudomonas aeruginosa, and ophthalmic S. aureus, Streptococcus pyogens, and E. coli (Ravikumar et al. 2016). Vasanthabharathi et al. (2011) studied the inhibitory potential of crude melanin obtained from Streptomyces against E. coli. Pigment extracted from Streptomyces hygroscopicus was effective against pathogens such as vancomycin and methicillin-resistant S. aureus, β-lactamase producing E. coli, P. aeruginosa and Klebsiella sp. (Berlanga et al. 2000; Selvameenal et al. 2009). Violacein obtained from C. violaceum and J. lividum showed promising antibacterial activities (Durán et al. 2007). Violacein reported from C. violaceum ATCC 12472 showed tremendous antimicrobial activity against S. aureus and inhibition of its biofilm formation (Batista and da Silva Neto 2017). Similarly, Antarctic bacterial violacein in lower concentrations showed activity against avirulent M. tuberculosis (Mojib et al. 2010). A combination of violacein with antibiotics such as kanamycin and cefadroxil minimize the inhibitory concentration of drugs against S. aureus (Subramaniam et al. 2014). Additionally, violacein extracted from J. lividum exhibited fungicidal activity against white root rot causing Rosellinia necatrix (Shirata et al. 1997). Prodigiosin obtained from Vibrio ruber DSM 14,379 exhibited bacteriostatic activity against E. coli and Bacillus subtilis (Danevčič et al. 2016). Furthermore, prodigiosin intracellularly produced by S. marcescens IBRL USM 84 inhibited the growth of tested bacteria (Ibrahim 2008). Prodigiosin revealed promising antibacterial activity against pathogens such as S. aureus, S. pyogenes, P. aeruginosa and Klebsiella pneumoniae (Nwankwo et al. 2017). Pigments extracted from endophytic fungi Monodictysc astaneae demonstrated antibacterial activity against Vibrio cholera, K. pneumoniae, and S. aureus (Visalakchi and Muthumary 2010). Suryawanshi et al. (2017) studied the strong antimicrobial activity of prodigiosin against E. coli, S. aureus, and Candida albicans. Similarly, several prodiginine compounds are reported having fungicidal activities against fungi such as Penicillium, Aspergillus, Candida, and Cryptococcus sp. (Stankovic et al. 2014).
Throughout human history, several viral outbreaks occurred and still occurring such as the Western African Ebola virus epidemic and the recent Coronavirus pandemic COVID-19 accompanied with moderate to high mortality rate. This urge that the discovery of novel antiviral drugs is critically important as many viral infections lack vaccines and effective treatments. Researches showed some initial successes using microbial pigments as antiviral agents. Violacein possesses antiviral potentials against poliovirus, simian rotavirus SA II, and herpes simplex virus (HSV) (Durán et al. 2007). In vitro effects of violacein are also evaluated against Acquired Immuno Deficiency Syndrome (AIDS) related lumphoma (Duran et al. 1996). Andrighetti-Fröhner et al. (2003) studied the antiviral activities of bacterial extracted violacein and inhibition of HSV-1 and Poliovirus-2 (PV-2) replication was observed. Similarly, an in-silico examination of prodigiosin by targeting proteins of Human Immunodeficiency Virus (HIV), Hepatitis B virus (HBV), influenza A virus (H1N1) and Hepatitis C virus (HCV) was performed (Suba et al. 2013). The outcome proposed potential antiviral activities of prodigiosin pigment against all tested viruses excluding HCV, where no binding interactions with active sites were reported. Moreover, compounds of quinone such as naphthoquinones, anthraquinones, and benzoquinones possess strong antiviral potential (Koyama 2006; Gessler et al. 2013). Several compounds obtained from Phoma species exhibited the inhibition of HIV integrase (Rai 2009). Phenazine compounds extracted from Streptomyces and Pseudomonas species have been demonstrated promising antiviral activities (Schneemann et al. 2011). Therefore, microbial pigments are potential agents to administered as a novel source of medications against pathogens.
Antioxidants activity
The rise of free radicals inside the body increases the risks of chronic diseases such as diabetes, autoimmune disorders, and cancer (Phaniendra et al. 2015). To evade this, antioxidant compounds are used which donate electrons to free radicals and neutralize them to protect cellular damage (Lobo et al. 2010). Antioxidants are obtained either from natural or synthetic sources, however, synthetic antioxidants are losing demand due to possible side effects on the human body (Ahmed et al. 2013). Therefore, microbial-based antioxidants are gaining ground in the pharmaceutical industry. Pigments from microorganisms such as carotenoid, xanthomonadin, and naphthaquinone showed antioxidant potential (Tuli et al. 2015). Carotenoids extracted from Antarctic bacterium Pedobacter exhibited solid antioxidant activity with protection against oxidative harm (Correa-Llantén et al. 2012). Osawa et al. (2010) demonstrated promising antioxidant abilities of rare C-50 carotenoids such as sarcinaxanthin monoglucoside, sarcinaxanthin diglucoside, and sarcinaxanthin, reported from a halophilic bacterium Micrococcus yunnanensis. Moreover, Shindo and Misawa. (2014) extracted rare and new carotenoids from the novel bacterial isolates and these pigments exhibited strong antioxidant activities. Similarly, flexirubin (red carotenoid), obtained from Fontibacter flavus showed antioxidant activity against 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), nitric oxide, hydroxyl radical, and inhibition of lipid peroxidation (Prabhu et al. 2013). Additionally, melanin reported from fungal isolate Streptomyces glaucescens (El-Naggar and El-Ewasy 2017) and anthraquinones obtained from endophytic Stemphylium lycopersici (Li et al. 2017) were characterized as antioxidants. Another valuable bacterial pigment violacein obtained from C. violaceum exhibited antioxidant protection in gastric ulceration (Antonisamy and Ignacimuthu 2010). Violacein can also safeguard the cellular lipid membranes from peroxidation due to hydroxyl radicals (Stafsnes and Bruheim 2013). Several studies reported melanin obtained from Pseudomonas sp, S. glaucescens NEAE-H, and Bifidobacterium infantis were found antioxidant agents (Huang et al. 2011; Tarangini and Mishra 2014; Zerrad et al. 2014). These reports recommend that pigments obtained from microorganisms could be used as antioxidant agents to prevent several chronic diseases.
Anticancer activity
Cancer is one of the most lethal diseases in human history. Till now, several anticancer medicines have been designed and are in the stage of the clinical trial. However, success limitations, adverse effects, and resistance towards treatments are the major challenges in cancer treatment (Foo and Michor 2014). Therefore, search for novel and effective anticancer agents with least or no side effects is of great interest. Several kinds of research conducted on microbial pigments as anticancer agents exhibited promising results. One such study on novel red pigment extracted from Athrobacter sp. G20 exhibited anticancer potential against the oesophageal cancer cell line (KYSE30) (Afra et al. 2017). A yellow pigment reported from Streptomyces griseoaurantiacus demonstrated strong cytotoxic activity against cervical cancer cells (HeLa) and HepG2 and resulted in a lower number of viable cells (Prashanthi et al. 2015). Carotenoid extracted from Kocuria sp. QWT-12 revealed anticancer potential against breast cancer cell lines and lung cancer cells (Rezaeeyan et al. 2017). Similarly, carotenoids from Haloferax volcanii killed 53.52% of human liver carcinoma cell lines (Sikkandar et al. 2013). Black melanin extracted from S. glaucescens exhibited significant cytotoxic activity against the HFB4 skin cancer cell line (El-Naggar et al. 2017). Dihydroxyphenylalanine melanin obtained from Streptomyces sp. MVCS6 exhibited dose–response anticancer activity against the cervical cancer cell line (Sivaperumal et al. 2015). Wang et al. (2012) studied prodigiosin obtained from Pseudoalteromonas sp. having a cytotoxic effect against U937 leukemia cells. Strong anticancer activity of prodigiosin obtained from S. marcescens is reported against human cervical cancer cells and laryngeal cancer cells (Maheswarappa et al. 2013). Cheng et al. (2017) evaluated the influence of prodigiosin reported from Vibrio sp. against human oral squamous carcinoma cells (OSCC) and found that the prodigiosin arrests their cell cycle. Recently, prodigiosin showed a reduction of the intracellular signaling pathway during the cell cycle and induced apoptosis in lung cancer cells and also showed in vivo tumoricidal activity (Chiu et al. 2018). Interestingly, a combination of natural compounds with chemotherapy drugs enhance its efficacy and reduce their toxicity. Prodigiosin when combined with paclitaxel against MCF7 breast cancer cells (Ho et al. 2009) and doxorubicin (Dox) against OSCC cell lines (Lin and Weng 2018) an efficient synergistic effect was reported. Violacein obtained from C. violaceum induced cytotoxicity and apoptosis activity in Chinese hamster lung fibroblast V79 cells (Melo et al. 2000) and leukemia cell lines (Melo et al. 2003; Ferreira et al. 2004). Additionally, violacein also increased apoptosis in colon cancer cells (Kodach et al. 2017) and human breast cancer cells (Alshatwi et al. 2016). Several anthraquinone derivatives from marine-derived fungi are capable of tumor cell inhibition (Fouillaud et al. 2016). Anthraquinone derivatives from fungus Alternaria sp. have been studied for anticancer activity against human breast cancer cell lines (Huang et al. 2011). In light of the above studies, the microbial pigment could be potential chemotherapeutic agents for cancer treatment.
Bio-indicators
Bioindicator bacteria are bacteria that can monitor environmental health and reveal the qualitative status of the environment by changing their behavior and specific physiological characteristics (Parmar et al. 2016). One such change is the production and/or alteration of certain pigments in pigmented bacteria that could be effectively used as bioindicators. For instance, violet pigmented bacteria along with Sporocytophaga and Flexibacter species were bioindicators of polluted drinking water (Schindler and Metz 1989). Indigoidine production by Vogesella indigofera is suppressed by Cr6+ in a concentration-dependent manner which is an indication of chromium concentration and toxicity in the environment (Gu and Cheung 2001). The existence of carotenoid in Lecanoraceae lichens has been confirmed to depend upon the pollution level in the atmosphere of the surrounding environment, where they reside by analyzing the carotenoid content of fungi acting as bioindicator (Ibarrondo et al. 2016). Using the gene clusters encoding prodigiosin biosynthesis in S. marcescens and violacein biosynthesis from C. violaceum demonstrate the implementation of reporter systems for the signal of biosynthetic gene expression (Domröse et al. 2017). The participation of melanin pigments in protection from environmental stress like UV radiation and potentially toxic metals is regarded as a bioindicator due to its overproduction in adverse conditions (Egorova et al. 2011). Similarly, cyanobacteria naturally present in water sources could act as excellent bioindicators for heavy metals, since in the presence of heavy metals, the carotenoids content in these cyanobacteria reduces (Wong and Teo 2014).
Miscellaneous applications
Pigment-producing microbes in cryospheric environments could act as a potential source of electrons and may be employed for developing dye-sensitized solar cells (DSSC) that would be a potential alternative of conventional photovoltaic-silicon cells based. Consequently, DSSC might signify a remarkable alternative that could somewhat solve the energy requirement at Antarctic regions. For instance, the orange-xanthophyll pigment extracted from UVC-resistant Hymenobacter sp. (Marizcurrena et al. 2017) was exploited for DSSC development (Órdenes-Aenishanslins et al. 2016; Montagni et al. 2018). Similarly, Pezzella et al. (2019) studied the eumelanin and graphene integration and observed improved electrical conductivity which shows the scope of eumelanin in bioelectronics. Bacterial pigments can be used as biodegradable ink on plastic materials. The hue and chroma values are observed in red and violet color suggestive of prodigiosin and violacein pigments isolated from S. marcescens and C. violaceum, respectively (Venil et al. 2017). Astaxanthin from radioresistant Deinococcus sp. exhibiting radio-protective and antioxidant activities can be incorporated in cosmetics including sunblock and sunscreen (Sajjad et al. 2017). The bacterial pigments prodigiosin and violacein exhibiting antioxidant and antimicrobial activities represent a new paradigm for sunscreens that utilize substances of biological origin (Suryawanshi et al. 2015). These pigments can be a potential ingredient in a range of commercial sunscreen products. Indigoidine can be used as an organic semiconductor with numerous applications in carbon dioxide capture devices, electrochemical cells, super capacitors, batteries, etc. (Yumusak et al. 2019).
Conditions optimization for pigment production
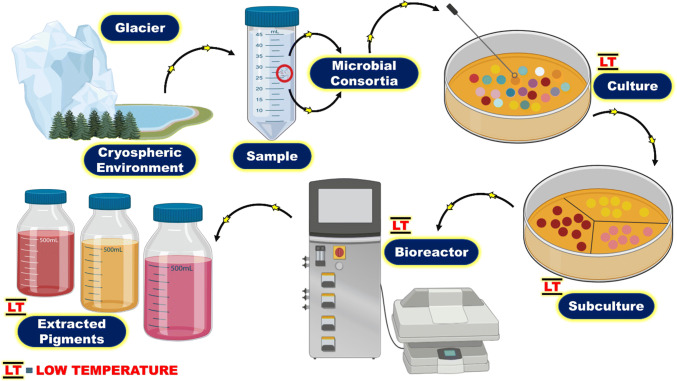
Microbial survival depends upon a wide range of nutrients and physicochemical factors that further control the production of metabolites illustrated in Fig. 3. In general, microbes could adapt three routes for metabolites production. These routes are; natural production of metabolites; metabolites production under strained environmental conditions; and stimulation of metabolites production by supplementing certain nutritional requirements along with physiological parameters (Ramesh et al. 2019). Detail description of pigment extraction has been reviewed from microalgae (Amaro et al. 2016), yeast (Yurkova et al. 2008), fungi (Rai 2009; Ahmad et al. 2015), and bacteria (Stafsnes and Bruheim 2013). Psychrophilic microorganisms produce several pigments to cope with the life-endanger challenges in the cryosphere. Cold-adapted algae are extensively studied for pigment production, however, little is known about the pigment production potential of cold-adapted bacteria and fungi due to several reasons such as their unique physiological and nutritional requirements, slow and unseen growth in the environment, and less exploration in axenic form. To obtain pigments from cold-adapted microorganisms identifying suitable substrates and evaluation of physicochemical factors are essential for better growth and higher pigment yield.
Fig. 3.
Schematic representation of pigment extraction from cold-adapted microbes at low temperature
Pigment production takes place intracellular or/and extracellular depending on pH, temperature, light, several media constituents (Buck et al. 1974), sampling sites and cultivation conditions (Stafsnes and Bruheim 2013). Under laboratory conditions, the microbial pigment production is ephemeral in nature. However, higher yield could be obtained if several factors such as medium components and environmental parameters are optimized in an understandable way (Ramesh et al. 2019). Such as a study conducted by Pandey et al. (2018) stated that additional supplementation of maltose as a source of carbon in potato dextrose broth boosted the orange pigment production by a psychrophilic strain of Penicillium sp. followed by fructose and glucose, however, supplementation of lactose inhibited this production. Pandey et al. (2018) found that extra nitrogen source could not enhance the pigment production, however, minerals salts like MgSO4 and KH2PO4 can enhance the production of pigment. Maximum pigment production was observed in Monascus sp. supplemented with maltose (Omamor et al. 2008). Pradeep and Pradeep (2013) reported maximum pigment production from Fusarium moniliforme by supplementing glucose in the medium. In the case of filamentous fungi, Carbon/Nitrogen (C/N) ratio is extremely essential in pigment production (Gmoser et al. 2017). A decrease in pigment production was reported by Cho et al. (2002) and Gunasekaran and Poorniammal (2008) during a higher C/N ratio. Similarly, high phosphate concentration and acidity caused the reduction of pigment production and a trace amount of sulfate vitiate pigment production (Reichenbach et al. 1974). Singgih et al. (2015) studied Neurospora intermedia N-1 for carotenoid production in the presence of maltose (2% w/v) as a carbon source. Moreover, minerals salts having different cations were found to improve the production of pigments (Pradeep and Pradeep 2013). Organic acid produced by Monascus ruber inhibits pigment production (Hajjaj et al. 2000). The addition of certain substrates such as wheat, rice meals, and light stimulation enhanced carotenoid production in yeasts and fungi (De Carvalho et al. 2014). ZoBell and Upham (1944) reported an increase in pigment production when bacteria were cultured in sea-water supplemented with neopeptone, bacto-tryptone, and beef extract at 4 °C. Ghosh et al. (2007) reported that methanol acted as a sole carbon source induced the production of pink pigment in Acinetobacter wofii. The yellow–green pigment was produced by Pseudomonas fluorescens supplemented with succinate as a sole carbon source, while this production was stopped with the addition of malic and citric acids as substrate (Margalith 1992). Moreover, the influence of light and dark on pigment production was studied and Myxococcus xanthus, Mycobacterium marinum, Rhodotorula glutinis, and Dacryopinax spathularia produced carotenoids during the presence of light. S. marcescens produced red pigment on solid peptone-glycerol agar plates, and unable to produce pigment in liquid medium except when supplemented with silica gel (Yamashita et al. 2001). Chen et al. (2013) reported that the prodigiosin production was enhanced when peptone and starch were supplemented as a source of carbon and calcium alginate beads were added as a porous carrier. Similarly, Lu et al. (2009) reported sucrose and casein as best carbon and nitrogen sources, respectively, for violacein production by J. lividum XT1 bacteria reported from the glacier in Xinjiang China.
To obtained higher pigment yield, pH and temperature optimization are essential as they affect the metabolic growth and physical activities of microorganisms. Being cold-adapted organisms, the temperature is a vital factor to be optimized for pigment production. Pandey et al. (2018) reported maximum pigment production at low temperature (15 °C) and acidic condition (pH 5) by Penicillium sp. Chattopadhyay et al. (1997) studied Antarctic psychrotrophic bacterium M. roseus that produced higher carotenoid pigment at 5 °C compared to 25 °C. Similarly, Lu et al. (2009) reported an optimal temperature of 15 °C for violacein production by psychrotrophic bacteria. M. tuberculosis produced carotenoid pigments under acidic stress (pH 5–6) (Saviola 2014), and different pigment production was reported in other Mycobacterium species (Robledo et al. 2011). Overproduction of pigment at lower temperatures shows the phenomenon of ecological resilience among cold-adapted microorganisms that combat the deadly environmental conditions. Similarly, enhancement of pigment production below optimum temperature range could be an adapting reaction that compensates the downregulation of metabolic processes at low temperatures (Gmoser et al. 2017). Several genes are responsible for the biosynthesis of pigments in microorganisms. To enhance the pigment production mutagens such as UV light, 1-methyl-3-nitro-1-nitrosoguanidine, and ethyl methanesulfonate were used in Haematococcus pluvialis and microwave in S. marcescens (Nigam and Luke 2016), which shows these mutagens could enhance the pigment yield when used precisely. In addition, alteration in genes (knockout or promotion of genes) and mutagenesis practices can increase the pigment production (Venil et al. 2014). Different substrates such as tryptophan, phenylalanine, tyrosine, and several physicochemical parameters can efficiently stimulate pigment production by microorganisms.
Essential pigments from fungal strains can be obtained under optimum growth conditions by solid, semi-solid, and submerged fermentation (Vendruscolo et al. 2010; Akilandeswari and Pradeep 2016) using a diverse range of sustainable substrates (Gupta and Aggarwal. 2014). However, parameters such as pH, temperature, agitation, aeration, and culture medium directly influence the production of pigment and should be kept at an optimized level (Medentsev et al. 2005). Moreover, several solvents such as acetone, chloroform, cyclohexane, acetonitrile, ethanol, dichloromethane, pyridine, hexane, and water are used to extract fungal pigments. The solvent choice is crucial for pigment extraction and depends upon the polarity of the studied molecules. After extraction and purification of pigments, it should be handled carefully and must be kept under optimum temperature, and pH to preserve its original colorimetric characteristics. Pigment storage in dry powder form is more preferable because of its higher stability and low water activity (De Carvalho et al. 2014).
Challenges in pigments obtained from psychrophiles
Antarctic region and the adjacent Southern Ocean is the rich source of psychrophilic microorganisms capable of pigment production. However, in 2014 the bioprospection is defined during XXXVII Antarctic Treaty Consultative Meeting held in Brasilia. According to this definition, bioprospection is “any activity of search, identification, description, collection, survey, monitoring, cultivation, replication, or any other scientific investigation processes, performed on indigenous biological species with the initial intention to consider potential industrial or commercial derived products or applications, notably through the development of patentable material or process.” According to this definition, research curiosity on the biological resources of Antarctica is growing for commercial use. However, several reports highlighted that due to the lack of clear governing and ownership rules for these resources causes appropriation by those who discover it and subsequently develop patent (UNU-UNEP 2009; Jabour 2010; UNEP 2012; Puig-Marcó 2014). In 2008, Antarctic Treaty Committee launched an online database for bioprospecting in Antarctica that provides up-to-date information about bioprospecting in Antarctica. This database also provides information about commercialized products obtained from biological samples indigenous to Antarctica. The records in the database contained many patents related to microbial metabolites having potential biotechnological applications (UNEP Report 2012). The increase in patents shows that Antarctic microorganisms are a precious source of marvelous potential profits. However, one of the leading challenges is the assurance of sustainable exploitation of indigenous biological resources of Antarctica which is yet to decide that how positive benefits of bioprospecting could achieve without inducing major damage to the indigenous environment. Since the uncontrolled prospecting actions that would include logistical labors to reach the biological resources and their definite outcomes could make the process unsustainable and compromise the indigenous microbial diversity (Hughes et al. 2015). In addition, the commercial based research activities and their secrecy could compromise the main pillar of the Antarctic Treaty System (Article III), viz., the unrestricted cooperation and free exchange of information among all parties (Yarzábal et al. 2016). Furthermore, the emerging profitable interest in biological resources raised several key policies, moral and ethical queries: Who would be the owner of these biological resources? How should they be exploited and used? And whom should be benefited and to what extent? Although, few of these questions are included in the 2014 SCAR1-sponsored Antarctic and Southern Ocean Horizon Scan (Kennicutt et al. 2014, 2015). However, several current uncertainty and absence of policies for these biological resources should be addressed to the territorial and commercial sensitivities to agree upon common agreement to protect the local environment. For instance, the Nagoya protocol that entered into force in October 2014 by over 70 countries under international law for the fair and equitable sharing of benefits derived from the utilization of genetic resources (Smith et al. 2017). Like Nagoya protocol, countries should be placed under an obligation to establish legislative, administrative and/or political measures regarding the access to common cold reservoirs such as Arctic and Antarctic. This will aim to provide equal chances of obtaining common cold resources for commercial or academic development by a country or organization. Moreover, to commercialize any kind of microbial pigments, huge investment along with research work for characterization, optimization processes, possible toxicological testing, regulatory approval, and penchant by the consumers are greatly essential (Ahmad et al. 2015; Dufossé et al. 2016; Carvalho et al. 2016). Similarly, optimized fermentation conditions, design and type of bioreactor to achieve desired productivity of microbial pigments (Venil et al. 2014) along with the downstream processing of product purification are quite essential to be considered especially for microorganisms from unusual environments such as cryosphere. Therefore, widespread research is essential to convey the microbial pigments from laboratories to markets, since its demand is higher than supply.
Conclusion
The demand for natural pigments obtained from microorganisms is now increasing due to the adverse effects causing by synthetic dyes. Therefore, new potential sources are crucial to explore for pigment-producing microorganisms. One such environment is the cryosphere which inhabits microorganisms that produce natural pigments as a protective shield against life-endangered ecological stresses. Exploration of these microorganisms would certainly provide promising sources of novel pigmented molecules having wider biotechnological applications. These unexplored microbes especially novel species could be the potential source for pigments that could be used to develop novel drug compounds as well as in the textile and food industries. Efforts should be done to produce cost-effective production of pigments from these microbes by optimization, and strain improvement through genetic engineering to get rid of toxic synthetic dyes. Sustainable exploitation of these biological resources from the cryosphere should be focused to prevent environmental disruption. Several cryospheric environments such as Antarctica should be considered a common legacy for mankind and the international community should declare a clear ethical and moral policy for certain questions including possession of these resources. It is obvious that due to unique qualities, the applications of these pigments obtained from cold-adapted microorganisms would be broader and will start many newer domains in biotechnology.
Acknowledgements
This study was supported by the second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0605), the Strategic Priority Research Programme of the Chinese Academy of Sciences, Pan-Third Pole Environment Study for a Green Silk Road (Pan-TPE) (XDA20040501), the National Natural Science Foundation of China (Grant 41630754). Wasim Sajjad is supported by a PIFI Fellowship from the Chinese Academy of Sciences (2020PC0052).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. WJDFS. 2011;6(1):71–78. [Google Scholar]
- Afra S, Makhdoumi A, Matin MM, Feizy J. A novel red pigment from marine Arthrobacter sp. G20 with specific anticancer activity. J Appl Microbiol. 2017;123(5):1228–1236. doi: 10.1111/jam.13576. [DOI] [PubMed] [Google Scholar]
- Agogué H, Joux F, Obernosterer I, Lebaron P. Resistance of marine bacterioneuston to solar radiation. Appl Environ Microbiol. 2005;71:5282–5289. doi: 10.1128/AEM.71.9.5282-5289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad WA, Venil CK, Aruldass CA. Production of violacein by Chromobacterium violaceum grown in liquid pineapple waste: current scenario. In: Liong MT, editor. Beneficial microorganisms in agriculture, aquaculture and other areas, microbiology monographs, 29. Berlin: Springer; 2015. pp. 45–58. [Google Scholar]
- Ahmed N, Singh J, Mudasir MA, Kour H, Gupta P, Chauhan H. Naturally occurring preservatives in food and their role in food preservation. Int J Pharm Bio Sci. 2013;4:22–30. [Google Scholar]
- Akilandeswari P, Pradeep BV. Exploration of industrially important pigments from soil fungi. Appl Microbiol Biotechnol. 2016;100:1631–1643. doi: 10.1007/s00253-015-7231-8. [DOI] [PubMed] [Google Scholar]
- Alihosseini F, Ju KS, Lango J, Hammock BD, Sun G. Antibacterial colorants: characterization of prodiginines and their applications on textile materials. Biotechnol Prog. 2008;24(3):742–747. doi: 10.1021/bp070481r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alija AJ, Sommerburg O, Langhans CD, Siems W, Eckl PM. Cyto- and genotoxic potential of beta-carotene and cleavage products under oxidative stress. BioFactors. 2005;24:159–163. doi: 10.1002/biof.5520240119. [DOI] [PubMed] [Google Scholar]
- Alshatwi AA, Subash-Babu P, Antonisamy P. Violacein induces apoptosis in human breast cancer cells through up regulation of BAX, p53 and down regulation of MDM2. Exp Toxicol Pathol. 2016;68:89–97. doi: 10.1016/j.etp.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Amaretti A, Simone M, Quartieri A, et al. Isolation of Carotenoid-producing Yeasts from an Alpine Glacier. Chem Eng Trans. 2014;38:217–222. [Google Scholar]
- Amaro HM, Sousa-Pinto I, Malcata FX, Guedes AC. Microalgae as a source of pigments extraction and purification methods. In: Leo MLN, editor. Marine microorganisms extraction and analysis of bioactive compounds. Boca Raton: CRC Press; 2016. pp. 99–128. [Google Scholar]
- Ambrožič Avguštin J, Žgur Bertok D, Kostanjšek R, Avguštin G. Isolation and characterization of a novel violacein-like pigment producing psychrotrophic bacterial species Janthinobacterium svalbardensis sp. nov. Anton Van Leeuwen. 2013;103:763–769. doi: 10.1007/s10482-012-9858-0. [DOI] [PubMed] [Google Scholar]
- Andrighetti-Fröhner CR, Antonio RV, Creczynski-Pasa TB, et al. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. The Memórias do Instituto Oswaldo Cruz. 2003;98:843–848. doi: 10.1590/S0074-02762003000600023. [DOI] [PubMed] [Google Scholar]
- Anesio AM, Laybourn-Parry J. Glaciers and ice sheets as a biome. Trends Ecol Evol. 2012;27:219–225. doi: 10.1016/j.tree.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Anesio AM, Lutz S, Chrismas NA, Benning LG. The microbiome of glaciers and ice sheets. NPJ Biofilms Microb. 2017;3(1):1–11. doi: 10.1038/s41522-017-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonisamy P, Ignacimuthu S. Immunomodulatory, analgesic and antipyretic effects of violacein isolated from Chromobacterium violaceum. Phytomedicine. 2010;17:300–304. doi: 10.1016/j.phymed.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Arcangeli C, Cannistraro S. In situ Raman microspectroscopic identification and localization of carotenoids: approach to monitoring of UV-B irradiation stress on Antarctic fungus. Biopolymers. 2000;57:179–186. doi: 10.1002/(SICI)1097-0282(2000)57:3<179::AID-BIP6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Arcangeli C, Zucconi L, Onofri S, Cannistraro S. Fluorescence study on whole Antarctic fungal spores under enhanced UV irradiation. J Photochem Photobiol B. 1997;39(3):258–264. doi: 10.1016/S1011-1344(97)00019-5. [DOI] [Google Scholar]
- Arcangeli C, Yu W, Cannistraro S, Gratton E. Two-photon autofluorescence microscopy and spectroscopy of an Antarctic fungus: a new approach for studying the effects of UV-B irradiation. Biospectroscopy. 2000;57:218–225. doi: 10.1002/1097-0282(2000)57:4<218::AID-BIP3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bakermans C, Tollaksen SL, Giometti CS, et al. Proteomic analysis of Psychrobacter cryohalolentis K5 during growth at subzero temperatures. Extremophiles. 2007;11:343–354. doi: 10.1007/s00792-006-0042-1. [DOI] [PubMed] [Google Scholar]
- Bar Dolev M, Bernheim R, Guo S, et al. Putting life on ice: bacteria that bind to frozen water. J R Soc Interface. 2016 doi: 10.1098/rsif.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona S, Yuivar Y, Socias G, et al. Identification and characterization of yeasts isolated from sedimentary rocks of Union Glacier at the Antarctica. Extremophiles. 2016;20:479–491. doi: 10.1007/s00792-016-0838-6. [DOI] [PubMed] [Google Scholar]
- Barauna RA, Freitas DY, Pinheiro JC, et al. A proteomic perspective on the bacterial adaptation to cold: integrating OMICs data of the psychrotrophic bacterium Exiguobacterium antarcticum B7. Proteomes. 2017 doi: 10.3390/proteomes5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista JH, da Silva Neto JF. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol. 2017;8:2213. doi: 10.3389/fmicb.2017.02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Hapak M, Troxtel E, Hoerter J, Eisenstark A. RpoS dependent overexpression of carotenoids from Erwinia herbicola in OXYR-deficient Escherichia coli. Biochem Bioph Res Co. 1997;239:305–309. doi: 10.1006/bbrc.1997.7469. [DOI] [PubMed] [Google Scholar]
- Benforte FC, Colonnella MA, Ricardi MM, et al. Novel role of the LPS core glycosyltransferase WapH for cold adaptation in the Antarctic bacterium Pseudomonas extremaustralis. PLoS ONE. 2018;13(2):e0192559. doi: 10.1371/journal.pone.0192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga M, Ruiz N, Hernandez-Borrell J, et al. Role of the outer membrane in the accumulation of quinolones by Serratia marcescens. Can J Microbiol. 2000;46:716–722. doi: 10.1139/w00-052. [DOI] [PubMed] [Google Scholar]
- Boger DL, Patel M. Total synthesis of prodigiosin. Tetrahedron Lett. 1987;28:2499–2502. doi: 10.1016/S0040-4039(00)95451-0. [DOI] [Google Scholar]
- Borić M, Danevčič T, Stopar D. Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb Ecol. 2011;62(3):528. doi: 10.1007/s00248-011-9857-0. [DOI] [PubMed] [Google Scholar]
- Bowman JP, Brown MV, Nichols DS. Biodiversity and ecophysiology of bacteria associated with Antarctic sea ice. Antarct Sci. 1997;9(2):134–142. doi: 10.1017/S0954102097000175. [DOI] [Google Scholar]
- Buck JD. Effects of medium composition on the recovery of bacteria from sea water. J Exp Mar Biol Ecol. 1974;15:25–34. doi: 10.1016/0022-0981(74)90060-4. [DOI] [Google Scholar]
- Burger SR, Bennett JW. Droplet enrichment factors of pigmented and nonpigmented Serratia marcescens: possible selective function for prodigiosin. Appl Environ Microbiol. 1985;50:487–490. doi: 10.1128/AEM.50.2.487-490.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Ojo-Fakunle VT, Woertman J, Veldhuizen EJ. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoSOne. 2014;9(4):e93414. doi: 10.1371/journal.pone.0093414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MJ, Day AW. Fungal melanins. Can J Microbiol. 1998;44:1115–1136. doi: 10.1139/w98-119. [DOI] [Google Scholar]
- Caruso C, Rizzo C, Mangano S, et al. Production and biotechnological potential of extracellular polymeric substances from sponge-associated Antarctic bacteria. Appl Environ Microbiol. 2018 doi: 10.1128/AEM.01624-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho JC, Bicas JL, Fernández DER, et al. Natural colorants from microorganisms. In: Bicas JL, Maróstica MR, Pastore GM, et al., editors. Biotechnological production of natural ingredients for food industry. 1. Sharjah: Bentham Science Publishers; 2016. pp. 288–321. [Google Scholar]
- Castro-Sowinski S, Matan O, Bonafede P, Okon Y. A thioredoxin of Sinorhizobium meliloti CE52G is required for melanin production and symbiotic nitrogen fixation. Mol Plant Microbe In. 2007;20:986–993. doi: 10.1094/MPMI-20-8-0986. [DOI] [PubMed] [Google Scholar]
- Cavicchioli R, Charlton T, Ertan H, et al. Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol. 2011;4(4):449–460. doi: 10.1111/j.1751-7915.2011.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion VB, Delforno TP, Lacerda-Júnior GV, et al. Unveiling resistome profiles in the sediments of an Antarctic volcanic island. Environ Pollut. 2019;255:113240. doi: 10.1016/j.envpol.2019.113240. [DOI] [PubMed] [Google Scholar]
- Chadni Z, Rahaman MH, Jerin I, et al. Extraction and optimisation of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology. 2017;8:48–57. doi: 10.1080/21501203.2017.1302013. [DOI] [Google Scholar]
- Chattopadhyay M, Jagannadham M. Maintenance of membrane fluidity in Antarctic bacteria. Polar Biol. 2001;24(5):386–388. doi: 10.1007/s003000100232. [DOI] [Google Scholar]
- Chattopadhyay MK, Jagannadham MV, Vairamani M, Shivaji S. Carotenoid pigments of an antarctic psychrotrophic bacteriumMicrococcus roseus: temperature dependent biosynthesis, structure, and interaction with synthetic membranes. Biochem Biophys Res Commun. 1997;239(1):85–90. doi: 10.1006/bbrc.1997.7433. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P, Chatterjee S, Sen SK. Biotechnological potential of natural food grade biocolorants. Afr J Biotechnol. 2008;7(17):2972–2985. [Google Scholar]
- Chen WC, Yu WJ, Chang CC, et al. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem Eng J. 2013;78:93–100. doi: 10.1016/j.bej.2013.02.001. [DOI] [Google Scholar]
- Cheng MF, Lin CS, Chen YH, et al. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar Drugs. 2017;15:224. doi: 10.3390/md15070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chequer FD, de Oliveira GAR, Ferraz ERA, et al. Textile dyes: dyeing process and environmental impact. Eco-friendly Textile Dye Finish. 2013;6:151–176. [Google Scholar]
- Chiu WJ, Lin SR, Chen YH, et al. Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in Doxorubin-sensitive and resistant lung cancer. J Clin Med. 2018;7:321. doi: 10.3390/jcm7100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YJ, Park JP, Hwang HJ, et al. Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett Appl Microbiol. 2002;35:195–202. doi: 10.1046/j.1472-765X.2002.01168.x. [DOI] [PubMed] [Google Scholar]
- Choi SY, Yoon KH, Lee JI, Mitchell RJ. Violacein: properties and production of a versatile bacterial pigment. Biomed Res Int. 2015 doi: 10.1155/2015/465056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyizhanska N, Beregova T (2009) Effect of melanin isolated from Antarctic yeasts on preservation of pig livestock after ablactation. Укpaїнcький aнтapктичний жypнaл.
- Collins T, Gerday C. Enzyme catalysis in psychrophiles. In: Margesin R, editor. Psychrophiles: from biodiversity to biotechnology. 2. Cham: Springer; 2017. pp. 209–235. [Google Scholar]
- Collins T, Margesin R. Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Appl Microbiol Biotechnol. 2019;103(7):2857–2871. doi: 10.1007/s00253-019-09659-5. [DOI] [PubMed] [Google Scholar]
- Contreras G, Barahona S, Sepúlveda D, et al. Identification and analysis of metabolite production with biotechnological potential in Xanthophyllomyces dendrorhous isolates. World J Microbiol Biotechnol. 2015;31(3):517–526. doi: 10.1007/s11274-015-1808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Llantén DN, Amenabar MJ, Blamey JM. Antioxidant capacity of novel pigments from an Antarctic bacterium. J Microbiol. 2012;50:374–379. doi: 10.1007/s12275-012-2029-1. [DOI] [PubMed] [Google Scholar]
- Corsaro MM, Casillo A, Parrilli E, Tutino ML. Molecular structure of lipopolysaccharides of cold-adapted bacteria. In: Margesin R, editor. Psychrophiles: from biodiversity to biotechnology. 2. Cham: Springer; 2017. pp. 285–303. [Google Scholar]
- Cristea D, Vilarem G. Improving light fastness of natural dyes on cotton yarn. Dyes Pigm. 2006;70(3):238–245. doi: 10.1016/j.dyepig.2005.03.006. [DOI] [Google Scholar]
- Cude WN, Mooney J, Tavanaei AA, et al. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl Environ Microbiol. 2012;78(14):4771–4780. doi: 10.1128/AEM.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico S, Gerday C, Feller G. Structural determinants of cold adaptation and stability in a large protein. J Biol Chem. 2001;276(28):25791–25796. doi: 10.1074/jbc.M102741200. [DOI] [PubMed] [Google Scholar]
- D’Amico S, Collins T, Marx JC, et al. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006;7(4):385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danevčič T, Borić Vezjak M, Tabor M, et al. Prodigiosin induces autolysis in actively grown Bacillus subtilis cells. Front Microbiol. 2016;7:27. doi: 10.3389/fmicb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PA, Villalba MS, Herrero OM, et al. Formation of indigoidine derived-pigments contributes to the adaptation of Vogesella sp. strain EB to cold aquatic iron-oxidizing environments. Antonie Van Leeuwenhoek. 2017;110(3):415–428. doi: 10.1007/s10482-016-0814-2. [DOI] [PubMed] [Google Scholar]
- De Carvalho JC, Cardoso LC, Ghiggi V, Woiciechowski AL, de Souza Vandenberghe LP, Soccol CR. Microbial Pigments. In: Brar S, Dhillon G, Soccol C, editors. Biotransformation of Waste Biomass into High Value Biochemicals. New York, NY, USA: Springer; 2014. pp. 73–98. [Google Scholar]
- De Maayer P, Anderson D, Cary C, Cowan DA. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15(5):508–517. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes GC, Godinho VM, Porto BA, et al. Antarctomyces pellizariae sp. nov., a new, endemic, blue, snow resident psychrophilic ascomycete fungus from Antarctica. Extremophiles. 2017;21:259–269. doi: 10.1007/s00792-016-0895-x. [DOI] [PubMed] [Google Scholar]
- Deming JW, Young JN. The role of exopolysaccharides in microbial adaptation to cold habitats. In: Margesin R, editor. Psychrophiles: from biodiversity to biotechnology. 2. Cham: Springer; 2017. pp. 259–284. [Google Scholar]
- Dharmaraj S, Ashokkumar B, Dhevendaran K. Food-gradepigments from Streptomyces sp. isolated from the marine sponge Callyspongia diffusa. Food Res Int. 2009;42:487–492. doi: 10.1016/j.foodres.2009.02.006. [DOI] [Google Scholar]
- Dieser M, Greenwood M, Foreman CM. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct Antarct Alp Res. 2010;42(4):396–405. doi: 10.1657/1938-4246-42.4.396. [DOI] [Google Scholar]
- Dillon JG, Castenholz RW. Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: implications for early photosynthetic life. J Phycol. 1999;35(4):673–681. doi: 10.1046/j.1529-8817.1999.3540673.x. [DOI] [Google Scholar]
- Dimitrova S, Pavlova K, Lukanov L, Zagorchev P. Synthesis of coenzyme q10 and β-carotene by yeasts isolated from Antarctic soil and lichen in response to ultraviolet and visible radiations. Appl Biochem Biotechnol. 2010;162:795–804. doi: 10.1007/s12010-009-8845-z. [DOI] [PubMed] [Google Scholar]
- Dimitrova S, Pavlova K, Lukanov L, et al. Production of metabolites with antioxidant and emulsifying properties by Antarctic strain Sporobolomyces salmonicolor AL1. Appl Biochem Biotechnol. 2013;169:301–311. doi: 10.1007/s12010-012-9983-2. [DOI] [PubMed] [Google Scholar]
- Domröse A, Weihmann R, Thies S, et al. Rapid generation of recombinant Pseudomonas putida secondary metabolite producers using yTREX. Synth Syst Biotechnol. 2017;2(4):310–319. doi: 10.1016/j.synbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downham A, Collins P. Colouring our foods in the last and next millennium. Int J Food Sci Tech. 2000;35:5–22. doi: 10.1046/j.1365-2621.2000.00373.x. [DOI] [Google Scholar]
- Dsouza M, Taylor MW, Turner SJ, Aislabie J. Genomic and phenotypic insights into the ecology of Arthrobacter from Antarctic soils. BMC Genomics. 2015;16(1):36. doi: 10.1186/s12864-015-1220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufossé L. Current and potential natural pigments from microorganisms (bacteria, yeasts, fungi, microalgae) In: Carle R, Schweiggert RM, editors. Handbook on natural pigments in food and beverages. Cambridge: Elsevier Ltd; 2016. pp. 337–354. [Google Scholar]
- Dufosse L. Natural and artificial flavoring agents and food dyes. USA: Academic Press; 2018. Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries; pp. 113–132. [Google Scholar]
- Durán N, Erazo S, Campos V. Bacterial chemistry-II: antimicrobial photoproduct from pigment of Chromobacterium violaceum. An Acad Bras Cienc. 1953;55:231–234. [Google Scholar]
- Duran N, Melo PS, Haun M (1996) In Vitro evaluation of violacein on AIDS-related lumphoma and human tumor cell lines. In: Proceedings of the 25th Annual Meetings of the Brazilian Society of Biochemistry and Molecular Biology, Sociedade Brasi-leira de Bioquimica e Biologia Molecular (SBBq), Caxambu, Brazil
- Duran N, Justo GZ, Ferreira CV, et al. Violacein: properties and biological activities. Biotechnol Appl Biochem. 2007;48:127–133. doi: 10.1042/BA20070115. [DOI] [PubMed] [Google Scholar]
- Egorova AS, Gessler NN, Belozerskaya TA. Melanin pigments in the fungus Paecilomyces lilacinus (thom) samson. Dokl Biochem Biophys. 2011;437(1):84. doi: 10.1134/S1607672911020086. [DOI] [PubMed] [Google Scholar]
- El-Naggar NE, El-Ewasy SM. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep. 2017;14:42129. doi: 10.1038/srep42129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert M, Deming JW. Sea ice microorganisms: environmental constraints and extracellular responses. Biology. 2013;2(2):603–628. doi: 10.3390/biology2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feofilova EP, Tereshina VM, Gorova IB. Changes in carbohydrate composition of fungi during adaptation to thermostress. Microbiology. 1994;63:442–445. [Google Scholar]
- Ferreira CV, Bos CL, Versteeg HH, et al. Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104:14464–14591. doi: 10.1182/blood-2004-02-0594. [DOI] [PubMed] [Google Scholar]
- Fleming ED, Castenholz RW. Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ Microbiol. 2007;9(6):1448–1455. doi: 10.1111/j.1462-2920.2007.01261.x. [DOI] [PubMed] [Google Scholar]
- Foght J, Aislabie J, Turner S, et al. Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb Ecol. 2004;47(4):329–340. doi: 10.1007/s00248-003-1036-5. [DOI] [PubMed] [Google Scholar]
- Fong NJC, Burgess ML, Barrow KD, Glenn DR. Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol. 2001;56:750–756. doi: 10.1007/s002530100739. [DOI] [PubMed] [Google Scholar]
- Foo J, Michor F. Evolution of acquired resistance to anti-cancer therapy. J Theor Biol. 2014;355:10–20. doi: 10.1016/j.jtbi.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillaud M, Venkatachalam M, Girard-Valenciennes E, et al. Anthraquinones and derivatives from marine-derived fungi: structural diversity and selected biological activities. Mar Drugs. 2016;14:64. doi: 10.3390/md14040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Schmidt F, Klockgether J, et al. Functional genomics of the initial phase of cold adaptation of Pseudomonas putida KT2440. FEMS Microbiol Lett. 2011;318:47–54. doi: 10.1111/j.1574-6968.2011.02237.x. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43(3):228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Fukuda W, Kimura T, Araki S, et al. Lysobacteroligotrophicus sp. Nov., isolated from an Antarctic freshwater lake in Antarctica. Int J Syst Evol Microbiol. 2013;63(9):3313–3318. doi: 10.1099/ijs.0.051805-0. [DOI] [PubMed] [Google Scholar]
- Füller JJ, Röpke R, Krausze J, et al. Biosynthesis of violacein, structure and function of l-Tryptophan oxidase VioA from Chromobacterium violaceum. J Biol Chem. 2016;291(38):20068–20084. doi: 10.1074/jbc.M116.741561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber NN. Prodigiosin-like pigments. CRC Crit Rev Microbiol. 1975;3:469–485. doi: 10.3109/10408417509108758. [DOI] [PubMed] [Google Scholar]
- Gessler NN, Egorova AS, Belozerskaya TA. Fungal anthraquinones. Appl Biochem Microbiol. 2013;49:85–99. doi: 10.1134/S000368381302004X. [DOI] [Google Scholar]
- Gessler NN, Egorova AS, Belozerskaya TA. Melanin pigments of fungi under extreme environmental conditions. Appl Biochem Micro. 2014;50(2):105–113. doi: 10.1134/S0003683814020094. [DOI] [PubMed] [Google Scholar]
- Ghobakhlou AF, Johnston A, Harris L, et al. Microarray transcriptional profiling of Arctic Mesorhizobium strain N33 at low temperature provides insights into cold adaption strategies. BMC Genomics. 2015;16(1):383. doi: 10.1186/s12864015-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Goyal A, Jain RK. Study of methanol-induced phenotypic changes in a novel strain of Acinetobacter lwoffii. Arch Microbiol. 2007;188:533–539. doi: 10.1007/s00203-007-0268-z. [DOI] [PubMed] [Google Scholar]
- Gmoser R, Ferreira JA, Lennartsson PR, Taherzadeh MJ. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol Biotechnol. 2017;4(1):4. doi: 10.1186/s40694-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Toril E, Amils R, Delmas RJ, et al. Diversity of bacteria producing pigmented colonies in aerosol, snow and soil samples from remote glacial areas (Antarctica, Alps and Andes) Biogeosci Discuss. 2008;5:1607–1630. doi: 10.5194/bgd-5-1607-2008. [DOI] [Google Scholar]
- Goordial J, Raymond-Bouchard I, Zolotarov Y, et al. Cold adaptive traits revealed by comparative genomic analysis of the eurypsychrophile Rhodococcus sp. JG3 isolated from high elevation McMurdo Dry Valley permafrost, Antartica. FEMS Microbiol Ecol. 2016 doi: 10.1093/femsec/fiv154. [DOI] [PubMed] [Google Scholar]
- Gorbushina A. Methodologies and techniques for detecting extraterrestrial (microbial) life microcolonial fungi: survival potential of terrestrial vegetative structures. Astrobiology. 2003;3:543–554. doi: 10.1089/153110703322610636. [DOI] [PubMed] [Google Scholar]
- Gounot AM. Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991;71:386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Gromek SM, Suria AM, Fullmer MS, et al. Leisingera sp JC1, a bacterial isolate from Hawaiian bobtail squid eggs, produces indigoidine and differentially inhibits vibrios. Front Microbiol. 2016;7:1342. doi: 10.3389/fmicb.2016.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JD, Cheung KH. Phenotypic expression of Vogesella indigofera upon exposure to hexavalent chromium, Cr6+ World J Microb Biot. 2001;17:475–480. doi: 10.1023/A:1011966725978. [DOI] [Google Scholar]
- Gunasekaran S, Poorniammal R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr J Biotechnol. 2008;7:1894–1898. doi: 10.5897/AJB2008.000-5037. [DOI] [Google Scholar]
- Gupta S, Aggarwal S. Novel bio-colorants for textile application from fungi. J Textile Assoc. 2014;74:282–287. [Google Scholar]
- Haddix PL, Jones S, Patel P, et al. Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. J Bacteriol. 2008;190(22):7453–7463. doi: 10.1128/JB.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjaj H, Blanc P, Groussac E, et al. Kinetic analysis of red pigment and citrinin by Monascus rubber as a function of organic acid accumulation. Enzym Microb Technol. 2000;27:619–625. doi: 10.1016/S0141-0229(00)00260-X. [DOI] [PubMed] [Google Scholar]
- Hakvag S, Fjaervik E, Klinkenberg G, et al. Violacein-producing Collimonas sp. from the sea surface microlayer of costal waters in Trondelag, Norway. Mar Drugs. 2009;7:576–588. doi: 10.3390/md7040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Williamson NR, Slater H, et al. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species and strain-dependent genome context variation. Microbiology. 2004;150:3547–3560. doi: 10.1099/mic.0.27222-0. [DOI] [PubMed] [Google Scholar]
- Hasan N, Hasan F, Nadeem S, et al. Community analysis and characterization of fungi from Batura glacier, Karakoram mountain range, Pakistan. Appl Ecol Environ Res. 2018;16(5):5323–5341. doi: 10.15666/aeer/1605_53235341. [DOI] [Google Scholar]
- Hassan N, Anesio AM, Rafiq M, et al. Cell membrane fatty acid and pigment composition of the psychrotolerant cyanobacterium Nodularia spumigena CHS1 isolated from Hopar glacier, Pakistan. Extremophiles. 2020;24(1):135–145. doi: 10.1007/s00792-019-01141-4. [DOI] [PubMed] [Google Scholar]
- He J, Yang Z, Hu B, et al. Correlation of polyunsaturated fatty acids with the cold adaptation of Rhodotorula glutinis. Yeast. 2015;32(11):683–690. doi: 10.1002/yea.3095. [DOI] [PubMed] [Google Scholar]
- Heider SA, Peters-Wendisch P, Wendisch VF. Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol. 2012;12(1):198. doi: 10.1186/1471-2180-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TF, Peng YT, Chuang SM, et al. Prodigiosin down-regulates survivin to facilitate paclitaxel sensitization in human breast carcinoma cell lines. Toxicol Appl Pharm. 2009;235:253–260. doi: 10.1016/j.taap.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hood DW, Heidstra R, Swoboda UK, Hodgson DA. Molecular genetic analysis of proline and tryptophan biosynthesis in Streptomyces coelicolor A3(2): interaction between primary and secondary metabolism—a review. Gene. 1992;115:5–12. doi: 10.1016/0378-1119(92)90533-U. [DOI] [PubMed] [Google Scholar]
- Huang HC, Chiu SH, Ke HJ, et al. Antimelanogenic and antioxidant activities of Bifidobacterium infantis. Afr J Microbiol Res. 2011;5:3150–3156. doi: 10.5897/AJMR11.021. [DOI] [Google Scholar]
- Huang JP, Mojib N, Goli RR, et al. Antimicrobial activity of PVP from an Antarctic bacterium, Janthinobacterium sp. Ant5–2, on multi-drug and methicillin resistant Staphylococcus aureus. Nat Products Bioprospect. 2012;2(3):104–110. doi: 10.1007/s13659-012-0021-4. [DOI] [Google Scholar]
- Hughes KA, Lawley B, Newsham KK. Solar UV-B radiation inhibits the growth of Antarctic terrestrial fungi. Appl Environ Microbiol. 2003;69:1488–1491. doi: 10.1128/AEM.69.3.1488-1491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KA, Cowan DA, Wilmotte A. Protection of Antarctic microbial communities—‘out of sight, out of mind’. Front Microbiol. 2015;6:151. doi: 10.3389/fmicb.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo I, Prieto-Taboada N, Martínez-Arkarazo I, Madariaga JM. Resonance Raman imaging as a tool to assess the atmospheric pollution level: carotenoids in Lecanoraceae lichens as bioindicators. Environ Sci Pollut. 2016;23(7):6390–6399. doi: 10.1007/s11356-015-5849-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. Production of carotenoids by a newly isolated marine Micrococcus sp. Biotechnology. 2008;7:469–474. doi: 10.3923/biotech.2008.469.474. [DOI] [Google Scholar]
- Jabour J. Biological prospecting: the ethics of exclusive reward from Antarctic activity. Ethics Sci Environ Polit. 2010;10:19–29. doi: 10.3354/esep00105. [DOI] [Google Scholar]
- Joshi VK, Attri D, Bala A, Bhushan S. Microbial pigments. Indian J Biotechnol. 2003;2:362–369. [Google Scholar]
- Kamla M, Jayanti T, Sneh G. A review on microbial pigment. Int J Microbial Res Technol. 2012;1(4):361–365. [Google Scholar]
- Kennicutt MC, Chown SL, Cassano JJ, et al. Polar research: six priorities for Antarctic science. Nature. 2014;512(7512):23–25. doi: 10.1038/512023a. [DOI] [PubMed] [Google Scholar]
- Kim SK. Marine biomaterials: characterization, isolation and applications. Boca Raton: CRC Press/Taylor & Francis; 2013. [Google Scholar]
- Kim SJ, Shin SC, Hong SG, Lee YM, Lee H, Lee J, Choi IG, Park H. Genome sequence of Janthinobacterium sp. strain PAMC 25724, isolated from alpine glacier cryoconite. J Bacteriol. 2012;194(8):2096–2096. doi: 10.1128/JB.00096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kang HJ, Yu BJ, et al. Planococcus faecalis sp. Nov., a carotenoid-producing species isolated from stools of Antarctic penguins. Int J Syst Evol Microbiol. 2015;65(10):3373–3378. doi: 10.1099/ijsem.0.000423. [DOI] [PubMed] [Google Scholar]
- Kimura T, Fukuda W, Sanada T, Imanaka T. Characterization of water-soluble dark-brown pigment from Antarctic bacterium, Lysobacter oligotrophicus. J Biosci Bioeng. 2015;120(1):58–61. doi: 10.1016/j.jbiosc.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Kitamoto D, Yanagishita H, Endo A, et al. Remarkable antiagglomeration effect of a yeast biosurfactant, diacylmannosylerythritol, on ice-water slurry forcold thermal storage. Biotechnol Prog. 2001;17(2):362–365. doi: 10.1021/bp000159f. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Nogi Y, Horikoshi K. New violet 3, 3′-bipyridyl pigment purified from deep-sea microorganism Shewanella violacea DSS12. Extremophiles. 2007;11(2):245–250. doi: 10.1007/s00792-006-0032-3. [DOI] [PubMed] [Google Scholar]
- Kodach LL, Bos CL, Durán N, et al. Violacein synergistically increases 5-fluoro-uracil cytotoxicity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis. 2017;27:508–516. doi: 10.1093/carcin/bgi307. [DOI] [PubMed] [Google Scholar]
- Konuray G, Erginkaya Z. Antimicrobial and antioxidant properties of pigments synthesized from microorganisms. In: Méndez-Vilas A, editor. The battle against microbial pathogens: basic science, technological advances and educational programs. Spain: Formatex Research Center; 2015. pp. 27–33. [Google Scholar]
- Koyama J. Anti-infective quinone derivatives of recent patents. Recent Pat Anti-Infect Drug Discov. 2006;1:113–125. doi: 10.2174/157489106775244073. [DOI] [PubMed] [Google Scholar]
- Kramar A, Llic Tomic T, Petkovic M, et al. Crude bacterial extracts of two new Streptomyces sp. isolates as bio-colorants for textile dyeing. World J Microbiol Biotechnol. 2014;30:2231–2240. doi: 10.1007/s11274-014-1644-x. [DOI] [PubMed] [Google Scholar]
- Krishna JG (2008) Pigment production by marine Serratia sp. BTW J8. Doctoral dissertation, Ph. D. Thesis. Department of Biotechnology, Cochin University of Science and Technology, Cochin, Kerala, India
- Kushwaha K, Saxena J, Tripathi BK, Agarwal MK. Detection of carotenoids in psychrotrophic bacteria by spectroscopic approach. J BioSci Biotechnol. 2014;3(3):253–260. [Google Scholar]
- Leiva S, Alvarado P, Huang Y, Wang J, Garrido I. Diversity of pigmented Gram-positive bacteria associated with marine macroalgae from Antarctica. FEMS Microbiol Lett. 2015;362(24):fnv206. doi: 10.1093/femsle/fnv206. [DOI] [PubMed] [Google Scholar]
- Li F, Xue F, Yu X. GC–MS, FTIR and Raman analysis of antioxidant components of red pigments from Stemphylium lycopersici. Curr Microbiol. 2017;74:532–539. doi: 10.1007/s00284-017-1220-3. [DOI] [PubMed] [Google Scholar]
- Liao L, Liu C, Zeng Y, Zhao B, Zhang J, Chen B. Multipartite genomes and the sRNome in response to temperature stress of an Arctic Pseudoalteromonas fuliginea BSW20308. Environ Microbiol. 2019;21(1):272–285. doi: 10.1111/1462-2920.14455. [DOI] [PubMed] [Google Scholar]
- Lim J, Thomas T, Cavicchioli R. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon Methanococcoides burtonii. J Mol Biol. 2000;297(3):553–567. doi: 10.1006/jmbi.2000.3585. [DOI] [PubMed] [Google Scholar]
- Lin SR, Weng CF. PG-priming enhances doxorubin influx to trigger necrotic and autophagic cell death in oral squamous cell carcinoma. J Clin Med. 2018;7:375. doi: 10.3390/jcm7100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu HC, Zhou YG, Xin YH. Microevolution and adaptive strategy of psychrophilic species Flavobacterium bomense sp. Nov. isolated from glaciers. Front Microbiol. 2019;10:1069. doi: 10.3389/fmicb.2019.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorv JS, Rose DR, Glick BR. Bacterial ice crystal controlling proteins. Scientifica. 2014 doi: 10.1155/2014/976895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang L, Xue Y, et al. Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China. Biochem Eng J. 2009;43(2):135–141. doi: 10.1016/j.bej.2008.09.009. [DOI] [Google Scholar]
- Madigan MT, Martinko JM, Stahl DA, Clark DP. Brock biology of microorganisms. 13. San Francisco: Pearson Education, Inc; 2012. [Google Scholar]
- Mageswari A, Subramanian P, Srinivasan R, et al. Astaxanthin from psychrotrophic Sphingomonas faeni exhibits antagonism against food-spoilage bacteria at low temperatures. Microbiol Res. 2015;179:38–44. doi: 10.1016/j.micres.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Maheswarappa G, Kavitha D, Vijayarani K, Kumanan K. Prodigiosin as anticancer drug produced from bacteria of termite gut. Indian J Basic Appl Med Res. 2013;3:257–266. [Google Scholar]
- Manikprabhu D, Lingappa K. Actinorhodin a natural and attorney source for synthetic dye to detect acid production of fungi. Saudi J Biol Sci. 2013;20:163–168. doi: 10.1016/j.sjbs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalith PZ. Pigment microbiology. London: Chapman & Hall; 1992. pp. 1–156. [Google Scholar]
- Margesin R, Collins T. Microbial ecology of the cryosphere (glacial and permafrost habitats): current knowledge. Appl Microbiol Biotechnol. 2019 doi: 10.1007/s00253-019-09631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margesin R, Schumann P, Spröer C, Gounot AM. Arthrobacter psychrophenolicus sp. Nov., isolated from an alpine ice cave. Int J Syst Evol Microbiol. 2004;54(6):2067–2072. doi: 10.1099/ijs.0.63124-0. [DOI] [PubMed] [Google Scholar]
- Marizcurrena JJ, Morel MA, Braña V, et al. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles. 2017;21(2):409–418. doi: 10.1007/s00792-016-0914-y. [DOI] [PubMed] [Google Scholar]
- Marizcurrena JJ, Cerdá MF, Alem D, Castro-Sowinski S. The ecological role of micro-organisms in the Antarctic environment. Cham: Springer; 2019. Living with pigments: the colour palette of Antarctic life; pp. 65–82. [Google Scholar]
- Martin-Cerezo ML, Garcia-Lopez E, Cid C. Isolation and identification of a red pigment from the Antarctic bacterium Shewanella frigidimarina. Protein Peptide Lett. 2015;22:1076–1082. doi: 10.2174/0929866522666150915122247. [DOI] [PubMed] [Google Scholar]
- Medentsev AG, Arinbasarova AY, Akimenko VK. Biosynthesis of naphthoquinone pigments by fungi of the genus Fusarium. Appl Biochem Microbiol. 2005;41:503–5076. doi: 10.1007/s10438-005-0091-8. [DOI] [PubMed] [Google Scholar]
- Medigue C, Krin E, Pascal G, et al. Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 2005;15(10):1325–1335. doi: 10.1101/gr.4126905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo PS, Justo GZ, de Azevedo MB, Duran N, Haun M. Violacein and its beta-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology. 2003;186:217–225. doi: 10.1016/S0300-483X(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Melo PS, Maria SS, de Campos VB, Haun M, Durán N. Violacein cytotoxicity and induction of apoptosis in V79 cells. In Vitro Cell Dev-Biol. 2000;36:5343–5395. doi: 10.1290/1071-2690(2000)036<0539:VCAIOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mojib N, Philpott R, Huang JP, Niederweis M, Bej AK. Antimycobacterial activity in vitro of pigment isolated from Antartica bacteria. Anton Van Leeuwen. 2010;98:531–540. doi: 10.1007/s10482-010-9470-0. [DOI] [PubMed] [Google Scholar]
- Mojib N, Farhoomand A, Anderson DT, Bej AK. UV and cold tolerance of a pigment- producing Antarctic Janthinobacterium sp. Ant5-2. Extremophiles. 2013;17:367–378. doi: 10.1007/s00792-013-0525-9. [DOI] [PubMed] [Google Scholar]
- Moliné M, Libkind D, Dieguez MC, van Broock M. Photoprotective role of carotenoid pigments in yeasts: experimental study contrasting naturally occurring pigmented and albino strains. J Photochem Photobiol B. 2009;95:156–161. doi: 10.1016/j.jphotobiol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Montagni T, Enciso P, Marizcurrena JJ, et al. Dye sensitized solar cells based on Antarctic Hymenobacter sp. UV11 dyes. J. Environ Sustain. 2018;1(1):89–97. doi: 10.1007/s42398-018-0007-1. [DOI] [Google Scholar]
- Mueller DR, Vincent WF, Bonilla S, Laurion I. Extremotrophs, extremophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. FEMS Microbiol Ecol. 2005;53(1):73–87. doi: 10.1016/j.femsec.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mykytczuk NCS, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. Bacterial growth at −15°C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7:1211–1226. doi: 10.1038/ismej.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Sawada T, Morita Y, et al. Isolation of a psychrotrophic bacterium from the organic residue of a water tank keeping rainbow trout and antibacterial effect of violet pigment produced from the strain. Biochem Eng J. 2002;12(1):79–86. doi: 10.1016/S1369-703X(02)00079-7. [DOI] [Google Scholar]
- Namazkar S, Ahmad WA. Spray-dried prodigiosin from Serratia marcescens as a colorant. Biosci Biotech Res Asia. 2013;10(1):69–76. doi: 10.13005/bbra/1094. [DOI] [Google Scholar]
- Nelis HJ, De Leenheer AP. Microbial sources of carotenoid pigments used in foods and feeds. J Appl Bacteriol. 1991;70(3):181–191. doi: 10.1111/j.1365-2672.1991.tb02922.x. [DOI] [Google Scholar]
- Nigam PS, Luke JS. Food additives: production of microbial pigments and their antioxidant properties. Curr Opin Food Sci. 2016;7:93–100. doi: 10.1016/j.cofs.2016.02.004. [DOI] [Google Scholar]
- Nwankwo IU, Itaman VO, Chidiebere OL, Nwachukwu MP. Evaluation of antimicrobial activity of prodigiosin produced from Serratia marcescens against some pathogenic bacteria. Futo J Ser. 2017;3:93–102. [Google Scholar]
- Ogugbue CJ, Sawidis T. Bioremediation and detoxification of synthetic waste water containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnol Res Int. 2011 doi: 10.4061/2011/967925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H, Orikasa Y, Nishida T. Significance of antioxidative functions of eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Appl Environ Microbiol. 2008;74(3):570–574. doi: 10.1128/AEM.02256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omamor IB, Eziashi EI, Adekunle AA. Carbon nutrition in relation to growth of three Monascus species isolated from decaying date fruits. Afr J Microbiol Res. 2008;2:153–155. [Google Scholar]
- Onofri S, Selbmann L, Zucconi L, Pagano S. Antarctic microfungi as models for exobiology. Planet Space Sci. 2004;52:229–237. doi: 10.1016/j.pss.2003.08.019. [DOI] [Google Scholar]
- Órdenes-Aenishanslins N, Anziani-Ostuni G, Vargas-Reyes M, et al. Pigments from UV-resistant Antarctic bacteria as photosensitizers in dye sensitized solar cells. J Photochem Photobiol B. 2016;162:707–714. doi: 10.1016/j.jphotobiol.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Osawa A, Ishii Y, Sasamura N, et al. Characterization and antioxidative activities of rare C50 carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside obtained from Micrococcus yunnanensis. J Oleo Sci. 2010;59:653–659. doi: 10.5650/jos.59.653. [DOI] [PubMed] [Google Scholar]
- Pagano MC, Dhar PP (2015) Fungal pigments: an overview. In: Gupta VK, Mach RL, Sreenivasaprasad S (eds) Fungal biomolecules: sources, applications and recent developments. 1st edn., Wiley, London. ISBN: 978-1-118-95829-2
- Pandey N, Rahul J, Anita P, Sushma T. Optimisation and characterisation of the orange pigment produced by a cold adapted strain of Penicillium sp. (GBPI_P155) isolated from mountain ecosystem. Mycology. 2018;9(2):81–92. doi: 10.1080/21501203.2017.1423127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantanella F, Berlutti F, Passariello C, et al. Violacein and biofilm production in Janthinobacterium lividum. J Appl Microbiol. 2007;102(4):992–999. doi: 10.1111/j.1365-2672.2006.03155.x. [DOI] [PubMed] [Google Scholar]
- Panwar AS, Molpa D, Joshi GK. Biotechnological potential of some cold-adapted bacteria isolated from North-Western Himalaya. Microbiology. 2019;88(3):343–352. doi: 10.1134/S002626171903007X. [DOI] [Google Scholar]
- Parmar M, Phutela UG. Biocolors: the new generation additives. Int J Curr Microbiol Appl Sci. 2015;4(7):688–694. [Google Scholar]
- Parmar TK, Rawtani D, Agrawal YK. Bioindicators: the natural indicator of environmental pollution. Front Life Sci. 2016;9(2):110–118. doi: 10.1080/21553769.2016.1162753. [DOI] [Google Scholar]
- Perfumo A, Banat IM, Marchant R. Going green and cold: biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018;36(3):277–289. doi: 10.1016/j.tibtech.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Pezzella A, Gargiulo V, Alfè M, et al. Eumelanin graphene-like integration: the impact on physical properties and electrical conductivity. Front Chem. 2019;7:121. doi: 10.3389/fchem.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals; properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette F, D’Amico S, Mazzucchelli G, et al. Life in the cold: a proteomic study of cold-repressed proteins in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Appl Environ Microbiol. 2011;77(11):3881–3883. doi: 10.1128/aem.02757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorniammal R, Parthiban M, Gunasekaran S, et al. Natural dye production from Thermomyces sp. fungi for textile application. Indian J Fiber Textile Res. 2013;38:276–279. [Google Scholar]
- Potera C. Diet and nutrition: the artificial food dye blues. Environ Health Perspect. 2010;118(10):A428–A428. doi: 10.1289/ehp.118-a428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S, Rekha PD, Young CC, et al. Zeaxanthin production by novel marine isolates from coastal sand of India and its antioxidant properties. Appl Biochem Biotech. 2013;171:817–831. doi: 10.1007/s12010-013-0397-6. [DOI] [PubMed] [Google Scholar]
- Pradeep FS, Pradeep BV. Optimization of pigment and biomass production from Fusarium moniliforme under submerged fermentation conditions. Int J Pharm Pharm Sci. 2013;5:526–535. [Google Scholar]
- Prashanthi K, Suryan S, Varalakshmi KN. In vitro anticancer property of yellow pigment from Streptomyces griseoaurantiacus JUACT 01. Braz Arch Biol Technol. 2015;58:869–876. doi: 10.1590/S1516-89132015060271. [DOI] [Google Scholar]
- Puig-Marcó R. Access and benefit sharing of Antarctica’s biological material. Mar Genomics. 2014;17:73–78. doi: 10.1016/j.margen.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Rafiq M, Hayat M, Anesio AM, et al. Recovery of metallo-tolerant and antibiotic resistant psychrophilic bacteria from Siachen glacier, Pakistan . PloS one. 2017;12(7):e0178180. doi: 10.1371/journal.pone.0178180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq M, Hayat M, Zada S, et al. Geochemistry and bacterial recovery from Hindu Kush Range glacier and their potential for metal resistance and antibiotic production. Geomicrobiology J. 2019;36(4):326–338. doi: 10.1080/01490451.2018.1551947. [DOI] [Google Scholar]
- Rai M. Advances in fungal biotechnology. New Delhi: I. K. International Pvt Ltd.; 2009. p. 545. [Google Scholar]
- Ramesh C, Vinithkumar NV, Kirubagaran R, et al. Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms. 2019;7(7):186. doi: 10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Rao N, Prabhu M, Xiao M, Li WJ. Fungal and bacterial pigments: secondary metabolites with wide applications. Front Microbiol. 2017;8:1113. doi: 10.3389/fmicb.2017.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar S, Uma G, Gokulakrishnan R. Antibacterial property of Halobacterial carotenoids against human bacterial pathogens. J Sci Ind (India) 2016;75:253–257. [Google Scholar]
- Raymond JA, Christner BC, Schuster SC. A bacterial ice-binding protein from the Vostok ice core. Extremophiles. 2008;12(5):713–717. doi: 10.1007/s00792-008-0178-2. [DOI] [PubMed] [Google Scholar]
- Reddy GS, Prakash JS, Prabahar V, et al. Kocuria polaris sp nov, an orange-pigmented psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int J Syst Evol Microbiol. 2003;53(1):183–187. doi: 10.1099/ijs.0.02336-0. [DOI] [PubMed] [Google Scholar]
- Reichenbach H, Kleinig H, Achenbach H. The pigments of Flexibacter elegans: novel and chemosystematically useful compounds. Arch Microbiol. 1974;101:131–144. doi: 10.1007/BF00455933. [DOI] [PubMed] [Google Scholar]
- Reverchon S, Rouanet C, Expert D, Nasser W. Characterization of indigoidine biosynthetic genes in Erwinia chrysanthemi and role of this blue pigment in pathogenicity. J Bacteriol. 2002;184(3):654–665. doi: 10.1128/JB.184.3.654-665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeeyan Z, Safarpour A, Amoozegar MA, et al. High carotenoids production by a halotolerant bacterium, Kocuria sp. strain QWT-12 and anticancer activity of its carotenoids. EXCLI J. 2017;16:840–851. doi: 10.17179/excli2017-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CH. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001;151(2):341–353. doi: 10.1046/j.1469-8137.2001.00177.x. [DOI] [Google Scholar]
- Robledo JA, Murillo AM, Rouzaud F. Physiological role and potential clinical interest of mycobacterial pigments. IUBMB Life. 2011;63:71–78. doi: 10.1002/iub.424. [DOI] [PubMed] [Google Scholar]
- Roos JC, Vincent WF. Temperature dependence of UV radiation effects on Antarctic cyanobacteria. J Phycol. 1998;34:118–125. doi: 10.1046/j.1529-8817.1998.340118.x. [DOI] [Google Scholar]
- Rosa LH, Vaz AB, Caligiorne RB, Campolina S, Rosa CA. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae) Polar Biol. 2009;32(2):161–167. doi: 10.1007/s00300-008-0515-z. [DOI] [Google Scholar]
- Ryazantseva IN, Andreyeva IN, Klementyeva GS, et al. Pigment-dependent light influence on the energetics of Serratia marcescens. Thermochim Acta. 1995;251:63–67. doi: 10.1016/0040-6031(94)02148-H. [DOI] [Google Scholar]
- Sabbe K, Hodgson DA, Verleyen E, et al. Salinity, depth and the structure and composition of microbial mats in continental Antarctic lakes. Freshw Biol. 2004;49(3):296–319. doi: 10.1111/j.1365-2427.2004.01186.x. [DOI] [Google Scholar]
- Sajjad W, Ahmad M, Khan S, et al. Radio-protective and antioxidative activities of astaxanthin from newly isolated radio-resistant bacterium Deinococcus sp. strain WMA-LM9. Ann Microbiol. 2017;67(7):443–455. doi: 10.1007/s13213-017-1269-z. [DOI] [Google Scholar]
- Santiago M, Ramírez-Sarmiento CA, Zamora RA, Parra LP (2016) Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front Microbiol 7(1408). 10.3389/fmicb.2016.01408 [DOI] [PMC free article] [PubMed]
- Saviola B. Pigments and pathogenesis. J Mycobact Dis. 2014;4:1068–2161. [Google Scholar]
- Schindler PR, Metz H. Bacteria of the Flexibacter/Sporocytophaga group and violet-pigmented bacteria as indicators for hygienically doubtful drinking water. Zentralblatt fur Hygiene und Umweltmedizin. Int J Hyg Envir Med. 1989;189(1):29–36. [PubMed] [Google Scholar]
- Schloss PD, Allen HK, Klimowicz AK, et al. Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol. 2010;29(9):533–541. doi: 10.1089/dna.2010.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann I, Wiese J, Kunz AL, Imhoff JF. Genetic approach for the fast discovery of phenazine producing bacteria. Mar Drugs. 2011;9:772–789. doi: 10.3390/md9050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seganish JL, Davis JT. Prodigiosin is a chloride carrier that can function as an anion exchanger. Chem Commun. 2005;46:5781–5783. doi: 10.1039/b511847f. [DOI] [PubMed] [Google Scholar]
- Segawa T, Yoshimura Y, Watanabe K, et al. Community structure of culturable bacteria on surface of Gulkana Glacier. Alaska Polar Sci. 2011;5(1):41–51. doi: 10.1016/j.polar.2010.12.002. [DOI] [Google Scholar]
- Selbmann L, Zucconi L, Isola D, Onofri S. Rock black fungi: excellence in the extremes, from the Antarctic to space. Curr Genet. 2015;61:335–345. doi: 10.1007/s00294-014-0457-7. [DOI] [PubMed] [Google Scholar]
- Selvameenal L, Radhakrishnan M, Balagurunathan R. Antibiotic pigment from desert soil actinomycetes; biological activity, purification and chemical screening. Indian J Pharm Sci. 2009;71:499–504. doi: 10.4103/0250-474X.58174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu Y, Wang N, et al. Variation with depth of the abundance, diversity and pigmentation of culturable bacteria in a deep ice core from the Yuzhufeng Glacier, Tibetan Plateau. Extremophiles. 2018;22(1):29–38. doi: 10.1007/s00792-0170973-8. [DOI] [PubMed] [Google Scholar]
- Shindo K, Misawa N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar Drugs. 2014;12:1690–1698. doi: 10.3390/md12031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirata A, Tsukamoto T, Yasui H, et al. Production of bluish-purple pigments by Janthinobacterium lividum isolated from the raw silk and dyeing with them. Nihon Sanshigaku Zasshi. 1997;66:377–385. [Google Scholar]
- Shirata A, Tsukamoto T, Yasui H, et al. Isolation of bacteria producing bluish-purple pigment and use for dyeing. Jpn Agric Res Q. 2000;34:131–140. [Google Scholar]
- Shivaji S, Ray MK, Kumar GS, et al. Identification of Janthinobacterium lividum from the soils of the islands of Scotia Ridge and from Antarctic peninsula. Polar Biol. 1991;11(4):267–271. doi: 10.1007/BF00238461. [DOI] [Google Scholar]
- Siddiqui KS, Williams TJ, Wilkins D, et al. Psychrophiles. Annu Rev Earth Planet Sci. 2013;41:87–115. doi: 10.1146/annurev-earth-040610-133514. [DOI] [Google Scholar]
- Siefirmann-Harms D. The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant. 1987;69:501–568. [Google Scholar]
- Sikkandar S, Murugan K, Al-Sohaibani S, et al. Halophilic bacteria—a potent source of carotenoids with antioxidant and anticancer potentials. J Pure Appl Microbiol. 2013;7:2825–2830. [Google Scholar]
- Silva TR, Tavares RS, Canela-Garayoa R, et al. Chemical characterization and biotechnological applicability of pigments isolated from Antarctic bacteria. Mar Biotechnol. 2019;21(3):416–429. doi: 10.1007/s10126-019-09892-z. [DOI] [PubMed] [Google Scholar]
- Singgih M, Andriatna W, Damayanti S, Priatni S. Carotenogenesis study of Neurospora intermedia N-1 in liquid. J Chem Pharm Res. 2015;7:842–847. [Google Scholar]
- Singh AK, Sad K, Singh SK, Shivaji S. Regulation of gene expression at low temperature: role of cold-inducible promoters. Microbiology. 2014;160(7):1291–1296. doi: 10.1099/mic.0.077594-0. [DOI] [PubMed] [Google Scholar]
- Singh A, Krishnan KP, Prabaharan D, Sinha RK. Lipid membrane modulation and pigmentation: a cryoprotection mechanism in Arctic pigmented bacteria. J Basic Microbiol. 2017;57(9):770–780. doi: 10.1002/jobm.201700182. [DOI] [PubMed] [Google Scholar]
- Sinha RP, Klisch M, Gröniger A, Häder DP. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B. 1998;47(2–3):83–94. doi: 10.1016/s1011-1344(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Sivaperumal P, Kamala K, Rajaram R. Bioactive DOPA melanin isolated and characterized from a marine actinobacterium Streptomyces sp. MVCS6 from Versova coast. Nat Prod Res. 2015;29:2117–2121. doi: 10.1080/14786419.2014.988712. [DOI] [PubMed] [Google Scholar]
- Smith D, da Silva M, Jackson J, Lyal C. Explanation of the Nagoya Protocol on access and benefit sharing and its implication for microbiology. Microbiology. 2017;163(3):289–296. doi: 10.1099/mic.0.000425. [DOI] [PubMed] [Google Scholar]
- Solano F. Melanin: skin pigments and much more-types, structural models, biological functions, and formation routes. New J Sci. 2014;2014:1–28. doi: 10.1155/2014/498276. [DOI] [Google Scholar]
- Soliev AB, Hosokawa K, Enomoto K. Bioactive pigments from marine bacteria: applications and physiological roles. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/670349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommaruga R, Libkind D, Broock M, Whitehead K. Mycosporine-glutaminol-glucoside, a UV-absorbing compound of two Rhodotorula yeast species. Yeast. 2004;21:1077–1081. doi: 10.1002/yea.1148. [DOI] [PubMed] [Google Scholar]
- Stafsnes MH, Bruheim P (2013) Pigmented marine heterotrophic bacteria. In: Kim S (ed) Marine biomaterials: characterization, isolation and applications, CRC Press/Taylor & Francis Group, London, pp 117–148
- Stankovic N, Senerovic L, Ilic-Tomic T, et al. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl Microbiol Biotechnol. 2014;98:3841–3858. doi: 10.1007/s00253-014-5590-1. [DOI] [PubMed] [Google Scholar]
- Starič N, Danevčič T, Stopar D. Vibrio sp. DSM 14379 pigment production—a competitive advantage in the environment? Microb Ecol. 2010;60:592–598. doi: 10.1007/s00248-010-9671-0. [DOI] [PubMed] [Google Scholar]
- Suba KP, Stalin A, Girija AS, Raguraman R. Homology modelling and docking analysis of prodigiosin from Serratia marcescens. Biotechnology. 2013;55:12897–12902. [Google Scholar]
- Subramaniam S, Ravi V, Sivasubramanian A. Synergistic antimicrobial profiling of violacein with commercial antibiotics against pathogenic micro-organisms. Pharm Biol. 2014;52:86–90. doi: 10.3109/13880209.2013.815634. [DOI] [PubMed] [Google Scholar]
- Suresh M, Renugadevi B, Brammavidhya S, et al. Antibacterial activity of red pigment produced by Halolactibacillus alkaliphilus MSRD1—an isolate from seaweed. Appl Biochem Biotech. 2015;176:185–195. doi: 10.1007/s12010-015-1566-6. [DOI] [PubMed] [Google Scholar]
- Suryawanshi RK, Patil CD, Borase HP, et al. Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int J Cosmet Sci. 2015;37(1):98–107. doi: 10.1111/ics.12175. [DOI] [PubMed] [Google Scholar]
- Suryawanshi RK, Patil CD, Koli SH, Hallsworth JE, Patil SV. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat Prod Res. 2017;31:572–577. doi: 10.1080/14786419.2016.1195380. [DOI] [PubMed] [Google Scholar]
- Sutthiwong N, Fouillaud M, Valla A, et al. Bacteria belonging to the extremely versatile genus Arthrobacter as novel source of natural pigments with extended hue range. Food Res Int. 2014;65:156–162. doi: 10.1016/j.foodres.2014.06.024. [DOI] [Google Scholar]
- Tarangini K, Mishra S. Production of melanin by soil microbial isolate on fruit waste extract: two step optimizations of key parameters. Biotechnol Rep. 2014;4:139–146. doi: 10.1016/j.btre.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashirev AB, Romanovskaia VA, Shilin SO, Chernaia NA. Screening of yeast-producers of melanin in the Antarctic terrestrial biotopes. Mikrobiol Zh. 2010;72:3–8. [PubMed] [Google Scholar]
- Thomson NR, Crow MA, McGowan SJ, et al. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol Microbiol. 2000;36:539–556. doi: 10.1046/j.1365-2958.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- Tian B, Hua Y. Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol. 2010;18(11):512–520. doi: 10.1016/j.tim.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Tribelli PM, Lopez NI. Reporting key features in cold-adapted bacteria. Life (Basel) 2018 doi: 10.3390/life8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribelli PM, Solar Venero EC, Ricardi MM, et al. Novel essential role of ethanol oxidation genes at low temperature revealed by transcriptome analysis in the Antarctic bacterium Pseudomonas extremaustralis. PLoS ONE. 2015;10(12):e0145353. doi: 10.1371/journal.pone.0145353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochine A, Turchetti B, Vaz ABM, et al. Description of Dioszegia patagonica sp. nov., a novel carotenogenic yeast isolated from cold environments. Int J Syst Evol Microbiol. 2017;67:4332–4339. doi: 10.1099/ijsem.0.002211. [DOI] [PubMed] [Google Scholar]
- Tuli HS, Chaudhary P, Beniwal V, Sharma AK. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2015;52:4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk M, Plemenitaš A, Gunde-Cimerman N. Extremophilic yeasts: plasma-membrane fluidity as determinant of stress tolerance. Fungal Biol. 2011;115(10):950–958. doi: 10.1016/j.funbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Umadevi K, Krishnaveni M. Antibacterial activity of pigment produced from Micrococcus luteus KF532949. Int J Chem Anal Sci. 2013;4:149–152. doi: 10.1016/j.ijcas.2013.08.008. [DOI] [Google Scholar]
- UNEP (2012) An update on biological prospecting in Antarctica and recent policy developments at the international level. ATCM XXXV Meeting. https://www.unep.org/dewa/Portals/67/pdf/ATCM35_ip063_e.pdf
- UNU UNEP (2009) Biological prospecting: an update on recent policy developments at the international level. ATCM XXXII meeting. https://www.unep.org/dewa/Portals/67/pdf/Atcm32_ip091_e.pdf
- Van Duin D, Casadevall A, Nosanchuk JD. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother. 2002;46(11):3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthabharathi V, Lakshminarayanan R, Jayalakhshmi S. Melanin production from marine Streptomyces. Afr J Biotechnol. 2011;10:11224–11234. doi: 10.5897/AJB11.296. [DOI] [Google Scholar]
- Vasileva-Tonkova E, Romanovskaya V, Gladka G, et al. Ecophysiological properties of cultivable heterotrophic bacteria and yeasts dominating in phytocenoses of Galindez Island, Maritime Antarctica. World J Microbiol Biotechnol. 2014;30:1387–1398. doi: 10.1007/s11274-013-1555-2. [DOI] [PubMed] [Google Scholar]
- Vaz AB, Rosa LH, Vieira ML, et al. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz J Microbiol. 2011;42(3):937–947. doi: 10.1590/S1517-83822011000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo F, Pitol LO, Carciofi BAM, et al. Construction and application a vanesystem in a rotational rheometer for determination of the rheological properties of Monascus ruber CCT 3802. J Biorheol. 2010;24:29–35. doi: 10.1007/s12573-010-0019-7. [DOI] [Google Scholar]
- Venil CK, Aruldass CA, Dufosse L, et al. Current perspective on bacterial pigments: emerging sustainable compounds with coloring and biological properties for the industry-an incisive evaluation. RSC Adv. 2014;4:39523–39529. doi: 10.1039/c4ra06162d. [DOI] [Google Scholar]
- Venil CK, Zakaria ZA, Ahmad WA. Bacterial pigments and their applications. Process Biochem. 2013;48:1065–1079. doi: 10.1016/j.procbio.2013.06.006. [DOI] [Google Scholar]
- Venil CK, Yusof NZ, Aruldass CA, et al. Application of violet pigment from Chromobacterium violaceum UTM5 in textile dyeing. Biologia. 2016;71(2):121–127. doi: 10.1515/biolog-2016-0031. [DOI] [Google Scholar]
- Venil CK, Wahidin MAB, Aruldass CA, Ahmad WA. Production of bacterial pigments in low cost medium and formulation of biodegradable ink. Indian J Exp Biol. 2017;55(7):441–447. [Google Scholar]
- Vila E, Hornero-Méndez D, Azziz G, et al. Carotenoids from heterotrophic bacteria isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol Rep. 2019;21:e00306. doi: 10.1016/j.btre.2019.e00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal P, Carrasco M, Barahona S, et al. Tolerance to ultraviolet radiation of psychrotolerant yeasts and analysis of their carotenoid, mycosporine, and ergosterol content. Curr Microbiol. 2016;72:94–101. doi: 10.1007/s00284-015-0928-1. [DOI] [PubMed] [Google Scholar]
- Vincent WF, Downes MT, Castenholz RW, Howard-Williams C. Community structure and pigment organisation of cyanobacteria-dominated microbial mats in Antarctica. Eur J Phycol. 1993;28(4):213–221. doi: 10.1080/09670269300650321. [DOI] [Google Scholar]
- Visalakchi S, Muthumary J. Antimicrobial activity of the new endophytic Monodictys castaneae SVJM139 pigment and its optimization. Afr J Microbiol Res. 2010;4:38–44. [Google Scholar]
- Voets IK. From ice-binding proteins to bio-inspiredantifreeze materials. Soft Matter. 2017;13(28):4808–4823. doi: 10.1039/C6SM02867E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackenroder H. Ueber das Oleum radicis Dauci aetherum, das Carotin, den Carotenzucker und den officinellen succus Dauci; so wie auch über das Mannit, welches in dem Möhrensafte durch eine besondere Art der Gahrung gebildetwird. Geigers Magazin Pharmazie. 1831;33:144–172. [Google Scholar]
- Wada N, Sakamoto T, Matsugo S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites. 2013;3(2):463–483. doi: 10.3390/metabo3020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford J, editor. “Historical development of food colouration”, in evelopments in Food Colours. London: Applied Science publishers; 1980. [Google Scholar]
- Wan A, Rozi AM, Bin MI et al (2014) A method for dyeing polyester fabrics using colorant extracted from Chromobacterium violaceum. MY150969 (A) (Patent).
- Wang Y, Nakajima A, Hosokawa K, et al. Cytotoxic prodigiosin family pigments from Pseudoalteromonas sp. 1020R isolated from the Pacific coast of Japan. Biosci Biotechnol Biochem. 2012;76:1229–1232. doi: 10.1271/bbb.110984. [DOI] [PubMed] [Google Scholar]
- Wehrs M, Gladden JM, Liu Y, et al. Sustainable bioproduction of the blue pigment indigoidine: expanding the range of heterologous products in R. toruloides to include non-ribosomal peptides. Green Chem. 2019;21(12):3394–3406. doi: 10.1039/C9GC00920E. [DOI] [Google Scholar]
- Wong LS, Teo SC (2014) Naturally occurring carotenoids in cyanobacteria as a bioindicator for heavy metals detection. In Proceedings of the international Conference on Advances. In: Applied Science and Environmental Engineering–ASEE.
- World Health Organization (WHO) (2018) The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top10-causes-of-death. Accessed 1 Jun 2018
- Wynn-Williams DD, Edwards HGM. Environmental UV radiation: biological strategies for protection and avoidance. In: Horneck G, Baumstark-Khan C, editors. Astrobiology. Heidelberg: Springer; 2002. [Google Scholar]
- Wynn-Williams DD, Edwards HGM, Garcia-Pichel F. Functional biomolecules of Antarctic stromatolitic and endolithic cyanobacterial communities. Eur J Phycol. 1999;34(4):381–391. doi: 10.1080/09670269910001736442. [DOI] [Google Scholar]
- Yamashita M, Nakagawa Y, Li H, Matsuyama T. Silica gel-dependent production of prodigiosin and serrawettins by Serratia marcescens in a liquid culture. Microbes Environ. 2001;16:250–254. doi: 10.1264/jsme2.2001.250. [DOI] [Google Scholar]
- Yarzábal LA. Microbial models: from environmental to industrial sustainability. Singapore: Springer; 2016. Antarctic psychrophilic microorganisms and biotechnology: history, current trends, applications, and challenges; pp. 83–118. [Google Scholar]
- Yoshida K, Hashimoto M, Hori R, et al. Bacterial long-chain polyunsaturated fatty acids: their biosynthetic genes, functions, and practical use. Mar Drugs. 2016;14(5):94. doi: 10.3390/md14050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Xu F, Valiente J, et al. An indigoidine biosynthetic gene cluster from Streptomyces chromofuscus ATCC 49982 contains an unusual IndB homologue. J Ind Microbiol Biot. 2013;40(1):159–168. doi: 10.1007/s10295-012-1207-9. [DOI] [PubMed] [Google Scholar]
- Yumusak C, Prochazkova AJ, Apaydin DH, et al. Indigoidine-biosynthesized organic semiconductor. Dyes Pigm. 2019;171:107768. doi: 10.1016/j.dyepig.2019.107768. [DOI] [Google Scholar]
- Yurkova AM, Vustin MM, Tyaglov BV, et al. Pigmented basidiomycetous yeasts are a promising source of carotenoids and ubiquinone Q10. Microbiology. 2008;77:1–6. doi: 10.1134/S0026261708010013. [DOI] [PubMed] [Google Scholar]
- Zerrad A, Anissi J, Ghanam J, et al. Antioxidant and antimicrobial activities of melanin produced by a Pseudomonas balearica strain. J Biotechnol Lett. 2014;5:87–94. [Google Scholar]
- Zhang X, Ma X, Wang N, Yao T. New subgroup of Bacteroidetes and diverse microorganisms in Tibetan plateau glacial ice provide a biological record of environmental conditions. FEMS Microbiol Ecol. 2008;67:21–29. doi: 10.1111/j.1574-6941.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Zobell C, Upham HC. A list of marine bacteria including descriptions of new species. BidL. ScrippsIns£. Oceanogr. 1944;5:n9–292. [Google Scholar]