Abstract
Introduction:
Various drugs affect liver problems caused by general hypoxia, including silymarin. Due to the fungal killer toxins, nowadays, silymarin (milk thistle) is used as an effective drug in the prevention and treatment of liver diseases and liver toxicity. In addition, silymarin protects the liver cells from solvents and chemical substances. The aim of this paper is to investigate the impact of silymarin on liver problems induced by general hypoxia.
Material and Methods:
This study was a double-blinded clinical trial on patients with hypoxia who referred to the hospital emergency department. Patients were randomly divided into case and control groups. The case group was treated with silymarin at a dose of 280 mg with orally gavage technique and the control group was treated with a placebo every 8 h for 3 days. To investigate the leukocytosis, liver enzymes levels of alanine transaminase (ALT), aspartate aminotransferase (AST), creatine phosphokinase (CPK), prothrombin time (PT), partial thromboplastin (PTT), international normalized ratio (INR), and white blood cell (WBC) were measured before and after the intervention. SPSS 21 software was used to analyze the data.
Results:
In the silymarin group, liver enzymes were lower than the placebo group on the third day after treatment (P < 0.05). There was no significant difference between the two groups in terms of coagulation factors and WBC count on the third day after treatment (P > 050). On the third day after treatment, the amount of GGT was lower in the silymarin group (P < 0.05).
Conclusion:
Silymarin decreased liver enzymes (ALT, AST, and CPK) and the level of GGT. Therefore, it is recommended to be used in patients with hypoxic liver injury.
Keywords: Hypoxia, liver disease, silymarin
Introduction
Hypoxia is a condition in which a part of the body does not receive sufficient oxygen. In vascular endothelial growth, factor hypoxia is activated by macrophages, neutrophils, and cells of various other genes.[1] Hypoxia increases the ability of cells to consume glucose by enhancing the activity of glycolytic and glucose transporter enzymes.[2] Hypoxia-inducible factors play an important role in regulating molecular response and much of the necessary cellular and physiological mechanisms to adapt to hypoxic conditions.[1] hypoxic liver injury is a severe but transient increase in liver transaminases because of an imbalance between the oxygen intake and the liver oxygen consumption in the absence of other acute causes of liver injury which leads to the centrilobular necrosis. The liver receives 20 to 25% of cardiac output by the portal vein (70%) and the common hepatic artery (30%).[3]
The most common cause of ischemia is because of congestion or chronic liver disease. Other factors, such as hypoxemia, poor blood oxygen uptake, inadequate oxygen uptake by hepatocytes, and increased metabolic needs contribute to its occurrence.[4] The clinical signs of this disorder include weakness, shortness of breath, upper abdominal pain, enlargement and hepatic congestion, and, specifically, the level of transaminases increased 20-fold, but they become normal within a few days. Various drugs have an effect on liver damage caused by general hypoxia including silymarin. Silymarin is an annual or biennial plant of the family Asteraceae which has been widely introduced outside its natural range, for example into Western Europe, Central and North India is today the most popular car in South Europe, Africa, China, Australia, South America and some parts of North America, and West and South Asia. This plant is distributed in Iran in the areas of Gonbade Kavus, Haraz Valley, Dashte Moghan, Poshte Koh, Malasani in Ahlaz, Shoosh, Hamidieh, Ramhormoz, Izeh, Karoun, and Kazeroun.[5]
Silymarin has been used for about 2,000 years as a traditional medicine for stimulating milk production, treating liver, kidney, spleen, gallstones, jaundice, and menstrual disorders.[6,7] Silymarin derived from thistle seeds is nowadays used as an effective drug in the prevention and treatment of liver diseases.[8] In addition, silymarin protects the liver cells from solvents and chemical substances.[9] The anticancer effects of silymarin on prostate, skin, breast, colon, ovarian, and brain cancer cells have been reported.[10,11] Silybin is the major active ingredient in silymarin, which reduces liver enzymes in patients with liver disease and improves liver fibrosis in 30% of mice. It has regulatory effects at the cellular and molecular levels,[12] and some human studies have shown that silibinin reduces insulin resistance, hepatic steatosis, and plasma levels of hepatic fibrosis which indicates the impact of silymarin.[13,14] Therefore, in this study, we aimed to investigate the effect of silymarin on liver hypoxia induced by general hypoxia.
Material and Methods
This study was designed as a double-blinded clinical trial. The subjects were selected from patients older than 18 years old who has hypoxia and referred to the emergency department of Valiasr Hospital in Arak, Iran. After 12 h, the demographic and clinical checklist of patients was completed for each patient in accordance with the study entry and exit criteria and with informed consent to participate in the study. Eligible patients who met the inclusion criteria were divided into case and control groups according to a random number table.
Inclusion criteria
Hypoxia patients over 18 years old who are selected from the categories of Post Cpr - Hypoxic Encephalopathy - Shock (hypotention + acidosis) who must complete one of the following criteria:
Cardiac or circulatory shock
A massive but reversible increase in serum aminotransferase levels
Right upper quadrant pain, weakness, shortness of breath
Centrilobular necrosis on liver biopsy
Exclusion of other causes of liver damage.
Obtain informed consent.
Exclusion criteria
Patient death
Patient nonparticipation
Patient conscious withdrawal.
Case and control groups were matched according to age, sex, and severity of liver dysfunction. The case group was treated with silymarin at a dose of 280 mg with orally gavage technique and the control group was treated with a placebo every 8 h for 3 days. Liver enzymes levels of alanine transaminase (ALT), aspartate aminotransferase (AST), creatine phosphokinase (CPK), prothrombin time (PT), partial thromboplastin (PTT), international normalized ratio (INR), and white blood cell (WBC) (for investigating leukocytosis) were measured before and after the intervention. SPSS 21 software was used to analyze the data. Enzyme activity was recorded in International Units per liter (IU/L). Finally, the information in the checklist was prepared, recorded, and analyzed.
Calculation of the sample size and its number
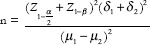
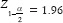
Z1–b= 1.28
δ1= 0.23
δ2= 0.12
μ1= 0.35
μ2= 0.18
According to the aforementioned formula and the calculation of the numbers obtained from it, the number of samples in each group was calculated as 45 persons.
Data analysis
After entering the data into SPSS software, descriptive statistics, central indices, dispersion, and graphs were used and the data were evaluated. T-test and Chi-square test were used to analyze the two groups, and P < 0.05 was considered significant.
Ethical considerations
A letter of introduction was received from respected university officials to introduce us to the research centers. The letter of introduction was obtained from the respected authorities of the selected research centers. The aim of the study was described for all research units and their written consent was obtained. The plan's administrator keeps all patients' information confidential. All Helsinki Research Ethics statements and ethics research committees are considered at all steps of research.
Results
In this study, 90 hypoxic patients were hospitalized. The minimum age was 15 years and the highest age was 89 years. The mean age of the patients was 56.31 ± 20.01 years. There were 59 (65.6%) males and 31 (34.4%) females. Comparison of the mean and standard deviation (SD) of age in the silymarin group was 52.86 ± 19.27 years, and in the placebo group, it was 59.75 ± 20.36. There was no statistically significant difference between the two groups in terms of age and P = 0.656 indicates that the two groups are approximately equal in age. Comparison of the number and percentage of sex was 30 males (66.66%) and 15 females (33.34%) in the silymarin group and 29 males (64.44%) and 16 females (35.56%) in the placebo group. There was no statistically significant difference between the two groups in terms of gender (P = 0.5).
Table 1 indicates the comparison of the mean and SD of liver enzymes on the first day. According to the results in the two groups, no significant difference was observed in liver enzymes on the first day (P < 0.05), which indicates that the liver enzymes did not differ in the two groups on the first day.
Table 1.
Comparison of the mean and SD of liver enzymes on the first day
| Variable group | Silymarin mean±SD | Placebo mean±SD | P |
|---|---|---|---|
| ALT | 409.93±80.83 | 383.37±45.88 | 0.849 |
| AST | 478.8±113.1 | 404.28±58.45 | 0.696 |
| CPK | 1312.45±342.07 | 1760.82±277.78 | 0.497 |
SD=Standard deviation, ALT=Alanine transaminase, AST=Aspartate aminotransferase, CPK=Creatine phosphokinase
Table 2 indicates the comparison of mean and SD of liver enzymes on the third day in silymarin and placebo groups. According to the results in the two groups, significant statistical difference was observed in liver enzymes on the third day after treatment (P < 0.05). In the silymarin group, the liver enzymes were lower than the placebo group.
Table 2.
Comparison of the mean and SD of liver enzymes on the third day
| Variable group | Silymarin mean±SD | Placebo mean±SD | P |
|---|---|---|---|
| ALT | 154.77±337.45 | 354.84±362.92 | 0.008 |
| AST | 184.17±603.28 | 405.62±469.77 | 0.05 |
| CPK | 624.17±143.20 | 1701.57±2361.35 | 0.011 |
SD=Standard deviation, ALT=Alanine transaminase, AST=Aspartate aminotransferase, CPK=Creatine phosphokinase
Table 3 indicates the comparison of the mean and SD of coagulation factors. According to the results in the groups, no statistically significant difference was observed for coagulation factors on the first day (P < 0.05).
Table 3.
Comparison of the mean and SD of coagulation factors
| Variable group | Silymarin mean±SD | Placebo mean±SD | P |
|---|---|---|---|
| PT | 14.55±3.06 | 14.75±1.47 | 0.689 |
| PTT | 32.08±8 | 31.41±3.97 | 0.617 |
| INR | 1.2±0.181 | 1.2±0.15 | 0.950 |
SD=Standard deviation, PT=Prothrombin time, PTT=Partial thromboplastin, INR=International normalized ratio
Table 4 indicates the comparison of mean and SD of coagulation factors on the third day. According to the results in the groups, no statistically significant difference was observed for coagulation factors on the third day (P < 0.05) which means that drugs have no impact on the coagulation factors.
Table 4.
Comparison of the mean and SD of coagulation factors on the third day
| Variable group | Silymarin mean±SD | Placebo mean±SD | P |
|---|---|---|---|
| PT | 14.06±2.84 | 14.58±1.4 | 0.269 |
| PTT | 31.58±8.67 | 31.11±3.67 | 0.739 |
| INR | 1.16±0.17 | 1.2±0.147 | 0.267 |
SD=Standard deviation, PT=Prothrombin time, PTT=Partial thromboplastin, INR=International normalized ratio
Comparison of mean and SD of WBC counts on the first and third days were evaluated in the silymarin and placebo groups. In the silymarin group, on the first day, the WBC count was (10.71 ± 3.53) 103. For the placebo group, it was (11.03 ± 2.83) 103 for the placebo group, and as P = 0.759, it was not statistically significant. On the third day, in the silymarin group, there were (10.65 ± 3.58) 103 WBCs, and in the placebo group, there were (11.86 ± 2.82) 103 WBCs. In addition, P = 0.08 was not statistically significant. According to the results, no significant difference was observed in the WBC count between the two groups in the first and third days. P > 0.05 indicates that silymarin had no impact on WBC count.
Comparison of mean and SD of gamma-glutamyl transpeptidase (GGT) for silymarin and placebo groups in the first and third days are 82.39 ± 75.35 and 73.34 ± 84.38, respectively. Due to the results of the two groups, no statistically significant difference was observed for GGT on the first day (P = 0.256). On the third day, the amount of GGT in the silymarin group was 50.25 ± 31.73 and in the placebo group it was 104.45 ± 93.33. According to the results obtained on the third day after treatment, the amount of GGT in the two groups was statistically significant (P = 0.0001). In the silymarin group, GGT was lower, which indicates the positive impact of silymarin on GGT.
Table 5 indicates the comparison of mean and SD of laboratory factors in the silymarin group on the first and third days after treatment. According to Table 5, there was a statistically significant difference in liver enzymes and GGT levels before and after treatment in the silymarin group (P < 0.05). After treatment with silymarin, ALT, AST, CPK, and GGT levels decreased.
Table 5.
Comparison of the mean and SD of laboratory factors in the silymarin group on the first and third days after treatment
| Silymarin Group Laboratory Factors | First Day mean±SD | Third Day mean±SD | P |
|---|---|---|---|
| ALT | 409.93±80.83 | 154.77±45.337 | 0.001 |
| AST | 478.80±113.10 | 184.17±603.28 | 0.008 |
| CPK | 1312.45±342.07 | 624.17±1437.20 | 0.028 |
| PT | 14.55±3.06 | 14.06±2.84 | 0.06 |
| PTT | 32.08±8.0 | 31.58±8.67 | 0.578 |
| INR | 1.2±0.181 | 1.16±0.17 | 0.09 |
| WBC | (10.71±3.53) 103 | (10.65±3.58) 103 | 0.291 |
| GGT | 82.75±39.35 | 50.31±25.73 | 0.0001 |
SD=Standard deviation, ALT=Alanine transaminase, AST=Aspartate aminotransferase, CPK=Creatine phosphokinase, PT=Prothrombin time, PTT=Partial thromboplastin, INR=International normalized ratio, WBC=White blood cell, GGT=Gamma-glutamyl transpeptidase
Discussion
The thistle is a dicotyledon species of Cassium. The scientific name of the plant is Silybummarianum, but it is also known as milk thistle. The flavonoids in milk thistle are silybin, silydianin, and silychristin. Together, they are called silymarin. Silymarin has potent antioxidant properties, membrane stabilization, antiinflammatory, and regenerative and cellular properties.[15] Silibinin is the main active ingredient is silymarin, which reduces liver enzyme levels in patients with liver disease, and in 30% of cases in mice it improves liver fibrosis. It has regulatory impact at the cellular and molecular levels and in some human studies it has been shown that the delays between hepatic steatosis and plasma fibrosis markers decrease.[12]
Accordingly, we decided to investigate the impact of silymarin on hypoxic liver injury. No significant differences were found in age, sex, liver enzymes, GGT, and coagulation factors between the two groups in silymarin and placebo (P > 0.05). However, after treatment with silymarin on the third day, the amount of GGT and liver enzymes in silymarin group decreased. There was a significant difference between the two groups (P < 0.05). There was no statistically significant difference between the two groups in the number of WBCs on the first and third days (P > 0.050). According to the results, it can be concluded that silymarin decreased liver enzymes and GGT levels.
Similar studies conducted in this area, including the study of Banaee et al., (2015) which investigated the protective effects of silymarin extract on animal samples of liver toxicity. They observed that liver enzyme levels were significantly reduced after taking silymarin. They reported that silymarin in hepatotoxicity could have an effect on the reduction of liver enzymes.[16] Solhi (2014) indicated that silymarin can be effective in reducing liver enzymes in patients with nonalcoholic fatty liver disease.[17] Their results were consistent with our study because, in our study, hepatic enzyme levels were significantly decreased by taking silymarin on the third day.
Iranikhah (2016) indicated that silymarin reduces the fatty liver grade and reduces liver enzymes in the nonalcoholic fatty liver disease in children.[18] Taghvaei (2013) indicated that silymarin improves liver enzymes in patients with alcoholic fatty liver disease, and it is effective in treating it.[12] The results of their study were in line with ours because in our study the amount of GGT and liver enzymes decreased with silymarin use.
Conclusion
According to the results of this study, it can be concluded that silymarin decreased liver enzymes (ALT, AST, and CPK) and GGT levels. However, it had no effect on WBC count and coagulation factors. Therefore, it is recommended to be used in patients with hypoxic liver injury. It has fewer side effects than other medicines because it is an herbal drug.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Weidemann A. Biology of HIF-1a. Cell death and differentiation. AaRJ. 2008;15:621. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 2.Lee YM. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: A possible signal for vessel development. Dev Dyn. 2001;220:175–86. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Dunn G. The liver in congestive heart failure. Am J Med Sci. 1973;265:174–90. doi: 10.1097/00000441-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Gibson PaF. Ischemic hepatitis:Clinical features, diagnosis and prognosis. Intern Med J. 1984;14:822–5. doi: 10.1111/j.1445-5994.1984.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 5.Kheiripour N, Karimi J, Khodadadi I, Tavilani H, Goodarzi M, Hashemnia M. Hepatoprotective effects of silymarin on liver injury via Irisin upregulation and oxidative stress reduction in rats with type 2 diabetes. Iranian J Med Sci. 2019;44:108–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Valson F. Modern phytotherapy and its uses in gastrointestinal conditions. Planta Med. 1991;5:48–52. [Google Scholar]
- 7.Salim L. A review of plants used in the treatment of liver disease:Part 1. Altern Med Rev. 1998;3:410–21. [PubMed] [Google Scholar]
- 8.Parish RC. Treatment of Amanita mushroom poisoning:A review. Vet Hum Toxicol. 1986;2:318–22. [PubMed] [Google Scholar]
- 9.Ghadermazi R, Khoshjou F, Hossini Zijoud SM, Behrooj H, Kheiripour N, Ganji M, et al. Hepatoprotective effect of tempol on oxidative toxic stress in STZ-induced diabetic rats. Toxin Rev. 2018;37:82–6. doi:10.1080/15569543.2017.1313277. [Google Scholar]
- 10.Manna MA, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–9. [PubMed] [Google Scholar]
- 11.Zanganeh N, Siahpoushi E, Kheiripour N, Kazemi S, Goodarzi MT, Alikhani MY. Brucellosis causes alteration in trace elements and oxidative stress factors. Biol Trace Elem Res. 2018;182:204–8. doi: 10.1007/s12011-017-1102-3. doi:10.1007/s12011017-1102-3. PubMed PMID:28735;383. [DOI] [PubMed] [Google Scholar]
- 12.Taghvaei T. Efficacy of silymarin on treatment of nonalcoholic steatohepatiti. J Mazandaran Univ Med Sci. 2013;23:164–71. [Google Scholar]
- 13.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children:A pilot study. J Pediatrics. 2000;136:734–8. [PubMed] [Google Scholar]
- 14.Saller R, Meier R. Brignoli. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–63. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ames BN, Shigenaga TM. Hagen, oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahdi B, Antoni S, Shima Sh, Nastaran F. Rotective effects of silymarin extract on malthion-induced zebra cichlid (Cichlasoma nigrofasciatum) hepatotoxicity. Hepatotoxicity. 2015;9:1240–6. [Google Scholar]
- 17.Solhi H. Silymarin in treatment of non-alcoholic steatohepatitis:A randomized clinical trial. Caspian J Intern Med. 2014;5:9–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Iranikhah A. Effects of silymarin on nonalcoholic fatty liver disease in children:A crossover clinical trial. J Mazandaran Univ Med Sci. 2017;26:119–26. [Google Scholar]


