Abstract
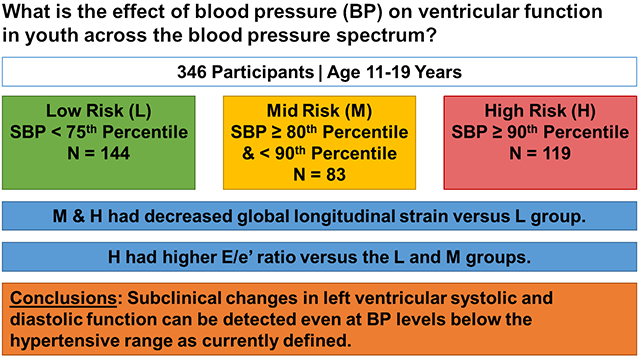
Hypertension is associated with cardiovascular events in adults. Subclinical changes to left ventricular strain and diastolic function have been found prior to development of decreased left ventricular ejection fraction (LVEF) and cardiovascular events. Our objective was to study effects of blood pressure (BP) on ventricular function in youth across the blood pressure spectrum. Vital signs and labs were obtained in 346 participants aged 11–19 years who had blood pressure categorized as low-risk (L = 144; SBP <75th percentile), mid-risk (M = 83; SBP ≥80th and <90th percentile), and high-risk (H = 119; SBP ≥ 90th percentile). Echocardiography was performed to assess left ventricular strain and diastolic function. Differences between groups were analyzed by ANOVA. General linear models were constructed to determine independent predictors of systolic and diastolic function. M and H participants had greater adiposity and more adverse metabolic labs (lower HDL, higher glucose, and higher insulin) than the L group. M and H participants had significantly lower left ventricular ejection fraction (LVEF) and peak global longitudinal strain (GLS) than the L group (both p≤0.05). The E/e’ ratio was higher in the H group versus the L and M groups, and the e’/a’ ratio was lower in the H versus the L group (both p≤0.05). Blood pressure and adiposity were statistically significant determinants of left ventricular systolic and diastolic function. Subclinical changes in left ventricular systolic and diastolic function can be detected even at BP levels below the hypertensive range as currently defined.
Keywords: Hypertension, elevated blood pressure, pediatrics, strain, systolic function, diastolic function
Summary
This study expands our understanding of the effect of blood pressure on ventricular function across the blood pressure spectrum demonstrating that subclinical changes in ventricular function were evident in youth at levels below current definitions of hypertension. Our data contributes to future risk stratification of these patients and emphasizes the importance of regular screening and management of elevated blood pressure.
Graphical Abstract
Introduction
Hypertension is known to be associated with cardiovascular (CV) disease in adults,1 and increases risk for stroke,2, 3 myocardial infarction,2, 3 and heart failure.4 Subclinical changes in left ventricular strain, a sensitive measure of systolic function, are seen in hypertensive adults prior to the development of these hard CV events.5, 6 Abnormalities in diastolic function are also seen in hypertension7 and in heart failure with preserved ejection fraction (HFpEF) prior to decreases in gross measures of ventricular function such as ejection fraction.6 Controlling BP in hypertensive patients is key to halting the progression from these early ventricular changes to heart failure.8
To date, data examining the relationship between blood pressure (BP) levels and left ventricular strain in youth are limited.9, 10 More pediatric studies are available examining subclinical changes in diastolic function in hypertensive youth.11–13 However, these studies have been small or used the older Fourth Report14 definition of childhood hypertension. Therefore, the primary aim of our study was to examine the relationship between left ventricular strain and diastolic function and BP in a large, multi-racial cohort and to determine if subclinical changes in ventricular function occur not only in hypertensive youth but also at lower BP levels. We suspected that increased abnormalities in ventricular function would be significantly related to hypertension, but that a significant relationship also might emerge with BP in the higher end of the normal distribution, after adjusting for specific sociodemographic and related medical covariates.
Methods
Study Design
The data that support the findings of this study are available from the corresponding author upon reasonable request. Participants in a multi-center study (5 sites) examining the cardiovascular effects of high BP on youth were included (N = 346, 57.5% male, 62.7% White, 27.2% Black, 4.3% Asian, and 5.8% Other).15 Participants were stratified by systolic blood pressure (SBP) into low risk (L = 144; SBP <75th percentile), mid-risk (M = 83; SBP ≥80th and <90th percentile), and high-risk (H = 119; SBP ≥ 90th percentile) groups according to the Fourth Report14 BP definitions as recruitment for the study started prior to release of the American Academy of Pediatrics (AAP) Clinical Practice Guidelines.16 We chose these thresholds because we hypothesized that changes to ventricular function would occur at BP levels <95th percentile. The participant BPs were then reanalyzed using the new BP guidelines16 to determine updated BP percentiles and for analyses in this study. Participants were kept in the original group into which they were recruited. None of the patients were on antihypertensive drug therapy at the time of recruitment. The ages of the participants ranged from 11–19 years (mean age = 15.5 ± 1.8 years). Demographic data, anthropometric data, vital signs, and lab values (fasting lipid panel, fasting glucose and insulin, creatinine, uric acid, C-reactive protein [CRP], and urine Na/K ratio) were obtained.
This study has undergone institutional review board review and approval at each participating institution. All participants and their parent/guardian provided written informed consent or assent according to local institutional review board requirements.
Study Assessments
Casual BP Measurement
Blood pressure measurements were obtained in the right arm by auscultation of the brachial artery using an aneroid sphygmomanometer (Mabis MedicKit5; Mabis Healthcare, Waukegan, IL). All study personnel responsible for BP measurement underwent standardized training and certification in the auscultatory BP measurement protocol. Four BP measurements at 30-second intervals were obtained on each of 2 separate visits, with the average of the 2nd, 3rd, 4th and 6th, 7th, 8th BP measurements used as the study BP value. Full details of BP measurement in this study were described previously.15
Cardiac Measurements
Cardiac sonographers were trained in the echocardiographic protocol, and standard cardiac ultrasound systems were used at each site. Parasternal long-axis, parasternal short-axis, apical 4-chamber, apical 2-chamber, high parasternal short-axis, and suprasternal notch views were obtained with 14 images collected with the subject supine.
A trained sonographer used the Cardiology Analysis System (Digisonics, Houston, TX) to measure offline the left ventricular (LV) end-diastolic dimension, LV end-systolic dimension, end-diastolic interventricular wall thickness, and LV end-diastolic and end-systolic posterior wall thickness.
LV mass was calculated using the Deveraux equation17 from 2-dimensional M-Mode images of the LV at end diastole.18, 19 LV mass index (LVMI) was defined as LV mass/ht2.7 as described by DeSimone20 to account for body size without overcompensating for obesity.
Systolic function was evaluated by tracing the endocardium from the 4-chamber view at peak systole and end diastole using TOMTEC software (TOMTEC Corporation, Chicago, IL) offline to quantify global longitudinal strain (GLS), determine strain rate, and calculate LV ejection fraction (LVEF).21 GLS measurements were obtained at native echo frame rates (40–60 frames per second). LV shortening fraction (LVSF) was also obtained. Diastolic function was assessed using pulse wave Doppler of the mitral inflow velocity in the apical 4-chamber view to determine E/A ratio. Tissue Doppler imaging (TDI) of the mitral annular inflow was recorded at the lateral and septal annulus with the e’/a’ ratios from both regions averaged.
Statistical Analysis
Statistical Analysis Software (SAS, version 9.4, Cary, North Carolina, USA) was used in analyses. Means and standard deviations or frequency and percentage were calculated for anthropometric, laboratory, hemodynamic, and echocardiogram data stratified by BP group.
Distributions of key variables were evaluated and variance stabilizing procedures were employed as needed. To address the primary research question, differences in mean values between groups were analyzed using analysis of variance or chi square as appropriate. Bonferroni correction was performed to adjust for multiple comparisons.
To determine independent predictors of systolic and diastolic function, bivariate correlations were calculated between covariates and echocardiogram parameters. A general linear model was constructed with the significant covariates used to construct a parsimonious model to test independent determinants of systolic and diastolic function. Nonsignificant covariates in the initial models were removed by stepwise regression until the remaining covariates were significant.
Results
Participant Characteristics
The M and H groups had a more adverse cardiovascular risk profile (table 1) including higher levels of adiposity, insulin, and lower HDL cholesterol (all p≤0.05). The H group had significantly higher levels of uric acid compared to the L group (p≤0.05). There were no differences in LDL, triglycerides, or CRP between groups.
Table 1.
Characteristics of low-, mid-, and high-risk BP groups
| Parameters | Low (L) N= 144 |
Mid (M) N=83 |
High (H) N=119 |
|---|---|---|---|
| Age (years)* | 15.6 ± 1.5 | 15.9 ± 1.9 | 15.1 ± 1.8 |
| Sex (% male)† | 73 (50.69) | 56 (67.47) | 70 (58.82) |
| Race (% Caucasian) | 95 (65.52) | 50 (60.24) | 72 (60.5) |
| Ethnicity (% Hispanic) | 21 (14.48) | 16 (19.28) | 19 (15.97) |
| Height (cm) | 168.2 ± 9.1 | 171.2 ± 11.2 | 168.0 ± 10.1 |
| Weight (kg)‡ | 74.7 ± 22.8 | 87.2 ± 29.5 | 83.4 ± 24.9 |
| BMI (kg/m2)‡ | 26.2 ± 6.8 | 29.4 ± 8.8 | 29.3 ± 7.6 |
| Waist/Height ratio§ | 0.50 ± 0.10 | 0.53 ± 0.11 | 0.55 ± 0.11 |
| Total cholesterol (mg/dl) | 151 ± 31 | 155 ± 36 | 156 ± 34 |
| LDL (mg/dl) | 85 ± 27 | 91 ± 30 | 92 ± 28 |
| HDL (mg/dl)|| | 48 ± 12 | 43 ± 11.6 | 43 ± 12 |
| Triglycerides (mg/dl) | 93 ± 62 | 104 ± 61 | 99 ± 50 |
| Glucose (mg/dl)§ | 88 ± 7 | 90 ± 11 | 91 ± 8 |
| Insulin (microIU/dl)‡ | 17 ± 12 | 22 ± 15 | 23 ± 15 |
| Creatinine (mg/dl)† | 0.71 ± 0.13 | 0.77 ± 0.19 | 0.73 ± 0.17 |
| Uric Acid (mg/dl)§ | 5.4 ± 1.6 | 5.8 ± 1.4 | 5.9 ± 1.5 |
| CRP (mg/dl) | 1.7 ± 2.3 | 1.6 ± 2.1 | 2.2 ± 2.5 |
| Urine Na/K ratio | 4.19 ± 3.02 | 4.23 ± 3.26 | 4.11 ± 2.70 |
| K1 SBP (mmHg)# | 111 ± 10 | 126 ± 6 | 133 ± 7 |
| K5 DBP (mmHg)# | 75 ± 10 | 82 ± 7 | 86 ± 9 |
| MAP (mmHg)# | 87 ± 6 | 97 ± 5 | 101 ± 7 |
| SBP percentile CPG# | 50.0 ± 27.2 | 86.0 ± 7.8 | 95.5 ± 3.3 |
| DBP percentile CPG‡ | 73.4 ± 24.4 | 90.1 ± 10.1 | 92.3 ± 13.5 |
| HR (bpm) | 72 ± 12 | 69 ± 12 | 73 ± 13 |
Data is expressed as mean and standard deviation or frequency and percentage where appropriate. BMI, body mass index; CPG, 2017 AAP pediatric hypertension clinical practice guideline, CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high density lipoprotein; HR, heart rate; K1, Korotkoff sound 1; K5, Korotkoff sound 5; LDL, low density lipoprotein; MAP, mean arterial pressure; SBP, systolic blood pressure.
P ≤ 0.05 for:
M>H
L<M
L<M&H
L<H
L>M&H
L<M<H using Bonferroni correction.
Systolic Function
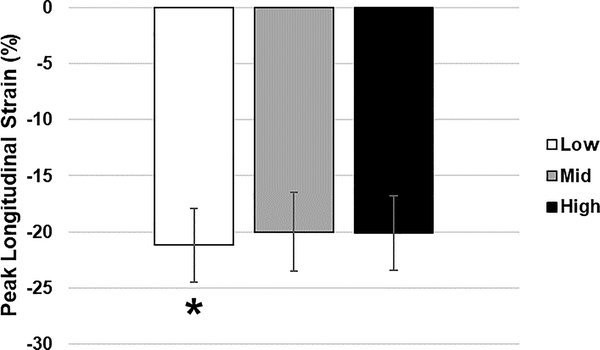
The M and H groups had lower (less negative) peak GLS compared to the L group that was significant (p=0.02) prior to Bonferroni correction but was less so after correction (p=0.06, Table 2 & Figure 1). GLS rose in a linear fashion with increasing SBP percentile without a threshold effect (R2=0.03, p=0.0044, data not shown). There was no significant difference in strain rate between groups (Table 2). LVEF was significantly higher in the L group versus the M group (p≤0.05), and LVMI was significantly higher in the M and H groups compared to the L group (p≤0.05).
Table 2.
Echocardiogram characteristics of low-, mid-, and high-risk BP groups
| Variable | Low (N=144) | Mid (N=83) | High (N=119) |
|---|---|---|---|
| LVMI (g/m2.7)* | 31 ± 7 | 34 ± 7 | 34 ± 7 |
| Peak Longitudinal Strain (%) | −21.2 ± 3.3 | −20.0 ± 3.5 | −20.1 ± 3.3 |
| Peak Longitudinal Strain Rate (/sec) | −1.05 ± 0.24 | −1.03 ± 0.25 | −1.03 ± 0.19 |
| LVSF (%) | 37.5 ± 4.5 | 36.2 ± 4.7 | 37.8 ± 4.5 |
| LVEF (%)† | 58.5 ± 7.1 | 55.9 ± 7.0 | 56.5 ± 6.7 |
| E/A ratio | 2.36 ± 0.71 | 2.20 ± 0.64 | 2.18 ± 0.65 |
| E/e’ ratio‡ | 6.0 ± 1.4 | 5.8 ± 1.3 | 6.5 ± 1.5 |
| e’/a’ ratio§ | 2.5 ± 0.8 | 2.3 ± 0.6 | 2.2 ± 0.6 |
Data is expressed as mean and standard deviation. LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVSF, left ventricular shortening fraction.
p ≤ 0.05 for:
L<M&H
L>M
L&M<H
L>H using Bonferroni correction.
Figure 1.
Peak longitudinal strain by blood pressure group. *P=0.02 (before Bonferroni correction; P = 0.06 after correction), L>M&H
Diastolic Function
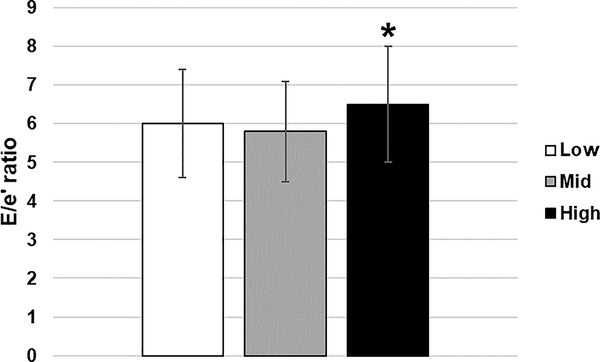
The E/e’ ratio in the H group was significantly higher than the L and M groups (p≤0.05, Table 2 & Figure 2). The e’/a’ ratio was also significantly lower in the H versus the L group (p≤0.05). Similar to GLS, diastolic function measures were also related to SBP percentile in a linear fashion (R2=0.02, p=0.01, data not shown).
Figure 2.
E/e’ ratio by blood pressure group. *P≤0.05, L&M<H
Multivariate Analysis
In multivariate models (Table 3), SBP percentile remained an independent determinant of E/e’ ratio and GLS while DBP percentile associated with LVSF, LVEF, log E/A, E/e’, and e’/a’. Adiposity (waist/height ratio) was associated with all measures of systolic and diastolic function except for LVSF.
Table 3.
Independent determinants of systolic and diastolic function
| Parameter | GLS | SF | EF | Log E/A | E/e’ | e’/a’ |
|---|---|---|---|---|---|---|
| Intercept | −28.17 | 40.00 | 63.66 | 1.57 | 1.95 | 2.06 |
| SBPpct | 0.019 | 0.0019 | ||||
| DBPpct | −0.032 | −0.081 | −0.0026 | −0.0021 | −0.0016 | |
| Age | 0.28 | −0.021 | −0.019 | |||
| Sex | −1.11 | 1.84 | 0.091 | |||
| Race | 1.95 | |||||
| Waist/Height | 6.80 | −9.73 | −0.36 | 0.38 | −0.92 | |
| HR | −0.0055 | −0.0067 | ||||
| R2 | 0.13 | 0.02 | 0.10 | 0.13 | 0.10 | 0.27 |
All model p ≤ 0.01 and all parameter estimates p ≤ 0.05.
DBPpct, diastolic blood pressure percentile; HR, heart rate; SBPpct, systolic blood pressure percentile.
Novelty and Significance
Discussion
Data from this study demonstrate that elevated BP, at levels below the current definition of hypertension in youth, are associated with subclinical changes in systolic and diastolic ventricular function in adolescents. Consistent with our hypothesis, reduction in systolic and diastolic ventricular function were associated in a linear manner with BP percentile. This finding is notable given that current pediatric hypertension guidelines use a statistical cut-point based on the BP distribution of healthy children and a single static cut-point (≥130/80 mmHg) in adolescents16 to define hypertension rather than linking BP thresholds with increased cardiovascular risk as in adult hypertension guidelines.1
Our study demonstrated that GLS decreases in the setting of elevated BP. Adult studies have shown reduced strain in the setting of hypertension5, 6, 22, 23 even with preserved LVEF. Kraigher-Krainer et al. found that GLS systematically decreases in a stepwise fashion when comparing controls to hypertensive adults to those with heart failure with preserved ejection fraction (HFpEF).6 These findings are important because strain may be a more sensitive measure of systolic function. In fact, compared to LVEF, GLS was found to be a superior predictor of cardiovascular outcomes,24 and subjects with lower GLS had higher risk for a composite endpoint of incident heart failure, acute myocardial infarction, or cardiovascular death.25 Additionally, hypertension appears to result in accelerated GLS reduction as Masugata et al. reported significantly reduced GLS in hypertensive subjects compared to controls with hypertensive group measurements being more in line with those seen in normal subjects at age 70–80s.22 Whether there are sex differences in the utility of strain as a predictor of CV risk is not clear, although Biering-Sorensen et al. found that GLS was an independent predictor of outcomes in men but not in women.25 Our findings also show that sex influences GLS with females having less of a decrease in strain compared to males in our general linear model (Table 3).
The increases in LVMI and diastolic dysfunction associated with hypertension7, 12, 16 do not necessarily explain the reduction of GLS in hypertension. A study comparing hypertensive young adults to normotensive competitive rowers and untrained controls showed decreased GLS in the hypertensive group compared to the other groups despite no differences in LVEF across groups and similar LVMI between rowers and hypertensive subjects.23 However, the E/A ratio was significantly lower in the hypertensive group compared to controls and the E/e’ ratio was significantly higher in the hypertensive group compared to both the rowers and control group. On the other hand, Szelenyi et al. found that GLS was reduced in hypertensive subjects regardless of having reduced or normal diastolic function compared to healthy controls.5 We found higher LVMI in our M and H group compared to the L group and worse diastolic function in the H group compared to the L and M groups (higher E/e’ ratio in H versus L & M and lower e’/a’ in H versus L group).
Few pediatric data exist relating BP levels to strain. Celik et al. recently reported that GLS and global radial strain was significantly decreased in treated hypertensive youth compared to controls despite both groups having similar office and 24 hour ambulatory BP.10 The study was small (N = 45 controls, N = 60 hypertensive) and focused on hypertensive subjects already on antihypertensive medication. Navarini et al. also reported that GLS was significantly reduced in adolescents with hypertension compared to controls, with no difference in LVEF or LV volumes between groups.9 However, this was a small study (N = 37 controls, N = 26 hypertensive), included predominantly Caucasian participants, and dichotomized the recruitment into normotensive and hypertensive (BP ≥ 95 percentile) subjects. Our study similarly found a significant reduction in GLS with higher BP. However, our study adds to the prior findings by including a larger study sample of multi-racial participants and by evaluating strain across the BP distribution. Our data demonstrate reductions in GLS that are associated with increasing BP percentile level without a threshold effect. Though LVEF for all patients were in the normal range, our participants with BP in the 80–90% range (M group) also had reduced GLS compared to truly normotensive youth (L mean SBP percentile = 50.0 ± 27.2). These findings suggest that subclinical changes to systolic function are evident even in patients with mild elevations in BP without hypertension.
More data are available relating diastolic dysfunction to hypertension in both adults7, 26 and children,9, 11–13 with pediatric studies showing significantly lower e’/a’ ratios and higher E/e’ ratios in hypertensive patients. The study by Agu et al.11 was multiethnic and had a similar age range as our study but had a smaller study population (N = 46 hypertensive, N = 34 controls) and used the Fourth Report14 for BP classification. Urbina et al.12 included a large proportion of subjects with diabetes mellitus (N = 258), included young adults up to age 23 years, and also used the Fourth Report14 for BP classification. The study by Zamojska et al.13 had a smaller study population (N = 34 hypertensive, N = 30 controls) that encompassed primarily male Polish children. Navarini et al.9 also had a smaller study population (N = 26 hypertensive, N = 37 controls) and used the Fourth Report14 for BP classification. Our study supports the findings of the previously mentioned studies in a large, multi-ethnic cohort with the e’/a’ ratio being significantly lower in the H group versus the L group and the E/e’ ratio being higher in the H versus the L and M groups. These early diastolic changes are important because the presence of diastolic dysfunction is a known precursor to HFpEF.8, 27
Limitations
This was a cross-sectional study so changes in echocardiogram findings over time are not able to be determined. Blood pressures were obtained in clinic rather than through ambulatory blood pressure monitors (ABPMs). Since not all clinical sites have access to ABPMs, this initial study was focused on the clinical setting. Future analyses may be performed with ABPMs. Adiposity contributes to left ventricular hypertrophy and diastolic dysfunction even in the absence of hypertension,28 and BMI is a known independent determinant of LVMI.29 Our study also found that adiposity (WHR) independently influenced parameters of systolic and diastolic function. Though the study aimed to balance adiposity across groups, the H group had slightly higher WHR than the L group. However, the differences are small and overall, the cohort is heavier in weight which may limit generalizability to a lean population. Additionally, our study used tissue Doppler imaging to assess global longitudinal strain rather than the speckle tracking technique. Although speckle tracking is less angle dependent, TDI has better temporal resolution30, 31 and does not require specialized software on the echo machines (can be read offline) thus facilitating multi-center studies.
Perspectives
Subclinical changes in left ventricular systolic and diastolic function are present even at levels below the current definition of hypertension. These findings support annual blood pressure screening and lifestyle modification for mild blood pressure elevation. Our study provides data to inform future guidelines in setting risk thresholds for children with elevated BP when considering therapy. The subclinical changes in systolic and diastolic function found in our study suggest that screening for these markers may eventually be a helpful adjunct in evaluating youth with elevated blood pressure.
Novelty and Significance.
What is New?
Subclinical changes in ventricular systolic and diastolic function are present in youth at blood pressure (BP) levels below the hypertensive range as currently defined.
BP and adiposity were independent determinants of ventricular systolic and diastolic function in youth.
What is Relevant?
These findings highlight the importance of regular screening and management of youth with elevated blood pressure and provide data to inform future BP guidelines in determining risk thresholds.
Acknowledgments
Sources of Funding: American Heart Association (AHA) grant 15SFRN23680000
Footnotes
Disclosures
Andrew H. Tran: None
Joseph T. Flynn: Silvergate Pharmaceuticals – modest support; AHA – significant support
Richard C. Becker: AHA – modest support
Stephen R. Daniels: None
Bonita E. Falkner: None
Michael Ferguson: None
Coral Hanevold: AHA – modest support
Stephen R. Hooper: AHA – modest support
Julie R. Ingelfinger: None
Marc Lande: AHA – modest support
Lisa Martin: AHA – modest support
Kevin Meyers: AHA – modest support
Mark Mitsnefes: AHA – modest support
Bernard Rosner: AHA – modest support
Joshua Samuels: AHA – modest support
Elaine M. Urbina: AHA – significant support
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, O’Leary DH, Bryan RN, Anderson M and Lumley T. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183–92. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB and Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Larson MG, Vasan RS, Kannel WB and Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 5.Szelenyi Z, Fazakas A, Szenasi G, Tegze N, Fekete B, Molvarec A, Hadusfalvy-Sudar S, Janosi O, Kiss M, Karadi I and Vereckei A. The mechanism of reduced longitudinal left ventricular systolic function in hypertensive patients with normal ejection fraction. J Hypertens. 2015;33:1962–9; discussion 1969. [DOI] [PubMed] [Google Scholar]
- 6.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD and Investigators P. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Schnohr P and Jensen JS. Tissue Doppler echocardiography in persons with hypertension, diabetes, or ischaemic heart disease: the Copenhagen City Heart Study. Eur Heart J. 2009;30:731–9. [DOI] [PubMed] [Google Scholar]
- 8.Messerli FH, Rimoldi SF and Bangalore S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017;5:543–551. [DOI] [PubMed] [Google Scholar]
- 9.Navarini S, Bellsham-Revell H, Chubb H, Gu H, Sinha MD and Simpson JM. Myocardial Deformation Measured by 3-Dimensional Speckle Tracking in Children and Adolescents With Systemic Arterial Hypertension. Hypertension. 2017;70:1142–1147. [DOI] [PubMed] [Google Scholar]
- 10.Celik SF, Karakurt C, Tabel Y, Elmas T and Yologlu S. Blood pressure is normal, but is the heart? Pediatr Nephrol. 2018;33:1585–1591. [DOI] [PubMed] [Google Scholar]
- 11.Agu NC, McNiece Redwine K, Bell C, Garcia KM, Martin DS, Poffenbarger TS, Bricker JT, Portman RJ and Gupta-Malhotra M. Detection of early diastolic alterations by tissue Doppler imaging in untreated childhood-onset essential hypertension. J Am Soc Hypertens. 2014;8:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR and Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. Journal of clinical hypertension (Greenwich, Conn). 2011;13:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamojska J, Niewiadomska-Jarosik K, Wosiak A, Lipiec P and Stanczyk J. Myocardial dysfunction measured by tissue Doppler echocardiography in children with primary arterial hypertension. Kardiol Pol. 2015;73:194–200. [DOI] [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program Working Group on High Blood Pressure in C and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 15.Mendizabal B, Urbina EM, Becker R, Daniels SR, Falkner BE, Hamdani G, Hanevold CD, Hooper SR, Ingelfinger JR, Lande M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA and Flynn JT. SHIP-AHOY (Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth). Hypertension. 2018;72:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Subcommittee On S and Management Of High Blood Pressure In C. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140:1–72. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I and Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 18.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ and American Society of E. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA and Laragh JH. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. Journal of the American College of Cardiology. 1995;25:1056–1062. [DOI] [PubMed] [Google Scholar]
- 21.Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N and Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Current cardiology reviews. 2009;5:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masugata H, Senda S, Goda F, Yamagami A, Okuyama H, Kohno T, Yukiiri K, Noma T, Hosomi N, Imai M and Kohno M. Influences of hypertension and diabetes on normal age-related changes in left ventricular function as assessed by tissue Doppler echocardiography. Clin Exp Hypertens. 2009;31:400–14. [DOI] [PubMed] [Google Scholar]
- 23.Galderisi M, Lomoriello VS, Santoro A, Esposito R, Olibet M, Raia R, Di Minno MN, Guerra G, Mele D and Lombardi G. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. Journal of the American Society of Echocardiography. 2010;23:1190–8. [DOI] [PubMed] [Google Scholar]
- 24.Stanton T, Leano R and Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circulation Cardiovascular imaging. 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 25.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, Sengelov M, Jorgensen PG, Mogelvang R, Shah AM and Jensen JS. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circulation Cardiovascular imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalaycioglu E, Gokdeniz T, Aykan AC, Gul I, Ugur M, Gursoy OM, Boyaci F and Celik S. The influence of dipper/nondipper blood pressure patterns on global left ventricular systolic function in hypertensive diabetic patients: a speckle tracking study. Blood Press Monit. 2014;19:263–70. [DOI] [PubMed] [Google Scholar]
- 27.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr., Jacobsen SJ and Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dusan P, Tamara I, Goran V, Gordana ML and Amira PA. Left ventricular mass and diastolic function in obese children and adolescents. Pediatric Nephrology. 2015;30:645–52. [DOI] [PubMed] [Google Scholar]
- 29.Crowley DI, Khoury PR, Urbina EM, Ippisch HM and Kimball TR. Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr. 2011;158:709–714 e1. [DOI] [PubMed] [Google Scholar]
- 30.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA and Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- 31.Schmid J, Kaufmann R, Grubler MR, Verheyen N, Weidemann F and Binder JS. Strain Analysis by Tissue Doppler Imaging: Comparison of Conventional Manual Measurement with a Semiautomated Approach. Echocardiography. 2016;33:372–8. [DOI] [PubMed] [Google Scholar]