Abstract
Women who carry pathogenic mutations in BRCA1 and BRCA2 have a lifetime risk of developing breast cancer of up to 80%. However, risk estimates vary in part due to genetic modifiers. We investigated the association of the RAD52 S346X variant as a modifier of the risk of developing breast and ovarian cancers in BRCA1 and BRCA2 mutation carriers from the Consortium of Investigators of Modifiers of BRCA1/2. The RAD52 S346X allele was associated with a reduced risk of developing breast cancer in BRCA2 carriers [per‐allele hazard ratio (HR) = 0.69, 95% confidence interval (CI) 0.56–0.86; P = 0.0008] and to a lesser extent in BRCA1 carriers (per‐allele HR = 0.78, 95% CI 0.64–0.97, P = 0.02). We examined how this variant affected DNA repair. Using a reporter system that measures repair of DNA double‐strand breaks (DSBs) by single‐strand annealing (SSA), expression of hRAD52 suppressed the loss of this repair in Rad52−/− mouse embryonic stem cells. When hRAD52 S346X was expressed in these cells, there was a significantly reduced frequency of SSA. Interestingly, expression of hRAD52 S346X also reduced the stimulation of SSA observed upon depletion of BRCA2, demonstrating the reciprocal roles for RAD52 and BRCA2 in the control of DSB repair by SSA. From an immunofluorescence analysis, we observed little nuclear localization of the mutant protein as compared to the wild‐type; it is likely that the reduced nuclear levels of RAD52 S346X explain the diminished DSB repair by SSA. Altogether, we identified a genetic modifier that protects against breast cancer in women who carry pathogenic mutations in BRCA2 (P = 0.0008) and to a lesser extent BRCA1 (P = 0.02). This RAD52 mutation causes a reduction in DSB repair by SSA, suggesting that defects in RAD52‐dependent DSB repair are linked to reduced tumor risk in BRCA2‐mutation carriers.
Keywords: BRCA2, breast cancer, double‐strand break repair, RAD52
We report that the hRAD52 S346X variant reduces risk of developing breast cancer in individuals carrying germline BRCA2 pathogenic variants. The reduced frequency of single‐strand annealing with this RAD52 variant resulted in a reduction in DNA double‐strand break repair. We observed reduced nuclear levels of RAD52 S346X, potentially explaining the reduced frequency of single‐strand annealing.
Abbreviations
- CI
confidence interval
- CIMBA
Consortium of Investigators of Modifiers of BRCA1/2
- DSB
DNA double‐strand break
- GFP
green fluorescent protein
- HDR
homology‐directed repair
- HR
hazard ratio
- MAF
minor allele frequency
- mESCs
mouse embryonic stem cells
- NLS
nuclear localization sequence
- PARP
poly(ADP‐ribose)polymerase
- RMD
repeat‐mediated deletion
- sgRNAs
single‐guide RNAs
- SSA
single‐strand annealing
- ssDNA
single‐stranded DNA
- WT
wild‐type
1. Introduction
The human DNA repair protein, RAD52 (hRAD52), is an important factor in several different aspects of genome maintenance (Jalan et al., 2019). One of the best‐defined roles of hRAD52 is as a key mediator of DNA double‐strand break (DSB) repair by single‐strand annealing (SSA) (Mendez‐Dorantes et al., 2018; Stark et al., 2004). SSA proceeds through the annealing and ligating together of complementary single strands of repetitive genomic sequences (repeats) flanking a DSB, providing a mechanism of DSB repair that does not conserve genome structure. SSA has been implicated in joining repeats separated by > 20 kbp, resulting in very large deletions of chromosomal DNA (Mendez‐Dorantes et al., 2018). The importance of hRAD52 in SSA is likely due to its various biochemical activities, including binding single‐stranded DNA (ssDNA) and stabilizing double‐stranded DNA (dsDNA) formed upon hybridization of complementary ssDNA (Brouwer et al., 2017). Importantly, while hRAD52 plays a major role in SSA, it is not part of the central apparatus controlling the repair of DSBs by homology‐directed repair (HDR) (Ceccaldi et al., 2016). Loss of multiple mechanisms of DSB repair may explain the synthetic lethality observed upon depletion of RAD52 in cells with hypomorphic BRCA2 mutations (Feng et al., 2011).
Because hRAD52 and BRCA2 play distinct roles in DSB repair, we examined the ability of the RAD52 S346X truncation variant (Fig. 1A) to act as a modifier of susceptibility to breast and ovarian cancers in BRCA1 and BRCA2 mutation carriers. Accordingly, we tested the association of RAD52 S346X with risk of developing breast or ovarian cancer in a large cohort of BRCA1 and BRCA2 mutation carriers from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) (Chenevix‐Trench et al., 2007). Based on the cellular function of hRAD52 in DNA repair, we tested the effect of RAD52 S346X on repair of DSBs (Mendez‐Dorantes et al., 2018) by SSA in conditions of both normal and reduced levels of BRCA2.
Fig. 1.
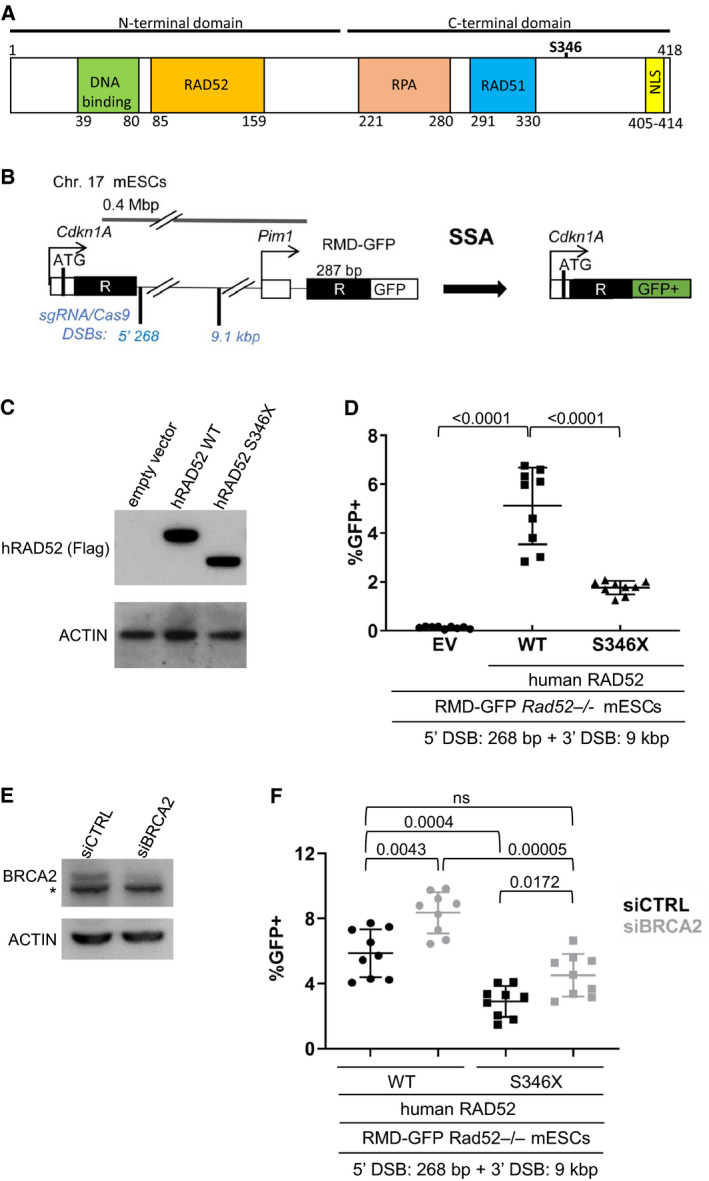
RAD52 S346X is dysfunctional in promoting SSA. (A) The domain map of human RAD52. The N‐terminal domain (1–212) contains DNA binding and self‐association regions. The C‐terminal domain (213–418) contains RPA and RAD51 interacting regions and a nuclear localization signal (NLS). (B) Diagram of the RMD‐GFP SSA reporter. Two tandem repeat (R) sequences are separated by 0.4 Mb and positioned such that SSA generates a Cdkn1a‐GFP fusion gene. SSA is induced by two DSBs between the repeats, with one DSB downstream from the 5′ repeat (5’268) and a second DSB 9.1 kbp upstream of the 3′ repeat. Shown are the single‐guide RNA (sgRNA)/Cas9 targeting sites for each DSB. Not to scale. (C) Flag immunoblot showing expression Flag‐hRAD52 WT and Flag‐hRAD52 S346X in RMD‐GFP Rad52−/− mESCs. (D) hRAD52 S346X is able to promote SSA but with a > 2‐fold decrease as compared to hRAD52 WT. Rad52−/− mESCs with RMD‐GFP were transfected with the 268 bp and 9 kbp sgRNA/Cas9 expression vectors along with a control EV, Flag‐hRAD52, or Flag‐hRAD52 S346X complementation vectors. Shown is the percentage of GFP+ cells from this experiment, normalized to transfection efficiency. n = 9. Lines represent the mean with SD. The numerical comparisons represent the P‐value determined using multiple t‐test with Holm–Sidak correction. (E) BRCA2 immunoblot showing depletion of BRCA2 in RMD‐GFP Rad52−/− mESCs, transfected with a pool of four BRCA2 siRNAs (siBRCA2). (∗) Nonspecific band. (F) Depletion of BRCA2 causes an increase in the ability of hRAD52 WT to promote SSA. RMD‐GFP Rad52−/− mESCs were transfected with the 268 bp and 9 kbp sgRNA/Cas9 expression vectors, either Flag‐hRAD52 WT or Flag‐hRAD52 S346X complementation vectors, along with a nontargeting siRNA (siCTRL) or siBRCA2. Shown is the percentage of GFP+ cells from this experiment, normalized to transfection efficiency. n = 9. Lines represent the mean with SD. The numbers shown above each comparison represent the P‐value determined using multiple t‐test with Holm–Sidak correction. (ns) Not significant.
2. Materials and methods
2.1. Association between RAD52 S346X and risk of developing breast and ovarian cancers in carriers of pathogenic BRCA1 and BRCA2 mutations
We initially identified the RAD52 S346X variant (NM_134424.3:c.1037C>A, rs4987207) in an African‐American breast cancer case while testing for mutations in DNA damage response genes (Fig. S1A). This variant was sufficiently common [minor allele frequency (MAF) of 0.017 in the ExAC database (Lek et al., 2016)] that we could investigate its association with cancer in BRCA1 and BRCA2 mutation carriers. In order to assess whether this mutation modified the risk of developing breast or ovarian cancer in women carrying pathogenic BRCA1 or BRCA2 mutations, we nominated this variant to the OncoArray project (Amos et al., 2017) for genotyping the BRCA1/2 mutation carriers in CIMBA (Chenevix‐Trench et al., 2007). All participants in CIMBA had been previously enrolled and consented to studies through their respective institutional review board‐approved protocols. The OncoArray also was run on samples in the Breast Cancer Association Consortium (BCAC) so we looked in the BCAC summary data for this specific SNP.
The association analysis of the RAD52 S346X variant with breast or ovarian cancer risk was carried out within a survival‐analysis framework. The time‐to‐event phenotype for each individual was defined by age at breast or ovarian cancer diagnosis or age at last follow‐up as described previously (Ding et al., 2012). Due to nonrandom sampling of BRCA1 and BRCA2 mutation carriers from different sites, a retrospective likelihood approach, developed by Antoniou et al. (2010) (Barnes et al., 2012) and implemented in the ‘retrolike1.0.3’ program, was used to model the retrospective likelihood of the observed S346X A allele conditional on age at cancer diagnosis or age at last follow‐up. In this model, breast or ovarian cancer incidence was assumed to depend on the underlying genotype through a Cox proportional hazards model; the magnitude of association was estimated as a per‐allele log‐relative hazards ratio (HR) in a multiplicative model where each individual has either zero, one, or two copies of minor allele A for the S346X variant.
2.2. Plasmids, cell lines, and siRNA
The Rad52−/−mouse embryonic stem cell (mESC) cell line harboring the repeat‐mediated deletion (RMD) green fluorescent protein (GFP)‐based assay reporter (RMD‐GFP) was previously reported (Mendez‐Dorantes et al., 2018). The RAD52KO human osteosarcoma U2OS cell line was previously reported (Kelso et al., 2019) and was derived from the U2OS Flp‐In T‐Rex cell line (Zhou et al., 2017). The plasmids for inducing DSBs in this reporter use single‐guide RNAs (sgRNAs) and Cas9, and are based on px330 (Ran et al., 2013). The 5’268 and 9.1 kbp sgRNA/Cas9 plasmids, pCAGGS‐BSKX empty expression vector (EV), and pCAGGS‐NZE‐GFP were described previously (Bennardo et al., 2008; Bennardo et al., 2009; Bhargava et al., 2017; Mendez‐Dorantes et al., 2018). The pCAGGS‐hRAD52 complementation vector was generated by amplifying the hRAD52 coding sequence from plasmid hRAD52‐GFP (from Simon Powell, Memorial Sloan Kettering Cancer Center) with the addition of a Kozak sequence, Flag tag, EcoRI site on the 5’ end, and an XhoI site on the 3′ end. The PCR product was digested and ligated into the EcoRI and XhoI sites of pCAGGS‐BSKX. The pCAGGS‐hRAD52 S346X plasmid was created by site‐directed mutagenesis using the Phusion Site‐Directed Mutagenesis Kit (Thermo Scientific, Waltham, MA USA). For depletion of BRCA2, we used either the nontargeting siRNA (siCTRL) (GE Dharmacon, Lafayette, CO, USA, D‐001810‐01) or a pool of four siRNAs of siBRCA2 (GE Dharmacon J‐042993‐05, J‐042993‐06, J‐042993‐07, and J‐042993‐08).
2.3. SSA DSB reporter assay
For the SSA reporter assay, 5.0 × 104 RMD‐GFP Rad52−/− mESCs were plated per well in a 24‐well plate. To compare wild‐type (WT) hRAD52 and hRAD52 S346X, each well was transfected with 200 ng of 5’268 and 9.1 kbp sgRNA/Cas9 plasmids and 200 ng of either pCAGGS, pCAGGS‐hRAD52, or pCAGGS‐hRAD52 S346X using 1.8 µL of Lipofectamine 2000. For the siRNA analysis, transfections included 5 pmol of siCTRL or siBRCA2 siRNAs. Transfection was performed in 0.5 mL of antibiotic‐free media for 4 h, after which the transfection media was replaced with 2 mL media containing antibiotics. The percentage of GFP+ cells was quantified by flow cytometry 3 days after transfection on a CyAn Advanced Digital Processing Analyzer (Dako, Carpinteria, CA, USA). For each experiment, the frequency of GFP+ cells was normalized to transfection efficiency, as described previously (Bhargava et al., 2018). Statistical analysis of the RMD reporter experiments was performed using the methods described in each figure legend. P‐values were adjusted for multiple comparisons.
2.4. Immunoblot analysis
To analyze cellular localization of hRAD52 and hRAD52 S346X, 2.0 × 105 RMD‐GFP Rad52−/− mESCs were plated per well in a 6‐well plate. Each well was transfected with 800 ng of either pCAGGS, pCAGGS‐hRAD52, or pCAGGS‐hRAD52 S346X using 7.2 µL of Lipofectamine 2000. Two days after transfection, the cells were collected and lysed using NETN buffer (20 mm Tris at pH 8.0, 100 mm NaCl, 1 mm EDTA, 0.5% Igepal, 1.25 mm DTT, Roche protease inhibitor, Basel, Switzerland) with multiple freeze/thaw cycles. Total cellular protein was fractionated by SDS/PAGE on NuPAGE 4–12% gradient gels (Invitrogen, Carlsbad, CA USA) and electrotransferred onto PVDF membranes. The membranes were probed with horseradish peroxidase (HRP)‐conjugated Flag (Sigma, A8592, St.Louis, MO, USA), BRCA2 (Abcam, ab27976, Cambridge, UK), actin (Sigma, A2066), and HRP goat anti‐rabbit secondary antibody (Abcam, ab205718) as appropriate, followed by addition of enhanced chemiluminescence reagent (Thermo Scientific) to develop horseradish peroxidase signals.
2.5. Immunofluorescence analysis
To assess the subcellular localization of hRAD52 WT and hRAD52 S346X, 2.0 × 105 RAD52KO U2OS cells (Kelso et al., 2019) were plated per well in a 12‐well plate. Each well was transfected with 400 ng of either pCAGGS‐hRAD52 or pCAGGS‐hRAD52 S346X using 3.6 µL of Lipofectamine 2000. Twenty‐four hours after transfection, the cells were plated onto chamber slides and then incubated for an additional 24 h. The slides were fixed with 4% paraformaldehyde and treated with 0.1 m glycine and 0.5% Triton X‐100 prior to probing with an antibody against Flag (Sigma, F3165), secondary Alexa Fluor 488r goat anti‐mouse antibody (Invitrogen, A11029), and DAPI using VECTASHIELD Mounting Medium (Vector Laboratories H1500, Burlingame, CA, USA). Confocal microscopy images were acquired at 63X magnification using the Zeiss LSM 880 (Oberkochen, Germany) Confocal Microscope along with ZEN Black image acquisition software.
3. Results
3.1. Association of RAD52 S346X with risk of breast or ovarian cancer in women carrying pathogenic BRCA1 or BRCA2 mutations
We tested the association of RAD52 S346X with risk of developing breast or ovarian cancer in a large cohort of BRCA1 and BRCA2 mutation carriers. The RAD52 S346X variant was genotyped on a custom Illumina array called OncoArray (Amos et al., 2017). We observed good separation of clusters for each of the three genotypes (Fig. S1B), and this variant passed the series of genotyping quality control steps developed for the OncoArray project (Amos et al., 2017). Of 15 679 BRCA1 mutation carriers, 459 were heterozygous and 3 were homozygous variant carriers (MAF = 0.017), and of 10 979 BRCA2 mutation carriers, 277 were heterozygous and 4 were homozygous variant carriers (MAF = 0.013). In assessing association of this variant with cancer, of the 15 679 BRCA1 carriers, 7889 and 2369 carriers were affected with breast and ovarian cancers, respectively; of 10 979 BRCA2 carriers, 5605 carriers and 2369 carriers were diagnosed with breast and ovarian cancers, respectively. RAD52 S346X was highly significantly associated with reduced risk of breast cancer for BRCA2 carriers (Table 1 and Table S1); each copy of the minor allele was estimated to confer a per‐allele HR of 0.69 (95% CI, 0.56 to 0.86, P = 0.0008). There also was evidence of association of RAD52 S346X with reduced breast cancer risk for BRCA1 carriers (Table 1 and Table S1). However, both the strength of association measured by P‐value of 0.02 and magnitude of association measured by per‐allele HR of 0.78 (95% CI of 0.64 to 0.97) were weaker compared to that observed for BRCA2 carriers. For ovarian cancers, although the HRs were of similar magnitude as for breast cancers in BRCA1 and BRCA2 carriers, respectively, the HRs were not significant (BRCA2 carriers, P = 0.10 and BRCA1 carriers, P = 0.11) likely reflecting the smaller number of events (Table 1 and Table S1). In the BCAC samples of 122 977 cases and 105 974 controls, the association was not significant (P‐value = 0.13); there were fewer carriers in the cases than the controls (regression coefficient = −0.047) (Michailidou et al., 2017).
Table 1.
Association of RAD52 S346X and risk of developing breast or ovarian cancer for BRCA1 and BRCA2 carriers. CI, confidence interval.
| Gene | Cancer | Sample size | Number of cases | P‐value | HR a | 95% CI |
|---|---|---|---|---|---|---|
| BRCA1 | Breast | 15 679 | 7889 | 0.02 | 0.78 | 0.64–0.97 |
| BRCA1 | Ovarian | 15 679 | 2369 | 0.11 | 0.79 | 0.60–1.05 |
| BRCA2 | Breast | 10 979 | 5605 | 0.0008 | 0.69 | 0.56–0.86 |
| BRCA2 | Ovarian | 10 979 | 848 | 0.10 | 0.71 | 0.47–1.07 |
The magnitude of association was estimated as a per‐allele hazards ratio (HR) in a multiplicative model where each individual has either zero, one, or two copies of minor allele A for the S346X variant.
3.2. Effect of RAD52 S346X on SSA frequency
The protective effect of RAD52 S346X in carriers of pathogenic variants of BRCA1 and BRCA2 may parallel the synthetic lethality observed upon simultaneous disruption of BRCA1/2 and hRAD52 (Feng et al., 2011; Lok et al., 2013). This synthetic lethality correlates with a synergistic reduction in the efficiency of DSB repair (Feng et al., 2011; Lok et al., 2013), suggesting that RAD52 S346X may decrease DSB repair. To test whether RAD52 S346X confers a defect in DSB repair, we used a recently developed reporter system to examine repair of DSBs by SSA, a mechanism supported by RAD52 in mammalian cells (Mendez‐Dorantes et al., 2018). This assay determines the frequency of repeat‐mediated deletion (RMD) in mouse embryonic stem cells (mESCs). Notably, mouse and human RAD52 proteins show substantial conservation (i.e., 69% identity and 80% similarity) (Muris et al., 1994), suggesting that hRAD52 could support SSA in Rad52−/− mESCs.
RMD by SSA is initiated by two DSBs positioned between a pair of nontandem repeats on the same chromosome. The DSBs are created at defined genomic locations through the catalytic activity of Cas9 programmed with specific single‐guide RNAs (sgRNAs) (Ran et al., 2013). The first DSB is introduced 268 bp downstream of the 5′ repeat, while the second DSB is introduced 9.1 kbp upstream of the 3′ repeat (Mendez‐Dorantes et al., 2018) (Fig. 1B). To study the function of hRAD52 in SSA, we cotransfected Rad52−/− mESCs containing a chromosomally integrated RMD‐GFP reporter with an empty expression vector (EV) or a vector containing either hRAD52 or hRAD52 S346X, as well as the sgRNA/Cas9 plasmids. Repair of the DSBs by SSA deletes the chromosomal DNA lying between the breaks and generates an intact GFP expression unit and GFP+ cells that can be scored by flow cytometric analysis. The frequency of RMD was calculated from the percentage of GFP+ cells observed three days after transfection.
As reported previously, the frequency of RMD was near the level of detection in EV‐transfected Rad52−/− mESCs, consistent with SSA being dependent on RAD52 (Mendez‐Dorantes et al., 2018). Importantly, transfection with the WT hRAD52 expression vector suppressed this defect to a similar degree (5.1% GFP+ cells) as expression of mouse RAD52, suggesting that human and mouse RAD52 possess similar capacities to support SSA in mESCs (Fig. 1D; Mendez‐Dorantes et al., 2018). Conversely, transfection with the hRAD52 S346X expression vector only partially suppressed the SSA defect, displaying RMD frequencies that were greater than twofold lower than in cells transfected with the WT hRAD52 expression plasmid (1.8% GFP+ cells, P < 0.0001; Fig. 1D). These findings suggest that hRAD52 S346X has a diminished capacity to promote SSA in mESCs. Immunoblot analysis indicated that similar levels of expression of the FLAG‐tagged WT and S346X proteins were expressed in these cells (Fig. 1C), suggesting that the differences in RMD frequency are unlikely to be due to different levels of WT and mutant hRAD52.
3.3. Depletion of BRCA2 causes an increase in RAD52‐dependent SSA
Because hRAD52 S346X was observed to exert a protective effect in carriers of pathogenic BRCA2 mutations (Table 1) and to diminish DSB repair (Fig. 1D), we examined how the interaction between the depletion of BRCA2 and expression of hRAD52 S346X affected DSB repair by SSA. Expressing siRNAs that depleted levels of BRCA2 in Rad52−/− mESCs (Fig. 1E) resulted in similarly increased frequencies of RMD in cells expressing either hRAD52 (adjusted P = 0.0043) or hRAD52 S346X (adjusted P = 0.0172) (Fig. 1F), indicating that BRCA2 exerts a suppressive effect on SSA that is consistent with previous findings (Stark et al., 2004). Further, the similar levels of stimulation observed upon depleting levels of BRCA2 in cells expressing WT and mutant hRAD52 indicate that the roles of BRCA2 and hRAD52 in determining levels of SSA are independent of one another. Additionally, depleting BRCA2 in conditions where hRAD52 S346X is expressed resulted in frequencies of SSA that were not significantly different than when hRAD52 is expressed without depletion of BRCA2 (siCTRL), suggesting that SSA would not become elevated upon inactivation of BRCA2 in the cells of hRAD52 S346X carriers.
3.4. RAD52 S346X is predominantly localized in the cytoplasm
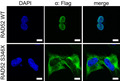
Because hRAD52 S346X is missing the C‐terminal nuclear localization sequence (NLS) of WT hRAD52 (Koike et al., 2013), we performed immunfluorescent staining and confocal microscopy to investigate the subcellular localization of the truncated protein. The Rad52KO U2OS human osteosarcoma cell line (Kelso et al., 2019) was transfected with expression vectors for WT or S346X hRAD52 tagged with a Flag epitope. Immunofluorescence analysis using anti‐Flag antibody showed that hRAD52 WT tagged with Flag localized to the nucleus, while hRAD52 S346X tagged with Flag predominantly localized to the cytoplasm (Fig. 2).
Fig. 2.
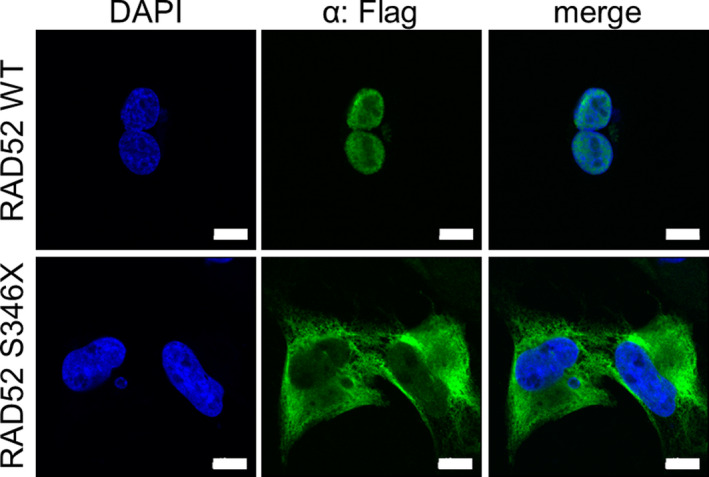
Subcellular localization of the WT and S346X RAD52 proteins tagged with Flag. RAD52KO cells transiently transfected with pCAGGS‐RAD52 WT or pCAGGS‐RAD52 S346X expression vectors were examined by immunofluorescence analysis with anti‐Flag antibody (green). Nuclei were stained with DAPI (blue). White scale bars, 10 µm.
4. Discussion
RAD52 is a protein that can mediate the annealing of complementary strands of DNA (Rothenberg et al., 2008; Symington, 2002) and has been shown to be important for repair of DSBs by SSA, an obligatorily mutagenic repair mechanism (Mendez‐Dorantes et al., 2018; Morales et al., 2015; Stark et al., 2004; Symington, 2002). hRAD52 S346X is a mutation that codes for a RAD52 protein with 17.2% of its amino acid sequence absent from its C terminus (Fig. 1A). We found that this mutation protected against the development of breast cancer in BRCA1/2 mutation carriers (Table 1). We further showed that hRAD52 S346X conferred a significantly reduced frequency of DSB repair by SSA in Rad52−/− mESCs compared to hRAD52. In contrast, attenuating levels of BRCA2 elevated the frequency of SSA, perhaps due to a reduction in the mediation of RAD51 recombinase function, which suppresses SSA by supporting HDR, a competitive mechanism of DSB repair that conserves genome structure (Bhargava et al., 2016). Importantly, hRAD52 S346X suppressed the elevated frequency of SSA caused by reducing the level of BRCA2. This suggests that hRAD52 S346X may suppress tumorigenesis in BRCA2‐deficient cells by suppressing the mutagenic effects of elevated SSA. Alternatively, the suppression of SSA by hRAD52 S346X may block tumor formation in BRCA2‐deficient cells because of their loss of two mechanisms of DSB repair, leading to the increased persistence of DSBs and apoptosis.
The last eight amino acids of mammalian RAD52 function as a nuclear localization sequence (NLS) (Koike et al., 2013). We hypothesized that the reduced DSB repair by SSA observed when hRAD52 S346X is expressed in Rad52−/− mESCs as compared to hRAD52 may be due to a lack of hRAD52 protein in the nucleus. Through immunostaining and confocal microscopy, we determined that hRAD52 S346X protein is predominantly localized to the cytoplasm. Therefore, we consider it likely that the reduction in nuclear hRAD52 S346X protein is responsible for the decreased repair of DSBs via SSA. Supporting our hypothesis, it has been shown that the protein encoded by the germline NEIL1 Q282X variant also lacks a C‐terminal NLS, resulting in reduced nuclear localization of the protein (Shinmura et al., 2015). The NEIL1 DNA glycosylase is involved in repair of oxidized bases via the base excision repair pathway, and the Q282X variant displays a diminished ability to suppress mutations (Shinmura et al., 2015). Since hRAD52 S346X still has all the other known functional domains (Fig. 1), it likely retains similar capabilities as that of the full‐length protein once in the nucleus. For example, hRAD52 S346X likely retains DNA‐binding capabilities based on previous findings that truncated hRAD52 peptides are as effective in strand annealing as that of full‐length hRAD52 (Kagawa et al., 2001; Ranatunga et al., 2001). Still, we acknowledge the possibility that the C‐terminal amino acids missing from hRAD52 S346X could play an as yet unknown role in SSA.
The Powell group made the important findings that reducing the cellular levels of RAD52 leads to synthetic lethality when levels of the HDR factors BRCA2, BRCA1, and PALB2, and the RAD51 paralogs are also reduced (Chun et al., 2013; Feng et al., 2011; Lok et al., 2013). The findings of Powell and others suggest that DSB repair by RAD52‐dependent HDR and/or SSA may act as backups to DSB repair by the canonical, BRCA‐mediated mechanism of HDR (Feng et al., 2011; Stark et al., 2004; Wray et al., 2008). Our results corroborate these findings, as we observed that the hRAD52 truncation variant is protective against development of cancer in women carrying pathogenic BRCA2 mutations and that this mutation confers a decrease in DSB repair by SSA.
We speculate that our observation that hRAD52 S346X confers both a loss of DSB repair and the protection of BRCA2‐mutation carriers against breast cancer will impact future cancer treatments. For instance, tumor cells from carriers of both pathogenic BRCA2 mutations and hRAD52 S346X should have a reduced ability to survive when exposed to chemotherapy‐induced DSBs. Recently, there has been a concerted effort to identify small molecular inhibitors of hRAD52 in the hope of clinically treating BRCA‐deficient breast and ovarian cancers (Chandramouly et al., 2015; Huang et al., 2016; Sullivan et al., 2016). Although no clinical trials have been reported for human cancers, a recent study showed that an inhibitor of RAD52 could reduce the growth of BRCA1‐deficient tumors in mice (Sullivan‐Reed et al., 2018). Interestingly, when these mice were treated with a combination of both RAD52 and PARP inhibitors, tumor growth was inhibited completely, suggesting a synergistic effect (Sullivan‐Reed et al., 2018). This finding is of particular interest due to the common phenomenon of acquired resistance to PARP inhibitors by BRCA‐deficient tumors (Lord and Ashworth, 2013). Ideally, future treatment of HDR‐deficient cancers will employ the synergistic effect of combining inhibitors of multiple DNA repair pathways. Further, evaluating the status of hRAD52 in BRCA‐deficient tumors may be advised as tumors that are defective for both hRAD52 and BRCA2 could show a more lasting response to PARP‐inhibitor treatment.
The physiological relevance of reduced SSA activity could affect other RAD52‐dependent events apart from the repair of DSBs by SSA per se. In addition to the repair of DSBs via SSA and HDR, hRAD52 has been implicated in break‐induced replication (BIR) (Sotiriou et al., 2016), transcription‐coupled homologous recombination (Teng et al., 2018), processing of stalled replication forks (Malacaria et al., 2019), mitotic DNA synthesis (MiDAS) (Bhowmick et al., 2016), and alternative lengthening of telomeres (Zhang et al., 2019), as well as several other pathways (Jalan et al., 2019). Thus, hRAD52 likely plays an important role in maintaining the viability of cancer cells under replication stress and could help explain the protective effect of hRAD52 S346X. Because replication stress is so prominent in cancer, it is possible that treatment with RAD52 inhibitors will have therapeutic effect even in HDR‐proficient tumor cells.
5. Conclusion
This is the first study to report the protective effect of a germline coding variant, hRAD52 S346X in individuals carrying germline pathogenic variants in the homologous recombination gene, BRCA2. This RAD52 mutation causes a reduction in DSB repair by SSA. There likely are additional variants in other DNA repair genes that by themselves do not significantly alter risk of developing cancer, but have profound effects in combination with a pathogenic variant in a second gene also involved in DNA repair.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
SNL and YCD conceived the project. SNL and JMS designed the project. AWA performed the experiments. YCD, AWA, and CMD analyzed the data. AWA, YCD, AMB, JMS, and SNL wrote and edited the manuscript. All authors approved the final manuscript.
Supporting information
Fig. S1. (A) Initial S346X mutation detection by Sanger sequencing; (B) Clustering of the S346X genotypes from the Illumina Oncoarray.
Table S1. Sample distribution by gene, cancer, and RAD52 S346X genotypes.
Acknowledgements
We thank Felicia Wednesday Lopezcolorado and Eva Jahanshir for technical assistance. We also thank Dr Simon Powell for the hRAD52‐GFP plasmid. This work was funded by the Morris and Horowitz Families Endowed Professorship (SLN), National Institutes of Health (NIH R01CA184585 to SNL, R01CA197506 to JMS, and R50CA211280 to AWA). Research reported in this publication included work performed in the Analytical Cytometry Core and the Light Microscopy Core supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA33572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Aaron W. Adamson and Yuan Chun Ding contributed equally to this article
References
- Amos CI, Dennis J, Wang Z, Byun J, Schumacher FR, Gayther SA, Casey G, Hunter DJ, Sellers TA, Gruber SB et al (2017) The OncoArray consortium: a network for understanding the genetic architecture of common cancers. Cancer Epidemiol Biomarkers Prev 26, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou AC, Wang X, Fredericksen ZS, McGuffog L, Tarrell R, Sinilnikova OM, Healey S, Morrison J, Kartsonaki C, Lesnick T et al (2010) A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor‐negative breast cancer in the general population. Nat Genet 42, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DR, Lee A, Investigators E, kConFab I, Easton DF and Antoniou AC (2012) Evaluation of association methods for analysing modifiers of disease risk in carriers of high‐risk mutations. Genet Epidemiol 36, 274–291. [DOI] [PubMed] [Google Scholar]
- Bennardo N, Cheng A, Huang N and Stark JM (2008) Alternative‐NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet 4, e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N, Gunn A, Cheng A, Hasty P and Stark JM (2009) Limiting the persistence of a chromosome break diminishes its mutagenic potential. PLoS Genet 5, e1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Carson CR, Lee G and Stark JM (2017) Contribution of canonical nonhomologous end joining to chromosomal rearrangements is enhanced by ATM kinase deficiency. Proc Natl Acad Sci USA 114, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Onyango DO and Stark JM (2016) Regulation of single‐strand annealing and its role in genome maintenance. Trends Genet 32, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Sandhu M, Muk S, Lee G, Vaidehi N and Stark JM (2018) C‐NHEJ without indels is robust and requires synergistic function of distinct XLF domains. Nat Commun 9, 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick R, Minocherhomji S and Hickson ID (2016) RAD52 facilitates mitotic DNA synthesis following replication stress. Mol Cell 64, 1117–1126. [DOI] [PubMed] [Google Scholar]
- Brouwer I, Zhang H, Candelli A, Normanno D, Peterman EJG, Wuite GJL and Modesti M (2017) Human RAD52 captures and holds DNA strands, increases DNA flexibility, and prevents melting of duplex DNA: implications for DNA recombination. Cell Rep 18, 2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Rondinelli B and D'Andrea AD (2016) Repair pathway choices and consequences at the double‐strand break. Trends Cell Biol 26, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouly G, McDevitt S, Sullivan K, Kent T, Luz A, Glickman JF, Andrake M, Skorski T and Pomerantz RT (2015) Small‐molecule disruption of RAD52 rings as a mechanism for precision medicine in BRCA‐deficient cancers. Chem Biol 22, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevix‐Trench G, Milne RL, Antoniou AC, Couch FJ, Easton DF, Goldgar DE and Cimba (2007) An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Buechelmaier ES and Powell SN (2013) Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1‐BRCA2‐dependent homologous recombination pathway. Mol Cell Biol 33, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YC, McGuffog L, Healey S, Friedman E, Laitman Y, Paluch‐Shimon S, Kaufman B, Swe B, Liljegren A, Lindblom A et al (2012) A nonsynonymous polymorphism in IRS1 modifies risk of developing breast and ovarian cancers in BRCA1 and ovarian cancer in BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 21, 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Scott SP, Bussen W, Sharma GG, Guo G, Pandita TK and Powell SN (2011) Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc Natl Acad Sci USA 108, 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Goyal N, Sullivan K, Hanamshet K, Patel M, Mazina OM, Wang CX, An WF, Spoonamore J, Metkar S et al (2016) Targeting BRCA1‐ and BRCA2‐deficient cells with RAD52 small molecule inhibitors. Nucleic Acids Res 44, 4189–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalan M, Olsen KS and Powell SN (2019) Emerging roles of RAD52 in genome maintenance. Cancers 11, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa W, Kurumizaka H, Ikawa S, Yokoyama S and Shibata T (2001) Homologous pairing promoted by the human Rad52 protein. J Biol Chem 276, 35201–35208. [DOI] [PubMed] [Google Scholar]
- Kelso AA, Lopezcolorado FW, Bhargava R and Stark JM (2019) Distinct roles of RAD52 and POLQ in chromosomal break repair and replication stress response. PLoS Genet 15, e1008319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Yutoku Y and Koike A (2013) The C‐terminal region of Rad52 is essential for Rad52 nuclear and nucleolar localization, and accumulation at DNA damage sites immediately after irradiation. Biochem Biophys Res Commun 435, 260–266. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell‐Luria AH, Ware JS, Hill AJ, Cummings BB et al (2016) Analysis of protein‐coding genetic variation in 60,706 humans. Nature 536, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok BH, Carley AC, Tchang B and Powell SN (2013) RAD52 inactivation is synthetically lethal with deficiencies in BRCA1 and PALB2 in addition to BRCA2 through RAD51‐mediated homologous recombination. Oncogene 32, 3552–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CJ and Ashworth A (2013) Mechanisms of resistance to therapies targeting BRCA‐mutant cancers. Nat Med 19, 1381–1388. [DOI] [PubMed] [Google Scholar]
- Malacaria E, Pugliese GM, Honda M, Marabitti V, Aiello FA, Spies M, Franchitto A and Pichierri P (2019) Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation. Nat Commun 10, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez‐Dorantes C, Bhargava R and Stark JM (2018) Repeat‐mediated deletions can be induced by a chromosomal break far from a repeat, but multiple pathways suppress such rearrangements. Genes Dev 32, 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou K, Lindstrom S, Dennis J, Beesley J, Hui S, Kar S, Lemacon A, Soucy P, Glubb D, Rostamianfar A et al (2017) Association analysis identifies 65 new breast cancer risk loci. Nature 551, 92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales ME, White TB, Streva VA, DeFreece CB, Hedges DJ and Deininger PL (2015) The contribution of alu elements to mutagenic DNA double‐strand break repair. PLoS Genet 11, e1005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris DF, Bezzubova O, Buerstedde JM, Vreeken K, Balajee AS, Osgood CJ, Troelstra C, Hoeijmakers JH, Ostermann K, Schmidt H et al (1994) Cloning of human and mouse genes homologous to RAD52, a yeast gene involved in DNA repair and recombination. Mutat Res 315, 295–305. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA and Zhang F (2013) Genome engineering using the CRISPR‐Cas9 system. Nat Protoc 8, 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga W, Jackson D, Lloyd JA, Forget AL, Knight KL and Borgstahl GE (2001) Human RAD52 exhibits two modes of self‐association. J Biol Chem 276, 15876–15880. [DOI] [PubMed] [Google Scholar]
- Rothenberg E, Grimme JM, Spies M and Ha T (2008) Human Rad52‐mediated homology search and annealing occurs by continuous interactions between overlapping nucleoprotein complexes. Proc Natl Acad Sci USA 105, 20274–20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Kato H, Kawanishi Y, Goto M, Tao H, Inoue Y, Nakamura S and Sugimura H (2015) NEIL1 p.Gln282Stop variant is predominantly localized in the cytoplasm and exhibits reduced activity in suppressing mutations. Gene 571, 33–42. [DOI] [PubMed] [Google Scholar]
- Sotiriou SK, Kamileri I, Lugli N, Evangelou K, Da‐Re C, Huber F, Padayachy L, Tardy S, Nicati NL, Barriot S et al (2016) Mammalian RAD52 functions in break‐induced replication repair of collapsed DNA replication forks. Mol Cell 64, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JM, Pierce AJ, Oh J, Pastink A and Jasin M (2004) Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol Cell Biol 24, 9305–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Cramer‐Morales K, McElroy DL, Ostrov DA, Haas K, Childers W, Hromas R and Skorski T (2016) Identification of a small molecule inhibitor of RAD52 by structure‐based selection. PLoS ONE 11, e0147230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan‐Reed K, Bolton‐Gillespie E, Dasgupta Y, Langer S, Siciliano M, Nieborowska‐Skorska M, Hanamshet K, Belyaeva EA, Bernhardy AJ, Lee J et al (2018) Simultaneous targeting of PARP1 and RAD52 triggers dual synthetic lethality in BRCA‐deficient tumor cells. Cell Rep 23, 3127–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington LS (2002) Role of RAD52 epistasis group genes in homologous recombination and double‐strand break repair. Microbiol Mol Biol Rev 66, 630–670, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Yadav T, Duan M, Tan J, Xiang Y, Gao B, Xu J, Liang Z, Liu Y, Nakajima S et al (2018) ROS‐induced R loops trigger a transcription‐coupled but BRCA1/2‐independent homologous recombination pathway through CSB. Nat Commun 9, 4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J, Liu J, Nickoloff JA and Shen Z (2008) Distinct RAD51 associations with RAD52 and BCCIP in response to DNA damage and replication stress. Can Res 68, 2699–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Yadav T, Ouyang J, Lan L and Zou L (2019) Alternative Lengthening of Telomeres through Two Distinct Break‐Induced Replication Pathways. Cell Rep 26, 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lee JH, Jiang W, Crowe JL, Zha S and Paull TT (2017) Regulation of the DNA damage response by DNA‐PKcs inhibitory phosphorylation of ATM. Mol Cell 65, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) Initial S346X mutation detection by Sanger sequencing; (B) Clustering of the S346X genotypes from the Illumina Oncoarray.
Table S1. Sample distribution by gene, cancer, and RAD52 S346X genotypes.


