Abstract
Introduction
Differential diagnosis of metaplastic squamous cell carcinoma of breast (MSCCB) is difficult. In particular, in terms of metastatic MSCCB, because of the low speciality of traditional markers such as mammaglobin, gross cystic disease fluid protein-15 (GCDFP-15) and GATA binding protein 3 (GATA3), the most common problem is differentiating the spread of MSCCB to the lung from a primary lung squamous cell carcinoma. It is urgently required to explore a novel marker to aid in differential diagnosis.
Aim
The aim of this study is to explore a novel marker to aid in the differential diagnosis of MSCCB from other squamous cell carcinomas (SCC) in other organs.
Methods
We tested the expression of SOX10 in 375 human SCC specimens with immunohistochemistry (IHC).
Results
In a series of 20 MSCCB, 9 (45%) were positive for SOX10. All of them were triple-negative MSCCB. Conversely, SOX10 was totally negative in another 205 SCC originating from lung, skin, cervix, oral mucosa, and esophagus. In a series of 150 triple-negative breast cancer and their metastatic foci, SOX10 labeling in the primary tumor and metastasis was 78% and 79.3%, respectively, and the agreement rate was 97.3% (P>0.05).
Conclusion
Our findings demonstrate that SOX10 was recommended for differentiating MSCCB from non-mammary metastasis to the breast, as well as for distinguishing primary SCC from metastatic MSCCB, and SOX10 may be valuable in the pathological diagnosis of breast-derived metaplastic squamous cell carcinoma.
Keywords: SOX10, squamous cell carcinoma, breast, lung, differential diagnosis
Introduction
Metaplastic breast cancer encompasses a heterogeneous group of malignant neoplasms having dominant areas of non-glandular (squamous, spindle cell, and/or mesenchymal) differentiation.1 Metaplastic squamous cell carcinoma of the breast (MSCCB) is an epithelial type of metaplastic breast cancer with poor prognosis.2,3 It accounts for 0.06–0.1% of all invasive breast carcinomas, affecting patients aged between 20 and 90 years with an average onset age of 54 years.1,4-6 The pathological diagnostic criteria of MSCCB include: a) more than 90% of the tumor cells are squamous, and other components could include spindle cells, ductal cells, osteocytes, chondrocytes, and striated muscle cells;6–8 b) the origin is independent of primary SCC in a distant site from the skin;1,9-11 and c) is possible after excluding the nipple or the overlying skin primary lesion.5,8,12,13
When SCC is found in the breast, the origin of the lesion should be sought due to the rarity of MSCCB. Extra-mammary SCCs have a tendency to metastasize to the breast; the most common primary sites are lung, esophagus, cervix, and urinary bladder.1 In addition, the lung is the most common organ of metastasis during the progression of MSCCB.14–16 Recently, some studies showed that a patient with a history of breast cancer has a higher risk of developing other primary non-mammary cancer types, particularly in the lung.17–19 When a lung nodule is found in a patient with a history of MSCCB, it can be difficult to diagnose definitively whether it is metastatic MSCCB or primary lung squamous cell carcinoma (LUSC). The morphologic features of MSCCB and LUSC seem to be similar. The management and prognosis are completely different for primary LUSC and MSCCB metastatic to the lung, so distinguishing the two scenarios are important. But until now, there has been no efficient marker to distinguish these two cases.
As traditional markers for breast cancer, mammaglobin, gross cystic disease fluid protein-15 (GCDFP-15), and GATA binding protein 3 (GATA3) demonstrate limited sensitivity; mammaglobin: 50–55%, GCDFP-15: 45–23%, GATA3: 67–72%; additionally, they are also expressed in lung squamous cell carcinoma at a lower proportion.20 Lung carcinomas usually express markers of squamous differentiation, such as p63, p40, and cytokeratin (CK) 5/6.21 However, these markers are also expressed in MSCCB to a lower extent.16 Therefore, it is essential to find a more effective immunohistochemical marker to assist in a definite diagnosis.
The Sry-related HMG box10 gene (SOX10) encodes a transcription factor that plays a crucial role in the survival, maturation, and differentiation of neural crest-derived melanocytes and glia.22,23 SOX10 was reported to be preferentially expressed in metaplastic breast carcinomas.24 Because of fewer cases (only 4 cases of metaplastic carcinomas mixed squamous with spindled and chondroid components), the expression of SOX10 in MSCCB remains unknown. Therefore, we investigated SOX10 labeling in MSCCB and SCC in other organs, and retrospectively reviewed the use of SOX10 immunohistochemistry (IHC) in the differential diagnosis of squamous cell carcinomas, including metastatic cases.
Patients and Methods
Patient Data
The Medical Ethics Committee of Xiangya Hospital and the Third Xiangya Hospital of Central South University, and Hunan Provincial People’s Hospital approved this study. Three hundred seventy-five cases of tumor samples between January 2010 and December 2018 were obtained from Xiangya Hospital and the Third Xiangya Hospital of Central South University, and Hunan Provincial People’s Hospital. The pathological diagnosis was confirmed by two senior pathologists.
Immunohistochemistry and Scoring
The slides for immunohistochemical analysis were produced by cutting ten histological sections to 3-μm thickness. Markers used for IHC experiments were SOX10 (EP-268, Fuzhou Maixin Biotechnologies, China), ER (EP1, Fuzhou Maixin Biotechnologies, China), PR (EP2, Fuzhou Maixin Biotechnologies, China), and HER2 (EP-3, Fuzhou Maixin Biotechnologies, China).
The paraffin-embedded sections were heated for 2 h at 65°C, deparaffinized by routine techniques, and subjected to high-PH epitope retrieval for 8 min. Sections were incubated overnight at 4°C with primary antibody and were subsequently incubated with anti-rabbit polymer (PV-9000, OriGene) for 30 min at room temperature. The slides were developed with DAB for 5 min, and followed by weakly hematoxylin counterstaining.
For ER, PR, and SOX10, the reactivity assessed was nuclear. For HER2, the reactivity assessed was membranous. The staining was considered positive when there was nuclear immunoreactivity in ≥1% of the tumor cells for ER and PR, whereas the staining intensity in the nuclei should be recorded and reported as weak, moderate, or strong.25 According to the American Society of Clinical Oncology guidelines, HER2 immunostaining was evaluated as 0, 1+, 2+, and 3+.26 For SOX10, <1% nuclear positivity was considered a negative result. The intensity and localization of the immunoreactivity were examined with a photomicroscope (Magscanner KF-PRO-005).
Fluorescence in situ Hybridization on Paraffin-Embedded Tissue Sections for HER2 Amplification
The paraffin-embedded tissues were cut into 3–5-µm sections using a microtome. One HE stained slide from each patient was examined by an expert pathologist to mark the malignant cell areas. The sections were placed on positive-charged slides, deparaffinized and rehydrated through an ethanol series, and were subsequently air-dried. The dual HER2/Cep17 probe was applied to malignant cells. HER2 signals were counted in at least 20 cell nuclei from at least two areas of the invasive tumor under a fluorescent microscope system. The interpretation criterion of FISH signals for positive was based on ASCO/CAP 2018 HER2 Test Guidelines and Recommendations for tumors with 2+ staining.26
Statistical Analysis
The findings were analyzed with statistical software SPSS for Windows, Version 18. Statistical significance was established at P<0.05.
Results
The Expression of SOX10 in MSCCB and Other Squamous Cell Carcinomas
We identified 20 MSCCB cases, including 3 luminal subtypes, 5 HER2 positive subtypes, and 12 triple-negative subtypes. All patients were female, with an average age at diagnosis of 49 years old (27–61 years). Of these 3 luminal A subtype cases, 2 cases were weakly positive for hormonal receptor (HR), and 1 case was moderate positive. The HER2 status was confirmed by IHC, and of those, 3 cases were positive for HER2 amplification by IHC, and 2 cases were positive (2+) on IHC and further confirmed positive by FISH. SOX10 expression in different molecular subtypes of MSCCB is presented in Table 1. Of 20 MSCCB cases, 12 (60%) of the MSCCB cases were negative for ER, PR, and HER2. In addition, the SOX10 labeling was only seen in TNBC, with labeling in 75% (9/12) of TNBC in contrast with luminal carcinomas (0/3) and HER2 positive carcinomas (0/5). SOX10 was totally negative in squamous cell carcinoma of lung (0/50, vs MSCCB, P<0.001), skin (0/55, vs MSCCB, P<0.001), cervix (0/30, vs MSCCB, P<0.001), oral cavity (0/25, vs MSCCB, P<0.001) and esophagus (0/45, vs MSCCB, P<0.001) (Table 2). The IHC staining patterns of representative cases are illustrated in Figure 1.
Table 1.
SOX10 Expression in Different Molecular Types in Metaplastic Squamous Carcinoma
| Molecular Subtypes | SOX10 Positive Ratio n/N |
|---|---|
| Lumina A | 0/3 (0%) |
| HER-2+ | 0/5 (0%) |
| TNBC | 9/12 (75%) |
| Total | 9/20 (45%) |
Abbreviations: SOX10, sex-determining region Y-related high mobility group-box 10; HER2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
Table 2.
Expression of SOX10 in Other Squamous Cell Carcinoma
| Tumor Type | SOX10 n/N (%) |
|---|---|
| Lung squamous cell carcinoma | 0/50 (0%) |
| Oral squamous cell carcinoma | 0/25 (0%) |
| Esophageal squamous cell carcinoma | 0/45 (0%) |
| Cervical squamous cell carcinoma | 0/30 (0%) |
| Skin squamous cell carcinoma | 0/55 (0%) |
Figure 1.
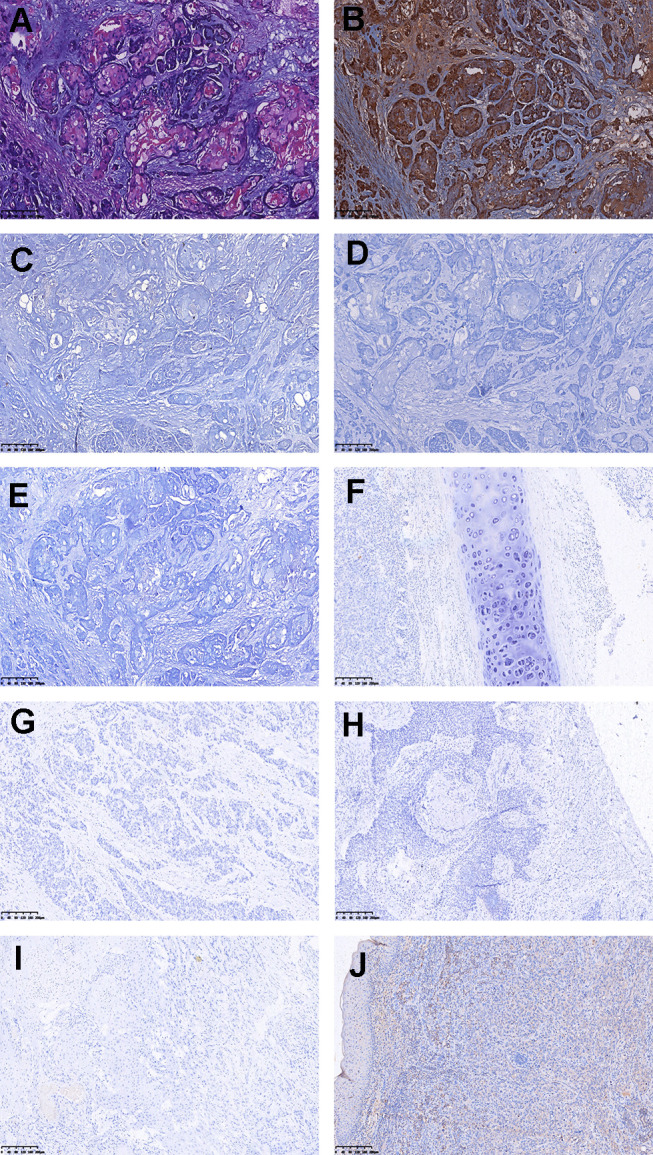
Representative immunohistochemical profiles of SCC. (A) Photomicrograph hematoxylin-eosin staining of MSCCB. (B) MSCCB is positive for SOX10. (C–E) MSCCB is negative for ER, PR, HER2. (F–J) Lung, esophageal, cervical, oral, and skin of SCC are negative for SOX10. (A–J) Original magnification × 10.
The Expression of SOX10 Between Primary Triple-Negative Breast Cancer and Their Metastatic Foci
To investigate the consistency of SOX10 expression between primary and metastatic foci, we analyzed 150 cases of TNBC with axillary or supraclavicular lymph node metastasis or with pulmonary metastasis, including 2 cases of MSCCB with pulmonary metastasis. SOX10 labeling in primary tumor and metastasis was 78% and 79.3%, respectively, and the agreement rate was 97.3% (P>0.05, Table 3). Both pulmonary metastatic lesions showed positive as well.
Table 3.
The Expression of SOX10 in Primary Triple-Negative Breast Cancer and Lymph Node Metastases
| Marker | Primary Tumor | Total | |
|---|---|---|---|
| Negative | Positive | ||
| SOX10 | |||
| Negative metastases | 30 | 1 | 31 |
| Positive metastases | 3 | 116 | 119 |
| Total | 33 | 117 | 150 |
Discussion
MSCCB is the most common histological type of metaplastic breast cancer.27,28 Most of them display morphological similarity, showing intracellular bridges, central keratinization, and pearl formation, which is similar to SCC in other organs.11 At the same time, the majority of MSCCBs are typically of the triple-negative breast cancer phenotype, so the diagnosis of MSCCB may be difficult due to reduced sensitivity of traditional markers, including GATA3, mammaglobin, and GCDFP-15 in breast cancer.29,30 Determining a lesion as MSCCB sometimes became very difficult, especially in the following situations. Firstly, it may be uncertain whether a superficial squamous cell carcinoma with an ulcer in the breast originates from skin squamous epithelium or from MSCCB. Secondly, some recent studies showed that patients with a history of breast cancer (especially HR-negative subtypes) have an increased risk of developing a second non-breast cancer, especially lung cancer,31 and lung metastasis can be a major site of tumor relapse among breast cancer patients.16 Therefore, distinguishing MSCCB metastatic to the lung from primary LUSC can be challenging for pathologists in a patient with MSCCB history. Thirdly, when a breast nodule is found in a patient with a history of other cancers, it may be difficult to determine whether it is primary breast cancer or just metastases, because some cancers might metastasize to breast too.
The Sry-related HMG box10 gene (SOX10) encodes a transcription factor that plays a crucial role in the survival, maturation, and differentiation of neural crest-derived melanocytes and glia.22,23 It is expressed in normal salivary gland tissue, breast myoepithelial cells, and bronchial cells.30,32 In clinical practice, SOX10 labeling was primarily used in melanoma, peripheral nerve sheath tumors, and salivary gland myoepitheliomas.24,32-34 Recently, SOX10 was also reported to be preferentially expressed in triple-negative breast carcinomas.24 As MSCCB often showed a triple-negative phenotype how will SOX10 function in this dilemma?
This study evaluated SOX10 expression in MSCCB and other primary SCCs. As a result, we found that SOX10 labeled 45% (9/20) of MSCCBs, and 0% of non-mammary SCCs, including lung squamous cell carcinoma, oral squamous cell carcinoma, esophageal squamous cell carcinoma, cervical squamous cell carcinoma, and skin squamous cell carcinoma.
Because the phenotype of ER/HER2 positive is more common in breast cancer, it is reasonable to speculate that a lung squamous carcinoma with ER/HER2 positive is a metastatic focus, especially in a patient with a history of breast cancer. In fact, SOX10 labeled 75% (9/12) triple-negative MSCCBs, if we ruled out ER/HER2 positive cases. This finding indicated that SOX10 labeling might be used to support MSCCB, especially in a “triple-negative” case, as well as to differentiate a metastasis from other SCCs in the breast. In addition, we found HR labeling in 15% (3/20) of MSCCBs, with HER2 labeling seen in 25% (5/20) of MSCCBs, all of which were negative for SOX10, which indicated that SOX10 might not be suitable to differentiate non-triple-negative MSCCBs. However, data from the literature indicated SOX10 expression was observed in a small fraction of SCC from lung (2/71, 2.8%) at the table,35 which is a little different from our results; we attribute this phenomenon to be related to the specificity of SOX10 antibodies from different manufacturers. However, its positive rate is far lower than that in triple-negative MSCCB (2.8% vs 75%). Hence, it is reasonable to speculate that combining SOX10 with ER, PR, and HER2 may be helpful to differentiate primary MSCCB from other SCCs, including metastases to the breast.
Metastasis is common in patients with breast cancer. Some studies indicated that gene expression might be altered during the course of this disease.30,36 In this study, we tested 150 TNBC cases with metastasis, including 2 cases of MSCCB with pulmonary metastasis to confirm the influence of metastasis on the expression of SOX10. We found that SOX10 labeling in primary TNBC and metastasis was 78% and 79.3%, respectively, and the coincidence rate of the results was 97.3%. Both pulmonary metastatic lesions were positive. This suggests that SOX10 does not show a significant loss of expression during metastasis of triple-negative breast cancer. So, we speculate that SOX10 might serve as a useful marker for triple-negative MSCCB, especially in a squamous lesion of unknown origin.
Conclusion
Positive of SOX10 in tumor cells supports the diagnosis of MSCCB, instead of primary SCC, as well as in newly lesion occur in local breast with a history of MSCCB. SOX10 staining can also be used to distinguish MSCCB from non-mammary metastasis to the breast, as well as to distinguish primary LUSC from MSCCB metastatic to the lung. SOX10 staining might serve as a useful marker for pathologic diagnosis of MSCCB, as it can help in deciding the appropriate treatment regimen and prognosis, and can even prevent unnecessary surgery.
Acknowledgment
The authors thank Xiangya Hospital of Central South University, the Third Xiangya Hospital of Central South University, and Hunan Provincial People’s Hospital for paraffin-embedded tumor samples.
Ethics Approval
The Medical Ethics Committee of Xiangya Hospital and the Third Xiangya Hospital of Central South University, and Hunan Provincial People’s Hospital approved this study. All patients provided written informed consent, in accordance with the Declaration of Helsinki.
Disclosure
The authors declare no conflict of interest.
References
- 1.Graziano L, Graziano Filho P, Bitencourt AGV, et al. Metaplastic squamous cell carcinoma of the breast: a case report and literature review. Rev Assoc Med Bras. 2016;62(7):618–621. doi: 10.1590/1806-9282.62.07.618 [DOI] [PubMed] [Google Scholar]
- 2.Benoist P, Mureau A, Joueidi Y, et al. Management and prognosis of pure primary squamous cell carcinoma of the breast. J Gynecol Obstet Hum Reprod. 2018;47(7):275–280. doi: 10.1016/j.jogoh.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 3.Honda M, Saji S, Horiguchi S-I, et al. Clinicopathological analysis of ten patients with metaplastic squamous cell carcinoma of the breast. Surg Today. 2011;41(3):328–332. doi: 10.1007/s00595-009-4276-2 [DOI] [PubMed] [Google Scholar]
- 4.Nayak A, Wu Y, Gilcrease MZ. Primary squamous cell carcinoma of the breast: predictors of locoregional recurrence and overall survival. Am J Surg Pathol. 2013;37(6):867–873. doi: 10.1097/PAS.0b013e3182877569 [DOI] [PubMed] [Google Scholar]
- 5.Punzo C, Fortarezza F, De Ruvo V, et al. Primitive squamous cell carcinoma of the breast (SCCB): case report of an uncommon variant of metaplastic carcinoma. G Chir. 2017;38(3):139–142. doi: 10.11138/gchir/2017.38.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oikawa M, Igawa A, Taguchi K, et al. Cytogenetic analysis of metaplastic squamous cell carcinoma of the breast inter- and intratumoral heterogeneity. Breast Cancer. 2017;24(6):733–741. doi: 10.1007/s12282-017-0768-x [DOI] [PubMed] [Google Scholar]
- 7.Seddik Y, Brahmi SA, Afqir S. Primary squamous cell carcinoma of the breast: a case report and review of literature. Pan Afr Med J. 2015;20:152. doi: 10.11604/pamj.2015.20.152.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macia M, Ces JA, Becerra E, et al. Pure squamous carcinoma of the breast. Report of a case diagnosed by aspiration cytology. Acta Cytol. 1989;33(2):201–204. [PubMed] [Google Scholar]
- 9.Gupta N, Nimbran V, Dhingra N, et al. Primary squamous cell carcinoma of the breast: case report and management decisions. J Cancer Res Ther. 2012;8(2):323–325. doi: 10.4103/0973-1482.99006 [DOI] [PubMed] [Google Scholar]
- 10.Behranwala KA, Nasiri N, Abdullah N, et al. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29(4):386–389. doi: 10.1053/ejso.2002.1422 [DOI] [PubMed] [Google Scholar]
- 11.Yasui M, Hasegawa Y, Kawahara T, et al. Squamous cell carcinoma of the breast as a clinical diagnostic challenge. Mol Clin Oncol. 2018;8(4):587–591. doi: 10.3892/mco.2018.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi R, Horii R, Maeda I, et al. Clinicopathologic study of 53 metaplastic breast carcinomas: their elements and prognostic implications. Hum Pathol. 2010;41(5):679–685. doi: 10.1016/j.humpath.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Shigekawa T, Tsuda H, Sato K, et al. Squamous cell carcinoma of the breast in the form of an intracystic tumor. Breast Cancer. 2007;14(1):109–112. doi: 10.2325/jbcs.14.109 [DOI] [PubMed] [Google Scholar]
- 14.Rayson D, Adjei AA, Suman VJ, et al. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999;10(4):413–419. doi: 10.1023/A:1008329910362 [DOI] [PubMed] [Google Scholar]
- 15.Barr JG, Jane Clayton ES, Sotheran W. A case of metaplastic breast cancer in a man. J Surg Case Rep. 2013;2013(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DN, Kawamoto S, Cimino-Mathews A, et al. Metastatic metaplastic breast carcinoma mimicking pulmonary squamous cell carcinoma on fine-needle aspiration. Diagn Cytopathol. 2015;43(10):844–849. doi: 10.1002/dc.23321 [DOI] [PubMed] [Google Scholar]
- 17.Evans HS, Lewis CM, Robinson D, et al. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84(3):435–440. doi: 10.1054/bjoc.2000.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26(8):1239–1246. doi: 10.1200/JCO.2007.11.9081 [DOI] [PubMed] [Google Scholar]
- 19.Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118(9):2285–2292. doi: 10.1002/ijc.21651 [DOI] [PubMed] [Google Scholar]
- 20.Hattori Y, Yoshida A, Yoshida M, et al. Evaluation of androgen receptor and GATA binding protein 3 as immunohistochemical markers in the diagnosis of metastatic breast carcinoma to the lung. Pathol Int. 2015;65(6):286–292. doi: 10.1111/pin.12278 [DOI] [PubMed] [Google Scholar]
- 21.Kriegsmann K, Cremer M, Zgorzelski C, et al. Agreement of CK5/6, p40, and p63 immunoreactivity in non-small cell lung cancer. Pathology. 2019;51(3):240–245. doi: 10.1016/j.pathol.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 22.Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene. 2003;22(20):3024–3034. doi: 10.1038/sj.onc.1206442 [DOI] [PubMed] [Google Scholar]
- 23.Rooper LM, McCuiston AM, Westra WH, et al. SOX10 immunoexpression in basaloid squamous cell carcinomas: a diagnostic pitfall for ruling out salivary differentiation. Head Neck Pathol. 2019;13:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimino-Mathews A, Subhawong AP, Elwood H, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44(6):959–965. doi: 10.1016/j.humpath.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond ME, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi: 10.1200/JOP.777003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 27.McCart Reed AE, Kalaw E, Nones K, et al. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications. J Pathol. 2019;247(2):214–227. doi: 10.1002/path.5184 [DOI] [PubMed] [Google Scholar]
- 28.Rakha EA, Tan PH, Varga Z, et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer. 2015;112(2):283–289. doi: 10.1038/bjc.2014.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis GH, Subhawong AP, Nassar H, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135(4):587–591. doi: 10.1309/AJCPMFR6OA8ICHNH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tozbikian GH, Zynger DL. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple-negative breast cancer. Hum Pathol. 2019;85:221–227. doi: 10.1016/j.humpath.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 31.Schonfeld SJ, Curtis RE, Anderson WF, et al. The risk of a second primary lung cancer after a first invasive breast cancer according to estrogen receptor status. Cancer Causes Control. 2012;23(10):1721–1728. doi: 10.1007/s10552-012-0054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32(9):1291–1298. doi: 10.1097/PAS.0b013e3181658c14 [DOI] [PubMed] [Google Scholar]
- 33.Nelson ER, Sharma R, Argani P, et al. Utility of Sox10 labeling in metastatic breast carcinomas. Hum Pathol. 2017;67:205–210. doi: 10.1016/j.humpath.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Ivanov SV, Panaccione A, Nonaka D, et al. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109(2):444–451. doi: 10.1038/bjc.2013.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miettinen M, McCue PA, Sarlomo-Rikala M, et al. Sox10–a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol. 2015;39(6):826–835. doi: 10.1097/PAS.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50(2):277–289. doi: 10.1016/j.ejca.2013.10.004 [DOI] [PubMed] [Google Scholar]


