Abstract
Introduction
Rapamycin has been considered as a potential treatment for osteoarthritis (OA). Drug carriers fabricated from liposomes can prolong the effects of drugs and reduce side effects of drugs. Low-intensity pulsed ultrasound (LIPUS) has been found to possess anti-OA effects.
Materials and Methods
The anti-osteoarthritic effects of liposome-encapsulated rapamycin (L-rapa) combined with LIPUS were examined by culture of normal and OA chondrocytes in alginate beads and further validated in OA prone Dunkin-Hartley guinea pigs.
Results
L-rapa with LIPUS largely up-regulated aggrecan and type II collagen mRNA in human OA chondrocytes (HOACs). L-rapa with LIPUS caused significant enhancement in proteoglycan and type II collagen production in HOACs. Large decreases in both MMP-13 and IL-6 proteins were found in the HOACs exposed to L-rapa with LIPUS. Intra-articular injection of 40 μL L-rapa at both 5 μM and 50 μM twice a week combined with LIPUS thrice a week for 8 weeks significantly increased GAGs and type II collagen in the cartilage of knee. Results on OARSI score showed that intra-articular injection of 5 μM L-rapa with LIPUS displayed the greatest anti-OA effects. Immunohistochemistry revealed that L-rapa with or without LIPUS predominantly reduced MMP-13 in vivo. The values of complete blood count and serum biochemical examinations remained in the normal ranges after the injections with or without LIPUS. These data indicated that intra-articular injection of L-rapa collaborated with LIPUS is not only effective against OA but a safe OA therapy.
Conclusion
Taken together, L-rapa combined with LIPUS possessed the most consistently and effectively anabolic and anti-catabolic effects in HOACs and the spontaneous OA guinea pigs. This study evidently revealed that liposome-encapsulation collaborated with LIPUS is able to reduce the effective dose and administration frequency of rapamycin and further stably reinforce its therapeutic actions against OA.
Keywords: liposome-encapsulation, rapamycin, osteoarthritis, low-intensity pulsed ultrasound, OA prone Dunkin-Hartley guinea pigs
Introduction
Osteoarthritis (OA) is a crucial degenerative joint disease in humans. OA is the most prevalent degenerative joint disease and globally a leading cause of disability linked with an increasing socioeconomic burden due to the elderly population. OA may affect one or several moveable joints, such as the knee and hip joints as well as small joints like joints in the hand.1,2 The long-standing challenge in OA pharmacological treatments is that the effective disease-modifying therapy is unavailable while commonly-used pharmacological interventions only manage pain and inflammation.1 Due to the complexity of etiopathogenesis and subsequent clinical course of OA,3 a single treatment is not likely to be effective and hence following and promising approaches should center on dealing with both symptoms and structural changes.4–6 Oral and injectable pharmacological agents are available for OA patients. However, investigations show that most OA patients have persistent pain regardless of taking their prescribed pharmacological therapies.2 Therefore, it is urgently important and necessary to develop and validate more efficacious pharmacological, physical and synergistic therapies for alleviation of symptoms and modification of structural changes in OA.
Rapamycin, a macrolide lactone, has been shown to possess anti-bacterial, anti-fungal, anti-tumor and immunosuppressive activities.7 The potential therapeutic effects of rapamycin are mammalian target of rapamycin (mTOR), a serine/threonine-protein kinase that importantly regulates many cellular processes such as growth, proliferation, and protein synthesis.8–10 Recent studies revealed that both pharmacological inhibition and genetic deletion of mTOR reduced the severity of OA in preclinical mouse models.8,10–12 However, it has been shown that mTOR possesses a negative feedback suppression on PI3K/Akt pathway so the inhibition of mTOR may lead to elevated activity of the PI3K/Akt/nuclear factor (NF)-κB pathway.8 This may enhance MMP production by chondrocytes. The possible side effects found in mTOR inhibitors may limit their use whereas reports also demonstrated that preventive and management measures during treatment course by combined therapies may resolve the issue.8 Liposomes have the distinctive feature in which they are biocompatible, biodegradable, non-toxic, inert and non-immunogenic lipids. The unique structures of liposomes are characterized by their aqueous compartments surrounded by one or more lipid bilayers, resembling the cell lipid membranes. With these advantages, liposomes can encapsulate and solubilize both hydrophilic and hydrophobic compounds and have ability to enhance stability via encapsulation of drug, improve pharmacokinetic effects and therapeutic index of drugs and reduce the toxicity.13–15 We have successfully fabricated beta-blocker propranolol-loaded liposomes and the liposomes-encapsulated propranolol exhibited significant anabolic effects on proliferation and differentiation in human osteoblastic cells in vitro and the prepared liposomes-encapsulated propranolol further enhanced tibial and spinal microarchitecture volumes in OVX rats in vivo.16,17 Anti-OA actions of pure and liposome-encapsulated rapamycin (L-rapa) were thus extensively assessed and evaluated as studies similarly have investigated and fabricated various intra-articular injective liposomal dosage forms to encapsulate NSAIDs with HA or other enhancers for effective treatment and management of arthritis.18,19
Among the alternative physical therapies, therapeutic ultrasound has been found to possess beneficial effects against OA-like reduction in pain and improvement of physical function of joints.10,20 Low-intensity pulsed ultrasound (LIPUS), in particular, has been shown to attenuate the regression of cartilage and essentially, has significant inhibitory actions on MMP-13 mRNA expression and proteins in vivo on the rabbit OA models.21–25 LIPUS is thus selected as an auxiliary therapy for rapamycin of different dosage forms in the current study. The histopathological findings of Dunkin-Hartley guinea pig as an OA model system are similar to those in the human disease. Moreover, OA in the guinea pigs and humans are both age-associated and subject to the OA risk factors shared in common with humans like body weight, mechanical load, and high bone turnover. Spontaneous knee OA Dunkin-Hartley guinea pigs have thus been considered an OA animal model corresponding to human non-traumatic idiopathic osteoarthritis26,27 and were used to further verify the potentially ameliorated anti-OA effects of L-rapa in collaboration with LIPUS in vivo in the current study. Therefore, herein we extensively investigated and examined the potential synergic anti-osteoarthritic effects of L-rapa in collaboration with LIPUS in human OA/normal chondrocytes and the spontaneous knee OA Dunkin-Hartley guinea pigs.
Materials and Methods
Materials
Cholesterol, octadecylamine, 1, 2-distearoyl, L-α-phosphatidylcholine (DSPC, MW: 790.15 Da) and safranin O-Fast Green were obtained from Sigma (St. Louis, MO, USA). Rapamycin was acquired from Biovision (Milpitas, CA, USA). BrdU Cell Proliferation Kit was from Merck Millipore (Burlington, MA, USA). Proteoglycan Measurement Kit was from Amsbio (Abingdon, UK). Type II collagen detection kit was obtained from Chondrex (Redmond, WA, USA). TRIZOL Reagent was from Invitrogen (Carlsbad, CA, USA). Reverse transcription Advantage RT-for-PCR Kit was from Takara (Kusatsu, Shiga, Japan). SYBR® Green Realtime PCR Master Mix was from Bio-Rad (Hercules, CA, USA). MMP-13 ELISA kit and horseradish peroxidase-diaminobenzidine detection immunohistochemistry kit were acquired from Abcam (Cambridge, UK). IL-6 ELISA kit was from R&D system (Minneapolis, MN, USA). C2C (type II collagen cleavage) ELISA kit was obtained from IBEX (Cumberland, MD, USA). CTX-II (C-terminal telopeptide of type II collagen) ELISA kit was from Lifespan Biosciences (Seattle, WA, USA).
Fabrication of DSPC Liposome-Encapsulated Rapamycin
The liposomes were prepared based on the evaporation sonication method with some modification.16 In brief, the phospholipids used for the liposome production were a mixture of DSPC and cholesterol in addition of octadecylamine (OCT). The powder form of DSPC, cholesterol and OCT was dissolved in methanol chloroform (1:1, v/v) and then the materials of liposomes were added to round bottom flasks. An equal volume of rapamycin solution was added and gently inverted with the mixture. The flask was placed in laminar flow hood and air dry for 24 h and a thin layer of film was formed. Nitrogen gas was then applied to remove any residues of organic solvent. Rehydration of the thin film containing rapamycin with deionized water was then carried out, followed by 20 minutes of sonication.16
Encapsulation Efficiency and in vitro Drug Release
The prepared rapamycin-loaded liposomes were separated from the free rapamycin using a Sephadex G-50 minicolumn centrifugation technique as we previously reported.16 The filtrate was then incubated with 0.25% Triton X-100 in 68 °C water bath for 5 minutes for complete release of the drug from the liposomes. For analysis of in vitro release of rapamycin from liposomes, at each scheduled time interval filtered rapamycin-loaded liposomes were transferred to a centrifuge tube and refilled with equal volume of PBS. The aliquots were filtered and diluted with 0.25% Triton X-100 tube and then placed in 37°C water bath and at a shaking rate of 40 rpm.16 The amount of encapsulated rapamycin was quantitated by high-performance liquid chromatography (Shimadzu LC-20AD prominence liquid chromatograph with Shimadzu SIL-10AD VP auto-injector and Shimadzu SPD-M10A VP diode array detector) (Shimadzu, Kyoto, Japan) based on the previous method with some modification.28,29 A reverse phase C18 column (5 μm, 100 Å, 250 × 4.6 mm) was used (Phenomenex, Torrance, CA USA). The mobile phase was 700 mL/L acetonitrile in H2O and the flow rate was at 1 mL/min. The release of rapamycin from rapamycin-loaded liposomes was measured using the following formula: In vitro release (%) = [(total amount of rapamycin − residue of rapamycin)/total amount of rapamycin] ×100%.30
Cell Culture Using Alginate Beads and LIPUS Treatment in vitro
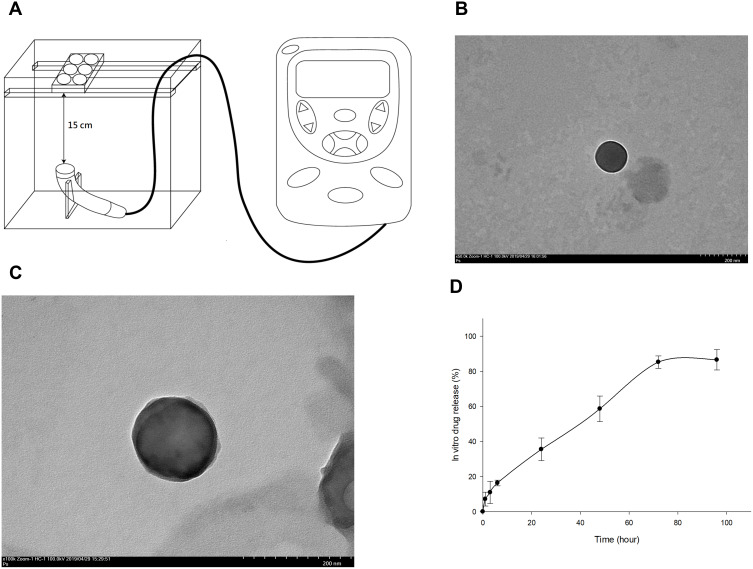
Human Chondrocytes – Osteoarthritis (HOACs, adult, 402OA-05A) derived from the cartilage of a 56-year-old Caucasian male osteoarthritis patient were obtained from Cell Applications Inc. (San Diego, CA, USA). These diseased chondrocytes were cryopreserved at first passage and can be cultured and propagated to fifth passage. Normal human chondrocytes were acquired from Lonza (CC-2550, Walkersville, MD, USA). These cells were isolated from the cartilage of the knee (Lonza) and used for comparison of the HOACs.31,32 Human OA and normal chondrocytes from the third passage were trypsinized, centrifuged and re-suspended for the culture in alginate beads. The chondrocytes were cultured in alginate beads using the classical methods reported previously.33–35 Chondrocytes at a density of 1 x 106 were centrifuged and the cell pellets were resuspended in alginate solution containing 1.2% alginate. The cell-alginate drops were released into 102 mM CaCl2 solution and washed by 0.9% NaCl solution. Alginate beads were cultured in DMEM/Ham’s F-12 medium (1:1) at 37°C in a humidified incubator with 5% CO2.33,34 All the procedures were sterilely performed. The chondrocyte beads were treated with LIPUS based on our previous study. The schematic diagram of LIPUS treatment is shown in Figure 1A.36 LIPUS was produced by an Intelect Mobile Ultrasound machine (Chattanooga Group, Intelect Legend, TN, USA) designed for treating musculoskeletal, neurological and soft tissue disorders. The LIPUS treatment (10 cm2 sound head, frequency 1.0 MHz, duty cycle 20% and intensity 0.5 W/cm2) started prior to the cells embedded in the beads for 48 h and was performed for 20 min/day at each other day. The alginate beads containing chondrocytes were incubated with L-rapa or pure rapamycin for 7 days with or without LIPUS treatment. The in vitro experiments were categorized into eight groups based on the treatment as below: Group A: control; Group B: 20 nM pure rapamycin; Group C: 20 nM L-rapa; Group D: 20 nM L-rapa + LIPUS; Group E: 2 nM pure rapamycin; Group F: 2 nM L-rapa; Group G: 2 nM L-rapa + LIPUS; Group H: LIPUS alone.
Figure 1.
(A) LIPUS was transmitted through a 15-cm water layer between the ultrasonic transducer and the bottom of culture plates. TEM image of prepared L-rapa showed an average of 135.17±28.3 nm at magnification 50,000× and 100,000× in (B) and (C), respectively (scale bar = 200 nm in both (B) and (C)). (D) In vitro release profile of rapamycin from rapamycin-loaded liposomes (N=3).
Determination of Effects of L-Rapa and LIPUS on Human Normal and OA Chondrocyte Proliferation
The effects of L-rapa in the presence or absence of LIPUS on proliferation of human normal and OA chondrocytes were assessed by BrdU assay (Millipore). Alginate beads were dissolved in 55 mM sodium citrate and cell pellets were prepared using Spin Fix Procedure for suspension cells suggested by the manufacturer’s instructions. Briefly, the cells in alginate beads were incubated with BrdU labeling solution for 2 h, fixed at room temperature for 30 min, and then treated with anti-BrdU IgG (peroxidase conjugate) for 30 min. The absorbance was measured at dual wavelength of 450/550 nm on a plate reader (Bio-Rad). The proteoglycan, type II collagen, MMP-13 and IL-6 protein productions of each experimental group were normalized by the respective amount of DNA synthesis determined by BrdU assay.
Quantification of mRNA Expression of Aggrecan, Type II Collagen MMP-13 and IL-6
Quantification of mRNA for aggrecan, type II collagen, MMP-13 and IL-6 in HOACs was carried out by real-time RT-PCR. The alginate beads were dissolved in a dissociation solution containing 55 mM sodium citrate. Trizol (Invitrogen) was added to the cell pellet after dissolving and gently centrifuge. The extraction was performed according to the manufacturer’s instructions. The concentration of RNA was measured using the NanoDrop spectrophotometer (Wilmington, DE, USA). For reverse transcription, RNA (1 μg) was treated with DNase-I (Invitrogen) and Moloney murine leukemia virus reverse transcriptase containing 5X reaction buffer, OligodT, dNTP and RNase inhibitor were incubated at 42 °C for 1h on a PCR thermal cycler (Takara). Human MMP-13 primers for real-time PCR were designed using the Primer3 program.37 Sequences of aggrecan, type II collagen, MMP-13 and IL-6 primers for real-time RT-PCR are shown in Table 1.38–42 Quantification of the target cDNA was accomplished using SYBR® Green Realtime PCR Master Mix (Bio-Rad) in the presence of human aggrecan, type II collagen, MMP-13 and IL-6 primers on CFX Connect Real-Time PCR Detection System (Bio-Rad). Relative quantitation of the genes was normalized based on GAPDH content using the ΔΔCt method.43,44
Table 1.
The Sequences of Real-Time PCR Primers
| Forward (5ʹ–3ʹ) | Reverse (5ʹ–3ʹ) | |
|---|---|---|
| Aggrecan | ACAGCTGGGGACATTAGTGG | GTGGAATGCAGAGGTGGTTT |
| Type II collagen | CAACACTGCCAACGTCCAGAT | TCTTGCAGTGGTAGGTGATGTTCT |
| MMP13 | CTTCCCAACCGTATTGATGCT | CTGGTTTCCTGAGAACAGGAG |
| IL-6 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
Measurement of Proteoglycan and Type II Collagen Produced by Human Normal and OA Chondrocytes
The proteoglycan synthesized by human normal and OA chondrocytes exposed to pure/liposomal rapamycin with or without LIPUS was quantified by colorimetric DMMB assay (Amsbio). The alginate beads were treated with 55 mM sodium citrate and centrifuged at 1500 rpm for 5 min. The supernatant was removed and then digested with papain solution (1.0 mL of 20 mM sodium phosphate buffer (pH 6.8) containing 1 mM EDTA, 2 mM dithiothreitol and 300 μg papain) before the DMMB detection according to the manufacturer’s instructions. The sulfated glycosaminoglycans, including chondroitin 4 and 6 sulfates produced by the chondrocytes were detected by the DMMB kit.
Type II collagen produced by human normal and OA chondrocytes was measured by collagen II ELISA kit (Chondrex) according to the manufacturer’s instructions. Briefly, the capture antibody was added to each well and incubated at 4°C overnight. The samples and standards were incubated with detection antibody at room temperature for 2 hours. After 3 washes, samples and standards were incubated with streptavidin peroxidase for 1 hour. The color reaction was carried out by adding o-phenylenediamine and urea hydrogen peroxide and incubating for 30 min. The reaction was terminated by the addition of sulfuric acid. The optical density (OD) values were determined at 490 nm on a microplate reader (Bio-Rad).
Determination of MMP-13 and IL-6 Proteins Produced by HOACs
Production of human MMP-13 and IL-6 proteins by the HOACs exposed to pure/liposomal rapamycin with or without LIPUS was assessed using the commercial ELISA kits according to the manufacturer’s instructions. Standards and cell culture supernatant of each group were incubated with human MMP13 antibodies overnight at 4°C or human IL-6 antibodies for 2 hours at room temperature. For assessment of MMP13 proteins, the wells were then incubated with biotinylated MMP13 Detection Antibody for 1 hour at room temperature after 4 washes, followed by 45 min incubation with HRP-Streptavidin solution for 1 hour. For determination of IL-6 proteins, the wells were incubated with HRP conjugate for 2 hours at room temperature. Color reaction was initiated by adding TMB reagent and stop solution was added to terminate the reaction. The OD values were measured at 450 nm on a microplate reader (Bio-Rad).
Experimental Animals and the Treatments
The animal (spontaneous OA Dunkin-Hartley guinea pigs) use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). The approval number is IACUC 106130. The animal experiments performed in this study were based on the Guideline for the Care and Use of Laboratory Animals amended by Council of Agriculture Executive Yuan Taiwan (No. 1070043010A on June 22, 2018). The guinea pigs were raised in a temperature controlled room (22~24◦C) and maintained on a cycle of 12 hours light and 12 hours dark in a laboratory animal center under artificial lighting. The animal center was certificated by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Food and water were provided ad libitum throughout the experiment. The stock solution (50 mM in DMSO) and dilution of pure rapamycin with phosphate-buffered saline (PBS) was performed as previously shown.11 Forty μL pure rapamycin or L-rapa diluted with PBS to a concentration of 50 μM or 5 μM or the same volume of PBS was injected into right knees twice a week (once every 3 days) for 8 weeks. LIPUS produced by a Mobile Ultrasound machine (Intelect) was applied to the guinea pigs as follows: on–off ratio of 20%, frequency of 3 MHz, irradiation intensity of 0.1 W/cm2, irradiation time of 20 min and treatment head 1 cm in diameter. Similar procedures were performed in sham LIPUS (sLIPUS) except the LIPUS dose was 0. LIPUS or sLIPUS was performed after sedation by Zoletil® (20 mg/kg, i.m.). Fifty-six 6 months old male spontaneous OA Dunkin-Hartley guinea pigs were received pure/liposomal rapamycin with LIPUS or sLIPUS for 8 weeks. All the right knees received the intra-articular injection of PBS, pure rapamycin or L-rapa with LIPUS or sLIPUS; the left knees were used as contralateral control. LIPUS was applied to the guinea pigs in Groups E, F, G and H thrice a week (once every other day) for 8 weeks. The spontaneous OA Dunkin-Hartley guinea pigs were randomly and assigned to eight groups: Group A: control (spontaneous knee OA guinea pigs with intra-articular injection of PBS) + sLIPUS; Group B: the spontaneous knee OA with intra-articular injection of 50 μM pure rapamycin + sLIPUS; Group C: the spontaneous knee OA with intra-articular injection of 50 μM L-rapa + sLIPUS; Group D: the spontaneous knee OA with intra-articular injection of 5 μM L-rapa+ sLIPUS; Group E: the spontaneous knee OA with intra-articular injection of 50 μM pure rapamycin + LIPUS; Group F: the spontaneous knee OA with intra-articular injection of 50 μM L-rapa + LIPUS; Group G: the spontaneous knee OA with intra-articular injection of 5 μM L-rapa + LIPUS; Group H: spontaneous knee OA guinea pigs with intra-articular injection of PBS + LIPUS.
Histology and Immunohistochemistry in vivo
The guinea pigs were euthanized by an overdose of CO2. The proximal tibiae were then collected and fixed with 10% neutral buffered formalin. The samples were decalcified by 10% formic acid. Subsequently, 5-μm sections in the coronary plane of the epiphyses were carefully cut. Glycosaminoglycans (GAGs) were stained with safranin O-Fast Green (1% safranin O counterstained with 0.75% hematoxylin and then 1% Fast Green; Sigma) and quantified using Image-Pro Plus 5.1 software (Media Cybernetics, Rockville, MD, USA). The ratio of relative density of the red-stained area to the total area (density/total area) was measured. Microscopic scoring recommended by Osteoarthritis Research Society International (OARSI) for histological assessment in guinea pigs was then calculated27 to assess the protective effects of the treatments and the improvement in the cartilage of knee.
For type II collagen and MMP-13 IHC staining, the endogenous peroxidase in tissues was firstly blocked by 3% H2O2 and the samples were digested by a mixture of 2.5% hyaluronidase and 1 mg/mL Pronase at 37°C for 1 h for epitope retrieval. The sections were blocked with fetal bovine serum for 1 h and incubated with type II collagen (Proteintech, Rosemont, IL, USA) or MMP-13 primary antibodies (Abcam) at 37°C for 4 h. Subsequently, the horseradish peroxidase-diaminobenzidine detection immunohistochemistry kit (Abcam) was applied. The sections were counterstained with hematoxylin. For type II collagen, the ratio of relative density of the brown-stained area to the total area (density/total area) was measured and quantified using Image-Pro Plus 5.1 software. For MMP-13, Image-Pro Plus 5.1 software was used to quantitate the data by defining the immunostaining of positive cells normalized with total cells (positive stain cell rate).
Determination of C2C and CTX-II in the Sera
Type II collagen biomarkers, serum C2C and CTX-II in the guinea pigs were assessed by commercial ELISA kits. For analysis of serum C2C, the samples were diluted one-half according to the manufacturers’ protocol (IBEX). Levels of serum CTX-II in the guinea pigs were determined by a sandwich ELISA (Lifespan Biosciences) to detect degradation products of C-terminal telopeptides of type II collagen in the sera according to the manufacturer’s instructions.
Examination of Complete Cell Count and Serum Biochemistry
Serum samples of guinea pigs were harvested and sent to Union Clinical Laboratories (UCL, Taipei, Taiwan) for complete cell count including the measurement of neutrophil segment, lymphocyte, monocyte, eosinophil, basophil, WBC and RBC, and analyses of serum biochemistry including AST (aspartate aminotransferase), ALT (alanine aminotransferase), BUN (blood urea nitrogen), creatinine, and electrolytes sodium, potassium, calcium, inorganic phosphorous as well as chloride.
Statistical Analysis
Each in vitro experiment was repeated at least three times and data were pooled from the repeated experiments. Results are expressed as means ± standard error of mean (SEM). Statistical comparisons were conducted by one-way analysis of variance (one-way ANOVA) followed by the Tukey-Kramer multiple comparisons test on GraphPad InStat 3 Software (San Diego, Calif., USA) to determine the existence of significant differences (P<0.05) between the experimental groups.45,46
Results
Observations of L-Rapa by TEM
The TEM images of liposomes-encapsulated rapamycin prepared in this study are shown in Figure 1. The TEM images were taken at magnification of 50,000× and 100,000×. The rapamycin-loaded liposomes presented with quite consistent, well-dispersed, and spherical shape with an average diameter of 135.17±28.3 nm by measuring the mean diameter of liposome from these TEM images (Figure 1B and C). The characteristics and size of rapamycin-loaded liposomes were similar with our previous findings.16
Encapsulation Efficiency and in vitro Drug Release
A standard curve of rapamycin with a sensitivity range of 25–5000 ng/mL and R2= 0.9998 was established by high-performance liquid chromatography (HPLC) (Shimadzu LC-20AD prominence liquid chromatograph) (data not shown). The encapsulation efficiency of liposomes-encapsulated rapamycin was approximately 77.94 ± 8.5%. The in vitro release of rapamycin from rapamycin-loaded liposomes presented with a relatively gradual and steady pattern within 72 hours. Rapamycin release rate from rapamycin-loaded liposomes was about 16% from 0 to 6 hours. The release from rapamycin-loaded liposomes was around 35% after 24 hours and reached to approximately 60% and 85% after 48 and 72-hour incubation, respectively (Figure 1D).
Effects of Pure and L-Rapa in the Presence or Absence of LIPUS on Human OA and Normal Chondrocyte Proliferation
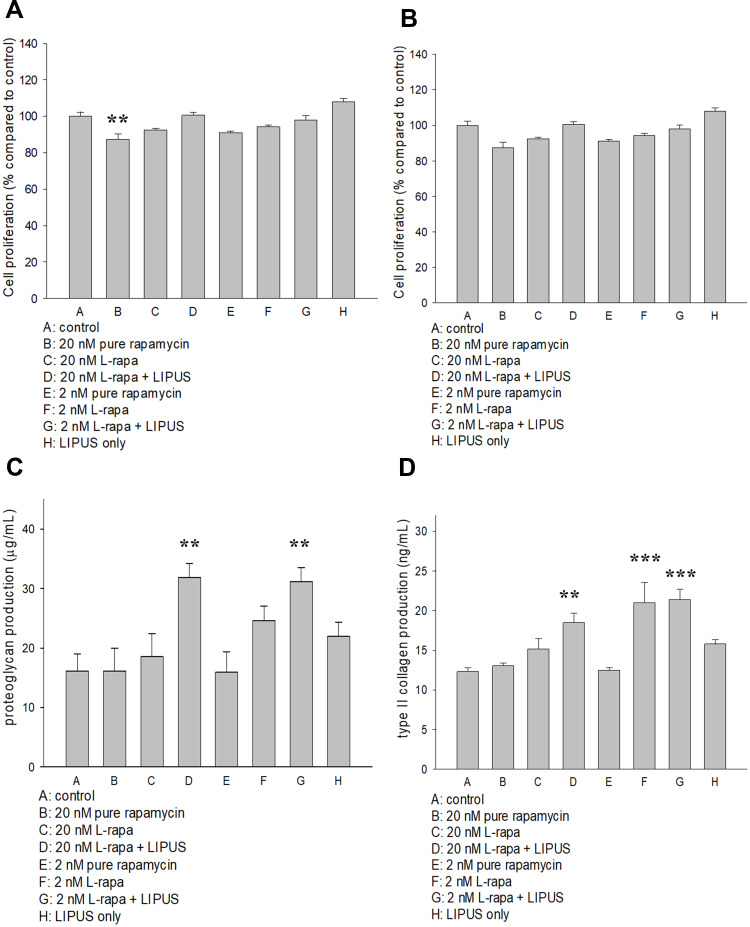
The effects of pure rapamycin and L-rapa with or without LIPUS on human chondrocyte proliferation were assessed by BrdU assay (Millipore). As a result, pure rapamycin at the high concentration (20 nM) reduced human normal chondrocyte proliferation (Figure 2A). L-rapa in the presence or absence of LIPUS did not cause any effects on DNA synthesis in human normal or OA chondrocytes cultured in alginate beads (Figure 2A and B). These data indicated that L-rapa with or without LIPUS does not significantly influence cell proliferation of human normal or OA chondrocytes cultured in alginate beads.
Figure 2.
(A) Pure rapamycin at 20 nM decreased human normal chondrocyte proliferation by about 15% while (B) pure rapamycin and L-rapa with or without LIPUS did not cause significant effects on HOAC proliferation. (C) L-rapa at 20 nM and 2 nM in collaboration with LIPUS approximately doubled proteoglycan production in human normal chondrocytes cultured in alginate beads. (D) L-rapa at 2 nM alone and in combination with LIPUS increased type II collagen production by around 1.7-folds in human normal chondrocytes cultured in alginate beads (PG: proteoglycan; **P<0.01; ***P<0.001; by one-way ANOVA, N=5).
L-Rapa in Combination with LIPUS Increased Proteoglycan and Type II Collagen Produced by Human Normal Chondrocytes
L-rapa at the low (2 nM) and high (20 nM) concentration combined with LIPUS significantly increased both proteoglycan and type II collagen production in human normal chondrocytes cultured in alginate beads. Low-concentration L-rapa alone also significantly increased type II collagen produced by human normal chondrocytes (Figure 2C and D).
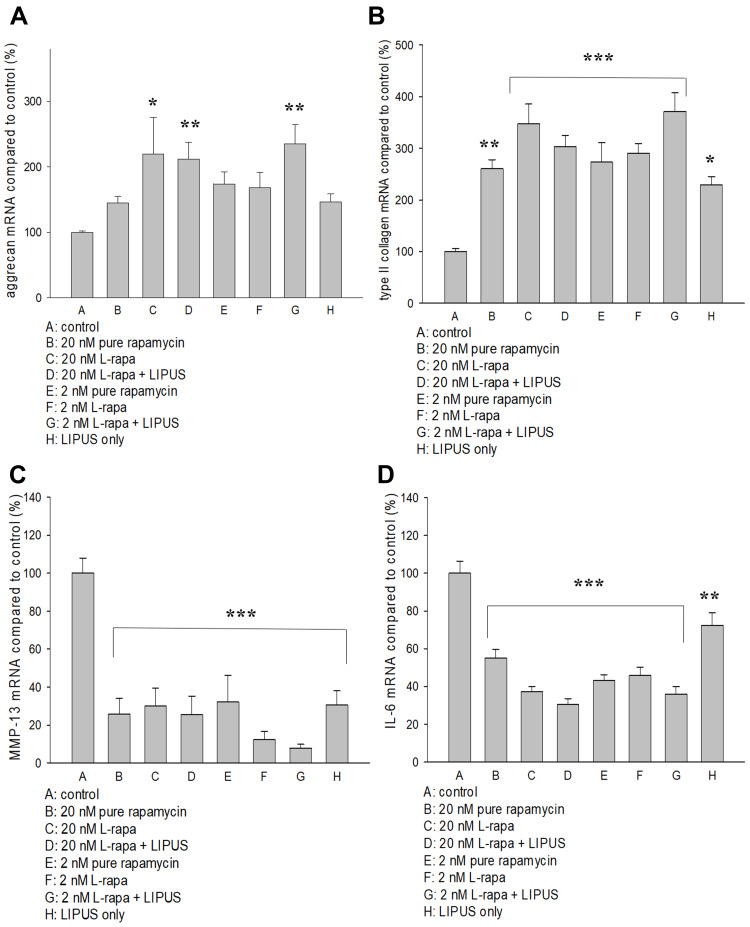
Regulations of L-Rapa in the Presence or Absence of LIPUS on mRNA for Aggrecan, Type II Collagen, MMP-13 and IL-6
The effects of pure rapamycin and L-rapa with or without LIPUS on aggrecan, type II collagen, MMP-13 and IL-6 mRNA expression in HOACs were further examined by real-time RT-PCR. As a result, high- and low-concentration L-rapa combined with LIPUS significantly up-regulated aggrecan mRNA expression by approximately 2.1- and 2.4-folds, respectively (Figure 3A). L-rapa in the presence or absence of LIPUS significantly elevated type II collagen mRNA in HOACs. L-rapa at the high concentration alone and at the low concentration combined with LIPUS showed the greatest stimulatory effects on type II collagen mRNA expression by around 3.5- and 3.7-folds, respectively (Figure 3B). An over 90% decrease of MMP-13 mRNA expression was found in the HOACs exposed to low-concentration L-rapa in combination with LIPUS. High-concentration L-rapa collaborated with LIPUS also caused around 75% decreases in MMP-13 mRNA expression. Meanwhile, L-rapa at the high or low concentration alone also exhibited about 70% and 88% inhibition on MMP-13 mRNA in the HOACs, respectively (Figure 3C). On the other hand, pure rapamycin and L-rapa in the presence or absence of LIPUS inhibited mRNA for IL-6 by approximately 54–70% while in particular, L-rapa at both high and low concentrations combined with LIPUS caused the largest decreases in IL-6 mRNA in HOACs by 70% and 64%, respectively (Figure 3D).
Figure 3.
(A) L-rapa at 20 nM and 2 nM combined with LIPUS caused 2.1-fold and 2.4-fold increases in aggrecan mRNA expression in HOACs, respectively. (B) Pure rapamycin and L-rapa with or without LIPUS significantly increased type II collagen mRNA expression by about 2.5~3.7-folds in HOACs. (C) Pure rapamycin and L-rapa with or without LIPUS, and LIPUS alone largely suppressed MMP-13 mRNA expression and (D) IL-6 mRNA expression was reduced by pure rapamycin and L-rapa in the presence or absence of LIPUS in HOACs (*P<0.05; **P<0.01; ***P<0.001 by one-way ANOVA, N=5).
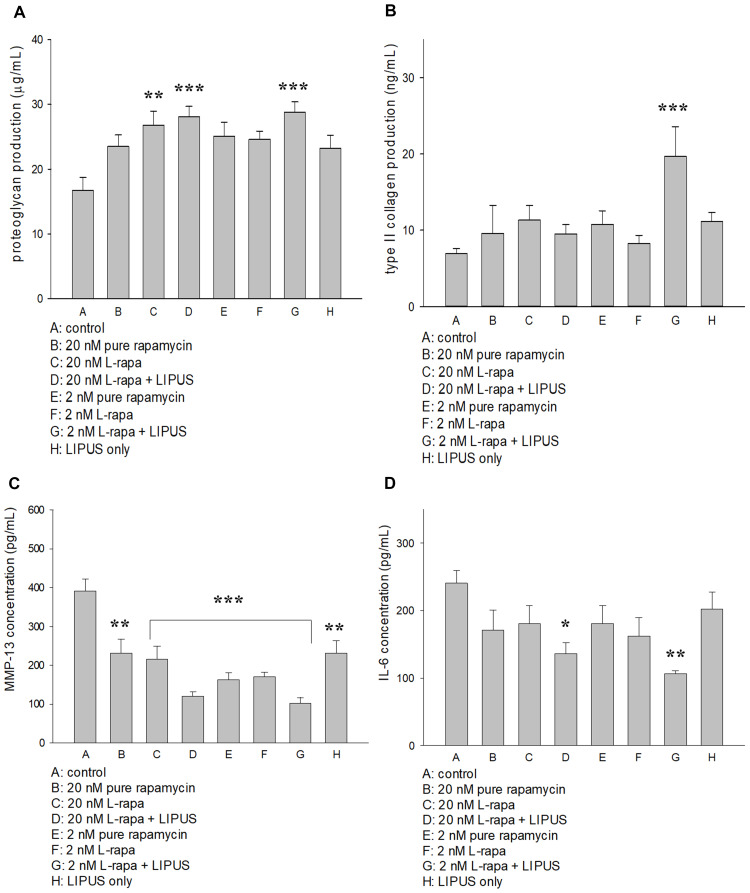
Effects of L-Rapa in Combination with LIPUS on Proteoglycan, Type II Collagen, MMP-13 and IL-6 Production in HOACs
L-rapa at the low concentration (2 nM) combined with LIPUS significantly increased both proteoglycan and type II collagen synthesis in HOACs cultured in alginate beads. At the high concentration (20 nM), L-rapa alone and in cooperation with LIPUS significantly enhanced proteoglycan produced by HOACs. However, L-rapa at the high or low concentration alone was unable to significantly increase type II collagen production in HOACs cultured in alginate beads (Figure 4A and B). The effects of L-rapa in the presence or absence of LIPUS on MMP-13 and IL-6 production were verified by commercial human MMP-13 (Abcam) and IL-6 (R&D system) ELISA kits. Treatments with L-rapa at the high and low concentrations in collaboration with LIPUS led to about 70% and 74% decreases in MMP-13 proteins produced by HOACs, respectively. High- and low-concentration L-rapa alone also caused reductions in MMP-13 production by around 45% and 56%, respectively. Pure rapamycin at the high concentration also had significantly inhibitory effects on MMP-13 production in HOACs in vitro (Figure 4C). These data are principally in accordance with the MMP-13 mRNA data obtained by real-time RT-PCR. On the other hand, L-rapa at both 2 nM and 20 nM collaborated with LIPUS significantly suppressed the production of IL-6 in HOACs whereas treatment with L-rapa at the high or low concentration, or pure rapamycin alone did not cause significant decreases in IL-6 production (Figure 4D), implicating that both liposome-encapsulation and LIPUS are essential for inhibition of IL-6 production in HOACs.
Figure 4.
(A) L-rapa at 20 nM and 2 nM combined with LIPUS significantly enhanced proteoglycan production in HOACs. (B) L-rapa at 2 nM collaborated with LIPUS enhanced type II collagen production by nearly 3-folds in HOACs. (C) Treatment with pure rapamycin and L-rapa in the presence or absence of LIPUS, and LIIPUS alone inhibited MMP-13 proteins and (D) L-rapa at 2 nM and 20 nM in the presence of LIPUS significantly suppressed IL-6 protein production in HOACs (*P<0.05, **P<0.01, ***P<0.001; by one-way ANOVA, N=5).
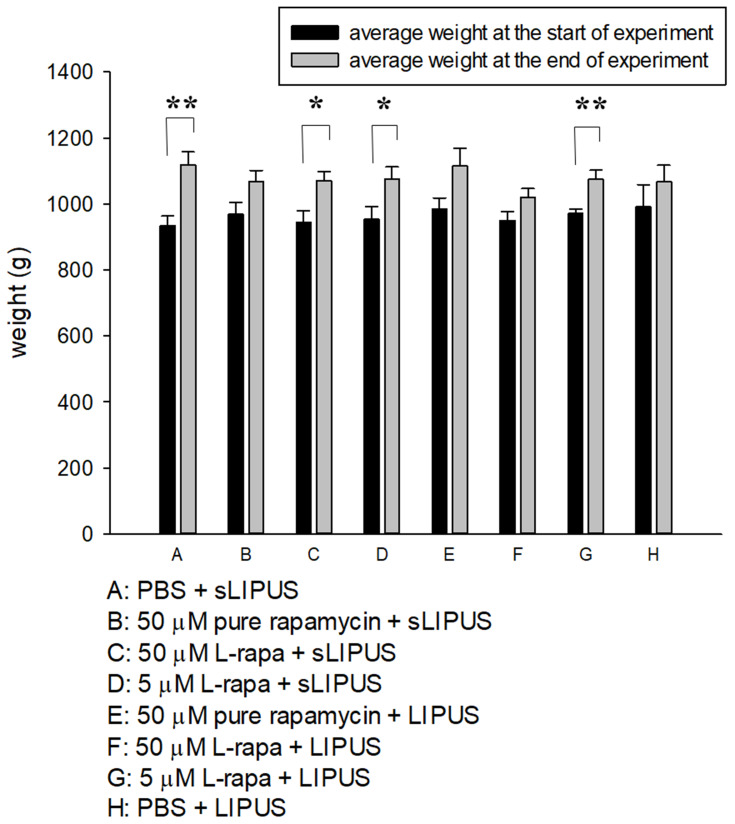
Measurement of Animal Weight
The weight of guinea pigs was measured at the beginning and end of the experiment (Figure 5). At the two time points, the mean body weight did not show statistically significant difference among groups. The end weight in each group was principally increased and significant enhancement in the end weight was found in Groups A, C, D and G compared to the start weight (Figure 5). During the 8-week administration period, no clinical signs of pain, salivation or abnormal behavior were found. Meanwhile, no significant changes in respiratory, physical responses to stimulation or neurological signs were observed in guinea pigs of all groups.
Figure 5.
The average weight of guinea pigs in 8 administrative groups measured at the start and end of experiment. The comparisons in the same group at the two different time points were analyzed by Student’s t-test. Eight-group comparisons at identical time point were performed using one-way ANOVA. No significant difference was found among experimental groups at the respective time point. At the end of experiment, significant increases in average weight were found in Groups A, C, D and G compared to the respective start weight (*P<0.05, **P<0.01; by Student’s t-test, Group A: n=5, Groups B~G: n=8, Group H: n=3).
Histological and Immunohistochemical Staining
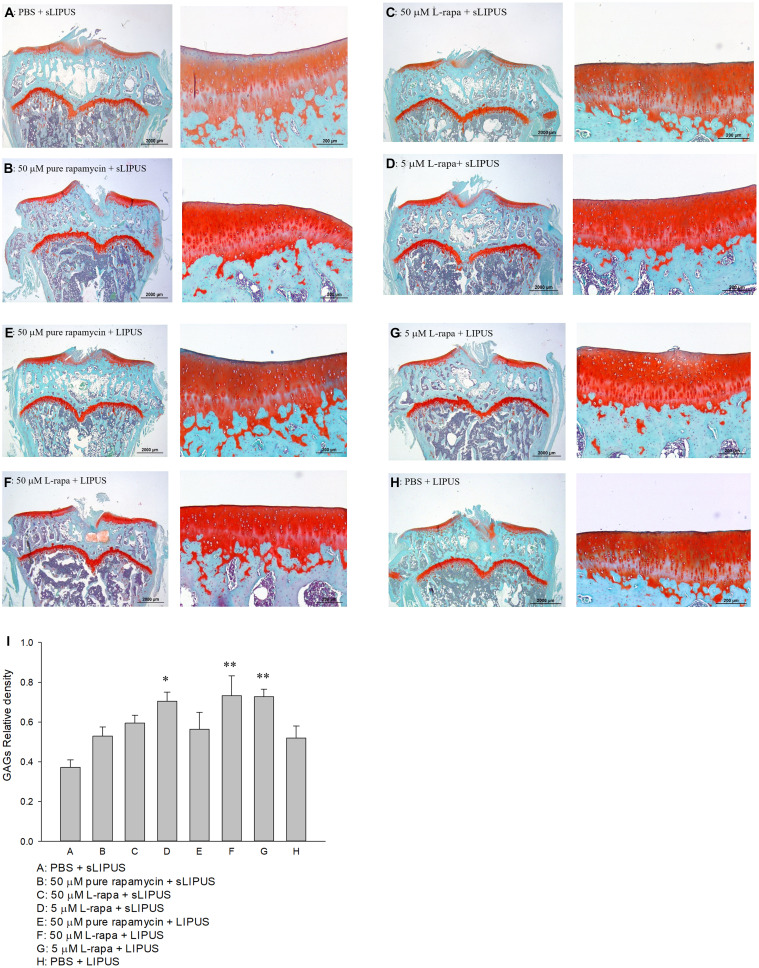
The representative histological appearance of safranin O-stained GAGs (relative density of the red-stained area to the total area) in articular cartilage is represented in Figure 6. Significant enhancement in GAGs was found in the groups treated with intra-articular injection of 40 μL of both high-dose (50 μM) and low-dose (5 μM) L-rapa twice a week combined with LIPUS thrice a week as well as low-dose L-rapa with sLIPUS (Figure 6D, F and G) compared to the GAGs in control (Figure 6A), indicating that GAGs-increasing effects were principally exerted by L-rapa combined with LIPUS in vivo. Furthermore, the OARSI score in the control (Group A: PBS + sLIPUS) group was the highest among experimental groups (7.08 ± 0.84) as the score was significantly decreased in low-dose L-rapa with LIPUS (3.38 ± 0.59; P<0.01), and L-rapa at the high dose (3.56 ± 0.82; P<0.05) or low dose with sLIPUS (3.71 ± 0.76; P<0.05) groups, implicating chondrocyte-protective and cartilage-ameliorating activities possessed by these treatments. Intra-articular injective pure rapamycin with LIPUS or sLIPUS could not display protective actions in the cartilage of spontaneous OA (Table 2).
Figure 6.
Six-month-old spontaneous OA Dunkin-Hartley guinea pigs were administered with pure rapamycin or L-rapa with LIPUS or sLIPUS for 8 weeks. GAGs in the cartilage of knee stained by safranin O-Fast Green and quantified using Image-Pro Plus 5.1 software. The GAGs in each experimental group were showed at 12.5 and 100 magnificence (A–H). (I) Intra-articular injection of 50 μM and 5 μM L-rapa combined with LIPUS thrice a week, and 5 μM L-rapa with sLIPUS (Groups D, F and G) significantly increased the content of GAGs compared to control (Group A: PBS + sLIPUS). 50 μM and 5 μM L-rapa with LIPUS showed the greatest GAGs-stimulating actions and nearly doubled GAGs density compared to that in control (L-rapa: liposomes-encapsulated rapamycin; LIPUS: low-intensity pulsed ultrasound; sLIPUS: sham LIPUS; *P<0.05; **P<0.01; by one-way ANOVA, Group A: n=5, Groups B~G: n=8, Group H: n=3).
Table 2.
OARSI Score Displayed That Intra-Articular Injection of 5 μM L-Rapa Combined with LIPUS (Group G) Has the Greatest Improvement in the Cartilage of Knee While Injection of 50 μM and 5 μM L-Rapa with sLIPUS (Groups C and D) Also Showed Protective Effects in the Cartilage of Spontaneous OA Guinea Pigs Compared to Control (Group A: PBS + sLIPUS)
| A: PBS + sLIPUS | B: 50 μM Pure Rapamycin + sLIPUS | C: 50 μM L-Rapa + sLIPUS | D: 5 μM L-rapa + sLIPUS | E: 50 μM Pure Rapamycin + LIPUS | F: 50 μM L-Rapa + LIPUS | G: 5 μM L-Rapa + LIPUS | H: PBS + LIPUS | |
|---|---|---|---|---|---|---|---|---|
| Articular cartilage structure | 1.79 ± 0.33 | 1.77 ± 0.31 | 0.92 ± 0.30 | 0.79 ± 0.21 | 1.04 ± 0.21 | 1.21 ± 0.27 | 0.98 ± 0.27 | 1.61 ± 0.42 |
| Proteoglycan content | 2.06 ± 0.28 | 1.69 ± 0.22 | 1.46 ± 0.29 | 1.08 ± 0.21 | 1.60 ± 0.29 | 1.00 ± 0.21# | 0.92 ± 0.18# | 1.56 ± 0.27 |
| Cellularity | 1.77 ± 0.28 | 1.21 ± 0.22 | 0.63 ± 0.27♦ | 0.92 ± 0.28 | 0.92 ± 0.25 | 0.77 ± 0.17 | 0.73 ± 0.21 | 1.00 ± 0.26 |
| Tidemark integrity | 0.94 ± 0.06 | 0.88 ± 0.13 | 0.38 ± 0.13♣ | 0.56 ± 0.13 | 0.69 ± 0.12 | 0.75 ± 0.11 | 0.44 ± 0.13 | 0.33 ± 0.21 |
| Additional features osteophyte | 0.52 ± 0.13 | 0.40 ± 0.12 | 0.19 ± 0.09 | 0.40 ± 0.12 | 0.40 ± 0.12 | 0.33 ± 0.12 | 0.29 ± 0.11 | 0.45 ± 0.21 |
| Mean | 7.08 ± 0.84 | 5.88 ± 0.73 | 3.56 ± 0.82* | 3.71 ± 0.76* | 4.63 ± 0.81 | 4.06 ± 0.62 | 3.38 ± 0.59** | 4.95 ± 0.81 |
Notes: All value presented as mean ± standard error (SE); L-rapa: liposomes-encapsulated rapamycin; LIPUS: low-intensity pulsed ultrasound; sLIPUS: sham LIPUS (*,#,♦,♣P<0.05; **P<0.01, by one-way ANOVA compared to the respective value of Group A (control), Group A: n=5, Groups B~G: n=8, Group H: n=3).
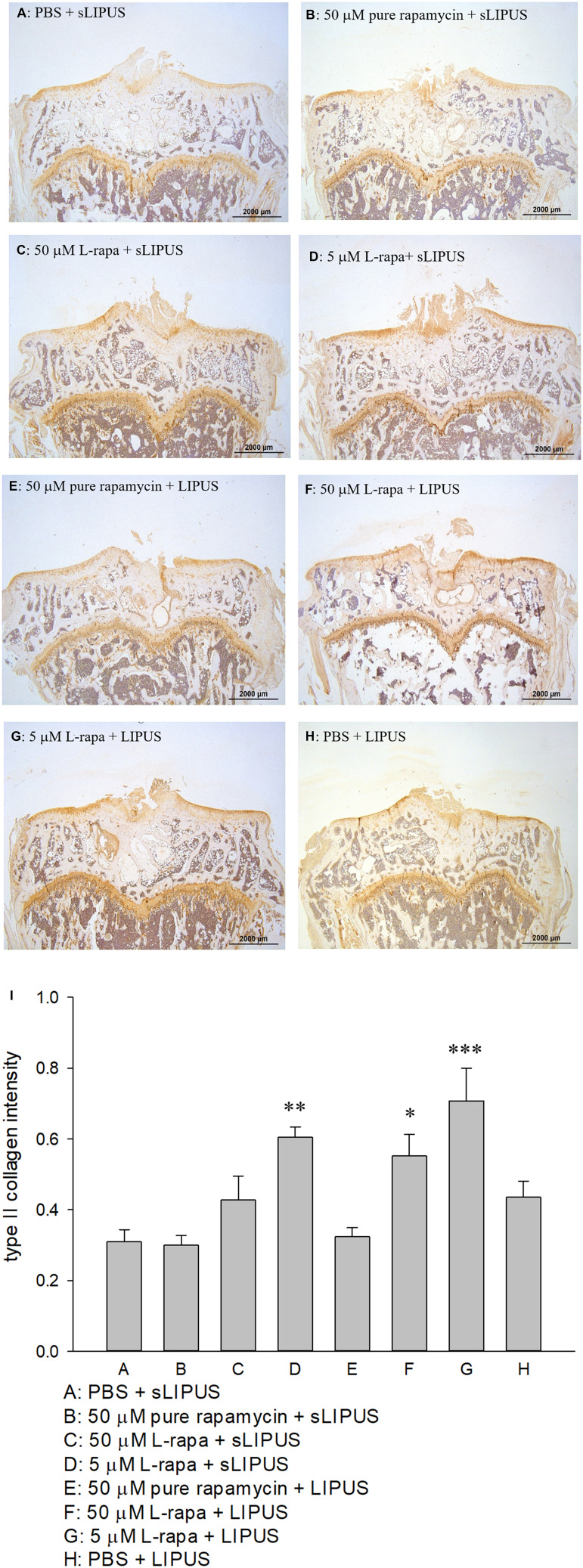
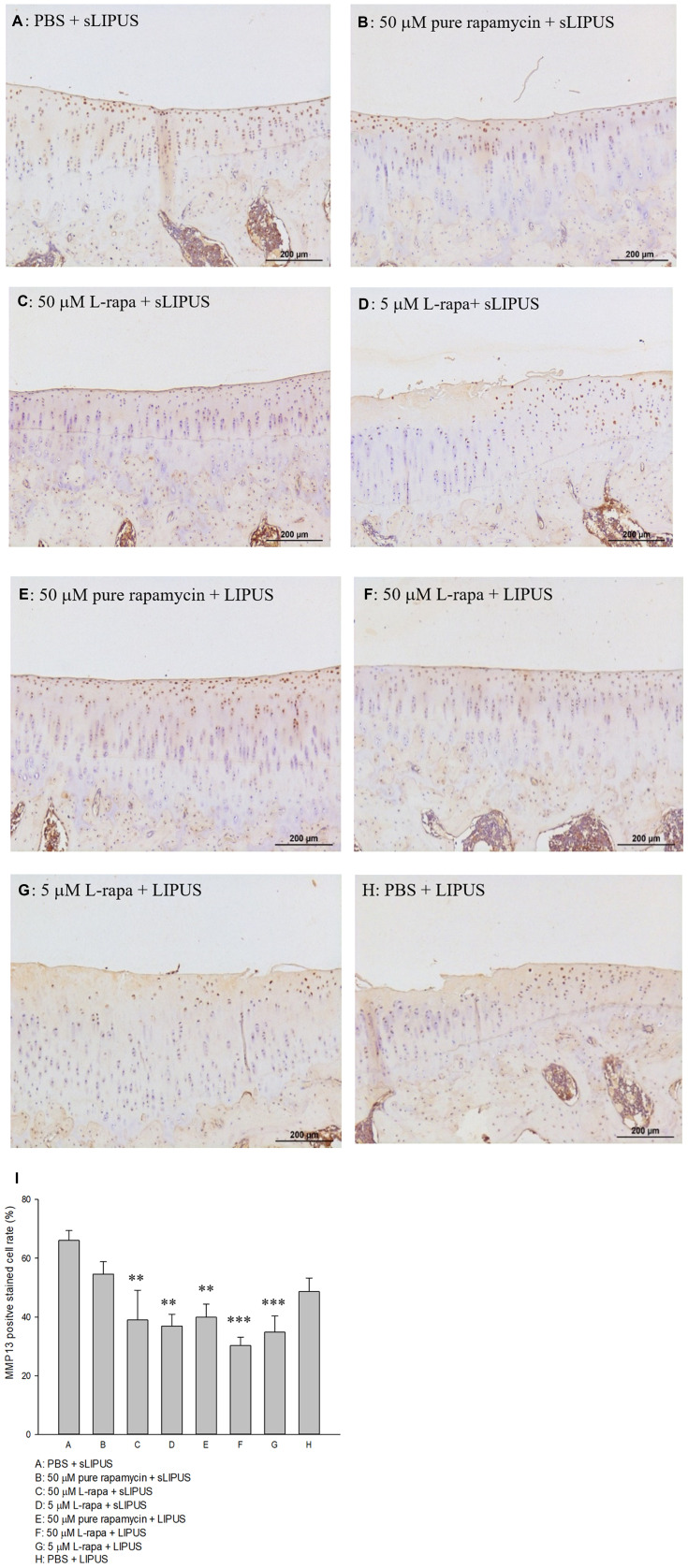
The representative appearance of the immunohistochemically stained type II collagen (relative density of the brown-stained area to the total area) and MMP-13 (positive brown-stained cell rate) in the articular cartilage of knee is presented in Figures 7 and 8, respectively. Similar to the data of in vivo GAGs, L-rapa at the high and low doses together with LIPUS, and low-dose L-rapa with sLIPUS significantly increased type II collagen intensity in the cartilage (Figure 7D, F and G) compared to control (Figure 7A). Pure rapamycin with LIPUS or sLIPUS was unable to significantly enhance the amount of GAGs or type II collagen in the cartilage (Figures 6B, E and 7B, E), suggesting that both liposome-encapsulation and LIPUS are highly crucial for low-dose rapamycin to exert anabolic effects on ECM in the cartilage of knee. L-rapa at high-dose with sLIPUS or PBS with LIPUS did not cause significant increases in GAGs or type II collagen, implicating that both liposome-encapsulation and LIPUS are essential for ECM-stimulating effects in the cartilage of knee when high-dose rapamycin and reduced administration frequency are applied (Figures 6C, H and 7C, H). Meanwhile, intra-articular injective L-rapa at both doses with LIPUS or sLIPUS largely and significantly reduced MMP-13 intensity stained by IHC in the cartilage of knee (Figure 8C, D, F and G) compared to control (Figure 8A). Pure rapamycin together with LIPUS moderately decreased MMP-13 intensity but pure rapamycin with sLIPUS or PBS with LIPUS could not display significant suppression in MMP-13, suggesting that without liposome-encapsulation, combination of high-dose rapamycin with LIPUS is necessary when lower administration frequency of both therapies is used (Figure 8B, E and H). The safranin O-stained GAG data and immunohistochemical findings on type II collagen and MMP-13 are principally consistent with those found in HOACs in vitro and further ascertained that the greatest anabolic and anti-catabolic activities against OA in vivo were possessed by L-rapa together with LIPUS.
Figure 7.
Intra-articular injection of L-rapa at 5 μM and 50 μM in collaboration with LIPUS enhanced type II collagen intensity stained by IHC in the cartilage of knee in spontaneous OA Dunkin-Hartley guinea pigs by approximately 1.8- and 2.8-folds compared to control (I) (Group A: PBS + sLIPUS). IHC intensity of type II collagen was quantified by Image-Pro plus 5.1 and the images were showed at 12.5 magnificence (A-H) (*P<0.05; **P<0.01; ***P<0.001; by one-way ANOVA, Group A: n=5, Groups B~G: n=8, Group H: n=3).
Figure 8.
Intra-articular injection of L-rapa at 5 μM and 50 μM with LIPUS or sLIPUS significantly and largely reduced MMP-13 stained by IHC in the cartilage of knee in spontaneous OA Dunkin-Hartley guinea pigs (I). L-rapa at 50 μM and 5 μM in combination with LIPUS exhibited the strongest inhibition on MMP-13 production in the cartilage by around 55.2% and 47.3%, respectively (Groups F and G). The intensity of IHC was measured by Image-Pro plus 5.1 and the IHC images of MMP-13 were showed at 12.5 magnificence (A-H) (**P<0.01, ***P<0.001; by one-way ANOVA, Group A: n=5, Groups B~G: n=8, Group H: n=3).
Changes in Serum C2C and CTX-II Level
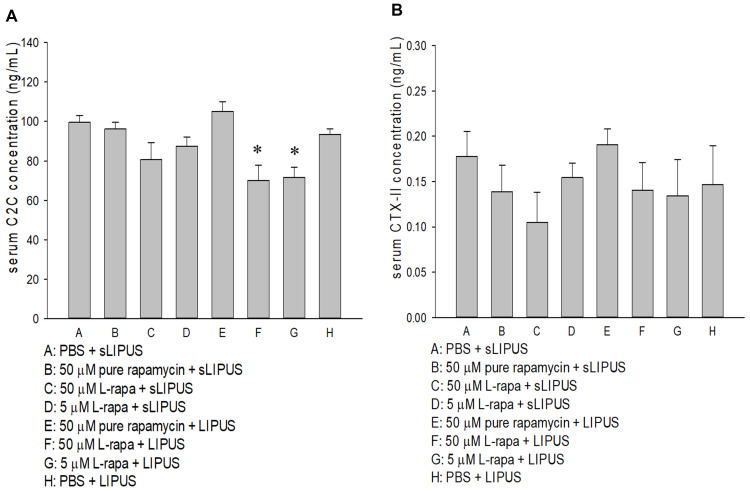
Serum C2C and CTX-II in each experimental group of spontaneous OA Dunkin-Hartley guinea pig were measured by commercial ELISA. As a result, serum C2C in the groups of L-rapa at the high and low doses collaborated with LIPUS was significantly decreased (Figure 9A). Pure rapamycin or L-rapa with or without LIPUS did not cause any significant changes on serum CTX-II between the experimental groups (Figure 9B).
Figure 9.
(A) L-rapa at 5 μM and 50 μM cooperated with LIPUS reduced serum C2C level in spontaneous OA Dunkin-Hartley guinea pigs. (B) Pure rapamycin and L-rapa with or without LIPUS did not significantly affect serum CPX-II level in the guinea pigs (*P<0.05, by one-way ANOVA; Group A: n=5, Groups B~G: n=8, Group H: n=3).
Effects on Complete Cell Count and Serum Biochemistry
Effects of intra-articular injection of pure rapamycin and L-rapa twice a week in the presence of LIPUS or sLIPUS thrice a week on complete cell count and serum biochemistry were further examined and evaluated. As a result, the values of complete cell count including neutrophil segment, lymphocyte, monocyte, eosinophil, basophil, WBC and RBC in all the administration groups were in the normal ranges of male guinea pigs47 (Table 3). For serum biochemistry, the levels of AST, ALT, BUN, creatinine and electrolytes sodium, potassium, calcium, inorganic phosphorous as well as chloride in all experimental groups were also in the normal range47,48 (Table 4).
Table 3.
The Values of Complete Blood Count Were Within the Reference Range47 After Administration with Intra-Articular Injection of Pure Rapamycin or L-Rapa Twice a Week with LIPUS or sLIPUS Thrice a Week, or PBS with LIPUS for 8 Weeks in Spontaneous OA Dunkin-Hartley Guinea Pigs (Group A: n=5, Groups B~G: n=8, Group H: n=3)
| Neutrophil Seg.(%) | Lymphocyte (%) | Monocyte (%) | Eosinophil (%) | Basophil (%) | WBC (103/μL) | RBC (106/μL) | |
|---|---|---|---|---|---|---|---|
| A: PBS + sLIPUS | 48.57 ± 6.59 | 43.76 ± 7.40 | 6.13 ± 1.34 | 1.26 ± 0.09 | 0.26 ± 0.07 | 6.33 ± 0.67 | 5.29 ± 0.10 |
| B: 50 μM Rapamycin + sLIPUS | 56.77 ± 4.74 | 35.75 ± 5.09 | 5.48 ± 0.94 | 1.53 ± 0.24 | 0.47 ± 0.21 | 5.72 ± 0.61 | 5.27 ± 0.07 |
| C: 50 μM L-rapa + sLIPUS | 52.85 ± 1.56 | 38.71 ± 1.80 | 6.78 ± 0.34 | 1.33 ± 0.24 | 0.34 ± 0.10 | 5.24 ± 0.37 | 5.35 ± 0.15 |
| D: 5 μM L-rapa + sLIPUS | 54.24 ± 4.54 | 37.89 ± 4.04 | 6.79 ± 0.69 | 0.74 ± 0.26 | 0.34 ± 0.13 | 5.79 ± 0.46 | 5.31 ± 0.22 |
| E: 50 μM Rapamycin +LIPUS |
46.55 ± 3.25 | 46.76 ± 3.43 | 5.43 ± 0.69 | 0.85 ± 0.17 | 0.41 ± 0.10 | 5.48 ± 0.38 | 5.26 ± 0.25 |
| F: 50 μM L-rapa +LIPUS |
50.46 ± 3.33 | 42.44 ± 3.41 | 5.33 ± 0.33 | 1.43 ± 0.41 | 0.34 ± 0.04 | 6.13 ± 0.91 | 5.41 ± 0.11 |
| G: 5 μM L-rapa +LIPUS |
50.65 ± 3.11 | 42.17 ± 2.92 | 6.12 ± 0.46 | 0.78 ± 0.16 | 0.28 ± 0.08 | 5.27 ± 0.51 | 5.09 ± 0.11 |
| H: PBS +LIPUS |
58.17 ± 2.72 | 34.47 ± 4.09 | 6.23 ± 1.18 | 0.63 ± 0.15 | 0.50 ± 0.25 | 8.07 ± 1.97 | 5.73 ± 0.24 |
Table 4.
The Values of Serum Biochemical Examination Were Within the Reference Range47,48 After Administration with Intra-Articular Injection of Pure Rapamycin or L-Rapa Twice a Week with LIPUS or sLIPUS Thrice a Week, or PBS with LIPUS in Spontaneous OA Dunkin-Hartley Guinea Pigs (Group A: n=5, Groups B~G: n=8, Group H: n=3)
| AST (U/L) |
ALT (U/L) |
BUN (mg/dL) |
Creatinin (mg/dL) |
Na (mg/dL) |
K (mg/dL) |
Ca (mg/dL) |
Cl (mg/dL) |
P (mg/dL) |
|
|---|---|---|---|---|---|---|---|---|---|
| A: PBS + sLIPUS | 162.4 ± 56.80 | 98.4 ± 10.39 | 17.6 ± 1.23 | 0.49 ± 0.05 | 138.2 ± 0.49 | 5.7 ± 1.08 | 10.8 ± 0.30 | 102.8 ± 0.58 | 4.5 ± 0.37 |
| B: 50 μM Rapamycin + sLIPUS | 126 ± 37.33 | 72 ± 17.05 | 17.1 ± 0.79 | 0.51 ± 0.02 | 137 ± 0.69 | 4.6 ± 0.16 | 10.2 ± 0.15 | 101 ± 0.92 | 4 ± 0.17 |
| C: 50 μM L-rapa + sLIPUS | 124 ± 44.03 | 60 ± 11.63 | 16.4 ± 1.14 | 0.47 ± 0.04 | 136 ± 1.18 | 4.5 ± 0.13 | 10.2 ± 0.27 | 102 ± 1.34 | 4 ± 0.46 |
| D: 5 μM L-rapa + sLIPUS | 94 ± 13.37 | 71 ± 15.66 | 17.3 ± 0.82 | 0.46 ± 0.02 | 138 ± 0.46 | 4.5 ± 0.13 | 10.3 ± 0.14 | 102 ± 0.58 | 4 ± 0.22 |
| E: 50 μM Rapamycin +LIPUS |
82 ± 9.61 | 54 ± 6.15 | 18.4 ± 0.95 | 0.53 ± 0.02 | 137 ± 0.61 | 5.0 ± 0.46 | 10.3 ± 0.09 | 102 ± 0.71 | 4 ± 0.42 |
| F: 50 μM L-rapa +LIPUS |
73 ± 9.74 | 47 ± 4.91 | 16.7 ± 0.62 | 0.43 ± 0.03 | 138 ± 0.63 | 4.4 ± 0.08 | 10.6 ± 0.13 | 103 ± 0.67 | 4 ± 0.21 |
| G: 5 μM L-rapa +LIPUS |
145 ± 28.55 | 77 ± 16.85 | 16.7 ± 0.56 | 0.52 ± 0.04 | 137 ± 1.13 | 5.0 ± 0.60 | 10.2 ± 0.14 | 103 ± 0.92 | 5 ± 0.52 |
| H: PBS +LIPUS |
152.7 ± 38.68 | 68.3 ± 29.95 | 21.1 ± 1.99 | 0.47 ± 0.05 | 137.7 ± 0.33 | 5.6 ± 0.80 | 10.9 ± 0.32 | 103 ± 1.16 | 4.7 ± 0.82 |
Discussion
OA is a highly prevalent joint disease in humans and related to epic medical and economic burden. This skeletomuscular disorder is characterized by its complicated pathogenesis, unclear molecular mechanisms as well as multiple risk factors such as aging, obesity, sport injury, inflammation and genetic factors.49,50 Autophagy has been found to play an essential role in cartilage deterioration in aging and OA.51 Rapamycin, a macrolide agent and mTOR inhibitor, is able to increase autophagy and prevent OA-like gene expression changes in human chondrocytes in vitro, revealing that the mTOR inhibitor is a potential treatment against OA.52 However, the negative feedback suppression on PI3K/Akt pathway caused by mTOR inhibitors may increase MMPs by chondrocytes. LIPUS is a physical therapy able to reduce the MMP production in OA. Moreover, due to the etiologic and pathological complexity of OA, single treatment is unlikely to be effective. We hence dedicated to prolonging and strengthening the pharmacological effects of rapamycin by liposome-encapsulation and combination with LIPUS to accomplish a collaborated therapy with steadily effective activities against OA. Liposome-encapsulation has been applied to enhance the therapeutic effects of drugs or potential compounds in vivo and in vitro in our previous studies16,17,53 as it was shown to further decrease the frequency of administration when combined with the transdermal delivery like iontophoresis.17 Meanwhile, a slower and steady in vitro release profile of L-rapa within 72 hours was found in the current study. This is reasonable for hydrophobic rapamycin encapsulated in liposomes with lipid bilayers. The slow and steady-release L-rapa in combination with LIPUS was therefore developed and established to efficiently treat OA. The combination of liposomes-encapsulated chemical compound with physical ultrasound is sensibly helpful for prolonging the therapeutic effects, decreasing frequency and dose of treatments and improving the anti-OA activities.
Previous investigation has shown that in ATDC5 chondrogenic cell line, 24~72 h treatment with rapamycin did not affect the cell proliferation.54 Yan et al showed that rapamycin at the low concentration of 1 nM did not affect mice chondrocyte proliferation but at higher concentrations of 10 and 50 nM, rapamycin caused inhibition in murine chondrocyte proliferation.55 In the current study, 7-day exposure of pure rapamycin to human normal chondrocytes caused slight and significant suppression on cell proliferation, which is consistent with the results found by Yan et al.55 We also found that L-rapa at 50 nM or 5 nM with or without LIPUS did not lead to cell proliferative inhibition in human normal chondrocytes or HOACs, suggesting that liposome-encapsulation is able to reduce the cytotoxicity of rapamycin. It should be noted that these previous reports did not apply alginate or agarose beads to encapsulate chondrocytes and thus the original phenotype of chondrocytes may not be maintained.35 Moreover, it has been shown that OA chondrocytes do not proliferate when encapsulated in scaffolding materials like alginate beads but the cells are still able to exert synthetic activities.35 This is probably why in the current study pure rapamycin slightly inhibited normal chondrocyte proliferation but did not affect that of HOACs in alginate beads.
In the current study L-rapa at low-concentration (2 nM) collaborated with LIPUS displayed the greatest proteoglycan and type II collagen production-enhancing as well as the strongest MMP-13- and IL-6-reducing effects in HOACs (Figure 4). This is consistent with our in vivo data that the intra-articular injective 40 μL of low-dose (5 μM) L-rapa with LIPUS exerted greatest and most consistent GAGs and type II collagen production-promoting and MMP-13-decreasing actions in the cartilage of knee in spontaneous OA guinea pigs (Figures 6G, 7G and 8G). At high-concentration (20 nM) together with LIPUS also significantly increased proteoglycan production and reduced MMP-13 in HOACs. Accordantly, high-dose 50 μM L-rapa with LIPUS also increased GAGs and reduced MMP-13 in the cartilage of knee in spontaneous OA guinea pigs (Figures 6F and 8F). These results strongly demonstrated that both liposome-encapsulation and LIPUS enable rapamycin to more effectively display anti-OA effects in HOACs and spontaneous OA knee of guinea pigs. Specifically, the reduced level of MMP-13 protein reached to the largest when HOACs and the cartilage of spontaneous OA exposed to L-rapa at the either concentration/dose in collaboration with LIPUS, indicating that for L-rapa LIPUS has potent auxiliary actions on MMP-13 reduction. Studies have also showed that LIPUS is effective to reduce MMP-13 in OA chondrocytes in vitro and on animal OA models in vivo.21–24 Our data are coherent with these previous findings. Also, previous study showed that the inhibition of mTOR may result in elevated activity of the PI3K/Akt/nuclear factor (NF)-κB pathway,8 which possibly enhance MMP production by chondrocytes. Interestingly, high-dose pure rapamycin alone did not significantly inhibit MMP-13 production in vivo (Figure 8B and I). The suppression on MMP-13 production was amplified and significant through liposome-encapsulation and LIPUS, suggesting that drug modification by liposome and LIPUS are capable of alleviating the undesirable effects of rapamycin on MMP-13 inhibition.
Previous investigation has shown that rapamycin inhibited mRNA expression of type I collagen in human skin fibroblasts.56 TGF-β1-induced type III collagen and fibronectin in lung fibroblasts were inhibited by rapamycin57 while in human urethral scar tissue fibroblasts, collagen content was reduced by rapamycin. However, in OA chondrocytes cultivated within a collagen type I hydrogel, treatment with 25 nM rapamycin for 14 days increased aggrecan and type II collagen gene expression, suggesting that inhibition in mTOR pathway may reduce the degradation of ECM components in OA status.58 Compared to the previous report, in the current study liposome-encapsulation combined with LIPUS is able to decrease the effective concentration (20 nM and 2 nM vs 25 nM) and shorten the treatment time (7 days vs 14 days) of rapamycin that increases aggrecan/proteoglycan and type II collagen in OA chondrocytes. On the spontaneous OA guinea pig model, steady increases in GAGs and type II collagen were also observed when intra-articular injection of low-dose L-rapa (40 μL of 5 μM) twice a week with or without LIPUS thrice a week was administered for 8 weeks (Figure 6D, G and 7D, G). Pure rapamycin at 20 nM or 2 nM, on the other hand, did not show proteoglycan- or type II collagen-stimulating effects in normal human chondrocytes or HOACs in alginate beads in vitro (Figures 2C, D and 4A, B). Intra-articular injection of pure rapamycin with or without LIPUS was unable to cause significant anabolic effects on GAGs, OARSI score or type II collagen in the cartilage of spontaneous OA guinea pigs. Also, intra-articular injection of 50 μM pure rapamycin with sLIPUS was unable to inhibit MMP-13 in vivo. These further verified the proposal that liposome-encapsulation collaborated with LIPUS is capable of reinforcing the anabolic effects of rapamycin on GAGs and type II collagen production as well as anti-catabolic actions on MMP-13 suppression in OA status. We also found that even though the mRNA expression of type II collagen was increased by nearly 2.7~3.1 folds in some groups in vitro (Figure 3B), the protein level of type II collagen was not significantly enhanced in HOACs (Figure 4B). This implicated that the production of type II collagen is harder than that of proteoglycan in OA status in vitro. Liposome-encapsulation in combination with LIPUS further successfully reduced the effectively type II collagen-increasing concentration of rapamycin to 2 nM in HOACs. Moreover, by liposome-encapsulation and LIPUS, the most potent type II collagen-stimulating dose for rapamycin was decreased to 5 μM (40 μL) in the spontaneous OA cartilage of guinea pigs (Figure 7G), indicating that LIPUS steadily and consistently possesses synergistic actions on type II collagen synthesis in vitro and in vivo. Previously, intra-articular injection of 10 μL of 10 μM rapamycin twice a week for 8 weeks showed therapeutic effects against OA in mice while daily intraperitoneal injections of rapamycin at 1 mg/kg body weight/dose for 10 weeks reduced severity of experimental OA on mice model.11,59 Compared to the rapamycin dose and administration frequency adopted on murine models in these previous studies, in our study an approximately 5~13-fold reduction in the dose of rapamycin by body weight was successfully attained.11,59 Moreover, dosing frequency of rapamycin was decreased by 3/4~2/3 compared to those in previous work.11,59 On the other hand, compared to the anti-OA results previously acquired by treatment with LIPUS alone in the guinea pigs,60 via collaboration with L-rapa, the LIPUS administration frequency was successfully decreased by 2/5 and duration of LIPUS administration was also shortened by around 1/2~2/3. The reduced administration frequency and duration of LIPUS (thrice a week for 8 weeks) in the current study is more appropriate for practically clinical therapy in the clinic or hospital. These pharmacological and physical therapeutic improvements strongly support that liposome-encapsulation together with LIPUS are crucially helpful for administration with rapamycin at lower dose (by body weight), and dosing frequency, to stably and evidently exhibit its therapeutic actions against OA.
IL-6 has been shown to be associated with pain, OA progression and OA-related depression in several animal and clinical studies61–63 so the inhibition of IL-6 has been found to be beneficial for pain management in OA.61 The results in the present work showed that pure rapamycin and L-rapa at the high and low concentrations decreased IL-6 mRNA as the combination of L-rapa with LIPUS further reinforced the inhibitory actions on IL-6 mRNA in HOACs. Interestingly, IL-6 protein production was significantly suppressed by low- and high-concentration L-rapa collaborated with LIPUS whereas the inhibitory effects of pure rapamycin or L-rapa alone on IL-6 production seemed to be mitigated (Figure 4D). The possible reason could be that the synthesis of IL-6 is regulated by other cytokines such as TNF-α and IL-1β in vitro64,65 and the accumulating influences are rationally more obvious at the protein level of cytokine. Therefore, a stronger suppressive action exerted by L-rapa together with LIPUS was essential for significant reduction in IL-6 synthesis in HOACs (Figure 4D). Combined with the findings on MMP-13, the current study for the first time showed that the anti-catabolic actions of LIPUS together with L-rapa in HOACs are principally resulted from the suppression of MMP-13 and IL-6.
It has been shown that the level of serum C2C is positively linked with cartilage structure degeneration of the medial tibial plateau in guinea pigs.66 The results in our study also revealed that serum C2C was significantly dropped in intra-articular injection of 40 μL of 5 μM and 50 μM L-rapa with LIPUS groups (Figure 9A) while in the 2 groups, both GAGs and type II collagen in the cartilage of knee were increased (Figures 6I and 7I). The previous and current data evidently support that serum C2C may be a valuable biomarker for monitoring ECM status of the cartilage in the progression of OA in Dunkin-Hartley guinea pigs. Serum CTX-II in guinea pigs reflected a profile most compatible with type II collagen turnover in the growth plate cartilage, being highest at 3 weeks of age but rapidly declining by 4 months,67 which is quite in accordance with the data found in current study. This suggested that serum CPX-II may not be appropriate for the biomarkers of diagnosis or monitoring the restoration of spontaneous OA in Dunkin-Hartley guinea pigs.
Inhibition of mTOR has been found to lead to age-delaying effects and an increase in autophagy.68 The combined therapies of rapamycin with other medications have also been considered and investigated for reduction of oxidative stress, deceleration of aging and increase of lifespan.69 Moreover, the autophagy-enhancing effects of rapamycin have been verified.8 Thus, in the current study for the first time L-rapa was combined with the physical therapy LIPUS to develop and establish of a more efficacious therapy for the aging and degenerative OA. As a result, compared to previous data, the effective concentration/dose and frequency of administration of rapamycin were successfully reduced and the anti-osteoarthritic effects of rapamycin were further stably reinforced by liposome-encapsulation in collaboration with LIPUS in vitro and in vivo. Low-concentration/low-dose L-rapa combined with LIPUS displayed the strongest proteoglycan/GAGs and type II collagen production-promoting as well as MMP-13-reducing effects in HOACs and spontaneous OA Dunkin-Hartley guinea pigs. The novel anti-OA actions exerted by the low-concentration/low-dose L-rapa combined with LIPUS demonstrated that cooperation of liposome-encapsulation with LIPUS is able to evidently strengthen the anti-osteoarthritic effects of rapamycin. L-rapa at 2 nM collaborated with LIPUS in vitro and intra-articular injection of 5 μM L-rapa with LIPUS in vivo showed the most consistently anabolic and anti-catabolic anti-OA actions. A combined treatment composed of liposomal mTOR inhibitor rapamycin and physical therapeutic ultrasound with augmented anti-OA effects, lower effective dose and decreased administration frequency was successfully developed and established in the current study. The current combined therapy comprising L-rapa and LIPUS is potentially a promising treatment against OA. Further clarification of whether the stronger inhibition in mTOR pathway is involved in the augmented anti-osteoarthritic effects may be helpful for practical verification of the combined therapy.
Acknowledgments
This work was funded by grants (MOST 106-2221-E-415-004 and MOST 107-2313-B-415-010) from the Ministry of Science and Technology, Taiwan. The authors express their appreciation to Ms Yi-Shan Lin and Prof. Yuan-Bin Cheng for their research support
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vinatier C, Dominguez E, Guicheux J, Carames B. Role of the inflammation-autophagy-senescence integrative network in osteoarthritis. Front Physiol. 2018;9:706. doi: 10.3389/fphys.2018.00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 3.Roman-Blas JA, Bizzi E, Largo R, Migliore A, Herrero-Beaumont G. An update on the up and coming therapies to treat osteoarthritis, a multifaceted disease. Expert Opin Pharmacother. 2016;17(13):1745–1756. doi: 10.1080/14656566.2016.1201070 [DOI] [PubMed] [Google Scholar]
- 4.Mobasheri A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr Rheumatol Rep. 2013;15(10):364. doi: 10.1007/s11926-013-0364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulsatelli L, Addimanda O, Brusi V, Pavloska B, Meliconi R. New findings in osteoarthritis pathogenesis: therapeutic implications. Ther Adv Chronic Dis. 2013;4(1):23–43. doi: 10.1177/2040622312462734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10(6):374–380. doi: 10.1038/nrrheum.2014.47 [DOI] [PubMed] [Google Scholar]
- 7.Seto B. Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med. 2012;1(1):29. doi: 10.1186/2001-1326-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal B, Endisha H, Zhang Y, Kapoor M. mTOR: a potential therapeutic target in osteoarthritis? Drugs R D. 2015;15(1):27–36. doi: 10.1007/s40268-015-0082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klionsky DJ. The autophagy connection. Dev Cell. 2010;19(1):11–12. doi: 10.1016/j.devcel.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Vasheghani F, Li YH, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–1440. doi: 10.1136/annrheumdis-2013-204599 [DOI] [PubMed] [Google Scholar]
- 11.Takayama K, Kawakami Y, Kobayashi M, et al. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther. 2014;16(6):482. doi: 10.1186/s13075-014-0482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki T, Matsushita T, Tabata Y, et al. Intra-articular administration of gelatin hydrogels incorporating rapamycin-micelles reduces the development of experimental osteoarthritis in a murine model. Biomaterials. 2014;35(37):9904–9911. doi: 10.1016/j.biomaterials.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 13.Shashi K, Satinder K, Bharat P. A complete review on: liposomes. Int Res J Pharm. 2012;7:10–16. [Google Scholar]
- 14.Romberg B, Oussoren C, Snel CJ, Hennink WE, Storm G. Effect of liposome characteristics and dose on the pharmacokinetics of liposomes coated with poly(amino acid)s. Pharm Res. 2007;24(12):2394–2401. doi: 10.1007/s11095-007-9393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamichhane N, Udayakumar TS, D’Souza WD, et al. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:2. doi: 10.3390/molecules23020288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teong B, Kuo SM, Chen CH, Chen YK, Cheng ZJ, Huang HH. Characterization and human osteoblastic proliferation- and differentiation-stimulatory effects of phosphatidylcholine liposomes-encapsulated propranolol hydrochloride. Biomed Mater Eng. 2014;24(5):1875–1887. doi: 10.3233/BME-140997 [DOI] [PubMed] [Google Scholar]
- 17.Teong B, Kuo SM, Tsai WH, Ho ML, Chen CH, Huang HH. Liposomal encapsulation for systemic delivery of propranolol via transdermal iontophoresis improves bone microarchitecture in ovariectomized rats. Int J Mol Sci. 2017;18:4. doi: 10.3390/ijms18040822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong J, Jiang D, Wang Z, Wu G, Miao L, Huang L. Intra-articular delivery of liposomal celecoxib-hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int J Pharm. 2013;441(1–2):285–290. doi: 10.1016/j.ijpharm.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 19.Pawar VA, Manjappa AS, Murumkar PR, et al. Drug-fortified liposomes as carriers for sustained release of NSAIDs: the concept and its validation in the animal model for the treatment of arthritis. Eur J Pharm Sci. 2018;125:11–22. doi: 10.1016/j.ejps.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 20.Loyola-Sanchez A, Richardson J, MacIntyre NJ. Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartilage. 2010;18(9):1117–1126. doi: 10.1016/j.joca.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 21.Xia LU, He H, Guo H, Qing Y, He CQ. Effects of ultrasound on estradiol level, bone mineral density, bone biomechanics and matrix metalloproteinase-13 expression in ovariectomized rabbits. Exp Ther Med. 2015;10(4):1429–1436. doi: 10.3892/etm.2015.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia P, Shen S, Lin Q, et al. Low-intensity pulsed ultrasound treatment at an early osteoarthritis stage protects rabbit cartilage from damage via the integrin/focal adhesion kinase/mitogen-activated protein kinase signaling pathway. J Ultrasound Med. 2015;34(11):1991–1999. doi: 10.7863/ultra.14.10016 [DOI] [PubMed] [Google Scholar]
- 23.Ji JB, Li XF, Liu L, Wang GZ, Yan XF. Effect of low intensity pulsed ultrasound on expression of TIMP-2 in serum and expression of mmp-13 in articular cartilage of rabbits with knee osteoarthritis. Asian Pac J Trop Med. 2015;8(12):1043–1048. doi: 10.1016/j.apjtm.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Li J, Cheng K, et al. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophys. 2011;61(2):427–434. doi: 10.1007/s12013-011-9206-4 [DOI] [PubMed] [Google Scholar]
- 25.He D, An Y, Li Y, et al. RNA sequencing reveals target genes of temporomandibular joint osteoarthritis in rats after the treatment of low-intensity pulsed ultrasound. Gene. 2018;672:126–136. doi: 10.1016/j.gene.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 26.McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52(5):803–818. doi: 10.1177/0300985815588611 [DOI] [PubMed] [Google Scholar]
- 27.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18(Suppl 3):S35–S52. doi: 10.1016/j.joca.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French DC, Saltzgueber M, Hicks DR, Cowper AL, Holt DW. HPLC assay with ultraviolet detection for therapeutic drug monitoring of sirolimus. Clin Chem. 2001;47(7):1316–1319. doi: 10.1093/clinchem/47.7.1316 [DOI] [PubMed] [Google Scholar]
- 29.Campanero MA, Cardenas E, Sadaba B, et al. Therapeutic drug monitoring for sirolimus in whole blood of organ transplants by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A. 2004;1031(1–22):265–273. doi: 10.1016/j.chroma.2003.10.121 [DOI] [PubMed] [Google Scholar]
- 30.Huang HH, Kuo SM, Wu YJ, Su JH. Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int J Nanomedicine. 2016;11:1237–1251. doi: 10.2147/IJN.S99911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alshenibr W, Tashkandi MM, Alsaqer SF, et al. Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis. Arthritis Res Ther. 2017;19(1):179. doi: 10.1186/s13075-017-1388-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aleksander-Konert E, Paduszynski P, Zajdel A, Dzierzewicz Z, Wilczok A. In vitro chondrogenesis of Wharton’s jelly mesenchymal stem cells in hyaluronic acid-based hydrogels. Cell Mol Biol Lett. 2016;21:11. doi: 10.1186/s11658-016-0016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otero M, Favero M, Dragomir C, et al. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol Biol. 2012;806:301–336. [DOI] [PubMed] [Google Scholar]
- 34.Chubinskaya S, Huch K, Schulze M, Otten L, Aydelotte MB, Cole AA. Gene expression by human articular chondrocytes cultured in alginate beads. J Histochem Cytochem. 2001;49(10):1211–1220. doi: 10.1177/002215540104901003 [DOI] [PubMed] [Google Scholar]
- 35.De Ceuninck F, Lesur C, Pastoureau P, Caliez A, Sabatini M. Culture of chondrocytes in alginate beads. Methods Mol Med. 2004;100:15–22. doi: 10.1385/1-59259-810-2:015 [DOI] [PubMed] [Google Scholar]
- 36.Tien YC, Lin SD, Chen CH, Lu CC, Su SJ, Chih TT. Effects of pulsed low-intensity ultrasound on human child chondrocytes. Ultrasound Med Biol. 2008;34(7):1174–1181. doi: 10.1016/j.ultrasmedbio.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 37.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365 [DOI] [PubMed] [Google Scholar]
- 38.Ng JJ, Wei Y, Zhou B, et al. Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc Natl Acad Sci U S A. 2017;114(10):2556–2561. doi: 10.1073/pnas.1611771114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aisenbrey EA, Bryant SJ. A MMP7-sensitive photoclickable biomimetic hydrogel for MSC encapsulation towards engineering human cartilage. J Biomed Mater Res A. 2018;106(8):2344–2355. doi: 10.1002/jbm.a.36412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111(50):E5429–E5438. doi: 10.1073/pnas.1421438111 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.El-Sayed KM, Paris S, Graetz C, et al. Isolation and characterisation of human gingival margin-derived STRO-1/MACS(+) and MACS(-) cell populations. Int J Oral Sci. 2015;7(2):80–88. doi: 10.1038/ijos.2014.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagappan A, Jung DY, Kim JH, Lee H, Jung MH. Gomisin N alleviates ethanol-induced liver injury through ameliorating lipid metabolism and oxidative stress. Int J Mol Sci. 2018;19:9. doi: 10.3390/ijms19092601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 45.Liao MH, Liu SS, Peng IC, Tsai FJ, Huang HH. The stimulatory effects of alpha1-adrenergic receptors on TGF-beta1, IGF-1 and hyaluronan production in human skin fibroblasts. Cell Tissue Res. 2014;357(3):681–693. doi: 10.1007/s00441-014-1893-x [DOI] [PubMed] [Google Scholar]
- 46.Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J Cell Physiol. 2009;220(1):267–275. doi: 10.1002/jcp.21761 [DOI] [PubMed] [Google Scholar]
- 47.Quesenberry KE, Donnelly TM, Mans C. Biology, husbandry, and clinical techniques of guinea pigs and chinchillas In: Quesenberry KE, Carpenter JW, editors. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery. 3 ed. St. Louis, MO: Elsevier/Saunders; 2012:279–294. [Google Scholar]
- 48.Rabe H. Reference ranges for biochemical parameters in guinea pigs for the Vettest®8008 blood analyzer. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2011;39(3):170–175. [PubMed] [Google Scholar]
- 49.Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Man GS, Mologhianu G. Osteoarthritis pathogenesis – a complex process that involves the entire joint. J Med Life. 2014;7(3):37–41. [PMC free article] [PubMed] [Google Scholar]
- 51.Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7(10):579–587. doi: 10.1038/nrrheum.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki H, Takayama K, Matsushita T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64(6):1920–1928. doi: 10.1002/art.34323 [DOI] [PubMed] [Google Scholar]
- 53.Wu YC, Wu GV, Huang HH, Kuo SM. Liposome-encapsulated farnesol accelerated tissue repair in third-degree burns on a rat model. Burns. 2019;45. doi: 10.1016/j.burns.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 54.Phornphutkul C, Wu KY, Auyeung V, Chen Q, Gruppuso PA. mTOR signaling contributes to chondrocyte differentiation. Dev Dyn. 2008;237(3):702–712. doi: 10.1002/dvdy.21464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan B, Zhang Z, Jin D, et al. mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation. Nat Commun. 2016;7:11151. doi: 10.1038/ncomms11151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279(22):23166–23175. doi: 10.1074/jbc.M401238200 [DOI] [PubMed] [Google Scholar]
- 57.Gao Y, Xu X, Ding K, Liang Y, Jiang D, Dai H. Rapamycin inhibits transforming growth factor beta1-induced fibrogenesis in primary human lung fibroblasts. Yonsei Med J. 2013;54(2):437–444. doi: 10.3349/ymj.2013.54.2.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Luna-preitschopf A, Zwickl H, Nehrer S, Hengstschlager M, Mikula M. Rapamycin maintains the chondrocytic phenotype and interferes with inflammatory cytokine induced processes. Int J Mol Sci. 2017;18:7. doi: 10.3390/ijms18071494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575–581. doi: 10.1136/annrheumdis-2011-200557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurkan I, Ranganathan A, Yang X, et al. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthritis Cartilage. 2010;18(5):724–733. doi: 10.1016/j.joca.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin Y, Liu L, Jiang H, Zhou J, Tang Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur J Pharmacol. 2017;811:260–267. doi: 10.1016/j.ejphar.2017.06.032 [DOI] [PubMed] [Google Scholar]
- 62.Radojcic MR, Thudium CS, Henriksen K, et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain. 2017;158(7):1254–1263. doi: 10.1097/j.pain.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 63.Shimura Y, Kurosawa H, Tsuchiya M, et al. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol. 2017;36(12):2781–2787. doi: 10.1007/s10067-017-3826-z [DOI] [PubMed] [Google Scholar]
- 64.Confalone E, D’Alessio G, Furia A. IL-6 induction by TNFalpha and IL-1beta in an osteoblast-like cell line. Int J Biomed Sci. 2010;6(2):135–140. [PMC free article] [PubMed] [Google Scholar]
- 65.Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J Neuroinflammation. 2010;7:16. doi: 10.1186/1742-2094-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huebner JL, Kraus VB. Assessment of the utility of biomarkers of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2006;14(9):923–930. doi: 10.1016/j.joca.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 67.Huebner JL, Williams JM, Deberg M, Henrotin Y, Kraus VB. Collagen fibril disruption occurs early in primary guinea pig knee osteoarthritis. Osteoarthritis Cartilage. 2010;18(3):397–405. doi: 10.1016/j.joca.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71(22):4325–4346. doi: 10.1007/s00018-014-1677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh AK, Garg G, Singh S, Rizvi SI. Synergistic effect of rapamycin and metformin against age-dependent oxidative stress in rat erythrocytes. Rejuvenation Res. 2017;20(5):420–429. doi: 10.1089/rej.2017.1916 [DOI] [PubMed] [Google Scholar]