Abstract
Cardiovascular diseases (CVDs) are one of the foremost causes of high morbidity and mortality globally. Preventive, diagnostic, and treatment measures available for CVDs are not very useful, which demands promising alternative methods. Nanoscience and nanotechnology open a new window in the area of CVDs with an opportunity to achieve effective treatment, better prognosis, and less adverse effects on non-target tissues. The application of nanoparticles and nanocarriers in the area of cardiology has gathered much attention due to the properties such as passive and active targeting to the cardiac tissues, improved target specificity, and sensitivity. It has reported that more than 50% of CVDs can be treated effectively through the use of nanotechnology. The main goal of this review is to explore the recent advancements in nanoparticle-based cardiovascular drug carriers. This review also summarizes the difficulties associated with the conventional treatment modalities in comparison to the nanomedicine for CVDs.
Keywords: cardiovascular diseases, nanoscience, nanoparticles, nanomedicine, nanocarriers, treatment
Introduction
Cardiovascular diseases (CVDs) are one of the leading causes of death worldwide. The World Heart Federation stated that the number of deaths occurring in a year due to CVDs is 17.3 million.1 Diagnosis and treatment costs of CVDs were rising at a higher rate and anticipated to escalate more in the next ten years. The high economic burden associated with CVDs is due to the increase in the risk factors of CVDs such as diabetics, obesity, and expansion of the geriatric population.2 Current statistics show that cardiovascular diseases have anticipated being the single foremost cause of deaths in the world. The number of death due to CVDs, especially by heart disease and stroke, will exceed 23.3 million by 2030.3 A review on ‘psychosocial factors and cardiovascular diseases’ investigates the effect of psychosocial factors on morbidity and mortality rate of CVDs. Negative emotional states (depression, anger, anxiety, and hostility), social ties, social support, social conflict, and chronic and acute psychosocial stressors have connected with the increased risk of cardiovascular morbidity and mortality. These psychosocial factors have a direct effect on pathophysiologic mechanisms that promote atherosclerosis and its clinical manifestations.4
Hypertension and CVDs
CVDs has characterized by destitute blood perfusion in the body.5 One of the most common incidents in non-communicable CVDs is hypertension or high blood pressure. Hypertension is responsible for the high ratio of mortality contributed by CVDs worldwide. Hypertension has observed in all groups of people irrespective of their age and sex. Generally, significant organs in the body such as heart, brain, blood vessels, eyes, and kidneys have damaged by high blood pressure. Hypertension also leads to the occurrence of different CVDs, such as ischemia, atherosclerosis, congestive heart failure, and cardiac arrest. According to a report published in 2015, approximately 1.13 billion people are affected by hypertension globally.6
The only solution to reduce the incidence and mortality rate of CVDs is to incorporate a healthy and preventive lifestyle as well as early diagnosis of the diseases. The initial diagnosis could save millions of lives annually and also improve the quality of life.7 The response of patients to the current therapeutic methods is limited and not practical.8 It has reported that a close link exists between diabetes mellitus and CVDs. CVDs are the most common cause of morbidity and mortality in diabetic populations in both man and woman populations. The mortality rate due to CVDs is higher in adult populations with diabetes than those without people with diabetes in the US. The higher mortality rates observed with diabetic patients are due to an increased risk of myocardial infarction and stroke.
Types of CVDs and Associated Risk Factors
Atherosclerosis is an essential factor of stroke and other CVDs. Retention of lipoproteins on the sub-endothelial extracellular matrix induces the formation of atherosclerotic plaques. The interaction of lipid accumulation and the inflammatory response results in the progression of plaques. Generally, plaques have destroyed through oxidative stress, proteases, and inflammation. Proteases directly breakdown the collagen network and rupture the plaque. The rupture of the plaque can lead to myocardial infarction, and stroke.9 Acute myocardial infarction and ischemic death of cardiomyocytes are one of the most severe types of atherosclerotic cardiovascular disease. Coronary artery disease is another leading cause of mortality globally, which can be life-threatening when it develops into acute myocardial infarction.10 Coronary artery diseases are caused by coronary atherosclerosis due to the coronary artery stenosis or occlusion. It has reported that mitochondria play an essential role in therapeutic strategies to reduce the size of myocardial infarct size and thus prevents heart failure in ischemic heart disease patients. Mitochondrial dysfunction can lead to acute myocardial perfusion injury followed by acute myocardial infarction.11
CVDs also include heart failure, which is a complex pathophysiological syndrome that arises due to the impaired function of the heart to fill or eject the blood. Different clinical manifestations have associated with heart failure along with myocardial insults such as genetic factors, hypertension, hypertrophy, and coronary artery disease. The incidence of symptomatic heart failure rises with the increasing age, and heart failure is reported as the primary cause of more than 55,000 deaths each year. Hypertrophied heart or myocardial hypertrophy occurs due to the increase in the cardiomyocyte size. Usually, adult mammalian cardiomyocytes tend to have reduced proliferative capacity, and the size of the heart starts to increase in response to the stress or increased workload such as hypertension, aortic stenosis, vascular heart disease, and myocardial infarction. It leads to the development of hypertrophied heart or myocardial hypertrophy. Myocardial hypertrophy is followed by the augmented synthesis of proteins, improved expression of embryonic genes, and assembly of sarcomeres, perivascular, and fibrosis, which eventually leads to heart failure. Another complication associated with the heart is restenosis, which has an incidence of 30–40% among the population.12
CVDs also include restenosis and aneurysm. Restenosis occurs as a serious complication of vascular interventional procedures, resulting in abnormal narrowing of the blood vessel. It is because vascular interventional procedures tend to focus on the restoration of blood flow across the obstructed arteries. Angioplasty and stent implants remove the occlusion and involve in the expansion of the inner diameter of the artery with an enhanced hemodynamic flow rate, which eventually leads to restenosis. Nonetheless, restenosis remains to be a reason for the overall ineffectiveness in vascular reactivity to control blood flow. An aortic aneurysm can be dangerous and causes death in case of rupture or dissection. Pathophysiology of aortic aneurysm includes the loss of smooth muscle cells in the aortic wall, chronic inflammation, and destructive connective tissue remodeling.13,14
One of the processes that predict the development of cardiovascular diseases is vascular calcification. Vascular calcification is common in individuals of 60 and above age, where an increase in the calcium deposits occurs in the arteries. Vascular calcification progressively decreases the arterial and aortic elastance and interferes with the cardiovascular hemodynamics. It can result in hypertension, cardiac hypertrophy, congestive heart failure, aortic stenosis, and ischemia. An increase in mineralization causes the formation of atherosclerotic plaques and envisage cardiovascular morbidity and mortality.15 There are no practical approaches available to reduce vascular calcification or arterial stiffness. It is very difficult to stop the pathophysiology of vascular calcification, and this demands early interference in targeting vascular calcification.16 Vascular calcification is not only linked with CVDs but also with several other diseases such as end-stage renal disease.17 Vascular calcification is one of the leading causes of mortality in patients with chronic kidney disease.18 There are several traditional and non-traditional risk factors associated with vascular calcification. Traditional risk factors are hypertension, diabetes, and dyslipidemia (elevation of lipids in the blood). Inflammation and abnormal mineral metabolism (Hypercalcemia and Hyperphosphatemia) are non-traditional risk factors. Early intervention of vascular calcification can prevent the progression of CVDs.19
In brief, cardiac diseases can define as the abnormalities in muscle repair, morphogenesis, cardiac function, and cardiac rhythm. In order to treat these diseases, an alternative and effective remedy needs to be employed, which involves the direct delivery of cardioprotective drugs to the cardiovascular system. The need to deliver these drugs into specific targets has led to the development of different methods for targeted drug delivery.20 Methods for preventing CVDs have recommended along with a healthy lifestyle, which includes non-smoking behavior, a healthy diet, regular physical activity, weight reduction, aerobic exercise training, maintaining blood pressure, and lowering blood lipids.21 However, several therapeutic methods and drugs available are based on the use of synthetic compounds. This condition may lead to different episodes of adverse effects on the body. This topic directs the researchers to focus on safer and promising therapeutic methods. In this context, nanotechnology has extended its heights to the treatment of CVDs. Nanoparticles proved their role as an effective and reliable platform for the controlled and targeted delivery of drugs to cure the lipid disorders, thrombosis, inflammation, and angiogenesis in atherosclerotic plaques. This review summarizes different nanoparticle-based drug carriers available so far for the treatment of cardiovascular diseases.
Different Treatment Strategies for CVDs
Even though there are recent advancements in the field of diagnosis, and management of many CVDs, mortality associated with CVDs remains much higher than cancer throughout the world. There are several ways to control and treat cardiovascular diseases. Treatment modalities of CVDs include control of blood pressure, cholesterol, diabetes, weight, and physical activity, depression, and selection of ideal medications. World Health Organization (WHO) has recommended population-wide and individual wise interventions to reduce the burden of CVDs. Tobacco control policies, applying taxes to decrease the amount of high fat, sugar and salted foods, tactics to lessen the alcohol intake, construction of cycle roads, and walking area to enhance physical exercises has included in the population interventions (Figure 1). Individual responses required for people at high risk of CVDs such as having hypertension and hypercholesterolemia, so that first heart attacks and strokes can prevent in them. Different kinds of medication can provide for the secondary prevention of CVDs associated with other diseases such as diabetes. These medications include beta-blockers, statins, aspirin, and angiotensin-converting enzyme inhibitors. Other than these medications, surgical operations, and medical devices are also preferred to treat CVDs, such as balloon angioplasty, heart transplantation, coronary artery bypass, valve repair, and replacement and artificial heart surgeries. Medical devices used to prevent CVDs are prosthetic valves, pacemakers, and patches for heart holes.22 Open aortic repair, thoracic endovascular aortic repair, hybrid aortic repair, and combination of open aortic repair and thoracic endovascular aortic repair are the known surgery approaches for treating thoracic and thoracoabdominal aortic diseases. Yet, a single treatment method has not customized for the treatment.23
Figure 1.
Current strategies included in CVD interventions.
The modern era of cardiac operations adopted invasive surgeries for treatment. But the paradigm is varying with the introduction of minimally invasive heart surgery with small incisions and without cardiopulmonary bypass. These methods received widespread attention due to safety, efficacy, and feasibility. Some of the minimally invasive procedures which reduced post-operative pain, hospital stay, blood flow, and good cosmesis are hemisternotomy and right anterior thoracotomy (aortic valve-based incisions) right minithoracotomy and robotic mitral valve surgery (mitral valve-based incisions), totally endoscopic coronary artery bypass grafting. Future technologies such as endoscopic, percutaneous, and robotic technologies will reduce the trauma associated with surgery and post-operative pain in CVD patients.24 Another promising treatment for CVDs is collectively known as vascular gene therapy. The possible targets of gene therapy are ischemia, restenosis, thrombogenesis, graft failures, in-stent restenosis, atherogenesis, and arterial cytoprotection—cardiovascular gene therapy aids in the overexpression of several therapeutically significant proteins and correct genetic problems. Transfer of vascular endothelial and fibroblast growth factor genes promoted blood flow and collateral development in ischemic limb and myocardium. In animal models of vein graft thickening or restenosis, vascular gene therapy of genes coding proteins such as vascular endothelial growth factor, thymidine kinase, retinoblastoma, and nitric oxide synthase, cyclin or cyclin-dependent kinase inhibitors, hirudin, growth arrest homoeobox, fas ligand and antisense oligonucleotides against transcription factors exhibited beneficial effects. Even though studies on gene transfer of vascular endothelial growth factor are available, further advancements in delivery methods, efficient vehicles, and valid targets are required.25
A large number of drugs are being introduced into the market for the prevention and treatment of hypertension and associated CVDs. Complementary and alternative medicine (CAM) is one of the conventional treatment modalities among patients with CVD. However, more than 95% of CAM has suggested to patients with hypertension. CAM includes the integral parts of Traditional Chinese Medicine (TCM) such as herbal medicine and acupuncture. Clinical trials showed that acupuncture could be employed to lower the high blood pressure and to improve the circadian rhythm of blood pressure of patients with hypertension. Among all available CAM, Chinese herbal therapy has gained more popularity. Many developments occur in the treatment of hypertension using Chinese herbs and formulations from theory to experiments and clinical trials. There are some Chinese patented drugs used for the treatment of hypertension in clinics such as Niuhuang Jiangya Pill, Yangxue Qingnao Granule, and Qing Gan JiangYa Capsule. The role of TCM is limited in the modern hypertension clinical practice and health care system, even though Chinese herbs can decrease the elevated blood pressure, improve the endothelial function, regulate the renin-angiotensin-aldosterone system, protect the target organs and reverse the risk factors of CVDs.26
TCM is a fascinating feature of Chinese culture, which combines western medicine as an integrative approach to treat CVDs. Scientists criticized TCM due to several reasons such as inadequate scientific evidence, lack of sufficient clinical trials, unknown therapeutic mechanisms, dozens of ingredients in TCM, making it challenging to study and availability of randomized controlled trials (RCTs) with small sample size and diverse outcomes.27 Results of some RCTs point out that some of the TCM therapies might be beneficial to control the risk factors of CVDs such as hypertension, diabetes, and dyslipidemia. Also, it indicated that some TCM medications might be useful as a complementary and alternative approach for primary and secondary prevention of CVDs.1
There are several single-dose, multiple-dose, or fixed-dose formulations available to decrease high blood pressure. The mechanism of action of the single-dose formulation is the inhibition of a single pathway, which leads to high blood pressure. Combination therapy has popularized among fixed-dose formulations, which increase the effectiveness of hypertension drugs by inhibiting multiple pathways. Combination therapy has suggested that patients who become resistant to single-dose formulations. Sampatrilat, lleptril, omapatrilat, gemopatrilat, and fasidotril are brand names of some hypertension drugs which inhibit multiple targets such as an angiotensin-converting enzyme (ACE) and neutral endopeptidase (NEP). These two enzyme inhibitors have used for the treatment of congestive heart failure (CHF), renal failure, and ischemic heart disease (IHD) through a combination of dose therapy.5 Precision medicine in CVDs is changing recently, whereas gene editing and gene-based therapeutics have applied in the clinics for the treatment of CVD patients.28
Conventional medicine and synthetic drugs are less in practice owing to their costs and associated cardiovascular complications. Natural products have received much attention as they slow down the progression of CVDs and are economically attractive. Natural products in CVD medicine have multi-targeted effects with fewer side effects than synthetic drugs.29 Many bioproducts from plants and phytotherapy are approved and marketed for the prevention and treatment of CVDs. Medications obtained from Ginko biloba, garlic, and Crataegus have suggested for patients with CVDs. Complementary and alternative medicine (CAM), along with phytotherapy, has directed for the efficient management of CVDs. CAM continues to control CVDs by embracing the folk medicine and legacies of our ancestors. Research in ethnopharmacological medicine would play a crucial role in CVDs in the future.30
Curcumin, a bioactive component in turmeric, has been widely studied for its broad therapeutic properties. Curcumin is known to treat jaundice, cold, hepatic disorders, cough, and inflammatory diseases. Besides, this phytochemical is famous for the treatment of CVDs owing to its pleiotropic actions. This excellent phytochemical suppresses the development of drug-induced cardiotoxicity, cardiac hypertrophy, aortic aneurysm, heart failure, diabetic cardiovascular complications, atherosclerosis, stroke, and myocardial infarction.31 Molecular targets of curcumin in CVDs include histone acetyltransferases (p300), Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), transcription factor (NF-κB), and angiotensin II type 1 receptor (AT1R). The application of curcumin for the treatment of CVDs has limited due to their low solubility and poor bioavailability in clinical trials. In this context, researchers had put effort into designing synthetic derivatives of curcumin or drug delivery systems, which improve bioavailability and solubility. Hence, curcumin has utilized as a routine food supplement by considering its safety level to prevent or treat CVDs.32
Policosanols are fatty alcohols that consist of primary aliphatic alcohols derived from the wax coating of sugar cane. These phytochemicals have reported being an essential alternative with enhanced prevention and treatment prospects to the common hypocholesterolemic agents. These are potential agents for patients who either suffer from medical problems or unwilling to take synthetic drugs. Approximately 5 to 20 mg/day doses of policosanols have found to be effective in reducing serum lipid levels, which can develop CVDs in later stages. In addition to the roles in CVDs, these plant chemicals deliver beneficial effects to liver function, platelet activity, and intermittent claudication. Advantages of policosanols such as enormous availability, low cost, and availability as food supplements make them to be a potential and promising alternative to non-natural hypolipemiant drugs.33
Likewise, polyphenols, the most abundant antioxidant phytochemicals, are widely studied for their ability to prevent degenerative diseases, especially CVDs. Altogether, polyphenols have considered for their protective role in CVDs using human trials.34 It has found that the inclusion of polyphenol-rich foods in daily meals alleviates the risk associated with CVDs. The biomarkers of CVDs have related to lipemia, inflammation, and oxidative stress. Consumption of polyphenol-rich foods such as tea extract, pomegranate juice, grape extract, green tea, black tea, wine polyphenols, red wine, and dealcoholized wine reduced the development of atherosclerotic lesions in apolipoprotein E-/- mice and hamsters.35 Other than the polyphenols, antioxidants phytochemicals as a whole can be considered as potent molecules for the treatment of CVDs. The intricate etiology of CVDs is characterized by the overproduction of reactive oxygen species and other free radicals, which may end up with the disruption of endothelial function and other deleterious vasodilator effects. There are some flavonoids from the pulp of Euterpe oleracea that exhibited atheroprotective effects in the laboratory. A flavonoid found in Flos chrysanthemi improved the vasodilator reactivity by alleviating the oxidative stress. Quercetin shows protective properties in animals with atherosclerosis through the interference with foam cell formation and pro-inflammatory response.36
Plant-based dietary patterns have always linked with the treatment of CVDs. A diet containing fruits have a positive impact on the treatment of CVDs. Epidemiological studies confirmed that the risk of cardiovascular pathologies is inversely related to the intake of fruits. Diet covering only a small amount of fruits is the third most imperative risk factor of CVDs after high blood pressure and cigarette smoking.37–39 Fruits which possess cardiovascular protecting properties include grape, pomegranate, blueberry, hawthorn, avocado, and apple. The mechanism of actions of these fruits includes reduction of lipid metabolism and elevated blood pressure, inhibition of thrombosis, oxidative stress and inflammatory response, modulation of signaling pathways, and molecular events associated with the correction of epithelial function, suppression of platelets function. It has always recommended studying the mechanism of action and the protective role of a more significant number of fruits in the future owing to their potential candidature for cardiovascular protection.40
CVDs such as hypertension and heart failure are related to mitochondrial dysfunction, which results in the overproduction of free radicals. Improper function of mitochondria results in the process of programmed cell death and finally to CVDs. Hence, control of mitochondrial dysfunction is a vital process in the design of drugs for CVDs. The current research and clinical trials are focused on identifying the role of antioxidant phytochemicals in controlling mitochondrial dysfunction. Interestingly, several studies in animals and humans proved that coenzyme Q10 could control mitochondrial dysfunction. This coenzyme present in the inner mitochondrial membrane is an anti-thrombolytic and antioxidant molecule, which can improve hypertension and hyperglycemia. This coenzyme can be administered alone or along with other drugs for the treatment of hypertension and heart failure in humans.41 Currently, berries are fetching attraction in the diet chart and functional foods not only for their pleasing aroma and appearance but also due to their medicinal properties. Natural berries such as mulberry, raspberry, blackberry, cranberry, bilberry, currants, and blueberry have both commercial and nutritional values owing to their protective and preventive properties for some chronic diseases. Antioxidant capacity of berries has attributed due to their high levels of anthocyanins and flavanols. These phytochemicals can scavenge ROS, which plays a vital role in the prevention of CVDs. Also, they play a potent role in the regulation of blood pressure, oxidative stress, endothelial function, whole-body metabolism, platelet aggregation, atherosclerosis and safeguards body from CVDs.42
Rapamycin is a natural product frequently used as an immunosuppressant after organ transplantation procedure. Rapamycin complex interacts with its mechanistic target of rapamycin (mTOR). Hence, the mTOR function has subdued. Rapamycin and its rapalogs have introduced into interventional cardiology and antitumor therapy after the discovery of their mechanism. Growing evidence shows that long-term treatment with rapalogs may lead to dyslipidemia after organ transplantation and immunosuppressive therapy in patients with high risk for CVDs. mTOR inhibitors, along with statins or other drugs, can be employed as a combination therapy by considering the role of dyslipidemia as a risk factor in cardiovascular disease.43 Resveratrol, a non-flavonoid polyphenolic compound, has beneficial effects on hypertension, stroke, heart failure, atherosclerosis, arrhythmia, chemotherapy-induced cardiotoxicity, ischemic heart disease, and diabetic cardiomyopathy. Some of its beneficial effects are mediated by the activation of cyclic adenosine monophosphate-activated protein kinase, silent information regulator 1 (SIRT1), an endogenous antioxidant enzymes. Besides, the anti-platelet, insulin-sensitizing, anti-inflammatory, and lipid-lowering properties of resveratrol have positive effects on CVDs. Clinical evidence of these drugs still lack which limited its use in clinics.44
Another possible target for the treatment of CVDs is Endoplasmic Reticulum stress (ER stress). It is a defense mechanism that identifies unfolded and misfolded proteins and helps in ER homeostasis. Once ER stress has extended, vascular cells and cardiomyocytes would become dysfunctional. It may result in apoptosis and further lead to the progression of CVDs. In the last five years, there was an exponential growth in the development of novel natural compounds (saponins, alkaloids, and polyphenols) to improve the ER stress along with their multiple pathogenic pathways, such as apoptosis, ROS production and inflammation to treat CVDs. Some of the examples of compounds which mitigate ER stress-mediated apoptosis, ER stress-mediated oxidative stress and ER stress-mediated inflammation are baicalein, resveratrol, berberine, quercetin, ginsenoside-standardized extract, anisodamine, notoginsenoside R1, and paeonol.45 A purine nucleoside, adenosine, which regulates a wide range of cellular and molecular functions of the body, has enormous therapeutic potential to treat a wide range of diseases from cancer to CVDs. Modulation of A2 adenosine receptor interaction with adenosine plays an important role in the regulation of blood pressure, heart rate, and heart rate variability. It has studied that the dysfunction of these receptors stimulates CVDs, but the exact pathological mechanisms behind these A2 receptors are not available so far.46
Stem cell transplantation has received much attention for balancing and regulating cardiac properties. Several studies indicated that stem cells could improve the heart functions through their release of paracrine signals and then networking with tissues or organs. Compared to the stem cells, stem cell-derived exosomes are more stable without immune rejection, and aneuploidy.47 Exosomes secreted by different cell lines possess cardioprotective property, direct angiogenesis, decrease apoptosis and respond to the stress. Stem cell transplantation possesses several limitations, such as the adequate source of stem cells, efficiency of in vitro amplification, determination of optimal implementation for the target stem cell transplantation, etc. Hence, exosomes derived from stem cells could serve as a promising alternative for stem cell transplantation. Exosomes can be loaded with drugs for the targeted delivery and effective gene therapy, which is not possible with stem cells. The exosome-mediated treatment has not widely implemented in the health care system due to some disadvantages such as complex and cumbersome extracting procedure of exosomes, a limited quantity of exosomes, and the presence of harmful compounds in exosomes. Also, only a few numbers of exosomes will stay in the site of injection after intravenous secretion.48
There are conventional drug carrier systems used for the effective delivery of cardioprotective drugs. Standard drug carriers for CVDs are tablets or their new liquid spray through lingual or sublingual administration. These oral tablets or transdermal patches can exhibit long time absorption through slow absorption via the digestive tract and skin. Even though some of the cardioprotective drugs maintain different types of tablet forms for efficient pharmacodynamic action. Drug delivery through a stent or balloon catheter helps to deliver drugs to a small local target area in coronary interventional therapy. But the drugs used to treat angina such as beta-blockers, calcium channel blockers, nitrates, and ranolazine are associated with adverse side effects. Medications like nitrates, calcium channel blockers, and β blockers used in the treatment of angina pectoris (Heart condition marked by chest pain due to the less supply of oxygen to the heart) produce adverse effects such as rash, constipation, nausea, drowsiness, edema, low blood pressure, headache.49 Available drugs and therapeutic methods for cardiovascular diseases possess significant side effects with long term usage irrespective of their therapeutic potentials such as rhabdomyolysis, renal failure, and hemorrhage.49
Protein conjugates are used for the visualization of thrombi and targeted thrombolytic therapy. Thrombus specific antibodies conjugated to a thrombolytic enzyme or therapeutic agent has recommended in targeting thrombus to manage inflammation and thrombolysis.50 Other disadvantages of conventional drug systems are due to the monolithic nature, which allows the loading of only one drug. Thus, multiple medications cannot be loaded on the same drug carrier, which decreases the overall outcome of cure.51 Overall, interventional therapy, stem cell transplantation, therapeutic drugs, and other treatment modalities are not ultimate to treat CVDs. We need to shift the restoration of heart functions through effective drug delivery and optimization of therapeutic strategies available for patients with CVDs. Hence, nanoparticles have introduced as an excellent and innovative carrier of cardioprotective drugs for enhanced efficacy with fewer side effects. The disadvantages of cardioprotective medications such as nonspecific effects, insolubility, and impermeability to blood-brain barriers can be eliminated by introducing nanotechnology.
Emergence of Nanomedicine in CVDs
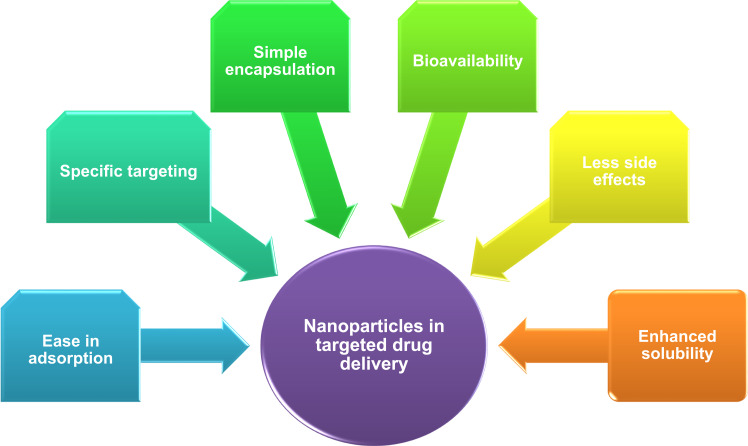
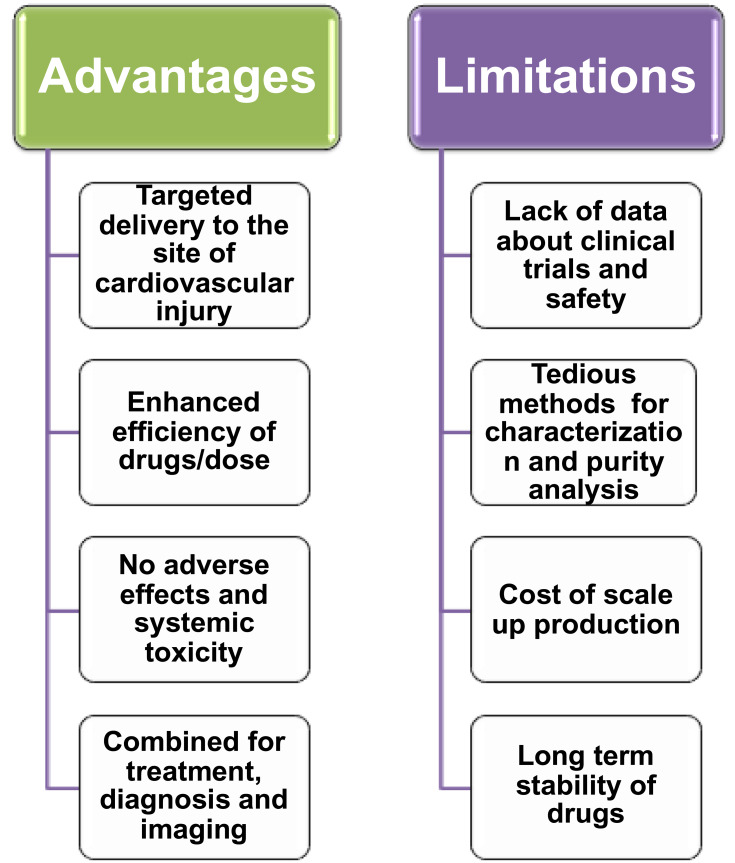
Nanoscience and nanotechnology are an emerging field in communication technology, information, biology, medical technology, biotechnology, and medicine. Nanoscience is the study of design, manipulation, production, and application of materials with a size of less than 100 nm. Nanotechnology offered numerous applications in the field of medicine such as for analytical purposes, imaging, and diagnosis, treatment procedures such as targeted drug delivery, gene delivery systems and scaffolds for tissue engineering.52 Nanoparticles have gained much attention in medicine owing to their physicochemical properties that improve biological function (reactivity, roughness, high surface energy and high surface to volume ratio). Nanomedicine is a promising field that allows the diagnosis, treatment, and control of diseases or disorders to improve the quality of life and health. Nanoparticles in medicine provided countless advantages over traditional and modern medical practices. These advantages include prolonged half-life of drugs, reduced toxicity, and enhanced biocompatibility of nanoparticles, and reduced side effects of drugs by altering the properties of nanoparticles (Figure 2). Moreover, targeted delivery of drugs using nanocarriers either by active or passive targeting appears to be a promising method of therapeutics. Active drug targeting requires the conjugation of drugs to the cell-specific ligand attached to the nanoparticle. In passive targeting, a high molecular weight polymer has employed for the delivery of drug which can permeate and retain in the tissue for better outcome.53
Figure 2.
Advantages of nanoparticle-mediated drug delivery.
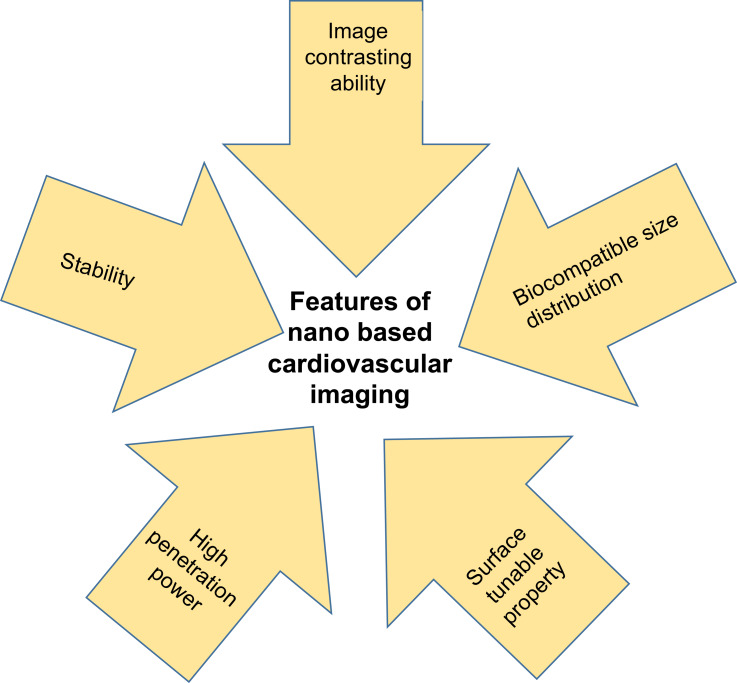
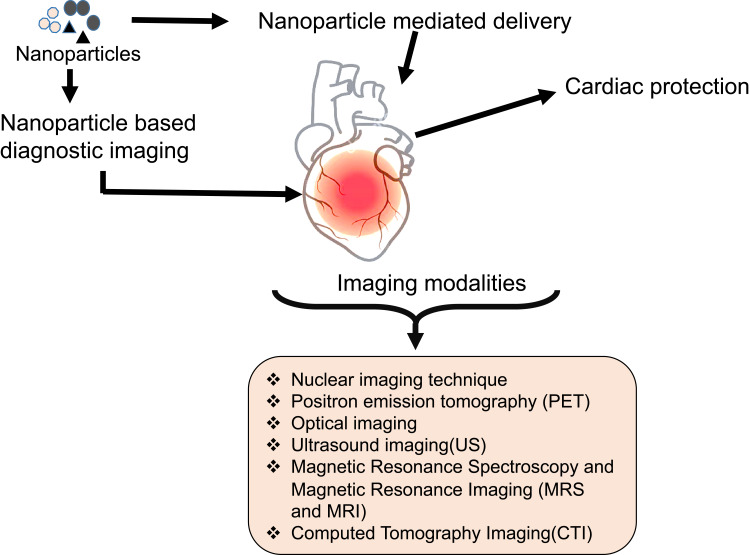
Nanoparticles of size 1 nm to 100 nm hold unique properties compared to their larger counterparts. They can cross biological membranes, cells, and tissues.54 The unique properties are nanosize, their distinctive quantum size, high surface to volume ratio, and shape. Nanoparticles can be surface modified to highlight their physical properties. Monodispersed nanoparticles have synthesized by controlling the size, shape, and aggregation of particles, which enables cell internalization. Nanomaterials have considered as a theranostic, diagnostic, drug carrier, and therapeutic agents in various biomedical applications owing to their unique properties. For example, high surface to volume ratio of nanoparticles allows the conjugation, absorption, or encapsulation of molecules for the delivery to the target site.55 Drug delivery vehicles are employed owing to their ability to deliver poorly soluble or highly toxic drugs to the target areas. Rapid growth occurred in the field of nanotechnology has answered all the problems associated with the therapeutic methods of CVDs. Nanotechnology helps in the early detection and effective treatment of CVDs, thus reducing the burden on the health care system. In short, the significant roles of nanoparticles in CVDs are superior medical imaging, targeted delivery of drugs, and targeted delivery of nanoparticles to kill diseased cells. As nanoparticles deliver drugs through blood and tissue, they can also be eliminated from there efficiently.56 Rapid developments in the field of nanotechnology enhance the imaging of CVDs through the design of capable cardiovascular molecular imaging probes based on nanotechnology in the personalized cardio-medicine. Unique features of nanoparticles that enable them as potential agents for cardiovascular imaging has provided in Figure 3. The promising applications of these imaging probes in CVD medicine are due to the ability of nanoparticles to cross the biological barriers and to accumulate at the target site.57 Nano-imaging in cardiology is an integrative approach for diagnosis and real-time monitoring during surgery and therapy. Nano-based cardiovascular imaging is interrelated to different fields of diagnosis, surgery, and therapy. Thus, the nano-based cardiovascular imaging has categorized into broad areas such as imaging of thrombus, stem cell, graft, and theranostic approach depending on the site of detection or the mode of mechanism.58 Different imaging modalities and methods used in cardiology has indicated in Figure 4.
Figure 3.
Special features of nanoparticles that made nanomaterials as indispensable in the cardiovascular imaging.
Figure 4.
Nanoparticle-mediated diagnosis and imaging of CVDs.
Theranostic application of nanoparticles in CVDs eliminates the gaps between experimental evidence and large-scale clinical trials. Theranostics in cardiology integrates imaging and therapeutics by enabling imaging-based therapeutic drug delivery systems. There are several nanoparticles employed for diagnostic imaging, drug delivery, and further assessment of drug efficacy. Nanoparticle-based drug delivery and its action in the target site has controlled by light, external magnetic field, or ultrasound, which minimizes local and systemic effects.59 Magnetic resonance imaging has employed for vascular intervention in a site-specific manner. It has reported that metallic nanoparticles attracted by the magnetic field identify and impede inflammatory processes in atherosclerotic plaques.60 Magnetic nanoparticles coated with natural compounds efficiently used in imaging of CVDs. For instance, ultra-small superparamagnetic iron oxide nanoparticles coated with fucoidan (a polysaccharide) had used for the molecular imaging of thrombus by Magnetic Resonance Imaging (MRI) arterial thrombus. This contrasting agent was able to image P-selection, adhesion molecule known as the molecular element of atherothrombotic disease in a rat model of elastase-induced vascular injury.61
Gold nanorods synthesized by a group of researchers had used to diagnose and attenuate macrophages through photodynamic therapy.62,63 Generally, nano theranostics are categorized into three distinct stages. In the stage I, nanoparticles-based imaging agents are used to evaluate the efficiency of conventional therapy. Stage II, imaging agents are integrated to study the efficacy of nanoparticle-mediated therapy. Stage III, nanoparticle-based imaging is used to evaluate the effectiveness of nanotherapy.64
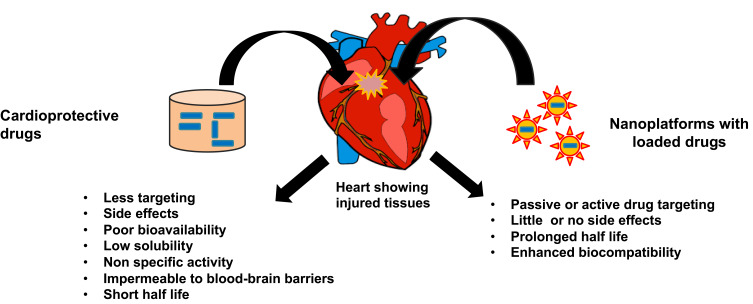
Commonly, nanomedicine in cardiology deals with four main areas that control and/or aids in the management of CVDs. These areas of nanotechnology are molecular imaging, targeted drug delivery, tissue engineering, and diagnostics. Nanocarriers hold several advantages such as the controlled release of drugs to the targets, enhanced bioavailability of therapeutic agents, and targeted delivery of drugs (Figure 5). There are several types of drug delivery systems or devices available for the management of CVDs. Some of the possible and widely studied drug carriers are liposomes, polymeric nanoparticles, micelles, dendrimers, etc. An ideal drug carrier has defined by its non-toxic nature, ability to escape from the host immune system, biodegradability, biocompatibility, non-immunogenic properties, and drug targeting properties.49 Also, the small size of nanoparticles allows them to pass through the cell membrane or blood-brain barriers for the targeted delivery of drugs of interest. Controlled and targeted delivery of drugs has achieved through optimizing several parameters such as temperature, enzyme activity, pH, stimuli (ultrasound, magnetic field, infra-red) etc.65 Some nanoparticles can be loaded with a huge number of drugs for a diagnostic or therapeutic approach such as in polymeric nanoparticles and liposomes. Other nanoparticles require additional functional ligands to conjugate the drugs. Nanoparticles have used as multifunctional agents for diagnosis and treatment of CVDs owing to their high surface ratio, which allows surface modification. Surface modification of nanoparticles with different functional moieties such as small molecules, peptides, aptamers, and antibodies allowed the modulation of their biodistribution, and their targeted delivery.66 Liposomal platforms are multifunctional nanomaterials which can be used as efficient drug carriers and molecular imaging probes. Ligand bound liposomes are employed in cardiovascular imaging.67 Nanocarriers allow the conjugation or encapsulation of drugs, which enhances the solubility of drugs, reduces systemic toxicity and protects from metabolism and excretion.68 The rational involved in the design of nanocarriers for cardiovascular medicine are multi-criteria design. This multi-criteria for design and development of nanocarriers include product physicochemistry, quality of the ingredient such as safety, pyrogen content and serializability, manufacturability including process cost, cost of goods, stability, biocompatibility, toxicity, efficacy, pharmacokinetics, biodistribution and finally clinical acceptability.69
Figure 5.
Schematic representation showing a general treatment method and targeted drug delivery using nanoplatforms to damaged cardiovascular tissues.
Some of the nanocarriers developed for the treatment of cardiovascular diseases are discussed here before going into detailed perspectives. Among all nanocarriers, polymeric nanoparticles are ideal nanodevices for targeted drug delivery.70 These smaller particles exhibit higher uptake in the walls of the artery with sustained release of the drugs to the target site. Sustained release of drugs has determined by their various characteristics such as cross-linking, molecular weight, and monomer ratio. Poly (lactic-co-glycolic acid) (PLGA) nanoparticles are the commonly used biodegradable polymer nanoparticles. This polymer nanoparticle degrades as soon as the drug has delivered to the site. For example, sirolimus delivered through PLGA nanoparticles (treated with gelatin) sustained in the system for 50 days. Delivery of nanoparticle coated with drugs using catheter helps in the penetration of nanoparticles to the arterial wall due to the pressure. It acts as the drug reservoir for the treatment, as the nanoparticle gets stuck in the wall once the pressure is released. The delivery of drugs using a catheter has an advantage over the drug-eluting stents. It is because inflammatory response is generated inside the system by the reaming polymer scaffold as soon as the drug has depleted. Drug-eluting stents have used for the delivery of drugs from polymer or stents71 (Figure 6). Different types of nanocarriers used for the targeted delivery of cardioprotective agents have described in Table 1.
Figure 6.
Advantages and disadvantages of nanoparticle-mediated drug delivery in cardiovascular diseases.
Table 1.
Nanocarriers Studied for the Efficient Treatment of Cardiovascular Diseases
| Types of Nanocarriers | Drugs Used in the Treatment of CVDs | Biological Functions | Model Organisms Used | Limitations of Drugs | Advantages of Nanoplatforms | Reference |
|---|---|---|---|---|---|---|
| Liposomes | ||||||
| Polyethylene glycol conjugated liposomal nanoparticles | [Pyr1]-apelin-13 polypeptide | Controls cardiac hypertrophy and hypertrophy-induced heart failure | Murine model of transverse aortic constriction | Short half-life in circulation | Prolonged apelin stability in the blood circulation Potentiated beneficial effects in cardiac function |
[72] |
| Liposomal nanoparticles coated with polyethylene glycol |
Prednisolone phosphate | Ideal for atherosclerotic disease | Clinical trials in humans | Short half-life in circulation | Prolonged the drug’s half-life to 45–63 hour in humans | [73] |
| Naked liposomes and water-soluble double emulsion polymer | Streptokinase (Streptase) | Plasminogen activators | Rabbits model of autologous carotid artery thrombosis | Shows immunogenic effect and severe bleeding complications | Reduced infarct size and reperfusion time and less hemorrhage | [74] |
| PEGylated Liposomes, with a peptide sequence of fibrinogen gamma-chain | Recombinant tissue plasminogen activator (rtPA, (alteplase)) | Plasminogen activator | Rats model of inferior vena cava thrombosis | Short half-life of rtPA | Enhanced thrombolytic activity | [75] |
| Metallic Nanoparticles | ||||||
| Gold | Vascular endothelial growth factor (VEGF) | To treat severe hindlimb ischemia | Murine ischemic hindlimb model | Short half-life of VEGF in circulation Less specific targeting | Highest targeting | [76] |
| Gold | Conjugated with miR155 | For the management of cardiovascular diseases in postmenopausal diabetic patients | Ovariectomized diabetic mouse model | Inefficient targeting of miR155 to macrophages | Efficient delivery of miR155 into macrophages via phagocytosis which in turn restores the cardiac function | [77] |
| Gold | Bone-marrow derived mesenchymal stem cells (BMSCs) | Potentiates the cardiogenic differentiation of stem Cells for infarcted myocardium regeneration | Nil | Decreased ability to differentiate into multiple lineages | Superior cardiomyogenic differentiation Enhanced biological and functional effects on the regeneration of infarcted myocardium | [78] |
| Gold | Levosimendan (Simdax) | Effective inotropic agent that increases myocardial contractility in patients with heart failure. | Heart failure Wistar rat model | Decreased preferential targeting Simdax to the target heart tissue | Showed significant cardioprotective effects in rats with doxorubicin-induced heart failure | [79] |
| Silica Nanoparticles | ||||||
| Mesoporous silica | Hydrogen sulfide (H2S) | New organ-preserving agent in the field of transplantation | Balb/c mice aged | Limited use due to the cytotoxic effects | Management of Cardiac allograft vasculopathy (CAV) which is the leading cause of death in heart transplant patients | [80] |
| Biodegradable porous silicon | Atrial natriuretic peptide | To treat an injured region of the myocardium in Ischemic heart disease patients | Ischemic Wistar rat model | Less targeting of peptide produced inside the body | Improved colloidal stability and greater cellular interactions with cardiomyocytes and non-myocytes with negligible toxicity | [81] |
| Polymeric superparamagnetic nano-silica | Quercetin | Antioxidant agent, quercetin is utilized to control atherosclerosis and other relative cardiovascular illnesses | Mice | Poor water solubility | Permitting cell enlistment, attachment, expansion, and articulation of heart proteins in local myocardium | [82] |
| PEGylated mesoporous silica | Puerarin | Chinese medicine used for the treatment of cardiovascular diseases | Male Sprague Dawley rats | Short elimination half-life in humans Intravenous administration of high doses of puerarin is needed Severe and acute side effects. | Improved blood compatibility with low hemolysis, Good candidate for intravascular drug delivery | [83] |
| Polymeric Nanoparticles | ||||||
| Poly (lactide-co-glycolide) (PLGA) | Heparin and glutathione | Anticoagulant and antioxidant agent used for vascular therapy | Nil | Systemic toxicity, Systemic coagulopathy and hemorrhage symptoms | Effective delivery to the site of an Ischemia/reperfusion injury | [84] |
| Dendrimer | Hirudine | Antithrombotic and anticoagulant agent | Antithrombotic effect evaluated in venous thrombosis model of Wistar rats | Short plasma half-life, generates irreversible hirudin thrombin complex | Gene transfer to thrombosis and thrombosis treatment | [85] |
| Micellar | Hirudine | Natural thrombin inhibitor | ApoE-null mice fed a high-fat diet | Short plasma half-life | Increased delivery of hirudine to the plaques and inhibited the formation of fibrin clots after coronary artery occlusion | [86] |
| Polymeric micelles | m-Tetra(hydroxyphenyl)chlorin (mTHPC) | Anti-inflammatory agent | Female Balb/c nude mice | Side effects and other off-target effects | Increased stability and thus allow accumulation of intact mTHPC- to macrophages of atherosclerotic lesions | [87] |
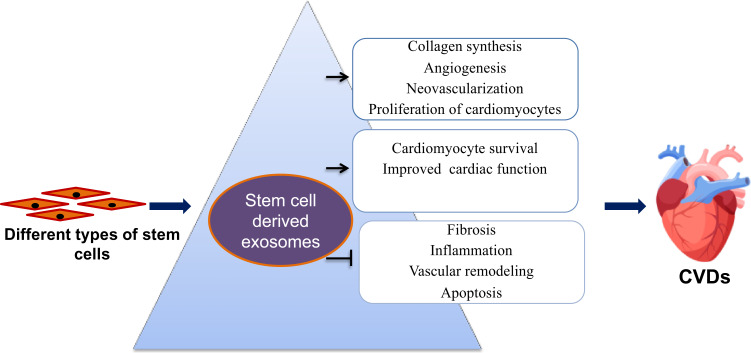
Exosomes derived from stem cells are nanosized vesicles exploited widely for their drug-carrying capacity. Stem cell-derived exosomes are essential in the delivery of mRNA, miRNA, and proteins to the recipient cells. The therapeutic potential of exosomes in CVDs includes their cardioprotection activity and drug delivery properties (Figure 7). In brief, these are called natural liposomes due to their similarity in tissue targeting and drug delivery as that of liposomal drug delivery systems. In addition, exosomes are used as diagnostic markers in CVDs.88 Researchers studied several biomimetic nanocarriers for the treatment of CVDs. These biomimetic nanoparticles are involved in the apoptosis of macrophages and smooth muscle cells, which will benefit in the rupturing of plaques. High-density lipoprotein mimicking nanoparticles can target the mitochondrial membrane potential that happens during apoptosis to identify the susceptible plaques. These biomimetic nanoparticles contain a core of biodegradable poly (lactic-co-glycolic acid), triphenylphosphonium, and cholesteryl oleate. Agents present in the nanoparticles can detect the collapse of mitochondrial membrane potential. A-I mimetic 4F peptide anchored to the lipid layer can enhance the passage of cholesterol from the lesions. Furthermore, quantum dots incorporated into the core improves optical imaging.89,90
Figure 7.
Applications of exosomes derived from stem cells in the treatment of cardiovascular diseases.
Synthetic High-Density Lipoprotein (HDL) -PLGA biomimetic nanoparticles have developed to accumulate in the macrophages and monocytes of the aorta. These HDL nanoparticles are having PLGA, apolipoproteins and lipids 1-stearoyl-2-hydroxysn-glycero-3-phosphocholine/1,2-distearoyl-sn-glycero-3-phosphocholine. These HDL nanoparticles for the quick detection of vulnerable plaques has used as preventive, therapeutic methods for atherosclerosis. Difficulty with such nanoparticles is associated with their scale up production for clinical/market trials due to their complex methods of construction.91 Hu et al, 2015 formulated nanocarriers using biomimetic nanoparticles covered with platelet membranes.92 These HDL nanoparticles of size 100 nm coated with platelet membranes have found to be promising drug carriers for the treatment of CVDs, especially atherosclerotic diseases. This type of nanoparticles utilizes the ability of HDL and platelet membranes to target natural atherosclerotic plaques and their selectivity to deliver to the biological motifs, damaged human and rodent vasculatures. Furthermore, they can direct the clearance of plaques from pre-atherosclerotic and atherosclerotic areas. Even though these nanoparticles have able to target plaques, their ability to eliminate the plaque is weak completely. Thus, diagnostics and therapeutics should be considered concurrently when designing these nanodevices for the elimination of plaques in the future.92,93 There are nanocarriers of thrombus-specific tissue plasminogen activator (tPA), which enhances the efficacy of thrombolysis and inhibits serious bleeding complications. Nanocarriers are developed to eliminate the shortcomings of this tPA, such as short life span (approximately 5 minutes), rapid inactivation by plasminogen inhibitors 1 in the blood, and requirement of large doses. For example, urokinase was delivered to a rat model of the autologous carotid artery and left jugular vein thrombosis through dextran-coated magnetic nanoparticles. Urokinase was conjugated to nanoparticles through primary amine ligands and showed a fivefold higher thrombolytic activity in model animals than the free plasminogen activator.94
The chelating agent, ethylenediaminetetraacetic acid (EDTA), can resorb calcium mineral deposits, but the systemic delivery of it may result in side effects. Nanoparticle-mediated delivery of chelating agents has recommended as it holds an effective treatment modality.95 In a study, targeted delivery of Matrix metalloproteinases (MMP) inhibitors has carried out using Poly Lactic Acid (PLA) nanoparticles conjugated with an anti-elastin antibody to a rat model of abdominal aortic aneurysm. Nanodelivery of batimastat (MMP inhibitor) prevented aneurysmal growth, vascular calcification, inhibited the activity of MMP, and degradation of elastin.96 Nosoudi and co-workers employed a dual therapy with targeted delivery of EDTA and pentagalloyl glucose for the removal of mineral deposits and to restore elastic lamina. The dual delivery of chelating agent and polyphenol reversed the aneurysm development through reduced macrophage recruitment, MMP activity, degradation of elastin and arterial calcification.97 Vascular calcification was reversed in a rat model of chronic kidney disease when supplemented them along with the targeted delivery of EDTA using anti-elastin conjugated albumin nanoparticles.95 One of the clinical trials of nano-drug platforms was successful and named as Abraxane (nanoparticles conjugated with albumin-bound paclitaxel). Abraxane treatment has found to be tolerated up to 30 mg/m2; however, additional studies are required to identify the effects of nanoplatforms delivered by intravenous and intracoronary administration.98 There are less clinically approved nanomedicine in the treatment of cardiovascular diseases. Solid lipid-based nanoparticles loaded with carvedilol (β adrenergic receptor blocker) and liprostin (a Phase 3 clinical trial drug) was clinically tested to improve their bioavailability and to treat peripheral artery disease. Dendrimer formulation of candesartan (clinically approved angiotensin receptor blocker) achieved an enhanced drug solubility extending its use in targeted delivery to injured cardiac tissues.2
As curcumin is a natural compound widely used for atherosclerosis from past so many years, curcumin nanoparticles have developed to enhance the efficacy of curcumin. There are several studies showing curcumin nanoparticles applied in the treatment of CVDs. But its clinical usage is limited by fast systemic exclusion, alkaline pH degradation, low gastrointestinal absorption, rapid metabolism, and low water solubility. Even though curcumin is the right candidate for suppressing myocardial tissue oxidative stress, curcumin nanoparticles showed enhanced cardioprotection than curcumin. Curcumin nanoparticles limit myocardial damage in acute myocardial infarction (AMI). These nanoparticles protected isoproterenol-induced myocardial infarction in rats by altering electrocardiogram and myocardial tissue oxidative stress.99 In a rat model of isoproterenol-induced MI, curcumin nanoparticles exerted a better cardioprotective effect than curcumin. It also showed improved myocardial function and reduced heart injury after MI. Enhanced effects of curcumin nanoparticles are in accordance with their enhanced antioxidant activity and reduced amount of pro-inflammatory cytokines and matrix metalloproteinases in serum.100 The impact and incidence of MI are higher in diabetic patients than in healthy individuals. Curcumin nanoparticles showed enhanced cardioprotective effects in a rat model of isoproterenol-induced AMI with diabetes mellitus when compared to curcumin. Also, these nanoparticles could be employed in the treatment of AMI developed in diabetic patients due to their antioxidant and anti-inflammatory nature.101
Nanoparticles for the Treatment of Coronary Artery Disease (CAD)
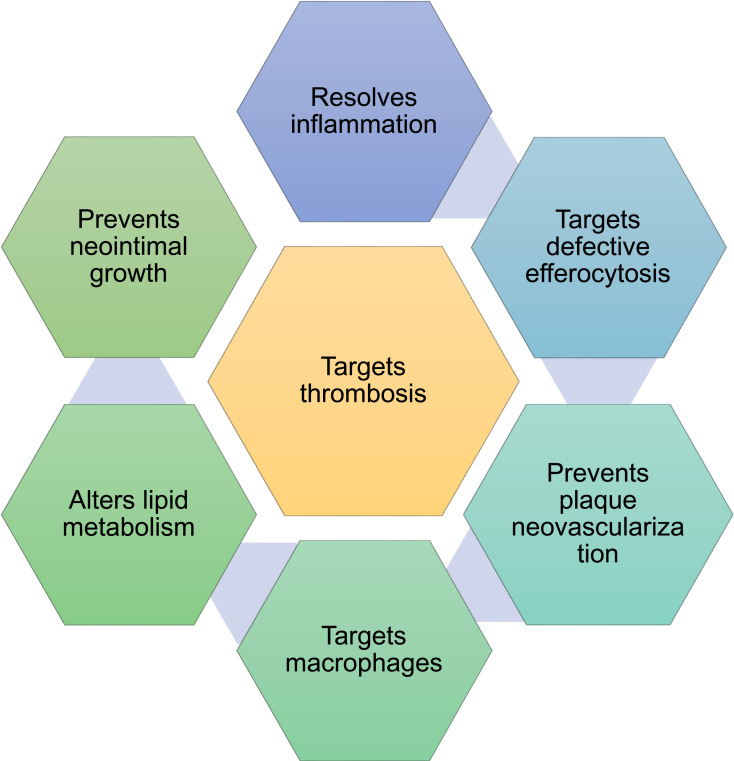
The clinical applications of nanomedicine in the field of cardiovascular diseases are limited and are in clinical trials. Coronary artery disease has occurred from the build-up of the atherosclerotic plaque on the inner wall of the coronary artery. It appears in the stenosis of the cavity, which reduces the compliance of the vascular wall. Thus, the blood supply to the myocardium has disrupted partially.102 A chronic disease, atherosclerosis has characterized by the thickening of the arterial wall and inflammation of plaques.103 Heart attack occurs when coronary arteries have obstructed by atherosclerosis. During this condition, a sequence of the intricate and interlocked physiological process take place involving many cells, extracellular matrix, and cytokines that are triggered by hypoxia of cardiomyocytes. It, in turn, directed to the loss of cardiac function and followed by the replacement of myocardium by fibrous scars.104 Coronary thrombosis happens during the shredding or rupture of atherosclerotic plaques. Plaques are vulnerable and shreds due to specific characteristics such as reduced smooth muscle cells and extracellular matrix, plaque bleeding and calcification and appearance of numerous inflammatory cells and large necrotic core with flimsy fibrous cap.105,106 Nanoparticles can be applied for the treatment of atherosclerosis by improving their circulation in the whole body, enabling better solubility of drugs, reducing the required amount of drugs, decreasing the cytotoxicity of drugs, enhancing the targeted delivery of drugs at specific concentrations and merging both the diagnostic and treatment methods to develop a better theranostics.107 Nanoparticle-mediated drug delivery and treatment for atherosclerosis and associated complications have focused in resolving inflammation and defective efferocytosis, preventing plaque neovascularization, targeting macrophages, altering lipid metabolism, preventing neointimal growth and targeting thrombosis (Figure 8).108 Liposomal preparation of bisphosphonate alendronate decreased the neointimal formation and then suppressed the circulating monocytes in rabbits with iliac artery stenting. Liposomal alendronate has found to be safer in early clinical trials for infusion at the time of percutaneous coronary intervention.109
Figure 8.
Several potential targets for the nanomedicines in the treatment of cardiovascular diseases.
Statins are commonly used drugs for the treatment of coronary artery diseases. In general, the use of statin is limited recently due to their high dose therapy associated with systemic side effects.110 Nanometer-sized vesicle loaded with pravastatin was prepared after functionalization by oligonucleotides to target the macrophages. These nanocarriers were found to be promising alternatives that allow a high dose therapy with enhanced efficacy and less toxicity of statins in the neighboring tissues.111 Likewise, paramagnetic nanoparticles are used for the delivery of fumagillin (antiangiogenic drug) targeted by integrin, which exhibits a decrease in the systemic adverse effects.112 Nanocarriers are thought to alter lipid metabolism through RNA interference. They directly target the metabolism of liver cholesterol. Targeting apolipoprotein B may benefit from lowering the LDL and total cholesterol levels.113,114 In monkeys and rodents, silencing of their apoB in hepatocytes using liposomal preparation of apoB-small interfering RNA resulted in the reduction of total cholesterol and LDL.115 LDL cholesterol can be reduced in a potent manner through the siRNA silencing of proprotein convertase subtilisin/Kexin type 9 (PCSK9) (inclusion). In the above study, second-generation lipid nanoparticles loaded with siRNA has targeted to PCSK9 in individuals with high LDL cholesterol levels. These lipid-based nanoparticles used for the silencing of RNA are called lipidoids. Lipidoids are used for the suppression of PCSK9 synthesis in the liver and cause rapid effects after the administration of a single dose.
Clinical trials of inclusion are ongoing on rodents and monkeys, where it reduced the LDL levels in the clinical phase for at least six months. Clinical Phase III trials are still in progress, and it is expected to bring revolution in cardiovascular medicine.116 ApoE-/- mice fed with the high-fat and induced atherosclerotic plaques have treated with antibodies conjugated to copper sulfide nanoparticles. Antibodies have synthesized against an ion channel from the transient receptor potential vanilloid subfamily 1 (TRPV1) expressed in vascular smooth muscle cells. These ion channels have opened with the increase in the temperature that causes the influx of calcium ions and activates autophagy in the smooth muscle cells. Also, ion channels decrease the accumulation of lipids and inhibit the formation of foam cells. Copper sulfide nanoparticles provide an effective and non-invasive treatment method for CVDs owing to their absorption in the near infra-red range and generate a photoacoustic signal that helps in treatment.117
Patients are suffering from peripheral artery disease display difficulty in revascularization procedures owing to the high rate of restenosis and re-intervention. Thus, nanotherapeutic methods are developed, which ultimately enhance the retention of drugs in the plaques and local vascular bed. These nanocarrier methods include the delivery of drugs through catheters fixed in stents. Paramagnetic nanoparticles loaded with rapamycin are used to treat balloon injured arteries of female rabbits. These nanocarriers enhance the targeted delivery of drugs and improve imaging through MRI due to the high contrast potential of nanoparticles. It also enables the local infusion of nanoparticles to the arterial wall with functional retention capacity and decreases the neointimal formation.118 Some researchers utilized the tunable properties of the nanoparticle to encapsulate with nitrogen gas and RNA interference compounds119 for the local delivery of drugs to the arterial wall. Nitric oxide is a gas with potential therapeutic effects of anti-inflammation, anti-proliferation, vasodilation, anti-thrombosis, and anti-atherogenesis. Systemic delivery of nitrogen gas provides less low bioavailability with adverse effects. Nitrogen gas loaded into echogenic liposomes with co-encapsulation of argon was used for controlled delivery of bioactive gas to inhibit intimal Hyperplasia (thickening of the blood vessel).120 Nanoparticle-mediated delivery of drugs has formulated to target thrombosis. Thrombus targeted nanoparticles are enabled by platelet activation, coagulation cascade, and fresh thrombus for the delivery of thrombolytic agents, direct thrombin inhibitors, urokinase, streptokinase, and anticoagulants (example tissue-type plasminogen activator, tPA). Intravenous injection of thrombus targeted nanoparticles (encapsulated with von Willebrand factor binding protein) with tPA results in the reperfusion and vessel recanalization in most of the swine. Nanoparticles have designed with a greater offloading, controlled release of tPA, and thrombolytic activity at the affected artery using transthoracic ultrasound.121 Thus, nanoparticles are potent nanodevices for anticoagulation and reperfusion therapy with diminished bleeding consequences. Theranostic nanoparticles loaded with anti-thrombin can be directly used for the attenuation of plaque coagulant activity in the damaged arteries of the apoE-/- mice model. These nanoparticles have able to retain in the plaques and cause prompt inactivation of locally produced thrombin. Anti-thrombin theranostic nanoparticles stimulate the stability of plaques. Besides, the expression of plaque inflammatory molecules has reduced, and damaged vascular endothelium has repaired through the inhibition of plaque thrombin.122
There are inflammatory macrophages or monocytes that perform a significant role in the suppression of atherosclerosis and rupture of plaques. Bioabsorbable nanoparticles can be utilized for the targeted delivery of pioglitazone to the circulating monocytes or macrophages. This causes the regulation of inflammatory reactions and further prevents the plaque ruptures.123 Immune cells always hold an endurable role in the progression of atherosclerosis. Nanoparticle library has generated for the treatments which utilize various physical and chemical properties of nanoparticles and different types of immune cells. The high density, endogenous lipoprotein-based nanoparticle library is prepared in such a way that it achieves the targeted delivery of drugs to the macrophages in the plaques.124 Hirulog (inhibitor of thrombin) obtained from hirudin has conjugated to the micellar nanoparticles, which inhibited the formation of fibrin clots after coronary artery occlusion. This coronary artery occlusion has mainly caused by atherosclerotic plaque degeneration and rupture.86 Pre-resolving nanomedicine is having promising applications in the treatment of atherosclerosis. Researchers synthesized nanoparticles containing anti-inflammatory peptides such as Ac2-26. They displayed enhanced resolution and assisted in neutrophil recruitment for an improved targeted delivery.125 Restenosis is carried out after coronary artery angioplasty and is influenced by mechanical injury, delayed endothelial healing, and inflammatory responses. Long term success of stent placement has determined by the inhibition of vascular thrombosis and restenosis.126 Nanoparticles such as PLGA has appeared to be a promising alternative for the improved intra-arterial localization of drugs in restenosis.127 These limitations can be addressed by the drug-eluting stents coated with nanoparticles. These particular stents can even enhance the therapeutic effects of ineffective drugs.128 Nanodevices has designed with a nanoparticle-drug eluting stent for the prevention of restenosis and improved endothelial recovery. For instance, sirolimus/pitavastatin nanoparticle-eluting stents are prepared and showed a reduction in in-stent restenosis. Early endothelial healing has occurred only in the case of sirolimus nanoparticle-eluting stents.129
A group of researchers has used polymeric nanoparticles (PLGA) for the treatment of plaque destabilization. They found that nanoparticles are partially delivered to the plaques by the phagocytosis of monocytes or macrophages. It has also confirmed that the nanoparticles have delivered to the lesions owing to their permeability. PLGA nanoparticles coated with pitavastatin (HMG CoA reductase inhibitor) reduced the infiltration of circulatory monocytes/macrophages and destabilization of plaques in the arteries. Liposomal delivery of siRNA against the CCR2 receptor to the spleen, liver, and bone marrow was successful.
Furthermore, it inhibited the infiltration of monocytes to the aorta and plaques.130 Pioglitazone, a clinically approved drug activates peroxisome proliferator-activated receptor gamma (PPARg). Oral administration of drugs causes a reduction in the macrophage content and matrix metalloproteinase activity in a murine model of atherosclerosis. Other than their anti-diabetic activity, pioglitazone has a beneficial effect on macrophage polarity. PLGA nanoparticles loaded with pioglitazone were employed in the murine model to study their effect on atherosclerosis. Activation of the receptor enhanced the differentiation of macrophages. Here, the targeted delivery of pioglitazone skews the monocyte and/or macrophage polarity and regulates inflammation in a murine model. Long term therapy (4 weeks) of the drug significantly reduced the number of buried fibrous caps and their thickness which is a surrogate marker of plaque rupture.130
Nanoparticles for the Treatment of Ischemic Heart Diseases
One of the severe ischemic heart diseases is myocardial infarction, which leads to heart failure and, ultimately, to death. Cardioprotective drugs that have applied in the treatment increase angiogenesis promote cardiomyocyte survival, improve heart function, restrain inflammation, and myocyte activation, and eventually prevent fibrosis. In short, all the treatment and therapeutic agents are involved in the protection and preservation of cardiac function. Virus mediated gene transfer and therapy are successful and well-known in promoting cardiomyocyte survival and cardio function in mice. Yet, virus-mediated gene therapy is limited to animal studies and clinical trials owing to their safety concerns. Safety issues related to the treatment are their immunogenicity, unpredicted insertion site in the genome of humans, and poor targeting efficiency.131 Nanoparticles mediated gene delivery to improve cardiac function provides a promising alternative in the field. Nanoparticles have used for gene delivery due to their small size that enables a smooth and efficient penetration to the cells. Specific tags can be attached to nanoparticles through a covalent bond for better absorption by the target cells. Another advantage of nanoparticles over viruses is their faster synthesis methods and their ability to cluster receptors for the activation of signaling pathways.132,133 There are entrapping systems and surface binding systems of nanoparticles that have used as carriers for DNA or RNA delivery. The entrapping system is a reservoir type nanosphere system and protects the gene of interest from degradation. The surface binding system supports the ionic interaction between the anionic nucleic acid and cationic polymer. PEGylation can be used for the delivery of a gene to vascular tissues. Researchers constructed PEG–POD/DNA by linking a peptide to the PEG for the ocular delivery. Reduction of PEG-POD/DNA results in the induction of a twenty-one-fold increase in the transgene expression. It ultimately leads to the decline (50%) in the choroidal neovascularization when tested in vivo.134 Mannosylated chitosan nanoparticles are excellent carriers for DNA and siRNA delivery and, instead, are readily taken up by the macrophages. Thus, the application and future perspectives of mannosylated chitosan nanoparticles for subduing inflammation and reducing myocardial infarction await further studies.135,136
Placental growth factor (PIGF) is useful to stimulate angiogenesis and improve cardiac function at the site of damage that occurred due to acute myocardial infarction. Chitosan-alginate nanoparticles have designed for the effective delivery of PIGF with biocompatibility, non-toxic and biodegradable properties. Chitosan-alginate nanoparticles have found to be an excellent vehicle for PIGF delivery by intramyocardial injection to improve the benefits of PIGF at the site of injury. These nanoparticles can protect the growth factor from enzymatic degradation before its delivery to the tissues.137 Endogenous angiotensin II is vital for the delayed phase of an injury, recruitment of monocytes, and finally, inflammation of myocardium occurs. Thus, intravenous injection of bioabsorbable nanoparticles (PIGA) loaded with irbesartan (antagonist of angiotensin II type 1 receptor) at the time of reperfusion causes the inhibition of monocyte recruitment and then reduces the myocardium infarct through anti-inflammatory mechanisms. Insufficient local drug accumulation during reperfusion time was observed in clinical studies and demanded the development of effective delivery systems for a better clinical outcome.138 Injectable hydrogels have formulated for the treatment of acute myocardial infarction. Delivery of drugs through injectable hydrogels formed by nanofibers is one of the non-invasive methods which reduce the recovery time required for patients and also the occurrence of infections. Hydrogels such as RAD16-II loaded with vascular endothelial growth factors has injected into animals with myocardial infarction for the intramyocardial drug delivery. Systemic delivery of vascular endothelial growth factor using liposomes resulted in the improvement of the heart function and vascular density.139
Mitochondrial dysfunction can lead to cardiac diseases. Hence mitochondria are considered as an important therapeutic target to prevent heart failure and to limit the myocardial infarct size in patients with ischemic heart disease. There are several mitoprotective drugs (cyclosporine-A (CsA) or TRO40303) designed to target cardiac mitochondria. The drawbacks of these mitoprotective drugs are less permeability through the plasma and mitochondria membrane. Nanoparticles have introduced along with mitoprotective drugs to solve the hurdle of reduced permeability. PLGA nanoparticles carrying mdivi-1 (small molecule inhibitor of mitochondrial fission protein) to mitochondria protected H2O2 mediated death of rat cardiomyocytes.140 Another drug, CsA encapsulated with PLGA nanoparticles reduced myocardial infarct size, protected mitochondrial permeability transition pore opening and prevented ventricular remodeling in a perfusion mice model.141
Nanoparticles as Drug Delivery Agents in the Treatment of CVDs
Gold Nanoparticles
Gold nanoparticles are one of the widely used nanocarriers for the delivery of cardioprotective drugs. The application of gold nanoparticles with existing clinical drugs has suggested as a novel approach with enhanced potential in the treatment of heart diseases. Gold nanocarriers have broadly used metal nanoparticles owing to their ease in synthesis, low toxicity, low immunogenicity, and stability.142 It has been reported that clinical drugs become more efficient and accurate in their conjugated form. Simdax is a clinically approved drug for the treatment of heart disease. Simdax conjugated on gold nanoparticles (AuNPs) exhibits cardioprotective effects in rats with doxorubicin-induced heart failure. The cardioprotective properties of gold nanoconjugates and simdax are superior to their counterparts owing to the enhanced targeting of gold nanoconjugates to the injured tissue.79 The β blockers exert beneficial effects on the hyperactivity of the sympathetic nervous system of the volume overloaded heart failure. Metoprolol is one of the widely used β blockers and conjugated with AuNPs for the improved delivery to the cardiac tissues. Metoprolol preferentially targets β1 receptors, and the conjugates show double efficiency in targeting the volume overloaded heart failure tissue. These conjugates can be employed in the clinical practice as it displays little adverse effects on other organs.143 Diabetic cardiomyopathy can be treated with miR155-AuNPs. It is a disease commonly found in women after menopause, where the disease symptoms worsen by estrogen deficiency.144 Generally, an excessive number of pro-inflammatory type 1 macrophages over anti-inflammatory type 2 macrophages were found in the hearts of diabetic mice. Additionally, an increase in the cell apoptosis, fibrosis, reactive oxygen species production, and cardiac hypertrophy had observed in the hearts of ovariectomized diabetic mice. In vivo delivery of miR155-AuNPs conjugates significantly increase anti-inflammatory type 2 macrophages, and decrease the intensity of inflammation. It is, in turn, reduces cell apoptosis and finally leads to the restoration of cardiac function.77
Cardiac tissue engineering is an emerging therapeutic method for the treatment of CVDs. Tissue repair or tissue regeneration combines different combinations of nanomaterial and biomaterials with engineering strategies. Generally, cardiac regenerative biomaterials have coated electrically active nanoparticles to target the cardiac tissues. In a study, the injection of hydrogels fabricated from electrically active gold and laponite nanoparticles loaded with extracellular matrix improved the biological and functional properties of cardiomyocytes. Gold or laponite nanoparticles with extracellular matrix-hydrogel enhanced the compatibility of cardiac cell and phenotypic maturation of cardiac-specific proteins. For example, nanoformulations used in the above study improved the expression of cardiac-specific markers such as SAC, Cx43, and cTnl. It confirms the potent applications these hydrogels in the targeted delivery of biologically active materials for the cardiac tissue engineering of infarcted myocardium. The functional properties and electrical conductivity of biomaterials are maintained by the incorporation of nanoformulations into the cardiac tissues. These nanoformulations decrease the porous structures in the hydrogel so that a favorable environment has provided for cardiomyocytes.145
Gold nanoparticles are proven promising nanomaterials for drug delivery without causing toxicity in the healthy tissues. Gold nanoparticles have used in the laboratory for the treatment of AMI; however, clinical trials and future experiments are still to be explored in the future. AMI may lead to other complications such as necrosis and apoptosis of cardiomyocytes, propagation of fibroblasts, conversion of cardiomyocytes to myofibroblasts, deposition of collagen in the myocytes, and cardiac hypertrophy. Hypertrophy of cardiomyocytes can lead to heart failure. However, gold nanoparticle conjugates or alone possess beneficial effects in AMI, which further prevents all other complications. PEGylated gold nanoparticles of size 10 nm protected have able to reduce the size of the infarcted heart through the inhibition of necrosis and apoptosis of cardiomyocytes.146 Moreover, they can decrease the collagen deposition and then inflammation. Thus, the study suggests the utilization of gold nanoparticles alone or its nanoconjugates for the treatment of CVDs.147 Photodynamic therapy and sonodynamic therapies are recommended for the treatment as they possess improved cure rates and reduced adverse effects. Certain drugs such as precursors of porphyrins with metal nanoparticles synthesized by photoreduction methods can target and destroy the pathogenic tissues. These nanoparticle formulations induce cell death by the production of reactive oxygen species and DNA damage. A group of researchers synthesized aminolevulinic acid (porphyrin IX precursor)-metal nanoparticles (gold nanoparticles or/and iron oxide nanoparticles) through the photoreduction method. Nanohybrids interfere with the selectivity of iron transport across the inner membrane of mitochondria. Thus, these nanohybrids act as a novel diagnostic and therapeutic method for CVDs, which selectively and effectively destroy macrophages.148
Ischemia causes hindrance in the blood flow and damages the downstream tissues owing to the less availability of oxygen and nutrients. Therapeutic angiogenesis occurs through the process of angiogenesis, where new blood vessels are built by evolving and diverging from the available blood vessels. Therapeutic angiogenesis is used to treat ischemia through the delivery of various growth factors such as vascular endothelial growth factors. Delivery of these growth factors have been shown to be less effective in the clinical trials such as short half-life of growth factors in the blood circulation, lack of specific targeting and the inability of growth factors to retain in the circulation for days to weeks, which is essential to avert the deterioration of new vessels. Gold nanoparticles act as an excellent payload for imaging and targeted delivery of vascular endothelial growth factors to ischemic muscle tissues in the murine hindlimb ischemic model. Murine model of ischemia is a typical model for peripheral artery disease. Gold nanoparticles of size below 200 nm are able to accumulate in the ischemic muscle. Hence, therapeutic targeting of exogenous growth factors to the tissues exhibits a rise (1.7-fold rise) in the blood perfusion. Gold nanoparticles target the ischemic muscle tissues through their enhanced permeability and retention effect. It promotes the recovery of a large volume of ischemic tissue along with enhanced angiogenesis due to the delivery of exogenous growth factors.76 Growth factors were successfully delivered through gold nanoparticles to modify the damaged heart tissue in coronary artery disease. Whereas traditional intravenous administration methods possess difficulties in targeting these growth factors to damaged tissue.77 Gradually, gold nanoparticles have proved as one of the most promising drug carriers for accurate delivery to injured heart tissue. Gold nanoparticles conjugated with clinically approved drugs enhanced the efficacy of these drugs to treat heart diseases.139,149
Liposomal Platforms
Liposomal delivery systems are robust platforms for site-specific delivery of therapeutic agents. Liposomes are spherical nanoparticles that possess a bilayer structure made of natural lipids or synthetic cholesterol. These spherical structures have a hydrophilic core surrounded by a hydrophobic membrane. They can be conjugated with proteins or peptides for improved delivery to the tissues or to produce biological benefits.150,151 The first therapeutic liposomal drug approved by the Food and Drug Administration (FDA) and prescribed for the patient is liposomal doxorubicin (Doxil). Doxil is used for the treatment of several cancers but minimizes the cardiotoxicity induced by doxorubicin. Liposomal preparation of drugs improves the cure rate by enhancing the half-life of doxorubicin from minutes to an hour.152 At present, there are no liposomal drug platforms available for the direct treatment of CVDs in humans. Several studies have conducted to exploit the possible benefits of liposomal platforms for drug delivery. Clinical trials have carried out where growth factors are delivered through a liposomal and adenoviral vector for the treatment of myocardial ischemia after angioplasty and stenting. They aim to prevent restenosis after the angioplasty and stenting.153 Some cases of liposomal delivery for gene transfer are found to be feasible and well-tolerated, yet treatments fail to reduce the frequency of restenosis. PEGylated liposomes encapsulated with therapeutic payloads of the AT1 receptor enhanced the recovery of cardiac cells after the incidence of a heart attack. In vivo studies revealed that this nano-receptor formulation is adequate for specific targeting, sustained release of the drug, increased accumulation in the damaged site without harming the healthy cardiac cells. The nano delivery system aided in the therapeutic delivery of payloads to the damaged myocardium with better therapeutic responses and excellent regeneration of cardiac cells.154 There are Liposomal alendronate platforms found as safer in early clinical trials for infusion at the time of percutaneous coronary intervention.109
Liposomes have targeted the heart vasculature, which contains several biomarkers. Vasculature targeting will enable the treatment of the affected areas of the cardiovascular system. C-Lipo, composed of a heart-targeting peptide, CRPPR, is an arginine-rich sequence biomarker that shows more than 300-fold selectivity for the endothelial cells of the heart. Liposomal carriers of size 71 to 110 nm conjugated with 18F-CRPPR are injected intravenously to the murine model for targeting healthy cardiac vasculature. It could help scientists to study the targeted delivery of peptides to heart vasculature. Liposomal platforms with conjugate are found in the heart within 100 seconds after the injection.155 CRPPR peptide is also used to conjugate with surface altered liposomes to target coronary endothelium in the myocardial infarction and ischemia-reperfusion animal models. Liposomal platforms are focused more on the injured tissue of heart vasculature than healthy tissue suggesting a better treatment rate.156 In a new study, a fluorescent-tagged PEGylated liposomal system is used for active and passive targeting to the infarcted heart. Passive targeting is carried out due to the ischemic dysfunctional blood vessels present in the left ventricle of the infarcted heart. Active targeting is performed using the liposomal carrier with ligands for the overexpressed angiotensin II type 1 receptor found in the infarcted heart. This study also confirms the active or passive targeting of liposomal platforms in the murine model of AMI within a week compared to the non-targeted liposomes.157 A study combining micellar and liposomal nanoformulations for treating AMI suggests liposomes and micelles as pro-angiogenic and cardioprotective drug delivery systems.158 Liposomes containing prednisone has used for the treatment of patients with atherosclerotic plaques. Small interfering RNA loaded onto polymer nanoparticles are delivered to atherosclerotic lesions to reduce the monocyte trans endothelial migration. The results have coincided with the reduced inflammatory activity in the atherosclerotic plaque.159
There are cell-based targets present in the infarcted site, which include macrophages. Phosphatidylserine (PS) is a ligand expressed on apoptotic cells and elicits anti-inflammation in the macrophages. Liposomal carriers with PS attached to the surface can be employed for the selective targeting to macrophages. A series of events occur after the engulfment of liposomes with PS such as the production of high amounts of anti-inflammatory cytokines, up-regulation of CD206 and downregulation of TNF alpha and CD86.160 Modified liposomes carrying an overall negative charge, nanogold, rhodamine and phospholipase A2 (an inflammatory marker) target activated macrophages in the atherosclerosis plaques. These liposomes target more to the lipid-laden and metabolically active areas of atheromas or atherosclerotic plaques.161 Saito and co-workers designed immunoliposome preparations to target carotid intimal hypertrophy in a rat model induced by balloon injury. A potent Rho-kinase inhibitor, fasudil, and a ligand decorated on the surface of liposomes had used to target LOX 1 on plaque lesions. LOX 1 is a lectin-like LDL receptor 1. The ligand used is an anti-LOX 1 antibody conjugated to the liposome. Targeted liposomes detected in the lesions decreased the expression of metalloproteinase-9 and intimal thickness.162 Cho et al worked on a different approach to treat atherosclerosis. They focused on solubilizing the bad cholesterol found in the plaques. Liposomes loaded or conjugated with phosphatidylcholine are aimed to enrich the lesion with high-density cholesterol. Typically, low-density cholesterol gathers at the walls of arteries, and oxidized cholesterol is taken up by foam cells. It resulted in the formation of atheroma. High-density cholesterol limits the inflammatory process by inhibiting the oxidation of LDL. Infusion of a liposomal formulation containing drug significantly reduced the plaque volume and cholesterol content on the walls. It suggests liposomal drug combinations as a promising substitute for the treatment of plaque regression.163,164 PEGylated Liposomes functionalized with peptide sequence of fibrinogen gamma-chain exhibited an efficient delivery of recombinant plasminogen activator (rtPA) into a rat model of inferior vena cava thrombosis for an improved thrombolytic activity.75
Liposomal preparation containing prednisolone has used to prevent in-stent restenosis in the rabbit model. Prednisolone possesses a high affinity for chondroitin sulfate proteoglycans present at the site of atheroma. It was able to reduce the percentage of stenosis and side effects in the atheroma model compared to the non-targeted liposomes.165 Liposomes loaded with nitric oxide and argon through freezing under pressure technique are utilized for the therapy of in-stent restenosis. In this method, high concentrations of gases dissolved in solution had used, which get entrapped in the liposomes during the freezing and thawing process. Huang and coworkers synthesized positively charged echogenic liposomes conjugated to dimyristoyl phosphatidylcholine (anti-proliferative agent). Charged liposomal formulations stimulate the relapse of lesions and reduction of hyperplastic development of smooth muscle cells and decrease the thickening of arterial walls in a balloon injured atheroma model of rabbits.119 Liposomal platforms are also proven as a successful therapeutic method of platelet targeting in CVDs. Liposomes with the modified surface are always attractive in the treatment of CVDs due to their improved and enhanced drug delivery to the injured cardiovascular tissues and surroundings.166 Liposomes loaded with oligodextran surfactants and arginine-glycine-aspartic acid can reduce the uptake of phagocytic cells of the reticuloendothelial system. Surfactant attachment has similar activities of cell glycocalyx and reduces the absorption of cells. Platelets are targeted more due to the binding of the peptide to the integrin present on the platelets. Thus, this study reported the camouflage activity of liposomes to target platelets, to diminish opsonization and the uptake of the endothelial system.167 Likewise, Srinivasan and coworkers determine the ability of liposomes to target platelets using arginine-glycine-aspartic acid in a rat model of restenosis with catheter induced carotid arterial injury. Results suggested that liposomal platforms containing peptides may be applied significantly to target platelets for the therapy of CVDs.168
Dendrimers
Unique properties of dendrimers such as their tunable size, multiple functional features, and outstanding biocompatibility, provide the opportunities to use polyamidoamine (PAMAM) and polyethyleneimine as gene delivery agents. These dendrimers interact with DNA/RNA and produce dendriplexes through electrostatic interactions. Dendrimers are promising nanocarriers with unique characteristics. Dendrimers are flexible gene delivery vehicles to the injured site as they carry a positive charge and smaller in size.169–171 Dendrimers have repeated branches with low polydispersity and high molecular uniformity. Drug moieties have loaded into the cavities and branch folding’s, which forms into the shape of channels and cages. The applications of dendrimers in targeted gene delivery to the injured cardiac tissues are infinite. In one study, the authors reported the use of starburst dendrimers for the transfer of genes (DNA or RNA) in cardiac grafts.172 Polyethylene glycol altered polyamidoamine was loaded with the recombinant plasmid, arginine-glycine-aspartic acid peptide, and hirudine. This dendrimer nanocomplex had used for the gene transfer and thrombosis treatment. Acute myocardial infarction and stroke can be prevented to an extent by treating the process of thrombosis. Dendrimer-DNA nanocomplex efficiently delivered the recombinant plasmid DNA at the site of thrombus formation and suggested for thrombosis therapy. Gene delivery system using dendrimers displayed little cytotoxicity in both in vitro and in vivo models by enabling excellent delivery of recombinant hirudine plasmid DNA to treat thrombosis.88
Hyperbranched PAMAM is more advantageous than PAMAM due to its ability to protect DNA from external damages, less cytotoxicity, and high gene transfection efficiency. A group of researchers synthesized hyper-branched dendrimers to transfer the hypoxia-controlled plasmid carrying human vascular endothelial growth factor-165 into the skeletal myoblasts. Transfer of the plasmid resulted in the expression of the growth factor gene in the myoblast cell. Skeletal myoblasts manipulated with the gene of interest have transplanted into the infarct myocardium for the repair of cardiac tissues in the AMI animal model. Transplantation of transfected skeletal myocardium with the gene of interest decreased the number of apoptotic cells, enhanced the survival of grafted cells, improved the blood vessel density, decreased the size of the infarct, and enhanced the cardiac function.173,174 Simvastatin, and nifedipine are two drugs used for the treatment of some CVDs. Simvastatin inhibits the enzyme hydroxyl methyl glutaryl CoA reductase and lowers the blood cholesterol levels. This drug is commonly used to treat hypercholesterolemia coronary events but displays some side effects like insoluble nature in water, less oral bioavailability, and irregular absorption. PAMAM dendrimer used to deliver the simvastatin has enhanced bioavailability, solubility, and pharmacokinetic properties and controlled release.175 Similarly, probucol cholesterol-lowering drug shows side effects if applied directly for the treatment. Thus, dendrimers can be loaded with the above drug to improve its pharmacokinetic properties and biological functions.176 Nifedipine is a calcium channel blocking drug used to treat hypotensive and angina patients. G0–G3 dendrimers could be used to enhance the solubility and beneficial effects for the treatment of CVDs. Amine and ester groups present in the dendrimers significantly improve the solubility and bioavailability of drugs for better treatment efficiency.177,178
Polymeric and Magnetic Nanoparticles
Polymeric nanoparticles gained much interest in cardiovascular nanomedicine due to their modifying nature and probable reabsorption in the body for maximum efficiency. Different routes of delivery of these nanoparticles are oral, dermal, mucosal, or transdermal, depending upon the site of action.52 The most commonly used polymers for the synthesis of biodegradable polymeric nanocarriers are poly lactic-co-glycolic acid (PLGA), polyglycolic acid (PGA) and polylactic acid (PLA). They are called biodegradable owing to their ease in elimination from the human body as carbon dioxide and water. Among these polymers, PLGA has widely tested as drug carriers for the treatment of CVDs.10 In a study, dextran-coated nanoparticles used for the dual delivery of two model drugs (poorly water-soluble drugs, SB431542 and CHIR99021) to reprogram the fibroblasts into cardiomyocytes. These drugs had encapsulated in the surface of the AcDX nanoparticles functionalized with spermine, which resulted in the pH triggered delivery into the fibroblasts. Cellular reprogramming of myofibroblasts and fibroblasts in the injured heart into healthy myocytes is always an attractive therapeutic method to replace the lost cardiac cells.179 In another work, polymeric nanoparticles fabricated with quercetin using the method of electrohydrodynamic atomization process for improved aqueous solubility and stability of the drug. This resulted in the prevention of atherosclerosis plaques. Encapsulation of this antioxidant agent on the PLGA nanoparticles enhanced the pharmacological effect of drugs due to the poor solubility and also reduced the production of reactive oxygen species.180 Polymeric nanocarriers are used for the targeted and controlled delivery of anti-proliferative agents to treat damaged vasculature and regarded as a safe approach to treat coronary artery disease also.181 It is clear that reactive oxygen species (eg, Hydrogen peroxide) generated due to the ischemia-reperfusion injury leads to vascular thrombosis and cardiac cell damage. Anticoagulant agents supplied directly to the patients showed a slow drug release rate and a short period of circulation in blood. Also, it causes adverse side effects with the faster dissolution of clots in the thrombo-occluded vessels. Hence, PLGA nanoparticles encapsulated with antioxidant (glutathione) and anticoagulant agents (heparin) exploited for the treatment of Ischemia/reperfusion (I/R) injury. Controlled and targeted delivery of drugs occurred within two hours indicating a potential platform to treat vascular diseases.84
Recent developments in the field of design and synthesis of multifunctional nanostructures comprising magnetic or plasmonic properties can be used as a powerful weapon to treat CVDs by employing magnetically assisted targeted drug delivery. Magnetic nanoparticles are being considered as safe MRI contrast agents and drug delivery agents to treat cardiovascular diseases. Drug loaded to magnetic nanoparticles can be easily tracked and used for targeted delivery, which depends on the contrasting agent employed. In this contest, iron oxide superparamagnetic nanoparticles are known as good nanocarriers to treat AMI. Magnetic nanoparticles loaded with tissue plasminogen activator enabled the magnetic orientation of the plasminogen activator in an embolic rat model to enhance local thrombolysis. It had found that the dose of drug bound to magnetic nanoparticles required to dissolve the clot was 100 times less than that applied as free drug. It implies the broad application of magnetic resonance tracking and delivery of drugs to many of the occlusive vascular diseases.182 Magnetoliposomes are synthesized using magnetic nanoparticles and liposomes and used for magnetic resonance imaging and targeted magnetic-based drug delivery in CVDs. Magnetolipsomes are potential multifunctional drug delivery systems. They are used to treat infarcted myocardium and for the delivery of growth factors, biomolecules, and cytokines to the degenerating cardiac cells.183 These nanoparticles efficiently targeted angiotensin receptor 1, which has overexpressed in infarcted myocardium.184 Nanoparticles encapsulated with p38 inhibitors minimized fibrotic region of affected heart.185
Even though nanoparticles possess enormous potential in cardiology, health risks, and safety associated with them should be considered. The effect of nanoparticles on both human health and the environment is to be assessed for safety measures. Impacts of nanoparticles on health have assessed by deposition and clearance of solid nanoparticles, systemic translocation, biocompatibility, has implications on the intestinal tract and central nervous system and bodily distribution. It is evident from studies that alteration in the structural and physicochemical properties of nanoparticles with reduced material size may lead to toxic effects (Figure 9). Hence, it is essential to bridge the gap between the toxic impact of nanoparticles and health benefits that have employed in CVDs.186
Figure 9.
Various types of nanoparticles widely used in the targeted delivery of drugs to treat CVDs.
Future Perspectives and Conclusions
It has evident that cardiovascular diseases and their associated disorders are one of the principal reasons for global morbidity and mortality. Even though some of the disorders are manageable, coronary artery diseases remain as a peril to biomedical researchers with rapid changes in the human lifestyle. There are numerous cardio-protective drugs available for the treatment and prevention of cardiovascular diseases, and newer therapeutic methods are gaining interest in the medical system. Among all treatment modalities available, natural compounds, herbal medications, and TCM found a place at the topmost strategies. These strategies and compounds are preferred over synthetic drugs as the side effects associated with them are very less. But these medications often failed to reach clinical settings, as they have not supported by scientific studies. Yet, researchers always demand new technologies and innovations in the field of diagnosis and treatment of CVDs more effectively and efficiently than older methods. Conventional treatment modalities that target different pathways such as lipid metabolism, blood volume, coagulation cascade, and arterial constriction exhibits disadvantages inefficient treatment of CVDs with side effects such as thrombosis in stents, unusual activation of the immune system and systemic toxicity. Thus, nano-drug delivery systems have employed as an alternative vehicle for efficient and controlled delivery of drugs into injured heart tissue. Likewise, nanoparticles have tremendous applications in the diagnosis and imaging of CVDs as they allow simple diagnosis of CVDs and real-time tracking during therapy. Nanoimaging in CVD exploited various multifunctional and multimodal imaging vehicle, which is not possible with conventional imaging techniques.
Nanotechnology and nanomedicine offer individual attention to the diagnostic and therapeutic fields of cardiovascular diseases as theranostic agents. Nanosized particles act as nanodevices or nanocarriers for the successful delivery of drugs to the injured site. Nanocarriers hold promising potential for the efficient, targeted delivery of drugs and genes that eliminate the problems associated with the solubility, bioavailability, and other pharmacokinetic properties. The current review delivers an overview of the different cardiovascular diseases and available therapeutic methods along with recent advancements in the field of treatment. It highlights and summarizes the relevant efforts and improvements made towards the targeted delivery of drugs to the cardiovascular tissues and their biomarkers for the treatment. Besides, a lacuna has observed in the field of nanomedicine for CVDs.
Although several cardiovascular nanoformulations are tested in both in vitro and in vivo in clinical trials, their clinical translation is still under development. It deals with the problems of pharmaceutical scale-up, release efficiency, substantial regulatory requirements exhibiting safety and Good Manufacturing Practice (GMP). Furthermore, in vivo and clinical trials are required to study the full effect of nanoparticles in the body and on cardiac tissues. Future designs and developments in the nanocarriers of CVDs require studies with much rigor. Several natural products derived from drugs and nanoformulations with cardioprotective activity are waiting for the research and clinical translations. The translational research of nanomedicine in CVDs has many challenges that prevent its successful implantation in clinics. These hurdles include institutional and industrial interests, unavailability of proper infrastructure, and skills to prepare patients for treatment, appropriate monitoring of patient outcomes, registration of patients in clinical trials, and country-specific compensation and affordability. Hence, translational medicine in CVDs using nanoparticles should be an integrative effort by bioengineers, pharmacists, chemists, biologists, and clinicians. Future research has targeted to use peptides and antibodies to detect the markers of CVDs and to develop selective nano delivery systems. Future nano-cardio medicine is oriented to evaluate the clinical effects of novel nanosystems to improve the quality of life.
Funding Statement
This research was funded by Congressionally Directed Medical Research Program (grant ID: PR130153), American Heart association (grant ID: 19IPLOI34730020) and the National Institutes of Health (grant ID: HL131577).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese Medicine for cardiovascular disease evidence and potential mechanisms. J Am Coll Cardiol. 2017;69(24):2952–2966. doi: 10.1016/j.jacc.2017.04.041 [DOI] [PubMed] [Google Scholar]
- 2.Iafisco M, Alogna A, Miragoli M, et al. Cardiovascular nanomedicine: the route ahead. Nanomedicine. 2019;14(18):2391–2394. doi: 10.2217/nnm-2019-0228 [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26(1):469–500. doi: 10.1146/annurev.publhealth.26.021304.144542 [DOI] [PubMed] [Google Scholar]
- 5.Leon BM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246. doi: 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav BK, Khatik GL, Haneef J, et al. Recent advancement over traditional drug in the treatment of hypertension. Int J Adv Res. 2019;4:58–61. [Google Scholar]
- 7.Karunathilake SP, Ganegoda GU. Secondary prevention of cardiovascular diseases and application of technology for early diagnosis. Biomed Res Int. 2018;2018:5767864. doi: 10.1155/2018/5767864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habetha J. The myheart project - fighting cardiovascular diseases by prevention and early diagnosis, in: 2006 International conference of the IEEE engineering in medicine and biology society. IEEE. 2006:6746–6749. [DOI] [PubMed] [Google Scholar]
- 9.Kim KS, Khang G, Lee D. Application of nanomedicine in cardiovascular diseases and stroke. Curr Pharm Des. 2011;17(18):1825–1833. doi: 10.2174/138161211796390967 [DOI] [PubMed] [Google Scholar]
- 10.Katsuki S, Matoba T, Koga J, et al. Anti-inflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med. 2017;4:87. doi: 10.3389/fcvm.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong SB, Lu S, Katwadi K, et al. Nanoparticle delivery of mitoprotective agents to target ischemic heart disease. Future Cardiol. 2017;13(3):195–198. doi: 10.2217/fca-2017-0012 [DOI] [PubMed] [Google Scholar]
- 12.Velagaleti RS, Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin. 2007;25(4):487–495. doi: 10.1016/j.ccl.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cury M, Zeidan F, Lobato AC. Aortic Disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med. 2013;2013:1–7. doi: 10.1155/2013/267215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473(7347):308–316. doi: 10.1038/nature10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng HM, Wang JJ, Chen CH. The role of vascular calcification in heart failure and cognitive decline. Pulse. 2017;5(1–4):1–4. doi: 10.1159/000484941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacheli GM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75(9):890–897. doi: 10.1038/ki.2008.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. JASN. 2009;20(7):1453–1464. doi: 10.1681/ASN.2008070692 [DOI] [PubMed] [Google Scholar]
- 19.Chen NX, Moe SM. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14(3):228–237. doi: 10.1007/s11906-012-0265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Li M, Wang G, et al. Heart-Targeted nanoscale drug delivery systems. J Biomed Nanotechnol. 2014;10(9):2038–2062. doi: 10.1166/jbn.2014.1894 [DOI] [PubMed] [Google Scholar]
- 21.Van Camp G. Cardiovascular disease prevention. Acta Clin Belg. 2014;69(6):407–411. doi: 10.1179/2295333714Y.0000000069 [DOI] [PubMed] [Google Scholar]
- 22.WHO. Facts sheets. cardiovascular diseases (CVDs); 17 May 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 17, February 2020.
- 23.Shimizu H, Hirahara N, Motomura N, Miyata H, Takamoto S. Current status of cardiovascular surgery in Japan, 2015 and 2016: analysis of data from Japan cardiovascular surgery database. 4―thoracic aortic surgery. Gen Thorac Cardiovasc Surg. 2019;67(9):751–757. doi: 10.1007/s11748-019-01163-x [DOI] [PubMed] [Google Scholar]
- 24.Langer NB, Argenziano M. Minimally invasive cardiovascular surgery: incisions and approaches. Methodist Debakey Cardiovasc J. 2016;12(1):4–9. doi: 10.14797/mdcj-12-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ylä-Herttuala S, Martin JF. Cardiovascular gene therapy. Lancet. 2000;355(9199):213–222. doi: 10.1016/S0140-6736(99)04180-X [DOI] [PubMed] [Google Scholar]
- 26.Xiong X, Yang X, Liu W, et al. Trends in the treatment of hypertension from the perspective of traditional Chinese medicine. evidence-based complement. Altern Med. 2013;2013:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartecchi CE. Complementary medicine has no place in cardiovascular medicine. J Am Coll Cardiol. 2006;47(7):1498–1499. doi: 10.1016/j.jacc.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 28.Dorn LE, Tual-Chalot S, Stellos K, et al. RNA epigenetics and cardiovascular diseases. J Mol Cell Cardiol. 2019;129:272–280. doi: 10.1016/j.yjmcc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell MS, Fleenor BS. The emerging role of curcumin for improving vascular dysfunction: a review. Crit Rev Food Sci Nutr. 2018;58(16):2790–2799. doi: 10.1080/10408398.2017.1341865 [DOI] [PubMed] [Google Scholar]
- 30.Ovidiu B, Felicia T, Burta O, et al. Phytotherapy in cardiovascular diseases: from ethnomedicine to evidence based medicine. J Biol Sci. 2008;8(2):242–247. doi: 10.3923/jbs.2008.242.247 [DOI] [Google Scholar]
- 31.Gupta SC, Patchva S, Koh W, et al. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Sureda A, Devkota HP, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2019;S0734–S9750:30010–30012. [DOI] [PubMed] [Google Scholar]
- 33.Haim D, Videla LA. Policosanols, protective natural compounds in cardiovascular disease. Agro Food Ind Hi Tech. 2008;19:60–63. [Google Scholar]
- 34.Giglio RV, Patti AM, Cicero AFG, et al. Polyphenols: potential use in the prevention and treatment of cardiovascular diseases. Curr Pharm Des. 2018;24(2):239–258. doi: 10.2174/1381612824666180130112652 [DOI] [PubMed] [Google Scholar]
- 35.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16(1):77–84. doi: 10.1097/00041433-200502000-00013 [DOI] [PubMed] [Google Scholar]
- 36.Zhang YJ, Gan RY, Li S, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgson JM, Prince RL, Woodman RJ, et al. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br J Nutr. 2016;115(5):860–867. doi: 10.1017/S0007114515005231 [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Hayasaka S, Shibata Y, et al. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the jichi medical school cohort study. J Epidemiol. 2011;21(3):169–175. doi: 10.2188/jea.JE20100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;369(10):954–964. doi: 10.1056/NEJMra1203528 [DOI] [PubMed] [Google Scholar]
- 40.Zhao CN, Meng X, Li Y, et al. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9(6):598. doi: 10.3390/nu9060598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siasos G, Tsigkou V, Kosmopoulos M, et al. Mitochondria and cardiovascular diseases—from pathophysiology to treatment. Ann Transl Med. 2018;6(12):256. doi: 10.21037/atm.2018.06.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Tian T, Wu D, et al. Prevention and treatment effects of edible berries for three deadly diseases: cardiovascular disease, cancer and diabetes. Crit Rev Food Sci Nutr. 2019;59(12):1903–1912. doi: 10.1080/10408398.2018.1432562 [DOI] [PubMed] [Google Scholar]
- 43.Kurdi A, Martinet W, De Meyer GRY. mTOR inhibition and cardiovascular diseases. Dyslipidemia and atherosclerosis. Transplantation. 2017;102:S44–S46. [DOI] [PubMed] [Google Scholar]
- 44.Zordoky BNM, Robertson IM, Dyck JRB. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta Mol Basis Dis. 2015;1852(6):1155–1177. doi: 10.1016/j.bbadis.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 45.Choy KW, Murugan D, Mustafa MR. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol Res. 2018;132:119–129. doi: 10.1016/j.phrs.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 46.Bahreyni A, Avan A, Shabani M, et al. Therapeutic potential of A2 adenosine receptor pharmacological regulators in the treatment of cardiovascular diseases, recent progress, and prospective. J Cell Physiol. 2019;234(2):1295–1299. doi: 10.1002/jcp.27161 [DOI] [PubMed] [Google Scholar]
- 47.Huang L, Ma W, Ma Y, et al. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 2015;11(2):238–245. doi: 10.7150/ijbs.10725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni J, Sun Y, Liu Z. The potential of stem cells and stem cell-derived exosomes in treating cardiovascular diseases. J Cardiovasc Transl Res. 2019;12(1):51–61. doi: 10.1007/s12265-018-9799-8 [DOI] [PubMed] [Google Scholar]
- 49.Singh B, Garg T, Goyal AK, et al. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. 2016;44(1):216–225. doi: 10.3109/21691401.2014.937868 [DOI] [PubMed] [Google Scholar]
- 50.Koren E, Torchilin VP. Drug carriers for vascular drug delivery. IUBMB Life. 2011;63(8):586–595. doi: 10.1002/iub.496 [DOI] [PubMed] [Google Scholar]
- 51.Lee WL, Wee P, Nugrahaa C, Loo SCJ. Gastric-floating microcapsules provide controlled and sustained release of multiple cardiovascular drugs. J Mater Chem B. 2013;1(8):1090–1095. doi: 10.1039/C2TB00495J [DOI] [PubMed] [Google Scholar]
- 52.Chandarana M, Curtis A, Hoskins C. The use of nanotechnology in cardiovascular disease. Appl Nanosci. 2018;8(7):1607–1619. doi: 10.1007/s13204-018-0856-z [DOI] [Google Scholar]
- 53.Logothetidis S. Nanotechnology in medicine: the medicine of tomorrow and nanomedicine. Hippokratia. 2006;10:7–21. [Google Scholar]
- 54.Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011 [DOI] [Google Scholar]
- 55.Mauricio MD, Guerra-Ojeda S, Marchio P, et al. Nanoparticles in medicine: a focus on vascular oxidative stress. Oxid Med Cell Longev. 2018;2018:6231482. doi: 10.1155/2018/6231482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong IY, Bhatia SN, Toner M. Nanotechnology: emerging tools for biology and medicine. Genes Dev. 2013;27(22):2397–2408. doi: 10.1101/gad.226837.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y, Zhu J, Wang J, et al. Functional probes for cardiovascular molecular imaging. Quant Imaging Med Surg. 2018;8(8):838–852. doi: 10.21037/qims.2018.09.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deb S, Ghosh K, Shetty S. Nanoimaging in cardiovascular diseases: current state of the art. Indian J Med Res. 2015;141(3):285. doi: 10.4103/0971-5916.156557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cattaneo M, Froio A, Gallino A. Cardiovascular imaging and theranostics in cardiovascular pharmacotherapy. Eur Cardiol Rev. 2019;14(1):62. doi: 10.15420/ecr.2019.6.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palekar RU, Jallouk AP, Lanza GM, Pan H, Wickline SA. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine. 2015;10(11):1817–1832. doi: 10.2217/nnm.15.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki M, Bachelet-Violette L, Rouzet F, et al. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine. 2015;10(1):73–87. doi: 10.2217/nnm.14.51 [DOI] [PubMed] [Google Scholar]
- 62.Shon SM, Choi Y, Kim JY, et al. Photodynamic therapy using a protease-mediated theranostic agent reduces cathepsin-b activity in mouse atheromata in vivo. Arterioscler Thromb Vasc Biol. 2013;33(6):1360–1365. doi: 10.1161/ATVBAHA.113.301290 [DOI] [PubMed] [Google Scholar]
- 63.Qin J, Peng Z, Li B, et al. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale. 2015;7(33):13991–14001. doi: 10.1039/C5NR02521D [DOI] [PubMed] [Google Scholar]
- 64.Tang J, Lobatto ME, Read JC, Mieszawska AJ, Fayad ZA, Mulder WJM. Nanomedical theranostics in cardiovascular disease. Curr Cardiovasc Imaging Rep. 2012;5(1):19–25. doi: 10.1007/s12410-011-9120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinstreuer C. Potential use of multifunctional nanoparticles for the treatment of cardiovascular diseases. J Cardiol Cardiovasc Sci. 2018;2(3):30–36. doi: 10.29245/2578-3025/2018/3.1134 [DOI] [Google Scholar]
- 66.McCarthy JR. Nanomedicine and Cardiovascular Disease. Curr Cardiovasc. Imaging Rep. 2010;3(1):42–49. doi: 10.1007/s12410-009-9002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18(1):26–30. doi: 10.1016/j.copbio.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 68.Kim DK, Dobson J. Nanomedicine for targeted drug delivery. J Mater Chem. 2009;2010:3731–3732. [Google Scholar]
- 69.Cicha I, Chauvierre C, Texier I, et al. From design to the clinic: practical guidelines for translating cardiovascular nanomedicine. Cardiovasc Res. 2018;114(13):1714–1727. doi: 10.1093/cvr/cvy219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Westedt U, Barbu-Tudoran L, Schaper AK, et al. Deposition of nanoparticles in the arterial vessel by porous balloon catheters: localization by confocal laser scanning microscopy and transmission electron microscopy. AAPS J. 2002;4(4):206–211. doi: 10.1208/ps040441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galvin P, Thompson D, Ryan KB, et al. Nanoparticle-based drug delivery: case studies for cancer and cardiovascular applications. Cell Mol Life Sci. 2012;69:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serpooshan V, Sivanesan S, Huang X, et al. [Pyr1]-Apelin-13 delivery via nano-liposomal encapsulation attenuates pressure overload-induced cardiac dysfunction. Biomaterials. 2015;37:289–298. doi: 10.1016/j.biomaterials.2014.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Valk FM, van Wijk DF, Lobatto ME, et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine. 2015;11(5):1039–1046. doi: 10.1016/j.nano.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varna M, Juenet M, Bayles R, et al. Nanomedicine as a strategy to fight thrombotic diseases. Future Sci OA. 2015;1(4):FSO46. doi: 10.4155/fso.15.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Absar S, Naha K, Kwon YM, et al. Thrombus-targeted nanocarrier attenuates bleeding complications associated with conventional thrombolytic therapy. Pharm Res. 2013;30(6):1663–1676. doi: 10.1007/s11095-013-1011-x [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Cao L, Shvartsman D, et al. Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett. 2011;11(2):694–700. doi: 10.1021/nl103812a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jia C, Chen H, Wei M, et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomedicine. 2017;12:4963–4979. doi: 10.2147/IJN.S138400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravichandran R, Sridhar R, Venugopal JR, et al. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol Biosci. 2014;14(4):515–525. doi: 10.1002/mabi.201300407 [DOI] [PubMed] [Google Scholar]
- 79.Spivak MY, Bubnov RV, Yemets IM, et al. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiology. EPMA J. 2013;4(1):1–23. doi: 10.1186/1878-5085-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Sun X, Zhang H, et al. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int J Nanomedicine. 2016;11:3255–3263. doi: 10.2147/IJN.S104604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X, Ding Y, Zhao B, et al. In vitro and in vivo evaluation of puerarin-loaded PEGylated mesoporous silica nanoparticles. Drug Dev Ind Pharm. 2016;42(12):2031–2037. doi: 10.1080/03639045.2016.1190742 [DOI] [PubMed] [Google Scholar]
- 82.Ferreira MPA, Ranjan S, Kinnunen S, et al. Drug-Loaded Multifunctional Nanoparticles Targeted to the Endocardial Layer of the Injured Heart Modulate Hypertrophic Signaling. Small. 2017;13(33):1701276. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, Feng M, Li Y, et al. Fabrication of superparamagnetic nano-silica@ quercetin-encapsulated PLGA nanocomposite: potential application for cardiovascular diseases. J Photochem Photobiol B Biol. 2019;196:111508. doi: 10.1016/j.jphotobiol.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 84.Lee PC, Zan BS, Chen LT, et al. Multifunctional PLGA-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int J Nanomedicine. 2019;14:1533–1549. doi: 10.2147/IJN.S174962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J, Lu Y, Cheng Y, et al. Novel Strategy of Gene Delivery System Based on Dendrimer Loaded Recombinant Hirudine Plasmid for Thrombus Targeting Therapy. Mol Pharm. 2019; 16:1648-1657 [DOI] [PubMed] [Google Scholar]
- 86.Peters D, Kastantin M, Kotamraju VR, et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci. 2009;106(24):9815–9819. doi: 10.1073/pnas.0903369106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wennink JWH, Liu Y, Mäkinen PI, et al. Macrophage selective photodynamic therapy by meta-tetra(hydroxyphenyl)chlorin loaded polymeric micelles: a possible treatment for cardiovascular diseases. Eur J Pharm Sci. 2017;107:112–125. doi: 10.1016/j.ejps.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 88.Zamani P, Fereydouni N, Butler AE, et al. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med. 2018;29(6):313–323. doi: 10.1016/j.tcm.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 89.Marrache S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci. 2013;110(23):9445–9450. doi: 10.1073/pnas.1301929110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon SM, Deng J, Lu LJ, et al. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9(10):5239–5249. doi: 10.1021/pr100520x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez-Gaytan BL, Fay F, Lobatto ME, et al. HDL-Mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26(3):443–451. doi: 10.1021/bc500517k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu CMJ, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park JH, Dehaini D, Zhou J, et al. Biomimetic nanoparticle technology for cardiovascular disease detection and treatment. Nanoscale Horiz. 2019;5(1):25–42. doi: 10.1039/C9NH00291J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bi F, Zhang J, Su Y, et al. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials. 2009;30(28):5125–5130. doi: 10.1016/j.biomaterials.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 95.Karamched SR, Nosoudi N, Moreland HE, et al. Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Sci Rep. 2019;9(1):2629. doi: 10.1038/s41598-019-39639-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nosoudi N, Nahar-Gohad P, Sinha A, et al. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles. Circ Res. 2015;117(11):e80–e89. doi: 10.1161/CIRCRESAHA.115.307207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nosoudi N, Chowdhury A, Siclari S, et al. Reversal of vascular calcification and aneurysms in a rat model using dual targeted therapy with EDTA-and PGG-loaded nanoparticles. Theranostics. 2016;6(11):1975–1987. doi: 10.7150/thno.16547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Margolis J, McDonald J, Heuser R, et al. Systemic Nanoparticle Paclitaxel (nab-Paclitaxel) for In-stent Restenosis I (SNAPIST-I): a First-in-Human Safety and Dose-finding Study. Clin Cardiol. 2007;30:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boarescu PM, Boarescu I, Bocșan IC, et al. Curcumin nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules. 2019;24(15):pii: E2802. doi: 10.3390/molecules24152802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boarescu PM, Chirilă I, Bulboacă AE, et al. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid Med Cell Longev. 2019;2019:1–13. doi: 10.1155/2019/7847142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boarescu I, Boarescu I, Bocșan IC, et al. Antioxidant and Anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants. 2019;8(10):504. doi: 10.3390/antiox8100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brito L, Amiji M. Nanoparticulate carriers for the treatment of coronary restenosis. Int J Nanomedicine. 2007;2(2):143–161. [PMC free article] [PubMed] [Google Scholar]
- 103.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146 [DOI] [PubMed] [Google Scholar]
- 104.Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ Res. 2003;92(10):1068–1078. doi: 10.1161/01.RES.0000073844.41372.38 [DOI] [PubMed] [Google Scholar]
- 105.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618–632. doi: 10.1111/joim.12296 [DOI] [PubMed] [Google Scholar]
- 106.Cominacini L, Garbin U, Mozzini C, et al. The atherosclerotic plaque vulnerability: focus on the oxidative and endoplasmic reticulum stress in orchestrating the macrophage apoptosis in the formation of the necrotic core. Curr Med Chem. 2015;22(13):1565–1572. doi: 10.2174/0929867322666150311150829 [DOI] [PubMed] [Google Scholar]
- 107.Lewis DR, Kamisoglu K, York AW, Moghe PV. Polymer‐based therapeutics: nanoassemblies and nanoparticles for management of atherosclerosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(4):400–420. doi: 10.1002/wnan.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flores AM, Ye J, Jarr KU, et al. Nanoparticle Therapy for Vascular Diseases. Arterioscler Thromb Vasc Biol. 2019;39(4):635–646. doi: 10.1161/ATVBAHA.118.311569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Danenberg HD, Golomb G, Groothuis A, et al. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108(22):2798–2804. doi: 10.1161/01.CIR.0000097002.69209.CD [DOI] [PubMed] [Google Scholar]
- 110.Cannon CP, Steinberg BA, Murphy SA, et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–445. doi: 10.1016/j.jacc.2006.04.070 [DOI] [PubMed] [Google Scholar]
- 111.Broz P, Ben-Haim N, Grzelakowski M, et al. Inhibition of macrophage phagocytotic activity by a receptor-targeted polymer vesicle-based drug delivery formulation of pravastatin.. J Ournal of Cardiovascular Pharmacology. 2008;51(3):246–252. doi: 10.1097/FJC.0b013e3181624aed [DOI] [PubMed] [Google Scholar]
- 112.Winter PM, Neubauer AM, Caruthers SD, et al. Endothelial α ν β 3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76 [DOI] [PubMed] [Google Scholar]
- 113.Zimmermann TS, Lee ACH, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688 [DOI] [PubMed] [Google Scholar]
- 114.Tadin-Strapps M, Peterson LB, Cumiskey AM, et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J Lipid Res. 2011;52(6):1084–1097. doi: 10.1194/jlr.M012872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA J Am Med Assoc. 2017;318(10):947–956. doi: 10.1001/jama.2017.11467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, Phase 1 trial. Lancet. 2014;383(9911):60–68. doi: 10.1016/S0140-6736(13)61914-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao W, Sun Y, Cai M, et al. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat Commun. 2018;9(1):231. doi: 10.1038/s41467-017-02657-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cyrus T, Zhang H, Allen JS, et al. Intramural Delivery of Rapamycin With α v β 3 -Targeted Paramagnetic Nanoparticles Inhibits Stenosis After Balloon Injury. Arterioscler Thromb Vasc Biol. 2008;28(5):820–826. doi: 10.1161/ATVBAHA.107.156281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li JM, Newburger PE, Gounis MJ, et al. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010;17(10):1279–1287. doi: 10.1038/gt.2010.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang SL, Kee PH, Kim H, et al. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol. 2009;54(7):652–659. doi: 10.1016/j.jacc.2009.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawata H, Uesugi Y, Soeda T, et al. A new drug delivery system for intravenous coronary thrombolysis with thrombus targeting and stealth activity recoverable by ultrasound. J Am Coll Cardiol. 2012;60(24):2550–2557. doi: 10.1016/j.jacc.2012.08.1008 [DOI] [PubMed] [Google Scholar]
- 122.Palekar RU, Jallouk AP, Myerson JW, et al. Inhibition of thrombin with PPACK-nanoparticles restores disrupted endothelial barriers and attenuates thrombotic risk in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(3):446–455. doi: 10.1161/ATVBAHA.115.306697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakashiro S, Matoba T, Umezu R, et al. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in apoE −/− Mice. Arterioscler Thromb Vasc Biol. 2016;36(3):491–500. doi: 10.1161/ATVBAHA.115.307057 [DOI] [PubMed] [Google Scholar]
- 124.Tang J, Baxter S, Menon A, et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci. 2016;113(44):E6731–E6740. doi: 10.1073/pnas.1609629113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamaly N, Fredman G, Subramanian M, et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci. 2013;110(16):6506–6511. doi: 10.1073/pnas.1303377110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cyrus T, Wickline SA, Lanza GM. Nanotechnology in interventional cardiology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(1):82–95. doi: 10.1002/wnan.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Labhasetwar V, Song C, Levy RJ. Nanoparticle drug delivery system for restenosis. Adv Drug Deliv Rev. 1997;24(1):63–85. doi: 10.1016/S0169-409X(96)00483-8 [DOI] [Google Scholar]
- 128.Nakano K, Egashira K, Masuda S, et al. Formulation of Nanoparticle-eluting stents by a cationic electrodeposition coating technology. efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc Interv. 2009;2(4):277–283. doi: 10.1016/j.jcin.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 129.Tsukie N, Nakano K, Matoba T, et al. Pitavastatin-Incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J Atheroscler Thromb. 2013;20(1):32–45. doi: 10.5551/jat.13862 [DOI] [PubMed] [Google Scholar]
- 130.Matoba T, Koga J, Nakano K, Egashira K, Tsutsui H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J Cardiol. 2017a;70(3):206–211. doi: 10.1016/j.jjcc.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 131.Yan C, Quan XJ, Feng YM. Nanomedicine for Gene Delivery for the Treatment of Cardiovascular Diseases. Curr Gene Ther. 2019;19(1):20–30. doi: 10.2174/1566523218666181003125308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sung KM, Mosley DW, Peelle BR, et al. Synthesis of monofunctionalized gold nanoparticles by fmoc solid-phase reactions. J Am Chem Soc. 2004;126(16):5064–5065. doi: 10.1021/ja049578p [DOI] [PubMed] [Google Scholar]
- 133.Fu A, Micheel CM, Cha J, et al. Discrete nanostructures of quantum dots/au with DNA. J Am Chem Soc. 2004;126(35):10832–10833. doi: 10.1021/ja046747x [DOI] [PubMed] [Google Scholar]
- 134.Dasari BC, Cashman SM, Kumar-Singh R. Reducible PEG-POD/DNA nanoparticles for gene transfer in vitro and in vivo: application in a mouse model of age-related macular degeneration. Mol Ther Nucleic Acids. 2017;8:77–89. doi: 10.1016/j.omtn.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma PL, Lavertu M, Winnik FM, et al. Stability and binding affinity of DNA/chitosan complexes by polyanion competition. Carbohydr Polym. 2017;176:167–176. doi: 10.1016/j.carbpol.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 136.Shilakari Asthana G, Asthana A, Kohli DV, et al. Mannosylated chitosan nanoparticles for delivery of antisense oligonucleotides for macrophage targeting. Biomed Res Int. 2014;2014:526391. doi: 10.1155/2014/526391 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Shum-Tim D, Paul A, Khan AA, et al. Intramyocardial sustained delivery of placental growth factor using nanoparticles as a vehicle for delivery in the rat infarct model. Int J Nanomedicine. 2011;2011:2667—2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakano Y, Matoba T, Tokutome M, et al. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci.Rep. 2016;6(1):29601. doi: 10.1038/srep29601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nguyen MM, Gianneschi NC, Christman KL. Developing injectable nanomaterials to repair the heart. Curr Opin Biotechnol. 2015;34:225–231. doi: 10.1016/j.copbio.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishikita A, Matoba T, Ikeda G, et al. Nanoparticle-mediated delivery of mitochondrial division inhibitor 1 to the myocardium protects the heart from ischemia-reperfusion injury through inhibition of mitochondria outer membrane permeabilization: a new therapeutic modality for acute myocardial infarction. J Am Heart Assoc. 2016;5:e003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeda G, Matoba T, Nakano Y, et al. Nanoparticle-mediated targeting of cyclosporine a enhances cardioprotection against ischemia-reperfusion injury through inhibition of mitochondrial permeability transition pore opening. Sci Rep. 2016;6(1):20467. doi: 10.1038/srep20467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang J, Ma A, Shang L. Conjugating existing clinical drugs with gold nanoparticles for better treatment of heart diseases. Front Physiol. 2018;9:1–5. doi: 10.3389/fphys.2018.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ni Y, Wang T, Zhuo X, et al. Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol Cell Biochem. 2013;384(1–2):95–103. doi: 10.1007/s11010-013-1785-5 [DOI] [PubMed] [Google Scholar]
- 144.Schmiegelow MD, Hedlin H, Stefanick ML, et al. Insulin resistance and risk of cardiovascular disease in postmenopausal women: a cohort study from the women’s health initiative. Circ Cardiovasc Qual Outcomes. 2015;8(3):309–316. doi: 10.1161/CIRCOUTCOMES.114.001563 [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Fan W, Wang K, et al. Novel preparation of Au nanoparticles loaded Laponite nanoparticles/ECM injectable hydrogel on cardiac differentiation of resident cardiac stem cells to cardiomyocytes. J Photochem Photobiol B Biol. 2019;192:49–54. doi: 10.1016/j.jphotobiol.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 146.Yang C, Tian A, Li Z. Reversible cardiac hypertrophy induced by PEG-coated gold nanoparticles in mice. Sci Rep. 2016;6(1):1–12. doi: 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian A, Yang C, Zhu B, et al. Polyethylene-glycol-coated gold nanoparticles improve cardiac function after myocardial infarction in mice. Can J Physiol Pharmacol. 2018;96(12):1318–1327. doi: 10.1139/cjpp-2018-0227 [DOI] [PubMed] [Google Scholar]
- 148.de Oliveira Gonçalves K, de Oliveira Silva FR, Vieira DP, Courrol LC. Synthesis and characterization of aminolevulinic acid with gold and iron nanoparticles by photoreduction method for non-communicable diseases diagnosis and therapy. J Mater Sci Mater Electron. 2019;30(18):16789–16797. doi: 10.1007/s10854-019-01337-6 [DOI] [Google Scholar]
- 149.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–1315. doi: 10.1016/j.addr.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 150.Mufamadi MS, Pillay V, Choonara YE, et al. A review on composite liposomal technologies for specialized drug delivery. J Drug Deliv. 2011;939851. doi: 10.1155/2011/939851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maurer N, Fenske DB, Cullis PR. Developments in liposomal drug delivery systems. Expert Opin Biol Ther. 2001;1(6):923–947. doi: 10.1517/14712598.1.6.923 [DOI] [PubMed] [Google Scholar]
- 152.Hamilton A, Biganzoli L, Coleman R, et al. 10968: a Phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx®, Doxil®) at a 6-week interval in patients with metastatic breast cancer. Ann Oncol. 2002;13(6):910–918. doi: 10.1093/annonc/mdf157 [DOI] [PubMed] [Google Scholar]
- 153.Bowey K, Tanguay JF, Tabrizian M. Liposome technology for cardiovascular disease treatment and diagnosis. Expert Opin Drug Deliv. 2012;9(2):249–265. doi: 10.1517/17425247.2012.647908 [DOI] [PubMed] [Google Scholar]
- 154.Dvir T, Bauer M, Schroeder A, et al. Nanoparticles Targeting the Infarcted Heart. Nano Lett. 2011;11(10):4411–4414. doi: 10.1021/nl2025882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H, Kusunose J, Kheirolomoom A, et al. Dynamic imaging of arginine-rich heart-targeted vehicles in a mouse model. Biomaterials. 2008;29(12):1976–1988. doi: 10.1016/j.biomaterials.2007.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H, Li N, Sirish P, et al. The cargo of CRPPR-conjugated liposomes crosses the intact murine cardiac endothelium. J Control Release. 2012;163(1):10–17. doi: 10.1016/j.jconrel.2012.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Serda R, Ruiz E, Flores-Arredondo J, et al. The physiology of cardiovascular disease and innovative liposomal platforms for therapy. Int J Nanomedicine. 2013;8:629–640. doi: 10.2147/IJN.S30599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paulis LE, Geelen T, Kuhlmann MT, et al. Distribution of lipid-based nanoparticles to infarcted myocardium with potential application for MRI-monitored drug delivery. J Control Release. 2012;162(2):276–285. doi: 10.1016/j.jconrel.2012.06.035 [DOI] [PubMed] [Google Scholar]
- 159.Duivenvoorden R, Senders ML, van Leent MMT, et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat Rev Cardiol. 2019;16(1):21–32. doi: 10.1038/s41569-018-0073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Harel-Adar T, Mordechai TB, Amsalem Y, et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci. 2011;108(5):1827–1832. doi: 10.1073/pnas.1015623108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walton BL, Leja M, Vickers KC, et al. Delivery of negatively charged liposomes into the atheromas of Watanabe heritable hyperlipidemic rabbits. Vasc Med. 2010;15(4):307–313. doi: 10.1177/1358863X10374118 [DOI] [PubMed] [Google Scholar]
- 162.Saito A, Shimizu H, Doi Y, et al. Immunoliposomal drug-delivery system targeting lectin-like oxidized low-density lipoprotein receptor–1 for carotid plaque lesions in rats. J Neurosurg. 2011;115(4):720–727. doi: 10.3171/2011.5.JNS10227 [DOI] [PubMed] [Google Scholar]
- 163.Cho BHS, Park JR, Nakamura MT, et al. Synthetic dimyristoylphosphatidylcholine liposomes assimilating into high-density lipoprotein promote regression of atherosclerotic lesions in cholesterol-fed rabbits. Exp Biol Med. 2010;235(10):1194–1203. doi: 10.1258/ebm.2010.009320 [DOI] [PubMed] [Google Scholar]
- 164.Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur Heart J. 2005;7(suppl_F):F4–F8. doi: 10.1093/eurheartj/sui036 [DOI] [Google Scholar]
- 165.Joner M, Morimoto K, Kasukawa H, et al. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol. 2008;28(11):1960–1966. doi: 10.1161/ATVBAHA.108.170662 [DOI] [PubMed] [Google Scholar]
- 166.Lestini BJ, Sagnella SM, Xu Z, et al. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J Control Release. 2002;78(1–3):235–247. doi: 10.1016/S0168-3659(01)00505-3 [DOI] [PubMed] [Google Scholar]
- 167.Holland NB, Qiu Y, Ruegsegger M, et al. Biomimetic engineering of non-adhesive glycocalyx-like surfaces using oligosaccharide surfactant polymers. Nature. 1998;392(6678):799–801. doi: 10.1038/33894 [DOI] [PubMed] [Google Scholar]
- 168.Srinivasan R, Marchant RE, Gupta AS. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J Biomed Mater Res. 2010;93:1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8(2):165–177. doi: 10.1016/j.apsb.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fang H, Guo Z, Lin L, et al. Molecular strings significantly improved the gene transfection efficiency of polycations. J Am Chem Soc. 2018;140(38):11992–12000. doi: 10.1021/jacs.8b05341 [DOI] [PubMed] [Google Scholar]
- 171.Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546(1–2):215–225. doi: 10.1016/j.ijpharm.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 172.Basu S. Novel drug delivery systems in management of cardiovascular diseases. Pharmatutor. 2018;6(6):19. doi: 10.29161/PT.v6.i6.2018.19 [DOI] [Google Scholar]
- 173.Wu D, Liu Y, Jiang X, et al. Evaluation of hyperbranched poly (amino ester) s of amine constitutions similar to polyethlenimine for DNA delivery. Biomacromolecules. 2005;6(6):3166–3173. doi: 10.1021/bm0504983 [DOI] [PubMed] [Google Scholar]
- 174.Zhu K, Guo C, Xia Y, et al. Transplantation of novel vascular endothelial growth factor gene delivery system manipulated skeletal myoblasts promote myocardial repair. Int J Cardiol. 2013;168:2622–2631. [DOI] [PubMed] [Google Scholar]
- 175.Kulhari H, Kulhari DP, Prajapati SK, et al. Pharmacokinetic and pharmacodynamic studies of poly(amidoamine) dendrimer-based simvastatin oral formulations for the treatment of hypercholesterolemia. Mol Pharm. 2013;10(7):2528–2533. doi: 10.1021/mp300650y [DOI] [PubMed] [Google Scholar]
- 176.Ma Q, Han Y, Chen C, et al. Oral absorption enhancement of probucol by pegylated g5 pamam dendrimer modified nanoliposomes. Mol Pharm. 2015;12(3):665–674. doi: 10.1021/mp500388m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Emara LH, Badr RM, Elbary AA. Improving the dissolution and bioavailability of nifedipine using solid dispersions and solubilizers. Drug De. Ind Pharm. 2002;28(7):795–807. doi: 10.1081/DDC-120005625 [DOI] [PubMed] [Google Scholar]
- 178.Devarakonda B, Hill RA, De Villiers MM. The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int J Pharm. 2004;284(1–2):133–140. doi: 10.1016/j.ijpharm.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 179.Ferreira MPA, Ranjan S, Kinnunen S, et al. Drug-loaded multifunctional nanoparticles targeted to the endocardial layer of the injured heart modulate hypertrophic signaling. Small. 2017;13(33):1701276. doi: 10.1002/smll.201701276 [DOI] [PubMed] [Google Scholar]
- 180.Giannouli M, Karagkiozaki V, Pappa F, et al. Fabrication of quercetin-loaded PLGA nanoparticles via electrohydrodynamic atomization for cardiovascular disease. Mater Today Proc. 2018;5(8):15998–16005. doi: 10.1016/j.matpr.2018.05.044 [DOI] [Google Scholar]
- 181.Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(2):271–299. doi: 10.1002/wnan.1364 [DOI] [PubMed] [Google Scholar]
- 182.Martín Giménez VM, Kassuha DE, Manucha W. Nanomedicine applied to cardiovascular diseases: latest developments. Ther Adv Cardiovasc. Dis. 2017;11(4):133–142. doi: 10.1177/1753944717692293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Namdari M, Cheraghi M, Negahdari B, Eatemadi A, Daraee H. Recent advances in magnetoliposome for heart drug delivery. Artif Cells Nanomed Biotechnol. 2017;45:1051–1057. [DOI] [PubMed] [Google Scholar]
- 184.Marcos-Campos I, Asín L, Torres TE, et al. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology. 2011;22(20):205101. doi: 10.1088/0957-4484/22/20/205101 [DOI] [PubMed] [Google Scholar]
- 185.Gamarra LF, Brito GES, Pontuschka WM, Amaro E, Parma AHC, Goya GF. Biocompatible superparamagnetic iron oxide nanoparticles used for contrast agents: a structural and magnetic study. J Magn Magn Mater. 2005;289:439–441. doi: 10.1016/j.jmmm.2004.11.123 [DOI] [Google Scholar]
- 186.Jin S, Ye K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol Prog. 2007;23(1):32–41. doi: 10.1021/bp060348j [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Facts sheets. cardiovascular diseases (CVDs); 17 May 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 17, February 2020.