Abstract
Opioid-induced bowel dysfunction (OIBD) is a common complication in long-term opioid users and abusers. It is a burdensome condition, which significantly limits quality of life and is associated with increasing health costs. OIBD affects up to 60% of patients with chronic non-cancer pain and over 80% of patients suffering from cancer pain and is one of the conditions of the most common symptoms associated with opioid maintenance. Given the continued use of opioids for chronic pain management in appropriate patients, OIBD is likely to persist in clinical practice in the coming years. We will herein review its underlying pathophysiological mechanisms and the available treatments. In the last years, pharmaceutical research has focused on the opportunity of targeting peripheral mu-opioid receptors without affecting their analgesic activity in the central nervous system, and several peripherally acting mu-opioid receptors antagonists (PAMORAs) drugs have been approved. We will mainly focus on naldemedine, discussing its pharmacological properties, its clinical efficacy and side effects. Head-to-head comparisons between naldemedine and the other PAMORAs are not available yet, but some considerations will be discussed based on the pharmacological and clinical data. As a whole, the available data suggest that naldemedine is a valid treatment option for OIBD, as it is a well-tolerated drug that alleviates constipation without affecting analgesia or causing symptoms of opioid withdrawal.
Keywords: PAMORAs, opioid-induced constipation, naldemedine, opioid-induced bowel dysfunction, analgesia, chronic pain
Opioid-Induced Bowel Dysfunction (OIBD)
Opioid-induced bowel dysfunction (OIBD) is a common complication in long-term opioid users and abusers. It is a burdensome condition, which significantly limits quality of life1 and it is associated with increasing health costs, due not only to pharmacological therapies but also to the management of possible complications.2 Given the persistent use of opioids for appropriate chronic pain management, OIBD is likely to be more commonly seen in clinical practice in the coming years and requires in-depth knowledge of the underlying pathophysiological mechanisms and appropriate management.3
Symptoms of OIBD involve the whole gastrointestinal (GI) tract, from the mouth to the anus, including xerostomia, gastro-oesophageal reflux, nausea, vomiting, and constipation, associated with bloating, abdominal distension and pain, hard dry stools, and incomplete bowel evacuation.4
Among all the signs and symptoms of OIBD, opioid-induced constipation (OIC) is the most common, affecting up to 60% of patients with chronic non-cancer pain (CNCP)5 and over 80% of patients suffering from cancer pain.6 OIC is also one of the most common symptoms associated with opioid maintenance treatment, where high doses of buprenorphine and methadone are used as substitution treatment.7
According to the Rome IV criteria for colorectal disorders, OIC has been classified among the “functional bowel disorders”, based on the frequent symptomatic overlap and shared underlying mechanisms.8 Diagnostic criteria include new or worsening symptoms of “functional constipation” when initiating, changing, or increasing opioid therapy (Table 1).9 However, these criteria are useful only for identifying patients with more severe OIC; hence they may underdiagnose patients with fewer or milder symptoms.10 Unlike other opioid-induced adverse effects, OIC may persist throughout the whole duration of the opioid treatment, since tolerance generally does not develop to this opioid adverse effect, potentially leading to opioid dosage reduction or withdrawal.1
Table 1.
Rome IV Criteria for Defining Opioid-Induced Constipation
| 1) New or worsening symptoms of constipation when initiating, changing or increasing opioid therapy |
| 2) Diagnostic criteria for functional constipation Must include two or more of the following symptoms*
|
| Abdominal pain and/or bloating may be present but are not predominant |
| Insufficient criteria for irritable bowel syndrome |
| Loose stools rarely present without laxatives |
Notes: Data from Simren et al.9 *With a frequency cut-off of 25% of defecations.
Opioid effects on the GI tract are dose-dependent, but they are already relevant at low doses.10 Patients using weak and strong opioids seem to describe a comparable degree of distress from OIC symptoms.10 Daily opioid consumption is more commonly associated with OIC. Some authors have stated that transdermal formulations seem to be protective towards OIC, compared to oral preparations, while other studies show opposite results.5,11 Conversely, atypical opioids, such as tapentadol, that have a dual analgesic mechanism of action, consisting of both mu-opioid agonism and inhibition of noradrenaline reuptake, are likely to be associated with a lower incidence of bowel dysfunction in chronic cancer and CNCP patients due to reduced opioidergic activity.12–14 Transdermal buprenorphine, as a partial mu-opioid agonist, has been associated with a reduced incidence of OIC in comparison with other opioids.15 Its less harmful effect on OIC could be related to the lower doses and to the transdermal formulation.
Pathophysiology of OIBD
Opioids exert their pharmacological effects through the interaction with specific 7- transmembrane G-protein-coupled receptors, namely delta- (DOR), kappa- (KOR), and mu- (MOR) receptors. Opioid receptors are widely distributed in the GI tract, where they modulate GI motility as a balance between excitatory and inhibitory neurotransmitters and neuromodulators. OIC is the result of opioid receptors activation, especially MOR, in the enteric nervous system (ENS),16 which is one of the main divisions of the autonomic nervous system.
Opioid Receptors Localization and Activity in the ENS
Opioid receptors are expressed throughout the whole GI tract, in different patterns among various animal species (rats, guinea pigs, pigs) and humans.5,11
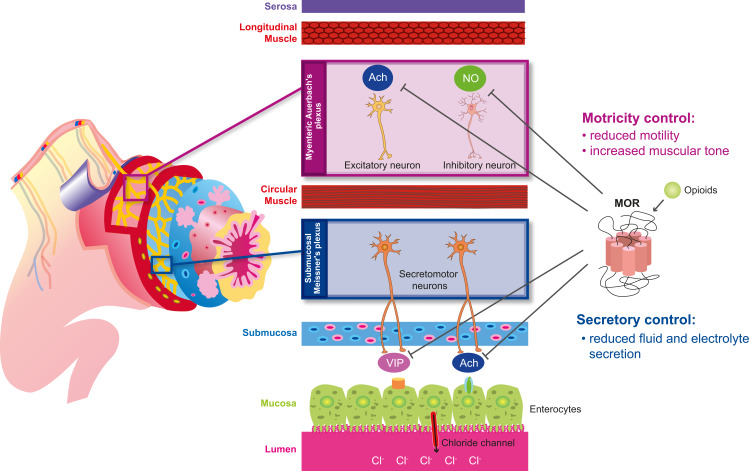
In humans, MOR is expressed throughout the small and large intestine, in both the myenteric and submucosal plexus11,17 MOR and DOR are also expressed in ileal villi and the lamina propria; however, DOR is mainly expressed on distal colonic epithelial cells18 and circular muscle.11 The myenteric and submucosal plexuses differently contribute to OIC along the different portions of the GI tract (Figure 1).
Figure 1.
Action of opioids on the MOR in the gastrointestinal tract. Schematic representation of the distinct roles of the myenteric and submucosal plexuses to opioid-induced constipation.
Abbreviations: Ach, acetylcholine; NO, nitric oxide; VIP, vasoactive intestinal polypeptide.
The myenteric plexus (Auerbach’s plexus), included in the muscularis propria, between longitudinal (outer layer) and the circular (inner layer) muscle, regulates the gut motility.1,5,11 Acetylcholine (Ach) and substance P, released by excitatory motor neurons, mediate muscle contraction17 by activating smooth muscle cells in the longitudinal layer; while nitric oxide (NO), and the vasoactive intestinal polypeptide (VIP), released by inhibitory motor neurons, facilitate relaxation of the circular muscle layer.5 Opioids inhibit the release of these neurotransmitters and alter the GI motility by increasing the muscular tone and decreasing physiological propulsive activity.
Opioid receptor activation inhibits the secretion of excitatory transmitters, especially Ach, thus hindering peristaltic movements in the colon, and, on the other hand, reduces the release of NO, hence resulting in increased activity of smooth muscle cells and resting muscle tone, non-propulsive movements and, therefore, spasms and cramps.11,19 The functional results of these effects are the increased gastric tone and delayed gastric emptying, altered motility of the esophagus and gallbladder, and finally reduced oro-caecal and colonic transit time, leading to OIBD.1,19
The submucosal plexus (Meissner’s plexus), located in the submucosa, is responsible for the secretion and absorption of water and electrolytes, especially chloride.1,5,17 The human GI tract produces about 10 liters of fluids per day. The submucosal plexus includes multiple populations of secretomotor neurons, which project to the gut mucosa, and vasodilator neurons, which act on the local microcirculation. Two-thirds of these neurons release Ach (cholinergic). The remaining neurons (non-cholinergic) release vasoactive intestinal peptide (VIP), nitric oxide synthase (NOS), serotonin, and somatostatin.5 In mucosal enterocytes expressing vasoactive intestinal polypeptide receptor 1 (VPAC1) and 2 (VPAC2) and cholinergic (muscarinic) receptors, chloride channels are activated, leading to chloride and water passage to the lumen via osmotic gradient.5,11,17
Opioids bind to receptors in secretomotor neurons and suppress Ach and VIP release, therefore reducing chloride and water secretion into the lumen, and increasing their absorption. The result is diminished fecal volume, hence decreasing gut motility.5 Magnetic resonance imaging (MRI) has been used to study opioid-induced altered colonic function, including the analysis of fecal volume and stool dryness.20 Additionally, wireless ingestible capsule systems have been used to record transit time under near‐normal physiologic conditions.21 Both these systems significantly contributed to elucidate the effects of opioid agonists in the GI tract.
Moreover, opioids increase the tone of the seven sphincters included in the GI tract (the upper and lower oesophageal sphincters, pylorus, sphincter of Oddi, the ileocecal valve and the internal and external anal sphincters).1,19
Opioids also hinder gallbladder contractions, which, together with opioid-induced increased tone of the sphincter of Oddi, may lead to reduced bile and pancreatic juice flow.5
Finally, opioids induce the recto-anal inhibitory reflex, thus hampering the relaxation of the internal anal sphincter, leading to increased rectal distension, incomplete evacuation, hemorrhoids and straining.5 Opioid antagonists have been shown to decrease the tone of the internal anal sphincter, without affecting resting anal pressure or distensibility.22 The altered motility may induce the loss of pelvic floor coordination during defecation.1
Peripherally-Acting Mu-Opioid Receptors Antagonists (PAMORAs)
In the last few years, pharmaceutical research has focused on the opportunity of targeting peripheral mu-opioid receptors without affecting their analgesic activity in the central nervous system (CNS).
Fixed-dose oral combination of oxycodone and naloxone have been studied for reducing the incidence of OIC. Naloxone is a competitive opioid antagonist that is commonly administered intravenous for reversing opioid overdoses. When naloxone is administrated orally it is extensively metabolized by the first-pass in the liver with a resultant bioavailability per os of approximately 2%. When the ratio between oxycodone and naloxone is fixed to 2:1, the incidence of OIC, as measured with bowel function index (BFI), has been shown to be significantly reduced.1,23
To obtain similar results with a molecule that could be used with opioids prescribed for severe chronic pain, the pharmacological effort was to find a way to prevent opioid antagonists from crossing the blood-brain barrier (BBB) and entering the CNS.
Methylnaltrexone bromide (Relistor®) was the first approved peripherally acting mu-opioid receptor antagonist (PAMORA) for subcutaneous use, to treat OIC in patients with advance illness receiving palliative care, after failing laxative therapies. It is a quaternary ammonium compound with a positive charge, which restricts its ability to cross the BBB.24
Naloxegol [Movantik® (US), Moventig ® (EU), previously NKTR‐118] was the first orally administered PAMORA. It is a polyethylene glycol (PEG) derivative of naloxone. Its PEG “tail” renders the drug a substrate for the p-glycoprotein (P-gp) efflux transporter, which physiologically prevents potentially dangerous substances or drugs from entering the BBB. Its effect is therefore limited almost exclusively to the opioid receptors in the GI tract, where naloxegol transits before absorption and entering in the bloodstream.25
Obviously, the peripheral activity of this kind of PAMORA is strictly related to the efficacy of the P-gp system, leading to the risk of a reduced activity related to drug–drug interaction (inhibition). Some chemotherapeutics, for example, are P-gp inhibitors; therefore, their use could affect the selectivity of the peripheral action of naloxegol.
The last approved PAMORA for the treatment of OIC is naldemedine [Symproic® (Japan, USA); Rizmoic® (EU)], an amide derivative of naltrexone, with limited ability to cross the BBB.26 A summary of the pharmacokinetics of the different available PAMORAs are shown in Table 2.27,28
Table 2.
Pharmacokinetics of PAMORAs
| Mechanisms Mechanism of Action | Route of Administration | Absorption Tmax (Hours) |
Distribution | Metabolism | Elimination T1/2 (Hours) | Side Effects | |
|---|---|---|---|---|---|---|---|
| Methylnaltrexone | Peripherally acting μ-opioid receptor antagonist | Oral; Subcutaneous | Oral: 1.5hr (delayed by 2 hrs with high-fat meals) Subcutaneous: 30 min |
Vss: 1.1L/Kg | - Sulfation (Phase II) to methylnaltrexone-3-sulfate - Carbonyl reduction (Phase I) to methyl-6-naltrexol and methyl-6β-naltrexol |
8 | Abdominal pain Flatulence Nausea Dizziness |
| Naloxegol | Peripherally acting μ-opioid receptor antagonist | Oral | <2hrs in most of subjects a secondary Cmax occurs approx 0.4–3 hrs after the first Cmax |
Vz/F: 968–2.140 L | CYP3A (Phase I) N-dealkylation O-demethylation Oxidation Partial loss of the PEG chain |
6–11 | Abdominal pain Diarrhea Nausea Flatulence |
| Naldemedine | Peripherally acting μ-, δ-, κ-opioid receptor antagonist | Oral | 0.75 hr; 2.5 hr (with food) |
Vz/F: 155 L | - CYP3A4 (Phase I) to nor-naldemedine - Glucuronidation (Phase II) to naldemedine-3-glucuronide |
11 | Abdominal pain Diarrhea Nausea Gastroenteritis |
Notes: Data from these studies.27,28
Abbreviations: Cmax, peak plasma drug concentration; Vz/F, apparent volume of distribution during terminal phase after non-intravenous administration; Vss, apparent volume of distribution at steady-state; t1/2, elimination half-life; Tmax, Time to reach maximum (peak) plasma concentration following drug administration at steady state.
Naldemedine: Pharmacology
Mechanism of Action
Naldemedine is a PAMORA with a high binding affinity for all recombinant human opioid receptors (MOR, KOR, and DOR).26 Naldemedine reduces OIC by blocking MOR located in the ENS in the GI tract.29 Since OIC is at least in part reversed through MOR antagonism, but not by KOR and DOR inhibition, the meaning of the affinity of naldemedine for those receptors is unclear.30 The role of DOR in human GI system is still poorly known, however, in animals, MOR and DOR activation results in inhibition of secretomotor neurons and reduced chloride ions and water passing into the colonic lumen.17
Naldemedine is the result of structural alterations of the opioid antagonist, naltrexone, to prevent its passage across the BBB. This result is obtained through two mechanisms: the increase of the molecular size and the binding with P-gp.
Naldemedine is an amide derivative of naltrexone, where the addition of a side chain (2-(3-phenyl-1,2,4-oxadiazol-5-yl)propan-2-yl)acetamide to the 7-position increases both the molecular weight and the polar surface (141.18 Å2) of the compound hampering its ability to cross the BBB, thus sparing opioid-induced analgesia. Furthermore, the number of H-bond of naldemedine (14) hinders its distribution to the brain.31
Naldemedine is also a P-gp substrate.1 The distribution of naldemedine in the whole body has been evaluated in rats and ferrets by using [14C]‐labeled naldemedine. One hour after a single oral administration in rats, radioactivity was widely distributed in the body: [14C]‐naldemedine was detected in submaxillary, adrenal and hypophysis glands, liver, renal cortex, and intestinal wall, but disappear in most tissue 72-hour post-dose. Conversely, brain (cerebellum, cerebrum) and spinal cord radioactivity were below limit of quantification or not detectable at any time of the 72 hours of observation, thus suggesting only a minimal penetration of naldemedine into the CNS.31 Ferrets have a well-developed BBB, and for this reason, have also been used for evaluating the distribution of [14C]‐naldemedine in the brain. [14C]‐naldemedine concentration was equal or higher than the plasma level at 0.5 hour in the area postrema, pituitary, and choroid plexus, which are regions not protected by the BBB.
Conversely, the concentration of [14C]‐naldemedine in all the other areas of the brain protected by the BBB (hippocampus, putamen, hypothalamus, thalamus and periaqueductal gray) was lower than plasma. This finding also suggests a potential role of naldemedine in the prevention of opioid-induced nausea and vomiting, as the area postrema, where the chemoreceptor trigger zone exists, can be reached by the antagonism of naldemedine, without affecting the opioid-induced analgesia.31 Finally, the contribution of P-gp on the brain distribution of naldemedine has been evaluated by using multidrug-resistant 1 a/b (mdr1a/b) knock out (KO) mice, an animal model with impaired activity of the P-gp-mediated efflux system. mdr1a/b KO mice showed a 4-fold increase in the brain concentration of naldemedine compared with wild type mice, suggesting a role for P-gp in brain distribution of naldemedine. However, the overall concentration in the brain was significantly lower than that in the plasma in both the groups. This finding suggests an alternative mechanism explaining the limited ability of naldemedine to cross the BBB.27 The clinical relevance of the minimal contribution of P-gp in reducing the brain distribution of naldemedine is that of a presumptive lower risk of drug–drug interactions between naldemedine and other drug substrates of the P-gp. When the unique mechanism to prevent the cross of the BBB is the P-gp efflux system, as for other PAMORAs, brain distribution could be altered by drug–drug interactions or by change of the P-gp activity.
Pharmacokinetics
After oral administration, naldemedine is rapidly absorbed in the GI tract and reaches peak plasma concentrations (Cmax) after about 45 min (Tmax) in the fasted state. The Cmax and the bioavailability, the area under the plasma concentration-time curve (AUC), are dose-proportional in the range of 0.1 to 100 mg of naldemedine.26,28 Naldemedine has a bioavailability of 20–56%. High-fat meals do not affect AUC but decrease Cmax by 35% and increase Tmax to 2.5 hours. Multiple-dose administrations in a 10 day-period, induced only a 1–1.2 and a 1–1.3-fold accumulation for AUC and Cmax.26
The protein binding of naldemedine is about 93–94%, mainly to albumin and less extensively to α1-acid-glycoprotein and gamma-globulin. Neither renal or hepatic dysfunction are related to reduced binding of naldemedine to plasma proteins.32 The volume of distribution of naldemedine is 155 L.26
Naldemedine is mainly metabolized by CYP3A4 to nor-naldemedine, via N-dealkylation of the methylcyclopropyl portion of the parent compound:33 nornaldemedine is responsible for 9–13% of systemic exposure to naldemedine.32 Naldemedine is also metabolized via glucuronidation through UGT1A3, forming the minor metabolite naldemedine 3-G: this metabolite accounts for about 3% of total exposure to naldemedine.1,28 Both nornaldemedine and naldemedine 3-G antagonize opioid receptors, but to a lesser extent than the parent compound.31 Another minor metabolite, naldemedine-(7R)-7-hydroxide, is formed after oxidation of naldemedine. In the GI tract, enterobacteria cleave the parent compound, starting by reducing its oxadiazole portion and hydrolyzing the remaining amide bond to form benzamidine and naldemedine carboxylic acid22,24 which are excreted in both urine and feces.
Naldemedine has an 8.4 L/h clearance and an 11 h terminal elimination half-life. After a single oral dose, 57% of naldemedine is excreted in the urine, with 16–20% of it being in the unchanged form, and 35% in the feces.28 Naldemedine does not require dose adjustments in patients with mild, moderate, or severe renal impairment, and in those with end-stage renal disease (glomerular filtration rate < 30 mL/min/1.73 m2). Similarly, in patients with mild or moderate hepatic impairment (Child-Pugh Class A and B), pharmacokinetics are comparable with those in normal healthy subjects. Conversely, in patients with severe liver failure data are not available, since opioid themselves may worsen this clinical condition.32
Drug–Drug Interactions
Naldemedine has not been reported to inhibit various CYP isoforms (4A11, 2E1, 2D6, 3A, 2C9, 2C8, 2C19, 1A2, 2B6, 2A6), OCT (1,2) or OATP (1B1, 1B3,3) ATPc (BCRP, P-gp), BSEP, MATE1 and MATE2-K transporters. Naldemedine is not a CYP3A4, 2B6, and 1A2 inducer.26
Since naldemedine is a CYP3A4 substrate, it should not be co-administrated with CYP3A4 inhibitors (ie, ketoconazole, itraconazole, saquinavir, clarithromycin), as these drugs could lead to increased naldemedine levels and adverse effects. On the other hand, co-administration with strong CYP3A4 inducers (ie, carbamazepine, phenytoin, rifampicin) may be associated with reduced naldemedine concentrations, thus hindering its clinical effects.26–28
P-gp inhibitors (ie, ciclosporin) could also increase the plasma concentration of naldemedine, however, according to a pre-clinical study, the contribution of the P-gp in limiting brain distribution of naldemedine is minimal. Therefore, the clinical effect of P-gp inhibitors is expected to be limited compared compared to that observed when using other PAMORAs.
Naldemedine: Clinical Data
Indications and Contraindications
Naldemedine is approved in many countries such as the EU, US, and Japan for the treatment of OIC in adults who were previously treated with laxatives without efficacy.1,26 Naldemedine has been used to treat OIC in adults with chronic cancer and non-cancer pain.
Following dose-finding studies, the recommended naldemedine dose was determined to be 0.2 mg to be administrated once daily,34,35 since this dosage was deemed to be the most effective and the safest one. Naldemedine can be co-administrated with other laxatives, since clinical trials demonstrated an additive effect, compared to placebo. In cases of opioid therapy interruption, naldemedine should also be suspended.26
There are no specific data regarding the use of naldemedine in pediatric patients, neither during pregnancy nor breastfeeding. Embryo-fetal development studies showed no malformations after naldemedine administration.28 However, naldemedine should only be given to pregnant women if strictly necessary,26 since opioid withdrawal is possible in the fetus36 and the neonate.1 Similarly, it is contraindicated during breastfeeding, since it passes into the human milk,26,28 however, breastfeeding may be resumed 3 days after the last naldemedine dose.33
Other contraindications include hypersensitivity to the drug, suspected or known GI perforation/obstruction and severe hepatic disease.1
Dose-Finding Studies
Two dose-finding studies have been conducted on naldemedine, one in CNCP patients34 and one in cancer patients.35 Both studies were multicenter, randomized, double-blind, and evaluated patients receiving 0.1, 0.2 or 0.4 mg naldemedine or placebo. Patients aged 18 years or older, with chronic pain for ≥ 3 months, using opioids at a stable dosage of ≥ 30 mg/day morphine equivalent for ≥ 1 month, with a diagnosis of OIC, were eligible. Regular use of laxatives at baseline could be continued during the study period, according to the investigator’s opinion. In both studies the primary outcome was change in the frequency of spontaneous bowel movements (SBMs) week from baseline during the last 14-day of the treatment period. Baseline was defined as the average number of SBMs/week during the 2 weeks before random assignment.
In 2017, Webster et al34 randomized 244 CNCP patients, in a dose-finding study. The only dosages of naldemedine that significantly (P=0.001) improved the primary outcome, compared with placebo, were 0.2 mg and 0.4 mg. The overall incidence of treatment-emergent adverse events (TEAEs) was similar among the groups; however, naldemedine 0.4mg/day significantly increased the incidence of TEAEs compared with placebo (39.3 vs 16.4; p<0.01). Diarrhea, abdominal pain, nausea and flatulence were the most common GI side effects. According to these findings, naldemedine 0.2 mg once daily had the best safety profile and this dosage was chosen for the Phase III trials.
In 2017, Katakami et al35 randomized 227 chronic cancer patients, in a dose finding study . All dosages of naldemedine significantly improved the primary outcome compared with placebo, but the difference was statistically significant (P=0.001) for naldemedine 0.2 mg and 0.4 mg. A dose-dependent increase in SBMs was observed, with a significant difference between these two dosages: 7.29 vs 4.75 (P=0.0083), respectively, in the 0.4 mg vs 0.2 mg group. These dosages were also significantly better than placebo in terms of change in SBMs frequency without straining and SBMs with a feeling of complete evacuation (CSBM) frequency. However, with regard to tolerability, the incidence of side effects was significantly higher in the naldemedine 0.4 mg group compared with naldemedine 0.2 mg (78.6 vs 67.2; P=0.005). In general, the incidence of treatment-emergent adverse events (TEAEs) was greater in all of the naldemedine groups compared with placebo, with a statistically significant difference between naldemedine 0.4 mg and placebo (78.6 vs 51.8%; P=0.005), and diarrhea being the most common (up to 51.8%). However, the number of AEs leading to treatment withdrawal was negligible (up to 7.1%). Therefore, even in this dose-finding study, the selected dose for phase III studies in cancer patients was 0.2 mg once daily.
In both these dose-finding studies, naldemedine did not affect opioid dosage, pain intensity and did not cause opioid withdrawal symptoms, as measured using the clinical opiate withdrawal scale (COWS). These findings confirmed its selective peripheral activity on MOR, without any effect in the CNS.
Clinical Trials in CNCP Patients
Two multicenter, randomized, double-blind, placebo-controlled phase III trials (COMPOSE 1 and COMPOSE 2) have been conducted in CNCP patients with OIC.37 Patients aged 18–80 years, with chronic pain for ≥3 months, using opioids at a stable dosage of ≥ 30 mg/day morphine equivalent for ≥ 1 month were eligible for receiving naldemedine 0.2 mg daily or placebo for a 12-week study period. Study outcomes were common for all these studies. The primary outcome was the percentage of responders. Responders were defined as patients with three or more spontaneous bowel movement (SBMs)/week and patients who had an increase from baseline of at least one SBM/week for at least 9 weeks out of the 12-week treatment period and at least 3 of the last 4 weeks of the 12-week treatment period. Baseline was defined as the average number of SBMs/week during the 2 weeks before random assignment.
Results are shown in Table 3. The percentage of responders was similar in the two studies (47.6% in the COMPOSE1 and 52.5% in the COMPOSE 2), and significantly higher than placebo (34.6% in the COMPOSE1 and 33.6% in the COMPOSE 2) (p<0.01). Similarly, the two Phase III clinical trials gave analog results in the secondary outcomes, where the efficacy of naldemedine resulted significantly better than placebo (Table 3).37 Secondary outcomes of efficacy were SBMs/week, SBMs with a feeling of complete evacuation (CSBMs)/week, and SBMs without straining/week.
Table 3.
Efficacy of Oral Naldemedine in Patients with Chronic Non-Cancer Pain
| Trial | Ref | Phase and Study Design | No of Patients | Treatment | Primary Endpoint | Secondary Endpoints (Mean Change from Baseline to the Last 2 wks) |
||
|---|---|---|---|---|---|---|---|---|
| Proportion of Responders (% pts) # | SBMs/wk | CSBMs/wk | SBMs/wk Without Straining | |||||
| V9231 – COMPOSE 1 | Hale et al37 | Phase III. Multicentre, double-blind, randomised, parallel-group trial |
Nal 274 Placebo 273 |
Nal 0.2 mg OD for 12 wks | Nal 47.6% Placebo 34.6% (p=0.002) |
Nal +3.42 Placebo +2.12 (p<0.0001) |
Nal +2.58 Placebo +1.57 (p<0.0001) |
Nal +1.46 Placebo +0.73 (p<0.001) |
| V9232 – COMPOSE 2 | Hale et al37 | Phase III. Multicentre, double-blind, randomised, parallel-group trial | Nal 277 Placebo 276 | Nal 0.2 mg OD for 12 wks | Nal 52.5% Placebo 33.6% (p<0.0001) |
Nal +3,56 Placebo +2.16 (p<0.001) |
Nal +2.77 Placebo +1.62 (p<0.001) |
Nal +1.85 Placebo +1.10 (p<0.01) |
Notes: #Responders had at least three SBMs per week with an increase from baseline of at least one SBM per week for at least 9 weeks of the 12-week treatment period and at least 3 of the last 4 weeks of the 12-week treatment period (baseline: the average number of SBMs/week during the 2 weeks before random assignment).
Abbreviations: wk, week; Nal, naldemedine; SBMs, spontaneous bowel movements; CSBMs, SBMs with a feeling of complete evacuation.
Both these studies included the safety evaluation of naldemedine in comparison to placebo. In both trials, the incidence of TEAEs was about 50% with naldemedine, without a significant difference with placebo (Table 4). No differences were observed in the incidence of serious TEAEs, TEAEs leading to discontinuation, and TEAEs of opioid withdrawal. GI side effects were the most common, including diarrhea, abdominal pain, and nausea. However, the incidence of these side effects was relatively low compared to the tolerability profile of other PAMORAs and even to that of traditional laxative drugs. In the COMPOSE 1 trial, the incidence of diarrhea was 7% and abdominal pain 6%.37
Table 4.
Safety of Oral Naldemedine in Patients with Chronic Non-Cancer Pain
| Trial | Ref | Phase and Study Design | No of Patients | Treatment | Primary Endpoint | Secondary Endpoints | ||
|---|---|---|---|---|---|---|---|---|
| TEAEs | Serious TEAEs | TEAEs Leading to Discontinuation | TEAEs of Opioid Withdrawal | |||||
| V9231 – COMPOSE 1 | Hale et al37 | Phase III Multicentre, double-blind, randomised, parallel-group trial | Nal 274 Placebo 273 |
Nal 0.2 mg OD for 12 wks | Nal 49% Placebo 45% # |
Nal 5% Placebo 2% |
Nal 1% Placebo 0% |
Nal 1% Placebo <1% |
| V9232 – COMPOSE 2 | Hale et al37 | Phase III. Multicentre, double-blind, randomised, parallel-group trial | Nal 277 Placebo 276 | Nal 0.2 mg OD for 12 wks | Nal 50% Placebo 48% # |
Nal 3% Placebo 5% |
Nal 1% Placebo1% |
Nal 0% Placebo 0% |
| V9235 – COMPOSE 3 | Webster et al38 | Randomized, double-blind, placebo-controlled study. | Nal 621 Placebo 619 | Nal 0.2 mg OD for 52 wks | Nal 68.4% Placebo 72.1% |
Nal 9.7% Placebo 11.8% |
Nal 6.3% Placebo 5.8% |
Nal 1.8% Placebo 1.1% |
| V9238 – COMPOSE 6 | Saito et al39 | Phase III. Single-arm, open-label study | Nal 43 | Nal 0.2 mg OD for 48 wks | Nal 88% | Nal 9% | Nal 7% | NA |
| V9239 – COMPOSE 7 | Saito et al39 | Phase III. Single-arm, open-label study | Nal 10 | Nal 0.2 mg once daily for 48 wks | Nal 90% | Nal 0% | Nal 10% | NA |
Note: #In COMPOSE 1 and 2 TEAEs is not the primary endpoint.
Abbreviations: Nal, naldemedine; TEAEs, treatment-emergent adverse events.
In both studies, naldemedine did not compromise the analgesic effect of opioids. Most patients had been on opioid therapy for over 1 year, at a mean total daily dose up to 128.4 mg morphine equivalents. This dose remained generally stable over time, and no meaningful differences were observed in the score for pain intensity from baseline through the study period. The onset of OIC was not assessed in these studies.37
Three long-term studies (from 48 to 52 weeks) have been conducted for evaluating the safety of naldemedine in CNCP patients.38,39 In these studies, the primary outcome was TEAEs, and secondary outcomes included serious TEAEs and TEAEs leading to discontinuation (Table 4).
In 2018, Webster et al published a long-term randomized, double-blind, placebo-controlled phase III trial (COMPOSE 3), where 1240 CNCP patients were randomized to receive naldemedine 0.2 mg (n=621) or placebo (n=619) for 52 weeks.38 The first meaningful result is that over 66% of patients completed the 52 week treatment period. No difference was observed in the incidence of TEAEs: 68.4% in the naldemedine group and 72.1% in the placebo group. Similarly, no significant differences were observed in the incidence of serious TEAEs (9.7 vs 11.8 respectively in the naldemedine and placebo group), TEAEs leading to discontinuation (6.3 vs 5.8 respectively in the naldemedine and placebo group), and TEAEs of opioid withdrawal (lower than 2%) among the two groups. Diarrhea was the most common TEAE in the naldemedine group (11%), followed by abdominal pain and nausea and vomiting.38
In terms of change from baseline in frequency of SBMs, the efficacy of naldemedine was consistent along the entire 52-week study period. Similarly, naldemedine induced a reduction in the Patient Assessment of Constipation Symptoms (PAC-SYM), which was significant compared with placebo for the entire study period.38
At baseline, the mean duration of the opioid therapy was 62.6 months for naldemedine and 57 months for placebo. However, over 60% of enrolled patients were taking less than 100 mg equivalent of morphine per day, a dosage that in general could be considered relatively low, particularly in the management of chronic cancer pain patients; however, in CNCP patients a low opioid dosage may be reasonable. Naldemedine did not affect opioid analgesia and the average total daily dose of opioids was stable in patients, as assessed every 4 weeks. The risk of precipitating an opioid-withdrawal syndrome was assessed by using a specific score, named Clinical Opiate Withdrawal Scale (COWS), which showed no differences between naldemedine and placebo over the course of 52 weeks.38
This was the first long-term study on a PAMORA to include a placebo group. Previous 48-week and 52-week studies evaluating methylnaltrexone bromide40 and naloxegol41 were open-label.
Finally, two open-label, single-arm, Phase III, supportive studies, have been conducted in patients using opioids for CNCP (COMPOSE 6) or specifically switched to a stable dose of PR oxycodone for 2 weeks before enrolling (COMPOSE 7).39 The primary aim of these studies was the long-term (48 weeks) safety of naldemedine, calculated as the summary measures of TEAEs. TEAEs were observed in about 90% of treated patients, but most of them were mild to moderate, with a percentage of TEAEs leading to discontinuation ≤10% (Table 4). Treatment-related adverse events (AEs) only occurred in 28% of patients in the COMPOSE 6 and 50% of patients in the COMPOSE 7. Gastrointestinal disorders were the most common (over 50%), with diarrhea ranging from 23 to 40% of treated patients. In terms of efficacy, in both studies a significant decrease in overall PAC-SYM scores was observed from baseline, with a significant improvement in the PAC-quality of life (PAC-QOL). No significant changes were observed in pain intensity and the opioid dose. However, in both these studies the mean daily dose of opioids at baseline was relatively low: 74.4 mg of morphine equivalents in the COMPOSE 6 and 45.3 mg of oxycodone in the COMPOSE 7.39 This is probably the main limitation in the interpretation of these results, together with the small patient populations (only 43 patients in COMPOSE 6 and 10 in the COMPOSE 7) and the lack of ethnic diversity, as all patients were Asian (Japanese). Naldemedine did not affect COWS scores at stable doses of oxycodone or other opioids.39 These results confirmed the safety of naldemedine in the management of OIC, without hindering the analgesic benefits of opioid therapy.
Clinical Trials in Chronic Cancer Pain Patients
In 2017, Katakami et al published a phase III, randomized, double-blind, placebo-controlled trial (COMPOSE 4),42 where 193 patients were randomized to receive naldemedine 0.2 mg or placebo for 2 weeks. The same authors simultaneously published an open-label, single-arm, extension study (COMPOSE 5),42 where 131 were treated for 12 weeks. All study centers were in Japan. Enrolled patients had an Eastern Cooperative Oncology Group performance status ≤2 and were on a stable dose of opioids for ≥ 2 weeks. Patients aged ≥18 years old were eligible if they had OIC, previously treated with laxative drugs, and the cancer type did not affect the GI tract. The primary endpoint of COMPOSE 4 was the proportion of SBM responders (Table 5), while the primary outcome of COMPOSE 5 was the safety of naldemedine (Table 6).
Table 5.
Efficacy Study of Oral Naldemedine in Patients with Chronic Cancer Pain
| Trial | Ref | Phase and Study Design | No of Patients | Treatment | Primary Endpoint | Secondary Endpoints (Mean Change from Baseline) |
||
|---|---|---|---|---|---|---|---|---|
| Proportion of Responders (% pts) # | SBMs/wk | CSBMs/wk | SBMs/wk Without Straining | |||||
| V9236 – COMPOSE 4 | Katakami et al42 | Phase III. Randomized, double-blind, placebo-controlled study | Nal 97 Placebo 96 |
Nal 0.2 mg OD for 2 wks | Nal 71.1% Placebo 34.4% (p<0.0001) |
Nal +5.16 Placebo +1.54 (p<0.0001) |
Nal +2.76 Placebo +0.71 (p<0.0001) |
Nal +3.85 Placebo +1.17 (p<0.001) |
Notes: #Responders had at least three SBMs per week with an increase from baseline of at least one SBM per week during the 2-week treatment period (Baseline: the average number of SBMs/week during the 2 weeks before random assignment).
Abbreviations: wk, week; Nal, naldemedine; SBMs, spontaneous bowel movements; CSBMs, SBMs with a feeling of complete evacuation.
Table 6.
Safety of Oral Naldemedine in Patients with Chronic Cancer Pain
| Trial | Ref | Phase and Study Design | No of Patients | Treatment | Primary Endpoint | Secondary Endpoints | ||
|---|---|---|---|---|---|---|---|---|
| TEAEs | Serious TEAEs | TEAEs Leading to Discontinuation | TEAEs of Opioid Withdrawal | |||||
| V9236 – COMPOSE 4 | Katakami et al42 | Phase III. Randomized, double-blind, placebo-controlled study | Nal 97 Placebo 96 |
Nal 0.2 mg OD for 2 wks | Nal 44.3% Placebo 26.0% # (p>0.01) |
Nal 13.4% Placebo 3.1% |
Nal 9.3% Placebo 1.0% (p>0.01) |
NA |
| V9237 – COMPOSE 5 | Katakami et al42 | Phase III. Single-arm, open-label study. (extension study of Study V9236) |
Nal 131* | Nal 0.2 mg OD for 12 wks | Nal 80.2% | Nal 30.5% | Nal 9.2% | NA |
Notes: * Hundred subjects completed 12 weeks of treatment. #In COMPOSE 4 TEAEs is not the primary endpoint.
Abbreviations: Nal, naldemedine; pts, patients; TEAEs, treatment-emergent adverse events.
Even in cancer patients naldemedine showed a significantly higher percentage of responders compared with placebo (71.1% vs 34.4%, respectively; p<0.0001), however the COMPOSE 4 study was only conducted for 2 weeks, that could be considered a too limited placebo-controlled double-blind treatment period. In the COMPOSE 4 study, patients were considered responders if they had at least three SBMs per week with an increase from baseline of at least one SBM per week during the 2-week treatment period. Baseline was defined as the average number of SBMs/week during the 2 weeks before random assignment. All the other secondary outcomes of efficacy (SBMs/week, CSBMs/week, and SBMs without straining/week) were significantly better in the naldemedine group (Table 5).42
In this study, unlike all other studies conducted on patients with CNCP, safety evaluation revealed that naldemedine was burdened with a significantly higher percentage of TEAEs compared with placebo (44.3% vs 26.0%, respectively; p>0.01), and TEAEs leading to discontinuation (9.3% vs 1.0%, respectively; p>0.01) (Table 6). The reason for the observed difference Ibetween cancer and non-cancer pain patients is still unclear, however we can postulate that the malignant disease by itself is a precipitating factor for any organ disorder. This higher percentage of TEAE was confirmed in the COMPOSE 5, where, after 12 weeks of treatment, the incidence of TEAEs was 80.2%, with apercentage of serious TEAEs higher than 30%.42
Even in these studies conducted in a cancer population, the mean daily dose of opioids was relatively low (below 70 mg per day). The main limitation in enrolling patients using relatively low opioid dose is the reliability of the results in terms of efficacy on OIC, analgesia and risk of withdrawal. In the COMPOSE 4 no difference was observed in the COWS score between naldemedine and placebo, suggesting that, at those opioid doses, naldemedine did not impede analgesia.42
In 2018, Katakami et al43 published other secondary outcomes of efficacy from COMPOSE 4 and 5. In the first 2 weeks, no differences were observed in the mean overall scores for PAC-SYM and the PAC-QOL domain. Conversely, naldemedine significantly improved the PAC-SYM stool domain and the PAC-QOL dissatisfaction domain, which reflect the main complaints reported by patients with OIBD: incomplete, hard, or small bowel movements.43
A recent subgroup analysis of the COMPOSE 1, COMPOSE 2 and COMPOSE 3, showed no difference in terms of tolerability (TEAEs) and gastrointestinal disorders between naldemedine and placebo in patients aged ≥ 65 years with CNCP. The results in older adults are consistent with those observed in the overall patient population.44
Naldemedine vs Other PAMORAs
No randomized trials have been conducted to directly compare naldemedine to other PAMORAs. A meta-analysis of 27 articles regarding pharmacological therapies for OIC identified naloxone as the safest and most cost-effective opioid antagonist. But naldemedine was considered the most effective compound to achieve an increase of ≥1 bowel movement per week over baseline and an average of at least 3 bowel movements per week, compared to placebo (RR 0.66): this was considered a restrictive definition of response rate.45
Conversely to naldemedine, the safety and efficacy of other PAMORAs may be influenced by the type of opioid administrated: for instance, naloxegol was associated with greater risk of GI adverse events when co-administrated with methadone.35 Moreover, long-term safety and tolerability studies on methylnaltrexone40 and naloxegol41 did not include a placebo group.
Naldemedine was only associated with inhibition of analgesia at 233-fold higher ED50 doses than doses used to reverse morphine-blocked small intestinal transit, while ED50 of methylnaltrexone only needed to be 2.24-fold times higher to affect analgesia.30
Expert Opinion and Future Perspectives
Naldemedine has been widely investigated for the management of OIC in patients not responding to traditional laxatives, which are still considered the first level treatment, together with the increase of dietary fibers. Dose-finding studies have been conducted in chronic cancer and non-cancer pain patients; the dose 0.2mg once per day has been selected for the treatment of OIC.34,35 The dose 0.1mg once per day did not offer adequate effect while increasing the dose to 0.4mg once per day did not increase efficacy.
Naldemedine 0.2 mg significantly improved symptoms of OIC and was more effective than placebo in all clinical trials.37–39,42 Clinical trials on CNCP patients have been conducted in different countries and populations,37–39 while clinical trials in cancer patients only included Japanese people and the duration of the double-blind treatment period was very short, only 2 weeks in all clinical trials.42 These could represent the main limitations in the correct interpretation of the clinical results. The other limitation is the relatively low dose of opioids used by enrolled patients, especially for the management of cancer pain. We cannot know if the same efficacy of naldemedine can be obtained when the opioid dose is increased, doubled or tripled. Further studies are warranted to investigate the role of naldemedine in patients treated with high doses of opioids.
By instance, it is not clear why the incidence of TEAEs was similar between naldemedine and placebo in CNCP patients,37–39 while in cancer patients, naldemedine treatment was associated with a higher percentage of TEAEs.42,43 Most TEAEs were mild to moderate and were tolerated by patients. Overall, clinical data support the use of naldemedine as a specific “target therapy” in patients chronically using opioids and suffering from OIC.
Head-to-head comparisons between naldemedine and other PAMORAs are not available yet. However, some considerations can be made based on pharmacological and clinical data. One of the advantages of naldemedine over the other PAMORAs could be the minimal contribution of the P-gp, as a mechanism limiting its brain diffusion.31 The clinical advantage is related to the relative lower risk of drug–drug interactions compared with the other PAMORAs. Secondly, this is the first PAMORA where the permeability of the drug to the area postrema has been experimentally demonstrated.31 This finding could mean an adjunctive mechanism of action in reducing opioid-induced nausea and vomiting (OINV), by blocking MOR in the CTZ. Further studies are warranted to support this hypothesis. According to a retrospective study, patients receiving morphine or oxycodone experienced less OINV when 0.2 mg oral naldemedine was co-administrated (36% vs 47.2% respectively), thus allowing a reduced utilization of rescue antiemetic drugs.46 This could partly be a secondary effect of the improvement of OIC, which by itself is responsible for nausea and vomiting, but it is probably related also to the MOR antagonism by naldemedine in the CTZ. Thirdly, for the first time since PAMORAs were developed, clinical studies on naldemedine used a specific test, such as the COWS, to evaluate the appearance of symptoms of opioid withdrawal.38,39,42 This measure is a further assessment of its safety and selective peripheral activity, without any influence of opioid dose or analgesia. Finally, by indirect comparison, naldemedine seems to have a better efficacy compared with methylnaltrexone and naloxegol.45 Although oral naloxone is still considered the most likely to be superior to placebo and the drug ranked first in terms of safety,45 its availability in fixed combination with oxycodone strongly limits its use. Moreover, according to a recent meta-analysis naldemedine was the most efficacious PAMORA when non-responders were defined as patients that failed to obtain an average of three or more SBMs per week with an increase of at least 1 SBM over baseline.45 Therefore, by considering the significant flexibility of naldemedine and the efficacy results, this drug could potentially represent overall a first choice when starting treatment with a PAMORA for the management of OIC, when laxatives fail.
Future perspectives could include a possible role in the management of OINV, if the clinical trials will confirm the selective activity on the area postrema, which is not protected from the BBB. Another possible area of investigation is the post-operative setting, where opioids still have a key role in the management of severe post-operative pain, but their use is well known to increase the post-operative ileus. Finally, clinical studies on naldemedine in the management of OIC in patients under opioid substitution treatment could be beneficial for patients that use doses of opioids significantly higher than those investigated in the COMPOSE trials. In general, more clinical trials are warranted to elucidate the efficacy of naldemedine in different diseases and clinical settings. Moreover, other results are pending on the safety profile of naldemedine in comparison with other PAMORAs, particularly on the risk of major adverse cardiovascular events in CNCP patients. Results are expected in 2025.47
Finally, recent findings using animal models suggest a potential role for opioid antagonists in increasing bone mass density.48,49 Clinical studies are needed to confirm the hypothesis that these drugs (naloxone and naltrexone) could act as bone promoters, leading to a new appealing area of therapeutic interest for PAMORAs, which could counteract the negative effects of the opioidergic system on bone density and bone healing, without affecting central analgesic effects of endorphin and opiates on the descending inhibitory pain pathway.50
Conclusion
Managing patients with OIBD is still a challenge for physicians and an economic burden for health care systems. Mechanism-based treatments are the cornerstone of the appropriate management of any disease. PAMORAs are a mechanism-based treatment of OIBD. For patients experiencing OIBD during chronic opioid therapy, naldemedine represents a valid available treatment option because it is a well-tolerated drug that improves SBMs without affecting analgesia or causing symptoms of opioid withdrawal.
Acknowledgments
Editorial assistance was provided by Content Ed Net, with the help of Giovanna Damia, MD.
Abbreviations
Ach, Acetylcholine; AE, adverse event; AUC, area under the plasma concentration-time curve; BBB, blood-brain barrier; BFI, bowel function index; Cmax, peak plasma concentrations; CNCP, chronic non-cancer pain; CNS, central nervous sistem; COWS, clinical opiate withdrawal scale; CSBMs, SBMs with a feeling of complete evacuation; CTZ, chemoreceptor trigger zone; DOR, delta opioid receptor; ENS, enteric nervous system; GI, gastrointestinal; KO, knock out; KOR, kappa opioid receptor; MOR, mu-opioid receptor; NO, nitric oxide; NOS, nitric oxide synthase; OIBD, opioid-induced bowel dysfunction; OIC, opioid-induced constipation; OINV, opioid-induced nausea and vomiting; PAC-SYM, Patient Assessment of Constipation Symptoms; PAMORAs, peripherally-acting mu-opioid receptors antagonists; PEG, polyethylene glycol; P-gp, p-glycoprotein; QOL, quality of life; SBMs, spontaneous bowel movements; TEAEs, treatment-emergent adverse events; Tmax, time to reach peak plasma concentrations; VIP, vasoactive intestinal peptide; VPAC1, vasoactive intestinal polypeptide receptor 1; VPAC2, vasoactive intestinal polypeptide receptor 2.
Author Contributions
All authors made substantial contributions to conception and design, data acquisition, or data analysis and interpretation, drafting the article or critically revising it for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
FC served as a speaker for Molteni, Grunenthal, Angelini, Malesci. JP reports personal fees from BDSI, Astra Zeneca, Salix, outside the submitted work; consultant/speaker and researcher for BDSI, Salix, Nuerana, Enalare, Scilex, and Neumentum. JP also declared he has no relationship with this specific research. The authors report no other conflicts of interest in this work.
References
- 1.Leppert W, Zajaczkowska R, Wordliczek J. The role of oxycodone/naloxone in the management of patients with pain and opioid-induced constipation. Expert Opin Pharmacother. 2019;20(5):511–522. doi: 10.1080/14656566.2018.1561863 [DOI] [PubMed] [Google Scholar]
- 2.Wan Y, Corman S, Gao X, Liu S, Patel H, Mody R. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8(2):93–102. [PMC free article] [PubMed] [Google Scholar]
- 3.Corsetti M, Pannemans J, Whorwell P. Targeting mu opioid receptors to modulate gastrointestinal function: what have we learnt so far from the studies in functional bowel disorders? F1000Res. 2019;8:257. doi: 10.12688/f1000research.15974.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes AM, Munkholm P, Simren M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-recommendations of the nordic working group. Scand J Pain. 2016;11(1):111–122. doi: 10.1016/j.sjpain.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Muller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18(10):1837–1863. doi: 10.1093/pm/pnw255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesia R, Virizuela Echaburu JA, Gomez J, Sauri T, Serrano G, Pujol E. Opioid-induced constipation in oncological patients: new strategies of management. Curr Treat Options Oncol. 2019;20(12):91. doi: 10.1007/s11864-019-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haber PS, Elsayed M, Espinoza D, Lintzeris N, Veillard AS, Hallinan R. Constipation and other common symptoms reported by women and men in methadone and buprenorphine maintenance treatment. Drug Alcohol Depend. 2017;181:132–139. doi: 10.1016/j.drugalcdep.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 8.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016;150(6):1393–407. [DOI] [PubMed] [Google Scholar]
- 9.Simren M, Palsson OS, Whitehead WE. Update on rome IV criteria for colorectal disorders: implications for clinical practice. Curr Gastroenterol Rep. 2017;19(4):15. doi: 10.1007/s11894-017-0554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andresen V, Banerji V, Hall G, Lass A, Emmanuel AV. The patient burden of opioid-induced constipation: new insights from a large, multinational survey in five European countries. United Eur Gastroent J. 2018;6(8):1254–1266. doi: 10.1177/2050640618786145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imam MZ, Kuo A, Ghassabian S, Smith MT. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology. 2018;131:238–255. doi: 10.1016/j.neuropharm.2017.12.032 [DOI] [PubMed] [Google Scholar]
- 12.Coluzzi F, Pergolizzi JV Jr., Giordan E, Locarini P, Boaro A, Billeci D. Tapentadol prolonged release for managing moderate to severe chronic neck pain with or without a neuropathic component. Curr Med Res Opin. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Coluzzi F, Polati E, Freo U, Grilli M. Tapentadol: an effective option for the treatment of back pain. J Pain Res. 2019;12:1521–1528. doi: 10.2147/JPR.S190176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kress HG, Coluzzi F. Tapentadol in the management of cancer pain: current evidence and future perspectives. J Pain Res. 2019;12:1553–1560. doi: 10.2147/JPR.S191543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pergolizzi JV Jr, Coluzzi F, Taylor R Jr. Transdermal buprenorphine for moderate chronic noncancer pain syndromes. Expert Rev Neurother. 2018;23:1–11. [DOI] [PubMed] [Google Scholar]
- 16.Farmer AD, Drewes AM, Chiarioni G, et al. Pathophysiology and management of opioid-induced constipation: european expert consensus statement. United Eur Gastroent J. 2019;7(1):7–20. doi: 10.1177/2050640618818305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galligan JJ, Sternini C. Insights into the role of opioid receptors in the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes PA, Costello SP, Bryant RV, Andrews JM. Opioidergic effects on enteric and sensory nerves in the lower GI tract: basic mechanisms and clinical implications. Am J Physiol Gastrointest Liver Physiol. 2016;311(3):G501–513. doi: 10.1152/ajpgi.00442.2015 [DOI] [PubMed] [Google Scholar]
- 19.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155(1–3):11–17. doi: 10.1016/j.regpep.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark EB, Bødker MB, Grønlund D, Østergaard LR, Frøkjaer JB, Drewes AM. MRI analysis of fecal volume and dryness: validation study using an experimental oxycodone-induced constipation model. J Magn Reson Imaging. 2019;50(3):733–745. doi: 10.1002/jmri.26628 [DOI] [PubMed] [Google Scholar]
- 21.Mark EB, Klinge MW, Grønlund D, et al. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system: effect of opioids. Neurogastroenterol Motil. 2020;32(3):e13753. doi: 10.1111/nmo.13753 [DOI] [PubMed] [Google Scholar]
- 22.Poulsen JL, Brock C, Grønlund D, et al. Prolonged-release oxycodone/naloxone improves anal sphincter relaxation compared to oxycodone plus macrogol 3350. Dig Dis Sci. 2017;62(11):3156–3166. doi: 10.1007/s10620-017-4784-7 [DOI] [PubMed] [Google Scholar]
- 23.Morlion BJ, Mueller-Lissner SA, Vellucci R, et al. Oral prolonged-release oxycodone/naloxone for managing pain and opioid-induced constipation: a review of the evidence. Pain Pract. 2018;18(5):647–665. doi: 10.1111/papr.12646 [DOI] [PubMed] [Google Scholar]
- 24.Mozaffari S, Nikfar S, Abdollahi M. Methylnaltrexone bromide for the treatment of opioid-induced constipation. Expert Opin Pharmacother. 2018;19(10):1127–1135. doi: 10.1080/14656566.2018.1491549 [DOI] [PubMed] [Google Scholar]
- 25.Bui K, Zhou D, Xu H, Floettmann E, Al-Huniti N. Clinical pharmacokinetics and pharmacodynamics of naloxegol, a peripherally acting micro-opioid receptor antagonist. Clin Pharmacokinet. 2017;56(6):573–582. doi: 10.1007/s40262-016-0479-z [DOI] [PubMed] [Google Scholar]
- 26.Blair HA. Naldemedine: a review in opioid-induced constipation. Drugs. 2019;79(11):1241–1247. doi: 10.1007/s40265-019-01160-7 [DOI] [PubMed] [Google Scholar]
- 27.Raffa RB, Taylor R Jr, Pergolizzi JV Jr. Treating opioid-induced constipation in patient taking other medications: avoiding CYP450 drug interactions. J Clin Pharm Ther. 2019;44(3):361–371. doi: 10.1111/jcpt.12812 [DOI] [PubMed] [Google Scholar]
- 28.Hu K, Bridgeman MB. Naldemedine (symproic) for the treatment of opioid-induced constipation. P T. 2018;43(10):601–627. [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota R, Fukumura K, Wajima T. Population pharmacokinetics and exposure-response relationships of naldemedine. Pharm Res. 2018;35(11):225. doi: 10.1007/s11095-018-2501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanemasa T, Koike K, Arai T, et al. Pharmacologic effects of naldemedine, a peripherally acting mu-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation. Neurogastroenterol Motil. 2019;31(5):e13563. doi: 10.1111/nmo.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watari R, Matsuda A, Ohnishi S, Hasegawa H. Minimal contribution of P-gp on the low brain distribution of naldemedine, a peripherally acting mu-opioid receptor antagonist. Drug Metab Pharmacokinet. 2019;34(2):126–133. doi: 10.1016/j.dmpk.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Fukumura K, Yamada T, Yokota T, Kawasaki A. The influence of renal or hepatic impairment on the pharmacokinetics, safety, and tolerability of naldemedine. Clin Pharmacol Drug Dev. 2020;9(2):162–174. doi: 10.1002/cpdd.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi S, Fukumura K, Kubota R, Wajima T. Absorption, distribution, metabolism, and excretion of radiolabeled naldemedine in healthy subjects. Xenobiotica. 2019;49(9):1044–1053. doi: 10.1080/00498254.2018.1536815 [DOI] [PubMed] [Google Scholar]
- 34.Webster LR, Yamada T, Arjona Ferreira JC. A phase 2b, randomized, double-blind placebo-controlled study to evaluate the efficacy and safety of naldemedine for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2017;18(12):2350–2360. doi: 10.1093/pm/pnw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katakami N, Oda K, Tauchi K, et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017;35(17):1921–1928. doi: 10.1200/JCO.2016.70.8453 [DOI] [PubMed] [Google Scholar]
- 36.Viscusi ER. Clinical overview and considerations for the management of opioid-induced constipation in patients with chronic noncancer pain. Clin J Pain. 2019;35(2):174–188. doi: 10.1097/AJP.0000000000000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hale M, Wild J, Reddy J, Yamada T, Arjona Ferreira JC. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, Phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol. 2017;2(8):555–564. doi: 10.1016/S2468-1253(17)30105-X [DOI] [PubMed] [Google Scholar]
- 38.Webster LR, Nalamachu S, Morlion B, et al. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain. 2018;159(5):987–994. doi: 10.1097/j.pain.0000000000001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito Y, Yokota T, Arai M, Tada Y, Sumitani M. Naldemedine in Japanese patients with opioid-induced constipation and chronic noncancer pain: open-label phase III studies. J Pain Res. 2019;12:127–138. doi: 10.2147/JPR.S175900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster LR, Michna E, Khan A, Israel RJ, Harper JR. Long-term safety and efficacy of subcutaneous methylnaltrexone in patients with opioid-induced constipation and chronic noncancer pain: a phase 3, open-label trial. Pain Med. 2017;18(8):1496–1504. doi: 10.1093/pm/pnx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther. 2014;40(7):771–779. doi: 10.1111/apt.12899 [DOI] [PubMed] [Google Scholar]
- 42.Katakami N, Harada T, Murata T, et al. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J Clin Oncol. 2017;35(34):3859–3866. doi: 10.1200/JCO.2017.73.0853 [DOI] [PubMed] [Google Scholar]
- 43.Katakami N, Harada T, Murata T, et al. Randomized phase III and extension studies: efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann Oncol. 2018;29(6):1461–1467. doi: 10.1093/annonc/mdy118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wild J, Webster L, Yamada T, Hale M. Safety and efficacy of naldemedine for the treatment of opioid-induced constipation in patients with chronic non-cancer pain receiving opioid therapy: a subgroup analysis of patients ≥ 65 years of age. Drugs Aging. 2020;37(4):271–279. doi: 10.1007/s40266-020-00753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luthra P, Burr NE, Brenner DM, Ford AC. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and network meta-analysis. Gut. 2018. [DOI] [PubMed] [Google Scholar]
- 46.Sato J, Tanaka R, Ishikawa H, Suzuki T, Shino M. A preliminary study of the effect of naldemedine tosylate on opioid-induced nausea and vomiting. Support Care Cancer. 2020;28(3):1083–1088. doi: 10.1007/s00520-019-04884-0 [DOI] [PubMed] [Google Scholar]
- 47.NCT03720613 CTg. Risk of major adverse cardiovascular events for naldemedine and other medications for opioid induced constipation in adults with chronic non-cancer pain.
- 48.Petrizzi L, Mariscoli M, Valbonetti L, Varasano V, Langhoff JD, Von Rechenberg B. Preliminary study on the effect of parenteral naloxone, alone and in association with calcium gluconate, on bone healing in an ovine “drill hole” model system. BMC Musculoskelet Disord. 2007;8(1):43. doi: 10.1186/1471-2474-8-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka K, Kondo H, Hamamura K, Togari A. Systemic administration of low-dose naltrexone increases bone mass due to blockade of opioid growth factor receptor signaling in mice osteoblasts. Life Sci. 2019;224:232–240. doi: 10.1016/j.lfs.2019.03.069 [DOI] [PubMed] [Google Scholar]
- 50.Coluzzi F, Scerpa MS, Centanni M. The effects of opiates on bone formation and bone healing. Curr Osteoporos Rep. 2020. doi: 10.1007/s11914-020-00585-4 [DOI] [PubMed] [Google Scholar]