Abstract
The success of protein, peptide and antibody based therapies is evident - the biopharmaceuticals market is predicted to reach $388 billion by 2024 [1], and more than half of the current top 20 blockbuster drugs are biopharmaceuticals. However, the intrinsic properties of biopharmaceuticals has restricted the routes available for successful drug delivery. While providing 100% bioavailability, the intravenous route is often associated with pain and needle phobia from a patient perspective, which may translate as a reluctance to receive necessary treatment. Several non-invasive strategies have since emerged to overcome these limitations. One such strategy involves the use of microneedles (MNs), which are able to painlessly penetrate the stratum corneum barrier to dramatically increase transdermal drug delivery of numerous drugs. This review reports the wealth of studies that aim to enhance transdermal delivery of biopharmaceutics using MNs. The true potential of MNs as a drug delivery device for biopharmaceuticals will not only rely on acceptance from prescribers, patients and the regulatory authorities, but the ability to upscale MN manufacture in a cost-effective manner and the long term safety of MN application. Thus, the current barriers to clinical translation of MNs, and how these barriers may be overcome are also discussed.
Key Words: drug delivery, Microneedle, peptide delivery, protein delivery, transdermal
Introduction
The increasing development and use of protein based therapies over the last few decades can be attributed to the improvement of protein expression and synthesis on the scale required for widespread manufacturing (1,2). Protein and peptide based drugs are now widely available and are considered first line treatments for a number of chronic health conditions, such as type I diabetes, rheumatoid arthritis, specific cancers and haemophilia (3). Proteins, peptides and antibody based therapeutics have the potential to treat diseases that were once thought incurable (4) and thus continue to be studied despite ongoing difficulties associated with their delivery.
Protein and peptide based drugs are primarily administered via the parenteral route, as this provides rapid drug delivery, and in the case of the intravenous route, 100% bioavailability. Such high bioavailability is required particularly for proteins and peptides because of the potential for rapid degradation and clearance once in the bloodstream (5,6). Although antibody therapies may be modified to extend their circulatory time in the body, very large doses are still required to provide a therapeutic effect, making the parenteral route the most practical route of delivery. Further properties typically associated with protein and peptide based drugs, such as a high molecular weight and poor tissue membrane permeability, limit the administration route and bioavailbility available via other routes (7,8). For example, delivery via the oral route is hindered by the presence of protease enzymes in the gastrointestinal tract, which readily denature proteins.
However, long-term administration of protein and peptide based drugs via the parenteral route is not without its disadvantages and complications, despite it being the traditional method. From a patient perspective, repeated intravenous drug delivery may be associated with needle phobia, pain, and more complex issues, for example phlebitis and tissue necrosis (1,9). The need for repeated administration due to rapid clearance from the blood increases the risk of toxic adverse effects (10,11). Proteins and peptides present in infusions may also trigger an immune response if the body recognises them as antigens (12,13).
To improve the bioavailability and stability of protein, peptide and antibody based drugs, alternative routes of administration have been sought. Ideally, these routes should allow patients to self-administer the drug and therefore should be minimally invasive and ideally, painless. Additionally, the route should allow rapid onset of drug action with potential for sustained drug delivery, to reduce the need for repeated administration. Alternative administration routes investigated include the pulmonary (14–16), ocular (17–19), nasal (14,20–22), rectal (22–24) and transdermal (25–27) routes. The justification for each of these administration routes have been summarised in detail elsewhere (28) and each possess advantages and disadvantages, which are summarised in Table I.
Table I.
Advantages and disadvantages of administration routes for protein, peptide and antibody based therapeutics. Created from information provided in (28)
| Route of administration | Advantages | Disadvantages |
|---|---|---|
| Parenteral |
Intravenous route offers 100% bioavailability Rapid delivery of drug into systemic circulation Viable alternative if oral route is not feasible |
Intravenous route is painful, invasive and poorly tolerated by patients Potential for toxic effects due to repeated administration |
| Oral |
Painless Convenient |
Potential for poor permeability across the intestinal epithelial membrane First pass metabolism Proteases present in the gastrointestinal tract may degrade drug |
| Pulmonary |
Painless Large surface area available for protein absorption Avoids first pass metabolism Low enzyme activity in the lungs |
Potential for poor permeability across epithelial lining fluid, epithelial cell layer and the endothelial membrane of capillary cells Proteins and peptides may be subjected to phagocytosis by the macrophages in the lungs |
| Ocular | Avoids first pass metabolism |
Potential for poor permeability, particularly of hydrophilic macromolecules, across eye membrane High enzyme activity, i.e. protease and aminopeptidase |
| Nasal |
Painless Large surface area available for protein absorption Avoids first pass metabolism Thin porous endothelial basement membrane of the nasal epithelium facilitates drug absorption |
Potential for poor permeability, particularly of large hydrophilic macromolecules, across nasal epithelium Rapid mucociliary clearance that reduces the available time for drug absorption Only small amounts of drug can be administered via the nasal route |
| Rectal | Offers partial bypass of first pass metabolism |
Potential for poor permeability across rectal epithelium Patient may consider this route distasteful |
| Transdermal |
Painless Convenient Large surface area available for protein absorption Avoids first pass metabolism Potential for adaptability to deliver both small and macromolecular therapeutics, e.g. by using microneedles |
Potential for poor permeability, particularly of large hydrophilic molecules, across the stratum corneum Potential for localised skin irritation |
Microneedles (MNs)
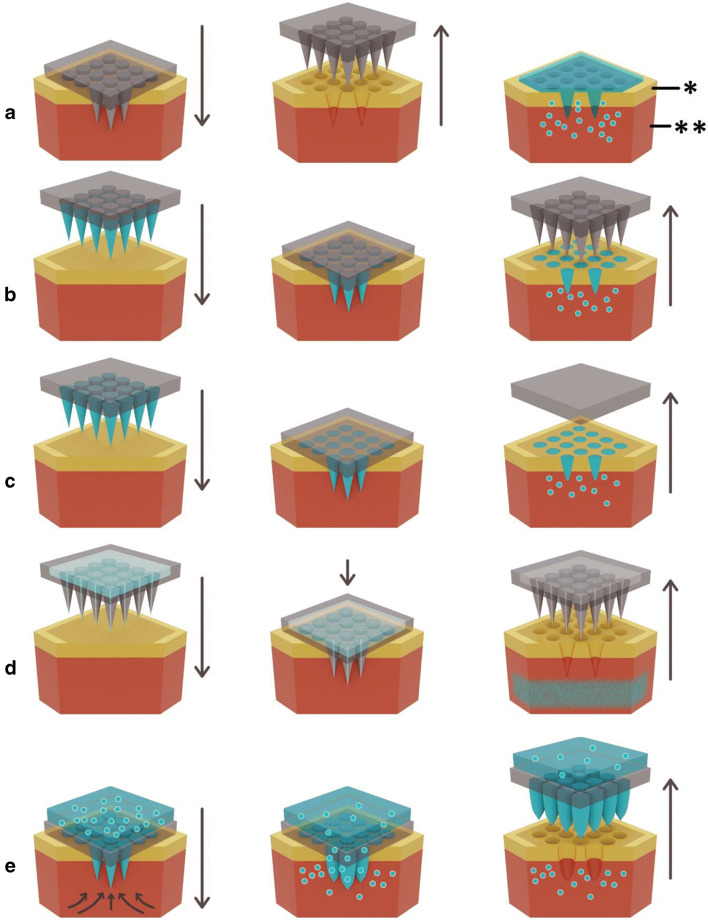
Microneedle (MN) arrays consist of multiple micro-projections assembled on one side of a supporting base, ranging in height from 25 to 900 μm. MN arrays effectively bypass the stratum corneum barrier by creating temporary microscopic aqueous channels within the epidermis, through which drug molecules can diffuse into the dense microcirculation, present in the dermis. MNs were first conceptualised by Gerstel and Place in 1971 (29), but were not practically realised until 1998, when manufacturing capabilities and microfabrication techniques became more advanced. Today, MN technology has developed further and they are traditionally placed in five different categories: solid, coated, hollow, dissolving and hydrogel-forming (Fig. 1).
Fig. 1.
Schematic representation of methods of MN application to the skin to achieve enhanced transdermal drug delivery, * stratum corneum, ** epidermis. (A) Solid MN that are applied and removed to create transient micropores, followed by application of the formulation. (B) Solid MN are coated with drug for instant delivery and to remove the two step process associated with solid MNs. (C) Drug is mixed with soluble polymeric/carbohydrate MNs that dissolve in skin interstitial fluid over time. (D) Hollow MNs puncture the skin, after which liquid drug can be actively infused through the needle bores. (E) Hydrogel-forming MNs imbibe skin interstitial fluid upon application to the skin. This induces drug diffusion through the swollen microprojections. Drug is often stored above the microprojections in a lyophilised wafer prior to interstitial fluid uptake
Each type of MN has its own distinct advantages and disadvantages and therefore it is important to determine the type of MN required for maximised transdermal delivery of a specific drug. Solid MNs may be combined with any conventional drug formulation for passive diffusion (i.e. transdermal patch, solution, cream or gel), however, the two-step application process is more impractical than other methods and may discourage patient use.
The use of coated MNs removes the two-step application process, however, the finite surface area of the needle array limits the amount of drug that can be applied. Thus, coated MNs are typically limited to use with potent drugs.
Dissolving MNs use biocompatible polymers mixed with the drug to form the needle tips. As the needle tips dissolve once applied to the skin, there is no risk of accidental re-piercing of the skin and no need for sharps disposal, a potential problem associated with solid, coated and hollow MNs. Additionally, dissolving MNs provide potential for controlled drug release - the release kinetics of the drug are dependent upon the constituent polymers’ dissolution rate (30). By adjusting the type of polymer and the polymer composition within the formulation, drug release may be controlled. Typical polymers used for the production of dissolving MNs include poly(vinyl alcohol) (PVA), poly(vinylpyrrolidone) (PVP), dextran, carboxymethyl cellulose (CMC), chondroitin sulfate and various sugars (31), all of which are low cost and therein lies the potential for cheap and straightforward mass production. The main limitation associated with dissolving MNs is the deposition of polymer, alongside the drug, into the skin. Although polymers discussed above are biocompatible, currently no long-term studies explore the effects of repeated polymer deposition into the skin. Further, long term research will be required to provide safety assurances to both prescribers and patients (32).
As an alternative, biodegradable polymers, such as poly(lactic acid), chitosan, poly(glycolic acid), or poly(lactide-co-glycolide) (PLGA), have been explored, which degrade, rather than dissolve, to release the drug. Carbohydrates have also been used as a dissolving MN material. They are cheap, safe, and can sufficiently pierce the skin (33–35). However, several problems associated with their processing and storage prevent their use clinically (36), primarily thermal treatment required during the manufacturing process which limits the number of drugs available for loading into the MN arrays.
Hollow MNs allow a greater volume of drug to be delivered into the skin, either by passive diffusion, or by infusion using pressure or electricity to drive the direction of drug flow into the skin (37). However, this may require bulky associated equipment (such as an electronic pump with associated electronics and microprocessor), reducing the convenience associated with MNs to a certain degree. The primary disadvantage associated with hollow MNs is the potential for drug flow resistance to occur – either by clogging of needle openings with skin tissue during insertion (38), or by compression of the MNs by dense dermal tissue (39). Limitations may be overcome somewhat by use of an alternative MN design (40), or by partially removing MNs immediately following insertion to reduce tissue clogging at the needle tips (41). Stability issues may arise when using hollow MNs, as the drug must be in liquid form for delivery. For biomolecules specifically, this would likely require a cold chain to be maintained from bench to bedside to ensure biomolecule stability. This removes the advantage associated with other MN types, for example, hydrogel-forming MNs, whereby the drug may sit in a compressed tabled or lyophilised wafer above the array until the array is applied.
Hydrogel-forming MNs are the most recent type of MN to be formulated (42,43). MNs used in this system do not contain drug, and instead integrate cross-linked polymeric MN projections with an attached drug reservoir. Figure 1E demonstrates that following interstitial fluid uptake, the drug may diffuse from the reservoir, through the swollen MNs, into the skin, to be up-taken by the dermal microcirculation. The most common types of polymeric materials used in the aqueous hydrogel blend include poly(methyl vinyl ether-co-maleic acid) crosslinked by esterification using poly(ethyleneglycol), chitosan, PLGA and PVA (32,42,44,45). Similarly to dissolving MNs, the delivery of drug may be controlled by the polymer blend, which affects the cross-linking ratio. Cross-linking hinders the mobility of the polymer chains and therefore reduces the swelling abilities of the hydrogel. There is the potential for interactions to occur between the hydrogel matrix and drug; therefore, drugs must be tested on an individual basis to determine their compatibility with the polymers used in the hydrogel-forming MN system. More recently, hydrogel-forming MNs have been made from light responsive polymer materials to control drug release (46). A further advantage of hydrogel-forming MN arrays is the fact that they are removed intact from the skin. Therefore, there is no concern with polymer left in the skin, as is the case with dissolving MNs. As the MNs are swollen, they cannot be re-inserted into the skin, removing the risk of accidental re-insertion and the need for sharps disposal.
Materials for MN Fabrication
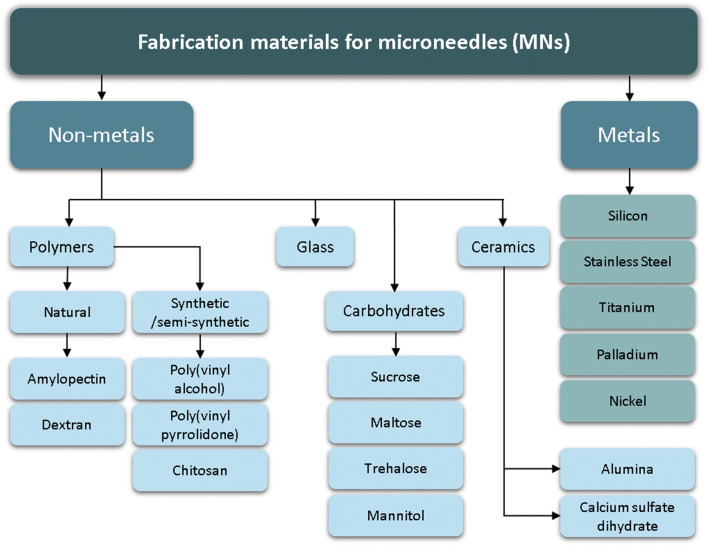
As briefly discussed above, the type of material used to make MNs in dissolving and hydrogel-forming systems will influence the drugs ability to diffuse into the skin. There has been numerous studies that explore the material types used to create MNs and their biocompatibilities. MNs were initially manufactured from silicon (47), but have since been made from materials such as stainless steel (48), silk (49) and various polymers (50,51) (Fig. 2).
Fig. 2.
Materials used for the preparation of MNs
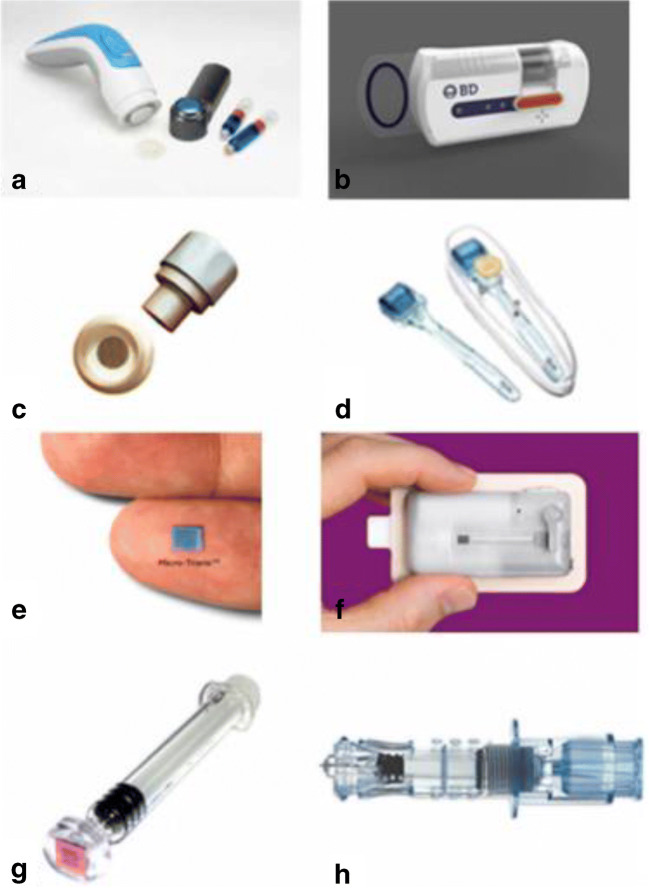
Silicon was the first material used for MN fabrication, prior to the development of more complex fabrication techniques (38,52). It is a versatile material, able to be fabricated into a range of MN shapes, with suitable strength to pierce the skin (53). The limitations associated with silicon MNs are the high cost associated with its use, including long fabrication times and multi-step processing. This increases the cost associated with silicon MN use, although MNs formed from this material can be made in batches to reduce costs (54). Furthermore, some concerns exist regarding the biocompatibility of silicon, as the needles may become brittle once fabricated, increasing the risk of material fracture when piercing the skin. The failure of the first MN device, Micronject® (Fig. 4G), may be attributed to some extent to its silicon needles, which are not biodegradable and may cause biofouling (56,57). Chen et al. (2008) produced silicon MNs with biodegradable tips in an attempt to negate this issue. Silica glass has also been investigated as a MN material (58). Although the material is inert and its transparency allows visualisation of fluid flow (41,59), the material is brittle and has similar fracture toughness to silica (54).
Fig. 4.
Current microneedle devices. A Microstructured Transdermal System (MTS). B Microinfusor. C Macroflux®. D Microneedle Therapy System (MTS Roller™). E Microtrans™. F h-patch™. G MicronJet. H Intanza®. Reproduced with permission from (55)
Various types of metals, such as stainless steel, titanium, palladium, palladium-cobalt alloys and nickel have been used to fabricate MNs (60). Metals are an attractive material for MN fabrication as their use is established in healthcare, for example, stainless steel hypodermic needles and titanium implants. Metals used for MN fabrication exhibit good biocompatibility and high fracture forces, reducing the risk of needles breaking off in skin tissue (61).
Ceramic MNs are typically fabricated by casting ceramic slurries into micromoulds (55), a low cost process with the potential for up-scaling. Types of ceramic used include alumina (Al2O3), calcium sulfate dihydrate (CaSO4·2H2O) and calcium phosphate dihydrate (CaHPO4·2H2O) (62,63). Alumina in particular is resistant to corrosion and adverse environmental conditions (64). Ceramics typically have good compression resistance, but can be brittle when exposed to tensile stress (62), and ultimately, have poorer strength than alternative materials such as metals.
To summarise, MNs are a minimally invasive drug delivery device, which combines the benefits of a transdermal patch with the drug delivery capabilities of a hypodermic needle. Their application is painless with minimalised skin trauma and bleeding compared to that of a hypodermic needle, a highly attractive attribute for patients (65,66). In addition to a reduction in needle phobia and reduced risk of infection, MNs can be self-administered, removing the need for healthcare staff support (67,68). Furthermore, dissolving and hydrogel-forming MNs eliminate the need for sharps waste disposal. It can be expected that these patient-friendly benefits of MNs may be translated into increased compliance. However, the benefits of MNs are not limited to the patient. There has been a large amount of research pertaining to the removal of the “cold chain” through vaccine-MN manufacturing, which would result in huge cost savings if accomplished (69,70). Numerous parameters can be changed to provide the most efficacious and controlled delivery of a specific drug, bypassing first pass metabolism and allowing the delivery of both small molecules and macromolecules. MNs hold the potential to transform transdermal drug delivery. There have been numerous studies demonstrating the drug delivery capabilities of solid (50,52,71–74), coated (75–78), dissolving (34,79–81), hollow (59,82,83), and hydrogel-forming (32,84–88) MNs. This review will focus on MN-mediated delivery of protein, peptide and antibody based therapies, and the hurdles that must be overcome for MNs to be accepted for clinical use.
MN Mediated Transdermal Delivery of Protein, Peptide and Antibody Based Therapeutics
Solid Microneedles
Diabetes affects 422 million people worldwide (89). The first line therapy for type 1 diabetics is daily subcutaneous insulin injections, and commonly becomes a later necessity for the treatment of type 2 diabetes. Therefore, alternative insulin delivery methods have become a popular route of scientific exploration. McAllister et al. (2003) fabricated solid silicon MNs to facilitate the delivery of insulin and bovine serum albumin (BSA) across human skin in vitro (50). Permeation of the two compounds was successful, and permeability of both was increased compared to when MNs were left in the skin, demonstrating that the compounds were able to diffuse through the aqueous channels created by the silicon MNs. The concentration of insulin delivered across the skin from a 1 cm2 patch containing 100 units/mL was deemed sufficient to meet the basal needs of many diabetics.
A secondary study exploring the ability of solid MNs to deliver insulin transdermally was completed by Martanto et al. (2004). Similarly to McAllister et al. (2003), MNs increased skin permeability to insulin, to an extent equal to a 0.05–0.5 units of insulin injected subcutaneously. Blood glucose levels in diabetic rats were lowered by as much as 80% (71).
Zhou et al. (2010) investigated the effects of differing metal MN lengths (250 μm, 500 μm and 1000 μm) on the transdermal delivery of insulin. For all three needle lengths, blood glucose levels rapidly decreased in 1 h and continued to decrease until 3 h. Glucose levels slowly increased thereafter, this was associated with the closure of the temporary micropores created by the MNs, confirmed by transepidermal water loss (TEWL). Furthermore, the rate of elevation in blood glucose levels was inversely proportional to the length of the needle (90).
More recently, Li et al. (2017) created solid MNs from poly(lactic acid) (PLA) to combine the advantages of MNs with the added benefit of a biodegradable system, an attractive prospect from a commercial point of view. The study systematically investigated the effects of MN dimensions, drug (insulin) concentration, viscosity of drug formulation and the administration time of drug on its transdermal delivery. Increasing insulin concentration increased the permeation amount, but not rate, of drug in vitro. Increasing formulation viscosity decreased permeation rate. In vivo studies were then conducted on diabetic mice, using solid PLA MNs with a height of 600 μm and a density of 100 MNs per cm2. The minimal blood glucose levels were found to be 29% at 5 h, compared to 19% at 1.5 h from a subcutaneous insulin injection. The authors concluded that the use of MNs may be beneficial when a delayed reduction in blood glucose is required (91).
Solid MN studies are not limited to the delivery of insulin. For example, Li et al. (2010) investigated the effects of solid MN (metal DermaRoller™ and maltose) pre-treatment on the transdermal delivery of human immunoglobulin G (IgG) in vivo (5 mg/mL applied concentration). Flux was recorded as 45.96 ng/cm2/h and 353.17 ng/cm2/h in vitro for maltose and metal MNs respectively. Cmax was recorded as 7.27 ng/mL and 9.33 ng/mL at 24 h for maltose and metal MNs respectively. The ability of the DermaRoller™ to create wider MN channels was attributed to the increase in both flux and Cmax (92).
Cui et al. (2011) evaluated the extent to which pre-treatment with MNs (DermaRoller™, 250 μm, 500 μm and 1000 μm) could enhance skin permeation of ovalbumin-conjugated nanoparticles in vitro and in vivo. For in vitro studies, MN pre-treatment increased ovalbumin permeation significantly more than the control. Furthermore, 28.3 ± 6.5% of ovalbumin was delivered transdermally from ovalbumin in solution compared to 13.6 ± 2.4% ovalbumin delivery from ovalbumin nanoparticles. This was attributed to the greater size of the ovalbumin nanoparticles hindering diffusion. When applied as a 70 μg/mouse dose, transcutaneous immunisation from ovalbumin nanoparticles following MN pre-treatment was greater than the same dose given subcutaneously (93).
Han and Das (2013) combined sonophoresis and MNs to enhance the delivery of BSA across porcine ear skin. Permeability of BSA was found to be 0.43 and 0.40 μm/s from MNs and sonophoresis alone, however, when the two physical methods of permeation enhancement were combined (1.5 mm MNs, 15-W ultrasound), permeability increased to 1 μm/s. This was reported as approximately 10 times higher than that achievable by passive diffusion of BSA (94).
Zhang et al. (2014) determined transdermal permeation of four model peptides following a 150 μm solid silicon MN pre-treatment across porcine ear skin. Similarly to Cui et al. (2011), molecular weights of the peptides influenced their ability to permeate transdermally. MN pre-treatment significantly enhanced permeation of all peptides, although increasing the molecular weight of the peptides decreased the amount delivered transdermally (95).
Coated MNs
Many studies that use coated MNs focus on the field of vaccination (96). Minimal amounts of vaccine delivered into the skin can still generate the required immune response, due to the high levels of Langerhans and dendritic cells within the skin (97). As minimal vaccine is sufficient, the reported disadvantage of limited drug loading on coated MNs does not apply, hence the popularity for using coated MNs for vaccine delivery. The use of MNs for vaccine delivery has been reviewed elsewhere in depth (31). This review will focus on delivery of proteins and peptides for therapeutic, rather than immunological benefits.
Saurer et al. (2010) successfully coated stainless steel MNs with DNA and protein-containing polyelectrolyte films in a layer-by-layer approach. The authors cited five key advantages of using these types of films – there is precise control over film thickness and therefore drug concentration; organic solvents are not required in the fabrication process, improving the safety of the MNs; fabrication of films provides control over the release of defined amounts of multiple different agents; auxiliary agents may be incorporated into the films (e.g. cationic polymers); and the fabrication process is able to coat objects having irregular shapes such as medical devices or implantable materials. In this study, the release of both protein and DNA from the coated MNs was characterised by fluorescence and optical microscopy following 2 h insertion into porcine cadaver skin. Post insertion fluorescence images demonstrate the capability of the coated layer to be released almost completely from the solid MNs and to be delivered into the epidermal and dermal layers of skin (98).
Acknowledging that MN mediated drug delivery focused mainly on hydrophilic molecules, Zhao et al. (2017) developed a novel formulation for the coating of MNs for delivery of hydrophobic auto antigen peptides, which are being investigated for antigen specific immunotherapy of type 1 diabetes. The formulation was comprised of three co-solvents (water, 2-methyl-2-butanol and acetic acid) and PVA 2000, which could dissolve both hydrophilic and hydrophobic peptide auto-antigens at relatively high, and clinically relevant, concentrations. The formulation coating and procedure did not adversely affect the biological activity of the peptides. Both in vitro (human skin) and in vivo (mouse skin) studies were completed to demonstrate the ability of hydrophobic peptides to be delivered via coated MNs. Delivery was maximised when electropolishing the underlying metal MN array, reducing the thickness of peptide coating and utilising peptides with greater aqueous solubility (99).
Caudill et al. (2018) utilised PEG MNs for the delivery of BSA in vitro and in vivo. MNs were inserted into a solution-filled coating mask device, then withdrawn and allowed to dry before piercing the skin. In vitro permeation of FITC-BSA loaded MNs (1000 μm, 64 needles/cm2) across full thickness porcine skin following 5 min MN insertion was found to be 45% at 24 h. FITC-BSA appeared to be concentrated in the epidermis, upper layers of the dermis, and around sites of microneedle penetration, with little fluorescent signal observed in the lower dermis. The effectiveness of the MNs was attributed to the needle density and needle length (100). The authors followed up the in vitro data with an in vivo study. MNs (700 μm in height) were coated with a 7% BSA solution and applied to the back of BALB/c mice for 2 min. Compared to a control subcutaneous dose, MN treated mice showed a more sustained retention of BSA at the site of administration. Furthermore, BSA was retained within the skin for a greater time period than the subcutaneous dose. MN treated mice had 79% and 19% fluorescence signal remaining at 6 h and 72 h, respectively. This is compared to the subcutaneous dose, which resulted in 14% and 4% fluorescence signal remaining at 6 h and 72 h, respectively. This depot effect was attributed to the presence of high molecular weight methylcellulose (MW 17,000 Da) within the MN formulation, which retained the coated BSA near the administration site for greater periods of time.
Li et al. (2018) coated the surface of individual metal MNs with various compounds (immiscible molecules, proteins, and nanoparticles) to allow delivery of a variety of therapies within the same MN patch. The compounds chosen represented drugs of different sizes and both particles and free drugs, in order to represent almost any type of therapy which may be utilised within MN systems. MNs were applied to full thickness porcine skin for 5 s and removed after 2 min. The protein used in the in vitro experiment was FITC-BSA, and was delivered alongside free fluorescein sodium dye and fluorescently labelled nanoparticles. Results showed that all three compounds were successfully delivered, but at differing rates. FITC-BSA delivery sat between the three compounds, with fluorescein sodium dye diffusing the fastest and fluorescently labelled nanoparticles diffusing the slowest. FITC-BSA fluorescent intensity declined to ~40% after 4 h and trace remains were left at the end of the 2 day experiment (101).
Dissolving MNs
Similarly to coated MNs, dissolving MNs have been investigated extensively for vaccine delivery (102–104), and their advantages, discussed earlier in this review, continue to support their exploration for delivery of protein and peptide drugs.
Mönkäre et al. (2015) developed monoclonal IgG loaded hyaluronan-based dissolving MNs for intradermal delivery in vitro. Following 10 min application to human skin (280 μm length), the majority of the original tip length (65%) was dissolved and IgG and hyaluronan were co-deposited until a depth of 150–200 μm in the skin. The authors noted that the low molecular weight of the hyaluronan likely improved the dissolution rate compared to other studies using hyaluronan for the basis of their dissolving MNs (105).
Chen et al. (2016) formulated interferon-α-2b containing dissolving MNs (680 μm length) for transdermal drug delivery. In vitro drug release efficiency was 49.2%. In vivo studies reported a Cmax and Tmax of 11.58 ng/mL at 40 min. The dissolving MNs showed sufficient stability for 2 months. The authors reported that the bioequivalence was similar between dissolving MNs and an intramuscular (IM) injection control, suggesting that IM injections of interferon-α-2b could be replaced with MNs for self-administration and increased patient compliance (106).
A dissolving MN system, comprised of PVA and trehalose to encapsulate active pharmaceutical peptides within the MN matrix was created by Dillon et al. (2017). Polymyxin B loaded MNs were applied to porcine ear skin for 30 s. The rate of drug delivery was found to be greater than the control (drug loaded disc without MNs) for the first 4 h post MN application, after which rate of permeation was equal to the control, but the percentage of drug delivered transdermally was significantly greater. At the end of the 22 h Franz cell experiment, 66.9 ± 11.59% of polymyxin B was delivered transdermally, compared to 54.14 ± 3.01% for the control (107).
To remove the two step application of solid MNs, Liu et al. (2018) fabricated insulin-loaded dissolving MNs for glucose regulation in diabetic rats. A two-step centrifuging and moulding process was used to form a dissolving composite containing insulin-loaded CaCO3 microparticles and PVP. Each patch contained 10 × 10 array of needles, 250 μm needle length. When compared to pure PVP MNs, mechanical strength was increased and solubility was slower, providing controlled release properties. Similarly to the study completed by Li et al. (2017) discussed above, delivery of insulin from the MNs was slower than that of a control subcutaneous injection. The 5 IU subcutaneous injection lowered blood glucose levels to 29.5 ± 5.2 mg/dL 2 h post injection, compared to 39.7 ± 7.5 mg/dL at 5 h post MN insertion containing the same units of insulin (108).
It is clear that numerous parameters can be changed to optimise the stability and activity of drugs encapsulated within a dissolving MN system. Lahiji, Jang, Huh, et al. (2018) and Lahiji, Jang, Ma, et al. (2018) evaluated the effects of polymer type, concentration, drying conditions and storage temperature on the activity of lysozyme (model protein) loaded in dissolving MNs. The activity of lysozyme was preserved up to 99.8 ± 3.8% for 12 weeks when fabricated at 4°C, allowed to dry naturally and when fabricated within the presence of stabilising agents such as trehalose (109,110).
Vora et al. (2020) acknowledged that there is a limited range of water soluble, biodegradable polymers that can be used to manufacture dissolving MNs. They therefore used a carbohydrate biopolymer (pullulan) for the first time to facilitate delivery of FITC-BSA across dermatomed neonatal porcine skin. After assuring stability of FITC-BSA remained intact in the formulation, in vitro studies were used to assess transdermal delivery of FITC-BSA from the novel dissolving MNs (600 μm needle length). FITC-BSA was detectable as soon as 15 min post-MN insertion, and at 28 h, 1105 ± 123 μg/cm2 was delivered from the dissolving MNs. Therefore, the authors demonstrated for the first time the potential of the carbohydrate biopolymer pullulan for fabrication of dissolving MNs for the successful delivery of high molecular weight compounds such as FITC-BSA (111).
Hollow MNs
Most studies regarding hollow MN arrays have focused on fabrication aspects, including design and characterisation studies. As a result, less attention has been given to their actual efficiency in delivering drug molecules across the skin (112). Again, focus has been given to intradermal delivery of vaccines, particularly those loaded in nanoparticles, which could not be delivered by other means i.e. coated MNs (113,114). Comparison with dissolving MNs has also occurred (115).
Delivery of high molecular weight compounds into the skin was questioned by Chen et al. (2010). The authors believed the answer to this question might lie in the combination of sonophoresis, a technique that uses low frequency ultrasound to induce acoustic cavitations in the lipid layers of the SC, and MNs. The transdermal delivery of calcein and BSA was measured passively, with either sonophoresis or hollow MNs (300 μm length) alone, or when the two methods were combined (SEMA). For both compounds, transdermal delivery was in the order of; SEMA > sonophoresis alone > hollow MNs alone > passive diffusion (116). Although the study effectively demonstrated that the two physical methods of permeation enhancement could increase transdermal delivery of macromolecules, the addition of sonophoresis to MNs removes some key advantages of MNs, namely the ability for self-administration and the convenience associated with the small array. The addition of sonophoresis also returns the device to a two-step process, similarly to the use of solid MNs.
A “pocketed” MN device design was created by Torrisi et al. (2013) for the intradermal delivery of botulinum toxin A to reduce pain, improve therapeutic targeting and to streamline the administration procedure. Pockets were cut into stainless steel MN shafts for liquid drug reservoir loading. Microneedle-mediated intradermal delivery of β-galactosidase and formaldehyde-inactivated botulinum toxoid revealed effective deposition and subsequent diffusion within the dermis (117).
In vitro intradermal delivery of synthetic mRNA using hollow MNs was demonstrated by Golombek et al. (2018). High levels of humanised Guassia luciferase (hGLuc) protein were detectable following hollow MN penetration. Levels after 24 h and 48 h were significantly higher than the control “naked mRNA” (118).
Hydrogel-Forming MNs
Hydrogel-forming MNs are a relatively newer type of MN compared to those discussed above (42). Thus, many studies have focused on changing parameters that may affect their swelling capabilities, and therefore, their ability to deliver drugs transdermally. Factors affecting transdermal drug delivery from hydrogel-forming MNs include polymer content (119), molecular weight of the cross-linking agent (120), concentrations of the cross-linking agent (121) and presence of foaming agent (122). Electrical modulation via iontophoresis (ITP) combined with hydrogel-forming MNs has also been shown to enhance transdermal delivery (40). Using this approach, Donnelly et al (2012) deemed it possible to facilitate on demand requirements, such as the delivery of insulin after a meal or rapid vaccine delivery. However, enhancing the delivery of proteins and antibody-based therapeutics is limited due to the approximated 13 kDa molecular limit associated with ITP delivery (121,122).
To improve adhesion to the skin, Seong et al. (2017) formulated double layered MN arrays with swellable needles inside a non-swelling patch, which are able to interlock upon skin insertion. This interlocking behaviour was attributed to the sustained release of insulin in vivo. Over 12 h, 60% of the applied insulin was delivered transdermally, 70% of which had a stable confirmation. The authors suggest this novel MN design could be used in the future where sustained release kinetics are required (123).
Courtenay et al. (2018) compared dissolving and hydrogel-forming MNs (500 μm needle length) for the transdermal delivery of bevacizumab in vivo. The dissolving MNs delivered a higher Cmax at a faster rate (488.7 ng/mL at 6 h) compared to the hydrogel-forming MNs (81.2 ng/mL and 358.2 ng/mL at 48 h for the hydrogel-forming MNs containing 5 mg and 10 mg of bevacizumab respectively). The differences in the pharmacokinetic profile was attributed to the molecular weight of bevacizumab (149,000 Da). It was suggested that diffusion of the large macromolecule through the tortuous hydrogel network was likely to have been the cause of the delayed Cmax compared to dissolving MNs (88). PVA is a hydrophilic polymer, and thus bevacizumab incorporation into PVA dissolving MNs would allow immediate dissolution and drug release upon MN insertion. As it appeared that the drug was released as a bolus from dissolving MNs, but a sustained release profile was observed from hydrogel-forming MNs, the MN type could be tailored to the desired pharmacokinetic profile for the delivery of high molecular weight macromolecules.
Safety and Clinical Translation of MN Based Products for Protein, Peptide and Antibody Based Therapeutics
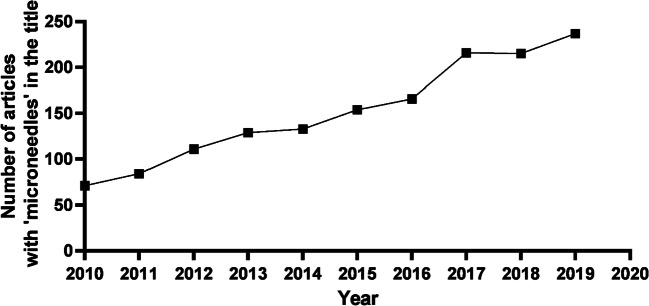
The field of MNs has grown immensely since they were first conceptualised by Gerstel and Place in 1971 (Fig. 3). Extensive studies have explored MN fabrication and drug loading techniques to optimise the system. In more recent years, delivery of high molecular weight, high dose and low potency protein and peptide based therapies has become more commonplace, allowing MNs to be considered for delivery of drugs which was previously thought unlikely or even impossible.
Fig. 3.
Number of journal articles published containing ‘microneedle’ in the title each year since 2010 (data acquired from PubMed)
The transdermal drug delivery market is predicted to grow by $1.79 billion between 2019 and 2023 (124), and the biopharmaceuticals market is predicted to reach $388 billion by 2024 (125). Biopharmaceuticals can technically modulate any physiological pathway that has been fully understood, thus, there is a huge growth potential. More than half of the current top 20 blockbuster drugs are biopharmaceuticals, illustrating the growth and interest in both the biopharmaceuticals and transdermal market. The next step for clinical success is clinical trials. A ClinicalTrial.gov search reports 106 studies for the keyword microneedle (March 2020), 67 of which have been completed worldwide. Studies that focus on intradermal vaccination, diabetes and anaesthesia are the most common. Of the completed studies, only four have reached Phase IV trials, one of which involves the intradermal delivery of the influenza vaccine. However, the use of protein, peptide and antibody based therapies transdermally faces numerous challenges that must be addressed before they can achieve their full potential.
Patient Safety
MNs now harbour the ability to deliver drugs that require high doses and are of low potency (126), as opposed to the traditional delivery of low dose, high potency therapies (127,128). Piercing the skin using MNs results in significantly lower microbial penetration than that produced by using a conventional hypodermic needle (72,129), and hydrogel-forming MNs have even demonstrated antimicrobial properties (130). Therefore, the likelihood of MNs inducing a skin or soft tissue infection is minimal. Furthermore, it is statistically unlikely that MNs will ever pierce the exact same points on the skin surface due to the small size of the device, increasing the likelihood of MNs having a favourable safety profile (131).
However, one must consider the implications of repeated use of MNs, particularly dissolving MNs, where deposition of polymer in the skin from the dissolving system is undesirable. For example, the dissolving MN used in the McCrudden et al. study (126) would deposit approximately 5–10 mg of polymer per cm2 in the skin. Assuming a patch size of 10 cm2, 50–100 mg of polymer would be deposited into the skin each time a patch is applied. This is unlikely to be a concern for vaccinations, however, most therapeutic agents, particularly biologics discussed in this review, require repeated administration. This supports the ongoing use of hydrogel-forming MNs (Fig. 1E) as the MN device can be removed from the skin intact, without polymer deposition. Dissolving MNs may be better placed for vaccine delivery, as their infrequent use reduces the issue of repeated polymer deposition within the skin.
In vivo studies revealed that repeat application of both dissolving (once daily for 5 weeks) and hydrogel-forming (twice daily for 3 weeks) MNs did not alter skin appearance or barrier function and caused no measurable disturbance of serum biomarkers of infection, inflammation or immunity (132). More recently, the clinical impact of repeated application of hydrogel-forming MN arrays was assessed by Al-Kasasbeh et al. (2020).The authors of this study repeatedly applied a hydrogel-forming MN array to the upper arm of human volunteers over a 5 day period. Safety of repeated MN application was assessed by measuring skin barrier integrity and the presence of systemic inflammatory biomarkers (C-reactive protein, interleukin 1-β, tumour necrosis factor-α, immunoglobulin G and immunoglobulin E) in blood. The results demonstrated that repeat hydrogel-forming MN application does not lead to prolonged skin reactions or prolonged disruption of skin barrier function.
Furthermore, concentrations of systemic inflammation biomarkers were all found to be within the normal range (133). It appears as though MNs may cause adverse events (such as skin irritation and intradermal granulomas) only when used inappropriately, i.e., when used in combination with cosmetic products that were not intended for application to MN-punctured skin (134,135).
However the question still remains, could repeated delivery of protein, peptide and antibody based therapies eventually result in an immune response from the host? Proteins and peptides present in infusions may trigger an immune response if the body recognises them as antigens (12,13), and the same may be the case for MN mediated delivery. Although biological therapies are typically designed to be non-immunogenic via “humanisation”, delivery via the dermal route allows the drug to come into contact with a wealth of immune cells involved in the skin’s innate immune response, namely Langerhans cells in the epidermis and dendritic cells in the dermis (136,137). These immune cells are present in much higher concentrations than those in subcutaneous tissue or muscle, the traditional target of protein based drugs. One study focused on the delivery of ovalbumin-loaded PLGA nanoparticles via electrohydrodynamic coating of MNs (138). In addition to showing extended release of ovalbumin over 28 days, the study also explored possible immunogenic effects of delivery of ovalbumin into dermal tissue. The coated MNs resulted in no significant increase in anti-OVA-specific IgG titres in C57BL/6 mice in vivo as compared to the untreated mice (p > 0.05), indicating that the formulations are nonimmunogenic.
Whilst it seems unwanted immunogenic effects from protein and peptide drugs delivered via MNs are unlikely, long-term studies exploring the immune response following repeated application of MNs containing biological drugs are required before clinical acceptance can be assured, and will likely have to be shown on a drug-by-drug basis.
Patient/Prescriber Acceptability
The success of MN based products is also dependent on the acceptability to patients and healthcare professionals. A prescriber must be willing to prescribe the product, and patients must accept the product and be able to apply the MN array correctly. Patient benefits, including reduced pain, blood, and needle stick injuries, increased acceptability by people with needle phobia and the potential for self-administration were the most important factors influencing the opinion of MNs in various groups such as in children and the elderly (66,139,140). Mild erythema post MN removal may be a concern for some patients, however barrier function will recover within hours and skin reddening will be transient (56). It is imperative for prescribers to properly educate users of MN devices, to prevent effects such as mild erythema from reducing patient compliance.
Appropriate MN application is of particular importance for cases such as global pandemics or bioterrorism incidents, where necessary treatment may be dependent on the ability to self-apply the device. Previous studies have shown that patients can successfully apply MNs to their own skin following instruction provided by pharmacist counselling in conjunction with a patient information leaflet (68). Furthermore, a “dosing indicator” has been developed to assure patients that application was successful (141). This is alongside appropriate instructions (68,142). This may be of particular use in the elderly, where declining motor function and manual dexterity may be an issue (143).
Qualitative studies demonstrate the complex and multifaceted nature of end-user acceptance. Thus, such studies will undoubtedly aid industry in taking the necessary action to address concerns and develop informative labelling and patient counselling strategies to ensure safe and effective use of MN devices. Marketing strategies will also be vital in achieving maximum market share relative to existing and widely accepted conventional delivery systems.
Regulatory Authority Acceptability
The likely considerations and potential requirements from a regulatory standpoint that must be addressed for MNs to be accepted for clinical use are summarised in Table II. One of the greatest concerns moving forward is whether MNs will be accepted as a drug delivery system, consumer product, or medical device. If MNs are to be considered closer to a traditional hypodermic injection than a transdermal patch, regulatory authorities are likely to request that the device is rendered sterile prior to use. Aseptic manufacture will be expensive and will present practical challenges if large-scale manufacturing is required. Furthermore, gamma irradiation, moist heat or microwave heating may damage the MNs or biomolecule cargoes, contaminating the delivery system. For example, McCrudden et al. (126) found that gamma radiation significantly reduced drug content (ovalbumin and ibuprofen) in dissolving MNs and the lyophilised wafer-type drug reservoirs, although the hydrogel-forming MNs (without drug) were unaffected (144). As equivalent manufacturing techniques are not currently available, any manufacturer wishing to develop MN products will need to make a substantial initial capital investment. Specifically for protein, peptide and antibody based therapies, stability of the formulation and potential immunological effects will be of particular concern.
Table II.
The likely considerations and potential requirements from a regulatory body that must be addressed for MNs to be accepted for clinical use. Replicated with permission from (131)
| Sterility of the MN dosage form |
MNs penetrate the skin surface rather than adhering to it as would a traditional transdermal patch MNs may be required to be rendered sterile depending on regulatory considerations A low bioburden may be sufficient if the system has inherent and demonstrable antimicrobial activity |
| Uniformity of content |
Either from the system as a whole, or potentially of individual drug loaded MNs within an array, depending on the system design Likely required as is the case with all other conventional transdermal patch dosage forms |
| Packaging |
Security of packaging, i.e., protection from water ingress Ease of removal from packaging by patients without accidental piercing of the skin prior to intended application |
| Potential for MN re-use |
Certain MN devices may be removed intact from the skin with the potential to re-pierce the skin e.g. silicon MNs Dissolving or hydrogel-forming MNs will likely be preferred as they are self-disabling |
| Disposal procedures |
MN materials that are not dissolvable or biodegradable may be a hazard Environmental aspects of disposal must be considered |
| Deposition of MN material into skin |
Of particular concern with dissolving MNs and those devices which would be used for chronic conditions Product may require alternating application site Potential for short term adverse effects, such as granuloma formation or local erythema, must be stated |
| Ease and reliability of MN application | Patients must be able to use the product properly, without significant inconvenience |
| Assurance of MN insertion |
Indication of correct application and delivery (particularly for vaccination applications) may be required Would be useful to assure patients they have applied the device correctly |
| Potential immunological effects |
Repeated insult of the skin, an immunologically active site, by MNs may result in an immunological reaction Assurances regarding immunological safety will be required |
These issues are somewhat intensified when considering the need for scale up manufacture of MNs, as the large scale requires further investment to overcome issues associate with formulating biologics alongside, and within, MNs. Laboratory-based processes are often difficult to scale-up initially, with problems of cost-efficiency of mass manufacture and turnaround time (145). Turnaround time will be dictated by Good Manufacturing Practice (GMP), Quality Assurance (QA) and Quality Control (QC) guidelines – the more stringent, the longer the turnaround time. These guidelines will also be influenced by the classification of MNs, discussed above. Rapid turnaround of MNs containing biologics, particularly vaccines, may be required during a pandemic, like severe acute respiratory syndrome (SARS) in 2003, the influenza H1N1 outbreak in 2009, and the coronavirus Covid-19 pandemic in 2020.
QC tests differ from those that are presented in Table II as they refer to those tests that might be performed during the manufacture of either the drug substance or drug product (146). Furthermore, QC additionally refers to the organisation, documentation and release procedures designed to ensure that the necessary and relevant tests are carried out, and that materials are not released for use, nor products released for sale or supply, until their quality has been judged satisfactory (145). However, without an understanding of the classification and acceptance criteria of MN arrays (speculated in Table II), one can only speculate on the QC tests required.
One must ask the question therefore: what are the basic requirements of MNs? The answer to this question dictates the acceptance criteria, and thus the QC tests which must be undertaken to assure the manufacturers that the MNs are meeting this basic requirement. MNs must adequately pierce the skin, penetrate, and be able to be removed intact. Where dissolving MNs are used, they must be able to sufficiently release their drug cargo within a reasonable period. Hollow MNs must remain “open” for the duration of drug delivery, and hydrogel-forming MNs must swell appropriately for delivery of the drug through the associated drug cargo. The MNs must not harm the patient. Lutton et al. (145) demonstrated that MNs fall within the scope of the International Conference of Harmonisation (ICH) Q6A guidelines (146) and used these criteria to set out a list of quality specifications applicable for all MNs. Therefore, QC tests that are likely to be performed on MNs during the manufacturing process include dissolution, disintegration, friability, uniformity of dosage, stability, water content where appropriate, microbial limits, sterility (if required by the manufacturer), particulate matter, antimicrobial preservative content, extractables, functionality of delivery system, and osmolarity. Furthermore, mechanical testing of MNs will be required – they must be hard enough to pierce the skin, but without being brittle, so as not to fracture, leaving the needle within the skin. Such tests include force of MN insertion/bending/fracture (i.e. axial, transvers, fracture force); insertion tests to ensure patients can manually insert the needles successfully, given that patients may apply needles over a range of forces and they cannot “calibrate” their application force; confirmation of MN insertion (i.e. via OCT (147)); and tests to ensure skin barrier function returns to baseline rapidly following MN removal (i.e. TEWL/TEER). Not only are such tests required, but a reasonable range of expected analytical and manufacturing variability must also be considered where appropriate. Even with these tests in place, further questions remain, such as, what is an appropriate model membrane to test for sufficient MN insertion? Skin cannot be used for QC testing, therefore any model membrane must be able to replicate the skin structure, which is challenging given that skin is not homogeneous.
Despite the ongoing questions surrounding MN manufacture and acceptance, it is interesting to note that the US FDA recently published draft guidance on “microneedling” for cosmetic applications (148), and PATH recently released a fact sheet illustrating a four-year initiative for accelerating the development of MNs for drug delivery and vaccines (149). Thus, the interest of regulators in the technology is clearly presented.
Conclusion
To conclude, numerous studies have illustrated the ability to deliver therapeutic doses of protein, peptide and antibody based therapies using MNs as an alternative drug delivery device. The advantages of MNs over traditional routes of drug delivery are apparent, and thus, it appears likely that MNs will be used as a drug delivery device within the next ten years. The use of these devices could vastly improve the quality of life for patients, improve public health, and increase the economic productivity of developing countries.
Future success of MNs will be contingent on their long-term safety profile, methods of manufacture, and their ability to comply with standardised GMP guidelines. Furthermore, marketing strategies will also be vital in achieving maximum market share relative to existing and widely accepted conventional delivery systems. In the meantime, academia and industry must work together to address concerns, and thereby push MN technology into the clinic, where its potential can be truly realised.
Expert Opinion
At present, MNs are primarily viewed as vaccine delivery systems for the developing world despite a plethora of published articles demonstrating their ability to deliver of a wide range of therapeutic molecules. Consequently, many pharmaceutical companies are unwilling to make investments in the field. For this reason, it is vitally important to change this mind set by proving that MNs offer far more than acting as simple vaccine delivery devices. Indeed, MNs have made important advancements in removing the need for needle and syringe in the treatment of diseases such as HIV, diabetes, Alzheimer’s and cancer (85,88,111,150,151). However, translating these findings from bench-top to bedside is proving extremely challenging. More recently, MNs have been tested for diagnostic fluid sampling (152,153). Evidently, this field has huge potential, with the possibility of MN sampling devices being delivered to patients’ homes and returned to the laboratory for analysis, without the patient having to enter a clinical setting. Perhaps, with further optimisation, a MN sampling device could be used in viral testing and, as a result, prove instrumental in the fight against future pandemics. As this MN design could be CE marked as a medical device rather than a drug product, reduced regulatory requirements could mean that pharmaceutical companies may be willing to first invest in a device such as this. Importantly, the initial upfront costs of scaled up manufacture could be offset by achieving faster market commercialisation. As a result, the infrastructure would be in place for the manufacture of drug containing MNs in the future.
Moving forward, pharmaceutical companies must view drug containing MNs as commercially viable. One way to achieve this is through focussed collaboration between academia, industry, healthcare professionals and patients. In particular, it is vitally important that the views and opinions of patients are respected and considered fully, as many products have failed in the past as they have forgotten about the end user. In this regard, MNs have shown to help overcome needle phobia through painless application, thus improving patient compliance (65,66). As a result, MN-mediated administration has the ability to reduce the frequency of repeat hospitalisation through minimising the number of missed doses. Furthermore, dissolving and hydrogel-forming MNs are designed to prevent needle re-use, to reduce the likelihood of needle-stick injury and to remove the need for dedicated sharps disposal. Therefore, the substantial benefits to both the patient and health care sector could outweigh the initial scale-up costs incurred by the industry. To this end, Zosano Pharma have developed a zolmitriptan based intracutaneous MN system for the treatment of migraine headaches. This MN device successfully produced a therapeutic effect in clinical studies and as result, is expected to receive FDA approval in 2021. As the first drug containing MN product to potentially achieve commercialisation, it appears many pharmaceutical companies are willing to delay MN development until its success becomes apparent. However, it is anticipated that this novel device will offer additional benefits to both patients and healthcare providers, thus opening the door for the next generation of transdermal delivery systems.
ACKNOWLEDGMENTS AND DISCLOSURES
Aaron R.J. Hutton is a PhD candidate funded by Department for the Economy (N. Ireland) studentship. Additionally, the authors would like to express their sincere gratitude to William Kerr for his assistance with the creation of Fig. I.
Abbreviations
- MN
Microneedle
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morales JO, Fathe KR, Li S, Montenegro-Nicolini M, Mousavikhamene Z, McConville JT, Prausnitz MR, Smyth HDC. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017;19:652–668. doi: 10.1208/s12248-017-0054-z. [DOI] [PubMed] [Google Scholar]
- 2.Pavlou AK, Reichert JM. Recombinant protein therapeutics - success rates, market trends and values to 2010. Nat Biotechnol. 2004;22:1513–1519. doi: 10.1038/nbt1204-1513. [DOI] [PubMed] [Google Scholar]
- 3.Tan ML, Choong PFM, Dass CR. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides. 2010;31:184–193. doi: 10.1016/j.peptides.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Brown LR. Commercial challenges of protein drug delivery. Expert Opin Drug Deliv. 2005;2:29–142. doi: 10.1517/17425247.2.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Jiskoot W, Randolph TW, Volkin DB, Russell Middaugh C, Schöneich C, Winter G, Friess W, Crommelin DJA, Carpenter JF. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery Systems for Sustained Release. J Pharm Sci. 2012;101:946–954. doi: 10.1002/jps.23018. [DOI] [PubMed] [Google Scholar]
- 6.Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Wu F, Yang S, Yuan W, Jin T. Challenges and strategies in developing microneedle patches for transdermal delivery of protein and peptide therapeutics. Curr Pharm Biotechnol. 2012;13:1292–1298. doi: 10.2174/138920112800624319. [DOI] [PubMed] [Google Scholar]
- 8.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006;11:905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Bashyal S, Noh G, Keum T, Choi YW, Lee S. Cell penetrating peptides as an innovative approach for drug delivery; then, present and the future. J Pharm Investig. 2016;46:205–220. [Google Scholar]
- 10.Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J Control Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31:53–59. doi: 10.1016/j.tips.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jani P, Manseta P, Patel S. Pharmaceutical approaches related to systemic delivery of protein and peptide drugs: an overview. Int J Pharm Sci Rev Res. 2011;12:42–52. [Google Scholar]
- 14.Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–430. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Hussain A, Arnold JJ, Khan MA, Ahsan F. Absorption enhancers in pulmonary protein delivery. J Control Release. 2004;94:15–24. doi: 10.1016/j.jconrel.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Rahhal TB, Fromen CA, Wilson EM, Kai MP, Shen TW, Luft JC, DeSimone JM. Pulmonary delivery of Butyrylcholinesterase as a model protein to the lung. Mol Pharm. 2016;13:1626–1635. doi: 10.1021/acs.molpharmaceut.6b00066. [DOI] [PubMed] [Google Scholar]
- 17.Vyas SP, Paliwal R, Paliwal SR. Ocular delivery of peptides and proteins. Boston: Academic Press; 2011. Ocular Delivery of Peptides and Proteins. [Google Scholar]
- 18.Mandal A, Pal D, Agrahari V, Trinh HM, Joseph M, Mitra AK. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95. doi: 10.1016/j.addr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahlumba P, Choonara Y, Kumar P, du Toit L, Pillay V. Stimuli-responsive polymeric Systems for Controlled Protein and Peptide Delivery: future implications for ocular delivery. Molecules. 2016;21:1002. doi: 10.3390/molecules21081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Hu L, Yang G, Hu Q. Current therapeutic strategy in the nasal delivery of insulin: recent advances and future directions. Curr Pharm Biotechnol. 2018;19:400–415. doi: 10.2174/1389201019666180619145429. [DOI] [PubMed] [Google Scholar]
- 21.Zheng C, Guo Q, Wu Z, Sun L, Zhang Z, Li C, Zhang X. Amphiphilic glycopolymer nanoparticles as vehicles for nasal delivery of peptides and proteins. Eur J Pharm Sci. 2013;49:474–482. doi: 10.1016/j.ejps.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 22.du Plessis LH, Kotzé AF, Junginger HE. Nasal and rectal delivery of insulin with chitosan and N-trimethyl chitosan chloride. Drug Deliv. 2010;17:399–407. doi: 10.3109/10717541003762888. [DOI] [PubMed] [Google Scholar]
- 23.de Boer AG, van Hoogdalem EJ, Heijligers-Feijen CD, Verhoef J, Breimer DD. Rectal absorption enhancement of peptide drugs. J Control Release. 1990;13:241–246. [Google Scholar]
- 24.de Boer AG, Breimer DD, Pronk J, Gubbens-Stibbe JM. Rectal bioavailability of lidocaine in rats: absence of significant first-pass elimination. J Pharm Sci. 1980;69:804–807. doi: 10.1002/jps.2600690716. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, Liu S, Zhang M, Wen L-P. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 26.Schuetz YB, Naik A, Guy RH, Kalia YN. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2005;2:533–548. doi: 10.1517/17425247.2.3.533. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A, Ibsen K, Iwao Y, Zakrewsky M, Mitragotri S. Transdermal protein delivery using choline and Geranate (CAGE) deep eutectic solvent. Adv Healthc Mater. 2017;6:1601411. doi: 10.1002/adhm.201601411. [DOI] [PubMed] [Google Scholar]
- 28.Ibraheem D, Elaissari A, Fessi H. Administration strategies for proteins and peptides. Int J Pharm. 2014;477:578–589. doi: 10.1016/j.ijpharm.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 29.Gerstel MS, Place VA. Drug delivery device. US3964482A, 1971.
- 30.Donnelly RF, Singh TRR, Morrow DIJ, Woolfson DA. Microneedle-mediated transdermal and intradermal drug delivery. John Wiley & Sons, Ltd: Sussex; 2012. [Google Scholar]
- 31.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly RF, McCrudden MTC, Alkilani AZ, Larrañeta E, McAlister E, Courtenay AJ, Kearney M-C, Singh TRR, McCarthy HO, Kett VL, Caffarel-Salvador E, Al-Zahrani S, Woolfson AD. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PLoS One. 2014;9:e11154. doi: 10.1371/journal.pone.0111547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368:109–115. doi: 10.1016/j.ijpharm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Lee K, Lee CY, Jung H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials. 2011;32:3134–3140. doi: 10.1016/j.biomaterials.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25:104–113. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly RF, Morrow DIJ, Singh TRR, Migalska K, McCarron PA, O’Mahony C, Woolfson AD. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm. 2009;35:1242–1254. doi: 10.1080/03639040902882280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55:1063–1071. doi: 10.1109/TBME.2007.906492. [DOI] [PubMed] [Google Scholar]
- 38.Gardeniers HJGE, Luttge R, Berenschot EJW, De Boer MJ, Yeshurun SY, Hefetz M, van’t Oever R, van der Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12:855–862. [Google Scholar]
- 39.Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112:357–361. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Griss P, Stemme G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst. 2003;12:296–301. [Google Scholar]
- 41.Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly RF, Singh TRR, Garland MJ, Migalska K, Majithiya R, McCrudden CM, Kole PL, Mahmood TMT, McCarthy HO, Woolfson AD. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly RF, McCarron P, Morrow D, Morrissey A, Woolfson D. Microneedles/Delivery Device and Method. WO2009040548, 2007.
- 44.Hong X, Wu Z, Chen L, Wu F, Wei L, Yuan W. Hydrogel microneedle arrays for transdermal drug delivery. Nano-Micro Lett. 2014;6:191–199. [Google Scholar]
- 45.Yang S, Feng Y, Zhang L, Chen N, Yuan W, Jin T. A scalable fabrication process of polymer microneedles. Int J Nanomedicine. 2012;7:1415–1422. doi: 10.2147/IJN.S28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy JG, Larrañeta E, Donnelly RF, McGoldrick N, Migalska K, McCrudden MTC, Irwin NJ, Donnelly L. McCoy CP. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol Pharm 2016;13:907–914. [DOI] [PubMed]
- 47.Hashmi S, Ling P, Hashmi G, Reed M, Gaugler R, Trimmer W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotechniques. 1995;19:766–770. [PubMed] [Google Scholar]
- 48.Verbaan FJ, Bal SM, van den Berg DJ, Groenink WHH, Verpoorten H, Lüttge R, Bouwstra JA. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117:238–245. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, Omenetto FG. Fabrication of silk microneedles for controlled-release drug delivery. Adv Funct Mater. 2012;22:330–335. [Google Scholar]
- 50.McAllister DV, Wang PM, Davis SP, Park J-HJ-H, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 53.Paul O, Gaspar J, Ruther P. Advanced silicon microstructures, sensors, and systems. IEEJ Trans Electr Electron Eng. 2007;2:199–215. [Google Scholar]
- 54.Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Reports. 2016;104:1–32. [Google Scholar]
- 55.Indermun S, Luttge R, Choonara YE, Kumar P, Du Toit LC, Modi G, Pillay V. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 56.Chow AY, Pardue MT, Chow VY, Peyman GA, Liang C, Perlman JI, Peachey NS. Implantation of silicon chip microphotodiode arrays into the cat subretinal space. IEEE Trans Neural Syst Rehabil Eng. 2001;9:86–95. doi: 10.1109/7333.918281. [DOI] [PubMed] [Google Scholar]
- 57.Voskerician G, Shive MS, Shawgo RS, Von Recum H, Anderson JM, Cima MJ, Langer R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials. 2003;24:1959–1967. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 58.Chen B, Wei J, Tay FEH, Wong YT, Iliescu C. Silicon microneedle array with biodegradable tips for transdermal drug delivery. Microsyst Technol. 2008;14:1015–1019. [Google Scholar]
- 59.Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O’Neal JM, Prausnitz MR. Microinfusion using hollow microneedles. Pharm Res. 2006;23:104–113. doi: 10.1007/s11095-005-8498-8. [DOI] [PubMed] [Google Scholar]
- 60.Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17:187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cahill EM, Keaveney S, Stuettgen V, Eberts P, Ramos-Luna P, Zhang N, Dangol M, O'Cearbhaill ED. Metallic microneedles with interconnected porosity: a scalable platform for biosensing and drug delivery. Acta Biomater. 2018;80:401–411. doi: 10.1016/j.actbio.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. Microelectron Eng. 2011;88:1681–1684. [Google Scholar]
- 63.Cai B, Xia W, Bredenberg S, Engqvist H. Self-setting bioceramic microscopic protrusions for transdermal drug delivery. J Mater Chem B. 2014;2:5992–5998. doi: 10.1039/c4tb00764f. [DOI] [PubMed] [Google Scholar]
- 64.Pignatello R. Biomaterials: applications for Nanomedicine. InTech: Rijeka; 2011. [Google Scholar]
- 65.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice - an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28:95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 67.Vicente-Pérez EM, Quinn HL, McAlister E, O’Neill S, Hanna L-A, Barry JG, Donnelly RF. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm Res. 2016;33:1–10. doi: 10.1007/s11095-016-2032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donnelly RF, Moffatt K, Alkilani AZ, Vicente-Pérez EM, Barry J, McCrudden MTC, Woolfson AD. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study Centred on pharmacist intervention and a patient information leaflet. Pharm Res. 2014;31:1989–1999. doi: 10.1007/s11095-014-1301-y. [DOI] [PubMed] [Google Scholar]
- 69.Mistilis MJ, Joyce JC, Esser ES, Skountzou I, Compans RW, Bommarius AS, Prausnitz MR. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 2017;7:195–205. doi: 10.1007/s13346-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. J Pharm Sci. 2015;104:740–749. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21:947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 72.Wei-Ze L, Mei-Rong H, Jian-Ping Z, Yong-Qiang Z, Bao-Hua H, Ting L, Yong Z. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389:122–129. doi: 10.1016/j.ijpharm.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 73.Stahl J, Wohlert M, Kietzmann M. Microneedle pretreatment enhances the percutaneous permeation of hydrophilic compounds with high melting points. BMC Pharmacol Toxicol. 2012;13:1–7. doi: 10.1186/2050-6511-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tas C, Mansoor S, Kalluri H, Zarnitsyn VG, Choi S-O, Banga AK, Prausnitz MR. Delivery of salmon calcitonin using a microneedle patch. Int J Pharm. 2012;423:257–263. doi: 10.1016/j.ijpharm.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Lee HS, Ryu HR, Roh JY, Park JH. Bleomycin-coated microneedles for treatment of warts. Pharm Res. 2017;34:101–112. doi: 10.1007/s11095-016-2042-x. [DOI] [PubMed] [Google Scholar]
- 78.Baek SH, Shin JH, Kim YC. Drug-coated microneedles for rapid and painless local anesthesia. Biomed Microdevices. 2017;19:1–11. doi: 10.1007/s10544-016-0144-1. [DOI] [PubMed] [Google Scholar]
- 79.Garland MJ, Caffarel-Salvador E, Migalska K, Woolfson AD, Donnelly RF. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J Control Release. 2012;159:52–59. doi: 10.1016/j.jconrel.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pamornpathomkul B, Ngawhirunpat T, Tekko IA, Vora L, McCarthy HO, Donnelly RF. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur J Pharm Sci. 2018;121:200–209. doi: 10.1016/j.ejps.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Ito Y, Murakami A, Maeda T, Sugioka N, Takada K. Evaluation of self-dissolving needles containing low molecular weight heparin (LMWH) in rats. Int J Pharm. 2008;349:124–129. doi: 10.1016/j.ijpharm.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 82.Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 83.Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11:329–337. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caffarel-Salvador E, Brady AJ, Eltayib E, Meng T, Alonso-Vicente A, Gonzalez-Vazquez P, Torrisi BM, Vincente-Perez EM, Mooney K, Jones DS, Bell SEJ, McCoy CP, McCarthy HO, McElnay JC, Donnelly RF. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS One. 2015;10:1–21. doi: 10.1371/journal.pone.0145644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kearney MC, Caffarel-Salvador E, Fallows SJ, McCarthy HO, Donnelly RF. Microneedle-mediated delivery of donepezil: potential for improved treatment options in Alzheimer’s disease. Eur J Pharm Biopharm. 2016;103:43–50. doi: 10.1016/j.ejpb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 86.Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MTC, Kearney MC, McAlister E, McCarthy HO, Donnelly RF. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018;285:142–151. doi: 10.1016/j.jconrel.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Courtenay AJ, Rodgers AM, McCrudden MTC, McCarthy HO, Donnelly RF. Novel hydrogel-forming microneedle Array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol Pharm. 2019;16:118–127. doi: 10.1021/acs.molpharmaceut.8b00895. [DOI] [PubMed] [Google Scholar]
- 88.Courtenay AJ, McCrudden MTC, McAvoy KJ, McCarthy HO, Donnelly RF. Microneedle-mediated transdermal delivery of Bevacizumab. Mol Pharm. 2018;15:3545–3556. doi: 10.1021/acs.molpharmaceut.8b00544. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organisation. Diabetes.; 2020. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1.
- 90.Zhou CP, Liu YL, Wang HL, Zhang PX, Zhang JL. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;392:127–133. doi: 10.1016/j.ijpharm.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 91.Li QY, Zhang JN, Chen BZ, Wang QL, Guo XD. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7:15408–15415. [Google Scholar]
- 92.Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques. J Pharm Sci. 2010;99:1931–1941. doi: 10.1002/jps.21981. [DOI] [PubMed] [Google Scholar]
- 93.Cui KA, Cui LX, Sandoval MA, Rodriguez LB, Sloat BR, Cui Z. Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomedicine. 2011;6:1253–1264. doi: 10.2147/IJN.S20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han T, Das DB. Permeability enhancement for transdermal delivery of large molecule using low-frequency sonophoresis combined with microneedles. J Pharm Sci. 2013;102:3614–3622. doi: 10.1002/jps.23662. [DOI] [PubMed] [Google Scholar]
- 95.Zhang S, Qiu Y, Gao Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm Sin B. 2014;4:100–104. doi: 10.1016/j.apsb.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vrdoljak A, McGrath MG, Carey JB, Draper SJ, Hill AVS, O’Mahony C, Crean AM, Moore AC. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release. 2012;159:34–42. doi: 10.1016/j.jconrel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing Langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976;25:137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- 98.Saurer EM, Flessner RM, Sullivan SP, Prausnitz MR, Lynn DM. Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules. 2010;11:3136–3143. doi: 10.1021/bm1009443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao X, Coulman SA, Hanna SJ, Wong FS, Dayan CM, Birchall JC. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release. 2017;265:2–13. doi: 10.1016/j.jconrel.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 100.Caudill CL, Perry JL, Tian S, Luft JC, DeSimone JM. Spatially controlled coating of continuous liquid Interface production microneedles for transdermal protein delivery. J Control Release. 2018;284:122–132. doi: 10.1016/j.jconrel.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 101.Li S, Li W, Prausnitz M. Individually coated microneedles for co-delivery of multiple compounds with different properties. Drug Deliv Transl Res. 2018;8:1043–1052. doi: 10.1007/s13346-018-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leone M, Mönkäre J, Bouwstra JA, Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34:2223–2240. doi: 10.1007/s11095-017-2223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leone M, Priester MI, Romeijn S, Nejadnik MR, Mönkäre J, O’Mahony C, Jiskoot W, Kersten G, Bouwstra JA. Hyaluronan-based dissolving microneedles with high antigen content for intradermal vaccination: formulation, physicochemical characterization and immunogenicity assessment. Eur J Pharm Biopharm. 2019;134:49–59. doi: 10.1016/j.ejpb.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Liu S, Zhang S, Duan Y, Niu Y, Gu H, Zhao Z, Zhang S, Yang Y, Wang X, Gao Y, Yang P. Transcutaneous immunization of recombinant staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine. 2019;37:3810–3819. doi: 10.1016/j.vaccine.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 105.Mönkäre J, Reza Nejadnik M, Baccouche K, Romeijn S, Jiskoot W, Bouwstra JA. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J Control Release. 2015;218:53–62. doi: 10.1016/j.jconrel.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Chen J, Qiu Y, Zhang S, Gao Y. Dissolving microneedle-based intradermal delivery of interferon-α-2b. Drug Dev Ind Pharm. 2016;42:890–896. doi: 10.3109/03639045.2015.1096282. [DOI] [PubMed] [Google Scholar]
- 107.Dillon C, Hughes H, O’Reilly NJ, McLoughlin P. Formulation and characterisation of dissolving microneedles for the transdermal delivery of therapeutic peptides. Int J Pharm. 2017;526:125–136. doi: 10.1016/j.ijpharm.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 108.Liu D, Yu B, Jiang G, Yu W, Zhang Y, Xu B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater Sci Eng C. 2018;90:180–188. doi: 10.1016/j.msec.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 109.Lahiji SF, Jang Y, Huh I, Yang H, Jang M, Jung H. Exendin-4-encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci Rep. 2018;8:1170. doi: 10.1038/s41598-018-19789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lahiji S, Jang Y, Ma Y, Dangol M, Yang H, Jang M, Jung H. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme. Eur J Pharm Sci. 2018;117:290–296. doi: 10.1016/j.ejps.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Vora LK, Courtenay AJ, Tekko IA, Larrañeta E, Donnelly RF. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–298. doi: 10.1016/j.ijbiomac.2019.12.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tuan-Mahmood TM, McCrudden MTC, Torrisi BM, McAlister E, Garland MJ, Singh TRR, Donnelly RF. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50:623–637. doi: 10.1016/j.ejps.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Groot AM, Du G, Mönkäre J, Platteel ACM, Broere F, Bouwstra JA, Sijts AJAM. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J Control Release. 2017;266:27–35. doi: 10.1016/j.jconrel.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, Koster AJ, Slütter B, Kros A, Jiskoot W, Bouwstra JA, Mönkäre J. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 115.Mönkäre J, Pontier M, van Kampen EEM, Du G, Leone M, Romeijn S, Nejadnik MR, O'Mahony C, Slütter B, Jiskoot W, Bouwstra JA. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm. 2018;129:111–121. doi: 10.1016/j.ejpb.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 116.Chen B, Wei J, Iliescu C. Sonophoretic enhanced microneedles array (SEMA)-improving the efficiency of transdermal drug delivery. Sensors Actuators B Chem. 2010;145:54–60. [Google Scholar]
- 117.Torrisi BM, Zarnitsyn V, Prausnitz MR, Anstey A, Gateley C, Birchall JC, Coulman SA. Pocketed microneedles for rapid delivery of a liquid-state botulinum toxin a formulation into human skin. J Control Release. 2013;165:146–152. doi: 10.1016/j.jconrel.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Golombek S, Pilz M, Steinle H, Kochba E, Levin Y, Lunter D, Schlensak C, Wendel HP, Avci-Adali M. Intradermal delivery of synthetic mRNA using hollow microneedles for efficient and rapid production of exogenous proteins in skin. Mol Ther - Nucleic Acids. 2018;11:382–392. doi: 10.1016/j.omtn.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh TRR, Woolfson AD, Donnelly RF. Investigation of solute permeation across hydrogels composed of poly(methyl vinyl ether- co -maleic acid) and poly(ethylene glycol) J Pharm Pharmacol. 2010;62:829–837. doi: 10.1211/jpp.62.06.0003. [DOI] [PubMed] [Google Scholar]
- 120.Garland MJ, Singh TRR, Woolfson AD, Donnelly RF. Electrically enhanced solute permeation across poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: effect of hydrogel crosslink density and ionic conductivity. Int J Pharm. 2011;406:91–98. doi: 10.1016/j.ijpharm.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 121.Wong R, Ashton M, Dodou K. Effect of crosslinking agent concentration on the properties of Unmedicated hydrogels. Pharmaceutics. 2015;7:305–319. doi: 10.3390/pharmaceutics7030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh TRR, Garland MJ, Migalska K, Salvador EC, Shaikh R, McCarthy HO, Woolfson AD, Donnelly RF. Influence of a pore-forming agent on swelling, network parameters, and permeability of poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: application in transdermal delivery systems. J Appl Polym Sci. 2012;125:2680–2694. [Google Scholar]
- 123.Seong KY, Seo MS, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, Yang SY. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 124.Technavio. Transdermal Drug Delivery Market .; 2019. Available from: https://www.technavio.com/report/transdermal-drug-delivery-market-industry-analysis?tnplus.
- 125.Mordor Intelligence. Biopharmaceuticals Market | Analysis | Overview (2019–2024).,2019. Available from: https://www.mordorintelligence.com/industry-reports/global-biopharmaceuticals-market-industry.
- 126.McCrudden MTC, Alkilani AZ, McCrudden CM, McAlister E, McCarthy HO, Woolfson AD, Donnelly RF. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J Control Release. 2014;180:71–80. doi: 10.1016/j.jconrel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Migalska K, Morrow DIJ, Garland MJ, Thakur R, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28:1919–1930. doi: 10.1007/s11095-011-0419-4. [DOI] [PubMed] [Google Scholar]
- 128.McCrudden MTC, Singh TRR, Migalska K, Donnelly RF. Strategies for enhanced peptide and protein delivery. Ther Deliv. 2013;4:593–614. doi: 10.4155/tde.13.31. [DOI] [PubMed] [Google Scholar]
- 129.Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26:2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Donnelly RF, Singh TRR, Alkilani AZ, McCrudden MTC, O’Neill S, O’Mahony C, Armstrong K, McLoone N, Kole P, Woolfson AD. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451:76–91. doi: 10.1016/j.ijpharm.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Donnelly RF. Clinical translation and industrial development of microneedle-based products. In: Donnelly RF, Singh TRR, editors. Microneedles Drug Vaccine Deliv. Patient Monit. 1st ed., Chichester: John Wiley and Sons; 2018, p. 307–322.
- 132.Vicente-Perez EM, Larrañeta E, McCrudden MTC, Kissenpfennig A, Hegarty S, McCarthy HO, Donnelly RF. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur J Pharm Biopharm. 2017;117:400–407. doi: 10.1016/j.ejpb.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Kasasbeh R, Brady AJ, Courtenay AJ, Larrañeta E, McCrudden MTC, O’Kane D, et al. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv Transl Res. 2020:1–16. [DOI] [PMC free article] [PubMed]
- 134.Soltani-Arabshahi R, Wong JW, Duffy KL, Powell DL. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatology. 2014;150:68–72. doi: 10.1001/jamadermatol.2013.6955. [DOI] [PubMed] [Google Scholar]
- 135.Daily Mail. Microneedle Therapy System “potentially lethal,” Chinese women warned | Daily Mail Online.; 2011. Available from: https://www.dailymail.co.uk/femail/article-2026700/Microneedle-Therapy-System-potentially-lethal-Chinese-women-warned.html.
- 136.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 137.Huang CM. Topical vaccination: the skin as a unique portal to adaptive immune responses. Semin Immunopathol. 2007;29:71–80. doi: 10.1007/s00281-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 138.Angkawinitwong U, Courtenay AJ, Rodgers AM, Larrañeta E, McCarthy HO, Brocchini S, Donnelly RF, Williams GR. A novel transdermal protein delivery strategy via Electrohydrodynamic coating of PLGA microparticles onto microneedles. ACS Appl Mater Interfaces. 2020;12:12478–12488. doi: 10.1021/acsami.9b22425. [DOI] [PubMed] [Google Scholar]
- 139.Mooney K, McElnay JC, Donnelly RF. Children’s views on microneedle use as an alternative to blood sampling for patient monitoring. Int J Pharm Pract. 2014;22:335–344. doi: 10.1111/ijpp.12081. [DOI] [PubMed] [Google Scholar]
- 140.Mooney K, McElnay JC, Donnelly RF. Paediatricians’ opinions of microneedle-mediated monitoring: a key stage in the translation of microneedle technology from laboratory into clinical practice. Drug Deliv Transl Res. 2015;5:346–359. doi: 10.1007/s13346-015-0223-5. [DOI] [PubMed] [Google Scholar]
- 141.Vicente-Pérez EM, Quinn HL, McAlister E, O’Neill S, Hanna L-A, Barry JG, Donnelly RF. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm Res. 2016;33:3072–3080. doi: 10.1007/s11095-016-2032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Quinn HL, Hughes CM, Donnelly RF. In vivo and qualitative studies investigating the translational potential of microneedles for use in the older population. Drug Deliv Transl Res. 2018;8:307–316. doi: 10.1007/s13346-017-0393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McCrudden MTC, Alkilani AZ, Courtenay AJ, McCrudden CM, McCloskey B, Walker C, Alshraiedeh N, Lutton REM, Gilmore BF, Woolfson AD, Donnelly RF. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv Transl Res. 2014;5:3–14. doi: 10.1007/s13346-014-0211-1. [DOI] [PubMed] [Google Scholar]
- 145.Lutton REM, Moore J, Larrañeta E, Ligett S, Woolfson AD, Donnelly RF. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res. 2015;5:313–331. doi: 10.1007/s13346-015-0237-z. [DOI] [PubMed] [Google Scholar]
- 146.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, “SPECIFICATIONS : TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR NEW DRUG SUBSTANCES AND NEW DRUG PRODUCTS : Q6A,” 1999.
- 147.Donnelly RF, Garland MJ, Morrow DIJ, Migalska K, Singh TRR, Majithiya R, Woolfson AD. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J Control Release. 2010;147:333–341. doi: 10.1016/j.jconrel.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 148.US Food and Drug Administration. Regulatory Considerations for Microneedling Devices: Draft Guidance for Industry and Food and Drug Administration Staff. U.S. Food & Drug Administration, Center for Devices & Radiological Health 2017.
- 149.PATH. The PATH Center of Excellence for Microarray Patch Technology.; 2019. Available from: https://www.path.org/resources/path-center-excellence-microarray-patch-technology/
- 150.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28:107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 151.Mc Crudden MTC, Larrañeta E, Clark A, Jarrahian C, Rein-Weston A, Creelman B, Moyo Y, Lachau-Durand S, Niemeijer N, Williams P, McCarthy HO, Zehrung D, Donnelly RF. Design, formulation, and evaluation of novel dissolving microarray patches containing Rilpivirine for Intravaginal delivery. Adv Healthc Mater. 2019;8:108510. doi: 10.1002/adhm.201801510. [DOI] [PubMed] [Google Scholar]
- 152.Xue P, Zhang L, Xu Z, Yan J, Gu Z, Kang Y. Blood sampling using microneedles as a minimally invasive platform for biomedical diagnostics. Appl Mater Today. 2018;13:144–157. [Google Scholar]
- 153.Samant PP, Prausnitz MR. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc Natl Acad Sci U S A. 2018;115:4583–4588. doi: 10.1073/pnas.1716772115. [DOI] [PMC free article] [PubMed] [Google Scholar]