Abstract
Stem cells are a powerful resource for many applications including regenerative medicine, patient-specific disease modeling, and toxicology screening. However, eliciting the desired behavior from stem cells, such as expansion in a naïve state or differentiation into a particular mature lineage, remains challenging. Drawing inspiration from the native stem cell niche, hydrogel platforms have been developed to regulate stem cell fate by controlling microenvironmental parameters including matrix mechanics, degradability, cell-adhesive ligand presentation, local microstructure, and cell–cell interactions. We survey techniques for modulating hydrogel properties and review the effects of microenvironmental parameters on maintaining stemness and controlling differentiation for a variety of stem cell types. Looking forward, we envision future hydrogel designs spanning a spectrum of complexity, ranging from simple, fully defined materials for industrial expansion of stem cells to complex, biomimetic systems for organotypic cell culture models.
Keywords: stem cell niche, hydrogel, engineered cellular microenvironments, matrix mechanics, cell-adhesive ligands, cell–cell interactions
1. INTRODUCTION
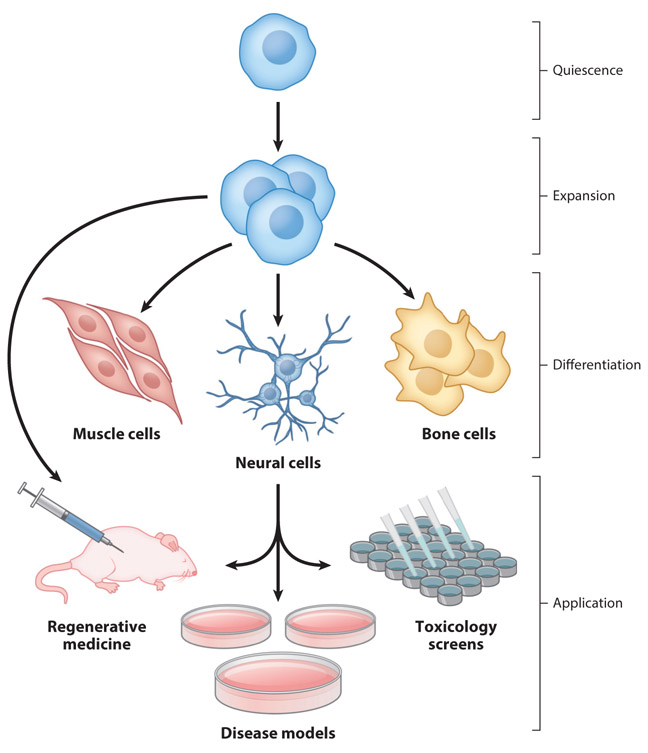
Stem cell research has sparked a revolution in the biomedical sciences, in applications ranging from regenerative medicine approaches to the repair or replacement of damaged tissue to patient-specific disease modeling and drug toxicology screening platforms (Figure 1). In all of these applications, maintaining control over the phenotype of the stem cells is paramount. Stem cells are characterized by their ability both to self-renew, generating more stem cells, and to differentiate into various mature cell types (Figure 1). The differentiation potential of these cells is dictated by the source of the cell. Embryonic stem cells (ESCs) are considered to be pluripotent, as ESCs can differentiate into mature cells from all three germ lines: ectoderm, endoderm, and mesoderm (1). Over the past decade, the advent of induced pluripotent stem cells (iPSCs) produced from terminally differentiated, patient-derived cells has dramatically expanded access to pluripotent stem cells (PSCs) and has opened the door to a myriad of personalized medicine applications (1). Other stem cell types investigated for clinical applications are somatic stem cells, such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and neural stem cells (NSCs). These somatic stem cells are considered to be multipotent, giving rise to a more restricted range of differentiated progeny than PSCs. For instance, NSCs are generally capable of differentiation into the three main neural lineages: neurons, astrocytes, and oligodendrocytes (2).
Figure 1.
Stem cell phenotypes and applications. Engineered hydrogels recapitulating aspects of the native stem cell niche can facilitate maintenance of stem cell quiescence, promote stem cell expansion, and direct stem cell differentiation. The stem cells and their differentiated progeny may be used for regenerative medicine applications, in vitro disease models, and toxicology screening.
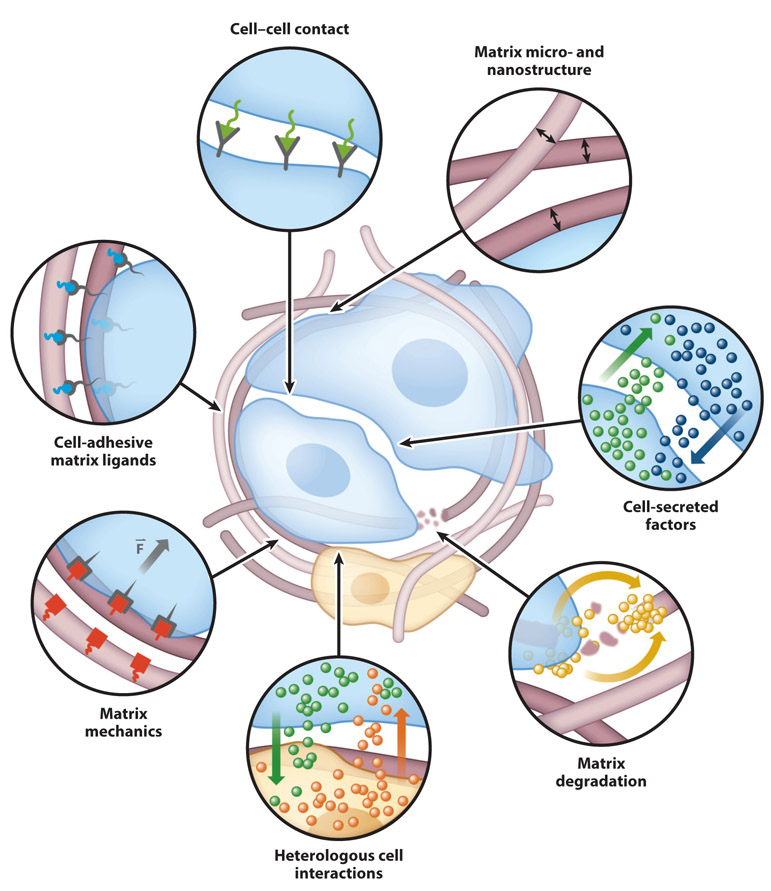
In vivo, stem cells reside in specialized microenvironments known as the stem cell niche (3, 4). The niche consists of both biophysical and biochemical factors that direct the fate of the resident stem cells. The stem cell niche is both dynamic and complex, with features on various time- and length scales that collectively affect stem cell phenotype (3, 4). Many of these cues are provided by the extracellular matrix (ECM). The biochemical composition, mechanical properties, and microstructure of the ECM are all known to modulate stem cell behavior, with optimal properties dependent on both the stem cell type of interest and the desired phenotypic output (Figure 2). In addition to matrix properties, cell–cell interactions dramatically affect the behavior of stem cells within the niche. Stem cells, their differentiated progeny, and other supporting cell types within the niche interact via secretion of soluble factors and direct cell–cell contact (Figure 2), modulating the biochemical signaling pathways that regulate maintenance of the stem cell pool and control differentiation into mature phenotypes (3, 4).
Figure 2.
Niche interactions known to modulate stem cell phenotype.
Engineering strategies to control stem cell fate can be grouped into two major categories: (a) strategies to maintain the stem cell phenotype, or “stemness,” and (b) strategies to differentiate the stem cells into desired mature cell types. Maintenance of stemness can be further subdivided into expansion of stem cells for clinical use and maintenance of stem cells in a quiescent state. Stem cell expansion is required in cases in which delivery of the naïve stem cell is required for therapeutic efficacy (e.g., HSC delivery to reconstitute a patient’s myeloid and lymphoid cells) or for further differentiation into a mature cell type that is nondividing (e.g., delivery of motor neurons derived from NSCs). Maintaining stem cell quiescence is required for long-term ex vivo culture of stem cells for disease models or drug screening. Using engineered niches to control stem cell differentiation is a popular strategy in the field of tissue engineering, with the goal of using the properties of scaffold materials to direct stem cells to mature into functional tissue constructs. In all of these cases, design principles learned from the native stem cell niche can be applied to elicit desired stem cell phenotypes.
This review focuses on the use of hydrogel biomaterials as engineered stem cell microenvironments. Hydrogels are water-swollen, cross-linked polymeric networks that can be composed of both naturally occurring and synthetic materials. The broad range of materials and processing techniques used to produce hydrogels affords tight control over many biophysical and biochemical properties including matrix mechanics, matrix degradability, cell-adhesive ligand presentation, microstructure, and costimulation with soluble factors and other cell types (5, 6). We review strategies for modulating these microenvironmental cues and discuss how these factors affect stemness maintenance and differentiation of various PSCs and somatic stem cells. Finally, we discuss future directions in stem cell niche engineering to improve the efficiency and accuracy of in vitro models and to scale up stem cell production for clinical therapies.
2. ENGINEERING HYDROGEL PROPERTIES TO RECAPITULATE THE NICHE
The stem cell niche consists of a myriad of interacting components (Figure 2), which may include the ECM, other stem cells, differentiated progeny, and heterologous cell types (e.g., endothelial cells) (3, 4). These components provide biophysical and biochemical inputs that regulate stem cell functions such as self-renewal, quiescence, and differentiation (3, 4). This section reviews engineering approaches to control these various aspects of the stem cell microenvironment.
2.1. Extracellular Matrix Mechanics
The native ECM is a hydrated network of proteins and polysaccharides that anchors cells within their specific microenvironment. Cells are mechanically coupled to the ECM through transmembrane proteins known as integrins (7). These integrins bind specific cell-adhesive ligands presented by ECM proteins, connecting the ECM to the intracellular actin cytoskeleton (7). The mechanical properties of the ECM alter the ability of cells to generate tension, modulating cell spreading, nuclear shape, and intercellular signaling pathways. For detailed discussions of mechanisms of cellular mechanosensing, the reader is directed to several excellent reviews (8-10).
2.1.1. Stiffness.
In the simplest approach, ECM mechanics can be described by a time-independent stiffness. For a cross-linked polymer network such as the ECM, stiffness is a metric of how easily a material deforms under an applied load. Stiffness is typically described by an elastic, or Young’s, modulus, which is defined as the ratio of the applied stress (i.e., force per area) to the strain (i.e., relative deformation) for small perturbations. Young’s moduli for mammalian tissues range from hundreds of pascals for nervous tissue (11) to tens of gigapascals for calcified bone (12). Despite this wide range of stiffnesses for native tissue, cells are commonly cultured on polystyrene plates, with an elastic modulus on the order of 1 GPa (13).
Initial attempts to elucidate the effects of matrix stiffness on stem cell fate utilized polyacrylamide hydrogels as two-dimensional (2D) cell culture platforms. Polyacrylamide gels are prepared by polymerizing acrylamide with a bisacrylamide cross-linker. Ideal elastic theory predicts that increasing the density of cross-links within the hydrogel will result in increased hydrogel stiffness. Accordingly, increasing the ratio of bisacrylamide to acrylamide, as well as increasing the total monomer content of the pregel solution, will increase the stiffness of the resultant hydrogels. This approach has been used to generate hydrogels with elastic moduli spanning a physiologically relevant range from tens of pascals to hundreds of kilopascals (14). To facilitate cell adhesion and mechanical coupling to the substrate, polyacrylamide gels can be covalently modified with ECM proteins or integrin-binding peptides (14, 15).
Whereas initial studies of stem cell mechanosensing were carried out on 2D substrates, the stem cell niche is a three-dimensional (3D) microenvironment. Polyacrylamide gels cannot be used for 3D cell encapsulation, as the monomer components are cytotoxic. More recent studies have utilized polymeric starting materials, such as poly(ethylene glycol) (PEG) (16), alginate (17), and hyaluronic acid (18), to prepare cell-encapsulating gels. These materials can be cross-linked with cell-compatible chemistries and are readily functionalized with cell-adhesive proteins and peptides. As with the 2D polyacrylamide gels, tuning the total polymer content or the network cross-link density permits stiffness modulation in these 3D systems. However, note that these strategies for modulating 3D stiffness often result in concomitant variation of other material properties, such as hydrogel mesh size and swelling, so carefully controlled experiments are necessary to disentangle the relative contributions of each of these properties to stem cell fate (19).
2.1.2. Viscoelasticity.
While the majority of stem cell mechanobiology studies to date have used elastic hydrogel systems that can be simply characterized by their Young’s modulus, natural ECM is not an ideal elastic solid. Rather, native tissues are viscoelastic, exhibiting time-dependent mechanical properties (20-22). Unlike purely elastic synthetic hydrogels, gels composed of reconstituted ECM proteins such as collagen (23) and fibrin (24) exhibit stress relaxation in response to a constant applied load. On a molecular level, the polymer chains that make up the network rear-range in response to the load to dissipate the applied force. Thus, in order to better recapitulate the mechanical properties of the native ECM, recent efforts have been directed toward designing hydrogels with tunable viscoelasticity (25).
Initial studies investigating the impact of matrix viscoelasticity on stem cell phenotype used 2D polyacrylamide gels. By carefully controlling the ratio of acrylamide to bisacrylamide used to prepare the gels, Cameron et al. (26) synthesized substrates with approximately the same storage moduli (elasticity) but varied loss moduli (viscosity). Thus, while these gels have the same initial stiffness, their time-dependent dissipation of force will be different. More recently, other hydrogel systems have been employed to facilitate 3D mechanobiology studies of encapsulated cells. Physically cross-linked hydrogels, such as calcium cross-linked alginate, are inherently viscoelastic due to the reversible nature of the cross-links (27). Chaudhuri et al. (28) developed a family of alginate hydrogels with independently tunable stiffness and stress relaxation rates. The concentration of calcium cross-linker was varied to tune the stiffness of gels, while decreasing the molecular weight of the alginate resulted in increased stress relaxation rates, due to decreased network connectivity and hence increased polymer chain mobility. Further increases in stress relaxation rate were achieved by coupling PEG spacers to low-molecular-weight alginate to further disrupt network architecture (28). Covalent cross-linking chemistries also have been developed recently to modulate hydrogel stress relaxation. McKinnon et al. (29) utilized reversible hydrazone linkages to prepare covalently cross-linked PEG hydrogels with tunable stress relaxation rates. Whereas aliphatic aldehydes form highly dynamic bonds with hydrazines in aqueous solution, aromatic aldehydes form much more stable bonds. Thus, hydrogels cross-linked with aliphatic aldehydes exhibit more rapid stress relaxation rates than gels cross-linked with aromatic aldehydes (29).
2.2. Engineered Matrix Degradation
In addition to exhibiting time-dependent mechanical properties, the native ECM can also be dynamically remodeled by resident cells. Cell-secreted enzymes can degrade the ECM, permitting cell spreading and migration through the matrix. Several approaches have been employed in engineered hydrogel systems to mimic this dynamic matrix degradation for 3D cell culture. Initial approaches took advantage of the passive hydrolysis of ester cross-links to prepare PEG hydrogels that degraded over time (30, 31). Subsequent studies sought to incorporate enzymatic degradability into synthetic hydrogel systems to permit cell-mediated remodeling. Lutolf et al. (32) cross-linked PEG hydrogels with peptides susceptible to cleavage by matrix metalloproteinases (MMPs). In gels cross-linked with the MMP degradable peptide, cells were free to spread and migrate (32). Furthermore, by altering the amino acid sequence of the cross-linking peptide, the affinity of MMPs for the peptides could be varied, thereby altering the kinetics of hydrogel degradation (33). More recent studies have extended this control to localized, on-demand material degradation. Kloxin et al. (34) developed a photodegradable PEG hydrogel system that permitted hydrogel cleavage by exposure to 365-nm light. Highly selective spatial and temporal resolution of hydrogel degradation in this system was achieved using two-photon laser scanning microscopy (34). Similar to methods modulating 3D stiffness, altering material degradability will necessarily result in changes in several hydrogel properties, including mechanics, mesh size, and swelling (19). This observation emphasizes the importance of designing carefully controlled experiments to isolate the effects of matrix degradation on stem cell phenotype.
2.3. Cell-Adhesive Ligands
Specific cell–matrix adhesion is required for cell spreading, migration, and mechanosensing. Cells adhere to native ECM via cell surface receptors. In particular, a class of heterodimeric receptors known as integrins link the intracellular cytoskeleton to specific cell-adhesive ligands on ECM proteins (7). The tripeptide arginine–glycine–aspartic acid (RGD) is found in multiple ECM proteins and binds to several different integrin dimers (35). Accordingly, peptides containing the RGD motif have been incorporated into hydrogel systems to permit adhesion of various cell types (36). Increasing the concentration of RGD peptide increases the adhesivity of the substrate, resulting in increased cell spreading (36). Cell motility exhibits a biphasic response to RGD concentration, with too little RGD preventing adhesion and too much RGD inhibiting cell detachment for migration (36, 37). Whereas the initial studies investigating the role of RGD peptide presentation on cell spreading and migration made use of 2D surfaces, presentation of RGD, in addition to cell-mediated hydrogel degradation, facilitates cell spreading and migration in 3D materials (32).
The native ECM is a mixture of proteins and polysaccharides that interact with cells through various cell surface receptors. In addition to RGD, other cell-adhesive peptide sequences have been identified from native ECM components, including the laminin-derived ligands YIGSR and IKVAV and the collagen-derived ligands GFOGER and DGEA (38). These ligands bind distinct receptors from the integrins engaged by RGD and can activate different intracellular signaling pathways. Although some studies have shown that these ligands can modulate stem cell behavior in isolation (39, 40), combinations of these other ligands with RGD are often necessary to elicit desired behaviors. Because the interactions among these ligands are difficult to predict and commonly nonlinear, combinatorial screens are often used to optimize ligand concentrations (41, 42).
In addition to concentration and identity, the nanoscale spacing of cell-adhesive ligands also regulates cell behavior. To form stable adhesions, integrin receptors must cluster together (36). Thus, when RGD is presented from a surface at a fixed global concentration, conditions with tighter local RGD packing permit greater adhesion (36). Local ligand density has been modulated by varying the number of ligands coupled per polymer chain, and global ligand concentration was controlled by blending in unmodified polymers. This strategy has been used with multiarm PEGs (43) and alginate hydrogels (44, 45) to study the effects of ligand clustering on cell migration, proliferation, and differentiation. Ligand clustering has also been identified as an important event in stem cell mechanosensing (17), with matrix properties such as stiffness (17), viscoelasticity (28), and degradation (46, 47) regulating ligand clustering and cytoskeletal tension generation. Additionally, hydrogels with mobile ligands have been developed to facilitate ligand clustering, wherein an RGD-coupled cyclodextrin is threaded onto a polymer chain that is later incorporated into a hydrogel network (48, 49). Stem cells encapsulated within these hydrogels were able to cluster ligands and differentiate efficiently with mobile ligands, but not with static ligands (49).
Given the importance of numerous functions regulated by cell–matrix adhesion, strategies to pattern adhesive ligands in hydrogels have been developed to control cellular access to adhesive cues. Techniques for patterning ligands on 2D surfaces have recently been reviewed (50), so we focus on techniques for achieving ligand patterning within 3 D hydrogels. Most of these approaches make use of focused lasers to selectively induce chemical reactions at defined coordinates, thereby controlling pattern position in three dimensions. For instance, Luo & Shoichet (51) prepared agarose hydrogels containing photocaged thiols. Wherever the gels were irradiated with UV light, thiols were exposed that could react with maleimide-modified RGD peptides. DeForest et al. (52) incorporated vinyl moieties into PEG hydrogels that allowed for patterning of thiol-containing RGD peptides via photoinitiated thiol–ene reactions. An alternative strategy has been to incorporate photocaged adhesive peptides into the gel that are initially inaccessible for cell binding (53, 54). In these systems, cells were able to adhere to the gel only in regions that had been exposed to UV light to uncage the adhesive peptides (54, 55).
The composition of the native ECM is dynamic, with matrix composition changing during development, aging, and disease progression. Thus, to recapitulate the time-dependent changes in adhesive ligand concentration and identity, various methods for dynamically controlling ligand presentation have been developed. Many of the photochemical approaches to hydrogel modification described above have been employed to temporally control ligand availability. Kloxin et al. (34) applied the same photoreactive group used for controlling hydrogel degradation to selectively release RGD peptides and decrease adhesivity after a defined culture interval. DeForest & Anseth (56, 57) combined this photodegradable functionality with the photoactivated thiol–ene reactions described above to develop gels that permit dynamic addition and removal of bioactive components. DeForest & Tirrell (58) later expanded the chemical tool kit for photoreversible hydrogel functionalization with the light-activated oxime ligation reaction. Mosiewicz et al. (59) used photocaged enzymatic substrates to selectively permit enzymatic ligation of bioactive molecules only after exposure to light. Other strategies have exploited molecular self-assembly to temporally alter ligand presentation (60, 61). For example, Boekhoven et al. (60) used host–guest interactions to display RGD peptides from alginate surfaces. Surfaces initially presenting cell-adhesive RGD peptides could be rendered nonadhesive by addition of a control peptide possessing a stronger host–guest binding partner (60). Other self-assembly driven approaches have used complementary leucine zipper peptides (62) and complementary DNA strands (63, 64) to achieve dynamic control over ligand presentation dynamics.
2.4. Microstructure
Most of the seminal research describing the impact of niche properties such as matrix mechanics and cell-adhesive ligand presentation described above was conducted on planar 2D surfaces. However, the native ECM is much more structurally complex. Most in vivo cellular microenvironments are 3D in nature, and scientists have recently begun to appreciate that biological processes observed on artificial 2D surfaces do not always translate to more biomimetic 3D contexts (19). Furthermore, the native ECM exhibits significant microscale heterogeneity. Many ECM components assemble into fibers that range in size from 0.1 μM to greater than 1 μM (65). Cells are capable of sensing changes in matrix topography, as cellular migration and protrusion elongation are increased along fibrous structures (66). Common strategies for generating biomimetic fibrous topographies include the use of electrospun fibers on the order of hundreds of nanometers to tens of micrometers (67) and self-assembly of proteins, peptides, or peptide amphiphiles to prepare nanofibrous gels (39, 68-72). Other research has focused on designing substrates with well-defined engineered features, such as grooves, pits, and pillars (73, 74). While the majority of studies reporting the impact of topography on cellular behavior have been empirical, some mechanistic insights are beginning to emerge, pointing to changes in cell–matrix adhesions and cell–cell junctions (73, 75).
Due to limitations in material fabrication techniques, most studies of matrix topography have been confined to the surfaces of 2D substrates. However, macroporous hydrogel scaffolds represent a notable class of 3D materials with engineered topographical variation. Common techniques for producing macroporous hydrogels include microparticle templating, freeze drying, and gas foaming (76). Cross-linking of hydrogel microribbons (77) and assembly of microgels (78) have recently been introduced as alternative techniques to prepare macroporous hydrogels. The size scale of the pores dictates whether cells experience a pseudo-2D microenvironment with freedom to spread and migrate or a more confining 3D environment. Altering pore size is known to change how cells respond to implanted hydrogel materials (79). Changing 3D architecture also modifies the transport properties of the cellular microenvironments, providing an additional mechanism to modulate the stem cell microenvironment. For instance, the geometric arrangement of cells dictates local concentrations of signaling molecules, in turn modulating tissue morphogenesis (80).
2.5. Cell–Cell Interactions
Beyond niche parameters directly controlled by the ECM, interactions among cells within the niche are crucial regulators of stem cell fate. Stem cells, their differentiated progeny, and heterologous cells communicate via secretion of soluble factors and direct cell–cell contact. Many studies have sought to optimize coculture of stem cells with other cell types to either maintain stem cells in a naïve state or trigger differentiation into desired lineages. These approaches have been reviewed in detail elsewhere (81, 82). In this section, we focus on engineering approaches to incorporate signals from niche cells into hydrogel systems.
Native ECM components contain binding motifs for many soluble signals such as growth factors. Several approaches to engineer desired bioactivity into artificial stem cell niches have been inspired by these natural interactions. Early studies revealed that growth factors tethered to solid substrates retained their bioactivity (83) and in some cases proved more efficacious than their soluble counterparts (84). The enhanced activity of tethered growth factors may be due to increased growth factor stability and sustained receptor activation by preventing cellular internalization and degradation. Recent efforts have been directed at incorporating dynamic mechanisms of growth factor tethering to temporally control presentation, including strategies employing supramolecular host–guest interactions (85) and enzymatic methods (86). To decrease costs associated with the use of full-length growth factors, small-peptide mimics capable of initiating growth factor signaling have been incorporated into several types of hydrogels (64, 87-89). Alternative methods for localizing cell-secreted factors have taken a more biomimetic approach, incorporating charged polysaccharides like heparan sulfate to sequester growth factors (90) or peptide sequences that bind and retain secreted ECM proteins (91). Conversely, growth factors have also been engineered to exhibit increased binding affinity to natural ECM components, increasing the potency of these factors through longer-lived interactions with the matrix (92). Many cellular processes, such as cell migration and tissue morphogenesis, are sensitive to gradients of soluble factors, rather than uniform presentation of the factors. In response, various engineering strategies, including microfluidic devices and spatial patterning of growth factor–sequestering molecules, have been employed to generate gradients in hydrogel systems (93).
Stem cells can also interact with other niche cells through direct cell–cell contact. Early efforts to recapitulate cell–cell contact in engineered systems used immunoglobulin Fc domains fused to the extracellular domain of E-cadherin to immobilize the cell adhesion molecules on a surface (94). More recently, HAVDI peptides that can engage N-cadherin were coupled to hyaluronic acid hydrogels (95, 96). Beyond cadherin-mediated contact, a peptide sequence mimicking the activity of neural cell adhesion molecule (NCAM) was incorporated into engineered elastin-like protein materials (97).
3. ENGINEERED MICROENVIRONMENTS TO PROMOTE STEMNESS
The previous section discusses engineering strategies for modulating various niche properties to control stem cell fate. Desired stem cell fate decisions can be grouped into one of two broad outcomes: maintenance of stemness or differentiation into mature lineages. This section reviews microenvironmental parameters to promote stemness maintenance in various stem cell types. To maintain stemness, a stem cell must continue to express proteins characteristic of the stem cell state, undergo self-renewing proliferation, and retain the capacity to differentiate into appropriate mature cell types. Table 1 summarizes the niche factors known to regulate stemness maintenance for each cell type discussed below.
Table 1.
Engineering niche properties to modulate stem cell phenotype
| Stem cell phenotype | Stem cell type | Engineered niche factor(s) | Reference(s) |
|---|---|---|---|
| Stemness maintenance: expansion | Pluripotent stem cells | Matrix stiffness | 104 |
| Matrix composition | 100, 101, 103 | ||
| Hematopoietic stem cells | Matrix stiffness | 110, 111 | |
| Matrix composition | 111, 112 | ||
| Cell–cell factors | 113 | ||
| Mesenchymal stem cells | Matrix stiffness | 116, 117 | |
| Matrix composition | 119 | ||
| Microstructure | 118 | ||
| Intestinal stem cells | Matrix stiffness, degradability, composition | 121 | |
| Stemness maintenance: quiescence | Muscle stem cells | Matrix stiffness | 124-126 |
| Microstructure, soluble factors | 126 | ||
| Differentiation | Pluripotent stem cells | Matrix stiffness | 129-133 |
| Matrix composition | 138-140 | ||
| Microstructure | 134-137 | ||
| Mesenchymal stem cells | Matrix stiffness | 14, 17, 142 | |
| Matrix viscoelasticity | 26, 28, 143, 144 | ||
| Matrix degradability | 46 | ||
| Cell-adhesive ligands | 40, 49, 145, 146 | ||
| Matrix composition | 148 | ||
| Cell-secreted factors | 88, 149 | ||
| Cell–cell contact | 95, 96, 151 | ||
| Neural stem cells | Matrix stiffness | 154-160 | |
| Matrix degradation | 161 | ||
| Microstructure | 162 | ||
| Cell-adhesive ligands | 15, 39, 42 | ||
| Cell-secreted factors | 163 |
3.1. Stem Cell Expansion
Expanding large numbers of stem cells is the most common goal when designing a culture platform to maintain stemness. Both clinical studies transplanting large numbers of naïve stem cells and tissue engineering approaches to produce functional tissue constructs from stem cells in vitro require large quantities of cells. Ineffective means for scaling up production of high-quality stem cells remains a significant barrier to clinical translation (98), so developing scalable platforms for stem cell expansion may be transformational for the field.
3.1.1. Pluripotent stem cells.
PSCs are capable of differentiation into all three germ lineages and are therefore able to differentiate into any adult tissue type (1). PSCs consist of both ESCs derived from the inner cell mass of blastocyst-stage embryos and iPSCs derived from terminally differentiated cells induced to express pluripotency factors (1). Such cells were traditionally cultured on feeder layers of mouse embryonic fibroblasts or on reconstituted basement membrane derived from mouse sarcoma (i.e., Matrigel) (99). The presence of these xenogeneic components often led to poor batch-to-batch consistency and would preclude clinical use of stem cells cultured in this manner (99). Thus, engineering xeno-free systems for PSC expansion would expand the utility of these stem cells.
Removing the xenogeneic components has been a major goal of attempts to engineer the PSC microenvironment for stem cell expansion (99). In 2010, three separate research groups identified fully defined engineered matrix compositions that permitted feeder-free expansion of ESCs: recombinant laminin 511 (100), acrylate surfaces presenting RGD peptides (101), and synthetic poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH) grafted surfaces (102). Since these reports, various other naturally derived and synthetic materials have been developed to support the culture of undifferentiated PSCs (103). In addition to controlling surface biochemistry, biophysical interactions with the substrate must also be considered. For instance, increased ESC self-renewal was observed on soft (E ~ 0.6 kPa) polyacrylamide gels compared with rigid plastic culture surfaces (104).
Although most research laboratory-scale culture of PSCs is conducted on 2D surfaces, 2D culture is less efficient in terms of space and energy requirements for industrial-scale production of PSCs. Transitioning to 3D hydrogel systems may facilitate industrial scale-up, as such culture systems would occupy considerably less space and require less energy to produce an equivalent number of cells than traditional 2D culture (105). To this end, hydrogels composed of hyaluronic acid (106), calcium cross-linked alginate (107), and thermoresponsive synthetic polymers (108) have been optimized to culture undifferentiated PSCs.
3.1.2. Hematopoietic stem cells.
HSCs are somatic stem cells that reside in the bone marrow and are capable of differentiating into all myeloid and lymphoid cell types (109). HSCs are responsible for reconstituting patients’ immune systems following a bone marrow transplant (109). Ex vivo expansion of these cells has the potential to increase the supply of these donor-limited cells and to improve the engraftment probability of transplants by delivering a greater number of multipotent stem cells to the patient. Recently, progress has been made in identifying the proper combination of biochemical and biophysical signals to promote expansion of naïve HSCs. A potential role of matrix stiffness in modulating HSC stemness was identified when substrates coated with more compliant tropoelastin materials promoted increased stem cell expansion relative to controls (110). In 2017, Choi & Harley (111) demonstrated that HSCs were best maintained in an undifferentiated state when cultured on hydrogel substrates with elastic moduli of ~40 kPa. Furthermore, this study revealed an interplay between stiffness and matrix composition, as matrices with high fibronectin content were required to maintain naïve HSCs (111). A role for matrix composition in maintaining HSC expansion potential was also demonstrated by Prewitz et al. (112), who reported increased HSC proliferation on MSC-derived ECM. The importance of other cell-produced niche factors has been studied using hydrogel microwell platforms presenting covalently immobilized proteins. Lutolf et al. (113) revealed that exposure to N-cadherin or Wnt3a increased HSC division or initiated HSC quiescence, respectively.
3.1.3. Mesenchymal stem cells.
Like HSCs, MSCs reside in the bone marrow (114). These cells are also known as bone marrow stromal cells (BMSCs) (114). MSC-like cells have additionally been isolated from adipose tissue (adipose-derived stem cells, or ASCs) (114). MSCs are functionally defined by the capacity to differentiate into bone, cartilage, and adipose tissue (115). Many of the studies on engineering MSC microenvironments have focused on controlling differentiation into bone or cartilage for orthopedic tissue engineering applications. This literature is reviewed in Section 4, below. Nevertheless, these tissue engineering applications commonly require expanding large numbers of MSCs prior to differentiation, so designing an engineered niche for stem cell expansion may facilitate the transition of MSC-based therapies to the clinic.
As discussed below, MSCs are known to bias their differentiation on the basis of the mechanical properties of their microenvironment (14, 17, 28). Yang et al. (116) further revealed that substrate stiffness modulates MSC stemness. The authors employed hydrogels that could be dynamically softened to investigate the temporal effects of matrix stiffness on MSC differentiation potential. MSCs cultured on stiff gels that were softened one day postseeding retained the ability to differentiate into both osteoblasts and adipocytes (116). In contrast, after one week on stiff gels, MSCs were committed to osteogenic differentiation, even after the gels were softened (116). A recent study by Li et al. (117) revealed that this mechanical memory was mediated by elevated transcription of miRNA-21 activated by culture on stiff substrates.
Additionally, matrix topography and biochemistry are known to alter MSC stemness. McMurray et al. (118) reported that nanopatterned substrates with uniform pit spacing maintained MSCs in an undifferentiated state, whereas more disordered substrates favored osteogenic differentiation. There is also evidence to suggest that binding of ECM components and soluble factors to engineered hydrogel matrices can alter MSC stemness. Bai et al. (119) demonstrated that fouling of hydrogels could result in spontaneous differentiation of encapsulated MSCs. By preparing hydrogels composed entirely of zwitterionic carboxybetaine monomers, these authors prepared nonfouling gels that maintained MSC multipotency in the presence of soluble differentiation factors (119).
3.1.4. Intestinal stem cells.
Intestinal stem cells (ISCs) are located in the base of the crypt–villus architecture of the adult intestine (4). ISCs give rise to the terminally differentiated cell types that constitute the intestinal epithelium, including Paneth cells, goblet cells, enteroendocrine cells, and enterocytes (120). In culture, isolated ISCs are capable of forming organoids reminiscent of the native intestinal epithelium in morphology and cellular composition. These organoids may find utility in drug screening and tissue engineering applications (120).
The first reported cultures of intestinal organoids derived from single ISCs used cells cultured within Matrigel-based hydrogels (120). Although effective at initiating and maintaining ISC organoid culture, Matrigel is animal derived, suffers from batch-to-batch variability, and exhibits limited tuning of microenvironmental properties, making both basic biological studies and translation to clinical applications challenging. Recently, Gjorevski et al. (121) published a systematic study using PEG-based hydrogels to optimize synthetic matrices for ISC organoid culture. The authors demonstrated that ISC expansion is mechanosensitive, with optimal organoid formation efficiency in matrices with shear moduli of ~1.3 kPa (121). ISC organoids are also sensitive to time-dependent variation in matrix mechanics, as dynamic softening of the PEG gels via hydrolysis was required to maintain the differentiation potential of the ISCs (121). Finally, ISC growth was shown to depend on interactions with cell-adhesive ECM components. Optimal organoid formation was observed in gels containing laminin and laminin-derived peptides, but also exhibited a dose-dependent response to RGD ligands (121). Although ISC organoid culture is still a relatively new application of hydrogel systems, other studies culturing intestinal organoids derived from explanted tissue suggest that ISC organoids may be cultured in other modular hydrogel platforms, such as those using engineered elastin-like proteins (122).
3.2. Maintaining Stem Cell Quiescence
Beyond harnessing the self-renewal capacity of stem cells to produce large numbers of cells for therapeutic use, there are several motivations for maintaining stem cells in a quiescent, slowly dividing state. These may include basic biological studies of stem cell biology as well as therapeutic strategies that rely on ex vivo manipulation of the stem cells, such as introduction of exogenous genes. This section discusses how niche properties can be optimized to maintain quiescence in the context of skeletal muscle stem cells (MuSCs).
MuSCs, also known as satellite cells, are located between the plasma membrane of myofibers and the basal lamina surrounding these fibers (123). MuSCs are critical for the maintenance of skeletal muscle tissue. Thus, culturing MuSCs ex vivo may provide insight into how dysfunction in the stem cell pool can lead to muscle wasting, and MuSC transplantation may eventually serve as an effective therapy for such disorders. However, for many years, MuSCs were unable to be cultured outside of their native niche, as plating the cells on standard tissue culture dishes rapidly led to a loss of stemness. Gilbert et al. (124) demonstrated that the relatively high stiffness of tissue culture plastic was largely responsible for this loss of stemness. MuSCs cultured on hydrogels with elastic moduli approximating the stiffness of native muscle tissue (~12 kPa) exhibited self-renewing divisions and did not differentiate, unlike cells cultured on plastic (124). Cosgrove et al. (125) later revealed that the regenerative capacity of aged MuSCs could be restored by culturing the aged cells on compliant hydrogels with simultaneous p38 kinase inhibition, suggesting a role for mechanotransduction pathways in aging-related MuSC dysfunction. Other properties of the niche also affect MuSC quiescence in vitro. Quarta et al. (126) developed an engineered muscle fiber niche with optimized stiffness, cell-adhesive proteins, and medium composition. MuSCs cultured in this engineered microenvironment retained the ability to engraft into muscle tissue in vivo following ex vivo manipulation (126).
4. ENGINEERED MICROENVIRONMENTS TO DIFFERENTIATE STEM CELLS
For many therapeutic and screening applications, pure populations of differentiated cells, rather than naïve stem cells, are required (127). Thus, in addition to expanding sufficient numbers of stem cells, protocols must be developed to guide the differentiation of the stem cells down a desired lineage. Many of the same microenvironmental properties that regulate stemness maintenance can bias stem cell differentiation. Table 1 summarizes the niche factors known to modulate stem cell differentiation for each cell type discussed below.
4.1. Pluripotent Stem Cells
Expansion of PSCs while maintaining stemness and preventing undesired differentiation is the first challenge of PSC culture (127). As discussed in the previous section, engineered hydrogels have been developed to maintain PSC stemness. The second challenge of PSC culture is controlling the differentiation of the cells into pure populations of mature cell types (127). High purity of differentiated cells is required for therapeutic applications, where the presence of undifferentiated PSCs may lead to tumor formation, and for in vitro assays, where contaminating cell populations may skew results. Much effort has been expended in engineering PSC microenvironments to direct differentiation. This section provides an overview of regulation of PSC differentiation by engineered niche cues. For more detailed discussions, the reader is referred to recent reviews on the topic (127, 128).
PSC differentiation is sensitive to matrix mechanics during both initial differentiation events and maturation into terminally differentiated cell lineages. Culturing ESCs on polydimethylsiloxane (PDMS) substrates with stiffness greater than 1 MPa increased expression of primitive mesodermal and endodermal genes (129). Interestingly, a recent study revealed a mechanism by which ESC culture on much more compliant hydrogels (E ~ 0.4 kPa) resulted in enhanced cell–cell contact and accumulation of β-catenin that primed the cells for mesodermal differentiation (130). Terminal differentiation of PSCs into neurons is also modulated by substrate stiffness, with significantly enhanced neuronal specification on relatively compliant substrates (131, 132). In a 3D context, Zoldan et al. (133) reported biased mesodermal differentiation for high-stiffness (E ~ 1.5–6 MPa) scaffolds, endodermal differentiation for intermediate-stiffness (E ~ 0.1–1 MPa) scaffolds, and ectodermal differentiation for low-stiffness (E < 0.1 MPa) scaffolds.
Topographical cues can also influence PSC fate decisions. ESCs cultured on smooth surfaces maintained an undifferentiated phenotype, whereas cells cultured on surfaces with nanoscale roughness underwent spontaneous differentiation (134). Neurogenic differentiation of ESCs was enhanced on surfaces with aligned nanoscale features, such as electrospun fibers (135) and grooved patterns (136). Substrates with regularly spaced nanopores increased the differentiation efficiency of iPSCs into pancreatic precursors (137).
Biochemical signals provided by the microenvironment are an additional parameter that can modulate PSC differentiation. Various studies have identified natural ECM components that can bias PSC fate decisions in 2D contexts and are reviewed by Dickinson et al. (138). In a 3D hydrogel context, Dixon et al. (139) prepared hydrogels that could dynamically switch from facilitating stemness maintenance to favoring mesodermal specification by altering hydrogel composition from alginate to collagen. Dextran hydrogels with covalently tethered RGD peptides and microencapsulated vascular endothelial growth factor (VEGF) enhanced the vascular differentiation capacity of encapsulated ESCs (140).
4.2. Mesenchymal Stem Cells
MSCs are commonly investigated for orthopedic tissue engineering applications due to their capacity to differentiate into bone and cartilage (114, 115). As for PSCs, the effects of microenvironmental parameters on MSC differentiation have been widely studied. Here, we highlight the major advances and direct the reader to relevant reviews for more detailed information (25, 141).
MSC differentiation is sensitive to the mechanics of the surrounding matrix. In a seminal study, Engler et al. (14) demonstrated that MSCs exhibited biased differentiation according to the stiffness of the polyacrylamide substrates on which the MSCs were cultured. MSCs cultured on gels with elastic moduli similar to that of precalcified bone (E ~ 25–40 kPa) differentiated down an osteogenic lineage, whereas MSCs on gels with elastic moduli similar to that of skeletal muscle (E ~ 8–17 kPa) differentiated down a myogenic lineage (14). MSCs on the most compliant gels with elastic moduli similar to that of nervous tissue (E ~ 0.1–1 kPa) exhibited a neuron-like phenotype (14). Huebsch et al. (17) confirmed that matrix stiffness can direct MSC differentiation in more biomimetic 3D microenvironments. The authors found that clustering of RGD cell-adhesive peptides was optimized in alginate hydrogels with elastic moduli of ~20 kPa, resulting in enhanced osteogenic differentiation. MSCs cultured in more compliant gels favored adipogenic differentiation (17). A later study by Huebsch et al. (142) demonstrated that MSC-mediated bone formation in vivo was also sensitive to the stiffness of the hydrogel in which the MSCs were delivered, with optimal regeneration observed in ~60-kPa hydrogels.
MSC differentiation is additionally sensitive to the time-dependent mechanical properties of the matrix. The first studies of matrix viscoelasticity affecting MSC behavior utilized polyacrylamide hydrogels with fixed storage moduli (elasticity) but variable loss moduli (viscosity) (26, 143). Substrates with increased loss moduli resulted in increased cell spreading and increased differentiation into myogenic, adipogenic, and osteogenic lineages (26). Increased myogenic differentiation on more viscoelastic gels was attributed to increased activation of Rac1 GTPase (143). Chaudhuri et al. (28) extended these studies to 3D materials by using alginate hydrogels with independently variable stiffness and viscoelastic stress relaxation rates. The authors demonstrated that increasing the stress relaxation rate of the material substantially enhanced the osteogenic differentiation of embedded MSCs, but only in matrices with an appropriate stiffness (E ~ 17 kPa). Similar to studies in predominantly elastic alginate gels (17), the increased osteogenic differentiation in rapidly stress-relaxing materials was correlated with increased RGD ligand clustering. Gels with rapid stress relaxation rates enhanced MSC-mediated bone formation in vivo (144).
Matrix degradation also plays a role in the mechanosensitive differentiation of MSCs. When MSCs were encapsulated in nondegrading, covalently cross-linked hyaluronic acid hydrogels, adipogenic differentiation was favored, regardless of matrix stiffness (46). However, when the gels were rendered degradable by cell-secreted MMPs, substantial osteogenic differentiation was observed (46). Khetan et al. (46) demonstrated that hydrogel degradation was required for tension generation by encapsulated MSCs to mediate osteogenic differentiation. Matrix degradation likely promotes ligand clustering to facilitate tension generation, in agreement with studies of mechanosensititve MSC differentiation in alginate gels (17, 28, 47). Accordingly, MSC differentiation was enhanced in nondegradable PEG hydrogels that presented mobile RGD ligands that could be clustered without hydrogel degradation (49).
In addition to ligand clustering to mediate mechanotransduction, the identity of cell-adhesive ligands can modulate MSC differentiation. For instance, the collagen-mimetic ligands DGEA and GFOGER increase osteogenic (40, 145) and chondrogenic (146) differentiation. The laminin-derived IKVAV motif also enhances osteogenic and adipogenic differentiation when presented in combination with RGD peptides (147). Beyond cell-adhesive ligands, other matrix components are known to alter MSC differentiation. Cartilage is rich in polysaccharides, and incorporation of chondroitin sulfate, hyaluronic acid, and heparan sulfate alters chondrogenic differentiation (148).
Mimicking cell–cell interactions is an additional approach to controlling MSC differentiation in engineered microenvironments. Bone morphogenetic proteins (BMPs) are potent activators of osteogenic differentiation. Incorporating peptide mimics of BMP-2 on implant surfaces (149) and within hydrogels (88) enhanced mineralization by MSCs. BMP-binding peptides that can sequester endogenous BMPs in hydrogel materials (150) may represent an alternative strategy to elicit osteogenic differentiation from MSCs. Direct cell–cell contact can also modulate MSC differentiation. Hydrogels presenting N-cadherin-mimicking HAVDI peptides altered the contractile state and mechanosensing of MSCs, in turn altering their mechanosensitive differentiation (95). Incorporation of HAVDI peptides into hyaluronic acid hydrogels also enhanced the chondrogenic differentiation of MSCs (96), likely through activation of β-catenin signaling (151).
4.3. Neural Stem Cells
NSCs are located within the subventricular zone (SVZ) and subgranular zone (SGZ) in the adult brain (2). NSCs can differentiate into neurons, astrocytes, and oligodendrocytes (2). Differentiation of NSCs into neurons makes these stem cells particularly attractive for clinical applications. Mature neurons are nondividing, so NSCs can be initially expanded and then differentiated to generate therapeutically relevant numbers of neurons. This section provides an overview of how engineered niche factors influence NSC fate. For greater detail, the reader is directed to other recent reviews (152, 153).
As for MSCs, the differentiation of NSCs is mechanosensitive. Studies using 2D polyacrylamide gels with grafted RGD cell-adhesion peptides revealed that neuronal differentiation was increased on compliant gels with elastic moduli from tens to hundreds of pascals, whereas astrocytic differentiation dominated on stiffer gels (154). Others have confirmed that neuronal differentiation is enhanced on compliant gels (E < 1 kPa) but that oligodendrocyte differentiation is enhanced on stiffer (E ~ 7 kPa) substrates (155). The discrepancy in the particular glial lineage favored at higher stiffness may be due to derivation of NSCs from different regions of the brain (SGZ versus SVZ). Keung et al. (156) revealed that mechanosensitive NSC differentiation is mediated by increased activation of the GTPases RhoA and Cdc42. A recent study also implicated Yes-associated protein (YAP) and β-catenin signaling in mechanosensitive neurogenesis (157). Although initial NSC differentiation decisions are sensitive to substrate stiffness, specification of mature neuronal subtype is not mechanosensitive (158). Although few studies have considered the role of matrix stiffness in 3D systems (152), neurogenesis was enhanced in gels with lower cross-link density (159) and lower polymer content (160), suggesting that neuronal differentiation is also favored within lower-stiffness 3D hydrogels.
Other biophysical parameters of the matrix also can influence NSC differentiation. Increasing the hydrolytic degradability of PEG hydrogels increased the expression of neurogenic markers by encapsulated NSCs (161). NSCs are sensitive to substrate topography, as culturing NSCs on aligned electrospun nanofibers resulted in enhanced neuronal differentiation (162).
In addition to biophysical niche signals, biochemical signals such as cell-adhesive ligands and growth factors can bias the differentiation of NSCs. Presenting high concentrations of the laminin-derived IKVAV ligand from nanofibrillar peptide gels enhanced the neuronal differentiation of NSCs (39), whereas RGD-containing peptides grafted to 2D polyacrylamide substrates supported better neuronal differentiation than IKVAV-containing peptides (15). More recently, combinatorial studies revealed that optimized concentrations of RGD, IKVAV, and YIGSR synergistically enhanced neuronal differentiation (42). Immobilizing platelet-derived growth factor AA (PDGF-AA) on agarose hydrogels enhanced the oligodendrocyte differentiation of NSCs (163).
5. FUTURE DIRECTIONS
Hydrogels are an attractive choice of material to serve as engineered stem cell niches due to the wide range of techniques that have been developed to modulate microenvironmental cues. Such systems have led to a greater understanding of how individual niche properties contribute to maintenance of stemness or to induction of stem cell differentiation. Future studies can apply these systems to further advance the use of stem cells and their differentiated progeny in regenerative medicine, patient-specific disease modeling, and toxicology screening applications.
5.1. Improving Reproducibility
The introduction of iPSC technologies has the potential to realize the goals of personalized medicine with patient-specific drug screening, disease modeling, and stem cell–mediated tissue repair. However, the use of iPSCs in these applications still faces several challenges. iPSCs must be generated from patient-derived cells such as fibroblasts, but traditional 2D culture methods yield very low reprogramming efficiencies, ranging from 0.1% to 10% depending on the starting cell type (164). Recently, engineering approaches to altering microenvironmental cues have resulted in significant improvements in iPSC generation efficiency. Cells reprogrammed on soft hydrogels (165) or hydrogels with aligned micropatterns (166) exhibited more efficient reprogramming than traditional methods. Caiazzo et al. (167) performed combinatorial studies investigating the role of matrix stiffness, degradation, and biochemistry in reprogramming within 3D PEG hydrogels. Reprogramming was more rapid and more efficient in the engineered hydrogels when compared with traditional 2D culture (167). Nutrient transport within cultures has also been demonstrated to play a significant role in reprogramming efficiency (168).
A second challenge in applying iPSC technologies is reproducibly generating pure populations of differentiated cells. Many differentiation protocols use poorly defined reagents and procedures, such as Matrigel and hanging-drop embryoid body formation, that can result in highly variable outcomes. Significant progress already has been made toward replacing Matrigel in iPSC maintenance and differentiation, as discussed in Sections 3 and 4, above. Techniques including microwell aggregation (169) and rotary suspension culture (170) have been employed to generate uniform-sized embryoid bodies with high differentiation potential. Other research groups have taken combined experimental and computational approaches to generating kinetic models of PSC differentiation to optimize cell culture conditions (171). Combining these disparate strategies may lead to significantly improved control of PSC differentiation.
5.2. Increasing Throughput and Sensitivity for Screening
The ability to derive essentially any mature cell type from stem cells provides an avenue to generate human-specific, and even patient-specific, platforms for drug screening and toxicology assays. The cellular microenvironment is known to alter how cells respond to external stimuli, so retaining the ability to modulate niche properties in parallel with drug or toxin treatment may provide results not easily recapitulated with traditional 2D culture techniques. High-throughput approaches will likely be required to adequately screen the large variable space for many of these studies. Microarray-based approaches have been developed to assess the effects of microenvironmental factors on stem cell behavior, including synthetic polymer coatings (172), ECM components (173) and cell–cell signaling factors (113, 174). The robot-assisted approaches used to generate these arrays have been applied to generate both 2D (175) and 3D (176) hydrogels with tunable mechanics and biochemistry to probe combinatorial effects of niche parameters on stem cell fate. Le et al. (177) recently developed a platform to generate tunable hydrogel arrays without robotic assistance by modulating the wettability of the surface. We direct the reader to a recent review for additional discussion of high-throughput techniques to probe stem cell fate decisions (178).
Another consideration when designing platforms for drug and toxicity screening is the sensitivity of the assay for detecting cellular responses to the drug or toxin. The highly variable composition of natural hydrogels such as Matrigel can result in significant variability among samples within the same condition. This high variance means that large sample sizes are required to provide sufficient statistical power to confidently assess the effect of treatment. Nguyen et al. (179) demonstrated that synthetic hydrogel microenvironments can replace Matrigel in vascular toxicity screens. The synthetic hydrogels provided enhanced sensitivity and reproducibility compared with Matrigel when assessing vascular network responses to known toxins (179).
5.3. Scale-Up for Clinical Use
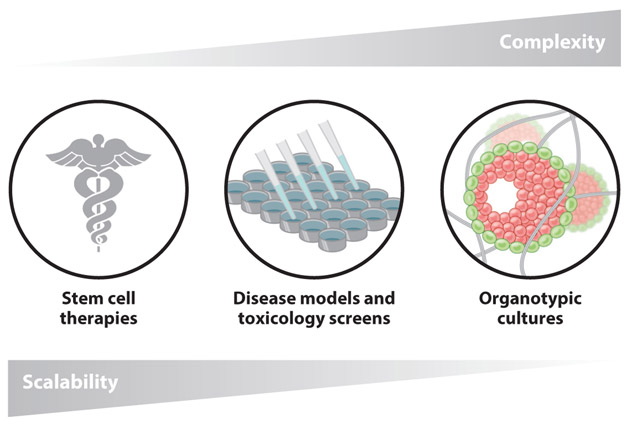
As discussed in Section 3, the translation of stem cell therapies to the clinic will require novel approaches to scale up stem cell expansion protocols. Engineered microenvironments have been demonstrated to promote stemness maintenance in various cell types, making these approaches attractive for future scale-up. Beyond maintaining stemness, such systems must also be amenable to industrial-scale processing and adhere to strict regulatory guidelines (105). Thus, hydrogel systems with complicated formulations or poorly defined components will likely not be useful for large-scale stem cell expansion (105). The identification of fully defined matrices for 2D expansion of PSCs was a significant advancement toward production of clinically useful cells (99). More recently, thermoresponsive 3D hydrogel platforms have been investigated for scaling up PSC production. Lei & Schaffer (108) developed a fully defined synthetic hydrogel that permits higher-density cell culture than traditional 2D methods and facile release of encapsulated cells, simplifying the processing steps necessary to expand and collect the cells. These studies suggest that a more minimalist approach to materials design is optimal to engineer niches for industrial-scale production of stem cells (Figure 3).
Figure 3.
The spectrum of required simplicity/complexity for engineered hydrogel niches designed for future stem cell applications.
5.4. Increasing Complexity for Better In Vitro Models
Engineering in vitro models to study basic biology or patient-specific disease states often employs a variety of niche factors to achieve more native-like behavior. Thus, in contrast to designing simplified materials for industrial-scale stem cell production, in vitro modeling may necessitate new strategies to incorporate additional complexity into hydrogel microenvironments (Figure 3). Two complementary approaches to address this challenge can benefit from advances in hydrogel systems for stem cell culture.
One strategy to generate more biologically relevant in vitro models takes a bottom-up approach, using stem or progenitor cells to self-organize into small tissue structures that recapitulate some functions of native organs. These organoid models have been developed for various human tissues, including intestine, kidney, brain, and retina (180). The protocols used to generate organoids require careful control of microenvironmental parameters such as soluble growth factors and Matrigel-based ECM (180). Several recent studies have demonstrated the utility of engineered microenvironments to generate various types of organoids. Fully defined hydrogels with tunable mechanics, degradation, and ligand presentation facilitated the maintenance of intestinal organoids (121). Microfibrillar templates enhanced the cortical development of brain organoids through geometric control over embryoid body formation (181). Generation of amnion-like tissue constructs required control over both stiffness and dimensionality (2D versus 3D culture protocols) (182).
A second strategy to generate more biologically relevant in vitro models employs a top-down approach, using additive manufacturing techniques to produce artificial tissue constructs. 3D bioprinting permits geometric control over larger length scales than self-organizing organoid approaches, enabling the introduction of perfusable vascular-like networks (183, 184). Thus, 3D bioprinting may yield larger tissue constructs that are not limited by nutrient diffusion. Many research groups are actively developing hydrogel formulations that maintain cell viability and enforce spatial cell arrangement throughout the printing process, while retaining control over microenvironmental cues that regulate stem cell phenotype (183, 184).
6. CONCLUSION
Stem cell fate is dictated by a complex interplay of biophysical and biochemical factors present in the native stem cell niche. Drawing inspiration from native stem cell microenvironments has led to engineering strategies to maintain stemness and direct differentiation ex vivo. Hydrogel materials afford control over critical regulators of stem cell fate, including matrix mechanics and biochemistry, microscale structure, and cell–cell interactions. Due to this level of control, engineered hydrogel niches have the potential to improve reproducibility and increase throughput for stem cell culture, facilitate production of stem cells for clinical use, and generate biomimetic tissue constructs.
ACKNOWLEDGMENTS
The authors thank Nicholas Suhar for assistance with figure preparation. The authors acknowledge funding support from the National Science Foundation (DMR 1508006), the National Institutes of Health (NIH) (U19 AI116484, R21 EB020235), the California Institute for Regenerative Medicine (RT3-07948), the Stanford Child Health Research Institute (UL1 TR001085), and the Ruth L. Kirschstein National Research Service Award (NRSA) Predoctoral (F31) and Siebel Scholars fellowships (to C.M.M.; NIH F31 EB020502 and Siebel Scholars Foundation).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Trounson A, DeWitt ND. 2016. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell. Biol 17:194–200 [DOI] [PubMed] [Google Scholar]
- 2.Goldman S 2005. Stem and progenitor cell–based therapy of the human central nervous system. Nat. Biotechnol 23:862–71 [DOI] [PubMed] [Google Scholar]
- 3.Scadden DT. 2006. The stem-cell niche as an entity of action. Nature 441:1075–79 [DOI] [PubMed] [Google Scholar]
- 4.Li L, Xie T. 2005. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol 21:605–31 [DOI] [PubMed] [Google Scholar]
- 5.Lee KY, Mooney DJ. 2001. Hydrogels for tissue engineering. Chem. Rev 101:1869–79 [DOI] [PubMed] [Google Scholar]
- 6.Ratner BD, Bryant SJ. 2004. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng 6:41–75 [DOI] [PubMed] [Google Scholar]
- 7.Barczyk M, Carracedo S, Gullberg D. 2010. Integrins. Cell Tissue Res. 339:269–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iskratsch T, Wolfenson H, Sheetz MP. 2014. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell. Biol 15:825–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons JT, Horwitz AR, Schwartz MA. 2010. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell. Biol 11:633–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorchak GR, Lammerding AKJ. 2014. Cellular mechanosensing: getting to the nucleus of it all. Prog. Biophys. Mol. Biol 115:76–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gefen A, Margulies SS. 2004. Are in vivo and in situ brain tissues mechanically similar? J. Biomech 37:1339–52 [DOI] [PubMed] [Google Scholar]
- 12.Rho JY, Ashman RB, Turner CH. 1993. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J. Biomech 26:111–19 [DOI] [PubMed] [Google Scholar]
- 13.Miyake K, Satomi N, Sasaki S. 2006. Elastic modulus of polystyrene film from near surface to bulk measured by nanoindentation using atomic force microscopy. Appl. Phys. Lett 86:031925 [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006Matrix elasticity directs stem cell lineage specification. Cell 126:677–89 [DOI] [PubMed] [Google Scholar]
- 15.Saha K, Irwin EF, Kozhukh J, Schaffer DV, Healy KE. 2007. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J. Biomed. Mater. Res. A 81:240–49 [DOI] [PubMed] [Google Scholar]
- 16.Lutolf MP, Hubbell JA. 2003. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 4:713–22 [DOI] [PubMed] [Google Scholar]
- 17.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, et al. 2010. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater 9:518–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. 2005. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6:386–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker BM, Chen CS. 2012. Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J. Cell Sci 125:3015–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levental I, Georges PC, Janmey PA. 2007. Soft biological materials and their impact on cell function. Soft Matter 3:299–306 [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Bilston L. 2000. On the viscoelastic character of liver tissue: experiments and modelling of the linear behaviour. Biorheology 37:191–201 [PubMed] [Google Scholar]
- 22.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. 2008. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology 45:677–88 [PubMed] [Google Scholar]
- 23.Knapp DM, Barocas VH, Moon AG. 1997. Rheology of reconstituted type I collagen gel in confined compression. J. Rheol 41:971–93 [Google Scholar]
- 24.Janmey PA, Amis EJ, Ferry JD. 1983. Rheology of fibrin clots. VI. Stress relaxation, creep, and differential dynamic modulus of fine clots in large shearing deformations. J. Rheol 27:135–53 [Google Scholar]
- 25.Haugh MG, Heilshorn SC. 2016. Integrating concepts of material mechanics, ligand chemistry, dimensionality and degradation to control differentiation of mesenchymal stem cells. Curr. Opin. Solid State Mater. Sci 20:171–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron AR, Frith JE, Cooper-White JJ. 2011. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32:5979–93 [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, et al. 2015. Substrate stress relaxation regulates cell spreading. Nat. Commun 6:6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, et al. 2016. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15:326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinnon DD, Domaille DW, Cha JN, Anseth KS. 2014. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater 26:865–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawhney AS, Pathak CP, Hubbell JA. 1993. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(α-hydroxy acid) diacrylate macromers. Macromolecules 26:581–87 [Google Scholar]
- 31.Bryant SJ, Anseth KS. 2001. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res 59:63–72 [DOI] [PubMed] [Google Scholar]
- 32.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. 2003. Cell-responsive synthetic hydrogels. Adv. Mater 15:888–92 [Google Scholar]
- 33.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, et al. 2003. Synthetic matrix metalloproteinase–sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. PNAS 100:5413–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. 2009. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruoslahti E, Pierschbacher MD. 1987. New perspectives in cell adhesion: RGD and integrins. Science 238:491–97 [DOI] [PubMed] [Google Scholar]
- 36.Hersel U, Dahmen C, Kessler H. 2003. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24:4385–415 [DOI] [PubMed] [Google Scholar]
- 37.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. 1997. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385:537–40 [DOI] [PubMed] [Google Scholar]
- 38.Rahmany MB, Van Dyke M. 2013. Biomimetic approaches to modulate cellular adhesion in biomaterials: a review. Acta Biomater. 9:5431–37 [DOI] [PubMed] [Google Scholar]
- 39.Silva G, Czeisler C, Niece K, Beniash E, Harrington D, et al. 2004. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303:1352–55 [DOI] [PubMed] [Google Scholar]
- 40.Mehta M, Madl CM, Lee S, Duda GN, Mooney DJ. 2015. The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. J. Biomed. Mater. Res. A 103:3516–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jongpaiboonkit L, King WJ, Murphy WL. 2009. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng. A 15:343–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam J, Carmichael ST, Lowry WE, Segura T. 2015. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater 4:534–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. 2000. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci 113:1677–86 [DOI] [PubMed] [Google Scholar]
- 44.Lee KY, Alsberg E, Hsiong SX, Comisar WA, Linderman JJ, et al. 2004. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Lett. 4:1501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. 2007. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials 28:4409–17 [DOI] [PubMed] [Google Scholar]
- 46.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. 2013. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater 12:458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent LG, Engler AJ. 2013. Stem cell differentiation: Post-degradation forces kick in. Nat. Mater 12:384–86 [DOI] [PubMed] [Google Scholar]
- 48.Seo J-H, Kakinoki S, Inoue Y, Yamaoka T, Ishihara K, Yui N. 2013. Inducing rapid cellular response on RGD-binding threaded macromolecular surfaces. J. Am. Chem. Soc 135:5513–16 [DOI] [PubMed] [Google Scholar]
- 49.Tong X, Yang F. 2016. Sliding hydrogels with mobile molecular ligands and crosslinks as 3D stem cell niche. Adv. Mater 28:7257–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekerdt BL, Segalman RA, Schaffer DV. 2013. Spatial organization of cell-adhesive ligands for advanced cell culture. Biotechnol. J 8:1411–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Shoichet MS. 2004. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater 3:249–53 [DOI] [PubMed] [Google Scholar]
- 52.DeForest CA, Polizzotti BD, Anseth KS. 2009. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater 8:659–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen S, Alonso JM, Specht A, Duodu P, Goeldner M, del Campo A. 2008. Phototriggering of cell adhesion by caged cyclic RGD peptides. Angew. Chem. Int. Ed 47:3192–95 [DOI] [PubMed] [Google Scholar]
- 54.Ohmuro-Matsuyama Y, Tatsu Y. 2008. Photocontrolled cell adhesion on a surface functionalized with a caged arginine–glycine–aspartate peptide. Angew. Chem. Int. Ed 47:7527–29 [DOI] [PubMed] [Google Scholar]
- 55.Lee TT, García JR, Paez JI, Singh A, Phelps EA, et al. 2015. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater 14:352–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeForest CA, Anseth KS. 2011. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem 3:925–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeForest CA, Anseth KS. 2012. Photoreversible patterning of biomolecules within click-based hydrogels. Angew. Chem. Int. Ed 51:1816–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeForest CA, Tirrell DA. 2015. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater 14:523–31 [DOI] [PubMed] [Google Scholar]
- 59.Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, et al. 2013. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater 12:1072–78 [DOI] [PubMed] [Google Scholar]
- 60.Boekhoven J, Pérez CMR, Sur S, Worthy A, Stupp SI. 2013. Dynamic display of bioactivity through host–guest chemistry. Angew. Chem. Int. Ed 52:12077–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neirynck P, Schimer J, Jonkheijm P, Milroy L-G, Cigler P, Brunsveld L. 2015. Carborane-β-cyclodextrin complexes as a supramolecular connector for bioactive surfaces. J. Mater. Chem. B 3:539–45 [DOI] [PubMed] [Google Scholar]
- 62.Liu B, Liu Y, Riesberg JJ, Shen W. 2010. Dynamic presentation of immobilized ligands regulated through biomolecular recognition. J. Am. Chem. Soc 132:13630–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, Chen N, Li S, Battig MR, Wang Y. 2012. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. J. Am. Chem. Soc 134:15716–19 [DOI] [PubMed] [Google Scholar]
- 64.Freeman R, Stephanopoulos N, Álvarez Z, Lewis JA, Sur S, et al. 2017. Instructing cells with programmable peptide DNA hybrids. Nat. Commun 8:15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ushiki T 2002. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol 65:109–26 [DOI] [PubMed] [Google Scholar]
- 66.Petrie RJ, Doyle AD, Yamada KM. 2009. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell. Biol 10:538–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sill TJ, von Recum HA. 2008. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006 [DOI] [PubMed] [Google Scholar]
- 68.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, et al. 2007. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. PNAS 104:7791–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banwell EF, Abelardo ES, Adams DJ, Birchall MA, Corrigan A, et al. 2009. Rational design and application of responsive α-helical peptide hydrogels. Nat. Mater 8:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, et al. 2006. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv. Mater 18:611–14 [Google Scholar]
- 71.Xia X-X, Xu Q, Hu X, Qin G, Kaplan DL. 2011. Tunable self-assembly of genetically engineered silk-elastin-like protein polymers. Biomacromolecules 12:3844–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koutsopoulos S 2016. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J. Biomed. Mater. Res. A 104:1002–16 [DOI] [PubMed] [Google Scholar]
- 73.Kulangara K, Leong KW. 2009. Substrate topography shapes cell function. Soft Matter 5:4072–76 [Google Scholar]
- 74.Unadkat HV, Hulsman M, Cornelissen K, Papenburg BJ, Truckenmüller RK, et al. 2011. An algorithm-based topographical biomaterials library to instruct cell fate. PNAS 108:16565–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mascharak S, Benitez PL, Proctor AC, Madl CM, Hu KH, et al. 2017. YAP-dependent mechanotransduction is required for proliferation and migration on native-like substrate topography. Biomaterials 115:155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, et al. 2010. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. B 16:371–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han L-H, Yu S, Wang T, Behn AW, Yang F. 2013Microribbon-like elastomers for fabricating macroporous and highly flexible scaffolds that support cell proliferation in 3D. Adv. Funct. Mater 23:346–58 [Google Scholar]
- 78.Griffin DR, Weaver WM, Scumpia PO, Carlo DD, Segura T. 2015. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater 14:737–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. 2014. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng 42:1508–16 [DOI] [PubMed] [Google Scholar]
- 80.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. 2006. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314:298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paschos NK, Brown WE, Eswaramoorthy R, Hu JC, Athanasiou KA. 2015. Advances in tissue engineering through stem cell–based co-culture. J. Tissue Eng. Regen. Med 9:488–503 [DOI] [PubMed] [Google Scholar]
- 82.Kaji H, Camci-Unal G, Langer R, Khademhosseini A. 2011. Engineering systems for the generation of patterned co-cultures for controlling cell–cell interactions. Biochim. Biophys. Acta Gen. Subj 1810:239–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuhl PR, Griffith LG. 1996. Tethered epidermal growth factor as a paradigm for growth factor induced stimulation from the solid phase. Nat. Med 2:1022–27 [DOI] [PubMed] [Google Scholar]
- 84.Fan VH, Au A, Tamama K, Littrell R, Richardson LB, et al. 2007. Tethered EGF provides a survival advantage to mesenchymal stem cells. Stem Cells 25:1241–51 [DOI] [PubMed] [Google Scholar]
- 85.Cabanas-Danés J, Rodrigues ED, Landman E, van Weerd J, van Blitterswijk C, et al. 2014. A supramolecular host–guest carrier system for growth factors employing VHH fragments. J. Am. Chem. Soc 136:12675–81 [DOI] [PubMed] [Google Scholar]
- 86.Cambria E, Renggli K, Ahrens CC, Cook CD, Kroll C, et al. 2015. Covalent modification of synthetic hydrogels with bioactive proteins via sortase-mediated ligation. Biomacromolecules 16:2316–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Webber MJ, Tongers J, Newcomb CJ, Marquardt K-T, Bauersachs J, et al. 2011. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. PNAS 108:13438–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madl CM, Mehta M, Duda GN, Heilshorn SC, Mooney DJ. 2014. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 15:445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai L, Dinh CB, Heilshorn SC. 2014. One-pot synthesis of elastin-like polypeptide hydrogels with grafted VEGF-mimetic peptides. Biomater. Sci 2:757–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hudalla GA, Murphy WL. 2011. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv. Funct. Mater 21:1754–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, et al. 2017. Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr. Biol 9:271–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, et al. 2014. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343:885–88 [DOI] [PubMed] [Google Scholar]
- 93.Nguyen EH, Schwartz MP, Murphy WL. 2011. Biomimetic approaches to control soluble concentration gradients in biomaterials. Macromol. Biosci 11:483–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagaoka M, Ise H, Akaike T. 2002. Immobilized E-cadherin model can enhance cell attachment and differentiation of primary hepatocytes but not proliferation. Biotechnol. Lett 24:1857–62 [Google Scholar]
- 95.Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, et al. 2016. N-Cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater 15:1297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bian L, Guvendiren M, Mauck RL, Burdick JA. 2013. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. PNAS 110:10117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Straley KS, Heilshorn SC. 2009. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front. Neuroeng 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen KG, Mallon BS, McKay RDG, Robey PG. 2014. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 14:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Azarin SM, Palecek SP. 2010. Matrix revolutions: a trinity of defined substrates for long-term expansion of human ESCs. Cell Stem Cell 7:7–8 [DOI] [PubMed] [Google Scholar]
- 100.Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, et al. 2010. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol 28:611–15 [DOI] [PubMed] [Google Scholar]
- 101.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, et al. 2010. Synthetic peptide–acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol 28:606–10 [DOI] [PubMed] [Google Scholar]
- 102.Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, et al. 2010. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol 28:581–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dzhoyashvili NA, Shen S, Rochev YA. 2015. Natural and synthetic materials for self-renewal, long-term maintenance, and differentiation of induced pluripotent stem cells. Adv. Healthc. Mater 4:2342–59 [DOI] [PubMed] [Google Scholar]
- 104.Chowdhury F, Li Y, Poh Y-C, Yokohama-Tamaki T, Wang N, Tanaka TS. 2010. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell–matrix tractions. PLOS ONE 5:e15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McDevitt TC. 2013. Scalable culture of human pluripotent stem cells in 3D. PNAS 110:20852–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. 2007. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. PNAS 104:11298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. 2008. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 29:3946–52 [DOI] [PubMed] [Google Scholar]
- 108.Lei Y, Schaffer DV. 2013. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. PNAS 110:E5039–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crane GM, Jeffery E, Morrison SJ. 2017. Adult haematopoietic stem cell niches. Nat. Rev. Immunol 17:573–90 [DOI] [PubMed] [Google Scholar]
- 110.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, et al. 2010. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol 28:1123–28 [DOI] [PubMed] [Google Scholar]
- 111.Choi JS, Harley BAC. 2017. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv 3:e1600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prewitz MC, Seib FP, von Bonin M, Friedrichs J, Stißel A, et al. 2013. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat. Methods 10:788–94 [DOI] [PubMed] [Google Scholar]
- 113.Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. 2009. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol 1:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chamberlain G, Fox J, Ashton B, Middleton J. 2007. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–49 [DOI] [PubMed] [Google Scholar]
- 115.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–47 [DOI] [PubMed] [Google Scholar]
- 116.Yang C, Tibbitt MW, Basta L, Anseth KS. 2014. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13:645–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, et al. 2017. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater 16:379–89 [DOI] [PubMed] [Google Scholar]
- 118.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, et al. 2011. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater 10:637–44 [DOI] [PubMed] [Google Scholar]
- 119.Bai T, Sun F, Zhang L, Sinclair A, Liu S, et al. 2014. Restraint of the differentiation of mesenchymal stem cells by a nonfouling zwitterionic hydrogel. Angew. Chem. Int. Ed 53:12729–34 [DOI] [PubMed] [Google Scholar]
- 120.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. 2009. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459:262–65 [DOI] [PubMed] [Google Scholar]
- 121.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, et al. 2016. Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–64 [DOI] [PubMed] [Google Scholar]
- 122.DiMarco RL, Dewi RE, Bernal G, Kuo C, Heilshorn SC. 2015. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci 3:1376–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin H, Price F, Rudnicki MA. 2013. Satellite cells and the muscle stem cell niche. Physiol. Rev 93:23–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, et al. 2010. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329:1078–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, et al. 2014. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med 20:255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quarta M, Brett JO, DiMarco R, Morree AD, Boutet SC, et al. 2016. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol 34:752–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tong Z, Solanki A, Hamilos A, Levy O, Wen K, et al. 2015. Application of biomaterials to advance induced pluripotent stem cell research and therapy. EMBO J. 34:987–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seale NM, Varghese S. 2016. Biomaterials for pluripotent stem cell engineering: from fate determination to vascularization. J. Mater. Chem. B 4:3454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, et al. 2009. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cells Mater 18:1–14 [DOI] [PubMed] [Google Scholar]
- 130.Przybyla L, Lakins JN, Weaver VM. 2016. Tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell 19:462–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun Y, Yong KMA, Villa-Diaz LG, Zhang X, Chen W, et al. 2014. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater 13:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, et al. 2014. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. PNAS 111:13805–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, Levenberg S. 2011. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 32:9612–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen W, Villa-Diaz LG, Sun Y, Weng S, Kim JK, et al. 2012. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 6:4094–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie J, Willerth SM, Li X, Macewan MR, Rade A, et al. 2009. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30:354–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, et al. 2010. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials 31:4360–66 [DOI] [PubMed] [Google Scholar]
- 137.Kim JH, Kim HW, Cha KJ, Han J, Jang YJ, et al. 2016. Nanotopography promotes pancreatic differentiation of human embryonic stem cells and induced pluripotent stem cells. ACS Nano 10:3342–55 [DOI] [PubMed] [Google Scholar]
- 138.Dickinson LE, Kusuma S, Gerecht S. 2011. Reconstructing the differentiation niche of embryonic stem cells using biomaterials. Macromol. Biosci 11:36–49 [DOI] [PubMed] [Google Scholar]
- 139.Dixon JE, Shah DA, Rogers C, Hall S, Weston N, et al. 2014. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. PNAS 111:5580–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. 2007. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28:2706–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reilly GC, Engler AJ. 2010. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech 43:55–62 [DOI] [PubMed] [Google Scholar]
- 142.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, et al. 2015. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell–mediated bone formation. Nat. Mater 14:1269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. 2014. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials 35:1857–68 [DOI] [PubMed] [Google Scholar]
- 144.Darnell M, Young S, Gu L, Shah N, Lippens E, et al. 2017. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthc. Mater 6:1601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, et al. 2010. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31:2574–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mhanna R, Öztürk E, Vallmajo-Martin Q, Millan C, Müller M, Zenobi-Wong M. 2014. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. A 20:1165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frith JE, Mills RJ, Hudson JE, Cooper-White JJ. 2012. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 21:2442–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang T, Lai JH, Han L-H, Tong X, Yang F. 2014. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng. A 20:2131–39 [DOI] [PubMed] [Google Scholar]
- 149.Lee JS, Lee JS, Murphy WL. 2010. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 6:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, et al. 2015. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv. Healthc. Mater 4:131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vega SL, Kwon M, Mauck RL, Burdick JA. 2016. Single cell imaging to probe mesenchymal stem cell N-cadherin mediated signaling within hydrogels. Ann. Biomed. Eng 44:1921–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stukel JM, Willits RK. 2016. Mechanotransduction of neural cells through cell–substrate interactions. Tissue Eng. B 22:173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yao S, Liu X, Wang X, Merolli A, Chen X, Cui F. 2013. Directing neural stem cell fate with biomaterial parameters for injured brain regeneration. Prog. Nat. Sci. Mater. Int 23:103–12 [Google Scholar]
- 154.Saha K, Keung AJ, Irwin EF, Li Y, Little L, et al. 2008. Substrate modulus directs neural stem cell behavior. Biophys. J 95:4426–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leipzig ND, Shoichet MS. 2009. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30:6867–78 [DOI] [PubMed] [Google Scholar]
- 156.Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S. 2011. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29:1886–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV. 2017. Dynamics of mechanosensitive neural stem cell differentiation. Stem Cells 35:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keung AJ, Dong M, Schaffer DV, Kumar S. 2013. Pan-neuronal maturation but not neuronal subtype differentiation of adult neural stem cells is mechanosensitive. Sci. Rep 3:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, et al. 2009. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30:4695–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aurand ER, Wagner JL, Shandas R, Bjugstad KB. 2014. Hydrogel formulation determines cell fate of fetal and adult neural progenitor cells. Stem Cell Res. 12:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lampe KJ, Bjugstad KB, Mahoney MJ. 2010. Impact of degradable macromer content in a poly(ethylene glycol) hydrogel on neural cell metabolic activity, redox state, proliferation, and differentiation. Tissue Eng. A 16:1857–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lim SH, Liu XY, Song H, Yarema KJ, Mao H-Q. 2010. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 31:9031–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aizawa Y, Leipzig N, Zahir T, Shoichet M. 2008. The effect of immobilized platelet derived growth factor AA on neural stem/progenitor cell differentiation on cell-adhesive hydrogels. Biomaterials 29:4676–83 [DOI] [PubMed] [Google Scholar]
- 164.Stadtfeld M, Hochedlinger K. 2010. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 24:2239–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Choi B, Park K-S, Kim J-H, Ko K-W, Kim J-S, et al. 2016. Stiffness of hydrogels regulates cellular reprogramming efficiency through mesenchymal-to-epithelial transition and stemness markers. Macromol. Biosci 16:199–206 [DOI] [PubMed] [Google Scholar]
- 166.Downing TL, Soto J, Morez C, Houssin T, Fritz A, et al. 2013. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater 12:1154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caiazzo M, Okawa Y, Ranga A, Piersigilli A, Tabata Y, Lutolf MP. 2016. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater 15:344–52 [DOI] [PubMed] [Google Scholar]
- 168.Sia J, Sun R, Chua J, Li S. 2016. Dynamic culture improves cell reprogramming efficiency. Biomaterials 92:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, et al. 2010The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials 31:1885–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carpenedo RL, Sargent CY, McDevitt TC. 2007. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 25:2224–34 [DOI] [PubMed] [Google Scholar]
- 171.Selekman JA, Das A, Grundl NJ, Palecek SP. 2013. Improving efficiency of human pluripotent stem cell differentiation platforms using an integrated experimental and computational approach. Biotechnol. Bioeng. 110:3024–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mei Y, Gerecht S, Taylor M, Urquhart AJ, Bogatyrev SR, et al. 2009. Mapping the interactions among biomaterials, adsorbed proteins, and human embryonic stem cells. Adv. Mater 21:2781–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Flaim CJ, Chien S, Bhatia SN. 2005. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2:119–25 [DOI] [PubMed] [Google Scholar]
- 174.Titmarsh DM, Ovchinnikov DA, Wolvetang EJ, Cooper-White JJ. 2013. Full factorial screening of human embryonic stem cell maintenance with multiplexed microbioreactor arrays. Biotechnol. J 8:822–34 [DOI] [PubMed] [Google Scholar]
- 175.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. 2011. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8:949–55 [DOI] [PubMed] [Google Scholar]
- 176.Ranga A, Gobaa S, Okawa Y, Mosiewicz K, Negro A, Lutolf MP. 2014. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun 5:4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le NNT, Zorn S, Schmitt SK, Gopalan P, Murphy WL. 2016. Hydrogel arrays formed via differential wettability patterning enable combinatorial screening of stem cell behavior. Acta Biomater. 34:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim HD, Lee EA, Choi YH, An YH, Koh RH, et al. 2016. High throughput approaches for controlled stem cell differentiation. Acta Biomater. 34:21–29 [DOI] [PubMed] [Google Scholar]
- 179.Nguyen EH, Daly WT, Le NNT, Farnoodian M, Belair DG, et al. 2017. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng 1:0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lancaster MA, Knoblich JA. 2014. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125. [DOI] [PubMed] [Google Scholar]
- 181.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, et al. 2017. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 35:659–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shao Y, Taniguchi K, Gurdziel K, Townshend RF, Xue X, et al. 2016. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater 16:419–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Murphy SV, Atala A. 2014. 3D bioprinting of tissues and organs. Nat. Biotechnol 32:773–85 [DOI] [PubMed] [Google Scholar]
- 184.Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. 2016. A review of three-dimensional printing in tissue engineering. Tissue Eng. B 22:298–310 [DOI] [PubMed] [Google Scholar]