Abstract
Purpose
Most patients with allergic rhinitis (AR) have moderate-to-severe disease, requiring complete and prompt relief when symptoms occur. The time course of fluticasone propionate (FP) penetration into nasal tissues after intranasal administration is not well characterized. The goal of this proof-of-concept study was to evaluate the mucosal penetration of FP from fixed-combination FP-azelastine nasal spray (MP-AzeFlu) compared with an FP-only nasal spray in an in vitro, 3-dimensional human bronchial tissue model.
Materials and Methods
Absorption of FP from MP-AzeFlu and FP nasal spray was modeled using EpiAirway™606 (MatTek Corporation; Ashland, MA, USA) tissue cultured in vertical diffusion cells. The dosing amount of MP-AzeFlu was optimized in a pilot study. Based on the results of the pilot study, 10 µL of MP-AzeFlu (3.65 µg; n = 8) and 10 µL of FP nasal spray (5.00 µg; n = 8) were evaluated for penetration of tissue. Tissue integrity was monitored with Lucifer yellow. FP in the receiving media was quantified for each sample using liquid chromatography with tandem mass spectrometry.
Results
MP-AzeFlu and FP nasal spray were associated with similar FP accumulation profiles in the receiving media, but the permeability of FP was greater for MP-AzeFlu during hours 0 to 6, suggesting faster absorption for MP-AzeFlu. No indications of compromised tissue integrity were found in any of the tested cells.
Conclusion
The higher and more rapid penetration of FP from MP-AzeFlu supports the use of MP-AzeFlu for patients with AR, particularly when prioritizing fast and pronounced symptom relief.
Keywords: absorption, allergic rhinitis, azelastine hydrochloride, mucosal penetration, pharmacology
Plain Language Summary
Most patients who have allergic rhinitis (AR) have a moderate-to-severe form of the disease. Because of this, many of them consider it important to have fast, complete relief from their symptoms. MP-AzeFlu, a nasal spray that contains a combination of azelastine hydrochloride and fluticasone propionate (FP) and is dosed in a single spray, has been shown in studies to be more effective than FP alone for treating patients with AR. The goal of this study was to determine how much FP absorbs into the mucus membranes from MP-AzeFlu compared with an FP-only nasal spray by using a lifelike model of human bronchial tissue. Using 10 µL of MP-AzeFlu (3.65 µg; n = 8) and 10 µL of FP nasal spray (5.00 µg; n = 8), the researchers found that although the 2 nasal sprays showed similar amounts of drug collected in the models, MP-AzeFlu absorbed into mucus membranes to a greater degree. This supports findings from other clinical studies showing greater effectiveness of MP-AzeFlu over FP-only nasal spray, as well as the use of MP-AzeFlu to provide fast, complete symptom relief for patients with AR.
Introduction
Most patients with allergic rhinitis (AR) have moderate-to-severe disease. In a study of 3052 patients with AR, 93% had moderate or severe disease on the basis of Allergic Rhinitis and its Impact on Asthma classification.1 Because of the severity of AR, many patients with AR prioritize complete and prompt relief when symptoms occur. In a study of adults with AR, patients reported they would consider a treatment ineffective if it did not provide rapid and long-lasting relief for their symptoms.2 Furthermore, 52% of patients reported they did not experience relief within 1 hour when using intranasal corticosteroids for AR.2 These data suggest a need for fast-acting, effective treatments for AR symptom relief.
Intranasal fixed-combination azelastine hydrochloride and fluticasone propionate (FP) delivered in a single spray (MP-AzeFlu) has demonstrated superior efficacy to intranasal FP (Flonase®) alone for treating AR. In a post hoc analysis of a double-blind, placebo-controlled study of people with moderate-to-severe AR, MP-AzeFlu resulted in a significantly better improvement (52%) in the combined total nasal and ocular symptom scores compared with FP nasal spray alone.3 Even in people with severe AR, MP-AzeFlu has been shown to provide relief for individual nasal symptoms at levels greater than those seen for FP alone.4
Despite patient reports of rapid relief, the time course of FP penetration into nasal tissues after intranasal administration of MP-AzeFlu or FP nasal spray alone is not well characterized. To evaluate the mucosal penetration of FP from MP-AzeFlu, an in vitro, 3-dimensional human bronchial tissue model was used. This model has features associated with epithelial airways, including a mixed-cell phenotype with ciliated, mucus-secreting goblet and basal cells, as well as tight junctions.5 Primary human epithelial cell culture models have been shown to be more sensitive to drug exposure than traditional immortalized cell culture models, and the permeable, 3-dimensional nature facilitates penetration studies.5 The EpiAirway model has been demonstrated to accurately assess drug permeability of new intranasal drug formulations.6 The cells were mounted in specially designed diffusion chambers to allow the measurement of drug absorption. We hypothesized that the enhanced efficacy of MP-AzeFlu may be attributable to differences in formulations between MP-AzeFlu and FP-only nasal spray.
Materials and Methods
Materials
MP-AzeFlu (0.1% solution azelastine hydrochloride and 0.037% suspension of micronized fluticasone propionate in an isotonic aqueous suspension containing glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, phenylethyl alcohol, edetate disodium, benzalkonium chloride, polysorbate 80, purified water) and FP spray were obtained from the manufacturers. Other reagents were obtained from commercial sources.
Study Design
EpiAirway™ EPI-606-X cells (MatTek Corporation; Ashland, MA, USA) were cultured in vertical diffusion cells at 37°C using 1-mL EpiAirway culture assay media (MatTek) with 4% bovine serum albumin (Fisher Bioreagents; Waltham, MA, USA). EpiAirway culture medium without phenol red (MatTek) was used as receiving medium in the bottom of the diffusion well.
The dosing amount of MP-AzeFlu was optimized in a pilot study. As a result, 10 µL of MP-AzeFlu (3.65 µg; n = 8) and 10 µL of FP (5.00 µg; n = 8) were evaluated for penetration of epithelial tissue. MP-AzeFlu placebo nasal spray was used as a control (n = 1).
MP-AzeFlu and FP-only formulations were applied dropwise to the ciliated apical surface (top) of the EpiAirway tissue. Samples were collected from the receiver fluid in the bottom of the chamber at 1, 2, 4, 6, 18, and 18.5 hours to determine the extent of FP penetration through the microporous membrane.
Tissue integrity was monitored with a Lucifer yellow assay at 18 hours for all replicates and at 0 hours as a control (n = 1). After the addition of 1.0 mL of 280 µM Lucifer yellow to each cell, the color change was observed for 30 minutes to evaluate the tissue integrity. After 30 minutes, 500 µL of receiving media was removed with a glass syringe for analysis.
Analytic Methods
FP and Lucifer yellow in the receiving media were quantified for each sample using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Chromatographic separation was performed with a Waters XBridge C18 Column (Waters Corporation, Milford, MA, USA; 3.0 mm x 50 mm, 3.5 µm) on a Shimadzu LC system with an LC-20AD pump and an SIL-20AC/HT autosampler (Shimadzu Scientific Instruments; Kyoto, Japan). The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The linear gradient elution program was as follows: 0.01 min, 95% A; 0.17 min, 95% A; 2.50 min, 0% A; 4.00 min, 0% A; 4.10 min, 95% A; and 5.00 min, 0% A. The flow rate was 0.5 mL/min, and the total run time was 5.0 min. MS was performed with an API 4000 (SCIEX; Framingham, MA, USA) and a TurboIonSpray interface (SCIEX) with a dwell time of 50 ms at a temperature of 700°C.
The parameters calculated based on the LC-MS/MS penetration profile were amounts of FP in receiver media (ng/cm2), flux of FP (ng/cm2/h), permeability of FP (nm/s), and Lucifer yellow tissue integrity.
Results
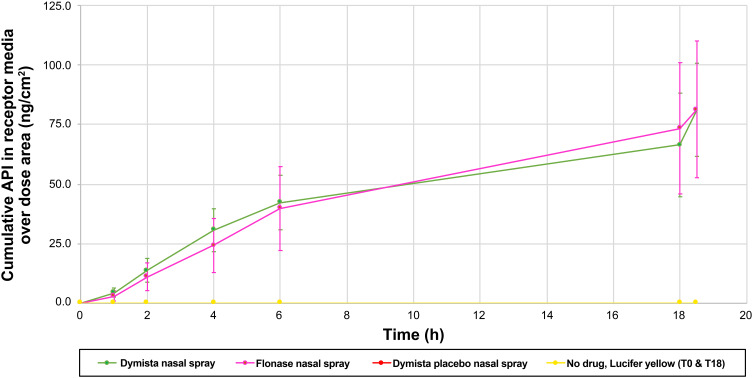
MP-AzeFlu and FP nasal spray were associated with similar FP accumulation profiles in the receiving media (Figure 1). On the basis of theoretical calculations, the drug accumulation profiles were suggestive of 2.5% drug delivery. However, because the concentration of FP differs between MP-AzeFlu and FP nasal spray, the permeability value differed despite the similar accumulation profiles. MP-AzeFlu placebo and the no-treatment control did not result in FP in the receiving media.
Figure 1.
Mean amount of fluticasone propionate released in receiving media (average of 8 cells + SD).
Abbreviations: API, active pharmaceutical ingredient; FP, fluticasone propionate; h, hour; MP-AzeFlu, combination FP-azelastine nasal spray; SD, standard deviation; T, time point.
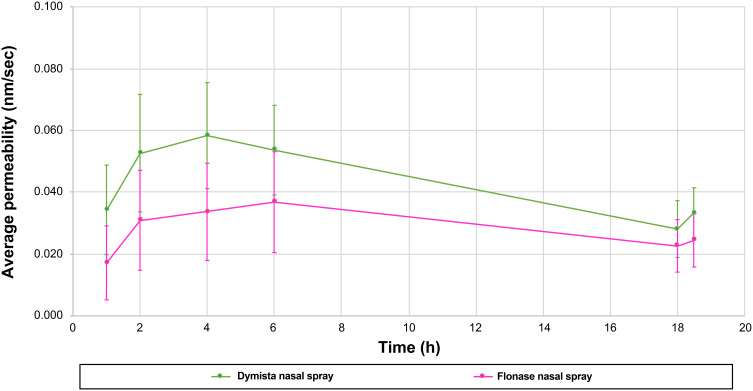
After controlling for differences in the concentration of FP in each product, FP flux and permeability were similar between MP-AzeFlu and FP nasal spray at 18 hours. Between 0 and 6 hours, MP-AzeFlu resulted in significantly higher FP penetration than FP nasal spray (P <0.05). Permeability profiles are shown in Figure 2.
Figure 2.
Permeability profiles of MP-AzeFlu and fluticasone propionate nasal spray (average of 8 cells + SD).
Abbreviations: FP, fluticasone propionate; h, hour; MP-AzeFlu, combination FP-azelastine nasal spray; SD, standard deviation.
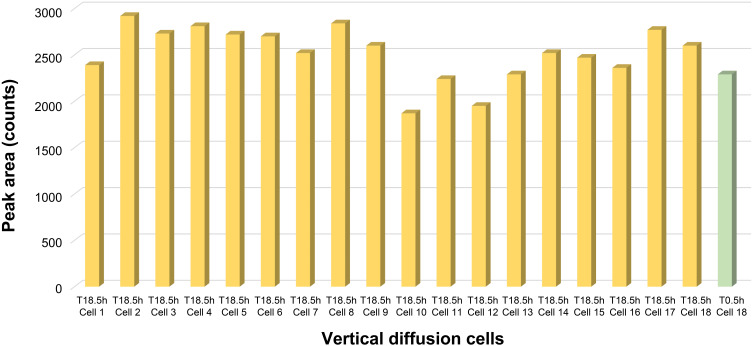
No indications of compromised tissue integrity were observed in any of the tested cells. The drug concentration in the receiving media increased gradually over the time points evaluated, suggesting maintenance of tissue integrity. Lucifer yellow content was uniform in all the cells at 18.5 hours, similar to the content reported for the control well at time point 0 hours, providing additional support for the maintenance of tissue integrity (Figure 3).
Figure 3.
Tissue integrity on the basis of Lucifer yellow testing after 18 hours.
Abbreviations: C, cell; h, hour; T, time.
Discussion
In this proof-of-concept study utilizing a 3-dimensional model of airway tissue, the application of both MP-AzeFlu and FP nasal spray resulted in FP penetration of the microporous membrane. This in vitro study was the first to evaluate the effect of the formulation on the penetration of only FP. Absolute amounts of accumulated FP were similar between treatments, but FP permeation occurred more quickly with MP-AzeFlu than with FP nasal spray. This would suggest that, compared with use of FP nasal spray, use of MP-AzeFlu may result in faster penetration of local nasal tissue, allowing for quicker local effects. These results are clinically important, because active ingredients of intranasal medications are removed from the nasal cavity by mucociliary clearance in a time-dependent manner.7
The results reported here are consistent with the findings from prior pharmacokinetic (PK) studies, as well as from a clinical trial comparing the onset of action of MP-AzeFlu and commercially available FP nasal spray.8,9 In the PK study, the maximum and total FP exposure among participants was 60% higher for MP-AzeFlu than for FP nasal spray despite their receiving the same nominal dose. However, MP-AzeFlu demonstrated limited systemic FP bioavailability (1.86%) and mean peak FP concentration of 10–12 pg/mL or less, well below the systemic concentrations that are likely to have clinical effects.8 Nasal delivery of the medication directly to the local site of action avoids clinically significant systemic corticosteroid exposure.8 In a randomized, controlled trial, AR symptoms were induced by exposure to ragweed pollen in an environmental exposure chamber. A single dose of MP-AzeFlu was associated with a significantly faster onset of action than the free combination of intranasal FP and oral loratadine (5 vs 150 minutes); however, differences in rates of absorption for FP may be attributable to the faster effect of intranasal versus oral antihistamines.9 Of note, the maximum approved daily dose for FP products (400 µg) is twice the intended daily dose of MP-AzeFlu (200 µg).10 Therefore, with regular use of MP-AzeFlu, patients will be exposed to less overall FP than with commercially available FP monotherapy products that have been established as safe.
Several potential explanations exist for the higher level of permeation of FP from MP-AzeFlu formulation compared with conventional FP nasal spray. The formulations of the products are different, which could lead to different solubilities of FP. Whereas both products contain FP as suspensions, MP-AzeFlu also contains azelastine in solution. It is possible that the differing formulation may lead to dissolution of FP to a greater extent in MP-AzeFlu than in FP nasal spray. The presence of a concomitant drug in the product may affect mucosal permeability and it is possible that azelastine acts as an absorption enhancer. Furthermore, excipients, including EDTA-Na2 (potential penetration enhancer, not present in conventional FP nasal spray) and lower concentration of benzalkonium chloride, may also contribute to the greater permeability of FP in MP-AzeFlu. While this study was not intended to evaluate the effect of different excipients on the level of permeation of FP into nasal tissues, given these results, future studies evaluating their effects would provide further insight into their potential impact. Finally, differences in droplet size distribution and spray pattern may influence the penetration of FP in vivo, and the difference in viscosity may influence the penetration of FP in vitro and in vivo.
Conclusions
Small differences in formulations may matter, and FP from MP-AzeFlu penetrated epithelial tissue in vitro more quickly than FP from conventional nasal spray. The higher and more rapid penetration of FP from MP-AzeFlu in these experiments provides support for observations made in clinical studies and for the use of MP-AzeFlu in patients with AR, particularly when prioritizing fast and pronounced symptom relief.
Acknowledgments
The abstract of this paper was presented at the AAAAI/WAO Joint Congress as a poster presentation in March 2018. The poster’s abstract was published in “Poster Abstracts” in The Journal of Allergy and Clinical Immunology: https://doi.org/10.1016/j.jaci.2017.12.957.
Funding Statement
This study was supported by Meda Pharma GmbH & Co. KG (a Mylan Company), Bad Homburg, Germany.
Abbreviations
AR, allergic rhinitis; FP, fluticasone propionate; LC-MS/MS, liquid chromatography with tandem mass spectrometry; PK, pharmacokinetic.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
WEB reports personal fees from AstraZeneca, ALK, Regeneron/Sanofi Genzyme, Optinose, Boehringer Ingelheim, and Kaleo, outside the submitted work. CB serves on the Speakers Bureau for Mylan Inc. RA was an employee of Meda Pharmaceuticals (now Mylan Inc.) at the time of the study. AK is an employee of Mylan Inc., USA. FK and JM are employees of Meda Pharma GmbH & Co. KG (a Mylan company), Germany. AD was an employee of Meda Pharmaceuticals (now Mylan Inc.) at the time of the study and is an independent consultant and president of Daddio Pharma Consulting LLC. The authors report no other conflicts of interest in this work.
References
- 1.Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117(1):158–162. doi: 10.1016/j.jaci.2005.09.047 [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012;33(Suppl 1):S113–S141. doi: 10.2500/aap.2012.33.3603 [DOI] [PubMed] [Google Scholar]
- 3.Meltzer E, Ratner P, Bachert C, et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161(4):369–377. doi: 10.1159/000351404 [DOI] [PubMed] [Google Scholar]
- 4.Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129(5):1282–1289. doi: 10.1016/j.jaci.2012.01.077 [DOI] [PubMed] [Google Scholar]
- 5.BéruBé K, Prytherch Z, Job C, Hughes T. Human primary bronchial lung cell constructs: the new respiratory models. Toxicology. 2010;278(3):311–318. doi: 10.1016/j.tox.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 6.Leonard AK, Sileno AP, Brandt GC, Foerder CA, Quay SC, Constantino HR. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int J Pharm. 2007;335(1–2):138–146. doi: 10.1016/j.ijpharm.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 7.Gizurarson S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol Pharm Bull. 2015;38(4):497–506. doi: 10.1248/bpb.b14-00398 [DOI] [PubMed] [Google Scholar]
- 8.Derendorf H, Munzel U, Petzold U, et al. Bioavailability and disposition of azelastine and fluticasone propionate when delivered by MP29-02, a novel aqueous nasal spray. Br J Clin Pharmacol. 2012;74(1):125–133. doi: 10.1111/j.1365-2125.2012.04222.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet J, Meltzer EO, Couroux P, et al. Onset of action of the fixed combination intranasal azelastine-fluticasone propionate in an allergen exposure chamber. J Allergy Clin Immunol Pract. 2018;6(5):1726–1732. doi: 10.1016/j.jaip.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 10.Dymista (azelastine hydrochloride and fluticasone propionate spray, metered) [prescribing information]. Somerset, NJ: Meda Pharmaceuticals Inc; 2018. [Google Scholar]