Abstract
Background
In population-based cancer survival studies, the event of interest is usually death due to cancer. However, other competing events may be present. Relative survival is a commonly used measure in cancer studies that circumvents problems caused by the inaccuracy of the cause of death information. A summary of the prognosis of the cancer population and potential differences between subgroups can be obtained using marginal estimates of relative survival.
Methods
We utilize regression standardization to obtain marginal estimates of interest in a relative survival framework. Such measures include the standardized relative survival, standardized all-cause survival and standardized crude probabilities of death. Contrasts of these can be formed to explore differences between exposure groups and under certain assumptions are interpreted as causal effects. The difference in standardized all-cause survival can also provide an estimate for the impact of eliminating cancer-related differences between exposure groups. The potential avoidable deaths after such hypothetical scenarios can also be estimated. To illustrate the methods we use the example of survival differences across socio-economic groups for colon cancer.
Results
Using relative survival, a range of marginal measures and contrasts were estimated. For these measures we either focused on cancer-related differences only or chose to incorporate both cancer and other cause differences. The impact of eliminating differences between groups was also estimated. Another useful way for quantifying that impact is the avoidable deaths under hypothetical scenarios.
Conclusions
Marginal estimates within the relative survival framework provide useful summary measures and can be applied to better understand differences across exposure groups.
Keywords: Relative survival, marginal measures, causal effects, avoidable deaths
Key Messages
Marginal measures provide population estimates in a relative survival framework with a simple interpretation.
Such measures are marginal relative survival, marginal all-cause survival and marginal crude probabilities of death.
Under certain assumptions, the difference in marginal estimates between subgroups can be interpreted as the average causal effect.
Using the relative survival framework enables us to focus on cancer-related differences instead of all-cause differences that are more difficult to explore.
The avoidable deaths under hypothetical scenarios can also be estimated.
Introduction
In population-based cancer survival studies the event of interest is usually death due to a specific cancer. However, death from other causes may prevent the event of interest occurring, i.e. there are so-called competing risks. To quantify cancer survival while accounting for differential other cause probability of death, net survival can be estimated. Net survival is the survival in a hypothetical world where the only possible cause of death is death due to cancer. It can be estimated using either the cause-specific or relative survival (excess mortality) approach.1,2 The cause-specific approach estimates cancer survival by censoring patients who died from other causes at the time of death. Information on cause of death is often inaccurate, particularly for the elderly. Relative survival is an alternative method to estimate net survival, but does not require information on cause of death through incorporating the expected mortality rates using population life tables.3–7 Information on appropriate expected mortality rates is essential for the interpretation of relative survival as net survival.
Within the relative survival framework, two probabilities of interest are the net probability of death, which is 1 minus relative survival, and the crude probability of death. Each approach yields a different estimand and the choice is based on the research question of interest.8 Net probabilities of death focus on a hypothetical world where the cancer of interest is the only possible cause of death. They provide a useful measure when comparing populations, such as countries or socio-economic groups, with differential other cause mortality rates as they focus on differences that are only due to the cancer of interest. Crude probabilities of death are more appropriate when making clinical decisions for a specific patient as they quantify survival in the presence of other possible causes of death.9 In the competing risks literature, the terminology is generally different where crude probabilities are often referred to as cause-specific cumulative incidence functions.10
To improve understanding of the mechanisms that drive associations, causal inference methods can be applied.11,12 The mathematical framework used for formulating statistical models and assumptions for causal inference is that of potential outcomes: the outcomes that would be observed if the patient received a specific level of the exposure.13 Causal effects are defined as contrasts of marginal effects of the potential outcomes and enable quantification of differences in prognosis of subgroups.14 In order to make causal statements certain assumptions need to hold and these are explored later in this paper in the context of the relative survival setting—with one main issue being the level of stratification in the lifetables that is adequate to achieve conditional exchangeability for other cause mortality. Marginal effects can be estimated using approaches such as inverse probability weights, but here our focus is on using regression standardization to obtain standardized survival and related functions.15,16 Their simple interpretation as a single measure for each time-point circumvents problems of communication of results from complex statistical models. Contrasts of standardized measures can also be utilized to investigate the impact of eliminating the observed differences between groups.
In this paper, we define various marginal measures using the relative survival framework and we define causal effects to compare population subgroups. To illustrate the measures we use the example of survival differences across socio-economic groups for colon cancer. Moreover, we estimate the potential avoidable deaths under a hypothetical scenario of eliminating survival differences between groups.
The remainder of the paper is organized as follows. First, we introduce the data to illustrate the methods and describe relative survival and excess mortality. Then, we define marginal measures of interest as well as contrasts between subgroups. Following, we describe contrasts within subsets of the population, including the avoidable deaths. Finally, we provide a summary of the methods.
Introducing the illustrative example
Data, made available by Public Health England, includes patients diagnosed with colon cancer in 2008 in England, with follow-up to the end of 2013 and information on gender, age at diagnosis and deprivation status. There were five deprivation groups, derived from national quintiles of the income domain of the area of patients’ residence at diagnosis.17,18 For simplicity we only included the least and most deprived groups, resulting in 7346 patients in total, 55% of whom are in the least deprived group. More details on the study population are available in Table 1.
Table 1.
Number of colon cancer patients (with proportions) for gender and age-groups by deprivation group
| Deprivation group |
||
|---|---|---|
| Least deprived | Most deprived | |
| Gender | ||
| Male | 2136 (52.37%) | 1772 (52.24%) |
| Female | 1943 (47.63%) | 1495 (45.76%) |
| Age group, years | ||
| 18–44 | 102 (2.50%) | 116 (3.55%) |
| 45–54 | 210 (5.15%) | 209 (6.40%) |
| 55–64 | 769 (18.85%) | 521 (15.95%) |
| 65–74 | 1149 (28.17%) | 972 (29.75%) |
| 75–84 | 1332 (32.66%) | 1045 (31.99%) |
| 85+ | 517 (12.67%) | 404 (12.37%) |
Excess mortality and relative survival
The underlying all-cause mortality rate of an individual , with covariate pattern , is given as the summation of the expected mortality rates if they did not have the cancer, and their excess mortality due to the cancer, :
with denoting the set of all covariates. and denote the covariates for expected and excess mortalities respectively. The expected mortality rate is obtained from available life tables on a comparable population in the general population matched by characteristics such as age, sex, calendar year and deprivation status.2 The survival analogue of excess mortality is relative survival. The relative survival of an individual, , is defined as their all-cause survival,, divided by their expected survival, . The all-cause survival is thus given as
| (1) |
Relative survival accounts for different background mortality rates in patients with different characteristics, without having to rely on the cause of death information and under assumptions is interpreted as net survival.7,19 Net survival has the interpretation of the probability of survival in a hypothetical world where the only possible cause of death is the cancer of interest. In order for this interpretation to be valid certain assumptions need to hold. These are (i) appropriate expected mortality rate that represents mortality due to other causes for the cancer population and (ii) the potential times to death from cancer and other causes are conditionally independent. When important variables that affect both cancer and other causes of deaths are not included in the available life tables for the expected mortality rates, then comparability between populations is lost and relative survival cannot be interpreted as net survival.19
There are several models for relative survival and the following sections in theory can be applied to any of these.20–23 We choose flexible parametric survival models, which use splines to model the effect of time and are preferable in our setting as they incorporate time-dependent effects (non-proportional hazards) easily.24,25
For the illustrative example, we fitted a flexible parametric model with 5 degrees of freedom for the baseline excess hazard. The model included deprivation status, gender and age. Age was included as a continuous non-linear variable using restricted cubic splines with 3 degrees of freedom. We also included time-dependent effects for age and deprivation. We then derived various predictions based on the fitted model. More details on Stata code are available in Supplementary Appendix A, available as Supplementary data at IJE online.
Marginal measures
Marginal measures provide population summaries with a simple interpretation.16 In the following subsections, we define the marginal relative survival function and describe how to obtain the marginal all-cause survival and marginal crude probabilities within the relative survival framework. Contrasts between subgroups are described in the section headed ‘Forming contrasts between population groups’.
Marginal relative survival
Let denote the conditional relative survival given covariates . The marginal relative survival is:
| (2) |
with the expectation over the marginal distribution of . After fitting a survival model, can be estimated by obtaining predictions of relative survival for each individual in the study population and taking an average of these predictions,
where is the number of patients in the population.
If interest is on the mortality scale, the standardized net probability of death can be obtained instead by .
Standardizing to an external population might also be applied and is particularly common when comparing relative survival across different countries.26 For instance, the externally age-standardized relative survival is calculated as
where is the ratio of the proportion within an age group in the reference population to the corresponding group in the study population. Weights higher than 1 are applied to groups that are underrepresented in the study population compared with the standard population.7
Marginal all-cause survival
To quantify survival in the presence of both cancer and other causes, the marginal all-cause survival function can be obtained. The estimand of interest in now defined as:
| (3) |
and is estimated by the standardized all-cause survival
The standardized all-cause probability of death can also be estimated as .
Marginal crude probabilities of death
Let the crude probability of dying from the cancer of interest by time in the presence of a competing risk of death due to other causes be and the probability of dying of causes other than the cancer of interest in the presence of cancer be 27 The marginal crude probability of dying from cancer is defined as
and is estimated by the standardized crude-probability of death due to cancer
Similarly, the marginal crude probability of dying of causes other than the cancer of interest is estimated by
Example
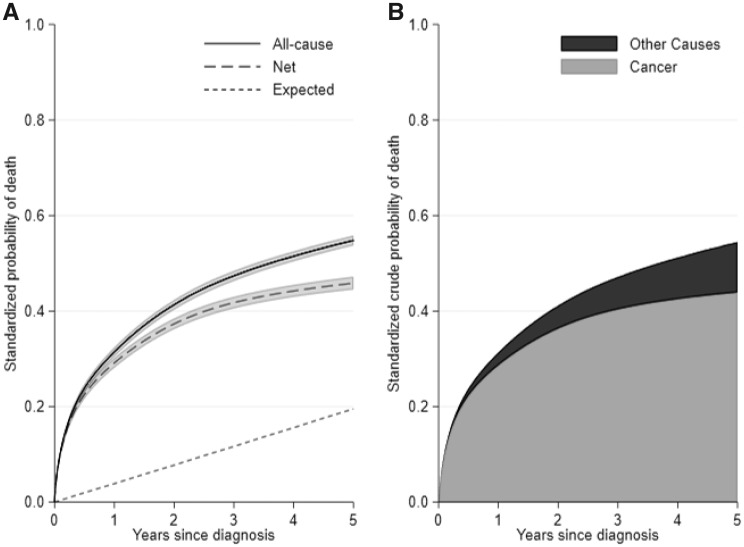
The marginal 5-year expected probability of death for a population without colon cancer is 20%. For the colon cancer population, the 5-year standardized net and all-cause probability of death was 46 and 55% respectively (Fig. 1). The net probability of death is lower as it is estimated in a hypothetical world where it is not possible to die from other causes. The all-cause probability of death can also be partitioned to that due to cancer and that due to other causes. The 5-year crude probability of death due to cancer in the presence of other risks was 44% and the crude probability of death due to other causes in the presence of cancer was 11% (Fig. 1).
Figure 1.
(A) Standardized all-cause and net probability of death, with 95% confidence intervals, as well as expected probability of death, and (B) stacked plot for the standardized crude probabilities for cancer and other causes, for the whole study population.
Forming contrasts between population groups
Let’s assume that we want to estimate the effect of exposure, , on the time-to-event outcome, while allowing for confounding . For simplicity, X will be a binary variable with for the exposed patients and for the unexposed. Let be the counterfactual survival function that we would have observed, had everybody in the population been exposed to level .
To form contrasts between the exposure groups the difference can be estimated and it has the advantage of being collapsible.28,29 The difference between levels and is defined as . The first term is the counterfactual survival function if everyone in our population had and the second term is the counterfactual survival function if everyone had .
Contrasts between the counterfactual outcomes can be estimated using the observed outcomes, under some assumptions.8,30 These are (i) conditional exchangeability meaning that the outcome and the exposure are independent given covariates, (ii) consistency i.e. an individual’s potential outcome under a specific exposure corresponds to the actual outcome of this person under this exposure level and (iii) positivity so that the probability of being in every level of the exposure group is positive for all levels of Z. Notice that the assumptions are now extended to both outcomes (death due to cancer and death due to other causes). The conditional exchangeability assumption for the other cause mortality can only be achieved by adjusting the available life tables of the general population for sufficient variables (see Discussion for further details).
Relative survival differences
The difference in marginal relative survival functions, comparing and , is defined as
| (4) |
and gives the difference in the hypothetical situation where the cancer of interest is the only possible cause of death. It is estimated by
Here everyone is first forced to be exposed () and then unexposed (). A key point is that the average over confounders, , is the same when estimating both marginal effects.
All-cause survival differences
The difference in marginal all-cause survival can also be defined by incorporating the expected survival in equation (4):
| (5) |
and is estimated by
All-cause survival differences move away from the hypothetical world of relative survival and take into account both cancer-related and other-causes-related survival. Equation (5) has also the interpretation of the potential impact of removing all-cause differences between exposed and unexposed.
Example
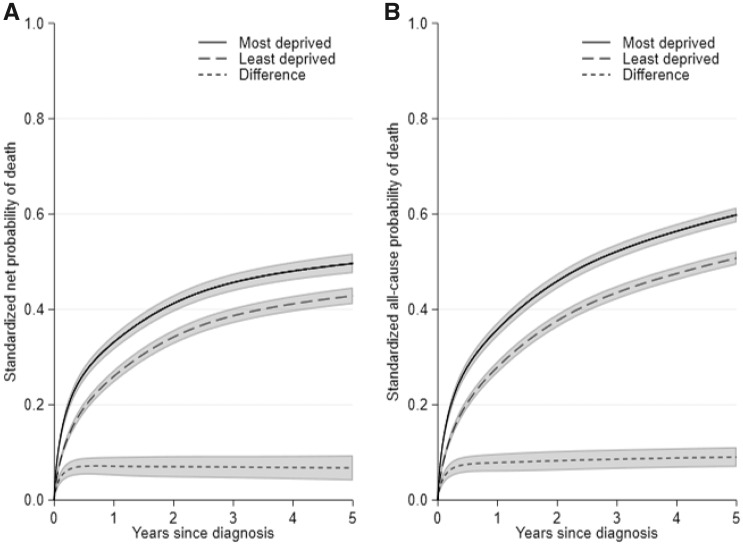
Figure 2 shows the standardized net and all-cause probabilities of death by deprivation. These are standardized over the combined age and sex distribution. The 5-year standardized net probability of death of the least and most deprived group was 43 and 50% respectively. The 5-year standardized all-cause probability of death was 51 and 60% for the least and most deprived. However, such a comparison does not distinguish whether the difference is due to cancer mortality, other cause mortality or both.
Figure 2.
(A) Standardized net probability of death, and (B) standardized all-cause probability of death, for the least and most deprived patients with 95% confidence intervals.
Forming contrasts within subsets of the population
It might also be of interest to estimate the measures and contrasts described earlier, within subsets of the whole population. For instance, the all-cause survival difference in the whole population, defined in equation (5), could also be defined among the exposed:
| (6) |
with and denoting the covariates for the exposed, for the expected and relative survival respectively. It can be estimated by standardizing only to patients of the exposed group, ,
Forming contrasts within subsets of the population is useful when estimating the potential impact of removing differences for groups with worse survival, under hypothetical scenarios.
Eliminating cancer-related differences
In practice, it might be difficult to remove all-cause survival differences as they are the result of complex mechanisms that involve both cancer-related and other cause mortality. A hypothetical scenario under which we eliminate cancer-related differences only may be easier to define. Contrasts of all-cause survival in which we only eliminate cancer-related survival differences between groups can be obtained using relative survival. For example, instead of equation (6), we could vary only relative survival between the two terms:
| (7) |
which is estimated as
In equation (7), we assume that under the hypothetical scenario, the other cause mortality rate remains unchanged.
Avoidable deaths
The impact of the hypothetical scenario described in the section ‘Eliminating cancer-related differences’ can also be estimated using avoidable deaths.31 Firstly, we need the predicted number of deaths for the exposed, which is given by multiplying the number of exposed patients diagnosed in a typical calendar year, , with the probability of death:
Secondly, we need the expected number of deaths under the hypothetical scenario that is derived by replacing the relative survival of the exposed with that of the unexposed:
The avoidable deaths are then estimated by:
| (8) |
Each of the terms is estimated by
| (9) |
The number of exposed patients in a typical year, may be different from the patients we standardize over, . For instance, can be calculated by the number of exposed patients diagnosed in the most recent year or by the total number of exposed patients divided by the number of years.
Equation (8) yields the all-cause avoidable deaths among the exposed and can be partitioned to cancer or other causes deaths. This can be estimated by multiplying the marginal crude probabilities of death by the number of patients, .
Example
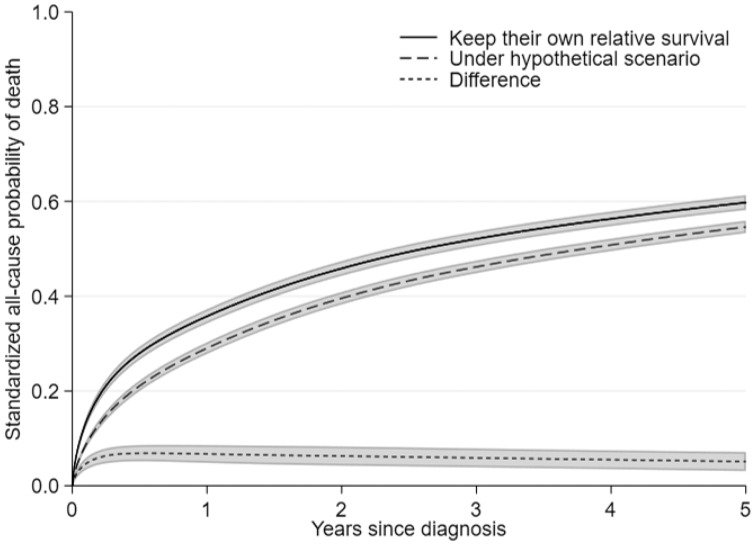
We estimated the impact on the standardized all-cause probability of death of the most deprived group under a hypothetical scenario of removing differences in relative survival between deprivation groups. To do so, we applied the relative survival of the least deprived, i.e. the most advantaged group, to the most deprived, but kept their expected survival unchanged. In such a scenario the 5-year standardized all-cause probability of death of the most deprived would decrease from 60 to 55% (Fig. 3).
Figure 3.
Standardized all-cause probabilities of death for the most deprived patients before and after the hypothetical scenario of removing differences in relative survival, with 95% confidence intervals.
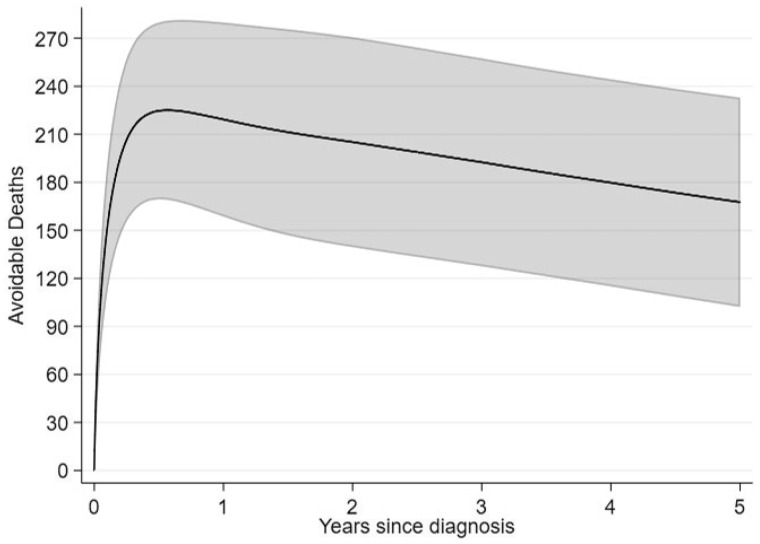
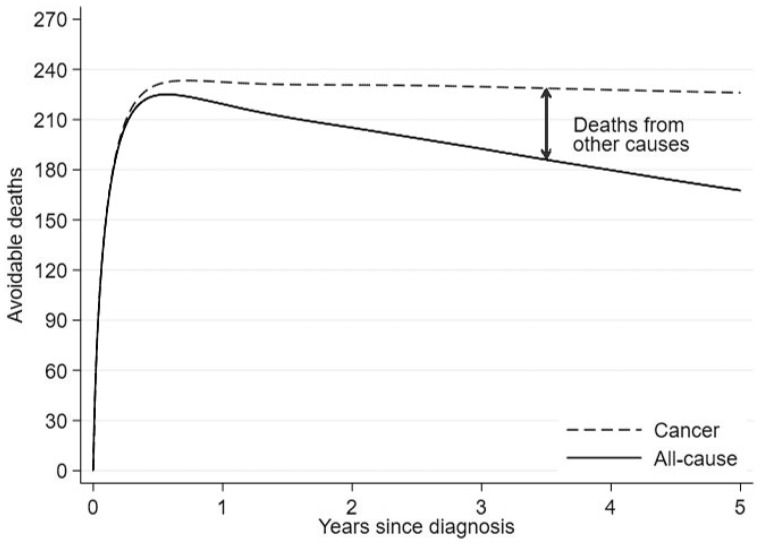
We also estimated the avoidable deaths for the most deprived patients under the same scenario. Five years after diagnosis 168 deaths could be avoided out of 3267 patients from the most deprived group diagnosed in 2008 (Fig. 4). In this example, and of equation (9) coincide (3267 patients) but this will not always be the case. Fig. 5 breaks down the all-cause avoidable deaths to cancer and other causes deaths. Even though the cancer avoidable deaths increase and finally stay constant with time, the all-cause avoidable deaths will decrease after the initial increase, as some patients that would die from the cancer will now die of other causes. That is why we observe an increase in other cause deaths.
Figure 4.
All-cause avoidable deaths under the hypothetical scenario of removing differences in relative survival between deprivation groups, with 95% confidence intervals.
Figure 5.
All-cause avoidable deaths partitioned to avoidable deaths due to cancer and increase in deaths due to other causes.
Discussion
We outlined marginal measures in a relative survival framework that can summarize the prognosis of a population. We also defined contrasts of these measures between subgroups of the population that under assumptions can be interpreted as causal effects. Most of these methods are used in practice, however in this paper we are formalizing them into a causal framework and providing software for their estimation. We also defined marginal crude probabilities as an additional useful measure. Marginal estimates were estimated using regression standardization. An advantage of these measures is that even after fitting a complex statistical model with interactions and time-dependent effects, a single number can be used to summarize the exposure effect at a given time point.16
In order to relate the counterfactual and the observed outcomes, some assumptions need to hold:8,30 conditional exchangeability, consistency and positivity. These have similar interpretation to that of an all-cause setting but here they are extended for both competing outcomes. Another assumption is that of well-defined interventions that would allow us to compute the causal effect in an ideal randomized experiment.14 As we evaluated the impact of an intervention that aims to eliminate deprivation differences one could argue that this is an ambiguous causal question. However, as others have argued, understanding the magnitude of disparities across deprivation groups in a formalized causal framework gives a firm basis to further unpick the reasons for the differences, even if the ideal randomized experiment would be difficult to precisely define.12,32–35 Our approaches can be extended to a mediation analysis setting to quantify measurable aspects that drive these inequalities, which will form part of future work.
In addition to standard causal inference assumptions, assumptions relevant to the relative survival need to hold. Information on expected mortality rates should be appropriate for the cancer population. Previous studies have assessed potential bias from including cancer patients in the general population. Bias was found to be negligible for individual cancers.36–38 Another assumption requires that the competing risks are conditionally independent.19 This means that there are no other factors to affect both competing events than the factors we have adjusted for. An example would be a strong effect of comorbidity that is likely to affect both cancer and other cause mortality rates.39 As the ability to adjust for confounders for other cause mortality depends on the available life tables, methods have been developed to do so when that information is not available.40–42 We appreciate that the estimates can only be interpreted as causal if this assumption is valid but, in principle, life tables can be constructed for any number of risk factors if there is available data to do so.
The interpretation of net survival in a hypothetical world where the only possible cause of death is the cancer of interest has received some criticism. Some proponents have argued that one should always ‘stick to this world’ for quantities of interest.43 Historically, relative survival has been used to account for differential mortality of competing events in population-based cancer survival.2,44 A hypothetical scenario of eliminating all-cause survival differences between exposures may not be straightforward in practice as many factors, which relate both to cancer and other causes, account for the differences. Relative survival allows to focus on cancer-related differences that may be easier to identify. Furthermore, we develop approaches for equalizing the excess mortality across population groups and then convert to ‘real’ world probabilities in the avoidable deaths measures we propose.
Avoidable deaths can be obtained to estimate the impact of eliminating survival differences between subgroups.31,45 The avoidable deaths depend on both the survival differences and the number of patients diagnosed with cancer and has the interpretation of postponable deaths as eventually all deaths will be observed. However, it helps to quantify the impact of removing survival inequalities for public health stakeholders.
We have demonstrated how to obtain marginal measures for the whole population or specific subsets as well as causal effects between exposure groups using the relative survival framework. Future work will focus on methods that will allow further investigation of observed differences. The relative survival framework could also be applied alongside mediation analysis to investigate the impact of potential mediators on the relationship between exposure and outcome.
Supplementary Material
Funding
This work was supported by a National Institute for Health Research Doctoral Research Fellowship to E.S. (Reference: DRF-2017–10-116). This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. P.C.L. was also funded by Cancer Research UK (Grant number C1483/A18262).
Conflict of interest: None declared.
References
- 1. Gamel JW, Vogel RL.. Non-parametric comparison of relative versus cause-specific survival in surveillance, epidemiology and end results (SEER) programme breast cancer patients. Stat Methods Med Res 2001;10:339–52. [DOI] [PubMed] [Google Scholar]
- 2. Dickman PW, Coviello E.. Estimating and modelling relative survival. The Stata Journal 2015;15:186–215. [Google Scholar]
- 3. Ederer F, Axtell L, Cutler S.. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr 1961;6:101–21. [PubMed] [Google Scholar]
- 4. Perme MP, Stare J, Estève J.. On estimation in relative survival. Biometrics 2012;68:113–20. [DOI] [PubMed] [Google Scholar]
- 5. Brenner H, Hakulinen T.. On crude and age-adjusted relative survival rates. J Clin Epidemiol 2003;56:1185–91. [DOI] [PubMed] [Google Scholar]
- 6. Brenner H, Arndt V, Gefeller O, Hakulinen T.. An alternative approach to age adjustment of cancer survival rates. Eur J Cancer 2004;40:2317–22. [DOI] [PubMed] [Google Scholar]
- 7. Lambert PC, Dickman PW, Rutherford MJ.. Comparison of approaches to estimating age-standardized net survival. BMC Med Res Methodol 2015;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young JG, Tchetgen Tchetgen EJ, Hernán MA, The choice to define competing risk events as censoring events and implications for causal inference. arXiv: 1806.06136v2.
- 9. Cronin KA, Feuer EJ.. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med 2000;19:1729–40. [DOI] [PubMed] [Google Scholar]
- 10. Klein JP, Moeschberger ML.. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer, 1997. [Google Scholar]
- 11. Maldonado G, Greenland S.. Estimating causal effects. Int J Epidemiol 2002;31:422–29. [PubMed] [Google Scholar]
- 12. Glymour MM, Spiegelman D.. Evaluating public health interventions: 5. causal inference in public health research—do sex, race, and biological factors cause health outcomes? Am J Public Health 2017;107:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubin DB. Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc 2005;100:322–31. [Google Scholar]
- 14. Hernán MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health 2004;58:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole SR, Hernán MA.. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 16. Sjölander A. Regression standardization with the R package stdReg. Eur J Epidemiol 2016;31:563–74. [DOI] [PubMed] [Google Scholar]
- 17.Neighbourhood Renewal Unit. The English Indices of Deprivation 2004 (Revised). London: Office of the Deputy Prime Minister, 2004. [Google Scholar]
- 18.Department for Communities and Local Government. The English Indices of Deprivation2010. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (December 2019, date last accessed).
- 19. Pavlic K, Pohar Perme M.. Using pseudo-observations for estimation in relative survival. Biostatistics 2019;20:384–99. [DOI] [PubMed] [Google Scholar]
- 20. Estève J, Benhamou E, Croasdale M, Raymond L.. Relative survival and the estimation of net survival: elements for further discussion. Stat Med 1990;9:529–38. [DOI] [PubMed] [Google Scholar]
- 21. Hakulinen H, Tenkanen L.. Regression analyses of relative survival rates. Appl Stat 1987;36:309–17. [Google Scholar]
- 22. Dickman PW, Sloggett A, Hills M, Hakulinen T.. Regression models for relative survival. Stat Med 2004;23:51–64. [DOI] [PubMed] [Google Scholar]
- 23. Cortese G, Scheike TH.. Dynamic regression hazards models for relative survival. Stat Med 2008;27:3563–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Royston P. Flexible parametric alternatives to the Cox model, and more. Stata J 2001;1:1–28. [Google Scholar]
- 25. Nelson CP, Lambert PC, Squire IB, Jones DR.. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 2007;26:5486–98. [DOI] [PubMed] [Google Scholar]
- 26. Corazziari I, Quinn M, Capocaccia R.. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16. [DOI] [PubMed] [Google Scholar]
- 27. Lambert PC, Dickman PW, Nelson CP, Royston P.. Estimating the crude probability of death due to cancer and other causes using relative survival models. Stat Med 2010;29:885–95. [DOI] [PubMed] [Google Scholar]
- 28. Aalen OO, Cook RJ, Røysland K.. Does cox analysis of a randomized survival study yield a causal treatment effect? Lifetime Data Anal 2015;21:579–93. [DOI] [PubMed] [Google Scholar]
- 29. Hernán MA. The hazards of hazard ratios. Epidemiology 2010;21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernán MA, Robins JM.. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdel-Rahman M, Stockton D, Rachet B, Hakulinen T, Coleman MP.. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer 2009;101:S115–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandenbroucke JP, Broadbent A, Pearce N.. Causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol 2016;45:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krieger N, Davey Smith. G. The tale wagged by the dag: broadening the scope of causal inference and explanation for epidemiology. Int J Epidemiol 2016;45:1787–808. [DOI] [PubMed] [Google Scholar]
- 34. Pearl J. Does obesity shorten life? Or is it the soda? On non-manipulable causes. J Causal Inference 2018;6:1–7. [Google Scholar]
- 35. Naimi AI, Kaufman JS.. Counterfactual theory in social epidemiology: reconciling analysis and action for the social determinants of health. Curr Epidemiol Rep 2015;2:52–60. [Google Scholar]
- 36. Ellison LF. Adjusting relative survival estimates for cancer mortality in the general population. Health Rep 2014;25:3–9. [PubMed] [Google Scholar]
- 37. Hinchliffe SR, Dickman PW, Lambert PC.. Adjusting for the proportion of cancer deaths in the general population when using relative survival: a sensitivity analysis. Cancer Epidemiol 2012;36:148–52. [DOI] [PubMed] [Google Scholar]
- 38. Talbäck M, Dickman PW.. Estimating expected survival probabilities for relative survival analysis - exploring the impact of including cancer patient mortality in the calculations. Eur J Cancer 2011;47:2626–32. [DOI] [PubMed] [Google Scholar]
- 39. Mariotto AB, Wang Z, Klabunde CN, Cho H, Das B, Feuer EJ.. Life tables adjusted for comorbidity more accurately estimate noncancer survival for recently diagnosed cancer patients. J Clin Epidemiol 2013;66:1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bower H, Andersson TML, Crowther MJ, Dickman PW, Lambe M, Lambert PC.. Adjusting expected mortality rates using information from a control population: an example using socioeconomic status. Am J Epidemiol 2018;187:828–36. [DOI] [PubMed] [Google Scholar]
- 41. Ellis L, Coleman MP, Rachet B.. The impact of life tables adjusted for smoking on the socio-economic difference in net survival for laryngeal and lung cancer. Br J Cancer 2014;111:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rubio FJ, Rachet B, Giorgi R, Maringe C, Belot A.. On models for the estimation of the excess mortality hazard in case of insufficiently stratified life tables. Biostatistics 2019. doi: 10.1093/biostatistics/kxz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andersen PK, Keiding N.. Interpretability and importance of functionals in competing risks and multistate models. Stat Med 2012;31:1074–88. [DOI] [PubMed] [Google Scholar]
- 44. Sarfati D, Blakely T, Pearce N.. Measuring cancer survival in populations: relative survival vs cancer-specific survival. Int J Epidemiol 2010;39:598–610. [DOI] [PubMed] [Google Scholar]
- 45. Rutherford MJ, Andersson TML, Møller H, Lambert PC.. Understanding the impact of socioeconomic differences in breast cancer survival in England and Wales: avoidable deaths and potential gain in expectation of life. Cancer Epidemiol 2015;39:118–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.