Why was the cohort set up?
Puberty marks the transition from childhood to adulthood and is a possible component cause in a number of adult diseases related to early life exposures. Previous investigations suggest a secular trend toward earlier timing of puberty, mainly based on research related to age at menarche.1–3 This shift has occurred during recent centuries in Europe and other Western countries. Yet, few have investigated whether age of puberty in boys has also decreased.4–7 Earlier pubertal development has been linked to increased risk of several frequent and severe diseases in adulthood, such as metabolic disorders, cardiovascular disease and certain types of cancer.8–11 Although the childhood obesity epidemic is considered an important cause of the temporal trend in timing of puberty,12 remaining causes continue to be scarcely understood.13,14 According to the theory of developmental origins of health and disease,15,16 exposures during fetal life may have long-lasting programming effects on offspring health, including pubertal development. This hypothesis has been consistently supported by data from animal studies,17 but large-scale epidemiological evidence is still limited.18–20
The Puberty Cohort, nested within the Danish National Birth Cohort (DNBC21, the DNBC Puberty Cohort), was set up to examine potential early life risk factors for altered pubertal timing, in addition to potential trajectories of health and disease after altered timing of pubertal development. A secondary aim of the DNBC Puberty Cohort was to examine current pubertal timing in Danish adolescents, in order to provide a starting point for continuous monitoring of pubertal timing.
A scientific management team consisting of senior researchers from several Danish academic institutions leads the DNBC and the DNBC Puberty Cohort. The scientific management team approves proposed projects, with input from an ‘oversee’ reference committee consisting of other experienced researchers and cohort representatives. Statens Serum Institut, Denmark, is responsible for the data in the DNBC. The Independent Research Fund Denmark and Aarhus Ideas funded the DNBC Puberty Cohort.
Who is in the cohort?
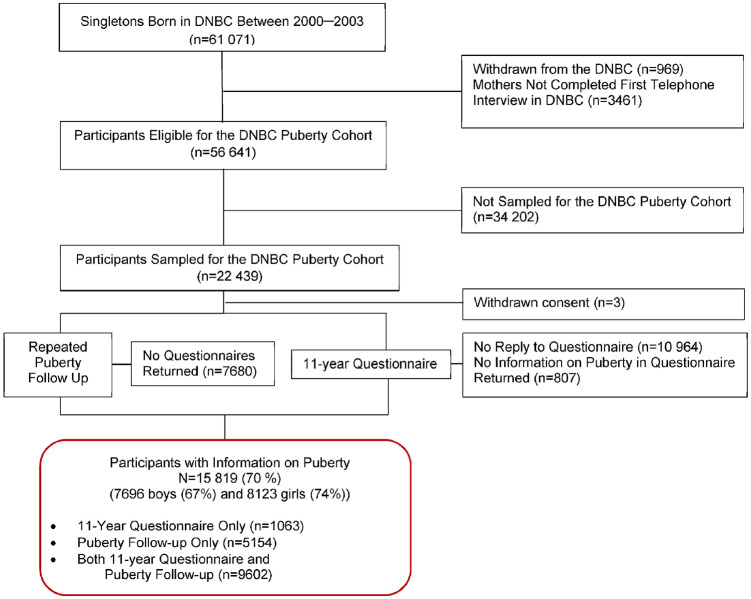
In 2012, the DNBC Puberty Cohort was established as a longitudinal sub-cohort within the DNBC. Children eligible for sampling were singletons born into the DNBC between 2000 and 2003, whose mothers participated in the first computer-assisted telephone interview (at ≈18 gestational weeks) and had not withdrawn their informed consent by May 2012. In total, 56 641 children fulfilled the eligibility criteria, of whom 22 439 children (11 446 boys and 10 993 girls, Figure 1) were sampled according to 27 sampling frames based on 12 pre- and perinatal exposures and a random sampling frame of 8000 children (Supplementary Table 1,22 available as Supplementary data at IJE online) The sampling strategy was applied to increase exposure contrasts by sampling children within categories of prenatal exposures relevant for pubertal timing, such as maternal smoking, pre-pregnancy body mass index and selected environmental exposures. Sampling weights can be provided, upon data extraction, which can reweight the selected DNBC Puberty Cohort to represent an unselected random sample of all those eligible for sampling. Further description of the sampling procedure and derivation of the sampling weights is available elsewhere.22
Figure 1.
Flow chart of participants in the DNBC Puberty Cohort, Denmark, 2000–18.
Since August 2012, the children sampled for participation in the DNBC Puberty Cohort have been invited every 6 months (either by e-mail or by hard copy letter) to complete a web-based questionnaire on current status of pubertal development, from 11.5 years of age until 17.5 years of age or full maturity. In case of no response, all invitations were followed by a sequence of up to three reminders forwarded 14 days apart. The invitations were sent to the mothers or another main legal guardian until the children turned 15 years of age, at which point the children were contacted directly. Children were encouraged to complete the questions on pubertal development themselves, regardless of age.
As part of a large 11-year follow-up of the entire DNBC, the children in the DNBC Puberty Cohort were also invited to complete questions on status of pubertal development identical to those included in the repeated puberty follow-up described above. These sources of data were combined to enrich data quantity and obtain information from the earliest point in time. Thus, information on pubertal development was available from 11 years of age onwards. Children were considered non-participants in the DNBC Puberty Cohort if they did not reply to the first two rounds of invitations and reminders in the repeated puberty follow-up and did not provide information on pubertal development in the 11-year questionnaire.
Altogether, 15 819 children of the 22 439 invited for participation [70%, 7696 boys (67%) and 8123 girls (74%)] provided information on pubertal development and are considered participants. In total, 5154 participants completed questionnaires in the repeated puberty follow-up only, 1063 completed the 11-year questionnaire only and 9602 completed both (Figure 1). Participating boys and girls were more often first-born and had slightly older parents from higher socioeconomic classes than non-participants (Supplementary Table 2, available as Supplementary data at IJE online). Children of mothers with later age at menarche and healthy lifestyle behaviour in pregnancy (except for light-to-moderate alcohol consumption during pregnancy, which was reported at a higher rate by participating mothers) were more likely to participate. Pregnancy and birth outcomes were generally comparable for participants versus non-participants among both boys and girls. Anthropometric measures of the children at 7 years of age were comparable between groups.
How often have they been followed up?
The last invitation in the repeated puberty follow-up in the DNBC Puberty Cohort will be at full sexual maturation of all participants or, at the latest, in December 2020 (Figure 2), when all have turned 17.5 years of age. Combining the data on pubertal development from the repeated puberty follow-up and the 11-year questionnaire, the median age at entry in the DNBC Puberty Cohort was 11.1 years [25th—75th percentile (p): 11.1—11.6 years] in boys, and 11.1 years (25th – 75th p: 11.0–11.6 years] in girls.
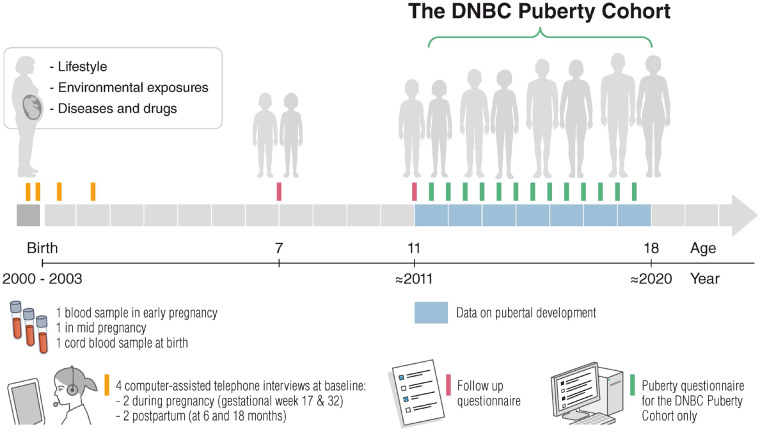
Figure 2.
The DNBC Puberty Cohort data collection timeline, Denmark, 2000–20.
In total, 94 625 questionnaires were returned by October 2018, ranging between 1 and 15 per participant, with a mean of 5.5 [standard deviation (SD): 3.4] for boys and 6.5 (SD: 3.7) for girls (Table 1). As the participants reported current pubertal stage (not specific ages, except for age at menarche and first ejaculation) in the questionnaires, the observations for each pubertal milestone are either left-, right- or interval-censored bounded by lower and upper limits. For example, an observation is interval-censored if a participant achieved a specific milestone between two consecutive questionnaires. The exact age at achieving the pubertal milestone remains unknown, but the lower and upper limit for the interval-censored observation correspond to the age span when the specific milestone was achieved.
Table 1.
Number of participants according to number of completed questionnaires on pubertal development stratified by sex and in total, the DNBC Puberty Cohort, Denmark, 2000–18
| Number of completed questionnaires | Participants, n (%) |
||
|---|---|---|---|
| Boys | Girls | Total | |
| 1 | 1351 (17.6) | 1117 (13.8) | 2468 (15.6) |
| 2 | 716 (9.3) | 583 (7.2) | 1299 (8.2) |
| 3 | 628 (8.2) | 531 (6.5) | 1159 (7.3) |
| 4 | 606 (7.9) | 500 (6.2) | 1106 (7.0) |
| 5 | 607 (7.9) | 558 (6.9) | 1165 (7.4) |
| 6 | 685 (8.9) | 673 (8.3) | 1358 (8.6) |
| 7 | 696 (9.0) | 700 (8.6) | 1396 (8.8) |
| 8 | 733 (9.5) | 671 (8.3) | 1404 (8.9) |
| 9 | 601 (7.8) | 752 (9.3) | 1353 (8.6) |
| 10 | 498 (6.5) | 732 (9.0) | 1230 (7.8) |
| 11 | 282 (3.7) | 555 (6.8) | 837 (5.3) |
| 12 | 174 (2.3) | 447 (5.5) | 621 (3.9) |
| 13 | 104 (1.4) | 274 (3.4) | 378 (2.4) |
| 14–15a | 15 (0.2) | 30 (0.4) | 45 (0.3) |
| Total participants | 7696 (100) | 8123 (100) | 15 819 (100) |
| Total questionnaires | 42 113 (100) | 52 512 (100) | 94 625 (100) |
The exact number of participants with 15 completed questionnaires cannot be given due to risk of disclosure (security rules at Statistics Denmark).
Among the 15 819 participants, 2529 boys (33%) and 2424 girls (30%) had reached full sexual maturation, defined as Tanner stage 5 for both pubic hair growth and breast or genital development, by October 2018. So far, 659 children (4%) have turned 17.5 years old without having achieved full maturity. Among the remaining 10 207 participants, we consider children as having incomplete follow-up if they have not reached full maturity (Tanner stages 5) and have not responded to the most recent questionnaire within 8 months of last invitation [n = 6797 (43%)]. However, in this group, considerably fewer have incomplete follow-up for the earlier pubertal milestones than the later. Further, if the available outcome data are analysed by a model for censored time-to-event data,23 all participants with a minimum one measure of pubertal development are available for statistical analyses, regardless of the number of returned questionnaires and follow-up status (Supplementary Table 3, available as Supplementary data at IJE online). Additionally, we do not suspect incomplete follow-up to introduce bias, if covariates related to the length of follow-up are adjusted for in the regression analyses. A total of 3410 children remain eligible for continued follow-up. When the repeated data collection for the DNBC Puberty Cohort is finalized, we can also assess progression of pubertal development.
What has been measured?
In the questionnaires, we asked the children to state height and weight and to specify if, when and to what extent he or she had achieved different pubertal milestones based on self-assessment. Tanner staging 1 to 524,25 captured the physical manifestation of pubic hair growth and genital or breast development in boys and girls. The children were also asked to report if age at menarche (girls) or first ejaculation of semen (boys) was achieved and when it had been achieved, specifying at what age (year and month). Boys were asked to report voice change in the following categories: ‘no’, ‘yes, sometimes’, ‘yes, definitive changes’ and ‘don’t know’. We defined ‘yes, sometimes’ as voice break and ‘yes, definitive changes’ as adult voice. In both sexes, the children stated the achievement of axillary hair growth and acne (no/yes). When the participants scored themselves as Tanner stage 5 or indicated that one of the other milestones was achieved, these questions were excluded from future questionnaires. The consistency of the self-reported information on Tanner staging has been explored by defining inconsistent reporting as a lower Tanner stage in one questionnaire than in any previous one returned. Among girls, Tanner breast stages and Tanner pubic hair stage were consistent in 86% and 81%, respectively. Among boys, Tanner genital stage and Tanner pubic hair stage were consistent in 77% and 87%, respectively. Further, exclusion of individuals with inconsistent information did not change the mean age for achieving the Tanner stages in the DNBC Puberty Cohort.26 Illustrations and short explanatory texts accompanied all Tanner stage questions. An English translation is available at [https://www.dnbc.dk/data-available].
In the DNBC, data on prenatal, postnatal and childhood factors were collected in four baseline computer-assisted telephone interviews with mothers at approximately gestational weeks 17 and 32, as well as at 6 and 18 months postpartum, and mailed or web-based questionnaires were completed at 7 (mothers) and 11 years (separate questionnaires for mother and child). Mothers provided a blood sample twice in pregnancy and umbilical cord blood samples were collected at birth (Figure 2). Among the children sampled for the DNBC Puberty Cohort, Table 2 presents frequencies of participation for each follow-up and jointly given participation in all previous follow-up waves with and without conditioning on participation in the DNBC Puberty Cohort. An overview of the collected information in these waves is available in Table 3. English translations of the code books are available at [https://www.dnbc.dk/data-available].
Table 2.
Frequency of participation in follow-up waves among sampled individuals and participants in the DNBC Puberty Cohort, Denmark, 2000–18
| Follow-up waves, n (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 1st interview ≈GW 17 | 2nd interview ≈GW 32 | 3rd interview ≈6 months postpartum | 4th interview ≈18 months postpartum | 7-year follow-up | 11-year follow-up, mothers | 11-year follow-up, children | DNBC Puberty Cohort follow-upb | |
| Frequency for each follow-up | 22 436 (100)a | 20 654 (92.1) | 18 648 (83.1) | 17 123 (76.3) | 13 556 (60.4) | 11 016 (49.1) | 10 665 (47.5) | 15 819 (70.5) |
| Frequency for each follow-up given participation in PC | 15 819 (100) | 14 742 (93.2) | 13 516 (85.4) | 12 701 (80.3) | 11 459 (72.4) | 10 649 (67.3) | 10 665 (67.4) | 15 819 (100) |
| Frequency for all previous follow-upc | 22 436 (100)a | 20 654 (92.1) | 17 401 (77.6) | 13 904 (62.0) | 9059 (40.4) | 6314 (28.1) | 5699 (25.4) | 5699 (25.4) |
| Frequency for all previous follow-up given participation in PCd | 15 819 (100) | 14 742 (93.2) | 12 658 (80.0) | 10 435 (66.0) | 7814 (49.4) | 6168 (39.0) | 5699 (36.0) | 5669 (36.0) |
GW, gestational week; PC, the DNBC Puberty Cohort.
Three mother-child dyads withdrew consent among the 22 439 originally sampled.
Information on pubertal development either from the 11-year questionnaire only, the repeated puberty follow-up only or both.
The frequency of participation given participation in all of the previous follow-up waves.
The frequency of participation given participation in all of the previous follow-up waves and participation in the DNBC Puberty Cohort.
Table 3.
Broad categories of collected information in each follow-up wave among participants in the DNBC Puberty Cohort, Denmark, 2000–18
| Broad categories a | 1st int ≈ GW 17 | 2nd int ≈GW 32 | 3rd int ≈6 months postpartum | 4th int ≈18 months postpartum | 7-year questionnaire | 11-year questionnaire, mothers | 11-year questionnaire, children | DNBC Puberty Cohort follow-up (11.5–18 years) |
|---|---|---|---|---|---|---|---|---|
| Sociodemographicsb | X | X | X | X | X | |||
| Index pregnancy and reproductive historyc | X | X | X | |||||
| Maternal overall health and comorbidity | X | X | ||||||
| Medicine use in pregnancy | X | X | X | |||||
| Maternal diet, vitamin intake and exercise in pregnancy | X | X | X | |||||
| Maternal lifestyle in pregnancy | X | X | X | |||||
| Maternal mental health | X | X | X | X | X | |||
| Prenatal environmental exposures | X | X | ||||||
| Breastfeeding and/or child’s diet | X | X | X | X | X | |||
| Maternal medicine use after pregnancy | X | X | ||||||
| Mother-child relationship | X | X | ||||||
| Child’s exposure to parental lifestyle factors | X | X | X | X | ||||
| Child care and family living conditions | X | X | X | X | X | |||
| Child’s diseases, vaccination and medication | X | X | X | X | X | |||
| Child’s growth and/or cognitive development | X | X | X | X | X | X | ||
| Parent’s anthropometric measures | X | X | ||||||
| Child’s physical activity | X | X | ||||||
| Child’s mental health | X | X | X | |||||
| Child’s gender identity | X | X | ||||||
| Child’s social relationships and well-being | X | |||||||
| Child’s school performance | X | |||||||
| Child’s smoking and alcohol consumption | X | |||||||
| Child’s overall health | X | |||||||
| Child’s view on physical appearance | X | |||||||
| Pubertal development | X | X |
Int, interview; GW, gestational week.
All questions included in the interviews or questionnaires were determined a priori.
Marital status; income, maternal and paternal education and current working conditions; and family and living conditions.
Includes a variety of diseases in pregnancy.
Upon birth or emigration, all Danish residents are assigned a unique Civil Registration Number, which is universally used in most registers within the Danish health care system.27 This identification number allows unequivocal individual-level linkage of data on parents and children in the DNBC Puberty Cohort to national Danish health and sociodemographic resources, including the most relevant: the Danish Birth Registry (information on pregnancy and birth outcomes),28 the Registry of Medicinal Product Statistics (information on redeemed prescriptions),29 the Patient Registry (diagnoses for all in- and outpatient hospital contacts),30 the Children’s’ Database (growth measures during childhood),31 the integrated Database for Labour Market Research (information on sociodemographics),32 the In Vitro Fertilization Registry (information on use of medically assisted reproduction and treatment outcomes),33 the Cancer Registry (information on type of cancer, TNM-classification and treatment)34 and the Registry of Causes of Death (diagnoses for causes of death).35
What has it found? Key findings and publications
Methodological issues related to non-participation in the DNBC Puberty Cohort (unpublished results) and misclassification of the pubertal milestones due to self-assessment36 have been addressed. Participation in the DNBC Puberty Cohort was associated with socioeconomic status and maternal lifestyle factors during pregnancy, but it was not associated with pubertal timing, measured by a surrogate puberty marker obtained from an unselected external register (unpublished results). Thus, non-participation is most likely not related to both exposure and outcomes in most aetiological studies on potential causes of altered pubertal timing, thereby most likely resulting in negligible risk of selection bias. Self-assessment of status of pubertal development was found to have a moderate to good agreement, with clinical examination showing no tendencies towards under- or overestimation.36
In a descriptive study, we report that mean ages at onset of puberty (Tanner genital or breast stage 2) in the DNBC Puberty Cohort were 11.1 and 10.5 years in boys and girls, respectively.26 Age at menarche occurred 3.6 months earlier in daughters than in mothers from the DNBC Puberty Cohort. In comparison, boys attained most pubertal milestones 6 to 12 months earlier than seen in previous Danish samples a few decades back.26
In aetiological studies published so far, we have found a strong association between maternal age at menarche and the pubertal timing in the children, supporting that the genetic heritage is a contributing factor in pubertal development.37 Further, maternal smoking during pregnancy,22 use of over-the-counter painkillers,38 prenatal exposure to perflourinated compounds,39 time to pregnancy and use of medically assisted reproduction,40 in addition to gestational diabetes,41 have been found to be potential risk factors for earlier pubertal timing in the DNBC Puberty Cohort. The DNBC continuously maintains a bibliography of the publications from the DNBC [https://www.dnbc.dk/dnbc-publications].
What are the main strengths and weaknesses?
Limitations
A large proportion of the participants had already reached early pubertal milestones at entry [boys: 65% at Tanner genital 2+; girls: 85% at Tanner breast 2+ (Supplementary Table 3, available as Supplementary data at IJE online)]. Analytical tools are able to account for the left-censoring of data23 and provide valid estimates under the assumption that the age at reaching the early pubertal milestones follows a normal distribution. Later milestones have high proportions of interval-censored data in the DNBC Puberty Cohort, and these data followed normal distributions. In addition, the age at achieving pubertal milestones is suggested to follow normal distributions in healthy populations such as the DNBC Puberty Cohort.42 Still, we cannot exclude that deviations from the normal distribution on early pubertal milestones may introduce error in descriptive studies, as recently shown by our research group.26 Briefly, in a simulated dataset with 90% left-censored observations corresponding to the empirical data for Tanner breast stage 2 and varying degrees of skewed distributions, the descriptive mean age was biased by approximately 4 months. However, the estimated bias for later milestones was negligible. A risk of error in aetiological studies is more unlikely, as the error introduced by deviation from normality is of approximately similar size among exposure groups, but caution is still recommended when using the data on early milestones.
The data in the DNBC Puberty Cohort can be analysed for each pubertal milestone separately. This strategy will capture associations between the exposure of interest and specific parts of pubertal development (12 outcomes in boys and 11 outcomes in girls), but it entails a risk of type 1 errors due to multiple testing of correlated outcomes. A way to account for multiple testing and the correlation structures is the Huber-White robust variance estimation.43,44 Using this estimator on all pubertal milestones, one can obtain a single estimate for the overall association with pubertal timing for each sex.39
The DNBC as a whole offers a breadth of measurements throughout pregnancy, infancy, childhood and adolescence, but risk of misclassification of baseline data due to self-reporting must be considered in most cases. However, the mothers were unaware of future pubertal timing in the offspring, which most likely results in non-differential misclassification. Despite relatively detailed information on shared demographic and health behaviours, fathers did not directly participate in the baseline data collection, and paternal age at pubertal onset, a contributor to offspring pubertal development,45 remains unknown. Misclassification due to self-assessment of Tanner stages is another limitation, which has been explored as mentioned.36 Mothers participating in the DNBC were mainly Caucasians, reflecting the Danish population, but on average from higher socioeconomic groups.46
Strengths
The main strength of the DNBC Puberty Cohort is the longitudinal design with repeated measurements in short intervals throughout sexual maturation (Figure 2). As opposed to most of the previous cohorts with pubertal measurements,1 the DNBC Puberty Cohort includes a large group of boys. Further, the longitudinal examination of a wide variety of pubertal milestones enables researchers to investigate both the onset of puberty and the progression of pubertal development, as requested by experts in this field.1 The DNBC Puberty Cohort is, to our knowledge, the largest cohort of its kind, providing a large study population for the examination of relatively infrequent exposures. The use of self-assessment of pubertal status made it possible to invite a large number of children within a reasonable economic frame and was crucial in achieving the relatively high participation rate of 70%. Lower participation rates with higher risk of selection bias have been an issue in earlier studies using clinical examination.3
Given the longitudinal design and detailed information on prenatal factors, this cohort presents an opportunity to explore important exposure time windows and apply complex exposure modelling (Tables 2 and 3). Detailed follow-up information from infancy and childhood foster rich opportunities for confounder adjustment and allow researchers to employ mediation analysis to dissect important pathways by which prenatal exposures may alter pubertal development, such as through pre-pubertal growth patterns. Furthermore, the unique Danish identification system allows data linkage with a wealth of registers and databases with additional exposure and outcome information covering the lifespan of both parents and children.
Furthermore, biological material from maternal and cord blood samples is available in smaller quantities and biochemical, genetic and epigenetic biomarker data can be obtained for future studies. Environmental monitoring data are available in smaller sub-sets of the cohort. Recurrent follow-up in the DNBC after full sexual maturation is ongoing (18-year questionnaire) and will continue throughout the life course of the participants. This extension will enhance the value of the DNBC data resource, enabling exploration of the links between exposures and numerous markers of long-term reproductive health such as puberty, semen quality and time to pregnancy. Last, with time, this extension facilitates investigation of life course risk of diseases in relation to altered pubertal development.
Can I get hold of the data? Where can I find out more?
The DNBC Puberty Cohort is considered an open access resource for researchers with projects that fall within the policy and overall aim of the DNBC [https://www.dnbc.dk/access-to-dnbc-data]. Applicants must submit a completed application form and a short protocol of the project to [dnbc-research@ssi.dk]. In addition, data access requires that applicants obtain permission from the Danish Data Protection Agency [https://www.datatilsynet.dk/]. The scientific management team reserves the right to prioritize ongoing projects and encourages applicants from outside the country to collaborate with Danish researchers, for example the principal investigator, the DNBC Puberty Cohort, Cecilia Høst Ramlau-Hansen [chrh@ph.au.dk]. A cost applies to access to data and initial construction of data and a yearly fee covers server connection, support and storage space. Further questions should be directed to the DNBC administrative office [dnbc-research@ssi.dk].
Profile in a nutshell
The longitudinal, population-based Danish National Birth Cohort (DNBC) Puberty Cohort was set up to examine potential early life causes and later consequences of altered timing of pubertal development.
From 2011, 22 439 children born into the DNBC during 2000–03 have been invited every 6 months to complete web-based questionnaires on current status of Tanner stage of pubic hair growth and breast or genital development, menarche, voice break and first ejaculation, from age 11 up until full maturity or 18 years.
A total of 15 819 children (70%) have returned at least one questionnaire on pubertal development (average: 6.0). By October 2018, 5612 had full follow-up and 3410 remained eligible for further follow-up. Participants were more often of higher socioeconomic background and exposed to a healthier maternal lifestyle in fetal life than non-participants.
In the DNBC, four comprehensive computer-assisted telephone interviews were completed (two in pregnancy and two postpartum), and children have been followed up with questionnaires at 7 and 11 years. Blood samples from mothers in early pregnancy and the umbilical cord are available. Data can be linked to Danish health registers.
Data access requires approval of projects that fall within the policy and aim of the DNBC.
Funding
The DNBC was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation. Follow-up of mothers and children have been supported by the Danish Medical Research Council (SSVF 0646, 271–08-0839/06–066023, O602-01042B, 0602-02738B), the Lundbeck Foundation (195/04, R100-A9193), The Innovation Fund Denmark (0603-00294B (09–067124)), the Nordea Foundation (02–2013-2014), Aarhus Ideas (AU R9-A959-13-S804), University of Copenhagen Strategic Grant (IFSV 2012) and the Danish Council for Independent Research (DFF – 4183–00594). This work was supported by the Danish Council for Independent Research (DFF 4183–00152).
Supplementary Material
Acknowledgements
The authors want to acknowledge the team of investigators and staff who were involved in the establishment of the DNBC. We are also particularly thankful to the daily management group of the DNBC as well as to the families who continuously have contributed with unique data to the DNBC and the DNBC Puberty Cohort.
Author contributions
The DNBC Puberty Cohort was conceptualized and designed by C.H.R. and J.O. with input from B.H.B., E.A.N., K.S-L., I.K.M and A-M.N.A. Principal investigator C.H.R. obtained funding for the establishment and the half-yearly collection of data on pubertal development in the DNBC Puberty Cohort. The planning of the overall analytical handling of the data in the DNBC Puberty Cohort, in addition to the validation studies in the cohort, was carried out by C.H.R., J.O., A.E., N.B., L.L.B.L. and E.T.P. Initial management of the data was conducted by A.E., N.B. and L.L.B.L. A.E., N.B. and E.T.P. analysed the data for this cohort description. A.E. drafted the article. All authors interpreted the results, reviewed the article critically for its intellectual content, approved the final version to be published and agree to be accountable for all aspects of the work.
Conflict of interest: None declared.
References
- 1. Euling SY, Herman-Giddens ME, Lee PA. et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 2008;121(Suppl 3):S172–91. [DOI] [PubMed] [Google Scholar]
- 2. Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A.. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics 2009;123:e932–39. [DOI] [PubMed] [Google Scholar]
- 3. Ong KK, Ahmed ML, Dunger DB.. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol 2006;254-255:8–12. [DOI] [PubMed] [Google Scholar]
- 4. Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE.. Age at voice break in Danish boys: effects of pre-pubertal body mass index and secular trend. Int J Androl 2007;30:537–42. [DOI] [PubMed] [Google Scholar]
- 5. Aksglaede L, Olsen LW, Sorensen TI, Juul A.. Forty years trends in timing of pubertal growth spurt in 157, 000 Danish school children. PLoS One 2008;3:e2728.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sorensen K, Aksglaede L, Petersen JH, Juul A.. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab 2010;95:263–70. [DOI] [PubMed] [Google Scholar]
- 7. Monteilh C, Kieszak S, Flanders WD. et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol 2011;25:75–87. [DOI] [PubMed] [Google Scholar]
- 8. Day FR, Elks CE, Murray A, Ong KK, Perry JR.. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep 2015;5:11208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V.. Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol 1986;124:39–52. [DOI] [PubMed] [Google Scholar]
- 10. Berkey CS, Frazier AL, Gardner JD, Colditz GA.. Adolescence and breast carcinoma risk. Cancer 1999;85:2400–09. [DOI] [PubMed] [Google Scholar]
- 11. Freedman DS, Khan LK, Serdula MK. et al. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagner IV, Sabin MA, Pfaffle RW. et al. Effects of obesity on human sexual development. Nat Rev Endocrinol 2012;8:246–54. [DOI] [PubMed] [Google Scholar]
- 13. Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE.. Role of environmental factors in the timing of puberty. Pediatrics 2008;121(Suppl 3):S167–71. [DOI] [PubMed] [Google Scholar]
- 14. Buck Louis GM, Gray LE Jr, Marcus M. et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics 2008;121(Suppl 3):S192–207. [DOI] [PubMed] [Google Scholar]
- 15. Barker DJ. Fetal origins of coronary heart disease. BMJ 1995;311:171–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillman MW. Developmental origins of health and disease. N Engl J Med 2005;353:1848–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zambrano E, Guzman C, Rodriguez-Gonzalez GL, Durand-Carbajal M, Nathanielsz PW.. Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol 2014;382:538–49. [DOI] [PubMed] [Google Scholar]
- 18. Bonde JP, Flachs EM, Rimborg S. et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update 2016;23:104–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caserta D, Mantovani A, Marci R. et al. Environment and women's reproductive health. Hum Reprod Update 2011;17:418–33. [DOI] [PubMed] [Google Scholar]
- 20. Roth CL, DiVall S.. Consequences of early life programing by genetic and environmental influences: a synthesis regarding pubertal timing. Endocr Dev 2016;29:134–52. [DOI] [PubMed] [Google Scholar]
- 21. Olsen J, Melbye M, Olsen SF. et al. The Danish National Birth Cohort - its background, structure and aim. Scand J Public Health 2001;29:300–07. [DOI] [PubMed] [Google Scholar]
- 22. Brix N, Ernst A, Lauridsen LLB. et al. Maternal smoking during pregnancy and timing of puberty in sons and daughters: a population-based cohort study. Am J Epidemiol 2019;188:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J. The Statistical Analysis of Interval-censored Failure Time Data. 1st edn.New York, NY: Springer, 2006. [Google Scholar]
- 24. Marshall WA, Tanner JM.. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall WA, Tanner JM.. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brix N, Ernst A, Lauridsen LLB. et al. Timing of puberty in boys and girls: a population-based study. Paediatr Perinat Epidemiol 2019;33:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedersen CB, Gotzsche H, Moller JO, Mortensen PB.. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 2006;53:441–49. [PubMed] [Google Scholar]
- 28. Knudsen LB, Olsen J.. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320–23. [PubMed] [Google Scholar]
- 29. Kildemoes HW, Sorensen HT, Hallas J.. The Danish National Prescription Registry. Scand J Public Health 2011;39(Suppl 7):38–41. [DOI] [PubMed] [Google Scholar]
- 30. Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH.. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–68. [PubMed] [Google Scholar]
- 31.Sundhedsdatastyrelsen. Children's Database 2018. https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/graviditet-foedsler-og-boern/boernedatabasen (23 October 2019, date last accessed).
- 32. Petersson F, Baadsgaard M, Thygesen LC.. Danish registers on personal labour market affiliation. Scand J Public Health 2011;39(Suppl 7):95–98. [DOI] [PubMed] [Google Scholar]
- 33. Andersen AN, Westergaard HB, Olsen J.. The Danish in vitro fertilisation (IVF) register. Dan Med Bull 1999;46:357–60. [PubMed] [Google Scholar]
- 34. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011;39(Suppl 7):42–45. [DOI] [PubMed] [Google Scholar]
- 35. Helweg-Larsen K. The Danish Register of causes of death. Scand J Public Health 2011;39(Suppl 7):26–35. [DOI] [PubMed] [Google Scholar]
- 36. Ernst A, Lauridsen LLB, Brix N. et al. Self-assessment of pubertal development in a puberty cohort. J Pediatr Endocrinol Metab 2018;31:763–72. [DOI] [PubMed] [Google Scholar]
- 37. Sorensen S, Brix N, Ernst A, Lauridsen LLB, Ramlau-Hansen CH.. Maternal age at menarche and pubertal development in sons and daughters: a Nationwide Cohort Study. Hum Reprod 2018;33:2043–50. [DOI] [PubMed] [Google Scholar]
- 38. Ernst A, Brix N, Lauridsen LLB. et al. Acetaminophen (paracetamol) exposure during pregnancy and pubertal development in boys and girls from a Nationwide Puberty Cohort. Am J Epidemiol 2019;188:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ernst A, Brix N, Lauridsen LLB. et al. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect 2019;127:017004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ernst A, Lauridsen LLB, Brix N. et al. Parental time to pregnancy, medically assisted reproduction and pubertal development in boys and girls. Hum Reprod 2019;34:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lauridsen LLB, Arendt LH, Ernst A. et al. Maternal diabetes mellitus and timing of pubertal development in daughters and sons: a nationwide cohort study. Fertil Steril 2018;110:35–44. [DOI] [PubMed] [Google Scholar]
- 42. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP.. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 2003;24:668–93. [DOI] [PubMed] [Google Scholar]
- 43. Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; June 21-July 18 1967 Berkeley CA, 1967.
- 44. White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817–38. [Google Scholar]
- 45. Wohlfahrt-Veje C, Mouritsen A, Hagen CP. et al. Pubertal onset in boys and girls is influenced by pubertal timing of both parents. J Clin Endocrinol Metab 2016;101:2667–74. [DOI] [PubMed] [Google Scholar]
- 46. Jacobsen TN, Nohr EA, Frydenberg M.. Selection by socioeconomic factors into the Danish National Birth Cohort. Eur J Epidemiol 2010;25:349–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.