Abstract
Background
Ionizing radiation is a known cause of female breast cancer, but there have been few studies of the risk after prolonged radiation exposure at low dose rates.
Methods
This population-based case-control study estimated breast cancer risk after ∼25 years’ exposure to radiation from the Chernobyl accident. Cases (n = 468) were women ≤55 years old when first diagnosed with invasive breast cancer during October 2008 through February 2013, who lived in Bryansk Oblast, Russia at the time of the accident and their diagnoses. Controls, individually matched to cases on birth year, administrative district of residence and urban vs non-urban settlement during the accident, were women without breast cancer who lived in Bryansk Oblast at the time of the accident and on their cases’ diagnosis dates (n = 468). Subjects were interviewed regarding residence, dietary and food source histories to support individualized estimation of their radiation doses to the breast, which ranged from 0.04 − 41 centigray (cGy) (mean 1.3 cGy).
Results
In multivariable analyses, the odds ratio for breast cancer risk was 3.0 [95% confidence interval (CI): 1.3, 7.0] and 2.7 (95% CI: 1.0, 7.3) in the seventh and eighth dose octiles, respectively, relative to the lowest octile. Analyses of dose effect modification suggested that radiation-related risk may have been higher in women who were younger at the time of the accident and/or at the time of diagnosis.
Conclusions
This study suggests that prolonged exposure to ionizing radiation at low dose rates can increase risk of breast cancer.
Keywords: Breast cancer, ionizing radiation, Chernobyl
Key Messages
Ionizing radiation is a known cause of female breast cancer, but there have been few studies of the risk after prolonged radiation exposures at low dose rates.
Residents of areas contaminated by fallout from the Chernobyl accident of 26 April 1986, have received prolonged, low dose rate, mixed internal and external radiation exposures.
In this case-control study of breast cancer incidence during October 2008 through February 2013 among women living in Bryansk Oblast, Russia, those in the two highest octiles of estimated Chernobyl-related radiation dose to the breast had elevated risk, with odds ratios of 3.0 (95% confidence interval 1.3, 7.0) and 2.7 (1.0, 7.3) relative to the lowest octile.
This study suggests that prolonged low dose rate radiation exposure may increase risk of female breast cancer.
Introduction
Ionizing radiation is a known cause of female breast cancer, based largely on studies including women exposed to high doses at high dose rates, e.g. Japanese atomic bomb (A-bomb) survivors and cohorts exposed medically.1,2 The A-bombs caused single acute exposures to a wide range of radiation doses, primarily from γ-rays. Breast dose estimates range up to 600+ centisievert (cSv) in the Life Span Study (LSS) cohort of A-bomb survivors, although an analysis of breast cancer incidence during 1950–1990 found a significant linear dose response when limited to women with DS86 breast doses ≤50 cSv.3 Studies of medical irradiation have addressed scenarios including varying numbers of low- or high-dose γ- or x-ray fractions, usually delivered at high dose rates. Preston et al.4 analysed breast cancer incidence in five cohorts of women who received such exposures to treat benign diseases, along with the LSS. They also included two cohorts in which most patients received ≥1 course (mean ∼1.5 courses), typically 2 h/course, of low dose rate γ-rays during 226Ra applicator treatment for skin haemangioma in infancy or early childhood. Relative risks associated with medical exposures were generally lower than those following A-bomb exposure, possibly due to the low baseline rate of breast cancer incidence among Japanese women: the seven other cohorts were all US or Swedish. Absolute excess risks were generally more similar, though notably smaller in the haemangioma cohorts when compared with the five other medically irradiated cohorts.
In contrast to the considerable evidence associating breast cancer with high dose or high dose rate ionizing radiation, few studies have investigated the effects of exposure to lower doses protracted over long intervals. Such exposure is of public health concern because it occurs in environmental and occupational settings. The Chernobyl Nuclear Power Station accident of 26 April 1986 contaminated a large area in the former Soviet Union and elsewhere. Residents of that area have been exposed to many radionuclides including 131I and 137Cs among others, through both external exposure and consumption of contaminated milk and other foods. As a result they have accumulated relatively low whole body and breast doses at low dose rates over decades. Pukkala et al.5 examined breast cancer incidence in contaminated areas of Ukraine and Belarus during 1979–2001, in relation to estimated district-average whole-body doses accumulated since the Chernobyl accident from external exposure and ingestion of long-lived radionuclides. Incidence rates increased after the accident, partly due to intensified screening, with larger increases in districts with higher average dose estimates. Although this is consistent with a radiation effect from Chernobyl, an ecological study such as this cannot account for individual women having doses above or below their district’s average.
We conducted a population-based case-control study investigating the occurrence and molecular characteristics of breast cancer in women resident in Bryansk Oblast, a 34 900 km2 region of Russia that was the most heavily contaminated area in the Russian Federation, especially in its western raions (comparable to counties in the USA). Cases were ≤55 years old when diagnosed, since studies suggest the radiation effect may be greatest for diagnoses at younger ages. Here we explore the dose–response relationship between radiation dose from the Chernobyl accident and subsequent breast cancer; analyses of molecular characteristics will be reported separately.
Methods
Identification of subjects
Eligible cases were women ≤55 years old when first diagnosed with invasive breast cancer between 20 October 2008 and 28 February 2013, who were residents of Bryansk Oblast at the time of the accident (ATA) on 26 April 1986 and at their breast cancer diagnosis. Cases were identified by the Bryansk Oncology Registry, a population-based registry of all newly diagnosed cancer cases among residents of Bryansk Oblast. Controls were living women with no diagnosis of breast cancer (no other disease criteria applied). Menopausal status did not affect eligibility, although women currently receiving hormonal therapy for postmenopausal symptoms were ineligible. One control, individually matched to her case on calendar year of birth, raion of residence and type of settlement (urban vs non-urban) ATA, was randomly selected from the essentially complete raion population roster maintained by the case’s local polyclinic.
Data collection
In-person interviews were conducted with each subject, in her home whenever possible. Eight participants born after 1968 were assisted by an older person(s) with direct knowledge of her life from the Chernobyl accident until age 18. Information collected included known or possible risk factors for breast cancer (e.g. age at menarche, pregnancy history, body mass index, radiation exposures other than Chernobyl, and family history of cancer), as well as residence and dietary history. For the case and her matched control, this information was collected for the interval from the Chernobyl accident until the reference date, i.e. the case’s breast cancer diagnosis date. Medical records of cases were reviewed and selected clinical and diagnostic information abstracted; slides and paraffin-embedded tissue blocks were sought; and all available pathology specimens and the original pathology reports were collected. When existing slides were inadequate for review, new slides were prepared from tissue blocks if available.
All procedures and data collection instruments were approved by Institutional Review Boards at the Fred Hutchinson Cancer Research Center and the Russian National Center for Hematology in Moscow. All study participants provided written informed consent to participate in the study prior to data collection.
Dose estimation
The sum of each participant’s external and internal radiation doses to the breast from the Chernobyl accident, accumulated through her diagnosis/reference date, was estimated from the following: her self-reported histories of residence location(s), of quantities and sources of milk and selected foods, and of pregnancy/lactation; soil contamination measurements or estimates for her residence and food source locations; and standard coefficients for external dose rates from soil contamination, and for soil-to-food transfer and ingestion-to-internal-dose conversion for selected radionuclides (see the Supplementary data, available at IJE online).
Statistical methods
Conditional logistic regression was used to estimate odds ratios (ORs) for breast cancer risk in relation to estimated radiation dose and other factors while accounting for the individual matching of cases and controls. For analyses treating radiation dose as a continuous variable, the linear model OR(d) = 1+ βd was used, where d denotes the estimated Chernobyl-derived radiation dose and β is the excess odds ratio (EOR). Generalizations of this model were used to test for confounding or effect modification, as described in the Supplementary data, available at IJE online. Parameter estimation was based on maximum likelihood methods.
Results
The Bryansk Oncology Registry identified 707 potential cases, of whom 476 were eligible for this study (Supplementary Table 1, available as Supplementary data at IJE online). Of these, eight were excluded: the diagnosis of breast cancer was not confirmed by study pathologists for three women, and interviews were not completed for five. Of the 468 included cases, 388 (83%) had ductal carcinoma and 54 (12%) had lobular or mixed ductal/lobular carcinoma. One eligible control was selected and interviewed for each case, for a total of 936 participants.
The average age of the cases at diagnosis was 46.7 years (range 25–55 years), and 60% lived in urban areas ATA (Table 1). The mean age at menarche was 13.7 years (range 9–18 years). Most women had at least one live birth (94%; Table 2). Among parous women the mean age at first live birth was slightly higher for cases than controls (22.9 vs 22.4 years). Cases were less likely than controls to be premenopausal at the diagnosis/reference date (43% vs 53%), due to a higher frequency with menopause related to medical interventions or diseases (21% vs 11%). Breast cancer in first degree relatives was more frequent in cases (9%) than controls (5%; P = 0.02).
Table 1.
Age and matching factors of breast cancer cases (n = 468) and matched controls (n = 468) from Bryansk Oblast, October 2008 to February 2013
| n | % | |
|---|---|---|
| Age at diagnosis/reference date, years | ||
| 25–29 | 12 | 1 |
| 30–39 | 131 | 14 |
| 40–49 | 424 | 45 |
| 50–55 | 369 | 39 |
| Birth year | ||
| 1954–59 | 294 | 31 |
| 1960–64 | 278 | 30 |
| 1965–69 | 182 | 19 |
| 1970–79 | 160 | 17 |
| 1980–85 | 22 | 2 |
| Settlement type ATA | ||
| Urban | 560 | 60 |
| Non-urban | 376 | 40 |
ATA, at the time of the Chernobyl accident (April 26, 1986).
Table 2.
Potential breast cancer risk factors of cases and controls from Bryansk Oblast, October 2008 to February 2013
| Cases (n = 468) |
Controls (n = 468) |
|||||
|---|---|---|---|---|---|---|
| Potential risk factor | n | % | n | % | ORa | 95% CI |
| Age at menarche, years | ||||||
| 9–12 | 77 | 16 | 86 | 18 | 1.00 | Ref |
| 13–14 | 262 | 56 | 264 | 56 | 1.09 | (0.78, 1.53) |
| 15–18 | 127 | 27 | 117 | 25 | 1.18 | (0.80, 1.74) |
| Unknown | 2 | 0.4 | 1 | 0.2 | − | − |
| Nulliparousb | 30 | 6 | 30 | 6 | 1.00 | (0.59, 1.69) |
| Age at 1st live birthc, years | ||||||
| <20 | 69 | 16 | 75 | 17 | 1.00 | Ref |
| 20–24 | 254 | 58 | 276 | 63 | 1.01 | (0.69, 1.50) |
| 25–29 | 89 | 20 | 62 | 14 | 1.73 | (1.04, 2.89) |
| ≥30 | 26 | 6 | 25 | 6 | 0.98 | (0.51, 1.88) |
| Number of live births | ||||||
| 0 | 30 | 6 | 30 | 6 | 1.00 | Ref |
| 1 | 162 | 35 | 151 | 32 | 1.08 | (0.62, 1.89) |
| 2 | 239 | 51 | 244 | 52 | 0.97 | (0.57, 1.67) |
| ≥3 | 37 | 8 | 43 | 9 | 0.84 | (0.42, 1.68) |
| Total number of pregnancies | ||||||
| 0 | 24 | 5 | 18 | 4 | 1.00 | Ref |
| 1–2 | 137 | 29 | 150 | 32 | 0.66 | (0.34, 1.31) |
| 3–4 | 181 | 39 | 179 | 38 | 0.75 | (0.38, 1.46) |
| 5–7 | 100 | 21 | 95 | 20 | 0.78 | (0.39, 1.55) |
| ≥8 | 26 | 6 | 26 | 6 | 0.74 | (0.32, 1.72) |
| BMI 1 year before diagnosis/ref. date, kg/m2 | ||||||
| <25 | 134 | 29 | 134 | 29 | 1.00 | Ref |
| 25–29 | 154 | 33 | 166 | 35 | 0.93 | (0.65, 1.32) |
| ≥30 | 180 | 38 | 168 | 36 | 1.08 | (0.76, 1.54) |
| Menopausal status at diagnosis/ref. date | ||||||
| Premenopausal | 200 | 43 | 246 | 53 | 1.00 | Ref |
| Natural menopause | 167 | 36 | 170 | 36 | 1.40 | (0.97, 2.02) |
| Other menopaused | 99 | 21 | 51 | 11 | 2.48 | (1.65, 3.73) |
| Unknown | 2 | 0.4 | 1 | 0.2 | − | − |
| Breast cancer in 1st degree relativeb | 41 | 9 | 22 | 5 | 1.90 | (1.12, 3.23) |
| Diagnostic radiological procedureb | 448 | 96 | 447 | 96 | 1.50 | (0.25, 8.98) |
| Breastb | 73 | 16 | 50 | 11 | 2.35 | (1.33, 4.15) |
| Chest/lung/heartb | 446 | 95 | 445 | 95 | 1.25 | (0.34, 4.65) |
| Cigarette smoking | ||||||
| Never smoked | 380 | 81 | 380 | 81 | 1.00 | Ref |
| Former smoker | 29 | 6 | 21 | 4 | 0.88 | (0.59, 1.30) |
| Current smoker | 59 | 13 | 67 | 14 | 1.39 | (0.77, 2.51) |
| Alcohol consumption | ||||||
| Never | 61 | 13 | 41 | 9 | 1.00 | Ref |
| ≤Monthly | 266 | 57 | 268 | 57 | 0.68 | (0.44, 1.04) |
| 2–4/Month | 130 | 28 | 146 | 31 | 0.62 | (0.39, 0.97) |
| ≥Weekly | 11 | 2 | 13 | 3 | 0.59 | (0.23, 1.47) |
| Subject’s education level | ||||||
| Grade 5–8 | 7 | 1 | 4 | 1 | 2.22 | (0.62, 7.87) |
| Grade 9–11 | 58 | 12 | 39 | 8 | 2.03 | (1.19, 3.45) |
| Technical school | 266 | 57 | 265 | 57 | 1.27 | (0.93, 1.73) |
| College/university | 137 | 29 | 160 | 34 | 1.00 | Ref |
| Parent’s education levele | ||||||
| Grade ≤4 | 99 | 21 | 76 | 16 | 1.89 | (1.13, 3.15) |
| Grade 5–8 | 112 | 24 | 109 | 23 | 1.41 | (0.89, 2.24) |
| Grade 9–11 | 65 | 14 | 45 | 10 | 1.95 | (1.14, 3.36) |
| Technical school | 129 | 28 | 162 | 35 | 1.01 | (0.65, 1.56) |
| College/university | 52 | 11 | 66 | 14 | 1.00 | Ref |
| Unknown | 11 | 2 | 10 | 2 | 1.40 | (0.52, 3.78) |
| Occupational historyb | ||||||
| Chemistry, petrochemistry | 12 | 3 | 5 | 1 | 2.75 | (0.88, 8.64) |
| Agriculture | 23 | 5 | 21 | 4 | 1.14 | (0.56, 2.34) |
| Metallurgy, mining | 14 | 3 | 6 | 1 | 2.60 | (0.93, 7.29) |
| Health care or research with exposure to radiation | 28 | 6 | 32 | 7 | 0.87 | (0.51, 1.47) |
| Any of the abovef | 75 | 16 | 63 | 13 | 1.27 | (0.86, 1.89) |
ATA, at the time of the Chernobyl accident (April 26, 1986); BMI, body mass index; CI, confidence interval; OR, odds ratio.
OR for each factor was estimated by conditional logistic regression, to account for matching on birth year, raion and type of settlement ATA; no other covariates were included.
OR is reported for Yes, relative to No, for the following variables: nulliparous, breast cancer in 1st degree relative, diagnostic radiological procedures (any, breast, chest/lung/heart) and each category of occupational history.
Nulliparous women excluded.
Other menopause includes menopause related to medical or surgical intervention, or resulting from any disease.
Highest level of mother’s and/or father’s education level.
Three women reported multiple occupational exposure categories.
Most participants (95%) had prior medical radiation exposures to the chest, heart or lungs, due largely to tuberculosis screening. Breast diagnostic imaging procedures, primarily mammography, were more common among cases (16%) than controls (11%), likely reflecting procedures related to the breast cancer diagnosis.
Cases were less likely than controls to have received secondary education (technical school, college or university; 86% vs 91%), and were also less likely to have a parent who received secondary education (39% vs 49%). Employment in chemical/petrochemical and metallurgy/mining industries, although infrequent, was somewhat more common among cases.
Mean age of cases ATA was 21.5 years (range 0–31 years). Estimated radiation doses ranged from 0.04 to 41 centigray (cGy) (mean 1.3 cGy; Table 3, Figure 1). These generally included significant contributions from both internal exposure (0.004–19 cGy, mean 0.73 cGy) and external exposure (0.01–23 cGy, mean 0.59 cGy). Total doses of the cases averaged 0.16 cGy larger than those of their matched controls.
Table 3.
Distributions of estimated total radiation doses to the breast from the Chernobyl accident, accumulated since April 26, 1986 by breast cancer cases and controls from Bryansk Oblast, October 2008 to February 2013
| Estimated total dose, cGy |
|||
|---|---|---|---|
| All women | Cases | Controls | |
| No. of women | 936 | 468 | 468 |
| Minimum | 0.04 | 0.04 | 0.05 |
| Median | 0.25 | 0.26 | 0.93 |
| Mean | 1.3 | 1.4 | 1.2 |
| Maximum | 41 | 41 | 21 |
cGy, centigray.
Figure 1.
Distributions of estimated total radiation dose to the breast from the Chernobyl accident among breast cancer cases and controls from Bryansk Oblast, October 2008 to February 2013.
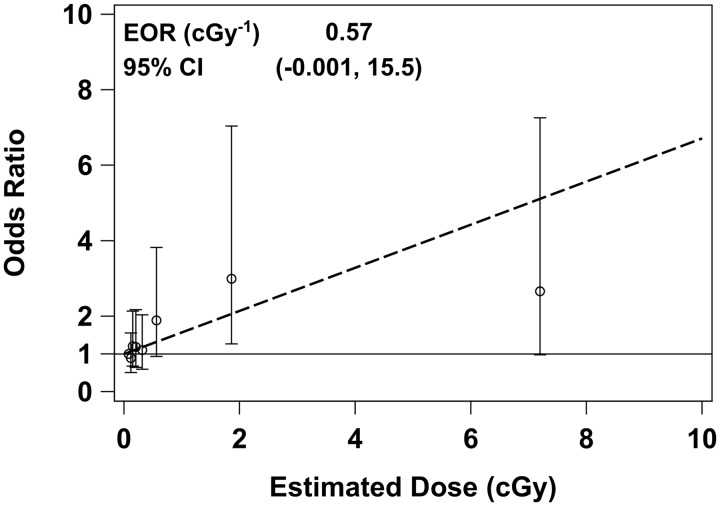
The ORs for breast cancer risk, estimated without adjustment for any covariates, were elevated in the upper dose octiles (Table 4, ‘Unadjusted’). As described in the Supplementary data, available at IJE online, investigation of possible confounding led to adjustment for six covariates: menopausal status at diagnosis/reference date (premenopausal vs natural menopause vs other menopause); breast cancer in any first degree relative (yes vs no); nulliparity (yes vs no); age at first live birth; subject’s education level (≤grade 11 vs technical school vs college/university); and history of employment in metallurgy/mining (yes vs no). The unadjusted and adjusted ORs in Table 4 are similar, suggesting that the apparent association with radiation dose did not arise from confounding by any of the available covariates. In analyses treating dose as a continuous variable, however, the EOR was quite sensitive to the inclusion of the covariates, with an unadjusted EOR = 0.18 cGy−1 [95% confidence interval (CI): –0.00, 1.78] and adjusted EOR = 0.57 cGy−1 (95% CI: –0.00, 15.5, see Figure 2).
Table 4.
Odds ratios for breast cancer risk, by estimated total radiation dose to the breast from the Chernobyl accident, among female residents of Bryansk Oblast, October 2008 to February 2013
| Dose octilea | Mean (min – max) dose, cGy | No. (%) of cases (n = 468) | No. (%) of controls (n = 468) | Odds Ratiob (95% CI) |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| 1 | 0.08 (0.04–0.10) | 57 (12) | 60 (13) | 1.0 | 1.0 |
| 2 | 0.12 (0.10–0.13) | 54 (12) | 63 (13) | 0.9 (0.5, 1.5) | 0.9 (0.5, 1.6) |
| 3 | 0.15 (0.13–0.17) | 59 (13) | 58 (12) | 1.1 (0.6, 1.9) | 1.2 (0.7, 2.1) |
| 4 | 0.20 (0.17–0.25) | 56 (12) | 61 (13) | 1.0 (0.6, 1.8) | 1.2 (0.6, 2.2) |
| 5 | 0.32 (0.25–0.41) | 58 (12) | 59 (13) | 1.2 (0.7, 2.2) | 1.1 (0.6, 2.0) |
| 6 | 0.56 (0.41–0.76) | 61 (13) | 56 (12) | 1.6 (0.8, 3.3) | 1.9 (0.9, 3.8) |
| 7 | 1.86 (0.79–3.57) | 64 (14) | 53 (11) | 2.4 (1.1, 5.6) | 3.0 (1.3, 7.0) |
| 8 | 7.28 (3.61–41.5) | 59 (13) | 58 (12) | 2.3 (0.9, 5.8) | 2.7 (1.0, 7.3) |
Octiles of estimated breast dose were calculated for all 936 cases and controls combined.
Odds ratios were estimated by conditional logistic regression, to account for individual matching on birth year and raion and type of settlement ATA, in a univariable model (‘Unadjusted’), or in a multivariable model (‘Adjusted’) that included the following covariates: menopausal status at diagnosis/reference date (premenopausal vs natural menopause vs other menopause), history of breast cancer in first degree relative (yes vs no), nulliparity (yes vs no), age at first live birth, subject’s education level (≤grade 11 vs technical school vs college/university) and history of employment in metallurgy or mining (yes vs no). The ‘Adjusted’ model excluded three case–control pairs in which menopausal status was unknown for one case or control. ATA, at the time of the Chernobyl accident (April 26, 1986); cGy, centigray; CI, confidence interval.
Figure 2.
Odds ratios for breast cancer among breast cancer cases and controls from Bryansk Oblast, October 2008 to February 2013, in relation to estimated total radiation dose to the breast from the Chernobyl accident. Point estimates and 95% CIs are shown for dose octiles 2–8, relative to the lowest octile (octiles are based on all n = 936 cases and controls combined). The dashed line shows the fitted linear odds ratio obtained by treating dose as a continuous variable. For both the categorical and continuous treatment of dose, multivariable conditional regression methods were used to account for the individual matching of cases and controls and for the following covariates: menopausal status (premenopausal vs natural menopause vs other menopause), breast cancer in any first degree relative (yes vs no), nulliparity (yes vs no), age at first live birth, subject’s education level (≤grade 11 vs technical school vs college/university) and history of employment in metallurgy or mining (yes vs no).
Analyses of radiation dose effect modification are described in detail in the Supplementary data, available at IJE online. When the radiation dose response was allowed to vary between subgroups defined by covariates, the resulting ORs or EORs had quite wide CIs for some or all subgroups, often due to small subgroup sizes. Only two covariates were identified as likely effect modifiers: age ATA and age at diagnosis/reference date. These two ages are very highly correlated in these data (Pearson correlation = 0.98), since all diagnoses occurred 22.5–26.8 years after the Chernobyl accident. The EOR for the women of age ATA <13 years was markedly higher than for older women, although the CIs for the two EORs are wide and overlapping (Table 5; similar analyses by dose octile are in Table D of the Supplementary data, available at IJE online). Allowing the EOR to vary as a continuous function of age ATA, it decreased by a multiplicative factor of 0.26 (95% CI: 0.09, 0.74) per 1-year increase in age ATA, suggesting that the radiogenic risk may be inversely associated with age ATA and/or at diagnosis.
Table 5.
Radiation dose effect modification of breast cancer risk by age ATAa, among female residents of Bryansk Oblast, October 2008 to February 2013
| Without radiation effect modification | With radiation effect modification | ||
|---|---|---|---|
|
|
|||
| Nos. of cases, controls | EORb (95% CI), cGy−1 | ||
| All Ages ATAa | 465, 465 | 0.57 (−0.00, 15.5) | – |
| Age ATAa <13 | 51, 51 | – | 9.59 (0.90, 99.8) |
| Age ATAa ≥13 | 414, 414 | – | 0.38 (−0.01, 11.5) |
| Covariates | ORb (95% CI) | ||
|
| |||
| Menopausal status | Premenopausal | 1.00 | 1.00 |
| Natural menopause | 1.43 (0.97, 2.10) | 1.45 (0.98, 2.14) | |
| Other menopause | 2.55 (1.65, 3.94) | 2.59 (1.68, 4.00) | |
| Breast cancer in 1st degree relative | Yes vs no | 2.24 (1.27, 3.95) | 2.24 (1.27, 3.96) |
| Participant’s education level | College/university | 1.00 | 1.00 |
| Grade ≤11 | 2.39 (1.37, 4.16) | 2.33 (1.34, 4.08) | |
| Technical school | 1.38 (0.99, 1.94) | 1.41 (1.01, 1.97) | |
| Nulliparous | Yes vs no | 0.93 (0.52, 1.66) | 0.91 (0.50, 1.64) |
| Age at 1st live birth | Per year | 1.05 (1.01, 1.09) | 1.05 (1.01, 1.09) |
| Occupation in mining/metallurgy | Yes vs no | 2.43 (0.83, 7.07) | 2.34 (0.80, 6.83) |
Each matched pair’s age ATA was based on the breast cancer case.
EORs and ORs were estimated by conditional logistic regression, to account for matching on birth year, raion and type of settlement ATA, in a multivariable model including the covariates. ATA, at the time of the Chernobyl accident (April 26, 1986); cGy, centigray; CI, confidence interval; EOR, excess odds ratio; OR, odds ratio.
Discussion
This study is the first to investigate breast cancer risk in relation to individual estimates of radiation dose to the breast from the Chernobyl accident. More generally, this is one of the few analytical epidemiological studies of breast cancer in women exposed to internal and external irradiation over a very prolonged period: most study participants accumulated their doses over more than two decades (much of the dose was accrued during the early years when exposure rates were highest). Our results suggest that risk was indeed elevated in women who accumulated the highest radiation doses (Table 4).
This is one of the few studies to investigate the association between breast cancer risk and exposure to radiation through environmental pathways. As described above, results of the ecological study of Pukkala et al.5 were consistent with a radiation-related increase in breast cancer incidence in areas of Ukraine and Belarus contaminated by Chernobyl fallout. Ostroumova et al.6 reported a dose response during 1956–2004 among women with chronic low dose rate exposures to external γ irradiation and internal 137Cs from waste discharged into the Techa-Iset River System from the Mayak nuclear weapons facility. In that study the estimated linear excess relative risk (ERR) was 0.05 cGy−1 (95% CI: 0.008, 0.13). However, in an updated analysis that extended the follow-up through 2007 and used an improved radiation dosimetry system, the dose response was smaller (ERR = 0.02 cGy−1; 95% CI: −0.01, 0.06).7 Bauer et al.8 reported a dose response for female breast cancer mortality during 1960–1999 in the Semipalatinsk historical cohort, with ERR = 0.01 cSv−1 (95% CI: 0.00, 0.16; P = 0.0047). Hwang et al.9 investigated breast cancer among Taiwanese women exposed to γ irradiation while living for prolonged periods in cobalt-contaminated buildings. With a loglinear model, the estimated ERR was 0.01 cGy−1 (90% CI: 0.00, 0.02), however the study’s limitations prevent a definitive conclusion.10
Most studies of radiogenic breast cancer risk have investigated A-bomb survivors or women exposed to radiation in occupational or medical settings. The risk of breast cancer was elevated by radiation exposure in studies of incidence and mortality among A-bomb survivors.11,12 The average ERR of breast cancer mortality during 1950–2003 in the LSS was 0.015 cGy−1 (95% CI: 0.009, 0.023).12 ERR was inversely associated with age at exposure: the average ERR for those exposed under age 20 was 0.033 cSv−1 (95% CI: 0.023, 0.044), compared with 0.010 (95% CI: 0.004, 0.016) for older women.13 Further analyses of A-bomb survivor data have suggested that this apparent effect of age at exposure may be due largely to age at diagnosis.3,11 In particular, for diagnoses at age <35 years the background breast cancer incidence rates (i.e. rates in the absence of A-bomb radiation exposure) were estimated to be lower than in previous analyses, which increased the apparent radiation-related excess risk.11 The most recent updated analysis of breast cancer incidence in A-bomb survivors suggests that ERR is higher for diagnoses at early ages and for young women with early menarche around the time of exposure.14 The results of our study are consistent with radiogenic risk being higher with younger age at exposure (Table 5) or at diagnosis/reference date, but cannot distinguish between effects of these two highly correlated age variables. Also, few women in our study were premenarchal ATA, so meaningful analysis of the timing of menarche and radiation exposure was not possible. Estimates of the EORs from our data (Table 5) are substantially larger that the ERRs seen in A-bomb survivors, however the EOR confidence intervals are very wide, indicating those estimates should be viewed cautiously.
The exposures addressed in the A-bomb, occupational or medical studies were primarily from external γ- or x-ray radiation, usually delivered over one or more very short periods of time, several seconds in the case of A-bombs, and in various modes for occupational and medical exposures. As a result, many women in those studies received relatively high doses delivered at high dose rates. In contrast, the radiation exposures from Chernobyl in this study were from a mix of external γ sources (primarily 137Cs) and internal β and γ sources from ingesting contaminated food and milk (primarily 131I and 137Cs), resulting in low doses for most women, delivered at lower dose rates over prolonged periods. Howe and McLaughlin15 found that effects of fractionated moderate-dose-rate exposures on breast cancer mortality in the Canadian Fluoroscopy Cohort were similar to those reported for A-bomb survivors. Preston et al.4 in a pooled analysis of breast cancer incidence in A-bomb survivors and seven medically exposed cohorts, found similar radiation effects from acute and fractionated high dose rate exposures, but possibly smaller effects from low dose rate exposures.
This study had several strengths. It was population-based, including essentially all eligible cases from Bryansk Oblast over a 52-month period. Breast cancer diagnoses were confirmed by study pathologists. Controls were individually matched to cases with respect to year of birth, raion of residence and type of settlement ATA, preventing confounding by age or factors that might be related to place and type of residence (about 45% of the oblast population lived in the city of Bryansk ATA). Each participant was extensively interviewed for information about known or possible breast cancer risk factors as well as factors that determined her radiation dose from Chernobyl. Each woman’s radiation dose to the breast due to Chernobyl was estimated, based on her residence and dietary histories. The impacts of pregnancy and lactation on dose from 131I during the first months after the accident were also accounted for. This study had adequate statistical power to detect associations similar to or smaller than those reported in other populations. The study protocol projected a total of 500 cases, and although only 468 were available, this caused very little loss of power: the dose-response coefficient detectable with a given level of power with n = 500 cases increases by only (500/468)1/2 = 1.033 or 3.3% compared with the actual accrual.
Potential limitations of the study include the following. Matching of cases and controls on raion of residence and settlement type may have forced their doses to be more similar than is typical for the population (overmatching). In particular, the largely rural raions in western or central Bryansk Oblast had few urban settlements, increasing the chance that a case from one of those urban settlements has a control from the same settlement. However, a woman’s radiation dose is influenced by her histories of residence locations and of the types, quantities and sources of the milk and foods she consumed. Therefore, women from the same settlement ATA can accumulate quite different doses. Data concerning dose-determining factors and other risk factors were obtained from interviews in which neither the participant nor the interviewer was blind to case–control status. Thus, the results may have been influenced by recall or reporting bias. There is no evidence that study participants were particularly knowledgeable about radiation exposure pathways or breast cancer risk factors, however this possibility cannot be excluded. This study included only cases diagnosed during a relatively short period of time 22.5–26.8 years after the Chernobyl accident. Consequently, ages ATA and at diagnosis/reference date were highly correlated and their effects on the radiation dose response could not be distinguished.
Further analyses will address whether and how molecular and other characteristics of the breast cancers are related to radiation dose. Additional development of the dose estimation system may enable analyses of risk in relation to features of dose accumulation, such as latency or maximum dose rate.
Funding
This work was funded by the National Cancer Institute at the U.S. National Institutes of Health [R01 CA118914].
Supplementary Material
Acknowledgements
The authors want to thank the following for their efforts in the conduct of this study: Theresa Taggart, Laurie Shields, Teri Kopp and Robert Day (Fred Hutchinson Cancer Research Center, Seattle, USA); Galina Pochtennaya (Bryansk Oncology Dispensary, Bryansk, Russian Federation); Galina Romanova (Bryansk Diagnostic Center, Bryansk, Russian Federation); Elena Iaskova, Tymofey Kolyzhenkov and Alexey Tyshkin (Medical Radiation Research Center, Obninsk, Russian Federation); Elena Maslova (Synovate Comcon, Moscow, Russia); and especially Andrey Vorobiev (Russian National Research Center for Hematology, Moscow, Russian Federation) and Anatoly Tsyb (Medical Radiation Research Center, Obninsk, Russian Federation). They also gratefully acknowledge the women of Bryansk Oblast who contributed their time and recollections as cases and controls.
Conflict of interest: None declared.
References
- 1. Ronckers CM, Erdmann CA, Land CE.. Radiation and breast cancer: a review of current evidence. Breast Cancer Res 2004;7:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert ES. Ionizing radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol 2009;85:467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Land CE, Tokunaga M, Koyama K. et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiat Res 2003;160:707–17. [DOI] [PubMed] [Google Scholar]
- 4. Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD.. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res 2002;158:220–35. [DOI] [PubMed] [Google Scholar]
- 5. Pukkala E, Kesminiene A, Poliakov S. et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer 2006;119:651–58. [DOI] [PubMed] [Google Scholar]
- 6. Ostroumova E, Preston DL, Ron E. et al. Breast cancer incidence following low-dose rate environmental exposure: Techa River Cohort, 1956–2004. Br J Cancer 2008;99:1940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis FG, Yu KL, Preston D, Epifanova S, Degteva M, Akleyev AV.. Solid cancer incidence in the Techa River Incidence Cohort: 1956-2007. Radiat Res 2015;184:56–65. [DOI] [PubMed] [Google Scholar]
- 8. Bauer S, Gusev BI, Pivina LM, Apsalikov KN, Grosche B.. Radiation exposure due to local fallout from Soviet atmospheric nuclear weapons testing in Kazakhstan: solid cancer mortality in the Semipalatinsk historical cohort, 1960-1999. Radiat Res 2005;164:409–19. [DOI] [PubMed] [Google Scholar]
- 9. Hwang SL, Guo HR, Hsieh WA. et al. Cancer risks in a population with prolonged low dose-rate γ-radiation exposure in radiocontaminated buildings, 1983-2002. Int J Radiat Biol 2006;82:849–58. [DOI] [PubMed] [Google Scholar]
- 10.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation. New York, NY: United Nations, 2017. [Google Scholar]
- 11. Preston DL, Ron E, Tokuoka S. et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- 12. Ozasa K, Shimizu Y, Suyama A. et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 13. United Nations Scientific Committee on the Effects of Atomic Radiation Sources and Effects of Ionizing Radiation. VolumeII: Effects New York, NY: United Nations, 2000. [Google Scholar]
- 14. Brenner AV, Preston DL, Sakata R. et al. Incidence of breast cancer in the Life Span Study of atomic bomb survivors: 1958-2009. Radiat Res 2018;190:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howe GR, McLaughlin J.. Breast cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with breast cancer mortality in the atomic bomb survivors study. Radiat Res 1996;145:694–707. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.