The angiotensin converting enzyme (ACE) 2 is a cell surface protein used for entry into type II pneumocytes and other tissues by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the infective agent of COVID-19.1 It has been demonstrated that ACE2 is upregulated on tissues by renin-angiotensin-aldosterone system (RAAS) inhibitors. This raised concerns that RAAS inhibitors may increase susceptibility and worsen prognosis in COVID-19. In contrast, ACE2 facilitates degradation of angiotensin II and has an anti-inflammatory function and may actually protect the lungs and other tissues from injury.1 Thus, the effect of RAAS inhibitors on susceptibility and prognosis of COVID-19 continues to be the subject of much debate.1 Individual observational studies in the area have yielded equivocal results; hence, we sought to conduct a meta-analysis of all available data to provide greater insight.
For this study, PubMed and Scopus were searched in May 2020 using the following keywords and their MeSH terms: “COVID-19,” “hypertension,” “ACE inhibitors (ACEIs),” and “Angiotensin receptor blockers (ARBs).” Studies were included if they:1 they reported the risk of testing positive for COVID-19 and/or the risk of mortality in COVID-positive patients; and2 compared hypertensive patients prescribed RAAS inhibitors to those not using these drugs. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) from each study were pooled using a random-effects model. A p-value <0.05 was considered significant.
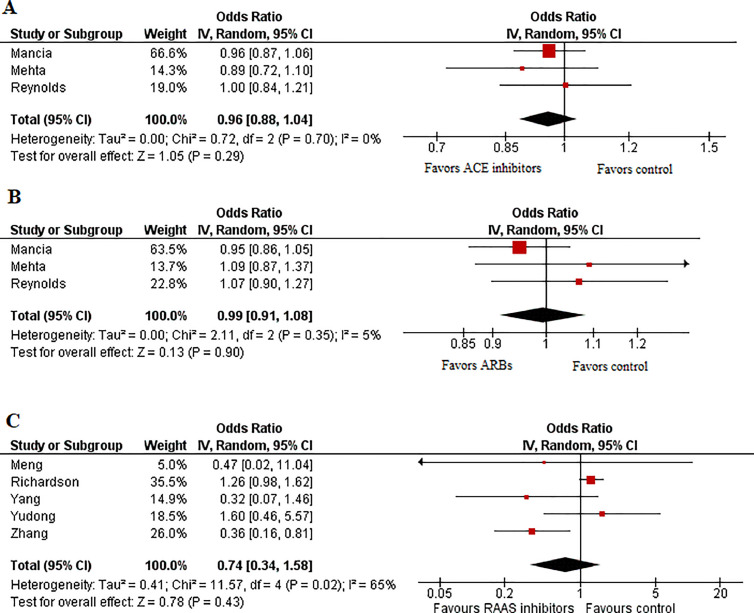
Our initial search yielded 950 potential studies. After exclusions, eight studies2, 3, 4, 5, 6, 7, 8, 9 with a total of 62,706 patients (n = 20,316 ACEI/ARB users and n = 42,390 nonusers) remained for analysis. Study and baseline characteristics are provided in Table 1 . Pooled analysis revealed no significant association between the likelihood of testing positive for COVID-19 and the use of ACEIs (OR 0.96 [0.88 to 1.04]; p = 0.29; I2 = 0%) (Figure 1 ) or ARBs (OR 0.99 [0.91 to 1.08]; p = 0.90; I2 = 5%) (Figure 1). Similarly, no significant difference was observed in mortality rate among hypertensive patients prescribed RAAS inhibitors compared with hypertensive patients not prescribed these medications (OR 0.74 [0.34 to 1.58]; p = 0.43; I2 = 65%) (Figure 1).
Table 1.
Baseline and study characteristics
| Study | Design | Country | Total patients | COVID-19 positive (%) | RAAS inhibitor group (Total, ACEi, ARB) | Non-RAAS inhibitor group (Total, non-ACEI, non-ARB) | Age | Male (%) | Adjustment |
|---|---|---|---|---|---|---|---|---|---|
| Studies reporting mortality | |||||||||
| Meng et al. | Cross-sectional | China | 42 | - | 17, -, - | 25, -, - | 64.5 (55.80 - 69.00) | 57.1 | - |
| Richardson et al. | Retrospective | USA | 2411 | - | -, 140, 194 | 2077, -, - | 63 (52 - 75) | 60.3 | - |
| Yang et al. | Retrospective | China | 126 | - | 43, -, - | 83, -, - | 66 (61 - 73) | 49.2 | - |
| Yudong et al. | Retrospective | China | 112 | - | 22, -, - | 90, -, - | 62 | - | - |
| Zhang et al. | Retrospective | China | 1128 | - | 188, -, - | 940, -, - | - | ACEIARB - 53.2 | - |
| Studies reporting risk of testing positive for COVID-19 | |||||||||
| Mancia et al. | Case-control | Italy | 37,031 | 16.9 | 15,375, 8071, 7304 | 21,656, -, - | 68 ± 13 | 63 | Multivariable adjustment for severity, sex, municipality, age at diagnosis, a number of treatment-related covariates and markers of patient clinical status |
| Mehta et al. | Cross-sectional | USA | 18472 | 9.4 | 2285, 1322, 982 | 16187, 17150, 17490 | ACEI - 63, ARB -64 | ACEI - 49, ARB - 59 | Propensity matched for age, sex, diabetes, coronary artery disease, hypertension, chronic obstructive pulmonary disease and heart failure |
| Reynolds et al. | Cross-sectional | USA | 3384 | 46.8 | 1692, 954, 1057 | 1692, 954, 1057 | ACEI - 64.7, ARB - 66 | ACEI - 56, ARB - 50 | Propensity matched for age; sex; race; ethnic group; body-mass index; smoking history; history of hypertension, myocardial infarction, heart failure, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and obstructive pulmonary diseases); and other classes of medication. |
RAAS inhibitor = Renin-angiotensin-aldosterone system inhibitor; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker.
Figure 1.
Forest plots displaying the odds of (A) testing positive for COVID-19 amongst patients using ACEI, compared to those not using ACEI; (B) testing positive for COVID-19 amongst patients using ARBs, compared to those not using ARBs; (C) mortality in COVID-19 patients using RAAS inhibitors, compared to those not using RAAS inhibitors.
The results of the current meta-analysis suggest that neither ACEI nor ARB use is significantly associated with the odds of testing positive with COVID-19. This result can be considered robust, as it was derived from 3 large-scale studies2 , 3 , 6 which adjusted for multiple potential confounding factors, including age, sex and co-morbidities. Our findings also show no significant association between RAAS inhibitor use and mortality in COVID-19 patients; however, this result must be viewed with caution as – due to the lack of data – we were unable to analyze ACEI users and ARB users separately, and adjusted data was reported by only one study. In this context, specific aspects of our analysis are notable. COVID-19 patients using RAAS inhibitors are older and have a higher burden of comorbidities, and this may have confounded our results. Adjustment for these factors could potentially shift the results in favor of RAAS inhibitors. Thus, our results support the consensus by multiple specialty societies, which recommend continued usage of RAAS inhibitors in COVID-19 patients and among the general public who have been prescribed these medications.
Disclosures
Javed Butler: is a consultant for Abbott, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Relypsa, Vifor. Stephen J Greene: has received a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis; has received research support from Amgen, Bristol-Myers Squibb and Novartis; has served on advisory boards for Amgen and Cytokinetics; and serves as a consultant for Amgen and Merck. Richard A Krasuski: is a consultant and receives research funding from Actelion Pharmaceuticals. He is also an investigator for Edwards Lifesciences and is an unpaid member of the scientific advisory board for Ventripoint.
References
- 1.Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, Milinovich A, Svensson LG, Jehi L, Young JB, Chung MK. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. Published online on May 05, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertens (Dallas, Tex: 1979) 2020;76:51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen Y, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]