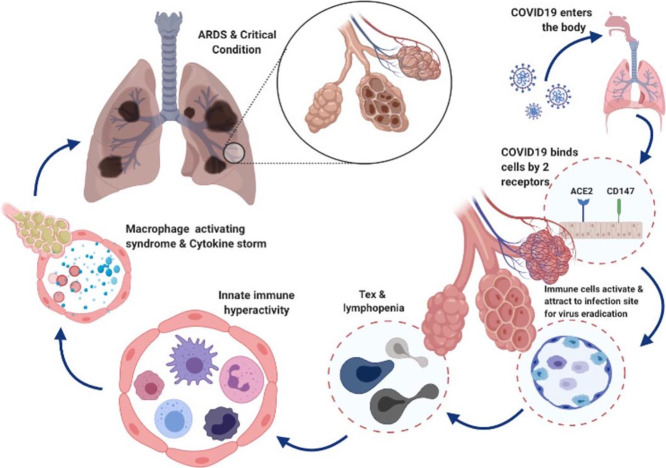
Abstract
Aim
Coronavirus disease 2019 (COVID-19) is a novel highly contagious infection caused by SARS-CoV-2, which has been became a global public health challenge. The pathogenesis of this virus is not yet clearly understood, but there is evidence of a hyper-inflammatory immune response in critically ill patients, which leads to acute respiratory distress syndrome (ARDS) and multi-organ failure.
Material and methods
A literature review was performed to identify relevant articles on COVID-19 published up to April 30, 2020. The search resulted in 361 total articles. After reviewing the titles and abstracts for inclusion, some irrelevant papers were excluded. Additional relevant articles were identified from a review of citations referenced.
Key findings
SARS-CoV-2, directly and indirectly, affects the immune system and avoids being eliminated in early stages. On the other hand, the secretion of inflammatory cytokines creates critical conditions that lead to multi-organ failure.
Significance
The immune system which is affected by the virus tries to respond via a cytokine storm and hyperinflammation, which itself leads to further multi-organ damage and even death.
Keywords: COVID-19, Immune system, Acute respiratory distress syndrome, Hyperinflammation, Cytokine storm
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the coronavirus disease 2019 (COVID-19), and has affected people's lives globally, since first observed in Wuhan, China in the last days of 2019 [1,2]. The main route of virus entry and transmission is respiratory droplets that are expelled and absorbed by the mucous membranes, especially the nasal and larynx mucosa. COVID-19 spreads readily via person-to-person contact [3]. The clinical spectrum of COVID-19 varies from an asymptomatic form to severe respiratory failure (SRF) that necessitates mechanical ventilation and support in an intensive care unit (ICU) and can lead to multi-organ failure. Pneumonia is the most frequent serious manifestation of COVID-19, characterized primarily by fever, dry cough, and dyspnea. Other less common symptoms are headaches, sore throat, and rhinorrhea. In addition to respiratory symptoms, gastrointestinal symptoms, myalgia, skin rashes, and neurological involvement have also been reported [1,[3], [4], [5], [6]].
2. SARS-CoV-2 and the immune system
2.1. SARS-CoV-2 pathology
SARS-CoV-2 belongs to the coronavirus family, members of which have caused two previous epidemics at the beginning of the 21st century; one named SARS-CoV and the other Middle East Respiratory Syndrome (MERS). Coronaviruses are large enveloped viruses with a positive sense RNA genome. The lipid bilayer envelope of the virus contains several proteins with different tasks. The spike or S glycoprotein (SP), has two domains of S1 and S2, is responsible for invasion, attachment, and entry into human cells. The receptor-binding domain (RBD) in S1 interacts with angiotensin-converting enzyme 2 (ACE2) on the human host cell surface, which is a similar entry mechanism to SARS-CoV; however, the S2 domain is responsible for virus-cell membrane fusion and viral entry with higher affinity [7]. Higher expression of the ACE2 receptor in adults compared to children may be a reason for the higher infection rate seen in adults [8,9]. Another noteworthy point is the increased level of enzymes in the liver, heart, and kidneys in COVID-19 patients with pneumonia, which is consistent with the tissue expression profile of the ACE2 receptor [10]; this could also explain the occurrence of multi-organ failure in some patients [11].
2.2. Effects of SARS-CoV-2 on the immune system
Since both SARS-CoV and SARS-CoV-2 have the same cell entry mechanism, the pathogenesis of both viruses could be the same, or at least very similar [12]. ACE2 is the common factor that binds to the superficial S glycoprotein on the envelope of the virus. It seems that this binding is sensed (essentially) by Toll-like receptor-7 (TLR-7), which is present in endosomes, and which then leads to the secretion of inflammatory cytokines [13,14]. ACE2 is highly expressed in some organs, like lung epithelial cells, especially type II pneumocytes, and in cells of the heart, kidneys, gastrointestinal tract, liver, and bladder [15,16]. Therefore these organs constitute the main target for the virus. Following entry of SARS-CoV-2 into the cell, the viral RNA genome is transferred from the envelope into the cytoplasm and the translation process begins. After replication of the RNA new viral particles are formed, by incorporating part of the host cell membrane in the new viral envelope. Although, SARS-CoV-2 buds from the infected cell, it does not lyse it directly [17]. Infected lung epithelial cells produce interleukin IL-8 which acts as a chemoattractant for neutrophils and T lymphocytes [18]. The innate immune response is initially triggered by lung epithelial cells, alveolar macrophages and neutrophils. In the next stage, adaptive immune responses are triggered involving T and B lymphocytes to complete the complete immune response [19]. Virus particles containing single-stranded ssRNA, act as pathogen-associated molecular patterns (PAMPs), and provoke a strong innate immune response after recognition by Toll-like receptor 7 (TLR7), which is expressed on monocyte-macrophages and dendritic cells (DC). TLR7 can activate several signaling pathways and transcription factors, such as Janus kinase transducers (JAK/STAT), nuclear factor κB (NF-κB), activator protein 1 (AP-1), interferon response factor 3 (IRF3), and IRF7. This signaling cascade leads to increased secretion of pro-inflammatory cytokines, like IL-1, IL-6, monocyte chemo attractant protein-1 (MCP-1), MIP-1A, tumor necrosis factor α (TNF-α) and ultimately interferon 1 (IFN1) [20]. Furthermore, neutrophils are rapidly recruited to sites of infection, where they kill viruses by an oxidative burst, defensin secretion, and neutrophil extracellular traps (NETs) [21]. Along with these events, antigen presentation subsequently stimulates the body's specific adaptive immunity (both humoral and cellular immunity) which culminates in approximately 7–14 days after infection. Following the representation of antigens by APCs to the CD4+ and CD8+ T-cells, pro-inflammatory cytokines are produced via the NF-κB signaling pathway. Activated B cells secrete virus-specific antibodies, while antigen-specific T cytotoxic cells kill virus-infected cells [17,22]. Additionally, Th17 cells, neutrophils, and granulocytes secrete IL-17, which in turn stimulates production of IL-1, IL-6, IL-8, MCP-1, Gro-a, G-CSF, GM-CSF, TNF-α, and PGE2. All these mediators can increase the recruitment of neutrophils, monocytes, and other immune cells. Besides, it has been reported that IL-17 expression is correlated with several inflammatory respiratory diseases [23]. All these immune signaling pathways are designed to create an inflammatory environment with the goal of eradicating SARS-CoV-2.
2.3. The immune system response
The pathology of SARS-CoV-2 is not yet completely understood; most of our knowledge has been based on research into SARS-CoV and MERS, which previously caused epidemics of acute respiratory syndromes. In this short duration of the present pandemic, studies have shown that SARS-CoV-2 has several defense mechanisms, which makes its eradication more difficult. The SARS-CoV-2 envelope includes attached proteins like M (membrane), S (spike), E (envelope), and N (nucleocapsid). Similar to other coronaviruses, the N protein of SARS-CoV-2 inhibits IFN1 by regulating IFN-β synthesis and signaling. On the other hand, the effectiveness of the innate immune response against viral infection depends mainly on IFN1 production and its downstream signaling that results in controlling viral replication and induction of an adequate adaptive immune response [7,20]. However the virus could avoid this attack due to the complex immune dysregulation caused by this infection. Chronic stimulation of T cells, resulting in a cytokine storm and T cell exhaustion, weakens the overall body defenses and puts the patient in a dangerous situation. High-grade chronic viral infections result in CD8+ T cell exhaustion (Tex) leading to a decreased effector function and lower proliferative capacity. Tex leads to over-expression of inhibitory receptors, including CD279 (PD-1), a lymphoid cell surface protein of the Ig superfamily, and a member of the extended CD28/CTLA-4 family of T cell regulators, which act as a mature T cell checkpoint for the modulation of apoptosis. PD-1 can bind to either of its ligands (PD-L1[CD274] and PD-L2[CD273]) both members of B7 family of T cell co-receptors. This binding causes significant suppression of the immune system by affecting T cells, as well as B cells and NK cells [7,20,24,25]. Another important observation is the strong correlation between inflammatory markers, including ESR, CRP and IL-6, and the relevant subset of lymphocytes [26]. Overall, general lymphopenia is seen in COVID-19 patients, especially in severe cases [27,28].
2.4. Disease in some particular groups of patients
Similar to all infectious diseases, the immune system plays an essential role in virus suppression. Therefore, it can be assumed that suppression of the immune defense system will make the situation worse, but it is not as simple as it sounds [29]. There has not yet been enough scientific evidence to generally employ immunosuppressive drugs in autoimmune diseases like rheumatoid arthritis (RA) [30,31]. On the other hand, the hyper-inflammatory and cytokine release syndrome (CRS) typical of COVID-19 causes tissue damage to the lung epithelium and ARDS [32]; therefore, immunosuppressive drugs may be useful as there is some evidence that an anti-IL-6 approach is effective in critically ill patients in the ICU [33]. Another high-risk group for COVID-19 is cancer patients, who are administered cytotoxic drugs. Although their immune function may be at an acceptable level, some cytotoxic drugs such as 6-MP used in chemotherapy, have shown destructive effects on virus replication in vitro [31]. On the other hand, the high number of critically ill patients and increased mortality in patients with underlying diseases (such as hypertension and diabetes) has been proven [34]. Diabetes mellitus type II can include a hyperinflammatory state with a low-grade inflammatory activity that causes long-term immune system stimulation [35], along with adipose tissue side effects on the immune system, which ultimately drives an immune imbalance [36]. Also, due to the overexpression of ACE2 in islet cells of the pancreas, SARS-CoV-2 may be a diabetogenic virus that causes severe instability in the blood glucose levels of diabetes patients, which worsens the inflammatory imbalance [37]. As a consequence, the condition of diabetic patients can be worsened after COVID-19 infection, as evidenced by the higher levels of multiple enzymes and inflammatory cytokines compared to nondiabetic individuals with COVID-19 pneumonia [38].
3. The immune system as a foe
3.1. An entry route for the virus
CD147, also known as Basigin or extracellular matrix metalloproteinase inducer (EMMPRIN), is also recognized as a red blood cell (RBC) receptor for the parasite that causes malaria in humans, and is a transmembrane protein of the immunoglobulin family. Its expression is induced in several conditions such as asthma, cancer stem cells, high glucose concentration in monocytes, and inflammatory processes [39]. Wang et al. recently demonstrated that the SARS-CoV-2 SP also binds to CD147 [40]; so, the immune system itself could be an entryway for SARS-CoV-2.
3.2. Macrophage activation syndrome and the cytokine storm
Patients with COVID-19 have increased levels of inflammatory cytokines and chemokines, such as IL-1, IL-6, IL-8, IL-17, IL-17, CCL-2, TNF-ɑ, G-CSF, IP-10, MCP-1, and MIP. The concentration of these markers fluctuates depending on the individual's condition; it seems that increased cytokine levels, especially IL-6, have a direct association with a worsened patient condition [41]. Furthermore, as higher levels of cytokines rapidly lead to deterioration of the patient's condition and death, they could be considered to be prognostic markers in the clinic [42]. The cytokine storm (CS) is typical of macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (sHLH). Consequently, tissue damage, lung injury and acute respiratory distress syndrome (ARDS) could be expected [43]. Furthermore, a study showed that the peripheral blood of a patient with severe COVID-19 had a strikingly high number of Th17 cells, which secrete IL-17, and are associated with autoimmune and inflammatory diseases. In the mucosal immune response, IL-22, IL-17, and TNF-α are known to induce antimicrobial peptides. Also, IL-22 upregulates mucins, fibrinogen, and anti-apoptotic proteins; therefore, IL-22 may contribute to the formation of life-threatening edema, and the lungs may become enriched with mucins and fibrin leading to the progression of ARDS, seen in COVID-19 patients [44]. All of the above-mentioned factors result in pneumonia and ARDS, besides hyper-ferritinemia, coagulopathy, and multi-organ failure in the liver, heart, and kidneys with elevated D-dimer, CRP, BUN, and Cr that are typical of MAS/sHLH [27,45]. However, NK cell reduction, suppression of antiviral defense, activation of aggressive tissue-damaging immune responses via increased IL-6 secretion, and secondary CS leads to a general picture of hyper-inflammatory immunodeficiency, are all in line with primary HLH. This is important in terms of the viral load, because of the correlation between the RNA viral load and ARDS severity [27,46]. Although the typical MAS/sHLH pathology that occurs in immunocompetent patients, may also be seen in COVID-19, but the differentiation between these two patterns is difficult.
3.3. Hyper-inflammation and severe respiratory failure
Although COVID-19 has numerous manifestations, lung injury and severe respiratory failure (SRF) are more common than others. The cornerstone of this condition is MAS/sHLH or immune dysregulation. Alveolar macrophages secret IL-6 that cause overproduction of pro-inflammatory cytokines by monocytes, and dysregulation of lymphocytes, characterized by CD4 lymphopenia and subsequently B cell lymphopenia. In parallel, the absolute natural killer (NK) cell count is depleted, probably as a result of the rapidly multiplying virus. Moreover, IL-6 decreases the expression of HLA-DR on the membrane and production of IFN-γ by CD4 cells [4]. In conclusion, it seems that innate immunity, characterized by neutrophils and proinflammatory cytokines in the format of the cytokine storm, is trying to limit the infection and overcome the virus; however, it leads to an excessive inflammatory response rather than harming the virus. This causes lung injury with tissue damage and life-threatening edema, enriched with mucins and fibrin, that unfortunately causes SRF and death in severely affected patients [22].
4. The immune system as a friend
4.1. Role of immunoglobulin and immunomodulation in recovery
It seems that lymphopenia, hyper-inflammatory state, and cytokines all play a role in the pathology of COVID-19; hence the hypothesis was proposed that immunomodulatory drugs may be beneficial to reverse the condition and treat the disease [47]. Considering the pivotal role of IL-6 in CS, tocilizumab as an IL-6 inhibitor could be effective in COVID-19 patients with a severe condition [33]. There are limited data about the effects of other immunosuppressive drugs, such as glucocorticoids, IL-1 inhibitors, mycophenolate mofetil, anti-TNF-αagents, methotrexate, and NSAIDs in COVIS-19, and further in vitro and in vivo studies are recommended [31]. Due to the T cell lymphopenia and reduced function, immunoglobulins produced by B cells represent the main arm of the immune system to combat the virus. Seroconversion and antibody production occurs by the first two weeks after infection [48]. Like other infections, specific IgM is the first defense to appear, and disappears after a short time, but specific IgG remains a long-term defense against the virus. Therefore, neutralizing IgGs play a major role in the patient recovery and control of infection. IgGs reach their peak in the serum during the convalescent phase, and tend to wane after recovery, but memory B cells could still survive to offer long-term protection [22,49].
4.2. Convalescent plasma therapy
There is a global effort to find an effective treatment for COVID-19. Although the benefits of some available drugs have been proven, they are not yet a certain specific cure for the disease, and controversies remain [50]. According to previous experience with other coronavirus family members, convalescent plasma therapy has been tested on some critically ill patients, with promising results. In this regard, several studies have confirmed the positive effects of this treatment, especially in critically ill patients in the ICU and with ARDS to shorten hospitalization time and reduce the mortality rate. The COVID-19 specific IgG antibodies act as passive immune therapy, and after administration by the transfused plasma might neutralize viral particles and activate the complement system, which could consequently lead to viral elimination. Despite its efficacy, this method has some difficulties such as concerns about causing an infection, and non-infectious transfusion reactions. Transfusion-induced infection is rare in industrial countries (unlike poor countries) while the latter is a global worry known as transfusion-related acute lung injury (TRALI) and allergic reactions. As an important point, IgG collected in one country may be different from another country, because lifestyle, diet, genetics, and the environment could play an important role in the development of specific antibodies against the virus. Furthermore, differences between strains of coronavirus should be considered in geographically distinct areas; so, treatment of infected cases with polyclonal IgG, collected from the same area may increase the chance of an effective response [[50], [51], [52], [53], [54], [55]]. As a result, specific monoclonal antibodies that recognize the virus particles to inhibit multiplication and cell entry may be more practical and reliable. Wang et al. reported some positive findings about creation of a monoclonal antibody against the S protein of the virus, that could inhibit cell attachment and entry [56].
4.3. Prospect of a reliable vaccine
The worldwide economy, culture, transport, sport, interpersonal relationships, education and each individual's lifestyle have been influenced by the COVID-19 pandemic [57]. However, the lethality of this virus is much lower than found in previous pandemics. What makes COVID-19 so significant and dangerous, is the rapid acceleration of the virus transmission. Despite all efforts to control and treat this disease, public quarantine and lockdown measures cannot be implemented forever, and all tested treatment methods have not been sufficiently effective. Therefore, along with trying to find more effective specific drugs, the effort to find a vaccine should be redoubled in order to gain herd immunity and allow the world to return to normal life and resume its routine. Because the process of vaccine preparation and testing is complicated and lengthy, validating and marketing vaccines can be tedious, but given the body of work by various research groups, there is a clear prospect of achieving this goal sooner rather than later [20,58,59].
5. Conclusion
The COVID-19 pandemic is an ongoing issue that affects the lives of most people around the world. Most countries are now semi-closed, strict travel regulations have been enacted, international relations have been affected, and humans are experiencing an unprecedented regime, which has changed ordinary life [60]. Therefore, it is of the utmost importance to understand the pathophysiology of disease and how the immune response to the pathogen affects the disease. Although the immune system plays an important role in fighting COVID-19, paradoxically it could also be harmful. Most critically ill patients in ICU, that develop ARDS, have high levels of inflammatory cytokines in their circulation, known as CRS. Considering all the reported data from observations and measurements, it seems that when the immune system is severely damaged and becomes inefficient by lymphopenia and Tex, it tries to compensate by triggering the CRS, which could potentially lead to complications like ARDS and multi-organ failure. It is necessary to find efficient drugs and vaccines to return to the normal situation and reduce the mortality rate.
Author contribution
Both FY and NR had role in concept and design of the paper. FY drafted the manuscript, while NR and MH critically revised it. All authors approved the final draft of manuscript.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanaei S., Rezaei N. COVID-19: Developing from an Outbreak to A Pandemic. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.04.021. published online ahead of print, 2020 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Public Health. 2020;17(8) doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahanshahlu L., Rezaei N. Central Nervous System Involvement in COVID-19 [published online ahead of print, 2020 May 22] Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infantino M., Damiani A., Gobbi F.L., Grossi V., Lari B., Macchia D. Serological assays for SARS-CoV-2 infectious disease: benefits, limitations and perspectives. Isr. Med. Assoc. J. 2020;22(4):203–210. [PubMed] [Google Scholar]
- 8.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezaei N. COVID-19 affects healthy pediatricians more than pediatric patients. Infect. Control Hosp. Epidemiol. 2020:1. doi: 10.1017/ice.2020.139. published online ahead of print, 2020 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 11.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus - a perspective. Expert. Rev. Clin. Immunol. 2020;16(5):465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Hai, Cao Xiaoguang, Ji Xiaoqi, Du Fangbing, Zhou Xuan, He Jiawei, Xie Yanghu, Zhu Yu. 2020. A Current Emerging Respiratory Infection: Epidemiological and Clinical Characteristics, Diagnosis and Treatments of COVID-19 (3/6/2020) Available at SSRN: [DOI] [Google Scholar]
- 14.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9(1):558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020:102463. doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groeneveld A.B.J. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vasc. Pharmacol. 2002;39(4):247–256. doi: 10.1016/s1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 19.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020;30(3) doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 21.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casillo G.M., Mansour A.A., Raucci F., Saviano A., Mascolo N., Jilani Iqbal A. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol. Res. 2020:104791. doi: 10.1016/j.phrs.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiappelli F. CoViD-19 Immunopathology & Immunotherapy. Bioinformation. 2020;16(3):219–222. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. 2020. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) medRxiv. 2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerging Microbes & Infections. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020:1–6. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spezzani V., Piunno A., Iselin H.-U. Benign COVID-19 in an immunocompromised cancer patient - the case of a married couple. Swiss Med. Wkly. 2020 doi: 10.4414/smw.2020.20246. [DOI] [PubMed] [Google Scholar]
- 30.Gianfrancesco M.A., Hyrich K.L., Gossec L. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries [published online ahead of print, 2020 Apr 16] Lancet Rheumatol. 2020;2(5):e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., Hoyt K.J., Han J., Grom A.A., Gattorno M., Ravelli A., De Benedetti F., Behrens E.M., Cron R.Q., Nigrovic P.A. On the Alert for Cytokine Storm: Immunopathology in COVID -19. Arthritis Rheum. 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Fang X., Cai Z., Wu X., Gao X., Min J. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzman-Flores J.M., Lopez-Briones S. Cells of innate and adaptive immunity in type 2 diabetes and obesity. Gac. Med. Mex. 2012;148(4):381–389. [PubMed] [Google Scholar]
- 36.Meshkani R., Vakili S. Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin. Chim. Acta. 2016;462:77–89. doi: 10.1016/j.cca.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. published online ahead of print, 2020 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulrich Henning, Pillat Micheli M. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev. Rep. 2020;16(3):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K., Chen W., Zhou Y.S., Lian J.Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020:988345. doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 41.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y., Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020;189:428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. 2020. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish Journal of Medical Sciences. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib [published online ahead of print, 2020 Mar 11] J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. published online ahead of print, 2020 Apr 12. S0091-6749(20)30495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84(106560):1–6. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Current Protocols in Microbiology. 2020;57(1) doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju B., Zhang Q., Ge X., Wang R., Yu J., Shan S. 2020. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection bioRxiv. 2020.03.21.990770. [DOI] [PubMed] [Google Scholar]
- 50.Md Insiat Islam R. Current drugs with potential for treatment of COVID-19: a literature review. J Pharm Pharm Sci. 2020;23(1):58–64. doi: 10.18433/jpps31002. [DOI] [PubMed] [Google Scholar]
- 51.Bloch E.M., Shoham S., Casadevall A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020;21(7):2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roback J.D., Guarner J. Convalescent Plasma to Treat COVID-19: Possibilities and Challenges. JAMA. 2020 doi: 10.1001/jama.2020.4940. published online ahead of print, 2020 Mar 27. [DOI] [PubMed] [Google Scholar]
- 54.Teixeira da Silva J.A. Convalescent plasma: A possible treatment of COVID-19 in India [published online ahead of print, 2020 Apr 15] Med J Armed Forces India. 2020;76(2):236–237. doi: 10.1016/j.mjafi.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye M., Fu D., Ren Y. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. published online ahead of print, 2020 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1):2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohamed K., Rodríguez-Román E., Rahmani F. Borderless collaboration is needed for COVID-19-A disease that knows no borders. Infect. Control Hosp. Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.162. published online ahead of print, 2020 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pawelec G., Weng N.-P. Can an effective SARS-CoV-2 vaccine be developed for the older population? Immun. Ageing. 2020;17(1) doi: 10.1186/s12979-020-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Momtazmanesh Sara. All Together to Fight COVID-19. The American Journal of Tropical Medicine and Hygiene. 2020;102(6):1181–1183. doi: 10.4269/ajtmh.20-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]