Abstract
Timely detection and diagnosis are urgently needed to guide epidemiological measures, infection control, antiviral treatment, and vaccine research. In this review, biomarkers/indicators for diagnosis of coronavirus disease 2019 (COVID-19) or detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the environment are summarized and discussed. It is concluded that the detection methods targeting antibodies are not suitable for screening of early and asymptomatic cases since most patients had an antibody response at about 10 days after onset of symptoms. However, antibody detection methods can be combined with quantitative real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) to significantly improve the sensitivity and specificity of diagnosis, and boost vaccine research. Fast, sensitive and accurate detection methods targeting antigens need to be developed urgently. Various specimens for diagnosis or detection are compared and analyzed. Among them, deep throat saliva and induced sputum are desired for RT-qPCR test or other early detection technologies. Chest computerized tomography (CT) scan, RT-qPCR, lateral flow immunochromatographic strip (LFICS) for diagnosis of COVID-19 are summarized and compared. Specially, potential electrochemical (EC) biosensor, surface enhanced Raman scattering (SERS)-based biosensor, field-effect transistor (FET)-based biosensor, surface plasmon resonance (SPR)-based biosensor and artificial intelligence (AI) assisted diagnosis of COVID-19 are emphasized. Finally, some commercialized portable detection device, current challenges and future directions are discussed.
Keywords: COVID-19, SARS-CoV-2, Biosensors for virus detection, Lateral flow immunochromatographic strip, AI assisted diagnosis
Highlights
-
•
Various biomarkers/indicators for diagnosis of COVID-19 or detection of SARS-CoV-2 are discussed.
-
•
Various specimens for diagnosis or detection are compared and analyzed.
-
•
CT scan, RT-qPCR, lateral flow immunochromatographic strip for diagnosis of COVID-19 are summarized and compared.
-
•
Potential EC biosensor, SERS-based biosensor, and AI assisted diagnosis of COVID-19 are emphasized.
1. Introduction
In December 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began to spread around the globe. The disease caused by the virus was named coronavirus disease 2019 (COVID-19). It has been demonstrated that the virus can spread through respiratory droplets, aerosols, and contact with the abiotic surface (Ong et al., 2020; van Doremalen et al., 2020). The virus may also be transmitted by the fecal route since live virus has been detected in feces (Wang et al., 2020b). More critically, asymptomatic infections and transmissions have been shown to occur (Hoehl et al., 2020; Yu et al., 2020c). Hence, there is an urgent need to develop sensitive, accurate, rapid and low-cost diagnostic tools to screen infected individuals so that proper isolation and treatment can be facilitated.
Currently, there are three types of COVID-19 diagnostics methods. (1) Chest CT scan combine with clinical symptoms; (2) RT-qPCR based ribonucleic acid (RNA) detection; (3) Lateral flow immunochromatographic strip (LFICS), fully automatic chemiluminescence assay and enzyme-linked immunosorbent assay (ELISA) based antibody detection. However, conducting CT scan is limited to larger central hospitals; normal clinics and test laboratories do not have access to the CT scan. Moreover, CT scan can neither distinguish different viruses nor identify specific viruses. On the other hand, the RT-qPCR method requires 1–3 days to report results (usually, the experiment time is about 4 h) and has a high false-negative rate. Individuals who initially test false-negative contribute greatly to the further spread of the virus and prevent proper control of infection. Meanwhile, detection methods that target the antibody are not suitable for screening of early and asymptomatic cases, as most patients had an antibody response at about 10 days after onset of symptoms. It is furthermore easy to generate a false positive result due to interference from other proteins in human blood or serum samples. Now, antibody test can only be used with RT-qPCR to increase the sensitivity and specificity of COVID-19 diagnosis.
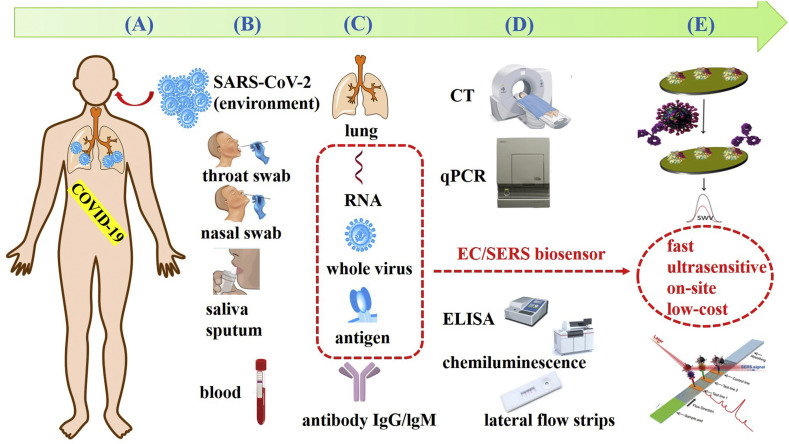
In addition to the limitations of detection techniques and methods, there are many factors that can affect test results, such as the choice of biomarkers, the taking of clinical specimens, etc. In this review, biomarkers, other biochemical indicators and specimens are comprehensively analyzed and discussed. Diagnostic cases of CT scan, various RNA detection methods and antibody detection methods are summarized and discussed. More interestingly, AI assisted diagnosis and potential ultrasensitive biosensor are emphasized. A comprehensive schematic diagram of current diagnostic methods and potential portable biosensors for COVID-19 is presented in Fig. 1 .
Fig. 1.
Schematic diagram of current diagnostic methods and potential portable biosensors for COVID-19. (A) A human being infected with SARS-CoV-2. (B) Sampling from suspected cases or patients and common specimens. (C) Biomarkers and other biochemical indicators for diagnosis of COVID-19. (D) Current detection methods to corresponding biomarkers or indicators. (E) Potential ultrasensitive biosensors, especially EC biosensor and SERS-based biosensors, for virus antigens detection.
2. Biomarkers or indicators for COVID-19
2.1. RNA
The positive-sense, single-stranded RNA genome of SARS-CoV-2 contains ~30 kilobases in size and encodes ~9860 amino acids. It is the most important biomarker for diagnosis of COVID-19. Usually, the preferred targets for RT-qPCR assays included the conserved and/or abundantly expressed genes such as the structural spike glycoprotein gene (S gene), envelop protein gene (E genes) and nucleocapsid protein gene (N genes), and the non-structural RNA-dependent RNA polymerase (RdRp) and replicase open reading frame 1a/b (ORF1a/b) genes (Chu et al., 2020; Corman et al., 2020). Designed primers targeting these genes are demonstrated to be specific and sensitive for the novel SARS-CoV-2 and ruled out most of the other coronaviruses (MERS, OC43 and 229E) and influenza viruses (H1N1, H3N2, H5N1, etc.) (Won et al., 2020). Among them, ORF1a/b and E genes are mostly reported (Pan et al., 2020). SARS-CoV-2 shares about 79% whole-genome sequence identity with SARS-CoV (Lu et al., 2020d; Wang et al., 2020a). Hence, design of the unique primers or guide RNAs (gRNAs) are needed. Park and coworkers designed two primer set for SARS-CoV-2 specific region which does not exist in SARS-CoV (Park et al., 2020a). A unique N gene gRNA was designed to distinguish SARS-CoV-2 with no cross-reactivity for SARS-CoV (Broughton et al., 2020a). A study from Lim and coworkers points out that targeting the N gene is more sensitive than targeting the RdRp gene by 7–43 fold (Kim et al., 2020b). Besides this, three novel RT-qPCR assays targeting the RdRp/helicase (Hel), S, and N genes of SARS-CoV-2 were developed and compared (Chan et al., 2020). The detection of RdRp/Hel shows excellent sensitivity using nasopharyngeal aspirates as specimens and it does not cross-react with other human-pathogenic coronaviruses and respiratory pathogens in cell culture and clinical specimens, whereas the RdRp-P2 assay cross-reacted with SARS-CoV in cell culture.
2.2. Whole virus and antigen
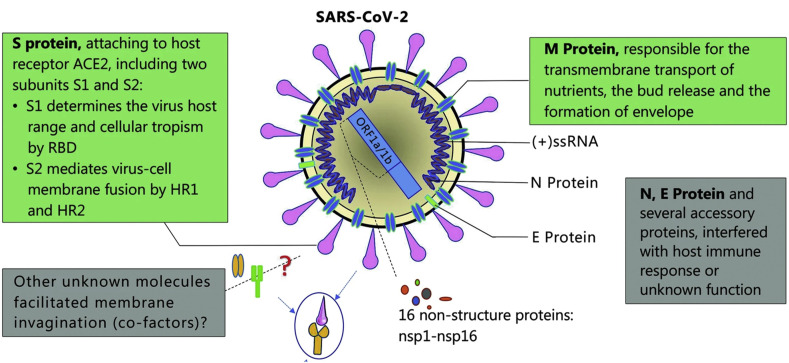
The whole virus SARS-CoV-2 and their structural proteins, including the spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein, nucleocapsid (N) protein, and also several accessory proteins, can theoretically be used as antigens for COVID-19 diagnosis (Fig. 2 ). It is predicted that the SARS-CoV-2 has 28 proteins (Wu et al., 2020). The particle size of the whole virus ranges from 50 to 200 nm (Kim et al., 2020a; Ramphul and Mejias, 2020). According to previous reports, the M and E proteins are necessary for virus assembly (Neuman et al., 2011; Nieto-Torres et al., 2014). The S protein is critical for adhering to host cells, where the receptor-binding domain (RBD) of S protein mediates the interaction with angiotensin-converting enzyme 2 (ACE2) (Zhou et al., 2020b). S and N proteins may be the most valuable antigen biomarkers for diagnosis of COVID-19, just like many detection methods have been reported for diagnosing SARS based on S and N proteins (Che et al., 2004; Woo et al., 2005). A proteome microarray with 18 out of the 28 predicted proteins was fabricated and used to investigate the antibody responses. The results show that the patients at the convalescent phase have 100% antibody responses to the proteome, especially to protein N, S1, ORF9b and NSP5 (Jiang et al., 2020). Recently, Lee Gehrke's group prepared to submit a lateral flow-based COVID-19 diagnostic technology to the United States Food and Drug Administration (FDA) for emergency use authorization. The detection targets are viral proteins. This technology appears in and is supported by their previously published work for dengue, yellow fever, and Ebola viruses (Yen et al., 2015). Our previous work discussed the Molecularly Imprinted Polymers (MIPs) and Surface Imprinted Polymers (SIPs)-based electrochemical biosensor for virus antigen detection (Cui et al., 2020). However, there are still few published articles for detection of the whole SARS-CoV-2 virus or their antigens.
Fig. 2.
Schematic diagram of the SARS-CoV-2 structure (reprint from ref (Guo et al., 2020b).). Structural proteins, including spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein, and nucleocapsid (N) protein, and also several accessory proteins.
2.3. Antibody
Detection of specific antibodies directed to SARS-CoV-2 in patient blood is another choice for diagnosis of COVID-19. It is widely accepted that immunoglobulin M (IgM) provides the first line of defense during viral infections, prior to the generation of adaptive, high affinity immunoglobulin G (IgG) responses which are responsible for immunological memory and long-term immunity (To et al., 2020a). Zhang et al. demonstrated that both IgM and IgG can be detected after 5 days of onset caused by SARS-CoV-2 using ELISA (Zhang et al., 2020b). A study conducted by Pan and coworkers shows that the sensitivity of colloidal gold-based immunochromatographic assay with IgM and IgG combinatorial detection in nuclear acid confirmed cases were 11.1%, 92.9% and 96.8% at the early stage (1–7 days after onset), intermediate stage (8–14 days after onset), and late stage (more than 15 days), respectively (Pan et al., 2020). For SARS infections, the IgM antibody could be detected in patients’ blood after 3–6 days, and IgG could be detected after 8 days (Lee et al., 2010; Wan et al., 2003). Therefore, detection technology targeting antibodies is not suitable for screening early COVID-19 cases. It is more helpful for the convalescent phase cases. In addition, the detection of antibody level is correlated with virus neutralisation titre, which is especially important for the design of vaccines (To et al., 2020a).
2.4. AI assisted discovery of other potential biomarkers
In the clinical laboratory, many samples or biomarkers can be tested, such as blood routine, urine routine, infectious index, hemagglutination index, blood gas analysis indicators, and cytokine indicators. When the patients with symptoms can not be confirmed by RT-qPCR tests, the discovery of other biomarkers or indexes is very important. It can assist the clinical diagnosis in avoiding delays in isolation and treatment. AI technology can easily process large sample studies and effectively reduce man-made decision, which can improve the accuracy of diagnosis and prediction. 174 patients’ characteristics were comprehensively analyzed by four types of AI algorithms (Peng et al., 2020), and the 18 most important diagnostic factors which are significantly related to positive COVID-19 diagnosis were revealed. Among them, white blood cell count (WBC), eosinophil count, eosinophil ratio, 2019 new Coronavirus RNA (2019n-CoV) and serum amyloid A (SAA) have the greatest clinical significance. Some cytokines were also be tested and analyzed and the results show their levels are abnormal, but the linkage to SARS/MERS is not so significant. However, the level of interleukin-6 (IL-6), IL-10, IL-2 and interferons-γ (IFN-γ) in the peripheral blood of the severe COVID-19 cases increased compared to those in the mild cases. The degree of lymphopenia and a proinflammatory cytokine storm is higher in severe COVID-19 patients than in mild cases.Cytokine storm is associated with the disease severity (Liu et al., 2020a; Wang et al., 2020c). Hence, cytokines can be indicators of prognosis for severe COVID-19. Biomarkers or indicators for diagnosis and prognosis of COVID-19 are listed in the Table 1 .
Table 1.
Biomarkers or indicators for diagnosis and prognosis of COVID-19.
| biomarkers or indicators | mechanism | specimens/equipment | comments |
|---|---|---|---|
| lung | clinical symptoms | chest/CT scan | limited to central hospital |
| RNA (ORF1ab, N genes etc.) | virus genome | list in Table 2 | high false negative rates |
| virus/antigen | virus proteins | ultrasensitive method needed | |
| antibody (IgG/lg M) | human immune responses | human blood | prone to false positive result |
| cytokine (IL-6) | immune storm/cytokine storm in the body | prognosis for severe COVID-19 |
3. Clinical and environmental specimens
Specimens are highly related to detection efficiency and accuracy. Now, specimens from the upper respiratory tract (contain throat swab, nasal swab and deep throat saliva), lower respiratory tract (contain sputum and bronchoalveolar lavage fluid), feces, fibrobronchoscope brush biopsy, and blood are being investigated. For the RT-qPCR based RNA detection, specimens from the lower respiratory tract induce a relatively less false-negative rate. For the upper respiratory tract specimens, the nasal specimen has higher viral loads than that of the throat (Zou et al., 2020). Although the nasopharyngeal or throat swabs are feasible for diagnosis, the sampling process can make the patient or suspected case uncomfortable and induce coughing and sneezing, which generates aerosol and creates a potential health hazard for medical staff. Another study reveals that sputum swabs (76.9%) showed a significantly higher positive rate than throat swabs (44.2%) (P = 0.001) (Lin et al., 2020). Because most patients have no sputum, sputum-inducing methods were used and studied in two cases (Han et al., 2020). The results demonstrated that sputum induction is more helpful than throat swabs for the detection of SARS-CoV-2 RNA in convalescent patients. For the health care workers, sputum induction is a simple, safe and non-invasive way for detecting many lung diseases (Fahy, 1998). Besides the respiratory tract, fecal specimens also have a similar diagnostic accuracy to pharyngeal swabs (Zhang et al., 2020a).
Timepoints of viral load study are important for choice of specimens. The viral load of SARS-CoV-2 is similar to that of influenza, which peaks occur shortly (within 3 days) after symptom onset, in contrast to that of SARS-CoV and MERS-CoV, which have peaks at around 10 and 14 days, respectively (Hayden et al., 1999; Oh et al., 2016; Peiris et al., 2003; Zou et al., 2020). Comparisons of viral loads or positive rates for different specimens are listed in Table 2.
Table 2.
Comparation of different clinical and non-clinical specimens.
| specimens | sites | viral loads or positive rates | process procedures | Ref. |
|---|---|---|---|---|
| throat/pharyngeal/oropharyngeal swab | upper respiratory tract | 32% a 44.2% b |
nasopharyngeal or throat swab specimens can induce coughing and sneezing, which generates aerosol and is a potential health hazard for health-care workers | NA |
| nasal swab | 63% a | |||
| posterior oropharyngeal saliva (deep throat saliva) | highest during the first week after symptom onset then declined with time | brought up by a throat-clearing manoeuvre |
To et al. (2020a) To et al. (2020b) To et al. (2019) |
|
| deep sputum induced sputum |
lower respiratory tract | 72% a 76.9% b |
induced sputum (ie, 10 mL of 3% hypertonic saline was inhaled through a mask with oxygen at a flow rate of 6 L/min for 20 min or until the sputum was produced) | Han et al. (2020) |
| BALF (bronchoalveolar lavage fluid) | 93% a | NA | NA | |
| feces | NA | 29% a | ||
| rectal swabs | NA | To et al. (2020a) | ||
| blood | 1% a | NA | ||
| urine | 0% a 73.6% c | |||
| fibrobronchoscope brush biopsy | 46% a | |||
| aerosols | environmental contamination | NA | generated with the use of a three-jet Collison nebulizer and fed into a Goldberg drum to create an aerosolized environment | Ong et al. (2020) |
| other abiotic material surface (copper, cardboard, plastic and stainless steel etc.) | NA |
Means RT-qPCR positive rates from ref. (Wang et al., 2020b).
Means RT-qPCR positive rates from ref. (Lin et al., 2020).
Means detected nucleocapsid protein in urine in 73.6% of diagnosed COVID-19 patients (Diao et al., 2020). NA: not available.
In conclusion, deep throat saliva and induced sputum are desired for RT-qPCR tests. However, developing methods which allow the patient to collect the samples by themselves are more desirable.
Environmental contamination as a route of transmission (Bin et al., 2016) must be considered. Stability of SARS-CoV and SARS-CoV-2 in aerosols and on different surfaces, such as plastic, stainless steel, copper, and cardboard, are currently being investigated (van Doremalen et al., 2020). SARS-CoV-2 remained viable in aerosols for 3 h. SARS-CoV-2 is more stable on plastic and stainless steel surfaces than on copper and cardboard, as viable viruses were detected up to 72 h after application to these surfaces. Developing biosensors for on-site environmental SARS-CoV-2 detection and long-term monitoring is urgently needed.
4. Diagnosis methods
4.1. Chest CT scan
A chest CT scan combines a series of X-ray images taken from different angles around the chest and uses computer processing to create cross-sectional images (slices) of the lung. The diagnosis of COVID-19 based on the radiologic features has been defined as a diagnostic criteria (Bai et al., 2020b; Li and Xia, 2020; Zhou et al., 2020c). However, CT scan is an experience-dependent diagnostic technique and requires large medical equipment. It can only be used in some central hospitals. What's more, CT scan is limited in terms of distinguishing different viruses and identifying specific viruses. It doesn't work for asymptomatic, pre-symptomatic infections, and even some mild symptomatic individuals without pneumonia. Every chest CT image needs to be read first by one radiologist and then needs to be checked by another radiologist. Recently, a 3D deep learning framework for the diagnosis of COVID-19 based on chest CT images was developed (Li et al., 2020a). The results showed that the reported model achieved a sensitivity of 90% and specificity of 96%. The AUC (area under the receiver operating characteristic curve) values for COVID-19 and community acquired pneumonia (CAP) were 0.96 and 0.95, respectively. It shows great potential as an automatic CT diagnostic tool.
4.2. RT-qPCR, dPCR, LAMP and CRISPR
Quantitative RT-qPCR is routinely used to detect the viruses and has been widely used by Center for Disease Control and Prevention and other relevant departments worldwide. (Won et al., 2020). However, the currently available RT-qPCR kits offering sensitivities ranging between 45% and 60%. Repeat testing may be required to make a definite diagnosis. The inconsistency and FNR of RT-qPCR can be attributed to many different factors, including the variation that occurs in viral RNA sequences, which subsequently affects results that use primers in the ORF1a/b gene and N genes. The influence of variation in viral RNA sequences can be minimized by the mismatch-tolerant amplification methods (Li et al., 2019; Zhou et al., 2019) which would be very helpful for improving the sensitivity and reliability of RNA detection. Another factor that thwarts the accuracy and consistency of RT-qPCR tests is sampling procedures, since the viral loads varies in different anatomic sites. The FNR was as high as 30%–50% from one-time testing in real COVID-19 cases (Wang et al., 2020c). To overcome the time-consuming and difficult operation of RNA extraction, Zhao and coworkers developed a carboxyl groups (PC)-coated magnetic nanoparticles (pcMNPs) for viral RNA extraction. Compared with traditional column-based nucleic acids extraction methods (>2 h), the pcMNPs-based method (~30 min) combines the virus lysis and RNA binding into one step, and the pcMNPs-RNA complexes can be directly introduced into subsequent RT-PCR reactions. It results in 10-copies sensitivity and the high linearity over 5 logs of gradient in SARS-CoV-2 viral RNA detection (Zhao et al., 2020b).
Digital PCR (dPCR) technology can significantly improve the sensitivity and accuracy of COVID-19 diagnosis. The limit of detection (LOD) of the optimized dPCR is at least 10-fold lower than that of RT-qPCR (Lu et al., 2020a). The overall sensitivity, specificity and accuracy of RT-dPCR protocol for RNA detection were 90%, 100% and 93%, respectively (Zhou et al., 2020a).
To overcome the obstacle of RT-qPCR's time-consuming and costliness but still be able to detect pathogens' nucleic acid, loop-mediated isothermal amplification (LAMP)-based method are developed (Lu et al., 2020b; Yu et al., 2020a). It provides nucleic acid amplification in a short time by using a DNA polymerase with chain displacement activity and 4–6 specially designed primers under a constant temperature of 60–65 °C (Tanner et al., 2015; Teoh et al., 2013). It has potential to be applied for point-of-care test (POCT) (Lu et al., 2020c). Moreover, unpurified samples could be detected directly using LAMP (Park et al., 2020a). This indicates that high-throughput test is possible when using unpurified specimen combined with non-instrumental (e.g. colorimetric) detection (Yan et al., 2020). Yu et al developed an isothermal LAMP based method for rapid colorimetric detection of COVID-19 (Yu et al., 2020b). The sensitivity was 97.6% (42/43) and read-out time was ~30 min. Besides LAMP, other isothermal amplification methods including rolling circle amplification (RCA), nucleic acid sequence-based amplification (NASBA), recombinase polymerase amplification (RPA), multiple displacement amplification (MDA) and helicase-dependent amplification (HDA) could be used for POCT-based nucleic acid detection (Zanoli and Spoto, 2013).
CRISPR (clustered regularly interspaced short palindromic repeats) based RNA detection can achieve an attomolar (10−18) level within 30 min (Bai et al., 2020a). A low-cost and accurate CRISPR-Cas12 based lateral flow assay for detection of SARS-CoV-2 was reported (Broughton et al., 2020b). Compared to the RT-qPCR, the sensitivity of this method was 90% and specificity was 100% for detection of respiratory swab samples. The entire time of this assay is 45 min, in contrast, the RT-qPCR needs 4 h. Ding et al. developed all-in-one dual CRISPR-Cas12a assay for detection of SARS-CoV-2 and human immunodeficiency virus (HIV). The LODs are as low as 1.2 copies mL−1 and 4.6 copies mL−1, respectively (Ding et al., 2020).
4.3. Current antibody/antigen detection method
Serological antibody test is important for symptomatic patients who present negative in RT-qPCR assays. Detection of COVID-19 related IgM tends to indicate a recent exposure to SARS-CoV-2, whereas detection of COVID-19 related IgG indicates virus exposure some time ago. Recombinant SARS-CoV-2 nucleocapsid protein (rN) and spike protein (rS) are evaluated using as antigens in ELISA for COVID-19 IgM/IgG detection. The results reveal that the rS-based ELISA has a significantly higher sensitivity than that of the rN-based ELISA (Liu et al., 2020b). This is different from sensitivity of SARS-CoV, where N-based IgG ELISA (94.7%) is significantly higher than that of S-based IgG ELISA (58.9%) (Woo et al., 2005).
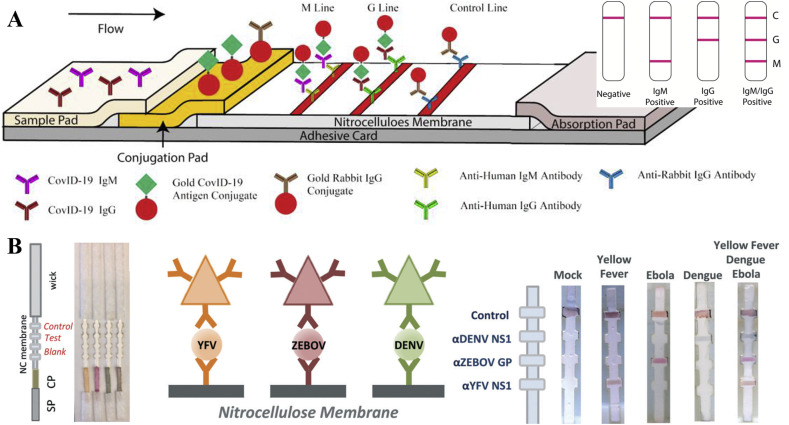
Lateral flow immunochromatographic strip (LFICS) (Huang et al., 2020) has been developed and approved in China for diagnosis of COVID-19. It can be a point-of-care disease diagnostic tool because it is portable, inexpensive, and without requiring power. The LFICS includes a sample pad (SP), conjugate pad (CP), nitrocellulose membrane (NC), and wick/absorbent pad and works similar to a pregnancy test (Fig. 3 A). Gold nanoparticles (Au NPs) colloid-based LFICS, which is also known as colloidal gold immunochromatographic assay (CGICA), can detect IgM and IgG antibodies simultaneously against SARS-CoV-2 virus in human blood within 15 min. A study conducted by Li shows that the overall testing sensitivity of LFICS assay is 88.66% and the specificity is 90.63% (Li et al., 2020b). Combining detection of RNA and total antibody can increase the diagnostic sensitivity to 99.4%, comparing with that of 67.1% for the RNA only test (Zhao et al., 2020a). Xiang et al., (2020) compared the enzyme-linked immunoassay (ELISA) and CGICA for detection of IgM and IgG. The sensitivity of the ELISA and CGICA for combined IgM-IgG test was 87.3% (55/63) and 82.4% (75/91), respectively. The specificity of both methods was 100% since they present negative for all healthy controls. It is demonstrated that there is no evident difference between ELISA and CGICA.
Fig. 3.
(A) Schematic diagram of the LFICS based on Au NPs colloid for COVID-19 IgM/IgG antibodies and an illustration of different testing results, C means control line, G means IgG line, M means IgM line (reprint from (Li et al., 2020b)). (B) Schematic of LFICS based on multi-colored silver nanoplates to distinguish dengue (DENV), yellow fever (YFV), and Ebola viruses (ZEBOV) (reprint from (Yen et al., 2015)).
A LFICS based on multi-colored silver nanoplates was used to distinguish dengue, yellow fever, and Ebola viruses. Their results demonstrated there is no cross-contamination for the three virus antigen proteins, the LODs of each disease were 150 ng ml−1 (Fig. 3B) (Yen et al., 2015). The multiplexing method offers great advantages for increasing speed and lowering costs for screening multiple virus simultaneously. This method is now being applied for SARS-CoV-2 protein detection. The performance of different detection methods are compared in Table 3 .
Table 3.
Comparation of different detection methods.
| methods | equipment | read-out time (entire protocol) | cost (each sample) | Sensitivity/specificity | LOD | Ref. |
|---|---|---|---|---|---|---|
| CT scan | CT machine | NA | NA | 97%, 25% | NA | Ai et al. (2020) |
| RT-qPCR | qPCR machine | 4 h | 15 $ | 71% (sensitivity) |
Won et al. (2020) Fang et al. (2020) |
|
| MNPs based RT-qPCR | qPCR machine | extraction ~30 min | NA | NA | 10-copy | Zhao et al. (2020b) |
| RT-digital PCR | PCR thermocycler | NA | 90%, 100% | NA | Zhao et al. (2020a) | |
| LAMP-based colorimetric method | PCR thermocycler or water bath | 20–30 min | 97.6% | NA | Yu et al. (2020b) | |
| 30 min | c | 100 copies | Park et al. (2020b) | |||
| LFICS-Au NPs colloid (IgM + IgG) | point-of-care strips | 15 min | 88.66%/90.63% | NA | Li et al. (2020b) | |
| ELISA (IgM + IgG) | fluorescent plate reader machine | ~2 h | 87.3%/100% | Xiang et al. (2020) | ||
| LFICS-Au NPs colloid (IgM + IgG) | point-of-care strips | 10 min | 82.4%/100% | |||
| LFICS-Au NPs colloid (IgM + IgG) | point-of-care strips | less than 15 min | 11.1%a/92.9% a 96.8% a |
Pan et al. (2020) | ||
| chemiluminescence (total Ab) | Fully automatic analyzer | NA | 86.9%/99.2% | Xia et al. (2020) | ||
| ELISA (total Ab) | fluorescent analyzer | ~2 h | 94.8%/100% | |||
| LFICS-Au NPs colloid (IgM) | point-of-care strips | less than 15 min | 96.2%/95.2% | |||
| LFICS- fluorescence (nucleocapsid protein) | point-of-care strips fluorescent analyzer |
within 10 min | 73.6% b | Diao et al. (2020) |
Means sensitivity was 11.1%, 92.9% and 96.8% at the early stage (1–7 days after onset), intermediate stage (8–14 days after onset), and late stage.
Means positive rat(more than 15 days), respectively. e. Ab: antibody. ELISA: Enzyme-linked immunosorbent assay. LFICS: lateral flow immunochromatographic strip. LAMP: Loop -mediated isothermal amplification.
Means specificity to SARS-CoV-2 versus alphacoronavirus (hCoV-229E), betacoronavirus (hCoV-OC43) and MERS-CoV. NA: not available.
4.4. Potential portable biosensors
Although RNA detection based on RT-qPCR and antibody detection based on ELISA and LFICS have been developed, both of these methods have certain practical limitations. Biosensors have the potential to be alternative tools since they can provide fast, accurate, sensitive early detection, especially the smartphone driven biosensors (Guo, 2017; Guo et al., 2018; Guo and Ma, 2017; Huang et al., 2018). These biosensors include EC biosensors, fluorescence based biosensor, colorimetric biosensor, localized surface plasmon resonance (LSPR), surface enhanced Raman scattering (SERS), quartz crystal microbalance (QCM) and piezoelectric microcantilever sensors (PEMS), etc (Cui et al., 2019; Kaya et al., 2020; Parkash et al., 2019; Ranjan et al., 2017). Among them, label-free electrical/EC biosensors and SERS are the most popular (Guo, 2016; Guo et al., 2020a). Electrical/EC biosensors possess the advantages of low cost, simplicity, and are more easily miniaturized and mass fabricated. They can also be used as point-of-care devices at home or at the doctor's office (Anusha et al., 2019; Xu et al., 2018). SERS is known as an ultra-sensitive molecular spectroscopy technique that has no interference from water, making it a distinct advantage in the identification of bio-samples. A SERS-based biosensor does not require extensive sample preparation steps and has high enough sensitivity to detect trace amounts of bioparticles, and under special circumstances, can even be capable of single-molecule detection (Patra, 2016). Most of the biomarkers of SARS-CoV-2 can be detected by biosensors.
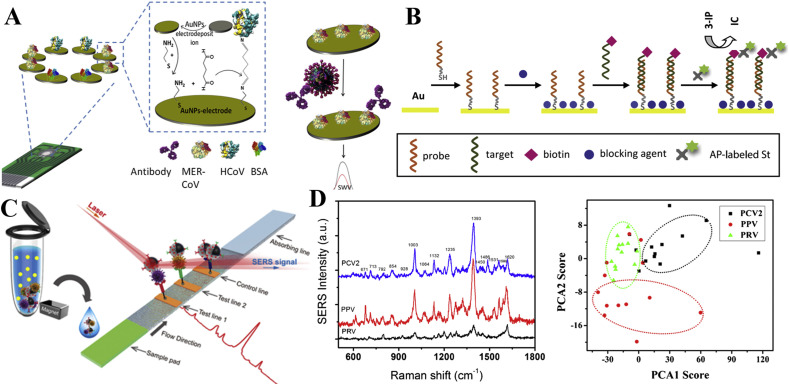
Recombinant spike protein S1 was used as a biomarker for two different coronavirus detection by EC immunosensor (Layqah and Eissa, 2019). The design of the electrode array enables the multiplexed detection (Fig. 4 A). The test can be completed within 20 min and the LOD was achieved as 0.4 pg mL−1 for human coronavirus (HCoV) and 1.0 pg mL−1 for MERS-CoV. The EC immunosensor was also successfully applied to spiked nasal specimen. The EC biosensor also be applied to detect the nucleic acid of the virus. González-López et al. developed a simple, cheap, and easy-to-handle EC genosensor for the detection of SARS-CoV gene (Fig. 4B (Abad-Valle et al., 2005).
Fig. 4.
(A) Schematic diagram of EC immunosensor for HCoV and MERS-CoV detection (reprint from (Layqah and Eissa, 2019)). (B) Schematic diagram of construction of EC genosensor for SARS-CoV gene detection (reprint from (Abad-Valle et al., 2005)). (C) Schematic diagram of the magnetic SERS based sensor for detection of H1N1 and HAdV viruses (reprint from (Wang et al., 2019)). (D) SERS spectra of three virus and PCA clustering plot of PC1 vs PC2 computed from the SERS spectra of three virus (reprint from (Luo et al., 2017)).
Field-effect transistor (FET)-based biosensor is one type of electrical biosensor. A graphene-based FET biosensor was reported to detect the SARS-CoV-2 and its spike protein in clinical samples (Seo et al., 2020). The results demonstrated that the LOD of spike protein was 1 fg mL−1 in phosphate-buffered saline (PBS) and 100 fg mL−1 clinical transport medium. The LOD of SARS-CoV-2 in culture medium was 1.6 × 101 pfu mL−1 and in clinical samples was 2.42 × 102 copies mL−1. The biosensor could discriminate the SARS-CoV-2 spike protein from that of MERS-CoV. The success of this biosensor confirmed the potential for COVID-19 diagnosis using antigen protein in the transport medium of nasopharyngeal swabs. It's also confirmed that the biosensor can detect the SARS-CoV-2 from clinical samples.
Two respiratory viruses, influenza A H1N1 virus and human adenovirus (HAdV), were detected by the SERS-based biosensor (Wang et al., 2019). The LOD for H1N1 and HAdV were 50 and 10 pfu mL−1, respectively, which are 2000 times more sensitive than those from the standard colloidal gold strip method (Fig. 4C). Porcine circovirus type 2 (PCV2), porcine parvovirus (PPV) and porcine pseudorabies virus (PRV) were detected by SERS based on a porous carbon substrate decorated with silver nanoparticles (Luo et al., 2017). The LOD of these three are as low as 1 × 107 copies mL−1. The principal components analysis (PCA) was used to discriminate the viruses based on the SERS spectra (Fig. 4D).
More recently, a plasmonic biosensor was reported to detected RNA of SARS-CoV-2 through nucleic acid hybridization (Qiu et al., 2020). The complementary cDNA sequences were fixed on the gold nanoislands (AuNIs) as receptors. Both localized surface plasmon resonance (LSPR) and plasmonic photothermal (PPT) effects were used collaboratively to enhance the signal. The LOD for detection of the RdRp gene was about 0.22 pM. With the in situ PPT enhancement on gold AuNIs chips, RdRp genes from SARS-CoV and SARS-CoV-2 can be accurately distinguished.
5. Conclusions and outlooks
Ultrasensitive and specific laboratory diagnostic method and portable devices are critical for controlling the rapidly evolving SARS-CoV-2-associated COVID-19 pandemic. Nowadays, CT scan, RT-qPCR, and LFICS based on Au NPs colloid (colloidal gold method) have been developed. Many diagnostic kits or strips have been cleared in China for laboratory detection of SARS-CoV-2 (Loeffelholz and Tang, 2020). Unfortunately, due to an overwhelming situation in local hospitals at the severe outbreak areas, many suspected cases cannot be efficiently confirmed. Hence, more reliable, rapid, low-cost and widely available diagnostic tools or detection strategies are needed. (1) For early diagnosis, on-site, fast, and ultrasensitive biosensors targeting virus antigen detection has a great potential. They can be used in hospitals, clinics, test laboratories, and even at home, airports or other high traffic areas. The nanomaterials and nanotechnologies should be applied to develop ultrasensitive biosensors for the detection of antigens. (2) For improving the accuracy of the diagnosis, combined detection of different biomarkers using multiplex biosensors may be one of alternative strategies. (3) For increasing the reliability and reproducibility of biosensor, machine learning-based signal process and direct result readout are needed to be developed. (4) Since SARS-CoV-2 can be transmitted by asymptomatic carriers, there is an immediate need for home-used biosensor which should be readily available for everyone to determine whether the person is negative or potentially positive for the presence of SARS-CoV-2. Colorimetric strips and smartphone-based biosensors targeting antibody/antigen own most potential as the home-used POCT.
CRediT authorship contribution statement
Feiyun Cui: Conceptualization, Writing - original draft. H. Susan Zhou: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Science Foundation (CBET-1805514) to HSZ.
References
- Abad-Valle P., Fernández-Abedul M.T., Costa-García A. Genosensor on gold films with enzymatic electrochemical detection of a SARS virus sequence. Biosens. Bioelectron. 2005;20(11):2251–2260. doi: 10.1016/j.bios.2004.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. 0(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anusha J., Kim B.C., Yu K.-H., Raj C.J. Electrochemical biosensing of mosquito-borne viral disease, dengue: a review. Biosens. Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111511. [DOI] [PubMed] [Google Scholar]
- Bai H., Cai X., Zhang X. OSF Preprints; 2020. Landscape Coronavirus Disease 2019 Test (COVID-19 Test) in Vitro--A Comparison of PCR vs Immunoassay vs Crispr-Based Test. [Google Scholar]
- Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., Pan I., Shi L.-B., Wang D.-C., Mei J., Jiang X.-L., Zeng Q.-H., Egglin T.K., Hu P.-F., Agarwal S., Xie F., Li S., Healey T., Atalay M.K., Liao W.-H. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. 0(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin S.Y., Heo J.Y., Song M.-S., Lee J., Kim E.-H., Park S.-J., Kwon H.-i., Kim S.m., Kim Y.-i., Si Y.-J. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin. Infect. Dis. 2016;62(6):755–760. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020:1–5. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Singh J., Streithorst J., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A. medRxiv; 2020. Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 Using a CRISPR-Based DETECTR Lateral Flow Assay. [Google Scholar]
- Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H., Fung A.Y.-F., Ng A.C.-K., Zou Z., Tsoi H.-W. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020;58(5) doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X.-y., Qiu L.-w., Pan Y.-x., Wen K., Hao W., Zhang L.-y., Liao Z.-y., Hua X., Cheng V.C., Yuen K.-y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004;42(6):2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Pan Y., Cheng S., Hui K.P., Krishnan P., Liu Y., Ng D.Y., Wan C.K., Yang P., Wang Q. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F., Zhou Z., Zhou H.S. Measurement and analysis of cancer biomarkers based on electrochemical biosensors. J. Electrochem. Soc. 2019;167(3) [Google Scholar]
- Cui F., Zhou Z., Zhou H.S. Molecularly imprinted Polymers and surface imprinted Polymers based electrochemical biosensor for infectious diseases. Sensors. 2020;20(4):996. doi: 10.3390/s20040996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wen K., Chen J., Liu Y., Yuan Z., Han C., Chen J., Pan Y., Chen L., Dan Y. medRxiv; 2020. Diagnosis of Acute Respiratory Syndrome Coronavirus 2 Infection by Detection of Nucleocapsid Protein. [Google Scholar]
- Ding X., Yin K., Li Z., Liu C. bioRxiv; 2020. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV Virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy J.V. A safe, simple, standardized method should be used for sputum induction for research purposes. Clin. Exp. Allergy. 1998;28(9):1047–1049. doi: 10.1046/j.1365-2222.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Radiology; 2020. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016;88(24):11986–11989. doi: 10.1021/acs.analchem.6b04345. [DOI] [PubMed] [Google Scholar]
- Guo J. Smartphone-powered electrochemical dongle for point-of-care monitoring of blood β-ketone. Anal. Chem. 2017;89(17):8609–8613. doi: 10.1021/acs.analchem.7b02531. [DOI] [PubMed] [Google Scholar]
- Guo J., Huang X., Ma X. Clinical identification of diabetic ketosis/diabetic ketoacidosis acid by electrochemical dual channel test strip with medical smartphone. Sensor. Actuator. B Chem. 2018;275:446–450. [Google Scholar]
- Guo J., Ma X. Simultaneous monitoring of glucose and uric acid on a single test strip with dual channels. Biosens. Bioelectron. 2017;94:415–419. doi: 10.1016/j.bios.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Guo J., Zeng F., Guo J., Ma X. Preparation and application of microfluidic SERS substrate: challenges and future perspectives. J. Mater. Sci. Technol. 2020;37:96–103. [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Luo Q., Mo F., Long L., Zheng W. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect. Dis. 2020;20(6):655–656. doi: 10.1016/S1473-3099(20)30174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Treanor J.J., Fritz R.S., Lobo M., Betts R.F., Miller M., Kinnersley N., Mills R.G., Ward P., Straus S.E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. Jama. 1999;282(13):1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., Behrens P., Böddinghaus B., Götsch U., Naujoks F. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 2020;382(13):1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Tian S., Zhao W., Liu K., Ma X., Guo J. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst. 2020;145(8):2828–2840. doi: 10.1039/c9an02485a. [DOI] [PubMed] [Google Scholar]
- Huang X., Xu D., Chen J., Liu J., Li Y., Song J., Ma X., Guo J. Smartphone-based analytical biosensors. Analyst. 2018;143(22):5339–5351. doi: 10.1039/c8an01269e. [DOI] [PubMed] [Google Scholar]
- Jiang H.-w., Li Y., Zhang H.-n., Wang W., Men D., Yang X., Qi H., Zhou J., Tao S.-c. medRxiv; 2020. Global Profiling of SARS-CoV-2 Specific IgG/IgM Responses of Convalescents Using a Proteome Microarray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S.I., Karadurmus L., Ozcelikay G., Bakirhan N.K., Ozkan S.A. Elsevier; 2020. Electrochemical Virus Detections with Nanobiosensors. Nanosensors for Smart Cities; pp. 303–326. [Google Scholar]
- Kim J.-M., Chung Y.-S., Jo H.J., Lee N.-J., Kim M.S., Woo S.H., Park S., Kim J.W., Kim H.M., Han M.-G. Identification of coronavirus isolated from a patient in korea with COVID-19. Osong Pub. Health Res. Perspect. 2020;11(1):3. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim D.-M., Lee B. 2020. Insufficient Sensitivity of RNA Dependent RNA Polymerase Gene of SARS-CoV-2 Viral Genome as Confirmatory Test Using Korean COVID-19 Cases. [Google Scholar]
- Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchimica Acta. 2019;186(4):224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-K., Lee B.-H., Seok S.-H., Baek M.-W., Lee H.-Y., Kim D.-J., Na Y.-R., Noh K.-J., Park S.-H., Kumar D.N. Production of specific antibodies against SARS-coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J. Vet. Sci. 2010;11(2):165–167. doi: 10.4142/jvs.2010.11.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., Lu Y., Fang Z., Song Q. Radiology; 2020. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. 200905. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wan Z., Hu Y., Zhou Y., Chen Q., Zhang C. A mismatch-tolerant RT-quantitative PCR: application to broad-spectrum detection of respiratory syncytial virus. Biotechniques. 2019;66(5):225–230. doi: 10.2144/btn-2018-0184. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus Disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am. J. Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W. Development and clinical application of A rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J. Med. Virol. 2020:1–7. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Xiang J., Yan M., Li H., Huang S., Shen C. medRxiv; 2020. Comparison of Throat Swabs and Sputum Specimens for Viral Nucleic Acid Detection in 52 Cases of Novel Coronavirus (SARS-Cov-2) Infected Pneumonia (COVID-19) [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S. Vol. 58. J. Clin. Microbiol.; 2020. (Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections—the state of the art. Emerg. Microb. Infect. 2020:1–26. doi: 10.1080/22221751.2020.1745095. just-accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Wang J., Li M., Wang Y., Dong J., Cai W. medRxiv; 2020. SARS-CoV-2 Detection Using Digital PCR for COVID-19 Diagnosis, Treatment Monitoring and Criteria for Discharge. [Google Scholar]
- Lu R., Wu X., Wan Z., Li Y., Jin X., Zhang C. A novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Int. J. Mol. Sci. 2020;21(8):2826. doi: 10.3390/ijms21082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Wu X., Wan Z., Li Y., Zuo L., Qin J., Jin X., Zhang C. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virol. Sin. 2020:1–4. doi: 10.1007/s12250-020-00218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Chen L., Liang C., Wei Q., Chen Y., Wang J. Porous carbon films decorated with silver nanoparticles as a sensitive SERS substrate, and their application to virus identification. Microchimica Acta. 2017;184(9):3505–3511. [Google Scholar]
- Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Castano-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-d., Park W.B., Choe P.G., Choi S.-J., Kim J.-I., Chae J., Park S.S., Kim E.-C., Oh H.S., Kim E.J. Viral load kinetics of MERS coronavirus infection. N. Engl. J. Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., Long X., Guo S., Zhao Z., Liu Y. medRxiv; 2020. Serological Immunochromatographic Approach in Diagnosis with SARS-CoV-2 Infected COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.-S., Ku K., Baek S.-H., Kim S.-J., Kim S.I., Kim B.-T., Maeng J.-S. Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2. J. Mol. Diagn. 2020;S1525–1578(1520):30090–30098. doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.-S., Ku K., Baek S.-H., Kim S.J., Kim S.I., Kim B.-T., Maeng J.-S. bioRxiv; 2020. Development of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Assays Targeting SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash O., Kumari P., Vasu Deva S.L., Ujjan J.A., Qadir S.M., Soomro F.M., Faryal R., Kanhar N.A. Dengue Fever: a Resilient Threat in the Face of Innovation. 2019. New tools for dengue diagnostics; p. 131. [Google Scholar]
- Patra P.P. 2016. Plasmofluidic Single-Molecule Surface-Enhanced Raman Scattering and Dynamic Assembly of Nanostructures. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.-M., Cheng V.C.-C., Chan K., Hung I., Poon L.L., Law K.-I., Tang B., Hon T., Chan C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Yang J., Shi Q., Ying L., Zhu H., Zhu G., Ding X., He Z., Qin J., Wang J. 2020. Artificial Intelligence Application in COVID-19 Diagnosis and Prediction. [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Ramphul K., Mejias S.G. Coronavirus disease: a review of a new threat to public health. Cureus. 2020;12(3) doi: 10.7759/cureus.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R., Esimbekova E.N., Kratasyuk V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017;87:918–930. doi: 10.1016/j.bios.2016.09.061. [DOI] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.C., Kim S.I. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Tanner N.A., Zhang Y., Evans T.C., Jr. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques. 2015;58(2):59–68. doi: 10.2144/000114253. [DOI] [PubMed] [Google Scholar]
- Teoh B.-T., Sam S.-S., Tan K.-K., Johari J., Danlami M.B., Hooi P.-S., Md-Esa R., AbuBakar S. Detection of dengue viruses using reverse transcription-loop-mediated isothermal amplification. BMC Infect. Dis. 2013;13(1):387. doi: 10.1186/1471-2334-13-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y., Chan K.-H., Wu T.-C., Chan J.M.-C., Leung W.-S., Chik T.S.-H., Choi C.Y.-C., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W.-S., Fung A.Y.-F., Hung I.F.-N., Cheng V.C.-C., Chan J.F.-W., Yuen K.-Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K., Yip C., Lai C., Wong C., Ho D., Pang P., Ng A., Leung K.-H., Poon R., Chan K.-H. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin. Microbiol. Infect. 2019;25(3):372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.C.-Y., Cai J.-P., Chan J.M.-C., Chik T.S.-H. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):515–516. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z., Zhang X., Yan X. IFA in testing specific antibody of SARS coronavirus. South China J. Prev. Med. 2003;29(3):36–37. [Google Scholar]
- Wang Y., Kang H., Liu X., Tong Z. Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J. Med. Virol. 2020;92:538–539. doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li X., Li T., Zhang S., Wang L., Wu X., Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020:1–7. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang C., Wang X., Wang K., Zhu Y., Rong Z., Wang W., Xiao R., Wang S. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl. Mater. Interfaces. 2019;11(21):19495–19505. doi: 10.1021/acsami.9b03920. [DOI] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J., Lee S., Park M., Kim T.Y., Park M.G., Choi B.Y., Kim D., Chang H., Kim V.N., Lee C.J. Vol. 29. Exp Neurobiol; 2020. (Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the coronavirus disease 2019 (COVID-19)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Tsoi H.-w., Fung A.M., Kao R.Y., Chan K.-h., Peiris J.M., Yuen K.-y. Differential sensitivities of severe acute respiratory syndrome (SARS) coronavirus spike polypeptide enzyme-linked immunosorbent assay (ELISA) and SARS coronavirus nucleocapsid protein ELISA for serodiagnosis of SARS coronavirus pneumonia. J. Clin. Microbiol. 2005;43(7):3054–3058. doi: 10.1128/JCM.43.7.3054-3058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N.-S., Wang G.-Q., Gong W.-F. 2020. Serological Test Is an Efficient Supplement of RNA Detection for Confirmation of SARS-CoV-2 Infection. [DOI] [Google Scholar]
- Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., Shen C. Evaluation of enzyme-linked immunoassay and colloidal gold-immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.27.20028787. [DOI] [Google Scholar]
- Xu D., Huang X., Guo J., Ma X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018;110:78–88. doi: 10.1016/j.bios.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Yan C., Cui J., Huang L., Du B., Chen L., Xue G., Li S., Zhang W., Zhao L., Sun Y., Yao H., Li N., Zhao H., Feng Y., Liu S., Zhang Q., Liu D., Yuan J. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020;26(6):773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.-W., de Puig H., Tam J.O., Gómez-Márquez J., Bosch I., Hamad-Schifferli K., Gehrke L. Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip. 2015;15(7):1638–1641. doi: 10.1039/c5lc00055f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V., Chen W.-H., Yin X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa102. hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wu S., Hao X., Li X., Liu X., Ye S., Han H., Dong X., Li X., Li J. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. medRxiv. 2020 doi: 10.1101/2020.02.20.20025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli L.M., Spoto G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors. 2013;3(1):18–43. doi: 10.3390/bios3010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Du R.-H., Li B., Zheng X.-S., Yang X.-L., Hu B., Wang Y.-Y., Xiao G.-F., Yan B., Shi Z.-L., Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microb. Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.02.22.961268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J. Clin. Infect. Dis.; 2020. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am. J. Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- Zhou J., Niu C., Wang Q., Pan Y., Wang X., Liu M., Dai X. medRxiv; 2020. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wan Z., Yang S., Li Y., Li M., Wang B., Hu Y., Xia X., Jin X., Yu N., Zhang C. A mismatch-tolerant reverse transcription loop-mediated isothermal amplification method and its application on simultaneous detection of all four serotype of dengue viruses. Front. Microbiol. 2019;10(1056) doi: 10.3389/fmicb.2019.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]