Abstract
There is currently no established therapy to treat or prevent Alzheimer’s disease. The ketogenic diet supplies an alternative cerebral metabolic fuel, with potential neuroprotective effects. Our goal was to compare the effects of a modified Mediterranean-ketogenic diet (MMKD) and an American Heart Association Diet (AHAD) on cerebrospinal fluid Alzheimer’s biomarkers, neuroimaging measures, peripheral metabolism, and cognition in older adults at risk for Alzheimer’s. Twenty participants with subjective memory complaints (n = 11) or mild cognitive impairment (n = 9) completed both diets, with 3 participants discontinuing early. Mean compliance rates were 90% for MMKD and 95% for AHAD. All participants had improved metabolic indices following MMKD. MMKD was associated with increased cerebrospinal fluid Aβ42 and decreased tau. There was increased cerebral perfusion and increased cerebral ketone body uptake (11C-acetoacetate PET, in subsample) following MMKD. Memory performance improved after both diets, which may be due to practice effects. Our results suggest that a ketogenic intervention targeted toward adults at risk for Alzheimer’s may prove beneficial in the prevention of cognitive decline.
Keywords: Alzheimer’s disease, Mild cognitive impairment, Ketogenic diet, CSF biomarkers, MRI, PET
1. Introduction
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder characterized by progressive deterioration of cognition, behavior, and ultimate functional decline (Alzheimer’s Association, 2018). With no established disease-modifying therapeutic or preventative strategy (Cummings et al., 2014), we must continue to explore unconventional interventions to combat this devastating disorder.
The traditional ketogenic diet (KD) was developed and first implemented to treat intractable childhood epilepsy in the 1920s (Freeman et al., 2007). The KD is a very low carbohydrate, adequate protein, and high fat diet that leads to a state of ketosis (increased systemic ketone bodies). During ketosis the main fuel used by the body shifts from glucose to favor ketone bodies (beta-hydroxybutyrate, acetoacetate [AcAc], acetone) and fatty acids (Hartman and Vining, 2007; Kossoff and Rho, 2009; Maalouf et al., 2009), an adaptation that also occurs with extended fasting. Importantly, ketone bodies may constitute up to 60%–70% of brain energy metabolism during this period, as has been documented during extended fasting (Cahill, 2006). Although the exact mechanism for the KD’s efficacy in suppressing seizures had not been definitively identified, several candidate mechanisms have been identified that also have relevance to AD, including inhibition of glutamatergic excitatory transmission with increased production of gamma-aminobutyric acid from glutamate, carbohydrate reduction and decreased glycolytic flux, and activation of adenosine triphosphate–sensitive potassium channels by mitochondrial metabolism (Danial et al., 2013; Maalouf et al., 2009; Stafstrom and Rho, 2012; Wallace et al., 2010). These mechanisms may mitigate neuronal hyperexcitability that has been described in preclinical and early clinical stages of AD and may be related to AD pathology (Vossel et al., 2017).
The KD supplies an alternative metabolic fuel to glucose, which may provide general neuroprotective effects and mitigate the impact of beta-amyloid (Aβ) on mitochondria, leading to increased efficiency of adenosine triphosphate production with decreased oxidative stress and glutamate toxicity (Danial et al., 2013; Maalouf et al., 2009; Stafstrom and Rho, 2012). Accumulating evidence in animal models and humans suggests that the implementation of an intervention aimed at inducing ketosis may have benefits in mild cognitive impairment (MCI) and AD dementia. In several mouse models of AD, consuming KD decreased brain Aβ content (Kashiwaya et al., 2013; Van der Auwera et al., 2005; Yao et al., 2011), although other studies have reported no impact of KD on Aβ (Beckett et al., 2013; Brownlow et al., 2013). Furthermore, KD has been shown to improve motor performance and learning/memory, while affecting amyloid beta precursor protein, phosphorylated-tau (tau-p181), gene expression of alpha/gamma-secretase, proteins involved in Aβ clearance/degradation, and genes involved in mitochondrial biogenesis (Beckett et al., 2013; Bough et al., 2006; Kashiwaya et al., 2013; Yao et al., 2011).
Despite the promising evidence of KD in mouse models of AD, there have been relatively few clinical studies exploring ketogenic dietary interventions in adults with cognitive impairment or AD (Brandt et al., 2019; Krikorian et al., 2012; Taylor et al., 2018). A 2012 study by Krikorian et al. (2012) showed that a ketogenic low carbohydrate diet enhanced verbal memory following a 6-week intervention relative to a high carbohydrate diet in older adults with MCI. This study also reported improvements in metabolic measures associated with the ketogenic intervention, including reductions in weight, waist circumference, and fasting glucose and insulin (Krikorian et al., 2012). Two recent small studies (Brandt et al., 2019; Taylor et al., 2018) have reported encouraging preliminary results on the feasibility and efficacy of ketogenic dietary interventions in MCI, but one lacked a dietary control group, and neither investigated diet effects on neuroimaging or cerebrospinal fluid (CSF) biomarkers.
Our goal was to study the effects of a modified Mediterranean-ketogenic diet (MMKD) and a control low-fat American Heart Association Diet (AHAD) on CSF AD biomarkers, neuroimaging measures, peripheral metabolic measures, and cognition. Because such interventions are promising tools for prevention, we examined the effects in adults at risk for AD dementia by virtue of systemic metabolic dysfunction (pre-diabetes) together with subjective memory complaints or amnestic MCI. We hypothesized that MMKD would lead to improvement in peripheral metabolic health, CSF AD biomarker profile, increased cerebral perfusion/ketone body uptake, and cognition.
2. Methods
2.1. Study participants
The protocol was approved by the Wake Forest Institutional Review Board (ClinicalTrials.gov Identifier: NCT02984540), and written informed consent was obtained from all participants and/or their study partners. Participants were medically supervised by clinicians, with safety monitoring overseen by the Wake Forest Institutional Data and Safety Monitoring Committee as detailed in Protection of Human Subjects. All participants had pre-diabetes defined by American Diabetes Association (2016) guidelines (hemoglobin A1c [HbA1c] of 5.7–6.4). Participants were further divided into 2 cognitive subgroups: adults with subjective memory complaints diagnosed using Alzheimer’s Disease Neuroimaging Initiative criteria (Risacher et al., 2015) (SMC; Cognitive Change Index score 16 on the first 12 items) and adults with MCI diagnosed by expert physicians and neuropsychologists using the National Institute on Aging at National Institutes of Health and the Alzheimer’s Association guidelines (Albert et al., 2011). Exclusion criteria included prior diagnosis of neurological or neurodegenerative illness (except MCI), major psychiatric disorder, stroke, use of diabetes and lipid-lowering medications, or medications with known central nervous system effects including anti-seizure medications, anti-psychotics, and opioids. Participants with well-controlled depression were allowed. Twenty-three adults were enrolled in the study with 3 participants discontinuing diet prior to completion of the study.
2.2. Procedure
The study consisted of a randomized crossover design in which participants consumed either MMKD or the control AHAD for 6 weeks, followed by a 6-week washout period in which participants were instructed to resume their pre-study diet, after which the second diet was consumed for 6 weeks. Prior to diet randomization, baseline characterization of cognitive status, lumbar puncture (LP), magnetic resonance imaging (MRI), and metabolic profiles were performed. Participants also completed a 3-day record in which they recorded all food consumed on 2 week-days and a week-end day at baseline and during weeks 3 and 6 of the diet intervention. Total calorie and macronutrient intake was calculated from the food records using ProNutra software (Viocare, Princeton, NJ). Cognitive function, LP, MRI, and metabolic parameters were re-assessed after each diet. Fig. S1 shows a representation of study design. Compliance and blood metabolic laboratory profiles were also assessed at the half-way point of each diet period.
2.3. Diet intervention and education
The experimental diet was MMKD, which is a very low carbohydrate diet aimed at inducing ketosis. Our control diet was AHAD, which is a low-fat diet (Krauss et al., 2001). Diets were eucaloric. The proportions of carbohydrates and fat were the main variables manipulated between the 2 diets. The target macronutrient composition (expressed as % of total calories) is approximately 5%–10% carbohydrate, 60%–65% fat, and 30% protein for MMKD; and 55%–65% carbohydrate, 15%–20% fat, and 20%–30% protein for AHAD.
Participants on MMKD worked closely with a registered dietitian to keep the amounts of carbohydrate, fat, and protein within the desired target ranges described above. Throughout the duration of the study, participants on MMKD were encouraged to avoid low-carbohydrate store-bought products and artificially sweetened beverages. MMKD was modeled on a Mediterranean diet in that emphasis was placed on protein sources low in saturated fat (fish, lean meats), healthy fats were emphasized, fruits and vegetable consumption was encouraged within limits, as were whole grains, and 1 glass of wine per day was allowed. Participants were supplied with 1 L of extra virgin olive oil during their Pre-Diet and Mid-Diet visits to use as a source of fat in their diet, and were encouraged to eat plentiful fish, lean meats, and nutrient-rich foods. The results of the PREDIMED trial have shown benefits with extra virgin olive oil supplementation on cardiovascular disease risk and mortality (Guasch-Ferre et al., 2014). Clearly, the carbohydrate restriction imposed for the study limited the amount of grains and fruits that might be associated with a classic Mediterranean diet. However, the dietary pattern was consistent with a Mediterranean-like diet. Participants on AHAD were encouraged to limit their amount of fat intake to <40 g/d, while eating plentiful fruits, vegetables, and carbohydrates containing adequate fiber, as well as proteins from healthy sources such as fish and lean meat. They did not receive supplementary olive oil.
A registered dietitian developed daily meal plans for each study participant based on their food preferences and caloric needs as determined by a 3-day food diary, activity level, and macronutrient requirements for the respective diets. Participants had weekly inperson and phone diet education/compliance visits starting 1 week prior to start of each diet and continuing throughout the remainder of the intervention. Participants maintained a food record that was reviewed at these visits. Capillary ketone body measures were collected at all major time points and during diet education visits using the Nova Max Plus (http://www.novacares.com/nova-max-plus/) capillary glucose and ketone body (beta-hydroxybutyrate) monitoring system. Prior studies have reported that less frequent blood ketone body measures are just as accurate a measure of ketosis as daily urine ketone body test strips (Gilbert et al., 2000). Subjective measures of compliance were also recorded by the study dietician. Participants were asked to keep their exercise and physical activity level stable throughout the study.
Participants were required to supply their own food based on a daily meal plan, food list, and other educational material provided. A food stipend of $25/wk was provided to help defray the cost of foods. Participants received a daily multivitamin supplement (Centrum Silver) while on diet. Moreover, participants were asked to discontinue the following supplements for the duration of the study: resveratrol, coenzyme Q10, coconut oil/other medium chain triglyceride-containing (e.g., Axona) supplements, or curcumin, as they may impact bioenergetic status and interfere with interpretation of study results.
2.4. Cognitive protocol
Study participants completed assessments of immediate and delayed memory at baseline and after each diet. Tests included the Free and Cued Selective Reminding Test (FCSRT) (Grober et al., 2010), story recall (modification of the episodic memory measure from the Wechsler Memory Scale-Revised) (Wechsler, 1987), and the ADAS-Cog12, a test of general cognition that includes items related to executive function, attention, verbal abilities, and memory (Rosen et al., 1984). Cognition was assessed before and after each diet intervention as seen in Fig. S1. Different versions of selected tests were utilized to mitigate the impact of learning on cognitive performance.
2.5. Plasma biomarkers
Fasting blood for metabolic measures was collected before and after each diet. Samples were immediately placed on ice and spun within 30 minutes at 2200 rpm in a cold centrifuge for 15 minutes. Plasma, serum, and red blood cells were aliquoted into separate storage tubes and flash frozen at 80 C until analyzed. Specific measures analyzed include the following: HbA1c, glucose, insulin, triglycerides, and total, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) cholesterol.
2.6. Lumbar puncture and cerebrospinal fluid biomarkers
Participants completed LP after 12-hour fast at baseline and after each diet for collection of CSF. Participants were placed in the seated or lateral decubitus position per study clinician preference. Using a 25-gauge needle, the L3–4 or L4–5 interspace was infiltrated with 1% lidocaine for local anesthesia. Using a 22-gauge Sprotte needle to drip, up to 25 mL of CSF was withdrawn into sterile polypropylene tubes. The first 3 mL was sent to the local laboratory for analysis to include cell count, protein, and glucose. CSF was then transferred in 0.2 mL aliquots into pre-chilled polypropylene tubes, frozen immediately on dry ice, and stored at −80 C until analysis. First thawed CSF was used for Aβ and tau measures. Aβ42, total tau, and phosphotau (tau-p181) were measured using a Luminex-based INNO-BIA Alzbio3 assay, and Aβ40 was measured by standard enzyme-linked immunosorbent assay (ELISA) according to manufacturer instructions, in each case using kits from Fujirebio (Malvern, PA; formerly Innogenetics, Belgium). We also quantified CSF neurogranin using an in-house sandwich ELISA, as previously described (Portelius et al., 2018), sTREM2 using an in-house Meso Scale Discovery assay, as previously described (Gisslén et al., 2019),YKL-40 using a commercial ELISA kit (R&D Systems, Minne-apolis, MN), and NF-Light using a commercial ELISA kit (Uman-Diagnostics, Umeå, Sweden) to assess the impact of diet on newly emerging CSF AD biomarkers (Blennow and Zetterberg, 2018). The measurements were performed by board-certified laboratory technicians who were blinded to clinical data. Baseline and follow-up samples were analyzed side-by-side on the same measurement plates and intra-assay coefficients of variation were below 10%.
2.7. Magnetic resonance imaging
Magnetic resonance images were acquired on a 3-Tesla Siemens Skyra scanner with a high-resolution 32-channel head coil (Erlangen, Germany) for the following sequences: (1) high-resolution T1-weighted images were used to provide gray matter and white matter volumes for the partial volume correction (Asllani et al., 2008) and warp perfusion images into a common space. High-resolution 3-dimensional T1-weighted images were obtained using a magnetization-prepared rapid gradient echo sequence: repetition time (TR) = 2300 ms; inversion time (TI) = 900 ms; echo time (TE) = 2.98 ms; slice thickness = 1 mm no gap; and in-plane resolution = 1 1 mm. (2) Perfusion imaging (pseudo continuous arterial spin labeling or PCASL) (Jung et al., 2010) was used to estimate cerebral perfusion. Quantitative data processing includes data cleaning (Tan et al., 2009), realignment, and quantification of cerebral blood flow into the physiological unit (mL/100 g tissue/min) using a kinetic model (Buxton et al., 1998). PCASL was collected with the following parameters: a single-shot echo-planar imaging (EPI) sequence; tagging duration = 1700 ms; post-labeling delay = 1500 ms; TR = 4000 ms; TE = 12 ms; repetitions = 80; field-of-view (FOV) = 22 22 cm; matrix size = 64 64; no. of slices = 24; slice thickness = 5 mm; and acquisition time = 5 minutes 24 seconds.
2.8. PET acquisition and processing
An indwelling catheter was placed into a forearm vein in both arms. A heating pad was wrapped around 1 arm 30 minutes prior to the positron emission tomography (PET) scan. About 10 mL of blood was obtained for glucose, AcAc, and beta-hydroxybutyrate quantification. This blood was stored on ice and immediately processed for analysis after the PET scan. Prior to PET acquisition, a low-dose computed tomography scan of the head was obtained for attenuation correction. For the first scan (acetoacetate or AcAc) participants were injected with an intravenous bolus of up to 5 mCi (±10%) of 11C-AcAc over 2 minutes and for the second scan [fluorodeoxyglucose (FDG)] participants were injected with an intravenous bolus of up to 5 mCi (370 MBq) (±10%) of 18F-FDG over 20 seconds, standard doses used in previous studies (Castellano et al., 2015; Nugent et al., 2014a,b). The AcAc scan acquisition began immediately after the tracer injection and lasted for a total of 30 minutes. Time frames used for analysis were from the first 10 minutes of scan acquisition, 12×10 seconds, 8×30 seconds, and 1×4 min. Immediately after the AcAc scan, a washout period occurred from 30 to 60 minutes post injection of the first tracer. This was used to ensure that most of the first tracer was excreted prior to injection of the 18F-FDG tracer and second part of the dual-tracer scan. The FDG scan acquisition began immediately after the tracer injection and lasted for a total of 60 minutes. Time frames for scan acquisition were 12×10 seconds, 8×30 seconds, 6×4 minutes, and 3×10 minutes.
Blood was drawn during the 11C-AcAc and 18F-FDG scans and processed for gamma radiation estimation immediately after the scan was completed. Up to 2 mL of blood was sampled from the forearm vein at 3, 6, 8, 12, and 20 minutes post 11C-AcAc tracer injection; and at 3, 8, 16, 24, 35, and 55 minutes after 18F-FDG tracer injection. Whole blood was spun at 6000 rpm for 5 minutes at room temperature. Three hundred microliters of plasma for each time point was placed in a counting tube. Plasma radiation estimation was performed in an automated fashion with a Wallac 1480 Wizard 3′ (Perkin Elmer) gamma counter. The estimates were used for calibration of brain counts. See Fig. S2 for representation of dual-tracer PET protocol.
PMOD software version 3.5 (PMOD Technologies Ltd, Zurich, Switzerland) was used to process PET data. Both AcAc and FDG data were first co-registered to each participant’s T1-weighted structural MRI using the PFUS tool in PMOD. Co-registered PET images were corrected for partial volume effects with the partial volume correction (brain-based) feature in PMOD using principles of the modified Müller-Gartner method. An arterial input function was determined by tracing 3–4 regions of interest (ROIs) within the internal carotid artery: first on the MR images and then applied to the PET images. The activity calculated in the internal carotid artery was then corrected using plasma radioactivity measures taken from each individual during the PET acquisition at time intervals indicated in the PET protocol. Cerebral metabolic rates (CMRs) were quantified with the Patlak model feature in the PKIN tool of PMOD. The lumped constant for determination of CMR AcAc was set to 1.0 and CMR glucose was set to 0.8, as previously published (Castellano et al., 2015).
2.9. Statistical analyses
CSF biomarker values, cognitive scores, and metabolic values were submitted to repeated measures analysis of covariance using Proc GLM from SAS v9.4. Time (Pre vs. Post) was the repeated factor, with group (SMC vs. MCI) as the independent factor, and age, apolipoprotein E4 allele (APOE4) carriage (yes or no), and order of diet intervention (MMKD first vs. AHAD first) as covariates. If covariates did not contribute significantly (p > 0.15) they were dropped from the model. Separate repeated measures analyses were conducted for pre and post MMKD data, and for pre and post AHAD data. Partial eta squared effect sizes and post hoc power estimates were determined with GPower. Partial eta squared values of 0.01, 0.06, and 0.14 are considered small, medium, and large (Cohen, 1988). For CSF values, analyses were conducted only for participants who completed LPs and had valid data for both baseline and post-diet LPs. Two CSF analytes (tau and neurofilament light) had non-normal distributions and were log-transformed prior to analysis. For MR gray matter volume values, a meta-ROI shown to discriminate between AD and controls was constructed as the average of the following regions: inferior temporal gyrus, caudate, paracentral lobule, superior temporal pole, posterior cingulate, amygdala, hippocampus, entorhinal cortex, angular gyrus, and mid-temporal pole (Rondina et al., 2018). Main effects of diet, cognitive group, and the interaction of diet by cognitive group on PCASL perfusion were examined utilizing a voxel-wise multiple regression in SPM12. An a priori anatomical mask was constructed using the WFU PickAtlas toolbox (Maldjian et al., 2003) and was applied to all analyses. The mask was composed of regions involved in memory and regions shown to be negatively affected by AD including the frontal, parietal, and temporal cortices, cingulum, postcentral gyrus, amygdala, parahippocampal gyri, and hippocampus (Fig. S3). Data were corrected for multiple comparisons with a voxel-wise threshold level of p < 0.005 holding alpha at 0.05 for a minimum cluster size of 112 contiguous voxels. This a priori threshold was derived via Monte Carlo simulations (3dClustSim, AFNI, http://afni/nimh/nih.gov). To further explore the regions of significance yielded from voxel-wise analyses, beta weights were extracted for pre and post MMKD diet PCASL for both cognitive groups utilizing the SPM12 toolbox MarsBaR (version 0.44).
For dual-tracer PET values, meta-ROIs that characterized prototypical AD hypometabolism were constructed for the 11C-AcAc and 18F-FDG scans from the average of the following regions: left and right angular gyrus, left and right temporal lobe, and posterior cingulate (Landau et al., 2011). Seven participants completed the dual-tracer PET at baseline and at the end of the MMKD and AHAD. For 2 participants, a scanner malfunction resulted in uninterpretable data. Exploratory analyses were conducted for the remaining 5 participants. The PET meta-ROI for 11C-AcAc and 18F-FDG tracers was subjected to repeated measures analysis of variance, with time (Pre-Diet, Post-MMKD, Post-AHAD) as the repeated measure.
3. Results
A total of 20 participants completed both diets. Regarding attrition, 3 of 23 participants discontinued the study early. Mean compliance rates assessed by dietician assessment of daily food records were 90% for the MMKD and 95% for AHAD. Baseline demographic characteristics are shown in Table 1. Our sample had a mean Mini-Mental State Examination score of 28.7, mean years of education of 16.1, and APOE4 positivity of 30%. No serious adverse events occurred, nor any adverse event deemed related to diet intervention by the study clinician.
Table 1:
Descriptive statistics: baseline descriptive statistics (means and standard deviations) for the total sample, and SMC and MCI groups
| Variable | All (n = 20) | SMC (n = 11) | MCI (n = 9) |
|---|---|---|---|
| Sex (male/female) | 5/15 | 2/9 | 3/6 |
| APOE4 (±) | 6/13 | 2/8 | 4/5 |
| Age (y) | 64.3 (6.3) | 64.9 (7.9) | 63.4 (4.0) |
| Education (y) | 16.1 (2.5) | 16.5 (2.3) | 15.7 (2.9) |
| BMI (kg/m2) | 28.4 (5.7) | 26.9 (6.2) | 30.3 (4.7) |
| MMSE (out of 30) | 28.7 (1.1) | 28.9 (1.0) | 28.3 (1.2) |
| Glucose (mg/dL) | 97.6 (18.3) | 93.9 (11.4) | 102.1 (24.4) |
| BHB (mmol/L) | 0.23 (0.27) | 0.35 (0.31)a | 0.1 (0.14)a |
| Insulin (μIU/mL) | 8.3 (6.1) | 5.2 (3.4)a | 12.0 (6.8)a |
| Hemoglobin A1c (%) | 5.9 (0.3) | 5.9 (0.2) | 6.1 (0.4) |
| Total cholesterol (mg/dL) | 215.2 (43.7) | 200.5 (42.1) | 233.2 (40.6) |
| HDL cholesterol (mg/dL) | 67.4 (25.8) | 69.5 (25.8) | 64.8 (27.1) |
| VLDL cholesterol (mg/dL) | 20.0 (11.1) | 15.2 (6.2)a | 25.8 (13.2)a |
| LDL cholesterol (mg/dL) | 121.5 (41.2) | 104.1 (45.6)a | 142.7 (24.2)a |
| Triglycerides (mg/dL) | 99.8 (55.7) | 75.5 (30.5)a | 129.4 (66.4)a |
Key: BHB, capillary beta-hydroxybutyrate; BMI, body mass index; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; SMC, subjective memory complaints; VLDL, very-low-density lipoprotein.
MCI different than SMC, p < 0.05.
3.1. Macronutrient, weight, and peripheral metabolic measures
Total calories and net carbohydrate, protein, and fat grams were calculated from the 3-day food record administered at baseline, as well as from data averaged across 2 on-diet time points. Calorie intake was reduced from baseline for both diets, and did not differ between diets (mean calorie intake prior to MMKD = 1981.6 802.6 [standard deviation, SD] vs. on-diet = 1681.6 5179, prior to AHAD = 1976.3 ± 753.9 vs. on-diet = 1456.5 ± 402.8; overall time effect p ± 0.0021, partial η2 ± 0.401, power = 0.907). Carbohydrate consumption (g) was significantly reduced for MMKD relative to AHAD (mean intake prior to MMKD = 191.1 ± 91.8 [SD] vs. on-diet = 40.2 ± 99.0, prior to AHAD = 191.4 ± 99.1 vs. on-diet = 170.5 ± 68.7; diet by time effect p = 0.0002, partial η2 = 0.538, power = 0.991). Fat consumption (g) was significantly increased for MMKD and reduced for AHAD (mean intake prior to MMKD = 86.6±45.9 [SD] vs. on-diet = 114.4 ± 29.3, prior to AHAD = 88.4 ± 51.6 vs. on-diet = 42.4 ± 33.3; diet by time effect p = 0.0001, partial η2 = 0.659, power = 0.999). No change in protein intake was observed with either diet. These results demonstrate that the dietary intervention successfully modulated macronutrient profiles.
Metabolic outcomes are presented in Table 2. MMKD successfully elevated fasting ketone body levels (p = 0.008, partial η2 = 0.424, power = 0.847), with the SMC group showing greater increases (time by group p = 0.015, partial η2 = 0.378, power = 0.774). Participants lost weight with MMKD, despite having calorie targets that were determined by pre-study caloric intake. The MCI group showed greater percent weight loss than the SMC group (p = 0.004, partial η2 = 0.427, power = 0.847). All participants had reduced fasting glucose and HbA1c levels, with no group differences noted (glucose and HbA1c by time p = 0.03, partial η2 = 0.287, power = 0.632 and p = 0.004, partial η2 = 0.514, power = 0.984). Fasting insulin levels were also reduced (p = 0.03, partial η2 = 0.241, power = 0.617). Total cholesterol was unchanged by the MMKD. VLDL cholesterol levels were reduced by MMKD (p = 0.02, partial η2 = 0.274, power = 0.693), particularly for the MCI group (time by group p = 0.02, partial η2 = 0.281, power = 0.709), who also showed a trend increase in HDL (time by group p = 0.096, partial η2 = 0.146, power = 0.382). Triglycerides were lowered with MMKD (p = 0.02, partial η2 = 0.273, power = 0.69) with the greatest reduction demonstrated by the MCI group (time by group p = 0.02, partial η2 = 0.288, power = 0.549).
Table 2:
Metabolic outcomes: mean change from baseline (post-pre diet) for metabolic outcomes after MMKD and AHAD for total sample and the SMC and MCI groups
| Metabolic indices | MMKD |
AHAD |
||||
|---|---|---|---|---|---|---|
| All (n = 20) | SMC (n = 11) | MCI (n = 9) | All (n = 20) | SMC (n = 11) | MCI (n = 9) | |
| Weight (lbs) | −8.5a | −3.9b | −14.2b | −4.8a | −3.2 | −6.8 |
| Glucose (mg/dL) | −13.5a | −13.9 | −13.1 | 0.3 | 2.4 | −2.1 |
| BHB (mmol/L) | 0.7a | 1.0b | 0.4b | 0 | 0 | 0 |
| Insulin (μIU/mL) | −2.1a | −0.5 | −4 | −1.6 | −0.2 | −3.3 |
| Hemoglobin A1c (%) | −0.1a | −0.2 | −0.1 | 0 | 0 | 0 |
| Total cholesterol (mg/dL) | 12.3 | 20.9 | 1.7 | −24.1 | −24.3 | −23.8 |
| HDL cholesterol (mg/dL) | 5.8 | 10.8c | −0.3c | −8.8a | −8.3 | −9.3 |
| VLDL cholesterol (mg/dL) | −4.6a | 0.1b | −10.2b | −0.8 | 0.1 | −1.8 |
| LDL cholesterol (mg/dL) | 17.4a | 21.6b | 12.2b | −14.6 | −16.1 | −12.7 |
| Triglycerides (mg/dL) | −23a | 1 | −52.3b | −3.4 | 0.6 | −8.3 |
Key: AHAD, American Heart Association Diet; BHB, capillary beta-hydroxybutyrate; MCI, mild cognitive impairment; MMKD, modified Mediterranean-ketogenic diet; SMC, subjective memory complaints.
Significant effect of diet, p < 0.05.
MCI different than SMC, p < 0.05.
MCI different than SMC, p < 0.10.
The AHAD did not affect peripheral ketone body, glucose, insulin, HbA1c, total cholesterol, VLDL cholesterol, or triglyceride levels. HDL cholesterol levels were lowered for both groups (p = 0.02, partial η2 = 0.277, power = 0.699), and both groups had reduced percent body weight after the diet (p = 0.008).
3.2. CSF biomarkers
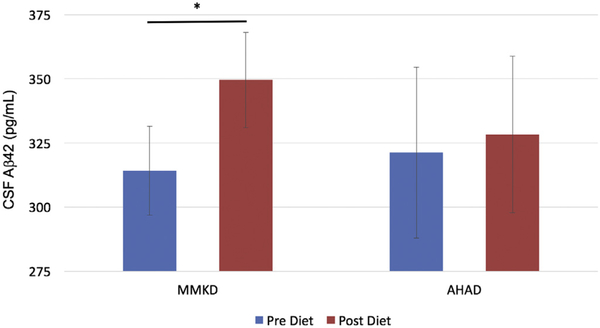
CSF Aβ42 levels increased following MMKD independent of group (time p = 0.04, partial η2 = 0.404, power = 0.700; Fig. 1). This effect was moderated by age (time by age interaction p = 0.04, partial η2 = 0.406, power = 0.704) such that older participants showed greater increases in Aβ42 following the diet (Spearman rho = 0.51, p = 0.06). No MMKD-associated changes were observed for Aβ0, or for the ratio of Aβ42/Aβ40 (data not shown).
Fig. 1.
MMKD effects on CSF Aβ42. CSF Aβ42 increased with MMKD independent of group (p = 0.04). Mean values, with error bars representing ±1 standard error from the mean. *Significant effect of MMKD, p < 0.05. Abbreviations: AHAD, American Heart Association Diet; CSF, cerebrospinal fluid; MMKD, modified Mediterranean-ketogenic diet.
MMKD-induced changes in CSF tau levels differed according to group (time by group interaction p = 0.007, partial η2 = 0.571, power = 0.935; Fig. 2). The MCI group showed decreased tau after MMKD and the SMC group’s tau levels were unchanged. Age also moderated this interaction (time by group by age interaction p = 0.008, partial η2 = 0.563, power = 0.927) with younger participants in the MCI group showing greater reductions (data not shown). No effects were observed for tau-p181 levels.
Fig. 2.
Diet effects on CSF tau. CSF tau decreased with MMKD in MCI (p = 0.007), and after AHAD independent of group (p = 0.02). Mean values, with error bars representing ±1 standard error from the mean. *Significant effect of diet in MCI, p < 0.05. Abbreviations: CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MMKD, modified Mediterranean-ketogenic diet; SMC, subjective memory complaints.
MMKD showed trend-level lowering of axonal injury marker NFL for the MCI group [time by group interaction p = 0.097, partial η2 = 0.23, power = 0.38; Pre vs. Post means with (standard error of the mean) for the SMC group were 2.71 (0.06) vs. 2.72 (0.06); and for the MCI group were 2.66 (0.08) vs. 2.61 (0.08)]. For the synaptic protein neurogranin, an age-moderated trend for reduced levels was observed following the MMKD independent of group (time by age interaction p = 0.09, partial η2 = 0.279, power = 0.386), reflecting greater reduction for older participants (Spearman rho = 0.65, p = 0.03; Fig. 3). Although sTREM2 levels were reduced following MMKD independent of group and age, this effect did not achieve significance (p = 0.12, partial η2 = 0.203, power = 0.303). YKL-40 levels were unchanged by MMKD.
Fig. 3.
MMKD effects on neurogranin by age. Neurogranin levels were reduced for older participants after MMKD independent of group (p = 0.03). Older participants showed greater reductions in NG levels (Spearman Rho = −0.65). Abbreviations: MMKD, modified Mediterranean-ketogenic diet; NG, neurogranin.
There were no changes in CSF Aβ42 or Aβ40 after AHAD for either group. AHAD reduced tau levels (p = 0.02, partial η2 = 0.511, power = 0.778), an effect that trended greater for the MCI group (time by group p = 0.056, partial η2 = 0.383, power = 0.559; Fig. 2), and was moderated by age (time by age p = 0.03, partial η2 = 0.465, power = 0.701), with younger participants showing greater reductions regardless of group (Spearman rho = 0.74, p = 0.01). AHAD did not affect CSF levels of NFL, neurogranin, YKL-40, or sTREM2.
3.3. Imaging measures
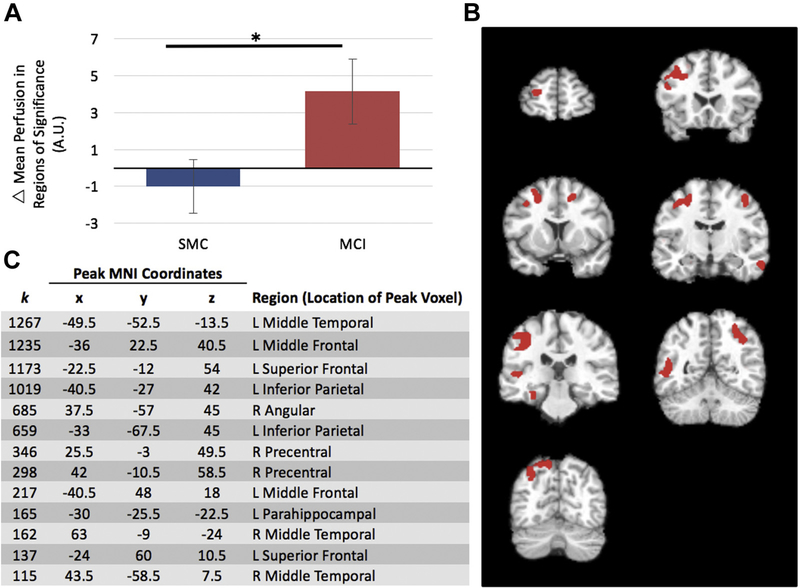
Gray matter volume meta-ROI values did not change following either diet for either group. Voxel-wise analyses detected increased perfusion in response to MMKD in the left parahippocampal and the right temporal lobe while there were no changes in response to AHAD. When this change in response to MMKD was divided by cognitive group, the MCI group displayed increased perfusion in left inferior parietal, medial and superior frontal, medial temporal, and parahippocampal as well as right medial temporal, precentral, and angular gyrus, compared to the SMC group (Fig. 4). The SMC group showed no regions of greater perfusion than the MCI group. The PCASL in these regions were extracted and demonstrated that the interaction was driven by an increased perfusion in the MCI group while the SMC group showed no change (Fig. 4).
Fig. 4.
(A) Change in mean perfusion (post-pre diet PCASL) across all regions of significance after the MMKD. Error bars represent ±1 standard error from the mean. *Significant at p < 0.05. (B) Regions where MCI showed greater perfusion compared to SMC in response to MMKD (p < 0.005, k = 112). All images presented in neurological orientation. (C) Regions where MCI showed greater perfusion than SMC in response to MMKD (p < 0.005, k > 112); k = number of contiguous voxels, and MNI coordinates for peak voxels within significant cluster. Abbreviations: MCI, mild cognitive impairment; MMKD, modified Mediterranean-ketogenic diet; MNI, Montreal Neurological Institute; PCASL, pseudo continuous arterial spin labeling; SMC, subjective memory complaints.
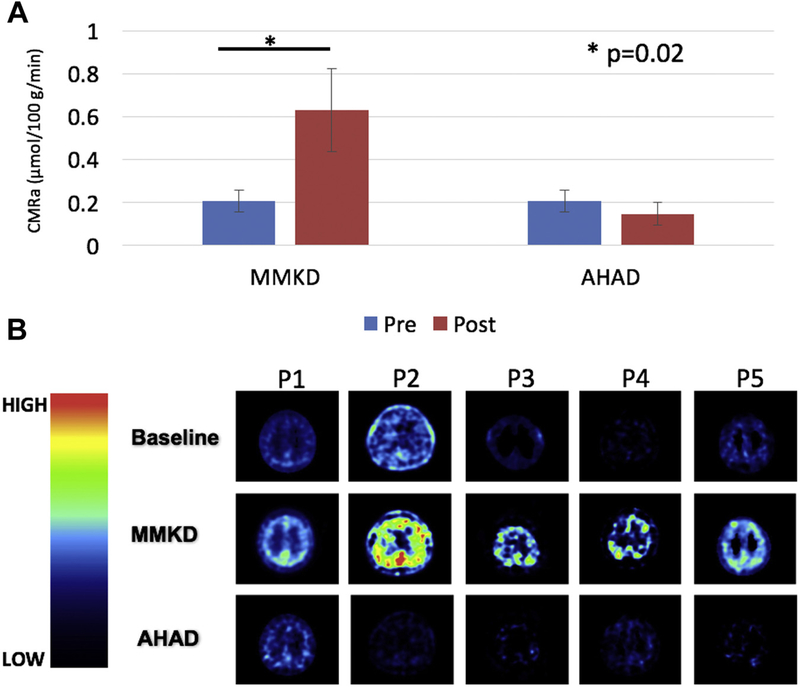
Baseline meta-ROI values were contrasted with each of the post-diet values. 11C-AcAc meta-ROI values increased for each of the 5 participants following MMKD, resulting in a significant effect of time (p = 0.02, partial η2 = 0.759, power = 0.552; Fig. 5). No significant changes in 11C-AcAc meta-ROI values were observed following AHAD. There were no differences in 18F-FDG after either diet (data not shown).
Fig. 5.
Dual tracer PET. (A) 11C-AcAc uptake increased after MMKD (p = 0.02), but not the AHAD independent of group. No effects were observed for FDG. CMRa or the mean cerebral metabolic rate of acetoacetate is plotted (μmol/100 g/min). (B) 11C-AcAc uptake at baseline, and after MMKD and AHAD for 5 individual participants. Abbreviations: AHAD, American Heart Association Diet; MMKD, modified Mediterranean-ketogenic diet.
3.4. Cognition
Cognitive test score differences are presented in Table S1. Both groups showed better performance on FCSRT after MMKD (time p = 0.03, partial η2 = 0.233, power = 0.6). No MMKD-associated changes were observed for total story recall or ADAS-Cog12 scores. For AHAD, the MCI group showed greater improvement on FCSRT (time by group p = 0.01, partial η2 = 0.3, power = 0.748). No changes were observed for total story recall or ADAS-Cog12 scores.
4. Discussion
The present study examined the effects of a modified KD intervention on CSF AD biomarkers, cerebral blood flow, cerebral metabolism, peripheral metabolic measures, and cognition in adults with metabolic and cognitive risk factors for AD. Both MMKD and AHAD diets achieved good mean compliance (>90%) and safety. Only MMKD improved peripheral metabolic profile, cerebral perfusion, and cerebral ketone body uptake. Both diets altered CSF AD biomarkers, but in different ways.
4.1. Ketogenic diet is associated with improvement in peripheral metabolic measures
The MMKD increased levels of capillary ketone bodies for all participants, confirming that the dietary intervention was successfully implemented. Interestingly, lower ketone levels were observed for the MCI group relative to the SMC group, despite similar dietary compliance as determined by daily food records. This pattern may reflect greater ketone uptake into target tissues in MCI, leading to lower circulating levels. This pattern would also be consistent with the possibility that adults with MCI are less able to generate ketone bodies as a result of ketogenic interventions. Additionally, the MCI group had higher fasting insulin and triglyceride levels at baseline, indicating they may have had greater insulin resistance, which may necessitate longer exposure to MMKD to generate ketone levels comparable to the SMC group.
Only MMKD had a beneficial impact on peripheral metabolic measures related to lipid and glucose metabolism; a significant reduction in HbA1c, glucose, insulin, triglycerides, and VLDL cholesterol were observed. These findings support metabolic data from other studies of dietary ketosis showing improvement in lipid profile and insulin sensitivity secondary to KD (Hu et al., 2012; Yancy et al., 2004). Our striking metabolic results are important as they show the potential for MMKD to beneficially impact metabolic dysfunction in older adults with and without cognitive impairment. The majority of published reports targeting ketosis have focused on supplementation of ketogenic compounds (medium chain triglycerides) (Croteau et al., 2018; Henderson et al., 2009; Reger et al., 2004). Although supplements may be easier to implement than dietary modification, our study demonstrates that a ketogenic dietary intervention is feasible in our target population–aided by robust education materials, meal plans, and the support of dieticians. Given that systemic metabolic dysfunction increases risk for the development of Alzheimer’s and cognitive decline (Craft, 2009; Di Paolo and Kim, 2011; Razay et al., 2007; Whitmer et al., 2005, 2008), the improvement in peripheral glucose and lipid metabolism through ketogenic interventions may provide a therapeutic tool for the prevention of age-related neurodegenerative disorders.
4.2. Ketogenic diet is associated with improvement in CSF biomarker profile
A primary goal of the study is to examine the effects of MMKD on CSF AD biomarkers. We observed that MMKD led to improvement in CSF AD biomarker profiles as evidenced by increased CSF Aβ42 (independent of group), decreased CSF tau (only in MCI), and increased CSF Aβ42/tau ratio after MMKD (greater in MCI). These promising results suggest that a targeted 6-week dietary intervention aimed at ketosis can positively affect CSF AD biomarker profile. To our knowledge, this is the first published report showing an impact of dietary ketosis on CSF amyloid and tau levels in humans. These results suggest that dietary interventions can have significant impact on AD pathological markers and as such support our previous work showing that a 4-week low saturated fat/low glycemic index diet also increased CSF Aβ42 in MCI (Bayer-Carter et al., 2011).
There is a general lack of knowledge concerning the impact of ketosis on the 2 main biomarkers of Alzheimer’s, with all current literature coming from animal models, and providing contradictory results. For example, several studies in mouse models of AD reported that ketosis leads to decreased brain Aβ content (Kashiwaya et al., 2013; Van der Auwera et al., 2005; Yao et al., 2011) and hyperphosphorylated tau (Kashiwaya et al., 2013), while other studies have shown no impact of ketosis on Aβ (Beckett et al., 2013; Brownlow et al., 2013). Mechanistically, ketone bodies may be neuroprotective by blocking Aβ entry into neurons (Yin et al., 2016), which may decrease oxidative stress, improve metabolic function, and decrease glutamate excitotoxicity (Hertz et al., 2015; Maalouf et al., 2009; Yin et al., 2016). In the present study, the fact that lowering of tau was only observed in adults with MCI raises the possibility that MMKD is affecting process relating to tau aggregation and neurodegeneration, which occurs around the time that clinical symptoms manifest. Supporting this possibility, we also found trend-level reduction of CSF neurogranin and NFL after MMKD, suggesting diet may have impact on measures of dendritic/synaptic injury and neurodegeneration (Blennow and Zetterberg, 2018; Zetterberg et al., 2016).
Intriguingly, AHAD also led to a reduction in CSF tau that was greater in MCI. We hypothesized that improvement in systemic metabolic health would promote a better CSF biomarker profile, yet only MMKD showed significant impact on peripheral metabolic measures. The positive change in CSF tau levels after AHAD may be driven by weight loss alone or other unknown diet-related effects, rather than the specific metabolic changes found after MMKD intervention. Another possible explanation for the post-diet changes in CSF measures could relate to a general diet-induced decrease in systemic inflammation, which might promote positive CSF AD biomarker outcomes. Unfortunately, this was not assessed in the present study and should be explored in future work.
4.3. Ketogenic diet is associated with increased cerebral perfusion
Cerebral blood flow or perfusion is an important measure of overall brain health (Chen et al., 2011, 2013) and has been shown to be negatively impacted on neurodegenerative disorders (Alsop et al., 2010; Heron et al., 2014; Zhang et al., 2017). In Alzheimer’s, decreased cerebral perfusion has been related to disease progression (Zhang et al., 2017), although there may be regional compensatory increases in cerebral blood flow early in the disease (Dai et al., 2009). In the present study, we used PCASL to determine how diet impacts cerebral perfusion. We found increased perfusion on the pre-specified meta-ROI only following MMKD (greater in the MCI group). Previous work has shown that metabolic dysregulation is associated with reduced perfusion (Birdsill et al., 2013; Zhang et al., 2017). Thus, it is possible that MMKD-induced metabolic improvement contributed to enhanced perfusion. We have an incomplete understanding of dietary impact on cerebral perfusion, especially in older adults at risk for Alzheimer’s. Prior reports have shown that dietary supplementation may impact cerebral perfusion (Presley et al., 2011; Taheri et al., 2016; Zerbi et al., 2014), while a study by Lin et al. (2015) reports that caloric restriction and ketosis preserved cerebral blood flow in aging rat brains. Our results provide important clinical data concerning diet effects on cerebral perfusion, and suggest that dietary ketosis may improve cerebral perfusion rather than a low-fat diet. The potential impact of diet on cerebral blood flow after a 6-week trial is intriguing but must be interpreted cautiously. There is a need for additional study of interventions aimed at ketosis to better understand their benefits and risks.
4.4. Ketogenic diet is associated with increased cerebral ketone body uptake
We used a novel dual-tracer PET imaging technique in a subset of participants to assess the impact of diet on both cerebral ketone body and glucose metabolism. We found that 11C-AcAc uptake increased after MMKD, while cerebral 18F-FDG uptake was unaffected. These results suggest that MMKD may increase peripheral ketones and thereby make greater concentrations available for uptake into the brain. These results complement previous work showing that a ketogenic intervention (medium chain triglyceride supplementation) increases cerebral ketone body uptake as assessed by 11C-AcAc PET, without impacting glucose uptake (Croteau et al., 2018). Although our sample size is too small to identify direct relationships among peripheral ketone bodies, cerebral ketone body uptake, and CSF biomarker/cognitive outcomes, or differences between SMC and MCI groups, our results clearly support the hypothesis that a ketogenic dietary intervention pro-motes cerebral ketosis. Future work should focus on larger samples to directly test this hypothesis.
4.5. Ketogenic diet is associated with better performance on FCSRT but not ADAS-Cog
Improved memory has been observed in some studies of ketogenic interventions in MCI and AD (Henderson et al., 2009; Krikorian et al., 2012; Reger et al., 2004; Van der Auwera et al., 2005). In a previous small uncontrolled pilot study, improvement on ADAS-Cog was observed following a 3-month KD intervention that was reversed following a washout period (Taylor et al., 2018). A second small controlled study did not find improved memory in an intent-to-treat analysis, although results were in a direction favoring the KD group (Brandt et al., 2019). We found that both diets were associated with improved memory performance as assessed by FCSRT, but not on story recall or ADAS-Cog12 scores. However, it is likely that improvement on FCSRT was affected by practice, a tendency that could have been exacerbated by the crossover design. Similarly, the 6-week intervention period may have been an insufficient length of exposure to impact global cognitive measures such as ADAS-Cog, particularly for adults with subjective memory complaints or MCI, whose scores are close to normal and thus have little room for improvement. Future controlled studies with parallel group designs, longer durations, more sensitive tests, and larger samples are needed to determine possible ketogenic-induced cognitive benefits.
4.6. Limitations and future directions
The present study has several key limitations, despite promising results. First, the small sample size limits generalizability, as well as the ability to examine subgroup response factors such as APOE genotype or sex. Second, our crossover design was susceptible to metabolic carry-over effects of diet and practice effects for cognitive tests. Furthermore, participant drop-outs, while infrequent, may potentially have biased results in this small sample. Although we saw significant diet-associated changes in CSF biomarkers, metabolic measures, imaging, and cognition after only 6 weeks, a longer study would likely strengthen our outcomes. The post-intervention weight loss may have contributed to diet-related effects. Although weight loss was observed with both diets, the MCI group showed greater weight loss following MMKD than did the SMC group. Participants in our study prepared their own food with close supervision by a registered dietician, so the dietary intervention may not have been as consistent as when food is supplied by a metabolic kitchen. However, this design improves applicability to a broader population. Finally, a potential drawback of ketogenic interventions is difficulty with compliance over prolonged periods. We observed good compliance in our study, but even minor lapses may have compromised results given the relatively brief intervention. Despite these limitations, we observed robust effects on CSF and brain imaging biomarkers that await replication in larger, longer studies.
5. Conclusions
In conclusion, our results demonstrate that the MMKD intervention was well-tolerated with good compliance, and associated with improved CSF AD biomarker profile, improved peripheral lipid and glucose metabolism, increased cerebral perfusion, and increased cerebral ketone body uptake. These effects suggest that further studies are warranted to determine whether a ketogenic intervention targeted toward adults at risk for AD dementia may prove beneficial in the prevention of cognitive decline. Future longer, larger studies may elucidate the mechanisms underlying therapeutic effects of ketogenic interventions, and may have broad applicability to Alzheimer’s and other neurodegenerative disorders.
Supplementary Material
Acknowledgements
Supported by the Wake Forest Alzheimer’s Disease Research Center (P30-AG049638), the Hartman Family Foundation, and the Roena B. Kulynych Center for Memory and Cognition Research. The authors thank Dixie Yow; Patricia Wittmer; Deborah Dahl, RN; Timothy Hughes, PhD; and Ben Wagner for technical assistance. The authors would also like to acknowledge the contributions of the Wake Forest Clinical and Translational Science Institute and the laboratory technicians at Sahlgrenska University Hospital, Mölndal, Sweden. The biomarker measurements were supported in part by grants from the Swedish Research Council and Swedish State Support for Clinical Research (ALFGBG).
Footnotes
Disclosure
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurobiolaging.2019.09.015.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Dai W, Grossman M, Detre JA, 2010. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J. Alzheimers Dis 20, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2018. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429. [Google Scholar]
- American Diabetes Association, 2016. Standards of Medical Care in Diabetes-2016 abridged for primary care providers. Clin. Diabetes 34, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, Brown TR, 2008. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn. Reson. Med 60, 1362–1371. [DOI] [PubMed] [Google Scholar]
- Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S, 2011. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch. Neurol 68, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett TL, Studzinski CM, Keller JN, Paul Murphy M, Niedowicz DM, 2013. A ketogenic diet improves motor performance but does not affect beta-amyloid levels in a mouse model of Alzheimer’s disease. Brain Res. 1505, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, Oh JM, Gallagher CL, Koscik RL, Jonaitis EM, 2013. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 21, 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, 2018. The past and the future of Alzheimer’s disease fluid biomarkers. J. Alzheimers Dis 62, 1125–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ, 2006. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol 60, 223–235. [DOI] [PubMed] [Google Scholar]
- Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC, 2019. Preliminary report on the feasibility and efficacy of the modified Atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J. Alzheimers Dis 68, 969–981. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Benner L, D’Agostino D, Gordon MN, Morgan D, 2013. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS One 8, e75713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR, 1998. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med 40, 383–396. [DOI] [PubMed] [Google Scholar]
- Cahill GF Jr., 2006. Fuel metabolism in starvation. Annu. Rev. Nutr 26, 1–22. [DOI] [PubMed] [Google Scholar]
- Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G,Imbeault H, Turcotte E, Fulop T, Cunnane SC, 2015. Lower brain 18F-fluo-rodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J. Alzheimers Dis 43, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH, 2011. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH, 2013. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PLoS One 8, e56733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. L. Erlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Craft S, 2009. The role of metabolic disorders in Alzheimer disease and vascular dementia. Arch. Neurol 66, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau E, Castellano C, Fortier M, Bocti C, Fulop T, Paquet N, Cunnane S, 2018. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp. Gerontol 107, 18–26. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K, 2014. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM, 2009. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250, 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Hartman AL, Stafstrom CE, Thio LL, 2013. How does the ketogenic diet work? Four potential mechanisms. J. Child Neurol 28, 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW, 2011. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat. Rev. Neurosci 12, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH, Hartman AL, 2007. The ketogenic diet: one decade later. Pediatrics 119, 535–543. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Pyzik PL, Freeman JM, 2000. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J. Child Neurol 15, 787–790. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson LM, Hagberg L, Spudich S, Fuchs D, Price RW, Zetterberg H, 2019. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol. Neuroimmunol. Neuroinflamm 6, e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sanders AE, Hall C, Lipton RB, 2010. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis. Assoc. Disord 24, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferre M, Hu FB, Martinez-Gonzalez MA, Fito M, Bullo M, Estruch R, Ros E, Corella D, Recondo J, Gomez-Gracia E, Fiol M, Lapetra J, Serra-Majem L, Munoz MA, Pinto X, Lamuela-Raventos RM, Basora J, Buil-Cosiales P, Sorli JV, Ruiz-Gutierrez V, Martinez JA, Salas-Salvado J, 2014. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 12, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Vining EP, 2007. Clinical aspects of the ketogenic diet. Epilepsia 48, 31–42. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC, 2009. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron CJL, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, Keenan RJ, Watts R, Dalrymple-Alford JC, Anderson TJ, 2014. Comparing cerebral perfusion in Alzheimer’s disease and Parkinson’s disease dementia: an ASL-MRI study. J. Cereb. Blood Flow Metab 34, 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Chen Y, Waagepetersen HS, 2015. Effects of ketone bodies in Alzheimer’s disease in relation to neural hypometabolism, beta-amyloid toxicity, and astrocyte function. J. Neurochem 134, 7–20. [DOI] [PubMed] [Google Scholar]
- Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS Jr., Kelly TN, He J, Bazzano LA, 2012. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol 176 (Suppl 7), S44–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Wong EC, Liu TT, 2010. Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn. Reson. Med 64, 799–810. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL, 2013. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Rho JM, 2009. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics 6, 406e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr., Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL, 2001. Revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. J. Nutr 131, 132–146. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ, 2012. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 33, 425.e19–425.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ, 2011. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 32, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Zhang W, Gao X, Watts L, 2015. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol. Aging 36, 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP, 2009. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev 59, 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Nugent S, Castellano CA, Goffaux P, Whittingstall K, Lepage M, Paquet N, Bocti C, Fulop T, Cunnane SC, 2014a. Glucose hypometabolism is highly localized, but lower cortical thickness and brain atrophy are widespread in cognitively normal older adults. Am. J. Physiol. Endocrinol. Metab 306, E1315–E1321. [DOI] [PubMed] [Google Scholar]
- Nugent S, Tremblay S, Chen KW, Ayutyanont N, Roontiva A, Castellano CA, Fortier M, Roy M, Courchesne-Loyer A, Bocti C, Lepage M, Turcotte E, Fulop T, Reiman EM, Cunnane SC, 2014b. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol. Aging 35, 1386–1395. [DOI] [PubMed] [Google Scholar]
- Portelius E, Olsson B, Hoglund K, Cullen NC, Kvartsberg H, Andreasson U, Zetterberg H, Sandelius A, Shaw LM, Lee VMY, Irwin DJ, Grossman M, Weintraub D, Chen-Plotkin A, Wolk DA, McCluskey L, Elman L, McBride J, Toledo JB, Trojanowski JQ, Blennow K, 2018. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathol. 136, 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD, 2011. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 24, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G, 2007. The metabolic syndrome and Alzheimer disease. Arch. Neurol 64, 93–96. [DOI] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S, 2004. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 25, 311–314. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Kim S, Nho K, Foroud T, Shen L, Petersen RC, Jack CR Jr., Beckett LA, Aisen PS, Koeppe RA, 2015. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 11, 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina JM, Ferreira LK, de Souza Duran FL, Kubo R, Ono CR, Leite CC, Smid J, Nitrini R, Buchpiguel CA, Busatto GF, 2018. Selecting the most relevant brain regions to discriminate Alzheimer’s disease patients from healthy controls using multiple kernel learning: a comparison across functional and structural imaging modalities and atlases. Neuroimage. Clin 17, 628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL, 1984. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 141, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM, 2012. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol 3, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Yu J, Zhu H, Kindy MS, 2016. High-sodium diet has opposing effects on mean arterial blood pressure and cerebral perfusion in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis 54, 1061–1072. [DOI] [PubMed] [Google Scholar]
- Tan H, Maldjian JA, Pollock JM, Burdette JH, Yang LY, Deibler AR, Kraft RA, 2009. A fast, effective filtering method for improving clinical pulsed arterial spin labeling MRI. J. Magn. Reson. Imaging 29, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH, 2018. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement. (N Y) 4, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Wera S, Van Leuven F, Henderson ST, 2005. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL, 2017. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 16, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Fan W, Procaccio V, 2010. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol 5, 297–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1987. Wechsler Memory Scale-Revised. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K, 2005. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K, 2008. Central obesity and increased risk of dementia more than three decades later. Neurology 71, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Yancy WS Jr., Olsen MK, Guyton JR, Bakst RP, Westman EC, 2004. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyper-lipidemia: a randomized, controlled trial. Ann. Intern. Med 140, 769–777. [DOI] [PubMed] [Google Scholar]
- Yao J, Chen S, Mao Z, Cadenas E, Brinton RD, 2011. 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PLoS One 6, e21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JX, Maalouf M, Han P, Zhao M, Gao M, Dharshaun T, Ryan C, Whitelegge J, Wu J, Eisenberg D, 2016. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 39, 25–37. [DOI] [PubMed] [Google Scholar]
- Zerbi V, Jansen D, Wiesmann M, Fang X, Broersen LM, Veltien A, Heerschap A, Kiliaan AJ, 2014. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer’s disease. Neurobiol. Aging 35, 600–613. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K, 2016. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 73, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, Goldberg TE, 2017. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci. Biobehav. Rev 72, 168–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.