Abstract
A method for the nucleophilic fluorination of heptamethyl aryl trisiloxanes to form fluoroarenes is reported. The reaction proceeds in the presence of Cu(OTf)2 and KHF2 as the fluoride source under mild conditions for a broad range of heptamethyltrisiloxyarenes with high functional group tolerance. The combination of this method with the silylation of aryl C–H bonds enables the regioselective fluorination of non-activated arenes controlled by steric effects following a two-step protocol.
Keywords: fluorination, aryl silanes, copper, nucleophilic fluoride, fluoroarenes
Graphical Abstract

The distinctive properties of aryl fluorides, which lead to applications in pharmaceuticals,[1] agrochemicals,[2] and positron emission tomography (PET) agents,[3] have prompted the development of synthetic methodologies for the formation of Csp2–F bonds over the last decade.[4] While classical methods for the formation of bonds between an aryl group and fluorine involve the reaction of highly electron-deficient aryl halides or aryl diazonium reagents with fluoride salts,[5] the use of transition metals has recently enabled the development of more general alternatives to these methods starting from prefunctionalized substrates, such as aryl halides,[6] aryl triflates,[7[ diaryl iodonium salts,[8] and phenols.[9] The regioselective direct fluorination of aryl C–H bonds has also been accomplished in the presence of transition metal complexes.[10] However, methods based on the functionalization of C-H bonds are typically limited to the preparation of ortho-fluorination products by coordination of the catalyst to a directing group. The undirected, electrophilic fluorination of aromatic C–H bonds has been realized with a palladium catalyst recently, but mixtures of constitutional isomers are often obtained.[11]
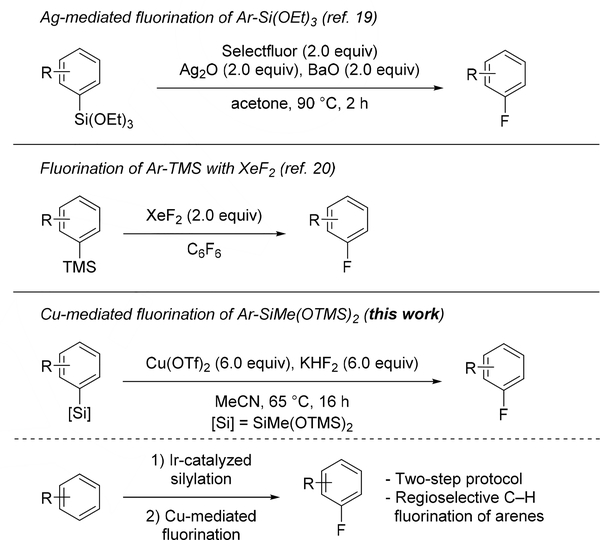
The direct fluorination of main group organometallic species, such as aryl stannanes, has been described with both electrophilic,[12] and nucleophilic fluorinating agents.[13] Nonetheless, the toxicity of the required organotin reagents limits the general applicability of these methods. The fluorination of boronic acid derivatives also has been developed with electrophilic fluorinating reagents in the presence of palladium,[14] silver,[15] and copper complexes,[16] and with nucleophilic fluorinating reagents in the presence of copper(II).[17] Arylsilanes represent an attractive alternative to arylboronic acid derivatives because they are derived from inexpensive reagents, are nontoxic, and are more stable than their organoboron counterparts. However, the direct fluorination of arylsilanes is less developed. The first examples of fluorination of arylsilanes were reported by Jolly in 1984 and Angelini in 1985, both with elemental fluorine for the labeling of arenes with 18F.[18] In 2011, the fluorination of triethoxysilanes was described in the presence of silver(I) (Scheme 1),[19] and the fluorination of simple aryltrimethylsilanes was reported to occur, albeit with narrow scope, with XeF2.[20]
Scheme 1.
Fluorination of Arylsilanes.
Our group has developed Rh- and Ir-catalyzed silylations of aromatic C–H bonds with the arene as limiting reagent,[21] and developed methods for the trifluoromethylation[22] and amination[23] of the resulting arylsilanes. If the fluorination of the type of arylsilane that forms by the silylation of C-H bonds, with common nucleophilic fluoride sources could be developed, then a process for the fluorination of aryl C-H bonds via silylarenes would result. Herein, we report a method for the fluorination of stable aryl heptamethyl trisiloxanes, which are readily accessible by C–H silylation. These Cu(OTf)2-mediated fluorinations occur with KHF2 as the fluorine source under mild conditions (65 °C, 16 h) and with a broad scope of aryl groups on the silane. When combined with C–H bond silylation, this protocol gives access to fluoroarenes directly from non-activated arenes in a regioselective fashion.
Earlier reports on the reductive elimination from high valent Cu(III) species[6a,b,8a,12b,16,24] prompted our search for copper reagents and fluoride sources that would enable the fluorination of arylsilanes. For the development of such a reaction, we selected 3,5-substituted arylsilane 1a, due to its high stability and ease of preparation on a multi-gram scale. A range of common fluoride sources were initially tested at 120 °C in the presence of an excess of Cu(OTf)2. While the use of alkali metal fluorides led exclusively to the protodesilylation of 1a, trace amounts of the fluorinated arene 2a were observed in the presence of KHF2 (Table 1, entry 1). Reactions at lower temperatures resulted in higher yields of 2a, which reached a maximum at 65 °C (Table 1, entries 2–4). Reactions conducted with various copper(I) and copper(II) sources as well as additives including bases, oxidants, or nitrogen-containing ligands occurred in the same or lower yield,[25] and low yields of the aryl fluoride were obtained in any solvent other than acetonitrile. In sharp contrast, reactions with amounts of Cu(OTf)2 and KHF2 up to 6.0 equiv (Table 1, entries 6–7) gave higher yields of 2a (74%). Reactions conducted with the same amount of other Cu(I) and Cu(II) sources (Table 1, entry 8–9) or 6.0 equiv of other fluoride sources under the same reaction conditions gave lower yields of the aryl fluoride 2a (Table 1, entries 10–11).[25] Like prior work on the fluorination of arylstannanes and boronates,[13, 17] superstoichiometric amounts of Cu(OTf)2 and the fluoride source were necessary for the fluorination of arylsilanes to proceed in satisfactory yields, suggesting that a similar reaction mechanism is followed.[25]
Table 1.
Effect of Reaction Parameters on the Fluorination of Arylsilanes.[a]
 | ||||
|---|---|---|---|---|
| Entry | [Cu] (equiv) | [F−] (equiv) | T [°C] | Yield (%)[b] |
| 1 | Cu(OTf)2 (3.0) | KHF2 (4.0) | 120 | trace |
| 2 | Cu(OTf)2 (3.0) | KHF2 (4.0) | 80 | 25 |
| 3 | Cu(OTf)2 (3.0) | KHF2 (4.0) | 65 | 40 |
| 4 | Cu(OTf)2 (3.0) | KHF2 (4.0) | 50 | trace |
| 5 | Cu(OTf)2 (2.0) | KHF2 (4.0) | 65 | trace |
| 6 | Cu(OTf)2 (6.0) | KHF2 (4.0) | 65 | 60 |
| 7 | Cu(OTf)2 (6.0) | KHF2 (6.0) | 65 | 74 |
| 8 | CuF2 (6.0) | KHF2 (6.0) | 65 | 0 |
| 9 | (tBuCN)CuOTf (6.0) | KHF2 (6.0) | 65 | 0 |
| 10 | Cu(OTf)2 (6.0) | AgF (6.0) | 65 | 0 |
| 11 | Cu(OTf)2 (6.0) | CsF (6.0) | 65 | 23 |
Reactions run on a 0.05 mmol scale.
Determined by 19F NMR spectroscopy with 1-fluoro-3-nitrobenzene as internal standard.
The scope of arylsilanes that form the corresponding fluoroarenes under the developed reaction conditions is shown in Table 2. The process tolerates variation in the electronic properties of the arene; both electron-rich and electron-deficient arylsilanes reacted to give the fluoroarene in moderate to good yields. Substrates functionalized with halides (2a,c,n), esters (2e,k,m,q), ketones (2f), aldehydes (2g), ethers (2h), nitriles (2j), sulfones (2l), trifluoromethyl groups (2i,o) and imides (2p) converted to the corresponding fluoroarenes. In the case of non-volatile fluoroarenes (2d-f, 2j-l, 2p), these products were isolated in pure form in yields comparable to those calculated by NMR spectroscopy using an internal standard. Furthermore, reactions conducted at 0.10 and 1.00 mmol scale provided comparable yields of the fluorinated arenes as illustrated for 2n. Unfortunately, the fluorination of heteroaromatic substrates did not occur.
Table 2.
Copper-mediated Fluorination of Arylsilanes.[a]
 |
Reactions were performed with 0.1 mmol of 1 to determine yields by 19F NMR spectroscopy with fluorobenzene as an internal standard added after the reaction.
Yield represents an average of two runs.
Isolated yield in parentheses.
Isolated with 4% of protodesilylated product.
Reaction was performed on 1.00 mmol scale.
With a procedure identified to convert aryl disiloxymethylsilanes to aryl fluorides, we considered that a tandem silylation-fluorination sequence could enable the overall fluorination of C–H bonds. To illustrate the potential of this two-step protocol, the fluorination of the natural product O-methylmellenine (3) was conducted. The reaction of this arene with heptamethyl trisiloxane in the presence of the recently reported[26] iridium catalyst containing 2,9-dimethyl phenanthroline in an open system for the silylation of more electron-rich arenes gave the silylarene intermediate, and treatment with KHF2 and Cu(OTf)2 formed the previously unreported fluorinated derivative 2q (Scheme 2a).[27] No purification of the arylsilane was required in this sequence. A similar two-step protocol for the fluorination of dimethyl naphthalene-2,3-dicarboxylate (1k), but with the first-generation iridium catalyst, gave the fluoroarene 2k in a yield over two steps that was comparable to that obtained for the individual steps combined (Scheme 2b). This sequence therefore represents a simple strategy for a sequential meta-selective fluorination of aryl C–H bonds.
Scheme 2.
Fluorination of C-H bonds by a combination of iridium-catalyzed arene silylation and fluorination of the arylsilane.
[a]Reaction was performed with 0.1 mmol of 1q. [b] Reaction sequence was performed with 0.3 mmol of dimethyl naphthalene-2,3-dicarboxylate. [c] Isolated yield in parentheses. [d] yields determined by 19F NMR spectroscopy with fluorobenzene as an internal standard.
In summary, we have developed an operationally simple and direct method for the copper-mediated fluorination of arylsilanes to fluoroarenes with KHF2. This reaction occurs with readily available reagents under mild conditions. Electron-rich, electron-deficient and diversely functionalized arylsilanes undergo fluorination in moderate to good yield. A sequential process allows the regioselective fluorination of aryl C–H bonds via the arylsilane with exquisite regioselectivity, which we also showed in the context of natural product derivatization. Methods for a related fluorination of heteroaryl silanes will be part of future studies.
Experimental Section
Inside a glovebox filled with N2, a 4 mL vial was charged with anhydrous KHF2 (46.9 mg, 0.600 mmol, 6.00 equiv), anhydrous MeCN (1.0 mL), arylsilane (0.1 mmol, 1 equiv), and anhydrous Cu(OTf)2 (217 mg, 0.600 mmol, 6.00 equiv). The vial was sealed with a Teflon-lined cap, and the suspension was heated at 65 °C for 16 h. The resulting mixture was allowed to cool to room temperature. 1-Fluoro-3-nitrobenzene was added as internal standard, and the reaction was analyzed by 19F NMR spectroscopy. For volatile products, the identity was confirmed by GC-MS. The reaction mixture of non-volatile products was diluted with Et2O (2 mL) and washed with a saturated solution of NH4OH (4 mL). The aqueous layer was extracted with CH2Cl2 (3 mL), and the combined organic layers were dried over Na2SO4. The organic layers were filtered through a short pad of SiO2, the SiO2 was flushed with CH2Cl2, and the resulting solution was concentrated under reduced pressure. The product was purified by preparative thin layer chromatography.
Supplementary Material
Acknowledgements
We thank the NIH (R35GM130387) for financial support of this work. We thank Ala Bunescu and Caleb Karmel for fruitful discussions. P. B. thanks the German National Academic Foundation (Studienstiftung) for support. D. P. S. thanks the German Academic Exchange Service (DAAD) for support.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) Purser S, Moore PR, Swallow S, Gouverneur V, Chem. Soc. Rev 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]; b) Hagmann WK, J. Med. Chem 2008, 51, 4359–4369. [DOI] [PubMed] [Google Scholar]
- [2].Jeschke P, ChemBioChem 2004, 5, 570–589. [DOI] [PubMed] [Google Scholar]
- [3].Ametamey SM, Honer M, Schubiger PA, Chem. Rev 2008, 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- [4].Selected Reviews:; a) O’Hagan D, Chem. Soc. Rev 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]; b) Campbell MG, Ritter T, Chem. Rev 2015, 115, 612–633. [DOI] [PubMed] [Google Scholar]; c) Champagne PA, Desroches J, Hamel JD, Vandamme M, Paquin JF, Chem. Rev 2015, 115, 9073–9174. [DOI] [PubMed] [Google Scholar]; d) Furuya T, Klein JEMN, Ritter T, Synthesis. 2010, 10, 1804–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Liang T, Neumann CN, Ritter T, Angew. Chem. Int. Ed 2013, 52, 8214–8264. [DOI] [PubMed] [Google Scholar]; f) Brown JM, Gouverneur V, Angew. Chem. Int. Ed 2009, 48, 8610–8614. [DOI] [PubMed] [Google Scholar]; g) Furuya T, Kamlet AS, Ritter T, Nature 2011, 473, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Hollingworth C, Gouverneur V, Chem. Commun 2012, 48, 2929–2942. [DOI] [PubMed] [Google Scholar]
- [5].a) Adams DJ, Clark JH, Chem. Soc. Rev 1999, 28, 225–231. [Google Scholar]; b) Swain CG, Rogers RJ, J. Am. Chem. Soc 1975, 97, 799–800. [Google Scholar]
- [6].a) Lee E, Hooker JM, Ritter T, J. Am. Chem. Soc 2012, 134, 17456–17458. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Casitas A, Canta M, Solà M, Costas M, Ribas X, J. Am. Chem. Soc 2011, 133, 19386–19392. [DOI] [PubMed] [Google Scholar]; c) Fier PS, Hartwig JF, J. Am. Chem. Soc 2012, 134, 10795–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee HG, Milner PJ, Buchwald SL, J. Am. Chem. Soc 2014, 136, 3792–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Watson DA, Su M, Teverovskiy G, Zhang Y, Garcia-Fortanet J, Kinzel T, Buchwald SL, Science 2009, 325, 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Maimone TJ, Milner PJ, Kinzel T, Zhang Y, Takase MK, Buchwald SL, J. Am. Chem. Soc 2011, 133, 18106–18109. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Noël T, Maimone TJ, Buchwald SL, Angew. Chem. Int. Ed 2011, 50, 8900–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Ichiishi N, Canty AJ, Yates BF, Sanford MS, Org. Lett 2013, 15, 5134–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang B, Qin L, Neumann KD, Uppaluri S, Cerny RL, DiMagno SG, Org. Lett 2010, 12, 3352–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Tang P, Wang W, Ritter T, J. Am. Chem. Soc 2011, 133, 11482–11484. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fujimoto T, Ritter T, Org. Lett 2015, 17, 544–547. [DOI] [PubMed] [Google Scholar]
- [10].a) Hull KL, Anani WQ, Sanford MS, J. Am. Chem. Soc 2006, 128, 7134–7135. [DOI] [PubMed] [Google Scholar]; b) Wang X, Mei TS, Yu JQ, J. Am. Chem. Soc 2009, 131, 7520–7521. [DOI] [PubMed] [Google Scholar]; c) Chan KSL, Wasa M, Wang X, Yu JQ, Angew. Chem. Int. Ed 2011, 50, 9081–9084. [DOI] [PubMed] [Google Scholar]; d) Lou SJ, Xu DQ, Xu ZY, Angew. Chem. Int. Ed 2014, 53, 10330–10335. [DOI] [PubMed] [Google Scholar]; e) Testa C, Gigot É, Genc S, Decréau R, Roger J, Hierso JC, Angew. Chem. Int. Ed 2016, 55, 5555–5559. [DOI] [PubMed] [Google Scholar]; f) Truong T, Klimovica K, Daugulis O, J. Am. Chem. Soc 2013, 135, 9342–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamamoto K, Li J, Garber JAO, Rolfes JD, Boursalian GB, Borghs JC, Genicot C, Jacq J, Van Gastel M, Neese F, Ritter T, Nature 2018, 554, 511–514. [DOI] [PubMed] [Google Scholar]
- [12].a) Tang P, Furuya T, Ritter T, J. Am. Chem. Soc 2010, 132, 12150–12154. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ye Y, Sanford MS, J. Am. Chem. Soc 2013, 135, 4648–4651. [DOI] [PubMed] [Google Scholar]
- [13].a) Gamache RF, Waldmann C, Murphy JM, Org. Lett 2016, 18, 4522–4525. [DOI] [PubMed] [Google Scholar]; b) Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH, Org. Lett 2016, 18, 5440–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Furuya T, Ritter T, J. Am. Chem. Soc 2008, 130, 10060–10061. [DOI] [PubMed] [Google Scholar]; b) Furuya T, Kaiser HM, Ritter T, Angew. Chem. Int. Ed 2008, 47, 5993–5996. [DOI] [PubMed] [Google Scholar]; c) Mazzotti AR, Campbell MG, Tang P, Murphy JM, Ritter T, J. Am. Chem. Soc 2013, 135, 14012–14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Furuya T, Ritter T, Org. Lett 2009, 11, 2860–2863. [DOI] [PubMed] [Google Scholar]
- [16].Fier PS, Luo J, Hartwig JF, J. Am. Chem. Soc 2013, 135, 2552–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ye Y, Schimler SD, Hanley PS, Sanford MS, J. Am. Chem. Soc 2013, 135, 16292–16295. [DOI] [PubMed] [Google Scholar]
- [18].a) Di Raddo P, Diksic M, Jolly D, J. Chem. Soc. Chem. Commun 1984, 159–160. [Google Scholar]; b) Speranza M, Shiue CY, Wolf AP, Wilbur DS, Angelini G, G. J. Fluorine Chem 1985, 30, 97–107. [Google Scholar]
- [19].Tang P, Ritter T, Tetrahedron 2011, 67, 4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lothian AP, Ramsden CA, Shaw MM, Smith RG, Tetrahedron 2011, 67, 2788–2793. [Google Scholar]
- [21].a) Chen C, Hartwig JF, Science 2014, 343, 853–857. [DOI] [PubMed] [Google Scholar]; b) Cheng C, Hartwig JF, J. Am. Chem. Soc 2014, 136, 12064–12072. [DOI] [PubMed] [Google Scholar]; c) Cheng C, Hartwig JF, J. Am. Chem. Soc 2015, 137, 592–595. [DOI] [PubMed] [Google Scholar]
- [22].Morstein J, Hou H, Cheng C, Hartwig JF, Angew. Chem. Int. Ed 2016, 55, 8054–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morstein J, Kalkman ED, Cheng C, Hartwig JF, Org. Lett 2016, 18, 5244–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Casitas A, Ribas X, Chem. Sci 2013, 4, 2301–2318. [Google Scholar]; b) Hickman AJ, Sanford MS, Nature 2012, 484, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].See Supporting Information for details.
- [26].Karmel C, Chen Z, Hartwig JF, J. Am. Chem. Soc 2019, 141, 7063–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Trita AS, Biafora A, Pichette Drapeau M, Weber P, Gooßen LJ, Angew. Chem. Int. Ed 2018, 57, 14580–14584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.