Abstract
Background:
BRCA genetic testing is recommended by the National Comprehensive Cancer Network (NCCN) in breast cancer patients who meet specific criteria. Limited data are available on the likelihood of detecting a mutation when these guidelines are followed.
Methods:
A retrospective chart review examined patients with breast cancer who underwent BRCA testing based on NCCN guidelines.
Results:
Twelve (6.0%) of the 199 patients had a deleterious BRCA mutation. Family history of BRCA mutations (50%, p = 0.019), age ≤45 at diagnosis (9.7%, p = 0.034) and meeting ≥3 NCCN criteria (13.3%, p = 0.03) yielded the highest rates of BRCA mutation. Having a family history of BRCA mutation and age ≤45 were associated with increased rate of BRCA mutation on multivariate analysis (OR 14.3, CI 1.2–166.3; OR 11.6, CI 1.2–108.6).
Conclusion:
Select NCCN criteria are associated with higher rates of BRCA mutations. Waiting for genetic testing results to guide surgical management may be warranted in this subset of patients.
Keywords: Breast cancer, BRCA, NCCN, Genetic testing, Family history
Summary for table of contents
Following NCCN guidelines for BRCA genetic testing in patients with breast cancer yielded a 6.0% detection rate of BRCA mutation. The criteria which yielded the highest rates of BRCA mutation were: family history of BRCA mutations (50%), age ≤45 at diagnosis (9.7%) and meeting ≥3 NCCN criteria (13.3%). Meeting only one criterion yielded a mutation rate of 2.3%.
Introduction
BRCA1 and BRCA2 are cancer susceptibility genes with strong links to hereditary breast cancer with reported rates in the overall population of approximately 0.07–0.6%1–3 and 2–6% in breast cancer patients.4–6 BRCA mutations increase a patient’s lifetime risk of breast cancer to 41–74%2,7,8 and provide a 11–40% risk of having a second, contralateral breast cancer.7,9 Genetic testing for BRCA is advised by the National Comprehensive Cancer Network (NCCN) in patients who meet one of 7 criteria based on age, sex, breast cancer type, and family history of cancer. Determining whether to recommend testing in individuals with a cancer history requires personalized risk assessment and genetic counseling. If NCCN criteria are met and the patient has undergone appropriate genetic counseling, either targeted BRCA or multi-gene testing can be considered.
NCCN criteria for BRCA1 and BRCA2 testing are based on previous retrospective studies and large meta-analyses. Specific clinical scenarios with a high risk for BRCA mutations have been identified. Breast cancers that do not express estrogen and progesterone receptors and do not overexpress HER2/neu [triple negative breast cancer (TNBC)] have been associated with rates of BRCA mutation between 7 and 28%.10–12 Men who develop breast cancer have a 4–16% rate of BRCA2 mutations.13 Women who develop breast cancer at a young age (≤45 years old) also have a greater likelihood of BRCA mutation.14 Additionally, family history of specific cancers including pancreatic, prostate, and ovarian cancers are also associated with increased rates of BRCA mutation,15–17 as is Ashkenazi Jewish ancestry, with a reported 12.5% rate in those with breast cancer.18 Risk prediction models exist, such as BRCAPRO and BOA-DICEA, which calculate the likelihood of an individual having a BRCA mutation and may guide the decision to undergo testing.19,20 Both models target a 10% risk of mutation as the threshold for testing. Current practice utilizes national guidelines to select patients for BRCA testing rather than requiring patients to meet predictive thresholds based on these models.21
Management of breast cancer may significantly change if a BRCA mutation is present. Detection of a mutation is ideally completed prior to undergoing definitive cancer management. When a patient with breast cancer undergoes BRCA testing, results may take several weeks and delay surgical planning. Delay to therapy in breast cancer has been shown to adversely affect survival outcomes.22,23 Anxiety related to a delay in treatment may also in part explain the well-documented association between genetic testing and choice of contralateral prophylactic mastectomy even when the results are negative.24,25
Recent data suggest that expansion of testing may be appropriate26,27 but currently NCCN guidelines are the most widely used criteria for testing and inform insurance coverage decisions. Limited data is available regarding the likelihood of detecting a BRCA mutation when the NCCN criteria are followed. We hypothesize that certain groups of patients who meet testing guidelines are more likely to have a mutation detected. The present study examines a population of patients with breast cancer at a single institution to determine which indications for BRCA testing correlate with higher rates of BRCA mutations.
Methods
Study population
After obtaining IRB approval, a retrospective, single institution review identified 363 sequential patients who underwent BRCA testing between January 2008 and July 2015. Patients eligible for genetic counseling were identified at a clinic visit or at a multi-disciplinary tumor board. Patients were seen by a genetic counselor and underwent testing based on NCCN guidelines.21 Of these 363 patients with BRCA testing, 199 patients presented with breast cancer prior to testing and comprised our cohort of interest. Results of mutations other than BRCA from panel testing were not examined, as the data were not available for all patients and there is currently a paucity of guidelines regarding surgical management of patients with breast cancer and a non-BRCA mutation. Demographic information, reasons for testing, family history, clinical stage, histological stage, hormone receptor status, and decisions in surgical and medical management were collected.
Definition of testing criteria
Of the 13 indications for BRCA genetic testing outlined in the NCCN guidelines, 7 criteria were identified that pertained to patients with a diagnosis of cancer (Table 1).21 A close relative was defined as any first, second, or third degree relative. Gleason scores for family members with prostate cancer were not available and all reports of close family members with prostate cancer were included. Criteria were first examined individually. Criteria were then separated into three categories of testing: young age (<45 years), family history, and high-risk breast cancer in order to identify whether specific combinations of risk factors were more predictive. Rates for BRCA mutations were gathered by individual testing criteria and type of reason for testing.
Table 1.
BRCA1/2 testing criteria in patients with breast cancer.
| Criterion | Reason for Testing | Category | |
|---|---|---|---|
| 1 | Family history of known deleterious BRCA1/2 gene mutation | Family History | |
| 2 | Diagnosed with breast cancer ≤ 45 years old | Young Age | |
| 3 | Diagnosed with breast cancer ≤ 50 years old and an additional breast cancer primary | High Risk Cancer | |
| 4 | Diagnosed with breast cancer | Family History of one of the following: | Family History |
| ≤50 years old and… | ≥1 close relative with pancreatic cancer | ||
| ≥1 close relative with prostate cancer An unknown or limited family history | |||
| 5 | Diagnosed with breast cancer ≤ 60 years old with TNBC | High Risk Cancer | |
| 6 | Diagnosed with breast cancer at any age and… | Family History of one of the following: | Family History |
| ≥1 close relative with breast cancer diagnosed at age ≤50 | |||
| ≥1 close relative with ovarian carcinoma | |||
| ≥1 close relative with male breast cancer | |||
| Ethnicity with higher mutation frequency (e.g., Ashkenazi Jewish) | |||
| 7 | Personal history of male breast cancer | High Risk Cancer |
Genetic testing and variant classification
Patients underwent genetic testing for hereditary breast and ovarian cancer syndrome through analysis of BRCA1 & BRCA2 alone or panel-based testing that included analysis BRCA1 & BRCA2. Germline DNA was isolated from peripheral blood or buccal swab and analyzed by a commercial laboratory (Myriad Genetic Laboratories or Invitae). Within this study, positive results were defined as having a pathogenic or likely pathogenic variant in BRCA1 or BRCA2. Negative results were defined as having no variant, a likely benign variant, or a variant of uncertain significance (VUS) in BRCA1 & BRCA2.
Statistical analysis
Variables were examined using Stata 15.1 (StataCorp, College Station, Texas). Chi-square and Fisher’s exact test were used to analyze categorical variables. Student t-test and ANOVA were used for continuous variables. Multivariate logistic regression was used to assess confounding variables. Significance was defined using α of 0.05. Variables with p-value ≤ 0.2 on univariate analysis were included in the backward stepwise multivariate logistic regression.
Results
Demographics
Of the 199 breast cancer patients who underwent testing,12 had a deleterious BRCA mutation detected (6%) and 187 did not (94%). Seven (3.5%) had a BRCA VUS detected. Patient demographics are detailed in Table 2. There was no significant difference in age, race, or histologic type. Compared to patients without a BRCA mutation, fewer patients with BRCA mutations had tumors that were ER positive (25% versus 66%, p = 0.003) or PR positive (25% versus 60%, p = 0.01). There was no significant difference in Her2 status between patients with BRCA mutation compared to those without (17% versus 11%, p = 0.144). Patients with TNBC comprised 18% of the entire cohort (36/199), and all but 5 of these were under the age of 60 at diagnosis. Three patients with TNBC had a BRCA mutation (8.3%) and 3 (8.3%) had a BRCA VUS.
Table 2.
Patient demographics.
| Variable | BRCA mutation (n = 12) | No BRCA mutation (n=187) | P value |
|---|---|---|---|
| Age at testing (years) | 0.222 | ||
| <30 | 1 (8.3%) | 3 (1.6%) | |
| 31–40 | 3 (25%) | 29(15.5%) | |
| 41–50 | 2 (16.7%) | 57 (30.5%) | |
| 51–60 | 2 (16.7%) | 54 (28.8%) | |
| >60 | 4 (33.3%) | 44 (23.5%) | |
| Gender (female) | 12(100%) | 186 (99.5%) | 1 |
| Race | 0.357 | ||
| White | 11 (91.2%) | 173 (92.5%) | |
| Hispanic | 0 (0%) | 4(2.1%) | |
| Asian | 0 (0%) | 3 (1.6%) | |
| Black | 1 (8.3%) | 1 (0.5%) | |
| Ashkenazi Jewish | 0 (0%) | 3 (1.6%) | |
| Native American | 0 (0%) | 2 (1.0%) | |
| Unknown | 0 (0%) | 1 (0.5%) | |
| Histology | 0.206 | ||
| DCIS | 2 (16.7%) | 28 (14.9%) | |
| Invasive Ductal Carcinoma | 8 (66.7%) | 135 (72.2%) | |
| Invasive Lobular carcinoma | 0(0%) | 13 (3.9%) | |
| Other | 0 (0%) | 6 (3.2%) | |
| Unknown | 2 (16.7%) | 5 (2.7%) | |
| ER positive | 3 (25.0%) | 124 (66.3%) | 0.003 |
| PR positive | 3 (25.0%) | 114(60.1%) | 0.01 |
| Triple Negative | 3 (25.0%) | 33 (17.6%) | 0.521 |
| Her2 positive | 2 (16.7%) | 20(10.7%) | 0.144 |
| Clinical TNM Stage | 0.155 | ||
| Stage 0 | 1 (8.3%) | 26 (13.9%) | |
| Stage I (A-B) | 2 (16.7%) | 79 (42.2%) | |
| Stage II (A-B) | 4 (33.3%) | 59 (31.5%) | |
| Stage III (A-C) | 0(0%) | 10 (5.3%) | |
| Stage IV | 2 (16.7%) | 2 (10.7%) | |
| Unknown | 2 (16.7%) | 13 (7.0%) | |
| Bilateral breast cancer | 0(0%) | 15 (8.0%) | 1 |
| Recurrent breast cancer | 0 (0%) | 10 (5.4%) | 1 |
| Completed panel testing | 3 (25%) | 67 (35.8%) |
BRCA mutation rates by NCCN testing criterion
Adherence to NCCN guidelines was confirmed in 98.5% of subjects. The most common NCCN guideline criteria for which patients were tested were being diagnosed with breast cancer at any age and having a strong family history (criterion 6, n = 118), being diagnosed with breast cancer at ≤50 years old and having a limited family history (criterion 4, n = 100), and being diagnosed with breast cancer at ≤45 years of age (criterion 2, n = 87). The number of patients meeting each criterion by mutation status is demonstrated in Fig. 1. These criteria were not mutually exclusive. Only one criterion for testing was identified in 84 patients (42%), while 62 (31%) had two criteria, and 45 (22%) had 3 or more.
Fig. 1.
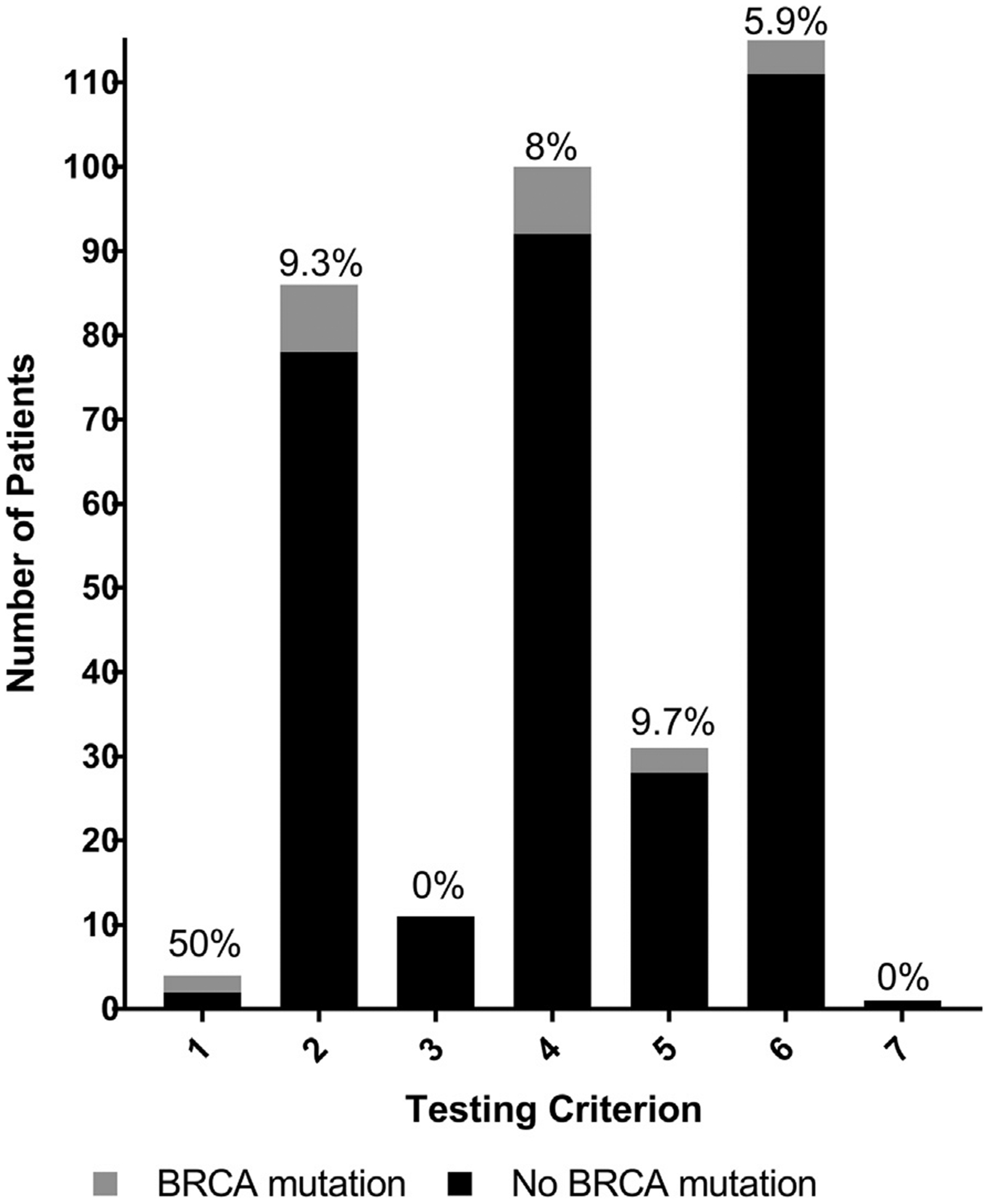
BRCA results by NCCN testing criteria. Rates of BRCA mutation represented by individual NCCN testing criterion. The percentage of patients within each criterion who had a BRCA mutation is represented on the graph; 115 patients met more than one criterion and are included more than once. Number of patients per criterion by mutation status are as follows: 1 - BRCA mutation n = 2, no mutation n = 2; 2 - BRCA mutation n = 8, no mutation n = 78; 3 - BRCA mutation n = 0, no mutation n = 11; 4 - BRCA mutation n = 8, no mutation = 92; 5 - BRCA mutation n = 3, no mutation n = 28; 6 - BRCA mutation n = 7, no mutation n = 111; 7 - BRCA mutation n = 0, no mutation n = 1.
Having a known family history of a BRCA mutation (criterion 1) yielded the highest rate of mutation (50%) and was significantly higher than the rate of mutation in the overall population (p = 0.019).
Criterion 5 (age ≤60 at time of diagnosis and TNBC) and criterion 2 (age ≤45 at time of diagnosis) had BRCA mutation rates of 9.7% and 9.3%, respectively, which were higher than the rate of BRCA mutation in the overall cohort (6%). Most of the patients tested under criteria 2 or 5 also met at least 1 other requirement for testing (78.1% for criterion 2 and 71.0% for criterion 5).
On multivariate analysis, having a known family history of a BRCA mutation (criterion 1) and being diagnosed with breast cancer at age ≤45 (criterion 2) remained statistically significant for the likelihood of detecting a BRCA mutation when compared to other NCCN criteria for undergoing genetic testing (Table 3).
Table 3.
Multivariate analysis by NCCN testing criteria.
| Criterion | BRCA1/2 Testing Criteria Met: | BRCA mutation (n = 12) | No BRCA mutation (n = 187) | Odds Ratio | 95% CIa | p value |
|---|---|---|---|---|---|---|
| 1 | Known family history of BRCA1/2 mutation | 2 (16.7%) | 2(1.1%) | 14.31 | 1.2–166.3 | 0.034 |
| 2 | Age ≤45 at diagnosis | 9 (75%) | 78 (41.7%) | 11.61 | 1.2–108.6 | 0.032 |
| 3 | Age ≤50 and two breast cancer primaries | 0 (0%) | 11 (5.9%) | – | – | – |
| 4 | Age ≤50 with high risk family history | 8 (66.7%) | 92 (49.2%) | 1.12 | 0.22–5.7 | 0.89 |
| 5 | Age ≤60 and TNBC | 3 (25%) | 28 (14.9%) | 1.84 | 0.37–9.2 | 0.458 |
| 6 | Any age with high risk family history | 7 (58.3%) | 111 (59.4%) | 1.25 | 0.28–5.5 | 0.769 |
| 7 | Male breast cancer | 0(0%) | 1 (0.5%) | – | – | – |
CI - Confidence Interval.
Rates of mutation by category of NCCN criteria
Types of reasons for genetic testing were analyzed in the three categories as identified in Table 1 - young age, high risk cancer, and family history. The rate of BRCA mutation was not significantly different among these 3 groups (7.8%, 7.8%, and 6.4% p = 0.877). High risk family history was the reason for testing in 140 patients (70.4%); young age (≤45y) at diagnosis in 128 (64.3%); and high-risk cancer [triple negative breast cancer (TNBC), male breast cancer or 2 primaries] in 50 (25.1%). Those who underwent BRCA testing for young age at diagnosis accounted for 75% of patients in whom a BRCA mutation was detected but only 41.7% of patients who did not have a BRCA mutation (p = 0.034). A similar proportion of patients had family history as a criterion for testing when comparing those who had a BRCA mutation and those who did not (83.3% versus 78.1%). Likewise, high risk cancer was present in a similar percentage of patients with a BRCA mutation versus those without (25% versus 20.9%). Meeting criteria in more than one category did not significantly increase rate of BRCA mutation. Thirteen patients (6.5%) had young age, high risk cancer, and family history all as reasons for testing, but only 1 patient in this subset had a BRCA mutation detected (7.2%) which was not significantly elevated compared to the overall population (p = 0.566).
Regardless of criteria type, meeting three or more individual NCCN criteria for testing was associated with a significantly increased rate of BRCA mutation, as shown in Table 4. 13.3% of patients with ≥3 criteria for testing had a BRCA mutation detected compared to 2.4% of patients with 1 criterion for testing. For patients meeting only 1 requirement for testing, the only criteria that identified a deleterious BRCA mutation were age <45 at diagnosis [criterion 2, BRCA mutation rate 1/19 (5.2%)] and family history of breast cancer at any age of diagnosis [criterion 6, BRCA mutation rate 1/49 (2.0%)].
Table 4.
Risk of mutation with multiple NCCN testing criteria.
| BRCA1/2 Testing Criteria met: | BRCA mutation (n = 12) | No BRCA mutation (n = 187) | p value |
|---|---|---|---|
| Meeting 1 testing criterion | 2 (16.7%) | 82 (43.9%) | 0.076 |
| Meeting 2 testing criteria | 4 (33.3%) | 58 (31.0%) | 1 |
| Meeting ≥3 testing criteria | 6 (50%) | 39 (20.9%) | 0.03 |
| Meeting 0 testing criteria | 0(0%) | 8 (4.2%) | – |
Discussion
We identified a 6% deleterious BRCA mutation rate in breast cancer patients who underwent genetic testing based on NCCN guidelines at our Midwestern academic institution. This rate is equivalent to the previously published rate of 2–6% BRCA mutation in all patients with breast cancer4–6 and less than the 10% targeted by BRCA testing risk models.19,20 Detecting BRCA mutations in patients who have breast cancer is important for pre-operative planning and may affect decisions for chemotherapy in TNBC.28–30 Because of the higher risk of ipsilateral recurrence and contralateral breast cancer in BRCA positive patients, counseling about bilateral mastectomy is appropriate.7 Surgical planning is commonly delayed until the results of BRCA genetic testing is available to avoid the need to return to the operating room for additional procedures. Delaying surgical planning for only the highest yield BRCA mutation testing may prevent delaying treatment for the majority of patients who have undergone genetic testing, thereby reducing the risk of adverse outcomes associated with the delay to breast cancer management. In our cohort, patients with ≥3 NCCN criteria for testing, a known family history of a BRCA mutation or age≤45 at time of diagnosis had the highest yield for identifying a mutation with testing.
A recent large single institution study by Cropper et al.31 examined the positive predictive value (PPV) of the NCCN criteria for BRCA testing. They performed a retrospective chart review of patients with breast cancer who were referred for genetic counseling and found 9.2% of patients had a BRCA mutation in their population. Like us, they showed that ≥2 NCCN criteria for testing improved the PPV from 3.2% to 12% but no specific combination of criteria improved PPV. Unlike our study however, they found age ≤45 as a sole reason for testing to have a low rate of BRCA mutation at 1.7%. We identified a significantly higher rate of BRCA mutation of 5.2% in those with age ≤45 as the sole NCCN criteria and 9.3% in all patients with age ≤45 as a reason for testing.
Both Cropper et al. and our study found a very low yield of deleterious BRCA mutation for patients meeting only one criterion for testing. The rate in this subgroup is equal to or less than that reported from testing all patients with breast cancer.4–6 Studies comparing BRCA mutation rates in women with breast cancer who do and do not meet NCCN guidelines consistently find that the yield of deleterious mutations is similar.26,27 Current concordance with NCCN guidelines for BRCA testing varies between 27 and 70%.32,33 Simplifying testing criteria may reduce disparities noted in genetic testing of patients with breast cancer as well as shorten the delay associated with waiting for genetic results by making it a standard part of breast cancer work up.34,35 This stance is supported by the recent American Society of Breast Surgeons consensus guideline on genetic testing.36 If testing were a standard part of the breast cancer work up, it must occur in the setting of appropriate genetic counseling and informed decision making on the part of the patient. Although not all institutions treating breast cancer have access to genetic counselors, a recent study suggests that most patients feel well informed with physician led counseling and testing.34
Limitations of this study include its retrospective nature and completion at a single institution with its own specific population. The retrospective nature of the study prevents us from identifying any patients who met NCCN criteria but were not tested. Additionally, the small number of BRCA mutations detected limits the power of the study, and the ability of certain NCCN criteria to detect mutations may have been missed. Generalizing findings from single institutions should be applied with caution. A large, prospective, multi-institutional study would be required to confirm the generalizability of our findings.
Conclusion
The rate of detection of deleterious BRCA mutations in patients with breast cancer tested based on NCCN guidelines varied widely depending on the type and number of criteria met. This study improves understanding of the relative probability of a patient having a BRCA mutation when using NCCN guidelines for testing. BRCA detection rates were significantly increased when the reasons for testing were age ≤45 at time of diagnosis, having a known family history of a BRCA mutation, and meeting ≥3 of NCCN criteria for testing. Waiting for genetic testing results to guide surgical management may be warranted in this subset of patients. Conversely, for the majority of patients who undergo genetic testing as a result of NCCN guidelines, results are unlikely to contribute to surgical management and should not delay surgical planning.
Financial disclosures
A.C.B. was supported by the NIH grant T32CA148062.
Footnotes
Conflicts of interest
No authors have conflicts of interest to disclose.
References
- 1.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Canc. 2004;4:665–676. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Canc. 2000;83:1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. J Clin Oncol. 2016;34:4183–4185. [DOI] [PubMed] [Google Scholar]
- 4.Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Facts & Figures. American Cancer Society; 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed February 20, 2019. [Google Scholar]
- 7.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Am Med Assoc. 2017;317:2402–2416. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Broek AJ, van ‘t Veer LJ, Hooning MJ, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol. 2016;34:409–418. [DOI] [PubMed] [Google Scholar]
- 10.Rummel S, Varner E, Shriver CD, Ellsworth RE. Evaluation of BRCA1 mutations in an unselected patient population with triple-negative breast cancer. Breast Canc Res Treat. 2013;137:119–125. [DOI] [PubMed] [Google Scholar]
- 11.Fostira F, Tsitlaidou M, Papadimitriou C, et al. Prevalence of BRCA1 mutations among 403 women with triple-negative breast cancer: implications for genetic screening selection criteria: a Hellenic Cooperative Oncology Group Study. Breast Canc Res Treat. 2012;134:353–362. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch FJ, Farid LM, DeShano ML, et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13:123–125. [DOI] [PubMed] [Google Scholar]
- 14.Young SR, Pilarski RT, Donenberg T, et al. The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Canc. 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran A, O’Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235–242. [DOI] [PubMed] [Google Scholar]
- 18.Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–1247. [DOI] [PubMed] [Google Scholar]
- 19.Euhus DM, Smith KC, Robinson L, et al. Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. J Natl Cancer Inst. 2002;94:844–851. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Canc. 2004;91:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genetic/familial high-risk assessment: breast and ovarian. National comprehensive cancer Network clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf; 2019. Accessed February 20, 2019. [Google Scholar]
- 22.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila). 2010;3:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Amara D, Peled AW, et al. Negative genetic testing does not deter contralateral prophylactic mastectomy in younger patients with greater family histories of breast cancer. Ann Surg Oncol. 2015;22:3338–3345. [DOI] [PubMed] [Google Scholar]
- 26.Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Axilbund JE, O’Leary E, et al. Underdiagnosis of hereditary breast and ovarian cancer in medicare patients: genetic testing criteria miss the mark. Ann Surg Oncol. 2018;25:2925–2931. [DOI] [PubMed] [Google Scholar]
- 28.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. [DOI] [PubMed] [Google Scholar]
- 29.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifton K, Gutierrez-Barrera A, Ma J, et al. Adjuvant versus neoadjuvant chemotherapy in triple-negative breast cancer patients with BRCA mutations. Breast Canc Res Treat. 2018;170:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cropper C, Woodson A, Arun B, et al. Evaluating the NCCN clinical criteria for recommending BRCA1 and BRCA2 genetic testing in patients with breast cancer. J Natl Compr Cancer Netw. 2017;15:797–803. [DOI] [PubMed] [Google Scholar]
- 32.Chun DS, Berse B, Venne VL, et al. BRCA testing within the Department of Veterans Affairs: concordance with clinical practice guidelines. Fam Cancer. 2017;16:41–49. [DOI] [PubMed] [Google Scholar]
- 33.Silver MI, Klein W, Samimi G, Minasian L, Loud J, Roberts MC. Concordance with BRCA1/2 testing guidelines among women in the Health of Women (HOW) Study((R)). Breast Cancer Res Treat; 2018. [DOI] [PubMed] [Google Scholar]
- 34.Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36: 1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White VB, Walsh KK, Foss KS, et al. Genetic testing for hereditary breast cancer: the decision to decline. Am Surg. 2018;84:154–160. [PubMed] [Google Scholar]
- 36.Consensus Guideline on Genetic Testing for Hereditary Breast Cancer. The American Society of Breast Surgeons; 2019. https://www.breastsurgeons.org/about/statements/PDF_Statements/Hereditary_Genetic_Testing_Patients_With_Without_Breast_Cancer.pdf. Accessed February 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


