Abstract
Background
Longer oral processing decreases food intake. This can be attributed to greater oro-sensory exposure (OSE) and a lower eating rate (ER). How these factors contribute to food intake, and the underlying physiological mechanisms, remain unclear.
Objectives
We aimed to determine the independent and simultaneous effects of OSE and ER on satiation and associated endocrine responses.
Methods
Forty participants in study 1 [mean ± SD age: 24 ± 4 y; BMI (in kg/m2): 22 ± 2] and 20 in study 2 (mean ± SD age: 23 ± 3 y; BMI: 23 ± 2) participated in a 2 × 2 randomized trial. In both studies, participants ate chocolate custard with added caramel sauce (low OSE) or caramel fudge (high OSE) and with short (fast ER) or long breaks (slow ER) in between bites, until fullness. In study 2, endocrine responses were measured during the meal.
Results
In study 1, participants ate (mean ± SEM) 42 ± 15 g less in the slow- than in the fast-ER condition, only within the high-OSE condition (P = 0.04). In study 2, participants ate 66 ± 21 g less in the high- than in the low-OSE condition and there were no intake differences between slow and fast ER (P = 0.35). Eight minutes after starting to eat, insulin concentrations increased by 42%–65% in all treatments compared with the control. At the end of the meal, insulin concentrations were 81% higher in the high-OSE, slow-ER than in the low-OSE, fast-ER condition (P = 0.049). Pancreatic polypeptide (PP) increased by 62%, 5 min after meal onset in the low-OSE, fast-ER condition (P = 0.005). Ghrelin concentrations did not change.
Conclusions
Greater OSE increases insulin responsiveness. In contrast, PP responses are stronger when OSE is reduced and ER is fast. Insulin and PP responses may mediate the independent effects of OSE and ER on food intake. These may be beneficial eating strategies, particularly for type 2 diabetic patients, to control food intake and maintain glucose homeostasis.
This trial was registered at trialregister.nl as NL6544.
Keywords: oro-sensory exposure, eating rate, eating behavior, insulin, pancreatic polypeptide, ghrelin, cephalic phase, satiation, human
Introduction
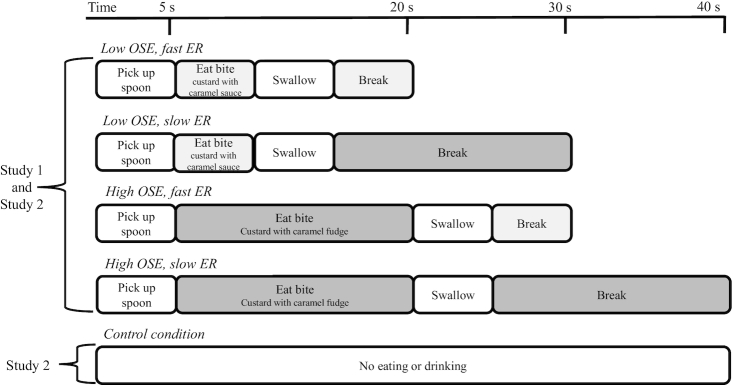
Eating behaviors such as eating rate and oral processing can prevent overconsumption within a meal if they slow down food intake (1). Both these behaviors affect oro-sensory exposure (OSE). OSE can be defined as the in-mouth sensory perception. The duration of the exposure is determined by the time needed to form a ready-to-swallow bolus (Figure 1) (2). The oral processing needed to form a ready-to-swallow bolus takes longer for hard- than for soft-structured foods. Because of this, hard foods induce higher OSE and are eaten at a slower rate than soft-structured foods, which leads to smaller meal sizes of hard than of soft foods (3, 4). This is exemplified by a study by Bolhuis et al. (5) where both lower eating rate (ER) and greater OSE reduced intake of tomato soup, by 22% and 8%, respectively. However, when a food requires mastication the contribution of OSE to the satiation process may exceed that of ER, because mastication may decrease meal size not only through increased OSE but also through increased effort (6, 7).
FIGURE 1.
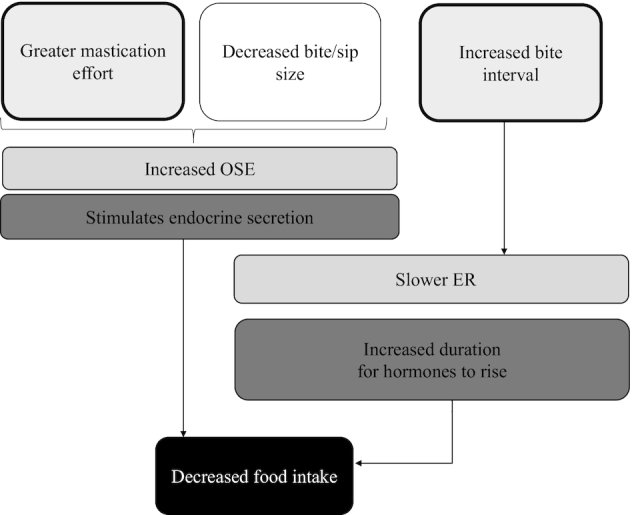
Proposed physiological mechanisms behind the OSE and ER effects on food intake. ER, eating rate; OSE, oro-sensory exposure.
The physiological mechanisms that mediate the effect of OSE and ER on food intake (4, 6, 8–11) are not known. Based on the literature, we hypothesize that OSE may stimulate endocrine responses through vagal afferent signaling (12, 13). In addition, longer OSE duration (slower ER) may result in more time for hormones to respond within a meal (Figure 1) (3, 14). Varying OSE may thus affect endocrine responses within a meal or early predigestive responses (15, 16).
Predigestive responses or cephalic-phase responses have been described for hormones such as insulin, pancreatic polypeptide (PP), and ghrelin. Such endocrine responses can be stimulated by sensory food cues such as smell and taste rather than through nutrient uptake (17–23). For example, insulin responses early after meal onset (5 min) have been described in the literature, without a concomitant rise in blood glucose concentration (24). The assumed function of this early rise in insulin is to maintain glucose homeostasis, although there are limited data underpinning this idea (19, 20). PP is an anorectic peptide inhibiting food intake and the early PP response may therefore be important for food intake control (21, 22, 25). Ghrelin concentrations increase before an expected meal time, signaling hunger, and drop after food consumption (26). Continued sensory stimulation through modified sham feeding decreases ghrelin concentrations within a meal, independently of actual food intake (23).
It has been hypothesized that these endocrine cephalic-phase responses may underlie the control of food intake by OSE (27–29). This is supported by studies by Teff and colleagues (12, 19) and Dhillon et al. (13), which found cephalic endocrine responses to solid foods that required oral processing (greater OSE duration) but not to liquids or soft-structured foods (limited OSE).
However, to our knowledge, cephalic-phase responses and the oro-sensory control of food intake have never been investigated simultaneously. Therefore, the objective of this study was to determine the independent and simultaneous effects of OSE and ER on satiation and associated endocrine responses. We expected that increased OSE and decreased ER would synergistically increase satiation (lower intake). In addition, we expected that increased OSE would lead to a higher initial (cephalic-phase) insulin and PP response and a steeper decrease of ghrelin concentrations, independent of ER.
Methods
Study design
Two studies (NL6544) with a 2 × 2 randomized crossover design with 4 test sessions were performed. In each study condition participants were asked to eat chocolate custard until they felt full. Study conditions were low or high OSE and a slow or fast ER (Figure 2). In study 2, blood samples were collected to determine insulin, PP, and ghrelin concentrations, and an additional control condition was included during which participants did not eat or drink anything. The order in which participants received the conditions was randomly assigned following a Latin square Williams design with 1 wk washout in between visits.
FIGURE 2.
Overview of study conditions. During the treatment sessions, the blocks were repeated for each bite until the participant decided to stop eating. ER, eating rate; OSE, oro-sensory exposure.
Participants
The study was performed at Wageningen University & Research, Netherlands. Healthy male participants between 18 and 35 y old with a BMI (in kg/m2) between 18.5 and 27 were recruited from Wageningen and the surrounding area using flyers and posters. In addition, emails were sent to volunteers in our database who previously had expressed an interest in participating in nutrition studies.
To be eligible, participants had to understand and speak English and commonly eat 3 meals/d at around the same times, weekends not included. Participants were excluded from participation if they reported having a lack of appetite or dental, chewing, or swallowing problems, if they followed an energy-restricted diet, if they had gained or lost >5 kg body weight during the past 2 mo, if they drank >21 glasses of alcohol per week, if they used medication that influenced appetite or taste or smell, if they did intensive exercise for >8 h/wk, or if they were high restrained eaters according to the Dutch Eating Behavior Questionnaire (DEBQ): cut off for men >2.9 (30). Personnel and thesis students of the Division of Human Nutrition were not allowed to participate.
During initial screening, participants were screened based on the inclusion and exclusion criteria and participants rated their liking of the test foods (chocolate custard with and without pieces) in duplicate. Participants were excluded from participation if they disliked 1 of the custards (defined as a score <4 on a 9-point hedonic Likert scale) or if they preferred 1 type of custard (defined as a >2-point difference in liking). During the meeting participants were also asked if they wanted to participate in study 1 or study 2. Participants that showed interest in participating in study 2 could only participate if they did not have a history of low blood pressure, did not recently donate blood (<1 mo before the first study day), and did not anticipate donating blood during the study period.
Potential eligible participants were invited to a training session during which they practised with the computerized ER instructions (see “Experimental manipulations”). In addition, participants were trained in scooping similar amounts of custard onto their spoon for each bite. Subsequently, height and body weight were measured. Participants were excluded if their measured BMI was outside the 18.5–27 range. In addition, participants for study 2 were screened by a nurse. Their antecubital veins were checked for placing an intravenous cannula and some measurements were taken. Participants were included if their hemoglobin was 8–11 mm/L (finger prick), fasting blood glucose >3.5 mmol/L (finger prick), and blood pressure >90/60 mm Hg. Participants eligible to participate in both studies were allocated to study 1 or 2 based on their preference.
The study was approved by the Medical Ethical Committee of Wageningen University, Netherlands (ABR: NL6215708117) and preregistered in the Dutch trial register as NTR6732 (www.trialregister.nl).All participants signed informed consent forms during the information meeting and received financial compensation.
Sample size calculations showed that a minimum of 34 participants was needed to show an effect of 20% between treatments on custard intake (study 1), and 20 participants were needed for the hormone outcomes (study 2). Before conducting the study it was unknown whether eating ad libitum while collecting blood samples would affect the amount eaten, therefore we decided to have both studies powered based on their main outcome. The calculations of study 1 were based on a (mean ± SD) 20% ± 41% expected difference in intake between treatments based on our previous study (6), a correlation of within-person measures of r = 0.5, and a 15% dropout rate. For the sample size calculation of study 2 we assumed a CV of 15% incremental area under the curve (iAUC) for the control condition and 20% iAUC for the experimental conditions for insulin (main outcome). A correlation of within-person measures of r = 0.6 was assumed and a dropout rate of 15% was allowed for (24, 31). For both studies the power was set at 1 − β = 0.80 at a significance level of α = 0.05.
In total, 122 participants joined the information meeting of which 97 potential participants were found eligible; 9 participants were lost to follow-up (Supplemental Figure 1). Finally, 88 participants joined the training session of which 60 participants were eligible for the test sessions. In study 1 (n = 40) participants were (mean ± SD) 24 ± 4 y old, had a BMI of 22 ± 2, and a DEBQ ‘restrained eating’ score of 2.0 ± 0.5 (30). Participants in study 2 (n = 20) were on average 23 ± 3 y old, had a BMI of 23 ± 2, and the DEBQ restrained eating score was 1.9 ± 0.4.
Experimental manipulations
OSE duration was manipulated by having participants eat chocolate custard with caramel sauce (melted caramel fudge, no mastication, low-OSE condition) or with caramel fudge pieces (to induce mastication, high-OSE condition). ER was manipulated by having long or short breaks between bites or spoonfuls (bite interval). Study conditions were single-blinded such that each sample/condition was given a random 3-number code by the researcher. Participants were instructed on a computer when to take a bite. Figure 2 visually represents the study manipulations.
Oral processing time and times between bites in the slow and fast conditions were determined based on the data obtained during the training sessions. During the training participants were asked to record their eating duration (using a stopwatch) for 1 bite eaten at a slow pace, 1 bite eaten at a fast pace, and 1 bite eaten at their normal ER for both chocolate custards. Eating chocolate custard with sauce in a slow manner, it took participants on average 12 ± 5 s to swallow 1 bite, when eating fast 4 ± 2 s, and when eating at their normal eating pace 7 ± 3 s. For the chocolate custard with pieces participants took 20 ± 10 s when instructed to eat in a slow manner, 10 ± 5 s when instructed to eat fast, and 15 ± 9 s when eating at their normal eating pace. Based on the average normal eating speed, we decided on a 10-s eating duration of the chocolate custard with sauce. Taking into account the large SD of the normal ER duration we decided on a 20-s eating duration for the chocolate custard with pieces to give participants sufficient time to chew.
To manipulate ER to the same extent as the OSE duration, the intervals between bites were either short (10-s break) or long (20-s break), including 5 s to pick up the spoon for the next bite.
The amounts of the first 5 bites of the custard meal were standardized. Participants were given preweighed appetizer spoons with 10 g custard that they had to consume in 1 bite.
Standardization of the first 5 bites was important for the endocrine cephalic phase triggered by early OSE and therefore the exposure had to be the same (i.e., no difference in bite size) for all participants. After these 5 standardized spoons, participants were given a bowl with 450 g chocolate custard and were instructed to eat until they were satiated. Participants were encouraged to ask for another portion if desired. In total they could receive 2 additional standard-sized bowls with 150 g after the first large 450-g bowl, i.e., 750 g in total (6239 kJ/1492 kcal). Because it appeared that in study 1 participants stopped eating when they finished their first bowl, we increased the portion size to 1 kg (8318 kJ/1990 kcal) in study 2.
Experimental procedures
Participants were instructed to eat a similar amount of the same meal (of their own choice) in the evening preceding the test sessions. They were instructed to eat their meal between 18:00 and 19:30 and not to eat or drink anything except water starting at 22:00 until their standardized breakfast. Participants received a breakfast yogurt (200 g, strawberry-flavored “Breaker,” Melkunie) that they had to consume at home 4 h before the start of their test meal, then they had to bring the empty package with them to the lab. Participants were instructed not to eat or drink anything but water after their breakfast yogurt. The yogurt package was covertly weighed to determine intake (mean ± SD: 191 ± 18 g). Participants were also instructed to avoid high-intensity exercise (everything besides regular walking and biking) and to use the same mode of transport every time they visited and to abstain from alcoholic drinks for 24 h preceding the test. To assess compliance participants were asked to keep a 24-h food and exercise diary.
Study 1
Participants arrived at the test location 30 min before the test meal to receive instructions and to drink 100 mL water. Before starting the researcher checked the participants’ food and exercise diary for compliance with the study rules (Table 1). After that, participants were seated in isolated sensory booths where they filled in the appetite questionnaires on a computer and ate the test meal according to 1 of the study conditions (Figure 2).
TABLE 1.
Overview of the experimental procedures of studies 1 and 2
| Timing | Study 1 | Study 2 |
|---|---|---|
| Previous day | No intensive exercise or alcoholic drinks | |
| Evening before test day | Same evening meal of own choice, not allowed to eat after 22:00 | |
| −240 min | Standard breakfast, not allowed to eat or drink anything but water | |
| −90 min | — | Arrival at test location using same mode of transport, placement of cannula |
| −30 min | Arrival at test location using same mode of transport, drinking 100 mL water | Drinking 100 mL water |
| −8 min | Appetite questionnaire | Appetite questionnaire |
| — | Start blood sample collection | |
| 0 min | Start meal | Start meal |
| Differs per participant | Finish meal, appetite questionnaire | Finish meal, appetite questionnaire, collection of last blood sample |
| 30 min | Stop meal if not finished eating before this time point | |
| 45 min | Last appetite questionnaire, leave test location, not allowed to eat | |
| 120 min | Allowed to eat | |
In study 2, participants alternated eating with their dominant and nondominant hands because of the intravenous cannula, which was placed in alternating arms to give time for recovery. To have conditions similar in study 1, we randomly assigned then alternated the hand of eating (left/right) over participants and sessions. To make sure participants would not automatically use their dominant hand, the noneating hand was lightly taped to the table.
Study 2
The participants of study 2 arrived 90 min before their test meal (Table 1). Upon their arrival their food and exercise diary and compliance with the study instructions were checked, after which a research nurse placed an intravenous cannula in an antecubital vein. The arm in which the cannula was placed was alternated every session such that there was a 2-wk recovery period (1 session/wk). After this participants waited for 1 h before the first baseline blood sample was collected to eliminate any adrenalin-related stress effects due to the insertion of the cannula (32). Thirty minutes before the start of the test session, participants drank 100 mL tap water. Participants were seated in a quiet room (alone together with the research nurse) and filled out the appetite questionnaire, after which they consumed the chocolate custard (except for in the control condition) until comfortably full, while following the computerized instructions (Figure 2). We wanted to ensure that participants ate until they were full, therefore participants were instructed before, and reminded after, the test session, not to eat anything ≤1 h after the end of the session.
Test product
Chocolate custard (“Chocolade vla,” Friesland Campina) with small 5 × 4 × 2-mm cubic caramel fudge pieces (fudge sprinkles, “Frusco Frisiana” caramel cubes, NiC Nederland BV) or with caramel sauce (same fudge sprinkles but melted) was used.
The composition of the chocolate custard/fudge (sauce or sprinkles) was 80% custard, 10% dairy butter (full-fat dairy butter, “Albert Heijn”), and 10% caramel fudge (by weight). The dairy butter was used to make a caramel sauce of the fudge pieces, and the same amount of dairy butter was melted and scooped through the chocolate custard with caramel fudge pieces to have isocaloric test foods.
Both custards contained 199 kcal/832 kJ, 2.7 g protein, 12.1 g fat, and 19.4 g carbohydrates per 100 g. The chocolate custard was stored overnight in a 4°C fridge and stored in a 6°C fridge close to the test room during the morning of the test sessions. Test meals were taken out of the fridge right before the start of the meal and the temperature was measured right before and after the meal. The increase in temperature pre- to postmeal was (mean ± SEM) 4 ± 2°C.
Supplemental Tables 1 and 2 provide an overview of all sensory ratings of the test foods. In both studies, the 2 study products were equally liked (both Ps > 0.10). Before the meal the mean ± SEM liking was 71 ± 4 mm [on a 100-mm visual analog scale (VAS)], which decreased to 50 ± 5 mm directly after the meal. There were no differences for desire to eat (DTE) the product before or directly after the meal between study products or conditions (all P values > 0.05) (Supplemental Tables 1 and 2).
Study measures
Food intake and eating behavior
In both studies the spoons and bowls were covertly weighed after the session to determine food intake. During eating, participants in both studies were video recorded to determine their eating behavior. A webcam was positioned in front of the participant (face-on) so that the lower frame was in line with the shoulders, the upper frame above the top of the cranium, and the sides at shoulder width. Video recordings were coded and analyzed with the use of Noldus Observer XT 11 (Noldus Information Technology). The behaviors of interest were oral processing duration (s), duration between bites (s), number of chews, and number of bites. From these variables, bite size was calculated by dividing the total amount eaten in grams by the total number of bites. ER was calculated by dividing the meal duration by the number of bites (bites/min) and was also expressed in g/min by dividing the amount eaten by meal duration.
Appetite ratings and well-being
At fixed time points participants rated their appetite, well-being, and the sensory characteristics of the test foods on a 100-mm VAS anchored by “not at all” and “extremely.” The appetite parameters questioned were hunger, fullness, thirst, DTE, DTE sweet, DTE savory, and prospective consumption. Participants rated their appetite after insertion of the cannula, right before the start of the meal (t−8), directly after food anticipation (t−3), at the end of the meal (tvariable), and 45 min after the start of the meal (t45).
The well-being questions asked about comfort, nausea, well-being, and light-headedness (see Supplemental Tables 1 and 2). No relevant differences (>10 mm on the VAS, P < 0.05) in well-being ratings were found between study conditions (33). The ratings of studies 1 and 2 were comparable.
In addition, participants rated liking of the custard after the first and last bites of the meal and DTE the custard after food anticipation (t−3), the first bite (t0), the last bite of the meal (tvariable), and 45 min after the start of the meal (t45). After the meal participants received a small (approximately 20 g) sample cup to rate the sensory characteristics of the custard. Participants were asked to rate the following sensory properties: liking, creaminess, chocolate flavor, caramel flavor, odor intensity, sweetness, chewiness, and smoothness.
After rating the sensory properties, participants were asked to indicate the main reasons why they stopped eating from the following options: “I was full,” “I got bored with the (texture/flavor/sweet taste),” “I got tired of chewing,” “I ate the portion I would normally eat,” “I got tired with the slow eating speed,” “I got tired with the fast eating speed” (Supplemental Tables 3 and 4).
Blood sampling and biochemical analysis
In study 2, blood samples were collected at 8 time points t(min): t−8 and t−6 (baselines), at t−4 participants anticipated eating the custard and this effect was measured at t−2, at t0 participants started to eat, and subsequent blood samples were taken at t2, t5, t8, and t10. The last sample was collected directly after the last bite of the meal.
Blood glucose concentrations were measured directly after sampling with a blood glucose meter (FreeStyle Freedom Lite) using a blood sample collected via the cannula with a syringe.
Insulin, ghrelin, and PP blood samples were collected in 2-mL EDTA-coated tubes. 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride blocker (Pefabloc, Sigma-Aldrich) was added to a final concentration of 1 mg/mL (4 mM) to the tubes intended for ghrelin concentration analysis. In addition, ghrelin plasma samples were acidified to a final concentration of 0.05 N. All samples were placed on ice after which they were centrifuged immediately for 15 min at 2500 × g at 4°C. Plasma samples were stored on dry-ice until all samples were collected, after which they were stored in a −80°C freezer until analysis.
Plasma insulin concentrations were measured using the Mercodia ultrasensitive insulin ELISA kit with a detection limit of ≤0.15 μIU/mL, an intra-assay CV of 5.4%, and an interassay CV of 10.3%.
Plasma PP was analyzed using a Human Pancreatic Polypeptide ELISA kit (Millipore) with a detection range of 12.3–3000 pg/mL, an intra-assay CV of 2.8%, and an interassay CV of 4.9%.
A Human Ghrelin Total ELISA kit (Millipore) was used to analyze total ghrelin concentrations with a detection range of 50–5000 pg/mL, an intra-assay CV of 2.9%, and an interassay CV of 7.1%.
Statistics
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc.). Participant characteristics are presented as means ± SDs. Results are presented as means ± SEMs unless otherwise stated. P values < 0.05 were considered statistically significant.
A mixed-model ANOVA (PROC MIXED) was used to test treatment effects (OSE, ER) on food intake, endocrine concentrations, and appetite and well-being ratings. In this model OSE, ER, and time and their interactions were added as fixed factors, subject as a random factor. Compound symmetry was used as a covariate structure because the variance was homogeneous and correlated consistently.
Normality of the data was checked by visual inspection of the distribution. Insulin, PP, and ghrelin concentrations were not normally distributed and therefore were log10 transformed before analysis. For these variables geometric means and back-transformed 95% CIs are reported. An ANOVA (PROC MIXED) model was used to test changes in endocrines over time. Differences are based on the geometric mean difference and reported with the 95% CI of the original distribution. Study condition was added as a fixed factor together with time and their interaction (condition × time). The repeated statement was used to indicate the repeated measures over time per participant. All outcomes were tested for an order effect. Significant order effects were found for the variables intake and eating behavior characteristics in study 1 and insulin concentrations in study 2. These outcomes were corrected for order by adding order as a covariate in the model. Post hoc Tukey t tests were used to compare means. Correlations between eating behavior characteristics and intake were calculated using Pearson partial correlations (PROC CORR), corrected for participant dependency.
Results
Intake and eating behavior—study 1
As intended, OSE duration was significantly different between the low- (6 ± 0.4 s) and high-OSE (12 ± 0.4 s) conditions (F(1,37) = 690, P < 0.001). Moreover, ER was successfully manipulated; participants ate on average 46 ± 4 g/min during the fast-ER conditions and 34 ± 4 g/min in the slow-ER conditions (P = 0.002). See Supplemental Table 5 for all eating behavior characteristics per study condition.
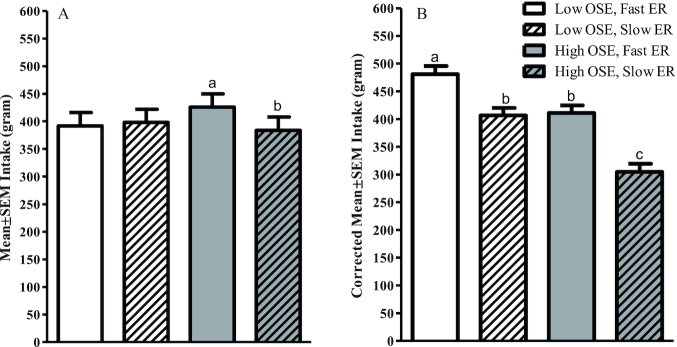
We found a significant interaction between ER and OSE for food intake (F(1,39) = 5.0, P = 0.03) (Figure 3A). Participants ate significantly less (42 ± 15 g) in the slow-ER, high-OSE than in the fast-ER, high-OSE condition (P = 0.04). No significant differences were found between ERs within the low-OSE conditions (P = 0.30).
FIGURE 3.
Food intake per study condition—study 1 (n = 40). Values are means ± SEMs, mixed-model ANOVA within subject. (A) Uncorrected intake, (B) intake corrected for meal duration and bite size by adding these variables as covariates to the model. Bars without a common letter differ significantly (Tukey's post hoc test, P < 0.05). ER, eating rate; OSE, oro-sensory exposure.
Total meal duration was significantly longer for the high- (12 ± 1 min) than for the low-OSE conditions (9 ± 1 min), and for the slow- (12 ± 1 min) than for the fast-ER conditions (10 ± 1 min) (P < 0.001) (Supplemental Table 5). A longer meal duration was associated with higher intake (r = 0.68, P < 0.001). Overall, larger bite sizes were associated with higher intake (r = 0.28, P = 0.001). However, bite size did not differ between conditions (P = 0.96).
When correcting for both meal duration as well as bite size, we observed a main effect of both OSE and ER on intake (F(1,39) = 62.3, P < 0.001; F(1,39) = 80.1, P < 0.001, respectively). Participants ate 86 ± 11 g (19%) less in the low- than in the high-OSE conditions (P < 0.001). In addition, participants ate 90 ± 10 g (20%) less in the slow- than in the fast-ER conditions (P < 0.001) (Figure 3B).
Appetite ratings—study 1
Supplemental Table 1 provides an overview of all appetite ratings. Hunger ratings were 7 ± 3 mm lower in the high-OSE exposures than in the low-OSE conditions (P = 0.008). Fifteen minutes post–meal intake DTE was 7 ± 3 mm lower (P = 0.014), DTE sweet was 4 ± 2 mm lower (P = 0.01), and prospective consumption was 6 ± 2 mm lower (P = 0.005) for the high- than for the low-OSE conditions. There were no differences in fullness ratings directly after the meal or 15 min postmeal. Supplemental Table 3 shows reasons to stop eating and their frequencies. The main reason to stop eating was feeling full; this was mentioned by 50% of the participants.
Intake and eating behavior—study 2
Eating behavior characteristics were similar to those described in study 1; OSE duration was 4 ± 0.7 s per bite for the low- and 12 ± 0.7 s per bite for the high-OSE conditions. ER was 51 ± 2 g/min for the fast- and 35 ± 2 g/min for the slow-ER condition within the low-OSE conditions. We found a significant interaction (P = 0.03): participants consumed 35 ± 2 g/min in the high- and 50 ± 2 g/min in the low-OSE conditions. See Supplemental Table 6 for eating behavior characteristics per study condition.
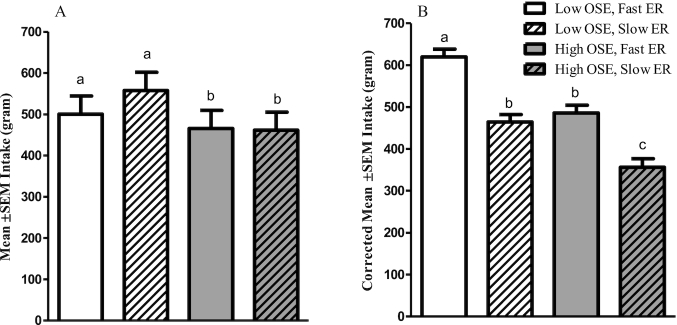
Participants ate 66 ± 21 g (14%) less in the high- than in the low-OSE conditions (P = 0.04). No differences in intake between fast and slow ERs were found (P = 0.6) (Figure 4A). Within the first 10 min of the meal, participants ate the most in the low-OSE, fast-ER condition compared with the other study conditions (Figure 5). Larger bite sizes (r = 0.51, P < 0.001) and longer meal duration (r = 0.63, P < 0.001) positively correlated with intake. Bite size and meal duration were not significantly different across sessions (P = 0.31 and P = 0.08, respectively). However, similar to study 1, when adjusting food intake for meal duration and bite size, there was a significant effect of both factors on food intake (both Ps < 0.001). Correcting for these factors participants consumed 120 ± 18 g (22%) less in the high- than in the low-OSE condition and 143 ± 19 g (26%) less in the slow- than in the fast-ER condition (Figure 4B).
FIGURE 4.
Food intake per study condition—study 2 (n = 20). Values are means ± SEMs, mixed-model ANOVA within subject. (A) Uncorrected intake, (B) intake corrected for meal duration and bite size by adding these variables as covariates to the model. Bars without a common letter differ significantly (Tukey's post hoc test, P < 0.05). Interaction effect ER × OSE, P = 0.03. ER, eating rate; OSE, oro-sensory exposure.
FIGURE 5.
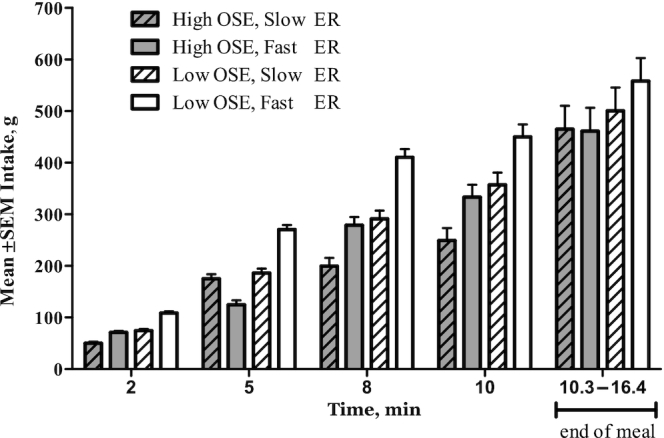
Intake per study condition per time point of study 2 (n = 20). Values are means ± SEMs, mixed-model ANOVA within subject (repeated measures). ER, eating rate; OSE, oro-sensory exposure.
Appetite—study 2
Supplemental Table 2 presents an overview of all appetite ratings. Directly after the meal we found significantly higher (by 5 ± 3 mm) hunger ratings in the low- than in the high-OSE conditions (P = 0.03). Likewise, prospective consumption ratings were 5 ± 3 mm lower for the high- than for the low-OSE conditions (P = 0.014). The main reason to stop eating was feeling full (57%). Supplemental Table 4 shows reasons to stop eating and their frequencies.
Endocrine and glucose responses
Blood glucose concentrations
In all treatment conditions glucose was significantly increased at the end of the meal (Figure 6); the “end of the meal” time point significantly differed from all other time points (Δ range: 0.84–0.92 mmol/L, all Ps < 0.009) and from the control (Δ range: 1.1–1.2 mmol/L, all Ps < 0.04). No differences were observed between treatment conditions.
FIGURE 6.
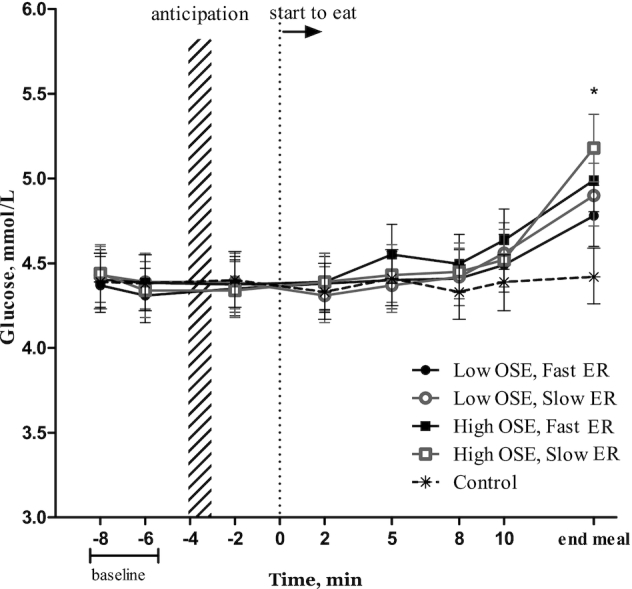
Glucose (mmol/L) per time point for each condition (n = 20). Values are means ± 95% CIs, ANOVA within subject (repeated measures). *“End meal” of each study treatment was significantly different to all other time points and compared with the control condition (P < 0.05, Tukey's post hoc test). ER, eating rate; OSE, oro-sensory exposure.
Plasma insulin concentrations
Eight minutes after starting to eat, insulin concentrations were increased by 1.3–2.0 μIU/mL in all treatment conditions compared with the control (all Ps < 0.001) (Figure 7). At 10 min this difference further increased to 4.1–6.3 μIU/mL above baseline (all Ps < 0.001) and to 12–25 μIU/mL at the end of the meal (all Ps < 0.001). At the end of the meal, the mean insulin concentration of the low-OSE, fast-ER condition was 12.2 μIU/mL (95% CI: 6.0, 13.4 μIU/mL) lower than in the high-OSE, slow-ER condition (P = 0.049).
FIGURE 7.
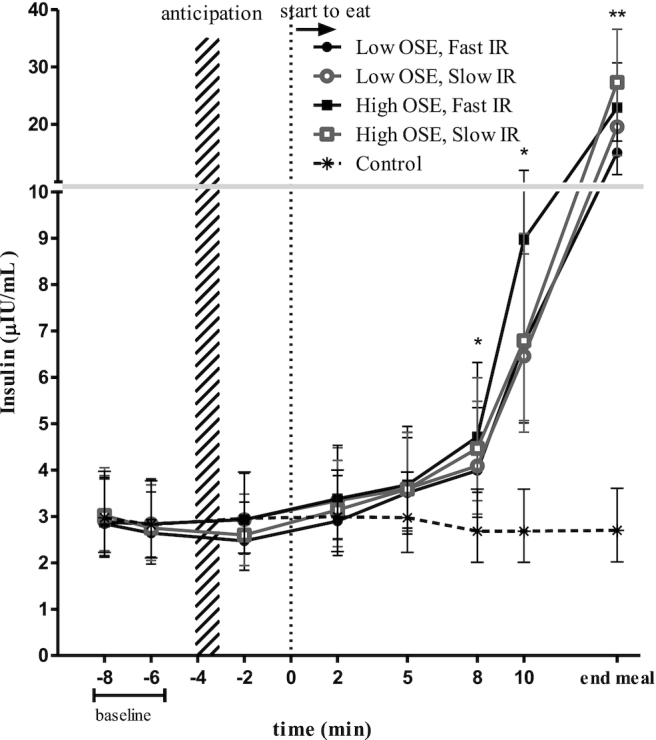
Insulin (μIU/mL) concentrations per time point for each study condition (n = 20). Values are geometric means ± 95% CIs, ANOVA within subject (repeated measures) *Time points 8 min, 10 min, and end of meal significantly differed from the control condition.. **Significant difference between the low-OSE, fast-ER and high-OSE, slow-ER conditions (Tukey's post hoc test, P < 0.05). ER, eating rate; OSE, oro-sensory exposure.
Plasma PP concentrations
Five minutes after starting to eat, PP concentrations were 49.6 pg/mL higher in the low-OSE, fast-ER condition than in the control (P = 0.005) (Figure 8). Eight minutes after the first bite, PP concentrations in all treatment conditions were between 78 and 123 pg/mL higher than in the control (F(4, 67) = 11.7, all Ps < 0.001). After 10 min PP concentrations in the treatment conditions further increased to 121–235 pg/mL and to 192–209 pg/mL at the end of the meal (all significantly different from the control, P < 0.001). Ten minutes after starting to eat, PP concentrations in the low-OSE, fast-ER condition were 114 pg/mL (95% CI: 19, 129 pg/mL) higher than in the high-OSE, slow-ER condition (P = 0.03).
FIGURE 8.
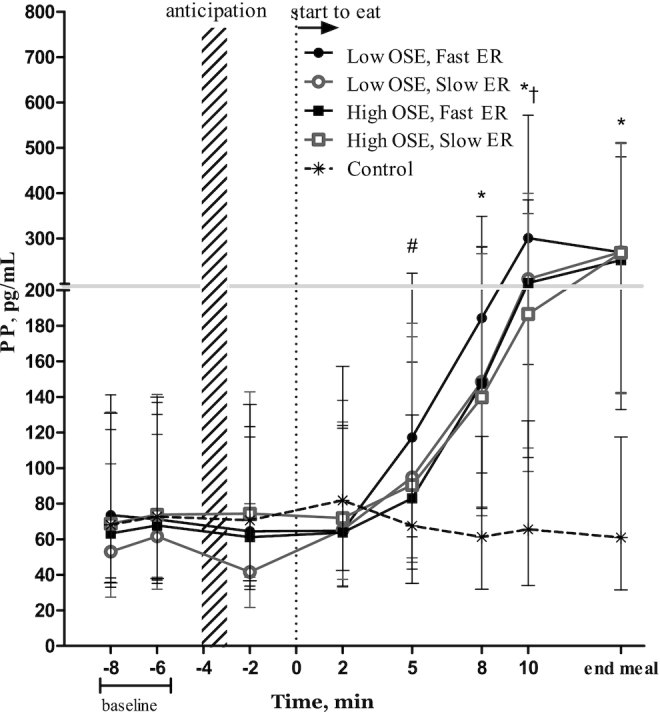
PP concentrations (pg/mL) over time, per condition (n = 20). Values are geometric means ± 95% CIs, ANOVA within subject (repeated measures). #Significant difference between low-OSE, fast-ER condition and control. *PP concentrations in all treatment conditions were significantly higher than in the control. †Significant difference between low-OSE, fast-ER and high-OSE, slow-ER conditions (P = 0.03). ER, eating rate; OSE, oro-sensory exposure; PP, pancreatic polypeptide.
Plasma ghrelin concentrations
No significant differences in ghrelin concentrations were found over time between any of the conditions (F(28,532) = 0.75, P = 0.82) (Figure 9).
FIGURE 9.
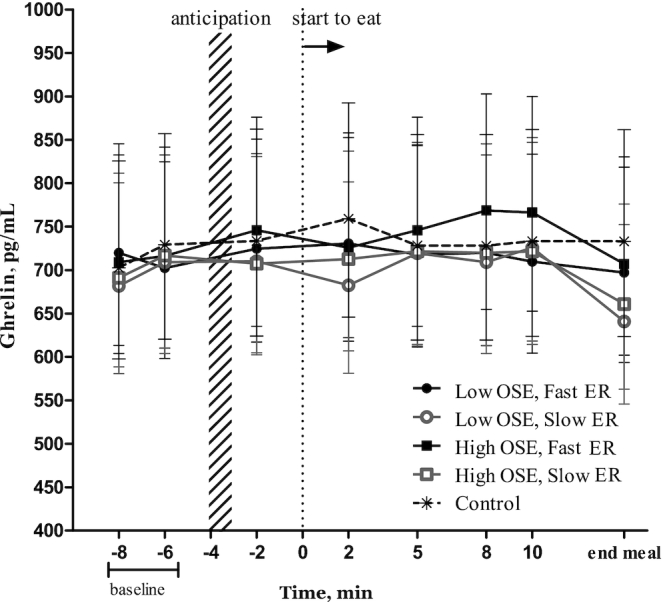
Ghrelin concentrations (pg/mL) over time per study condition (n = 20). Values are geometric means ± 95% CIs, ANOVA within subject (repeated measures). No significant differences over time between study conditions were found. ER, eating rate; OSE, oro-sensory exposure.
Discussion
The results of this study show clear effects of OSE and ER on satiation and endocrine responses. In study 1 we found that a reduced ER in the high-OSE condition led to a 42-g (10%) decrease in food intake. In study 2 greater OSE led to a 66-g (14%) decrease in intake but with no effect of ER on intake.
In both studies, meal duration and bite size significantly affected food intake. When correcting intake for these factors we found that a reduced ER led to a 20% (study 1) and 26% (study 2) decrease in food intake. Moreover, greater OSE independently led to a 19% and 22% decrease in food intake, in studies 1 and 2, respectively. Differences in intake could not be attributed to differences in liking, because the chocolate custard was equally liked and wanted in all 4 conditions, in both studies.
Both studies together suggest that a slower ER and higher OSE decrease food intake, but this may depend on the portion size that is given. When we gave 450-g portions (study 1) participants ate less when ER was reduced, in the high-OSE condition. This is in line with our hypothesis and with findings of previous studies of our laboratory (14, 34, 35). However, when we gave participants a larger portion of 1 kg, they ate less in the high- than in the low-OSE condition, but without an effect of ER on intake. This is similar to findings of Mosca et al. (36) that participants ate significantly less when granola with larger particles was added to yogurt to induce longer OSE. In both studies appetite was rated significantly higher with longer OSE duration, supporting the finding that greater OSE reduces intake. Although differences in appetite ratings were small, they were consistent across the different appetite questions. Based on this we conclude that OSE increases subjective satiation.
We were successful in creating the 4 experimental conditions (high and low OSE and slow and fast ER); our data showed that in both studies participants ate 15 g/min less in the slow-ER condition than in the fast-ER condition, and participants consumed a spoonful of chocolate custard in half the time in the low- compared with the high-OSE condition. In both studies we observed that bite size and meal duration significantly correlated with food intake. This has also been described by Spiegel et al. (37) where ER was notably manipulated through smaller bite sizes and participants increased their meal duration by 4 min. As participants compensated for ER by increasing meal duration, no differences in intake between the slow and fast ERs were observed in this study. When we corrected the intake data in this study for differences in meal duration and bite size, greater OSE and slower ER independently decreased food intake by ∼20% in both studies. This decrease is substantial but is similar to what previous studies that manipulated eating behavior to slow down the eating process and reduce food intake have observed (4, 7, 33). It may be that unconsciously manipulating oral processing and ER through, for example, food texture may not lead to compensatory eating strategies. This would then lead to a larger decrease in food intake than found in this study where participants were aware of the eating manipulation (i.e., instructed when to take a bite).
Next to the changes in eating behavior, we were interested in the underlying endocrine responses. To study these, we used a highly controlled study setting with a relatively large sample size (n = 20) compared with other cephalic-phase response studies (generally ∼15 participants) (24). Our pretesting conditions were strict and participants were highly compliant to enable us to detect small effects as underscored by Teff (24). Early insulin increases of 42%–65% (1.3–1.9 μIU/mL) were found at 8 min after meal onset in all treatment conditions compared with the control. Glucose concentrations did not start to increase until 10 min after the first bite. This suggests that exogeneous glucose did not reach the bloodstream before we measured the increase in insulin. However, whether or not the 8-min increase can be classified as a cephalic-phase response is debatable. Previously, Teff and colleagues (24, 38, 39) defined a cephalic insulin response in normal-weight participants as a 23% increase above baseline, corresponding to a 2-μIU/mL insulin increase within 5 min after food exposure. However, Morey et al. (40) defined it as a 5-μIU/mL increase in insulin from baseline, whereas Lucas et al. (41) defined it as a positive increase greater than twice the SD of spontaneous insulin fluctuations, ranging between 1 and 17 μIU/mL (20). In the current study we found an insulin increase of 42%–65% (1.2–1.9 μIU/mL) above baseline, 8 min after meal onset. This would only qualify as a (late) cephalic-phase insulin response according to the definition of Teff and colleagues (24, 38, 39). Therefore, we consider the insulin increase observed in this study as a response to early glucose absorption rather than as a conditioned insulin response.
At the end of the meal insulin concentrations were 12.2 μIU/mL (81%) higher in the high-OSE, slow-ER than in the low-OSE, fast-ER condition. To determine the response independent of the amount eaten, responsivity was determined. This was done by dividing the increase in insulin concentration (percentage difference from baseline) by the amount eaten. Based on this, we indeed found that insulin was more responsive in the high-OSE than in the low-OSE conditions (Supplemental Tables 7 and 8). This confirms the findings of previous studies (15, 42). For example, a study by Zhu et al. (15) found higher insulin concentrations directly after the meal when participants chewed 40 compared with 15 times per bite. Another study found higher insulin concentrations after consuming soup with chunks (chewing, higher OSE) than after consuming pureed soup (lower OSE) 10 min after meal onset (42). This indicates that for insulin to rise sufficiently (i.e., induce satiation) within a meal, OSE combined with a slow ER are important. However, the higher insulin concentrations at the end of the meal in the high-OSE, slow-ER condition may in part be due to the 6-min longer meal duration than in the low-OSE, fast-ER condition. This resulted in more time for insulin to rise before the end of the meal, which may promote meal termination (43). This is also supported by our data because participants ate a smaller meal (average: 465 ± 44 g) within a longer time frame in the high-OSE, slow-ER than in the low-OSE, fast-ER condition (average: 558 ± 44 g).
PP concentrations were significantly higher in the low-OSE, fast-ER than in the high-OSE conditions. This is not in line with our hypotheses, and also not in line with our aforementioned insulin results. A statistically significant PP increase of 62% was found at 5 min after meal onset in the low-OSE, fast-ER condition, albeit this is smaller than the 100% above baseline previously described as a cephalic PP response (31). In addition, this early steep rise in PP concentration continued and led to a 114-pg/mL (61%) significantly higher PP concentration than in the high-OSE, slow-ER condition, 10 min after meal onset. This finding is contrary to findings of Yeomans et al. (44) where ingestion of a thick and creamy drink (greater OSE) led to a stronger increase in PP concentrations than ingestion of a thin drink. An explanation for the early increase in PP in the low-OSE, fast-ER condition observed in this study might be the endocrine “break” function. This “break” function has been described for insulin when food is infused intragastrically and is thought to prevent rapid food intake before the body is able to process the incoming food (45). This hypothesis is also in line with the function of PP to slow down gastric emptying (46).
Ghrelin concentrations tended to be lower at the end of the meal; however, no significant increases relative to baseline or the control were observed. This contradicts the literature about ghrelin which often describes ghrelin as a cephalic-phase responder (23, 26, 47–49). Based on this and our previous study (50) we suggest that ghrelin responds slowly to food intake and may therefore not be involved in satiation. Summarizing findings of previous studies that do find a cephalic ghrelin response, it seems that ghrelin follows a circadian rhythm and is involved in meal initiation (i.e., can be independent of a food cue) and thus plays a role in satiety and hunger (23, 26, 47–49).
In conclusion, greater OSE and reduced ER can independently decrease food intake. In addition, this study is the first to our knowledge to study early meal insulin and PP responses simultaneously with eating behavior and food intake. The results show that greater OSE increases insulin but not PP responsiveness. In contrast, PP responses are greater when OSE is lower and ER is higher. Early PP responses may therefore have a “break” function to prevent rapid consumption of food. Together, insulin and PP may thus mediate the effects of OSE and ER on satiation. Increasing oral processing duration and slowing down ER may especially be beneficial eating strategies for type 2 diabetic patients to control food intake and maintain glucose homeostasis.
Supplementary Material
Acknowledgments
We thank Henriette Fick, Els Siebelink, Jantien Takens, Diana Emmen-Benink, Carmen Griffioen, and Sarah Bond for their help with conducting the study.
The authors’ responsibilities were as follows—ML, PAMS, and MM: wrote the paper; ML: conducted the research (hands-on conduct of the experiments and data collection); and all authors: designed the research (project conception, development of the overall research plan, and study oversight) and read and approved the final manuscript. PAMS is an Editor on The American Journal of Clinical Nutrition and played no role in the Journal’s evaluation of the manuscript. The authors report no conflicts of interest.
Notes
This work was carried out as part of a public–private partnership funded by Netherlands Organization for Scientific Research (NWO) grant 057-14-001 (to CdG).
Supplemental Figure 1 and Supplemental Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: DEBQ, Dutch Eating Behavior Questionnaire; DTE, desire to eat; ER, eating rate; OSE, oro-sensory exposure; iAUC, incremental area under the curve, PP, pancreatic polypeptide; VAS, visual analog scale.
References
- 1. Hopkins M, Blundell J, Halford J, King N, Finlayson G. The regulation of food intake in humans. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp Pet al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- 2. Jalabert-Malbos M-L, Mishellany-Dutour A, Woda A, Peyron M-A. Particle size distribution in the food bolus after mastication of natural foods. Food Qual Preference. 2007;18(5):803–12. [Google Scholar]
- 3. Robinson E, Almiron-Roig E, Rutters F, de Graaf C, Forde CG, Tudur Smith C, Nolan SJ, Jebb SA. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am J Clin Nutr. 2014;100(1):123–51. [DOI] [PubMed] [Google Scholar]
- 4. Krop EM, Hetherington MM, Nekitsing C, Miquel S, Postelnicu L, Sarkar A. Influence of oral processing on appetite and food intake – a systematic review and meta-analysis. Appetite. 2018;125:253–69. [DOI] [PubMed] [Google Scholar]
- 5. Bolhuis DP, Lakemond CM, de Wijk RA, Luning PA, Graaf C. Both longer oral sensory exposure to and higher intensity of saltiness decrease ad libitum food intake in healthy normal-weight men. J Nutr. 2011;141(12):2242–8. [DOI] [PubMed] [Google Scholar]
- 6. Lasschuijt MP, Mars M, Stieger M, Miquel-Kergoat S, de Graaf C, Smeets P. Comparison of oro-sensory exposure duration and intensity manipulations on satiation. Physiol Behav. 2017;176:76–83. [DOI] [PubMed] [Google Scholar]
- 7. de Wijk RA, Zijlstra N, Mars M, de Graaf C, Prinz JF. The effects of food viscosity on bite size, bite effort and food intake. Physiol Behav. 2008;95(3):527–32. [DOI] [PubMed] [Google Scholar]
- 8. Campbell CL, Wagoner TB, Foegeding EA. Designing foods for satiety: the roles of food structure and oral processing in satiation and satiety. Food Structure. 2017;13:1–12. [Google Scholar]
- 9. Hogenkamp PS, Schiöth HB. Effect of oral processing behaviour on food intake and satiety. Trends Food Sci Technol. 2013;34(1):67–75. [Google Scholar]
- 10. Mattes RD, Considine RV. Oral processing effort, appetite and acute energy intake in lean and obese adults. Physiol Behav. 2013;120:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Graaf C. Why liquid energy results in overconsumption. Proc Nutr Soc. 2011;70(2):162–70. [DOI] [PubMed] [Google Scholar]
- 12. Teff KL. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav. 2010;99(3):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhillon J, Lee JY, Mattes RD. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol Behav. 2017;181:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrade AM, Greene GW, Melanson KJ. Eating slowly led to decreases in energy intake within meals in healthy women. J Am Diet Assoc. 2008;108(7):1186–91. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Y, Hsu WH, Hollis JH. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br J Nutr. 2013;110(2):384–90. [DOI] [PubMed] [Google Scholar]
- 16. Cassady BA, Hollis JH, Fulford AD, Considine RV, Mattes RD. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. 2009;89(3):794–800. [DOI] [PubMed] [Google Scholar]
- 17. Brand JG, Cagan RH, Naim M. Chemical senses in the release of gastric and pancreatic secretions. Annu Rev Nutr. 1982;2:249–76. [DOI] [PubMed] [Google Scholar]
- 18. Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010;68(11):643–55. [DOI] [PubMed] [Google Scholar]
- 19. Teff KL, Devine J, Engelman K. Sweet taste: effect on cephalic phase insulin release in men. Physiol Behav. 1995;57(6):1089–95. [DOI] [PubMed] [Google Scholar]
- 20. Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation?. Appetite. 2008;51(3):622–7. [DOI] [PubMed] [Google Scholar]
- 21. Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88(8):3989–92. [DOI] [PubMed] [Google Scholar]
- 22. Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124(5):1325–36. [DOI] [PubMed] [Google Scholar]
- 23. Simonian HP, Kresge KM, Boden GH, Parkman HP. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil. 2005;17(3):348–54. [DOI] [PubMed] [Google Scholar]
- 24. Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34(2):206–13. [DOI] [PubMed] [Google Scholar]
- 25. Adamska E, Ostrowska L, Gorska M, Kretowski A. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Prz Gastroenterol. 2014;9(2):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287(2):E297–304. [DOI] [PubMed] [Google Scholar]
- 27. Hetherington MM, Boyland E. Short-term effects of chewing gum on snack intake and appetite. Appetite. 2007;48(3):397–401. [DOI] [PubMed] [Google Scholar]
- 28. Lavin JH, French SJ, Ruxton CHS, Read NW. An investigation of the role of oro-sensory stimulation in sugar satiety?. Int J Obes Relat Metab Disord. 2002;26(3):384–8. [DOI] [PubMed] [Google Scholar]
- 29. Zijlstra N, de Wijk RA, Mars M, Stafleu A, de Graaf C. Effect of bite size and oral processing time of a semisolid food on satiation. Am J Clin Nutr. 2009;90(2):269–75. [DOI] [PubMed] [Google Scholar]
- 30. Van Strien T. Nederlandse Vragenlijst voor Eetgedrag (NVE). Handeleiding. [Dutch Eating Behavior Questionnaire. Manual.]. Amsterdam, Netherlands: Boom Test Publishers; 2005; [in Dutch]. [Google Scholar]
- 31. Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50(2–3):194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alvarez MA, Portilla L, Gonzalez R, Ezcurra E. Insulin response to a short stress period. Psychoneuroendocrinology. 1989;14(3):241–4. [DOI] [PubMed] [Google Scholar]
- 33. Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H et al.. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hogenkamp PS, Mars M, Stafleu A, de Graaf C. Intake during repeated exposure to low- and high-energy-dense yogurts by different means of consumption. Am J Clin Nutr. 2010;91(4):841–7. [DOI] [PubMed] [Google Scholar]
- 35. Zijlstra N, Mars M, de Wijk RA, Westerterp-Plantenga MS, de Graaf C. The effect of viscosity on ad libitum food intake. Int J Obes. 2008;32(4):676–83. [DOI] [PubMed] [Google Scholar]
- 36. Mosca AC, Torres AP, Slob E, de Graaf K, McEwan JA, Stieger M. Small food texture modifications can be used to change oral processing behaviour and to control ad libitum food intake. Appetite. 2019;142:104375. [DOI] [PubMed] [Google Scholar]
- 37. Spiegel TA, Kaplan JM, Tomassini A, Stellar E. Bite size, ingestion rate, and meal size in lean and obese women. Appetite. 1993;21(2):131–45. [DOI] [PubMed] [Google Scholar]
- 38. Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav. 2011;103(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teff KL, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism. 1993;42(12):1600–8. [DOI] [PubMed] [Google Scholar]
- 40. Morey S, Shafat A, Clegg ME. Oral versus intubated feeding and the effect on glycaemic and insulinaemic responses, gastric emptying and satiety. Appetite. 2016;96:598–603. [DOI] [PubMed] [Google Scholar]
- 41. Lucas F, Bellisle F, Di Maio A. Spontaneous insulin fluctuations and the preabsorptive insulin response to food ingestion in humans. Physiol Behav. 1987;40(5):631–6. [DOI] [PubMed] [Google Scholar]
- 42. Bolhuis DP, Lakemond CM, de Wijk RA, Luning PA, de Graaf C. Effect of salt intensity in soup on ad libitum intake and on subsequent food choice. Appetite. 2012;58(1):48–55. [DOI] [PubMed] [Google Scholar]
- 43. Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes. 2012;61(4):782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeomans MR, Re R, Wickham M, Lundholm H, Chambers L. Beyond expectations: the physiological basis of sensory enhancement of satiety. Int J Obes. 2016;40(11):1693–8. [DOI] [PubMed] [Google Scholar]
- 45. Spetter MS, Mars M, Viergever MA, de Graaf C, Smeets PAM. Taste matters – effects of bypassing oral stimulation on hormone and appetite responses. Physiol Behav. 2014;137:9–17. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt PT, Näslund E, Gryback P, Jacobsson H, Holst JJ, Hilsted L, Hellström PM. A role for pancreatic polypeptide in the regulation of gastric emptying and short-term metabolic control. J Clin Endocrinol Metab. 2005;90(9):5241–6. [DOI] [PubMed] [Google Scholar]
- 47. Arosio M, Ronchi CL, Beck-Peccoz P, Gebbia C, Giavoli C, Cappiello V, Conte D, Peracchi M. Effects of modified sham feeding on ghrelin levels in healthy human subjects. J Clin Endocrinol Metab. 2004;89(10):5101–4. [DOI] [PubMed] [Google Scholar]
- 48. Monteleone P, Serritella C, Martiadis V, Maj M. Deranged secretion of ghrelin and obestatin in the cephalic phase of vagal stimulation in women with anorexia nervosa. Biol Psychiatry. 2008;64(11):1005–8. [DOI] [PubMed] [Google Scholar]
- 49. Massolt ET, van Haard PM, Rehfeld JF, Posthuma EF, van der Veer E, Schweitzer DH. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul Pept. 2010;161(1–3):81–6. [DOI] [PubMed] [Google Scholar]
- 50. Lasschuijt MP, Mars M, de Graaf C, Smeets PAM. Exacting responses: lack of endocrine cephalic phase responses upon oro-sensory exposure. Front Endocrinol (Lausanne). 2018;9(Jun):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.