Abstract
Background
Vitamin K-dependent proteins in vascular tissue affect vascular stiffness and calcification, which is associated with cardiovascular disease (CVD) and all-cause mortality.
Objective
To determine the association of circulating vitamin K concentrations with CVD and all-cause mortality by conducting a participant-level meta-analysis.
Methods
We obtained individual participant-level data from the Health, Aging, and Body Composition Study, the Multi-Ethnic Study of Atherosclerosis, and the Framingham Offspring Study, known cohorts with available measures of fasting circulating phylloquinone (vitamin K-1) and confirmed CVD events and mortality. Circulating phylloquinone was measured in a central laboratory from fasting blood samples and categorized as ≤0.5 nmol/L, >0.5–1.0 nmol/L, and >1.0 nmol/L. Multivariable Cox proportional hazard regression with multiple imputations was used to evaluate the association of circulating phylloquinone with incident CVD and all-cause mortality risk.
Results
Among 3891 participants (mean age 65 ± 11 y; 55% women; 35% nonwhite), there were 858 incident CVD events and 1209 deaths over a median of 13.0 y. The risk of CVD did not significantly differ according to circulating phylloquinone [fully adjusted HR (95% CI) relative to >1.0 nmol/L: ≤0.5 nmol/L, 1.12 (0.94, 1.33); >0.5–1.0 nmol/L, 1.02 (0.86, 1.20)]. Participants with ≤0.5 nmol/L circulating phylloquinone had an adjusted 19% higher risk of all-cause mortality compared with those with >1.0 nmol/L [fully adjusted HR (95% CI): 1.19 (1.03, 1.38)]. Mortality risk was similar in participants with >0.5–1.0 nmol/L compared with >1.0 nmol/L [fully adjusted HR (95% CI): 1.04 (0.92, 1.17)].
Conclusions
Low circulating phylloquinone concentrations were associated with an increased risk of all-cause mortality, but not of CVD. Additional studies are needed to clarify the mechanism underlying this association and evaluate the impact of increased phylloquinone intake on cardiovascular and other health outcomes in individuals with low vitamin K status.
Keywords: cardiovascular disease, mortality, vitamin K, phylloquinone, meta-analysis
Introduction
A role for vitamin K in cardiovascular disease (CVD) has been proposed because vascular tissue contains vitamin K–dependent proteins such as matrix gla protein (MGP) (1). MGP, when posttranslationally carboxylated via a vitamin K–dependent process, inhibits calcification in vascular and other tissues (2). When vitamin K status is low, less MGP is carboxylated, a scenario linked to more arterial calcification in preclinical models (3). Coronary artery calcification (CAC), an indication of atherosclerotic CVD (4), and peripheral artery calcification, which contributes to arteriosclerosis and arterial stiffening (5), are associated with increased risk of incident clinical CVD and all-cause mortality (6, 7).
In the Multi-Ethnic Study of Atherosclerosis (MESA), participants with low serum phylloquinone, the primary circulating form of vitamin K, had a statistically nonsignificant 34% increased odds of CAC progression compared with those with adequate serum phylloquinone [OR (95% CI): 1.34 (0.94, 1.90)]. However, the association differed according to hypertension status, such that low serum phylloquinone was associated with a 2-fold higher odds of CAC progression in participants treated for hypertension [OR (95% CI): 2.37 (1.38, 4.09)], but was not associated with CAC progression in those not treated for hypertension [OR (95% CI): 0.79 (0.47, 1.30)] (8). This finding was replicated in a post hoc analysis of a randomized controlled trial that tested the effect of phylloquinone supplementation on CAC progression (8, 9). In the Health, Aging, and Body Composition Study (Health ABC), low plasma phylloquinone was associated with a >2-fold higher risk of incident CVD in participants treated for hypertension (10). Although these results might suggest a unique association between circulating vitamin K and CVD in people with hypertension, an alternative explanation is that vitamin K might be more relevant to CVD in individuals with higher baseline cardiovascular risk, because hypertension is a risk factor for CVD.
To better assess the association of circulating phylloquinone with incident CVD and all-cause mortality, we conducted an individual participant-level meta-analysis using data from MESA, Health ABC, and Framingham Offspring, 3 population-based cohort studies in which fasting circulating phylloquinone measures and confirmed CVD events were available. We evaluated the association overall and in a priorispecified subgroups defined according to baseline CVD risk and standard risk factors.
Methods
Participants
We included participants in the MESA (n = 780), Health ABC (n = 1858), and Framingham Offspring (n = 1976) cohorts for whom measures of fasting circulating phylloquinone and triglycerides were available. To our knowledge, these 3 studies are the only cohorts with available measures of fasting circulating phylloquinone, triglycerides, and confirmed CVD events. (Phylloquinone is transported on triglyceride-rich lipoproteins in circulation, so triglycerides are an essential covariate.) In MESA and the Framingham Offspring, participants with circulating phylloquinone measures did not differ from those without such measures with respect to any pertinent demographic or clinical characteristics (11, 12). Health ABC participants with plasma phylloquinone measurements had higher LDL cholesterol, were more likely to be black and/or female, and less likely to smoke compared with those without plasma phylloquinone measurements (10). Warfarin users were excluded because warfarin antagonizes vitamin K–dependent protein carboxylation (n = 63 Health ABC, n = 41 Framingham Offspring). Participants with CVD events prior to vitamin K measurement were also excluded (n = 411 Health ABC, n = 207 Framingham Offspring). All MESA participants were free of clinical CVD at baseline, and serum phylloquinone was measured only in participants not taking warfarin. Although our original MESA analysis used a case-cohort design, with cases selected based on a high rate of CAC progression (8), only MESA participants in the randomly chosen subcohort were included in the current meta-analysis (Supplemental Figure 1). In Health ABC, plasma phylloquinone was measured at the 12-mo follow-up clinic visit (10). In Framingham Offspring, plasma phylloquinone was measured at exam 6/7 (12). Health ABC and Framingham Offspring participants who had a CVD event prior to their circulating phylloquinone measurement were not included. Additional details about participant characteristics, follow-up, and events in each cohort are provided in Supplemental Table 1.
The study was approved by the Tufts University Health Sciences Institutional Review Board. All participants of MESA, Health ABC, and the Framingham Offspring Study gave written informed consent.
Circulating phylloquinone
In all 3 cohorts, circulating phylloquinone was measured from fasting samples using HPLC by the Vitamin K Laboratory at Tufts University, as described (13). The laboratory participates in the Vitamin K External Quality Assurance (KEQAS) program (14). In >15 y of KEQAS participation, the laboratory has consistently generated serum/plasma phylloquinone data within the acceptable range of expected values (analyses occur every 4 mo, for >30 cycles of verification) (14). Low and high control specimens had average values of 0.56 and 3.1 nmol/L, with intra- and interassay CVs of 4.2% and 4.9%, respectively.
Outcomes
Because the proposed mechanism linking vitamin K to CVD involves vascular calcification, which is associated with all-cause mortality, not just CVD (6, 7), we evaluated incident CVD and all-cause mortality as separate outcomes in our main analysis. CVD was defined as first nonfatal coronary heart disease (CHD) event (myocardial infarction, resuscitated cardiac arrest, revascularization, angina), first nonfatal stroke, or fatal CHD or stroke. Confirmation methods of CVD and mortality events in each study have been described (15–18) and are provided in the Supplemental Methods. Because death is a competing risk for incident CVD (19), a composite outcome of incident CVD or all-cause mortality was analyzed as a secondary outcome.
Covariates
In each cohort, demographic characteristics, including age, sex, race/ethnicity, and education were ascertained using questionnaires. For statistical purposes, race was categorized as white or nonwhite, ethnicity was categorized as Hispanic or non-Hispanic, and education as less than high school, high school graduate, or college graduate. Race, ethnicity, and education variables also included a not reported category. BMI was calculated from height and weight measures obtained at clinic visits. Triglycerides, glucose, and C-reactive protein (CRP) were analyzed from fasting blood samples as described (20–23). LDL cholesterol was calculated from total and HDL cholesterol using the Friedewald equation. The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Modification of Diet in Renal Disease Study (MDRD) equation in Health ABC (24) and the Chronic Kidney Disease Epidemiology Collaboration formula in MESA and the Framingham Offspring (25). Estimated GFR was categorized as <60 or ≥60 mL · min−1 · 1.73 m-2. Smoking status (current yes/no) and alcohol intake (drinks per week) were self-reported. In MESA and the Framingham Offspring, medication use was also self-reported. In Health ABC, participants brought all medications taken within the past 2 wk to the clinic visit. Medication use was recorded and coded by using the Iowa Drug Information system. In MESA and the Framingham Offspring, leisure-time physical activity was estimated using self-administered questionnaires (26, 27). In Health ABC and MESA, physical activity was based on self-reported time spent walking per week. In the Framingham Offspring physical activity was based on minutes of moderate activity per week. All 3 cohorts administered FFQs to estimate dietary intakes, as described (28–30). We included energy-adjusted β-carotene intake as an indicator of diet quality as a covariate in the fully adjusted models. Circulating phylloquinone can reflect healthy diets because the primary dietary sources of phylloquinone are green leafy vegetables and vegetable oils (31). β-Carotene intake also reflects healthy diet patterns (32), but the dietary sources of β-carotene are not all the same as the dietary sources of phylloquinone.
In MESA all covariates were measured at the baseline visit. In Health ABC, covariates were measured at the 12-mo clinic visit, except for triglycerides, alcohol intake, and smoking, which were assessed 1 y prior, at baseline. In the Framingham Offspring, covariates were measured at exam 6 or 7, except for physical activity, which was evaluated at exam 8.
Statistical approach
Participants were categorized according to circulating phylloquinone as ≤0.5 nmol/L, >0.5–1.0 nmol/L, and >1.0 nmol/L. These categories are based on the results of controlled metabolic feeding studies demonstrating that circulating phylloquinone is ∼1.0 nmol/L when the recommended Adequate Intakes for vitamin K are met (33, 34). Accordingly, a concentration ≤0.5 nmol/L reflects dietary vitamin K intake that is ∼50% or less of the recommended Adequate Intakes.
HRs and 95% CIs for incident CVD and all-cause mortality were calculated according to circulating phylloquinone category using Cox proportional hazard regression models, in a 1-stage individual participant-level meta-analysis with fixed cohort effects. Censoring occurred at latest follow-up date available for participants without an event. HRs and 95% Wald CIs for incident CVD and all-cause mortality across circulating phylloquinone categories were calculated. We evaluated the associations overall, then verified the consistency of the results by stratifying the models by cohort, sex, baseline CVD risk [based on the Framingham Risk Score (35)], and standard CVD risk factors, including diabetes (defined as being treated with diabetes medication), hypertension (defined as being treated with antihypertensive medication), and hyperlipidemia (defined as being treated for hyperlipidemia or having total cholesterol >200 mg/dL or triglycerides >150 mg/dL). P values for joint tests of the interaction terms were calculated using an SAS macro (SAS v9.4, SAS Institute) to combine chi-square statistics from imputed data sets (36).
Models were adjusted for study (unless stratified) and clinic/site (model 0), then for age, sex, race (white/nonwhite), Hispanic ethnicity, education, BMI, triglycerides, LDL cholesterol, fasting glucose, CRP, systolic blood pressure, eGFR, antihypertensive medication use, diabetes medication use, lipid-lowering medication use, anti-inflammatory medication use (model 1), and additionally for smoking status, alcohol intake, physical activity, and energy-adjusted β-carotene intake (model 2). The proportional hazard assumption was assessed by plotting the log of the negative log of the estimated survival function against time (on log scale) for categorical variables and assessed by examining Schoenfeld residuals for continuous variables. All variables showed no evidence of proportional hazards violation, with the exception of the race variable. To test robustness of the models, a race by time interaction term was included and found to be nonsignificant (P = 0.521) and did not change the reported HRs.
For the main analyses, multiple imputation procedures were used based on the joint normal model and implemented with the SAS MI and MIANALYZE procedures. Missingness patterns were examined and showed evidence to support assumption of an arbitrary pattern of missingness. All covariates in the fully adjusted model were included in the multiple imputation analysis. Among these variables, β-carotene, glucose, triglyceride, and CRP were log-transformed prior to imputation to account for skewness. Auxiliary variables included vitamin K status, age, sex, race, ethnicity, education, and study site. An expectation-maximization algorithm and a Markov chain Monte Carlo procedure were specified and the number of imputations was 30. After imputations, BMI, Framingham Risk Score, hyperlipidemia, and eGFR imputed values were categorized and then used in the Cox regression models. Imputed categorical variables were rounded to the nearest integer. The frequencies of missing data are shown in Supplemental Table 2.
In a secondary analysis, the association of circulating phylloquinone with a composite outcome of incident CVD or all-cause mortality was evaluated using a similar approach. In sensitivity analyses, we conducted complete case analyses (using only participants with complete covariate data) and 2-step meta-analyses using complete cases for the overall model. Heterogeneity in the 2-step meta-analyses was quantified with τ-squared and I-squared measures using the meta package in R version 3.6 (R Foundation). All other analyses used SAS v. 9.4 (SAS Institute). Statistical significance was set at the 2-sided 0.05 α level.
Results
We included 3891 total participants: 779 from MESA, 1384 from Health ABC, and 1728 from Framingham Offspring. Baseline characteristics according to circulating phylloquinone categories are shown in Table 1. Twenty-eight percent had circulating phylloquinone ≤0.5 nmol/L, and 43% had circulating phylloquinone >1.0 nmol/L. Overall, the mean ± SD age was 65 ± 11 y, 55% of participants were female, and 35% were nonwhites. Circulating phylloquinone was significantly positively correlated with triglycerides, LDL cholesterol, systolic blood pressure, hypertension, and β-carotene intake (all P ≤ 0.03) and inversely correlated with CRP (P < 0.001).
TABLE 1.
Combined participant characteristics (Health ABC, MESA, Framingham Offspring), according to circulating phylloquinone at baseline1
| ≤0.5 nmol/L (n = 1081) | >0.5–1.0 nmol/L (n = 1112) | >1.0 nmol/L (n = 1698) | |
|---|---|---|---|
| Age,2 y | 65 ± 11 | 66 ± 11 | 64 ± 10 |
| Female,3n (%) | 631 (58) | 616 (55) | 907 (53) |
| Nonwhite,3n (%) | 431 (40) | 357 (32) | 583 (34) |
| Non-Hispanic ethnicity,2n (%) | 918 (85) | 1007 (91) | 1513 (89) |
| BMI,3 kg/m2, n (%) | |||
| ≥30.0 | 302 (28) | 306 (27) | 509 (30) |
| 25–29.9 | 426 (39) | 441 (40) | 733 (43) |
| <24.9 | 353 (33) | 365 (33) | 452 (27) |
| Framingham Risk Score,3n (%) | |||
| >20% | 385 (36) | 467 (42) | 711 (42) |
| >10–20% | 297 (27) | 279 (25) | 447 (26) |
| ≤10% | 399 (37) | 364 (33) | 536 (32) |
| Hypertension medication use,3n (%) | 369 (34) | 429 (39) | 691 (41) |
| Systolic blood pressure,3 mmHg | 128 ± 20 | 130 ± 20 | 130 ± 20 |
| Diabetes medication use, n (%) | 82 (8) | 90 (8) | 132 (8) |
| Fasting glucose,3 mg/dL | 99 ± 26 | 100 ± 24 | 102 ± 26 |
| LDL cholesterol,2 mg/dL | 119 ± 32 | 123 ± 34 | 124 ± 34 |
| Triglycerides,2 mg/dL | 110 ± 49 | 122 ± 60 | 157 ± 97 |
| Hyperlipidemia,3,4n (%) | 508 (50) | 609 (58) | 1008 (65) |
| Anti-inflammatory medication use,2n (%) | 292 (27) | 322 (29) | 390 (23) |
| CRP,2 mg/dL | 5.6 ± 12.5 | 4.6 ± 7.9 | 4.2 ± 7.2 |
| eGFR <60 mL · min−1 · 1.73 m-2, n (%) | 387 (36) | 440 (40) | 625 (37) |
| Cohort,2n (%) | |||
| Health ABC | 387 (36) | 477 (43) | 520 (30) |
| MESA | 254 (23) | 144 (13) | 381 (22) |
| Framingham Offspring | 440 (41) | 491 (44) | 797 (47) |
| Current smoker,2n (%) | 190 (18) | 110 (10) | 178 (11) |
| Alcohol intake,2 drinks/wk, n (%) | |||
| >7 | 153 (14) | 186 (17) | 334 (20) |
| 1–7 | 292 (27) | 312 (28) | 499 (30) |
| <1 | 630 (59) | 606 (55) | 852 (50) |
| β-Carotene intake,2 µg/d | 3798 ± 2882 | 4044 ± 3152 | 4325 ± 3081 |
| Phylloquinone intake,2 µg/d | 114 ± 110 | 120 ± 93 | 135 ± 112 |
| Energy intake, kcal/d | 1828 ± 727 | 1841 ± 755 | 1776 ± 707 |
| Physical activity, min/wk | 241 ± 355 | 219 ± 328 | 244 ± 351 |
Data are presented as n (%) or mean ± SD. CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; Health ABC, Health, Aging, and Body Composition Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Overall difference across groups P ≤ 0.001, based on 1-factor ANOVA for continuous variables or chi-square test for categorical variables.
Overall difference across groups P < 0.05 (but >0.001), based on 1-factor ANOVA for continuous variables or chi-square test for categorical variables.
Defined as being treated for hyperlipidemia or having total cholesterol >200 mg/dL or triglycerides >150 mg/dL.
During a median follow-up of 13.0 y (12.1 y in MESA, 11.5 y in Health ABC, 15.1 y in the Framingham Offspring) from the time of phylloquinone measurement, there were 858 incident CVD events, 1209 deaths, and 1570 composite outcome events (incident CVD or all-cause mortality).
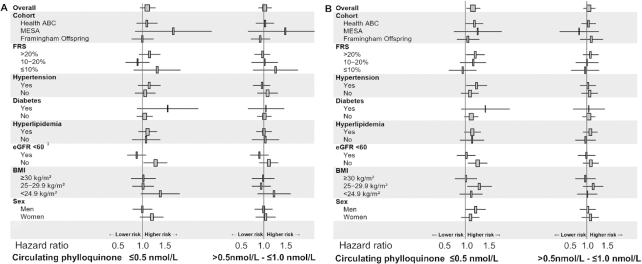
Participants with ≤0.5 nmol/L circulating phylloquinone had an adjusted 12% higher risk of incident CVD compared with those with >1.0 nmol/L, but this difference was not statistically significant [fully adjusted HR (95% CI): 1.12 (0.94, 1.33)]. The risk of CVD was similar in those with >0.5–1.0 nmol/L compared with those with >1.0 nmol/L [fully adjusted HR (95% CI): 1.02 (0.86, 1.20)]. The association of low circulating phylloquinone with incident CVD differed between those with low kidney function (eGFR <60 mL · min−1 · 1.73 m-2) and those with normal kidney function (eGFR ≥60 mL · min−1 · 1.73 m-2) (interaction P = 0.01), but no other significant interactions were detected (all interaction terms P > 0.09) (Figure 1, Supplemental Table 3).
FIGURE 1.
HR (95% CI) for incident CVD (A) and all-cause mortality (B) in persons with ≤0.5 nmol/L (n = 1081) and with >0.5–1.0 nmol/L (n = 1112) circulating phylloquinone, compared with those with >1.0 nmol/L (n = 1698), overall and according to baseline risk factors Values were obtained using Cox proportional hazard regression with multiple imputations adjusted for study, clinic/site, age, sex, race, Hispanic ethnicity, education, BMI category, triglycerides, LDL cholesterol, fasting glucose, C-reactive protein, systolic blood pressure, eGFR (<60 or ≥60 mL · min−1 · 1.73 m-2), antihypertensive medication use, diabetes medication use, lipid-lowering medication use, anti-inflammatory medication use, smoking status, alcohol intake, physical activity, and energy-adjusted β-carotene intake. Rectangles represent the HRs and horizontal lines represent the corresponding 95% CIs. 1Interaction P value = 0.01; all other interaction P values >0.09. CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; Health ABC, Health, Aging, and Body Composition Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Participants with ≤0.5 nmol/L circulating phylloquinone had an adjusted 19% higher risk of all-cause mortality compared with those with >1.0 nmol/L [fully adjusted HR (95% CI): 1.19 (1.03, 1.38)]. Mortality risk was similar in participants with >0.5–1.0 nmol/L compared with >1.0 nmol/L [fully adjusted HR (95% CI): 1.04 (0.92, 1.17)]. The association between circulating phylloquinone and all-cause mortality did not significantly differ according to any baseline risk factors tested (all interaction terms P ≥ 0.10 in fully adjusted models) (Figure 1, Supplemental Table 4).
In our secondary analysis, participants with circulating phylloquinone concentrations ≤0.5 nmol/L had an 18% higher risk for the composite outcome (incident CVD or all-cause mortality) compared with those with concentrations >1.0 nmol/L [fully adjusted HR (95% CI): 1.18 (1.04, 1.34)] (Supplemental Table 5). The risk of the composite outcome was similar in participants with >0.5–1.0 nmol/L and >1.0 nmol/L [fully adjusted HR (95% CI): 1.04 (0.92, 1.17)].
In our complete case sensitivity analyses, participants with circulating phylloquinone ≤0.5 nmol/L had an adjusted 15% higher risk of incident CVD compared with those with >1.0 nmol/L, but the difference was also not statistically significant [HR = 1.15 (0.95, 1.40)]. The adjusted risk did not differ between those with >0.5–1.0 nmol/L and those with >1.0 nmol/L [HR = 0.99 (0.83, 1.19)]. The adjusted risk of all-cause mortality was 28% higher in participants with ≤0.5 nmol/L phylloquinone, compared with those with 1.0 nmol/L [fully adjusted HR: 1.28 (1.08, 1.51)], but the adjusted all-cause mortality risk did not differ between those with >0.5–1.0 nmol/L and those with >1.0 nmol/L [fully adjusted HR: 1.05 (0.89, 1.23)].
When analyzed using a 2-step approach, the HR for incident CVD in participants with ≤0.5 nmol/L circulating phylloquinone, compared with those with >1.0 nmol/L, was 1.14 (0.92, 1.40), whereas among those with >0.5–1.0 nmol/L circulating phylloquinone, the HR for CVD was 1.01 (0.79, 1.30) in adjusted models. The HR for all-cause mortality in participants with ≤0.5 nmol/L circulating phylloquinone, compared with those with >1.0 nmol/L, was 1.30 (1.09, 1.54), whereas among those with >0.5–1.0 nmol/L circulating phylloquinone, the HR for all-cause mortality was 1.05 (0.87, 1.30). Heterogeneity was nonsignificant for both outcomes (I2 ≤ 33%; P ≥ 0.22) (Supplemental Figures 2 and 3).
Discussion
In a participant-level meta-analysis of adults without clinically apparent CVD from 3 well-defined, community-based, multiethnic cohorts, the risk of incident CVD did not significantly differ according to circulating phylloquinone status. However, individuals with circulating phylloquinone concentrations ≤0.5 nmol/L had a 19% higher risk of all-cause mortality, compared with those with >1.0 nmol/L, independent of known risk factors and potential confounders. The hypothesis that vitamin K status is associated with CVD and all-cause mortality is biologically based on the presence of vitamin K–dependent proteins in vascular tissue that inhibit calcification. CAC is indicative of subclinical atherosclerotic CVD (4) and is positively associated with all-cause mortality risk (6, 7). However, arterial calcification is not confined to coronary arteries and systemic arterial calcification is associated with all-cause mortality as well. In the Rotterdam cohort, a 1 SD increase in calcification in the aortic arch, in the extracranial and intracranial carotid arteries, and in the coronary arteries, was associated with a 29–49% higher risk of total mortality (7). These findings were consistent with the associations of coronary and noncoronary artery calcification with total mortality in a clinic-based US cohort (6). The all-cause mortality outcome may better capture the wide spectrum of events associated with worse systemic vascular health, and could explain why we found low circulating phylloquinone to be associated with a higher risk of all-cause mortality but not of incident CVD.
Motivated by prior observations that low circulating phylloquinone was associated with a higher odds of CAC progression (8) and risk of CVD (10) in persons taking antihypertensive medications, we explored the association of circulating phylloquinone with incident CVD and all-cause mortality in subgroups defined according to baseline CVD risk and risk factors. In contrast to our previous study (10), the association of circulating phylloquinone with incident CVD did not differ based on hypertension status in this meta-analysis. However, we detected an interaction between circulating phylloquinone and kidney function such that circulating phylloquinone was significantly associated with incident CVD in participants with normal kidney function, but not in participants with low kidney function. Because hypertension is a main risk factor for impaired kidney function, this finding is also inconsistent with what we found previously (10). In evaluating 8 participant characteristic subgroups across 3 circulating phylloquinone categories at the 0.05 α level, ≥1 significant P value could be due to chance alone. The results of subgroup analyses should be cautiously interpreted until substantiated in future studies.
Several observational studies have evaluated the association between vitamin K intake and CVD events and mortality, with equivocal results. Most studies did not find a significant association between phylloquinone intake and incident CVD (37, 38). Higher intakes of menaquinones (vitamin K-2) have been associated with a lower risk for CHD in several studies (37, 39), but not with stroke or mortality (40). Dietary menaquinones are vitamin K forms found in meat, dairy, and fermented foods, whereas phylloquinone is found primarily in green leafy vegetables and vegetable oils. Although both vitamin K forms are in the food supply, phylloquinone is the primary form in circulation (41). Although there is an enthusiasm among some for supplemental menaquinones in reducing CVD risk based on the current evidence in humans (42), the relative importance of menaquinones compared with phylloquinone to cardiovascular health and other health outcomes is unknown. Filling this gap will require randomized controlled trials designed to compare the effects of the different vitamin K forms on CVD and non-CVD outcomes.
Although circulating phylloquinone is considered a global indicator of vitamin K status, other biomarkers exist. For example, circulating dephosphorylated uncarboxylated MGP [(dp)ucMGP] decreases in response to vitamin K supplementation (43) and is thought to be a functional indicator of vitamin K status in vascular tissue when corrected for the total amount of MGP in circulation (44). Higher plasma (dp)ucMGP (indicative of lower vitamin K status) has been associated with a higher risk of CVD and mortality in some (45, 46), but not all (47, 48), studies. We did not find any association between plasma (dp)ucMGP and incident CVD in Health ABC (10). Higher (dp)ucMGP concentrations also reflect higher circulating total MGP (measured regardless of carboxylation status) (49), and MGP synthesis is regulated by factors other than vitamin K, including calcium (50). Therefore, studies linking vitamin K status to CVD based only on circulating (dp)ucMGP are prone to confounding unless the total MGP is accounted for. Unfortunately (dp)ucMGP measures are not available in MESA or the Framingham Offspring, so we were unable to include them in our meta-analysis.
To the best of our knowledge, this is the largest study of circulating phylloquinone and clinical events, which is a notable strength. In each cohort, circulating phylloquinone was measured using the same assay by the same laboratory, which minimized the interlaboratory variation with respect to the exposure of interest. All samples were obtained in a fasted state, and we also adjusted our statistical models for triglycerides. This is important because circulating phylloquinone is carried on triglyceride-rich lipoproteins and varies postprandially. In a substudy of the Dutch Prospect cohort, postmenopausal women with higher plasma phylloquinone were more likely to have prevalent CAC (51). However, the plasma samples from which phylloquinone was measured were not obtained in a fasted state and triglyceride measures were unavailable. Because it was not possible to minimize the confounding by triglycerides or postprandial elevations in circulating phylloquinone, we did not include this cohort in our meta-analysis.
The following limitations merit consideration. Even though we combined participant-level data from 3 separate cohorts, our available sample size was small compared with other participant-level meta-analyses of nutritional status (52). In all 3 cohorts, circulating phylloquinone was measured from a single blood draw. Repeated measures over time would be more reflective of long-term status and reduce misclassification bias, but were not available. Higher circulating phylloquinone concentrations can reflect healthier diets and lifestyles. We adjusted for β-carotene intake—which also reflects healthy diets (32)—and physical activity, but acknowledge that residual confounding might exist. There were fewer incident CVD events, compared with total deaths, which might have also reduced our ability to detect significant differences in CVD risk across circulating phylloquinone categories. Our statistical power to evaluate CVD subtypes and cause-specific mortality was also limited. Because the majority of participants were white, generalizability to other racial or ethnic groups is uncertain. Our results are also not applicable to patients taking warfarin because warfarin users were excluded. Given the observational design, causation cannot be inferred.
In conclusion, our results suggest that individuals with <0.5 nmol/L circulating phylloquinone are at higher risk for all-cause mortality. Additional studies are needed to clarify the mechanism underlying the association of circulating phylloquinone with all-cause mortality and evaluate the impact of increased phylloquinone intake on cardiovascular and other health outcomes in individuals with low vitamin K status.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MKS, SLB, DEW: responsible for study conception and design; MKS: obtained funding and provided oversight; KB: conducted the data analysis; GM: contributed to the data analysis; MKS, KB: drafted the manuscript; SLB, EJB, MC, SBK, DEW: provided critical feedback on manuscript revisions; and all authors: read, provided critical revisions on, and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the National Heart, Lung, and Blood Institute (R21HL133421) and the USDA ARS Cooperative Agreement (58-1950-7-707). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Health ABC was supported by the Intramural Research Program of the NIH, National Institute on Aging, and contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; National Institute on Aging (R01-AG028050, P30-AG21332); the National Institute of Nursing Research (R01-NR012459); the National Institute of Arthritis Musculoskeletal and Skin Diseases (R21AR062284); and the Arthritis Foundation. MESA was supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169), the National Center for Advancing Translational Sciences (grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420), and the American Heart Association (09CRP2070013). The Framingham Offspring Study was supported in part by the National Heart, Lung, and Blood Institute's Framingham Heart Study Physical Examination, Testing, and Surveillance (HHSN26818HV00006R) and the National Institute of Aging (R01AG14759); EJB is supported by R01HL128914; 2R01 HL092577; 1R01 HL141434 01A1; and 2U54HL120163. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data described in the article and the code book will not be made publicly available. Further information including the procedures to obtain and access data can be found: for the Multi-ethnic Study of Atherosclerosis at https://www.mesa-nhlbi.org; for the Health, Aging, and Body Composition Study at https://healthabc.nia.nih.gov/; for the Framingham Offspring at https://www.framinghamheartstudy.org/. The analytic code will be available to qualified researchers upon request.
Supplemental Methods, Supplemental Tables 1–5, and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CAC, coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease; CRP, C-reactive protein; (dp)ucMGP, dephosphorylated uncarboxylated matrix gla protein; eGFR, estimated glomerular filtration rate; Health ABC, Health, Aging, and Body Composition Study; KEQAS, Vitamin K External Quality Assurance program; MESA, Multi-Ethnic Study of Atherosclerosis; MGP, matrix gla protein.
References
- 1. Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost. 2004;2(12):2118–32. [DOI] [PubMed] [Google Scholar]
- 2. Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;5(12):2503–11. [DOI] [PubMed] [Google Scholar]
- 3. Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–31. [DOI] [PubMed] [Google Scholar]
- 4. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35(23):1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–6. [DOI] [PubMed] [Google Scholar]
- 7. Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, Vernooij MW, Ikram MA. Comparison of atherosclerotic calcification in major vessel beds on the risk of all-cause and cause-specific mortality: the Rotterdam Study. Circ Cardiovasc Imaging. 2015;8(12):e003843. [DOI] [PubMed] [Google Scholar]
- 8. Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98(1):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89(6):1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, Simonsick EM, Wassel CL, Vermeer C, Kritchevsky SB. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the Health, Aging, and Body Composition Study (Health ABC). J Nutr. 2017;147(5):888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shea MK, Cushman M, Booth SL, Burke GL, Chen H, Kritchevsky SB. Associations between vitamin K status and haemostatic and inflammatory biomarkers in community-dwelling adults. The Multi-Ethnic Study of Atherosclerosis. Thromb Haemost. 2014;112(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89(10):4904–9. [DOI] [PubMed] [Google Scholar]
- 13. Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 14. Card DJ, Shearer MJ, Schurgers LJ, Harrington DJ. The external quality assurance of phylloquinone (vitamin K(1)) analysis in human serum. Biomed Chromatogr. 2009;23(12):1276–82. [DOI] [PubMed] [Google Scholar]
- 15. Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, Newman AB, Visser M, Kritchevsky SB; Health ABCS . Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis. 2011;21(6):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kritchevsky SB, Tooze JA, Neiberg RH, Schwartz GG, Hausman DB, Johnson MA, Bauer DC, Cauley JA, Shea MK, Cawthon PM et al.. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the Health ABC Study. J Clin Endocrinol Metab. 2012;97(11):4156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Seaghdha CM, Tin A, Yang Q, Katz R, Liu Y, Harris T, Astor B, Coresh J, Fox CS, Kao WH et al.. Association of a cystatin C gene variant with cystatin C levels, CKD, and risk of incident cardiovascular disease and mortality. Am J Kidney Dis. 2014;63(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter CE, Katz R, Kramer H, de Boer IH, Kestenbaum BR, Peralta CA, Siscovick D, Sarnak MJ, Levey AS, Inker LA et al.. Influence of urine creatinine concentrations on the relation of albumin-creatinine ratio with cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2013;62(4):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puddu PE, Amaduzzi PL, Ricci B. Coronary heart disease incidence and competing risks: an important issue. J Geriatr Cardiol. 2017;14(7):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB et al.. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–22. [DOI] [PubMed] [Google Scholar]
- 21. Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF Jr, Wang TJ et al.. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol. 2013;33(7):1728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313–20. [DOI] [PubMed] [Google Scholar]
- 23. Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2010;33(4):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T et al.. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K et al.. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 27. Dannenberg AL, Keller JB, Wilson PW, Castelli WP. Leisure time physical activity in the Framingham Offspring Study. Description, seasonal variation, and risk factor correlates. Am J Epidemiol. 1989;129(1):76–88. [DOI] [PubMed] [Google Scholar]
- 28. Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR Jr.. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83(6):1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 30. Anderson AL, Harris TB, Tylavsky FA, Perry SE, Houston DK, Lee JS, Kanaya AM, Sahyoun NR. Dietary patterns, insulin sensitivity and inflammation in older adults. Eur J Clin Nutr. 2012;66(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 2002;132(6):1329–34. [DOI] [PubMed] [Google Scholar]
- 32. Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA Jr, Bogle ML, Tucker KL. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. J Am Diet Assoc. 2008;108(12):2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133(8):2565–9. [DOI] [PubMed] [Google Scholar]
- 34. Institute of Medicine Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press;2001. [PubMed] [Google Scholar]
- 35. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 36. Schafer JL. Analysis of incomplete multivariate data. London, UK: Chapman and Hall;1997. [Google Scholar]
- 37. Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer I, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134(11):3100–5. [DOI] [PubMed] [Google Scholar]
- 38. Erkkila AT, Booth SL. Vitamin K intake and atherosclerosis. Curr Opin Lipidol. 2008;19(1):39–42. [DOI] [PubMed] [Google Scholar]
- 39. Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19(7):504–10. [DOI] [PubMed] [Google Scholar]
- 40. Zwakenberg SR, den Braver NR, Engelen AIP, Feskens EJM, Vermeer C, Boer JMA, Verschuren WMM, van der Schouw YT, Beulens JWJ. Vitamin K intake and all-cause and cause specific mortality. Clin Nutr. 2017;36(5):1294–300. [DOI] [PubMed] [Google Scholar]
- 41. Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;963:128–33. [DOI] [PubMed] [Google Scholar]
- 42. Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. 2019;20(4):E896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shea MK, O'Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141(8):1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104(4):811–22. [DOI] [PubMed] [Google Scholar]
- 45. Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36(11):3766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen HG, Sheng LT, Zhang YB, Cao AL, Lai YW, Kunutsor SK, Jiang L, Pan A. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58(6):2191–205. [DOI] [PubMed] [Google Scholar]
- 47. Zwakenberg SR, van der Schouw YT, Vermeer C, Pasterkamp G, den Ruijter HM, Beulens JWJ. Matrix Gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology. 2018;141(1):32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Circulating desphospho-uncarboxylated matrix gamma-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost. 2014;12(7):1028–34. [DOI] [PubMed] [Google Scholar]
- 49. Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8(1):E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109(6):697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dalmeijer GW, van der Schouw YT, Booth SL, de Jong PA, Beulens JW. Phylloquinone concentrations and the risk of vascular calcification in healthy women. Arterioscler Thromb Vasc Biol. 2014;34(7):1587–90. [DOI] [PubMed] [Google Scholar]
- 52. Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, Streppel M, Gardiner J, Ordonez-Mena JM, Perna L et al.. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.