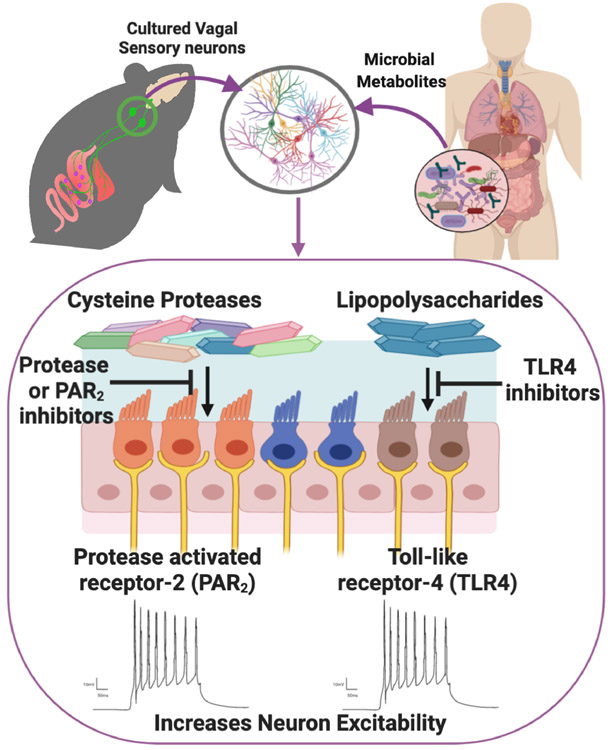
Our gastrointestinal tract contains more than 1014 commensal bacteria exhibiting a considerable diversity, with as many as 1000 distinct bacterial species. The commensal bacteria that reside in the gut have emerged as key regulators of host physiology and behavior (Cryan et al., 2019), yet we understand little of the mode of communication that regulates microbiota-gut-brain signaling. Understanding the mechanisms underlying microbiota-brain communication is important to fully comprehend its role in health and disease. In this issue of Journal of Physiology, Pradhananga et al. (2020) identify separate cellular mechanisms by which different bacterial metabolites excite sensory vagal neurons (Figure 1). This finding provides direct evidence that bacterial metabolites can affect vagal signaling and thus starts to shed new light on how bacteria could influence essential aspects of host physiology and behavior.
Figure 1.
Schematic representation of mechanism of vagus nerve excitation by bacterial metabolites.
80-90% of axons in the vagus nerve convey afferent information to the CNSand can detect mechanical, nutrient, gut metabolites and immune factors. Anatomical studies have demonstrated that vagal sensory afferent neurons with cell bodies in the nodose ganglia innervate the whole length of the gut and are involved in controlling satiety, gut homeostasis, and inflammatory reflexes. There is growing evidence that microbiota can signal through the vagus nerve to modulate physiology and behavior. Ingestion of the probiotic bacteria Lactobacillus rhamnosus (JB1) caused extensive neurochemical changes in the brain and reduced anxiety behavior, both of which are abolished by vagotomy (Bravo et al, 2011). Furthermore, recording from the mesenteric bundle innervating the jejunum increased in response to JB1 probiotic bacteria and this was abolished by vagotomy (Perez-Burgos et al., 2013). Chronic intraperitoneal administration of a bacterial byproduct inhibited vagally-mediated satiety (de La Serre et al. 2015). These studies indicate a role for the vagus nerve in mediating microbiota signaling to the brain, however, whether this occurs through direct or indirect mechanisms is unclear. Pradhananga et al. report that supernatant from MET-1, a defined population of 33 different commensal bacteria cultured from a healthy human, directly increased NG neuron excitability in culture by causing a hyperpolarizing shift in the voltage dependence.
Pathogenic (Wang et al., 2002) and non-pathogenic (Perez-Burgos et al., 2013) bacteria activate different brain nuclei and have opposing effects on behavior. All these effects are abolished by vagotomy (Wang et al., 2002; Bravo et al. 2011). Furthermore exposure of an ex vivo section of the distal colon to peptidoglycan, (main cell wall component of gram-positive bacteria) but not lipopolysaccharide (LPS, main cell wall component of gram-negative bacteria), evoked vagal nerve firing (Buckley & O’Malley, 2018). These previous studies alluded to the idea that vagal sensory neurons can distinguish between bacterial signals and convey contrasting information to the brain. Pradhananga et al. expand on these previous findings by demonstrating that pathogenic LPS and MET-1 supernatant both excite vagal sensory neurons through different intracellular signaling mechanisms. MET-1 supernatant increased the excitability of sensory NG neurons by acting on protease-activated receptor-2 (PAR-2), while LPS excites vagal sensory neurons through a TLR-4 mediated NF-κB intracellular signaling pathway (Figure 1). Interestingly, despite an abundance of gram-negative bacteria, MET-1 supernatant induced excitability of vagal sensory neurons was not inhibited by TLR-4 antagonist. Although, the reason for the absence of TLR-4 signaling by MET-1 supernatant was not assessed by Pradhananga et al., it is plausible that pathogenic and commensal bacteria produce different quantities or variants of LPS with different virulence depending on the environment niche (Maldonado et al., 2016).
Collectively, Pradhananga et al identify novel intracellular pathways by which gut microbiota influences the excitability of vagal afferent neurons, which leads to numerous interesting questions. What other bacterial products are capable of activating vagal sensory neurons? Does the same neuronal population convey different information in response to separate stimuli, or do distinct vagal populations respond to separate stimuli? Furthermore, do the same neurons express PAR-2 and TLR-4, and which organs do these neurons innervate? Are circulating bacterial metabolites in vivo sensed at the level of the nodose ganglia or by gut vagal terminals? Do non-neuronal cell populations in the nodose ganglia also respond to bacterial products to subsequently alter neuronal signaling? How important are the colonic bacteria in vagal signaling to the brain? Sparse vagal innervation undermines the signaling ability of abundant bacterial products produced in the colon. Does the abundance of metabolites result in too much “noise” for individual signals to be conveyed to the brain or do vagal neurons only sense above a certain threshold? Thus, more studies are warranted to elucidate the extent of bacterial metabolites, the range of vagal afferent neurons and intracellular pathways that are recruited as well as the physiological and behavioral consequences of activating these pathways.
References
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G & Dinan TG. (2019). The microbiota-gut-brain axis. Physiol Rev 99,1877–2013. [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J & Kunze W. (2013). Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 304,G211–220. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, de Lartigue G & Raybould HE. (2015). Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav 139,188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ & Ju G. (2002). Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol 8, 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J & Cryan JF. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MM & O’Malley D. (2018). Development of an ex Vivo Method for Multi-unit Recording of Microbiota-Colonic-Neural Signaling in Real Time. Front Neurosci.12, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RF, Sá-Correia I & Valvano MA. (2016). Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev.40, 480–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]