Abstract
Background/Objective:
Lingering morbidities including physical, cognitive, emotional, and psychosocial sequelae, termed the Post-Intensive Care Syndrome, persist years after pediatric neurocritical care (PNCC) hospitalization. Sleep disturbances impact other Post-Intensive Care Syndrome domains and are under-evaluated to date due to a lack of appropriate measurement tools. The present study evaluated the validity of the Sleep Disturbance Scale for Children (SDSC) to address the growing need for assessing sleep problems after PNCC.
Methods:
We conducted a prospective observational study of youth aged 3–17 years with acquired brain injury (N = 69) receiving care through longitudinal PNCC programs at two tertiary academic-medical centers. Parents completed the SDSC and provided proxy reports of internalizing symptoms, health-related quality of life (HRQOL), fatigue, pain behavior, and cognitive function within 3 months of hospital discharge. Evidence for the validity of the SDSC was established by utilizing the full sample for psychosocial measure comparisons and by comparing SDSC outcomes by severity (Low Risk, Mild-Moderate Risk, and High Risk defined by reported standardized T-scores).
Results:
Internal consistency of the SDSC was good (α = .81). Within the full sample, increased sleep disturbances on the SDSC were significantly correlated with Post-Intensive Care Syndrome measures, including worse physical (r = .65), psychological (r = .62), and cognitive (r = −.74) sequelae. Youth in the High Risk group evidenced greater dysfunction in mental acuity, pain behavior, internalizing symptoms, and social engagement. Findings revealed both statistically and clinically significant impacts of sleep disturbances as measured by the SDSC on HRQOL.
Conclusions:
The SDSC is a valid and reliable measure for assessing sleep disturbances in children after PNCC. Results support the use of the SDSC to measure sleep disturbances after PNCC. Targeted interventions for sleep disturbances may be key to overall patient recovery.
Keywords: Critical care, sleep, quality of life, hospitalization, pediatric, outcomes, brain injury
INTRODUCTION
Over 50,000 children are admitted to the pediatric intensive care unit for a primary neurologic diagnosis each year.1 Beyond the billions of dollars generated in hospital expenditures, children requiring pediatric neurocritical care (PNCC) experience high rates of hospital death and poor functional outcomes at discharge.1, 2 Advancement in PNCC have significantly reduced mortality,3 yet, lingering morbidities often require ongoing assessment and intervention. Physical, cognitive, emotional, and psychosocial sequelae, termed the Post-Intensive Care Syndrome, are present months to years after hospitalization.4 Among these, pervasive sleep problems are reported by more than half of children and may compound other Post-Intensive Care Syndrome morbidities, leading to impaired quality of life.5, 6
During the acute recovery period after injury, sleep is essential to the healing process and can reduce secondary injury caused by inflammation.7 However, given the number of arousal systems routinely disrupted in PNCC patients,8 it is not surprising that children display disturbances in their sleep-wake regulation even after hospital discharge. Injuries to the sleep-arousal system often interfere with onset latency, maintenance, and wakefulness,8–10 making it difficult for children with brain injury to obtain the recommended quantity and quality of sleep. Research indicates prolonged sleep disturbances beyond the acute recovery period further complicates recovery and worsens psychosocial outcomes (Figure 1).11
Figure 1.
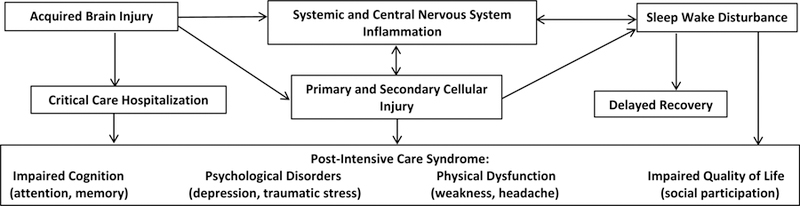
Proposed relationship between acquired brain injury, inflammation, sleep wake disorders, and Post-Intensive Care Syndrome.
Adapted from Williams, C. N., Lim, M. M., & Shea, S. A. (2018). Sleep disturbance after pediatric traumatic brain injury: critical knowledge gaps remain for the critically injured. Nature and Science of Sleep, 10, 225.
High rates of sleep disturbances spanning a range of ages and injury severity during recovery have been described;8, 9, 11, 12 however, the incidence, risk factors, and severity of specific sleep disorders after discharge remains unknown in PNCC patients. A notable challenge compounding this gap is the lack of validated measures of sleep behavior sensitive to the nuances of an acute PNCC population. Taken together, the limited evidence base creates a barrier to adequately assessing and treating SWD in these vulnerable children. Validation of subjective assessments with the ability to capture changes in sleep patterns post-injury may spark routine screening and therapeutic interventions for children in a critical recovery phase. Advancements in PNCC outcomes signal the need to identify problematic sleep disturbances early to understand how poor sleep may be impacting recovery, functioning, and adherence to treatment recommendations.
Numerous sleep-related questionnaires have been developed in response to the growing trend of more frequent assessment of children’s sleep behavior (see 13 for review). Despite these advancements, very few measures have undergone a rigorous validation and evaluation process and even fewer can also be used across the span of child development while maintaining reliability, internal consistency, and an invariant factor structure.13 Among these, the Sleep Disturbance Scale for Children (SDSC)14 has emerged as the most widely studied.
The SDSC, a 26-item parent-report measure, provides a multidimensional assessment of children’s sleep behavior. Internal consistency, validity, and reliability of the SDSC has been described in a range of pediatric populations. Capable of distinguishing between clinical and control groups, the SDSC has demonstrated good diagnostic accuracy.14 To date, the SDSC has been utilized in several clinical samples across an assortment of pediatric neurological conditions and demonstrated evidence of discriminant validity.12, 15–17 When compared to controls, elevated SDSC total scores have been documented among children in the acute recovery phase following traumatic brain injury (TBI).12 Similarly, children with pineal cyst endorsed higher SDSC scores than the control groups. Although these findings lend preliminary support to the sensitivity of the SDSC, a revised factor structure has been proposed based on a sample of children with heterogeneous neurological conditions.16 Further, the utility of the SDSC has not been documented in children presenting with higher acuity and more severe brain injury, such as those receiving PNCC. Therefore, it remains to be explored whether the SDSC performs similarly within a PNCC sample in which there is an increased likelihood for greater disruptions to the sleep-arousal system.8, 9
We sought to conduct a preliminary validation of the SDSC in a clinical sample of youth presenting to a PNCC clinic for an initial follow-up appointment post-discharge from the hospital. Our first aim was to explore the psychometric properties of the SDSC in an acute PNCC sample. Second, we sought to evaluate the validity of the SDSC using a battery of well-established psychosocial measures. In addressing this aim, we hypothesized a valid measure of sleep disturbance would show significant relationships with Post-Intensive Care Syndrome morbidities including physical, cognitive, and psychosocial sequelae. Our final aim was to provide preliminary guidelines for clinical interpretation of the SDSC in a sample of PNCC patients in an acute recovery phase.
Methods
Procedure
We conducted a prospective observational study of consecutive children receiving care through longitudinal PNCC programs at Oregon Health & Science University and St. Louis Children’s Hospital, two metropolitan tertiary academic-medical centers, between January 2018 and March 2019. Prior studies have described referral patterns and follow-up rates within each program.5, 18 Diagnoses were grouped as TBI, infectious and inflammatory (e.g., meningitis, encephalitis, demyelinating), hypoxic ischemic injury (e.g., cardiac arrest, extracorporeal life support), refractory status epilepticus, stroke (e.g., hemorrhagic, ischemic), and other (e.g., hemolytic uremic syndrome, carbon monoxide, severe sepsis, hippocampal necrosis after polypharmacy ingestion). Participants were (1) between the ages of 3 and 17 years, (2) admitted to the Pediatric Intensive Care Unit (PICU) for ≥ 24 hours with a neurologic injury or illness, and (3) attended a follow-up appointment with the PNCC team within 3 months of hospital discharge. Study procedures were approved by the Institutional Review Board at both institutions with a waiver of informed consent. Double data entry for all measures was used to ensure data quality during collection for this study.
Participants
Participants included 69 youth ages 3–17 years (Mage = 9.70, SD = 4.03) and their parent or caregiver. Primary diagnosis upon PICU presentation was TBI/trauma (57%) with motor vehicle traffic (and related) accidents as the most prevalent mechanism of injury (59%). Average length of time since injury/illness was 53.7 days (SD = 22.7). See Table 1 for complete demographic information.
Table 1.
Descriptive statistics of study sample.
| Demographic Variables (N = 69) | M (SD) or n (%) |
|---|---|
| Child age (years) at injury | 9.7 (4.0) |
| Male Gender | 31 (45) |
| Insurance Type | |
| Private/Commercial | 40 (58.0) |
| Medicaid | 27 (39.1) |
| Race | |
| White/Caucasian | 52 (75.4) |
| African American | 6 (8.7) |
| Asian American | 2 (2.9) |
| American Indian | 1 (1.4) |
| Multiracial | 5 (7.2) |
| Not Reported | 3 (4.3) |
| Ethnicity | |
| Non-Hispanic or Latino | 60 (87) |
| Hispanic or Latino | 8 (11.6) |
| Not Reported | 1 (1.4) |
| Admission Diagnosis | |
| Trauma/Traumatic Brain Injury | 39 (56.5) |
| Stroke | 9 (13.0) |
| Infection/Inflammation | 8 (11.6) |
| Hypoxic Ischemic | 5 (7.2) |
| Other | 5 (7.2) |
| Status Epilepticus | 3 (4.3) |
| Critical Care Interventions | |
| Intubation | 32 (50.8) |
| Arterial Line | 23 (36.5) |
| Central Venous Line | 22 (34.9) |
| Hemodynamic Intervention | 18 (28.6) |
| Neurosurgical Intervention | 17 (27) |
| Non-invasive Ventilation | 8 (12.7) |
| External Ventricular Drain | 5 (7.9) |
| Bolt | 3 (4.8) |
| Functional Status Score | |
| Baseline* | 6.0 (.15) |
| Discharge* | 7.2 (3.3) |
| Clinic | 6.2 (.8) |
| Pediatric Index of Mortality-2 | −3.5 (.90) |
| Length of Hospitalization (Days) | 15.5 (21) |
| PICU Length of Stay (Days) | 9.3 (13.8) |
| Pre-admission Chronic Condition | 20 (31.7) |
| SDSC Total Scoret | 54.5 (12.3) |
| Disorders of Initiating and Maintaining Sleep | 61.4 (16.0) |
| Sleep Breathing Disorders | 49.5 (8.8) |
| Disorders of Arousal | 50.0 (7.8) |
| Sleep-Wake Transition Disorders | 50.4 (10.0) |
| Disorders of Excessive Somnolence | 50.9 (9.6) |
| Sleep Hyperhydrosis | 49.0 (7.3) |
Note:
Functional status score at baseline and discharge collected in OHSU sample only
SDSC values reported as T-scores.
Measures
Demographic information and clinical data were extracted from the electronic medical record. Information collected included age, sex, socioeconomic factors, mechanisms of injury or disease, imaging findings, and initial Glasgow Coma Scale in TBI patients. We recorded pre-injury comorbidities at admission, which varied with admission diagnosis and included epilepsy, attention deficit disorder, asthma, congenital heart disease, etc. We dichotomized comorbidities for analysis due to the large number of separate conditions, consistent with prior studies.2, 6 Pediatric Index of Mortality-2 score and need for critical care interventions measured severity of illness. Functional Status Scale score assigned in clinic visits measured functional outcomes.19
The Sleep Disturbance Scale for Children (SDSC)14 is a 26-item standardized assessment of child and adolescent sleep behaviors. Parents reported about their child’s sleep behavior across six domains of sleep disturbances (Disorders of Initiating and Maintaining Sleep, Sleep Breathing Disorders, Disorders of Arousal, Sleep-Wake Transition Disorders, Disorders of Excessive Somnolence, and Sleep Hyperhydrosis) using a 5-point Likert scale with anchors “Never” to “Always (Daily).” Item responses were summed to calculate each factor score and converted to T-scores for ease of interpretation, with higher scores indicating more sleep disturbance. The SDSC has been shown to differentiate across six groups of sleep disorders in children and adolescents.13, 14, 20 However, the utility of the SDSC has not yet been evaluated in an acute PNCC population.
The Epworth Sleepiness Scale for Children and Adolescents (ESS-CHAD)21 is an 8-item measure of daytime sleepiness. Parents were asked to report on their child’s likelihood of failing asleep in a range of scenarios using a 4-point Likert scale. Raw scores were summed to create a total score, with higher scores indicating greater daytime sleepiness. Preliminary investigations into the validation of the ESS-CHAD lend support to a unidimensional factor structure with strong test-retest reliability and acceptable internal consistency, but have not been evaluated in the PNCC population.22
The Pediatric Quality of Life Inventory (PedsQL)23 4.0 Generic Core Scales is a parent-report measure of health-related quality of life (HRQOL) in youth ages 2 to 18 years. The measure provides four subscale scores: Physical, Emotional, Social, and School Functioning, as well as a Psychosocial Health Summary Score and Total Score. Consisting of a 5-point Likert scale, response options range from “Never a problem” to “Almost always a problem.” Responses are reverse scored to a “0” to “100” scale and the subscale scores are derived by summing items and dividing by the number of items answered with higher scores signifying better HRQOL. The PedsQL has been widely used across general populations, disease-specific groups, and in sleep research 24–27 and internal consistency statistics are consistently in or above the acceptable range for research use (α > .80).28 Internal consistencies for the present study are provided in Table 2.
Table 2.
Descriptive statistics and correlation matrix for SDSC and psychosocial scales.
| M (SD) | α | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | SDSCt | 54.54 (12.28) | .81 | -- | ||||||||
| 2. | ESS-CHAD | 4.17 (3.55) | .69 | .24 | -- | |||||||
| 3. | Fatigue | 74.75 (16.57) | .92 | −.65* | −.21 | -- | ||||||
| 4. | HRQOL | 70.17 (20.15) | .93 | −.52* | −.37 | .82* | -- | |||||
| 5. | Cognitive Functiont | 50.66 (9.80) | .92 | −.74* | −.17 | .62* | .55* | -- | ||||
| 6. | Anxietyt | 47.17 (11.80) | .94 | .63* | .09 | −.63* | −.77* | −.77* | -- | |||
| 7. | Depressiont | 48.66 (11.46) | .92 | .62* | .07 | −.57* | −.58* | −.71* | .74* | -- | ||
| 8. | Pain Behaviort | 34.30 (18.78) | .95 | .65* | .27 | −.59* | −.42* | −.59* | .67 | .50* | -- | |
| 9. | Pain Intensity | 1.73 (2.10) | -- | .68* | .07 | −.57* | −.25 | −.65* | .63* | .56* | .71* | -- |
Notes:
p < .05
Values reported as T-scores; Anxiety, PROMIS Parent Proxy Anxiety; Cognitive Function, PROMIS Parent Proxy Cognitive Function; Depression, PROMIS Parent Proxy Depressive Symptoms; ESS-CHAD, Epworth Sleepiness Scale – Child and Adolescent; Fatigue, PedsQL Multidimensional Fatigue Scale Total score; HRQOL, PedsQL Total score; Pain Behavior, PROMIS Parent Proxy Pain Behavior; Pain Intensity, PROMIS Parent Proxy Pain Intensity; SDSC, Sleep Disturbance Scale for Children Total score.
The PedsQL Multidimensional Fatigue Scale (PedsQL MFS)29, 30 is an 18-item scale assessing three dimensions of child fatigue over the past month: General (e.g., “Feeling tired,” “Feeling physical weak,” “Feeling too tired to spend time with his/her friends”), Sleep/Rest (e.g., “Sleeping a lot,” “Taking a lot of naps”), and Cognitive (e.g., “Difficulty remembering what he/she just heard,” “Difficulty keeping his/her attention on things”). Parents report on their child’s fatigue over the past month using a 5-point Likert scale. Similar to the PedsQL, items are reverse scored and transformed to a “0” to “100” scale with higher scores indicating fewer symptoms of fatigue. Excellent internal consistency, validity, and reliability of the PedsQL Multidimensional Fatigue Scale has been established in both general 31 and disease-specific populations 29, 32, 33, including pediatric TBI samples 34–36.
PROMIS Parent Proxy Reports.
Parents completed the pain intensity (1 item), pain behavior (8 items), anxiety (8 items), depressive symptoms (6 items), and cognitive function (7 items) subscales of the Proxy Report for Pediatric Patient-Reported Outcomes Measurement Information System (PROMIS)37. Developed by the National Institutes of Health, all PROMIS measures have strong psychometric properties and are publicly accessible (online via http://www.healthmeasures.com). The pain intensity subscale was used to capture children’s average pain intensity on a “0” (“No pain”) to “10” (“Worst pain you can think of”) numerical scale. The pain behavior subscale assessed the frequency of child pain behavior displays on a 6-point Likert scale with anchors “Had No Pain” to “Almost Always.” On the anxiety, depressive symptoms, and cognitive function subscales, parents reported on their child’s experience of related core symptoms using a 5-point Likert scale with anchors “never” to “almost always”. For each PROMIS scale with greater than 50% of items completed, a prorated total raw score was computed based on the number of items completed and converted to T-scores based on the data tables provided through scoring manuals in the Assessment Center.
Data Analytic Approach
Preliminary analyses were conducted to assess for departures from statistical assumptions (e.g., normality, outliers) and elucidate sample characteristics. Independent samples t-tests and one-way ANOVA were used to examine differences between diagnosis and clinical characteristics in SDSC scores. Internal consistency of the SDSC total score was assessed using Cronbach α coefficient. Validity of the SDSC was examined in the full sample and with group comparisons related to sleep disturbance severity. We hypothesized a valid measure of sleep disturbance would show significant relationships with Post-Intensive Care Syndrome morbidities including physical, cognitive, and psychosocial sequelae.
Primary analyses including the full sample explored the construct validity of the SDSC total score and six subscale scores. Bivariate correlations were performed to evaluate convergent and divergent validity between the subscale scores and parent-proxy PROMIS psychosocial ratings (i.e., depressive symptoms, anxiety, cognitive function). The Holm-Bonferroni Method was used to correct for multiple comparisons.38
Secondary analyses included evaluation of subgroups of the study sample. The full sample was divided into three groups based on clinical risk of sleep disorders using the T-scores derived from the SDSC: Low Risk= T-scores < 50; Mild-Moderate Risk= T-scores 50–69; High Risk= T-scores ≥70. See Figure 2 for distribution of scores by admission diagnosis. Kruskal-Wallis tests were conducted to determine if there were differences in psychosocial measures across SDSC severity groups. Distributions of dependent variables were not similar across groups, as assessed by visual inspection of a boxplot. Due to non-normal distributions, Kruskal-Wallis H tests were used to examine group differences across demographic characteristics and psychosocial measures. Pairwise comparisons were performed using Dunn’s39 procedure with a Bonferroni correction for multiple comparisons. Adjusted p-values are presented.
Figure 2.
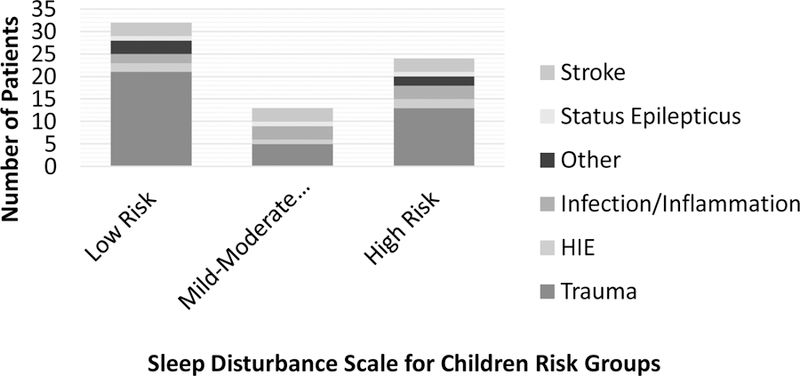
Distribution of admission diagnosis by Sleep Disturbance Scale risk group.
Finally, two clinical metrics, minimal clinically importance difference [MCID] and at-risk status cut-point score, were considered to determine whether statistical group differences translated to observable and meaningful changes in subjective HRQOL.40–42 Previous research has determined that the MCID for PedsQL is 4.5-point change in Total Scale score for parent proxy-report.43 Furthermore, a cut-off point score of ≤ 65.4 can be used to signify whether a child is at-risk for experiencing impairments in HRQOL.31
RESULTS
Means and standard deviations and a correlation matrix for the measures are presented in Table 2. No significant differences in sleep disturbances across gender, diagnosis, or age (continuous) were detected in the full sample. Bivariate correlations were performed to determine strength and significance of SDSC score to measures of physical (i.e., PROMIS pain behavior & intensity, ESS-CHAD), cognitive (i.e., PROMIS cognitive function), and psychosocial sequelae (i.e., PROMIS anxiety & depression, PedsQL HRQOL & Multidimensional Fatigue Scale). All correlations were significant and in the expected direction with the exception of the ESS-CHAD, which was not significantly associated with any study variables.
Reliability
Internal consistency for the SDSC Total score was good (α = .81), showing the SDSC to be a reliable measure.
Convergent and Divergent Validity
Significant associations were found between parent proxy reports of child anxiety and the Disorders of Initiating and Maintaining Sleep (r = .42, p = .016), Sleep Breathing Disorders (r = .46, p = .008), Sleep-Wake Transition Disorders (r = .50, p < .003), Disorders of Excessive Somnolence (r = .58, p < .001), and Sleep Hyperhydrosis (r = .40, p = .02) subscales. Parent proxy reports of worse cognitive function (lower scores) were correlated with Disorders of Initiating and Maintaining Sleep (r = −.72, p < .001) and Disorders of Excessive Somnolence (r = −.74, p < .001) subscales. Significant associations were also detected between depressive symptoms and Disorders of Initiating and Maintaining Sleep (r = .50, p = .004), Sleep-Wake Transition Disorders (r = .43, p = .01), and Disorders of Excessive Somnolence (r = .61, p < .001) subscales. Finally, significant relationships between parent report of child fatigue (i.e., lower PedsQL MFS Score) and Disorders of Initiating and Maintaining Sleep (r = −.59, p < .001), Sleep-Wake Transition Disorders (r = −.35, p = .047), and Disorders of Excessive Somnolence (r = −.61, p < .001) subscales were observed.
Construct Validity
Kruskal-Wallis tests produced statistically significant differences in psychosocial outcomes across the Low, Mild-Moderate, and High Risk sleep disturbance groups. Age, gender, and admission diagnosis were not significantly different across groups. Patients in the High Risk group had higher rates of pre-admission comorbidity. Findings are presented in Table 3.
Table 3.
Descriptive statistics and nonparametric comparisons across SDSC risk group.
| Median (Interquartile Range) or n (%) | |||||
|---|---|---|---|---|---|
| Low RiskA (n = 32) |
Mild-ModerateB (n = 13) |
HighC (n = 24) |
Adjusted p-value | Post Hoc Comparisons | |
|
Demographic Variables | |||||
| Age (years) | 8.0 (6, 13) | 9.0 (7.5, 12.5) | 11.0 (6.25, 14) | .462 | -- |
| Gender, Female (n [%]) | 14 (43.8) | 7 (53.8) | 10 (41.7) | .764 | -- |
| Pre-admission Chronic Condition | 5 (16.7) | 4 (30.8) | 11 (55.0) | .017 | C>A&B |
| Diagnosis (n [%]) | -- | -- | |||
| Infection/Inflammation | 2 (6.3) | 3 (23.1) | 3 (12.5) | -- | -- |
| Trauma | 21 (65.6) | 5 (38.5) | 13 (54.2) | -- | -- |
| Status Epilepticus | 1 (3.1) | 1 (7.7) | 1 (4.2) | -- | -- |
| Hypoxic Ischemic | 2 (6.3) | 1 (7.7) | 2 (8.3) | -- | -- |
| Stroke | 3 (9.4) | 3 (23.1) | 3 (12.5) | -- | -- |
| Other | 3 (9.4) | - | 2 (8.3) | -- | -- |
| Admission GCS | 12.0 (8, 15) | 12.0 (6, 15) | 9.5 (7.3, 13.8) | .689 | -- |
| Length of Hospitalization (Days) | 7.4 (1.7, 29.3) | 6.3 (2.1, 27) | 6.9 (2.6, 10.4) | .966 | -- |
| Functional Status Score (Clinic) | 6 (6, 6) | 6 (6, 6) | 6 (6, 6) | .686 | -- |
|
Psychosocial Measures | |||||
| SDSCt | 45.0 (42, 46) | 53.0 (50.5, 57.5) | 66.0 (63.3, 71.5) | -- | -- |
| HRQOL | 87.0 (65.2, 94.6) | 78.3 (44.6, 79.7) | 55.4 (43.5, 68.7) | .029 | A > C |
| Fatigue | 92.4 (71.9, 100) | 73.6 (62.8, 83.7) | 58.3 (52.8, 69.4) | .002 | A > C |
| ESS-CHAD | 2.7 (1, 5) | 6.0 (2.5, 9.5) | 4.0 (1, 8) | .366 | -- |
| PROMIS Cognitive Functiont | 57.2 (52.9, 63) | 63.0 (57.2, 63) | 43.9 (39.2, 46.5) | .001 | A&B>C |
| PROMIS Anxietyt | 34.6 (34.6, 47.8) | 43.9 (34.6, 52.8) | 57.4 (50.4, 62.8) | .006 | C > A |
| PROMIS Depressiont | 39.2 (36.2, 52.1) | 45.1 (36.2, 51.6) | 58.9 (55.6, 68.4) | .001 | C>A&B |
| PROMIS Pain Behaviort | 15.6 (10, 41.6) | 37.3 (22.1, 47.6) | 49.0 (43.6, 51.2) | < .001 | C > A |
| Pain Intensity | 0.0 (0, 0.25) | 0.0 (0, 2.8) | 4.0 (3, 5) | .001 | C>A&B |
Notes: Groups were compared using X2 for categorical variables and Kruskal-Wallis H tests for continuous variables
Values reported as T-scores; Admission GCS, Glasgow Coma Score; ESS-CHAD, Epworth Sleepiness Scale – Child and Adolescent; Fatigue, PedsQL Multidimensional Fatigue Scale Total score; HRQOL, PedsQL Total score; PROMIS Anxiety, Parent Proxy Anxiety; PROMIS Cognitive Function, Parent Proxy Cognitive Function; PROMIS Depression, Parent Proxy Depressive Symptoms; PROMIS Pain Behavior, Parent Proxy Pain Behavior; PROMIS Pain Intensity, Parent Proxy Pain Intensity; SDSC, Sleep Disturbance Scale for Children Total score.
Fatigue and Sleepiness.
Higher PedsQL Multidimensional Fatigue Total Scores were endorsed by parents among children in the High Risk group (mean rank = 9.27), compared to the Low Risk group (mean rank = 23.11, p = .001). This difference was also observed at the subscale level with parents noting greater General Fatigue (mean rank = 10.83, p = .006), Sleep-Rest (mean rank = 11.42, p = .003), and Cognitive Fatigue (mean rank = 9.27, p = .001) among children in the High Risk group, relative to the Low Risk group. There was no statistically significant difference in fatigue total score or subscales between the Low and Mild-Moderate or the Mild-Moderate and High Risk groups. Group differences also did not emerge for the ESS-CHAD, but as reported in Table 2, the ESS-CHAD performed poorly in our sample.
Cognitive Function.
Children in the High Risk group (mean rank = 7.12) displayed significantly worse impairments in cognitive function compared to both the Low (mean rank = 17.17; p = .003) and Mild-Moderate Risk (mean rank = 20.0; p = .013) groups, revealing children with the highest levels of sleep disturbances evidenced greater parent-perceived dysfunction in mental acuity, concentration, and related deficits. No significant difference in cognitive function was detected between the Low and Mild-Moderate Risk groups.
Pain Behavior.
Children in the High Risk group engaged in more parent-reported displays of pain behavior (mean rank = 36.39) than the Low Risk group (mean rank = 18.42, p < .001). Furthermore, they experienced higher pain intensity (mean rank = 25.41), relative to their peers in the Low (mean rank = 11.79, p < .001) and Mild-Moderate Risk (mean rank = 14.55, p = .027) groups.
Psychosocial Health.
Anxiety, depressive symptoms, and HRQOL were also associated with group membership. Relative to both the Low (mean rank = 12.0, p = .001) and Mild-Moderate Risk (mean rank = 13.89, p = .023) groups, increased depressive symptoms were endorsed for children in the High Risk group (mean rank = 25.64). Similarly, more anxiety symptoms were reported for the High Risk group (mean rank = 24.32) relative to the Low Risk group (mean rank = 12.32, p = .005), signaling decreased positive affect, lower engagement, and greater hyperarousal with more sleep disturbances. Similar findings were detected when examining parent proxy HRQOL Total scores. Within specific HRQOL domains, children in the High Risk group yielded more significant impairments in Emotional (mean rank = 7.39, p = .002), compared to their peers in the Low Risk group (mean rank = 18.87). However, no significant group differences were observed on the Physical, Social, and School Function subscales.
Identifying Clinically Meaningful Significant Difference
Two clinical metrics (i.e., minimal clinically importance difference [MCID], at-risk status cut-point score) were considered to determine whether statistical group differences translated to observable and meaningful changes in HRQOL.40–42 The difference in HRQOL between the Low and High Risk groups (MDifference = 23.25) was 5.2 times the suggested MCID value for HRQOL.43 A clinically significant difference above the MCID value also emerged between the Low and Mild-Moderate Risk groups (MDifference = 12.6). Further, the mean HRQOL Total Score of children in the High Risk group (MHRQOL = 55.97 ± 13.1) falls well below the suggested cut-off point score (≤ 65.40; 31) signifying children in the High Risk group are at increased risk of HRQOL impairments.
DISCUSSION
Advancements in PNCC have significantly reduced pediatric mortality rates following neurological injury.3 Emergence of the ongoing injury/illness related sequelae of Post-Intensive Care Syndrome during the recovery process has resulted in increased recognition of the importance of addressing these needs through targeted interventions. As a result, evidence-based assessment of morbidities has risen to the forefront as providers seek new ways to assess, track, and optimize outcomes. Pervasive sleep disturbances are of paramount importance. Restful and complete sleep is essential to the healing process for children recovering from neurological injury across the entire recovery period; it is especially important during the acute/post-acute phase.44 Additionally, sleep disturbances place children at risk for physical, emotional, and cognitive sequelae. Therefore, it is essential for clinicians to have psychometrically sound measurement tools to assess sleep disturbances within this patient population so that appropriate and targeted treatments can be applied. The dearth of research evaluating the performance of established measurement tools within PNCC populations remains a significant gap in propelling patient care forward. Our study shows the SDSC is a reliable and valid measure in the PNCC population that can be used to address the growing need for assessing sleep problems in the acute recovery phase.
The customary areas explored to examine reliability include the evaluation of internal consistency, test-retest reliability, standard errors of measurement, and inter-rater scoring statistics (when appropriate). We examined the internal consistency of the SDSC in our PNCC population. Because there were multiple items for several subscales that may not be related, internal consistency was not evaluated for individual indices. For instance, a child who grinds teeth while sleeping may not startle or jerk while falling asleep, but together these items indicate greater concern for sleep-wake transition disorders. Consistent with previous research,12, 15–17 the current study showed good internal consistency among the items loading to the SDSC Total score, suggesting the SDSC is a reliable measure for evaluation of sleep disturbances in the PNCC population.
Convergent or criterion-related validity for the SDSC was established by utilizing the full sample for measure comparisons. Given available knowledge of the negative impacts of sleep disturbances in children,27, 45 we expected a valid measure of sleep disturbance to correlate well with measures of Post-Intensive Care Syndrome in physical, cognitive, emotional, and psychosocial domains. The SDSC total score showed significant correlations in the expected direction for pain, fatigue, cognitive impairments, and mood disturbances. Evaluation of sleep phenotypes using the SDSC domain scores additionally showed expected findings -- children who struggle to fall asleep, maintain sleep, or experience parasomnias, and children with greater daytime sleepiness also exhibit more symptoms of fatigue, mood disturbances, and cognitive dysfunction.46 Overall, findings suggest that the SDSC is a valid measure in the PNCC population as it performed well when compared to psychometrically sound measures of physical, psychosocial, cognitive, and emotional outcomes.
Interestingly, the SDSC did not show correlation with the ESS-CHAD, a tool for measuring daytime sleepiness. However, the ESS-CHAD has not been previously evaluated in the PNCC population. In our sample, the ESS-CHAD performed poorly with α=.69 and did not correlate with measures of physical, cognitive, emotional, or psychosocial health. As above, daytime sleepiness or somnolence would be expected to show negative associations with physical, emotional, and cognitive function. Our findings suggest the ESS-CHAD is not a valid tool among the PNCC population, but additional research regarding this tool is needed.
Construct validity for the SDSC was established by comparing SDSC outcomes by severity (i.e., Low Risk, Mild-Moderate Risk, and High Risk defined by reported standardized T-scores) across multiple parent proxy measures of child focused health and wellbeing, collectively considered to be HRQOL. HRQOL is a comprehensive approach to measuring health outcomes that evaluates an individual’s psychosocial, emotional, and physical wellbeing;47 further, it is a subjective term that emphasizes adjustment and overall well-being in the context of a health issue. The assessment of HRQOL is well-suited to conditions that have a multi-dimensional impact, such as those within our PNCC sample and related to Post-Intensive Care Syndrome. As expected, the youth that were placed in the High Risk group consistently showed more struggles (in comparison to their PNCC peers in the Low and Moderate Risk groups) across measures related to fatigue/sleepiness, cognitive functioning, pain behavior, and emotional health. In other words, consistent with research on the impact of sleep disruption in children,48–51 the youth in our sample with the highest levels of sleep disturbances on the SDSC evidenced greater parent-perceived dysfunction in mental acuity, concentration, displays of pain behavior, pain intensity, anxiety and depressive symptoms, emotional regulation, and social engagement. We interpret these findings to mean that children with sleep disturbance in the High Risk group may be experiencing the morbidities of Post-Intensive Care Syndrome and impairments in HRQOL at a higher level than their PNCC peers. Without targeted intervention, deficits in HRQOL may result in ongoing, poorer perceptions about their health and promote negative health appraisals and behaviors as children age.52, 53
Patient-reported outcomes in health care is of growing importance.54 As such, the final component of our SDSC psychometric investigation was to investigate whether or not SDSC scores have any clinically meaningful significance in our sample of children in an acute recovery phase. We found the differences in HRQOL between the Low, Mild-Moderate, and High Risk groups were much greater than the identified MCID value.43 Further, the High Risk group fell well below the suggested cut-off point score (≤ 65.4) for impaired HRQOL. Taken together, these findings provide clinically-meaningful support for the effects of sleep disturbances as measured by the SDSC on the overall well-being of children in our sample. Our findings suggest interventions to improve sleep may be a modifiable target to improve Post-Intensive Care Syndrome comorbidities and quality of life after PNCC. While these results support our hypothesis regarding the validity of the SDSC, more research into the complex interactions of sleep disturbances and HRQOL with respect to patient comorbidity, diagnosis, and functional outcomes are needed.
There are limitations to acknowledge. Analyses were limited to parental perceptions of the child’s sleep disturbance and psychosocial functioning at a single time point, without assessment of sleep behavior prior to illness/injury or concurrent objective measures of sleep. Relationships between SDSC scores and other health outcomes were limited to short-term follow-up, but future studies could assess how these relationships vary over time from discharge. We recruited from critical care follow-up programs at large tertiary children’s hospitals. Our patient population represents a large proportion of PNCC survivors at our institutions that are likely similar to those of other large children’s hospitals, but there is known institutional and regional variability in PNCC that may limit generalizability. While the primary aim of this study was to determine the reliability and validity of the SDSC, future research should consider in-depth evaluation of the relationships between sleep disturbance and other outcomes when controlling for patient and disease characteristics in a broader sample. The SDSC performs well in the PNCC sample and could facilitate this future work, however consideration of child self-report, objective measures of sleep (e.g., actigraphy), multiple assessments of functional status, and longitudinal methodology among a broader population and age range of PNCC survivors should also be considered.
CONCLUSIONS
Sleep disturbances are important sequelae of acquired brain injury in children that have been under-evaluated to date partly due to a lack of appropriate measurement tools. We found the SDSC is a valid and reliable measure for assessing sleep disturbances in children after PNCC. Worse sleep disturbance as measured by the SDSC was significantly associated with measures of Post-Intensive Care Syndrome including worse physical, emotional, psychological, and cognitive sequelae. Our results support the use of the SDSC to measure sleep disturbances after PNCC and clinicians using this tool should be mindful of sleep disturbances’ impact on other Post-Intensive Care Syndrome morbidities and HRQOL. Targeted interventions for sleep disturbances are needed and may be key to overall patient recovery.
Acknowledgments
All authors have read, approved, and contributed to this paper. Dr. Cordts was involved in all aspects of manuscript preparation, including conceptualization, conducting statistical analyses, and writing of the manuscript. Drs. Hall and Wagner were involved in the study conceptualization, data collection, and writing of the manuscript. Dr. Williams supervises Ms. Madison Luther and Ms. Jalane Jara who assisted with data collection, entry, and writing of the manuscript. Dr. Williams was involved in all aspects of the research from conceptualization of the study to editing the manuscript. Drs. Piantino, Hartman, Guilliams, Guerriero assisted in data collection and editing the manuscript. Dr. Williams is supported by the Agency for Healthcare Research and Quality, grant number K12HS022981. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Guilliams is supported by the National Institute of Neurologic Disorders and Stroke grant number K23NS099472. Dr. Piantino is supported by the National Heart, Lung and Blood Institute grant number K12HL133115. The current work has not been published elsewhere, nor is it under consideration at another journal. Informed consent was obtained from all individual participants included in the study. The authors declare that they have no conflicts of interest. A completed STROBE checklist has been included with the submission documents.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Williams CN, Piantino J, McEvoy C, Fino N, Eriksson CO. The Burden of Pediatric Neurocritical Care in the United States. Pediatr Neurol 2018;89:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CN, Eriksson CO, Kirby A, Piantino J, Hall TA, Luther M, et al. Hospital mortality and functional outcomes in pediatric neurocritical care. Hospital Pediatrics Accepted 9/2019, In press. [DOI] [PMC free article] [PubMed]
- 3.Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, Delzoppo C, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatric Critical Care Medicine 2010;11(5):549–55. [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, et al. Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. The Journal of pediatrics 2018;198:16–24. [DOI] [PubMed] [Google Scholar]
- 5.Williams CN, Kirby A, Piantino J. If you build it, they will come: initial experience with a multi-disciplinary pediatric neurocritical care follow-up clinic. Children 2017;4(9):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams CN, Hartman ME, McEvoy CT, Hall TA, Lim MM, Shea SA, et al. Sleep wake disturbances after acquired brain injury in children surviving critical care. Pediatric Neurology, Accepted 8/2019, In Press. [DOI] [PMC free article] [PubMed]
- 7.Williams CN, Lim MM, Shea SA. Sleep disturbance after pediatric traumatic brain injury: critical knowledge gaps remain for the critically injured. Nat Sci Sleep 2018;10:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beebe DW, Krivitzky L, Wells CT, Wade SL, Taylor HG, Yeates KO. Brief report: parental report of sleep behaviors following moderate or severe pediatric traumatic brain injury. Journal of pediatric psychology 2007;32(7):845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tham SW, Palermo TM, Vavilala MS, Wang J, Jaffe KM, Koepsell TD, et al. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. Journal of neurotrauma 2012;29(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandsmark DK, Elliott JE, Lim MM. Sleep-Wake Disturbances After Traumatic Brain Injury: Synthesis of Human and Animal Studies. Sleep 2017;40(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shay N, Yeates KO, Walz NC, Stancin T, Taylor HG, Beebe DW, et al. Sleep problems and their relationship to cognitive and behavioral outcomes in young children with traumatic brain injury. Journal of neurotrauma 2014;31(14):1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer JT, Hannay HJ, Alfano CA, Swank PR, Ewing-Cobbs L. Sleep disturbances and internalizing behavior problems following pediatric traumatic injury. Neuropsychology 2018;32(2):161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep medicine reviews 2011;15(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res 1996;5(4):251–61. [DOI] [PubMed] [Google Scholar]
- 15.McCann M, Bayliss DM, Pestell C, Hill CM, Bucks RS. The relationship between sleep and working memory in children with neurological conditions. Child Neuropsychology 2018;24(3):304–21. [DOI] [PubMed] [Google Scholar]
- 16.Marriner AM, Pestell C, Bayliss DM, McCann M, Bucks RS. Confirmatory factor analysis of the Sleep Disturbance Scale for Children (SDSC) in a clinical sample of children and adolescents. J Sleep Res 2017;26(5):587–94. [DOI] [PubMed] [Google Scholar]
- 17.DelRosso LM, Martin K, Bruni O, Ferri R. Sleep disorders in children with incidental pineal cyst on MRI: a pilot study. Sleep medicine 2018;48:127–30. [DOI] [PubMed] [Google Scholar]
- 18.Dodd JN, Hall TA, Guilliams K, Guerriero RM, Wagner A, Malone S, et al. Optimizing Neurocritical Care Follow-up through the Integration of Neuropsychology. Pediatric Neurology 2018. [DOI] [PMC free article] [PubMed]
- 19.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics 2009;124(1):e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatric sleep measures. Journal of pediatric psychology 2011;36(7):780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns M The assessment of sleepiness in children and adolescents. Sleep Biol Rhythms 2015;13(Suppl 1):97. [Google Scholar]
- 22.Janssen KC, Phillipson S, O’Connor J, Johns MW. Validation of the Epworth sleepiness scale for children and adolescents using Rasch analysis. Sleep medicine 2017;33:30–5. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39(8):800–12. [DOI] [PubMed] [Google Scholar]
- 24.Magee CA, Gordon R, Caputi P. Distinct developmental trends in sleep duration during early childhood. Pediatrics 2014;133(6):e1561–7. [DOI] [PubMed] [Google Scholar]
- 25.Price AMH, Quach J, Wake M, Bittman M, Hiscock H. Cross-sectional sleep thresholds for optimal health and well-being in Australian 4–9-year-olds. Sleep medicine 2016;22:83–90. [DOI] [PubMed] [Google Scholar]
- 26.Price AM, Wake M, Ukoumunne OC, Hiscock H. Outcomes at six years of age for children with infant sleep problems: longitudinal community-based study. Sleep medicine 2012;13(8):991–8. [DOI] [PubMed] [Google Scholar]
- 27.Quach J, Hiscock H, Canterford L, Wake M. Outcomes of child sleep problems over the school-transition period: Australian population longitudinal study. Pediatrics 2009;123(5):1287–92. [DOI] [PubMed] [Google Scholar]
- 28.Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. Journal of personality assessment 2003;80(1):99–103. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Burwinkle TM, Szer IS. The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. The Journal of rheumatology 2004;31(12):2494–500. [PubMed] [Google Scholar]
- 30.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer: reliability and validity of the pediatric quality of life inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer 2002;94(7):2090–106. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Beaujean AA, Limbers CA. Factorial invariance of pediatric patient self-reported fatigue across age and gender: a multigroup confirmatory factor analysis approach utilizing the PedsQL Multidimensional Fatigue Scale. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2013;22(9):2581–94. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Limbers CA, Bryant WP, Wilson DP. The PedsQL Multidimensional Fatigue Scale in type 1 diabetes: feasibility, reliability, and validity. Pediatric diabetes 2009;10(5):321–8. [DOI] [PubMed] [Google Scholar]
- 33.Marcus SB, Strople JA, Neighbors K, Weissberg-Benchell J, Nelson SP, Limbers C, et al. Fatigue and health-related quality of life in pediatric inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2009;7(5):554–61. [DOI] [PubMed] [Google Scholar]
- 34.Limond J, Dorris L, McMillan TM. Quality of life in children with acquired brain injury: parent perspectives 1–5 years after injury. Brain injury 2009;23(7):617–22. [DOI] [PubMed] [Google Scholar]
- 35.Crichton AJ, Babl F, Oakley E, Greenham M, Hearps S, Delzoppo C, et al. Prediction of Multidimensional Fatigue After Childhood Brain Injury. J Head Trauma Rehabil 2017;32(2):107–16. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy ML, MacKenzie EJ, Durbin DR, Aitken ME, Jaffe KM, Paidas CN, et al. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Archives of physical medicine and rehabilitation 2005;86(10):1901–9. [DOI] [PubMed] [Google Scholar]
- 37.Fries JF, Bruce B, Cella D. The promise of PROMIS: using item response theory to improve assessment of patient-reported outcomes. Clinical and experimental rheumatology 2005;23(5):S53. [PubMed] [Google Scholar]
- 38.Holm S A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics 1979:65–70.
- 39.Dunn OJ. Multiple comparisons using rank sums. Technometrics 1964;6(3):241–52. [Google Scholar]
- 40.Copay AG, Subach BR, Glassman SD, Polly DW Jr., Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7(5):541–6. [DOI] [PubMed] [Google Scholar]
- 41.Wyrwich KW, Tierney WM, Wolinsky FD. Using the standard error of measurement to identify important changes on the Asthma Quality of Life Questionnaire. Quality of life research 2002;11(1):1–7. [DOI] [PubMed] [Google Scholar]
- 42.Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. Journal of pediatric psychology 2007;32(9):1151–63. [DOI] [PubMed] [Google Scholar]
- 43.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory pediatrics 2003;3(6):329–41. [DOI] [PubMed] [Google Scholar]
- 44.Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics 2016;13(2):403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quach J, Hiscock H, Wake M. Sleep problems and mental health in primary school new entrants: Cross-sectional community-based study. Journal of paediatrics and child health 2012;48(12):1076–81. [DOI] [PubMed] [Google Scholar]
- 46.Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep medicine reviews 2014;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 47.Fayers P, Machin D. Clinical interpretation. Quality of life, the assessment, analysis and interpretation of patient-reported outcomes 2007:427–55.
- 48.Paavonen EJ, Raikkonen K, Pesonen AK, Lahti J, Komsi N, Heinonen K, et al. Sleep quality and cognitive performance in 8-year-old children. Sleep medicine 2010;11(4):386–92. [DOI] [PubMed] [Google Scholar]
- 49.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child development 2002;73(2):405–17. [DOI] [PubMed] [Google Scholar]
- 50.Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. Journal of developmental and behavioral pediatrics : JDBP 1999;20(4):244–52. [DOI] [PubMed] [Google Scholar]
- 51.Poppert Cordts KM, Steele RG. Trajectories of pediatric sleepiness and their associations with health-related quality of life. Bulletin of the Menninger Clinic 2019;83(2):175–97. [DOI] [PubMed] [Google Scholar]
- 52.Piko BF, Keresztes N. Self-perceived health among early adolescents: Role of psychosocial factors. Pediatrics International 2007;49(5):577–83. [DOI] [PubMed] [Google Scholar]
- 53.Vingilis ER, Wade TJ, Seeley JS. Predictors of adolescent self-rated health. Analysis of the National Population Health Survey. Can J Public Health 2002;93(3):193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Food and Drug Administration. Patient reported outcome measures: Use in medical product development to support labelling claims. Guidance for industry URL: http://wwwfdagov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282pdf [accessed 2013-08-15][WebCite Cache ID 6ItVJsak1] 2009.


