Myeloablative allogeneic hematopoietic cell transplantation (HCT) is commonly used in adults with acute myeloid leukemia (AML) in morphologic remission.1,2 Numerous conditioning regimens are available for this purpose, some containing total body irradiation (TBI).3 Despite several prospective randomized trials and large retrospective analyses, the benefit of high-dose TBI (HD-TBI; TBI doses ≥ 12 Gy) remains controversial.4 Moreover, whether the relative value of HD-TBI- versus non-HD-TBI-based myeloablative conditioning differs depending on the pre-HCT measurable residual disease (MRD) status is unknown.
These questions prompted us to compare outcomes among adults ≥18 years with AML (based on 2016 WHO criteria5) who underwent a first allogeneic transplant in first or second morphologic remission at our institution after myeloablative conditioning using peripheral blood or bone marrow as a stem cell source. We included all transplants between 4/2006, when a refined multiparameter flow cytometry (MFC)-based MRD assay was introduced, and 1/2019. Patients were treated on Institutional Review Board-approved research protocols or standard treatment protocols and gave consent in accordance with the Declaration of Helsinki. Follow-up was current as of April 29, 2019.
We used the refined MRC/NCRI criteria6 to assign cytogenetic risk. Three-tube, 10-color MFC was performed routinely on marrow aspirates before HCT as described.7–11 MRD was identified as a cell population showing deviation from the normal patterns of antigen expression found on specific cell lineages at specific stages of maturation as compared with either normal or regenerating marrow. The assay detects MRD in the large majority of cases down to a level of 0.1% and in progressively smaller subsets of patients as the level of MRD decreases below that level. Any measurable level of MRD was considered MRDpos.7–11 MRD-test results were available to transplant teams.
Unadjusted probabilities of relapse-free survival (RFS) and overall survival (OS) were estimated using the Kaplan-Meier method, and probabilities of relapse and non-relapse mortality (NRM) were summarized using cumulative incidence estimates. NRM was defined as death without prior relapse and was considered a competing risk for relapse, while relapse was a competing risk for NRM. Cox regression and competing risk sub-distribution regression models assessed outcome associations with the following covariates: remission status (remission 1 vs. remission 2), pre-HCT MRD (yes vs. no), conditioning regimen (HD-TBI vs. other), cytogenetic risk at diagnosis (favorable/intermediate vs. adverse), type of AML (secondary vs. de novo), pre-HCT karyotype (normalized vs. not normalized for patients presenting with abnormal karyotypes), blood counts before HCT (recovered vs. not recovered), age at time of HCT, and white blood cell (WBC) count at diagnosis. Categorical and quantitative characteristics were compared using Fisher’s exact tests and Wilcoxon rank sum tests, respectively. Two-sided p-values are reported. Statistical analyses were performed using STATA 16.0 and R.
Four hundred twenty-three patients met study inclusion criteria. Excluding patients who did not agree to their data being used for research purposes (n=6), those who did not undergo pre-HCT MRD testing at our institution (n=6), and those who received radiolabeled antibodies with conditioning (n=24), our study cohort was comprised of 387 patients, of whom 58 (15%) had HD-TBI-based conditioning with 12.0 or 13.2 Gy TBI. Regimens for the other 329 patients included 4 days of busulfan plus cyclophosphamide (BU/CY, n=160), 4 days of busulfan with fludarabine (BU/FLU, n=72), and treosulfan combined with fludarabine with or without low-dose (LD) TBI (2.0 Gy; Treo/FLU/±LD-TBI, n=97; see Table 1 for basic patient characteristics and HCT details). There were several statistically significant differences between patients who had HD-TBI-based conditioning and those who did not. Specifically, patients who had HD-TBI conditioning were younger (P<0.001), had a higher WBC at diagnosis (P<0.001), less often had secondary AML (P<0.001), and more often had a history of CNS (P=0.005) or extramedullary (P=0.001) disease. Moreover, their remission duration before HCT was shorter (P<0.001) and they less likely had an unrelated donor as stem cell source (P<0.001). On the other hand, there was no difference in the proportion of patients transplanted in first (vs. second) remission (P=0.31) and in the proportion of patients with MFC evidence of MRD (P=0.86).
TABLE 1.
Demographic and clinical characteristics of study cohort, stratified by type of conditioning therapy
| HD-TBI Conditioning (n=58) | Non-TBI Conditioning (n=329) | All patients (n=387) | P-value | |
|---|---|---|---|---|
| Median age at diagnosis (range), years | 33 (18-57) | 50 (18-70) | 48 (18-70) | <0.001 |
| Median age at transplant (range), years | 33 (18-58) | 51 (18-70) | 49 (18-70) | <0.001 |
| Male gender, n (%) | 36 (62) | 167 (51) | 203 (52) | 0.12 |
| Median WBC at diagnosis (range), ×103/µL | 33.2 (0.6-280) | 8.7 (0.2-297) | 11.3 (0.2-297) | <0.001 |
| CNS disease, n (%) | 0.005 | |||
| No | 51 (88) | 320 (97) | 371 (96) | |
| Yes | 7 (12) | 9 (3) | 16 (4) | |
| Extramedullary disease, n (%) | 0.001 | |||
| No | 48 (83) | 314 (95) | 362 (94) | |
| Yes | 10 (17) | 15 (5) | 25 (6) | |
| Cytogenetic risk, n (%) | 0.35 | |||
| Favorable | 8 (14) | 23 (7) | 31 (8) | |
| Intermediate | 35 (63) | 204 (66) | 239 (65) | |
| Adverse | 13 (23) | 84 (27) | 97 (26) | |
| Missing | 2 | 18 | 20 | |
| Secondary AML, n (%) | <0.001 | |||
| No | 55 (95) | 248 (75) | 303 (78) | |
| Yes | 3 (5) | 81 (25) | 84 (22) | |
| Median CR duration before HCT (range), days | 69 (7-197) | 108 (11-485) | 98 (7-485) | <0.001 |
| Remission status, n (%) | 0.31 | |||
| First remission | 42 (72) | 258 (78) | 300 (78) | |
| Second remission | 16 (28) | 71 (22) | 87 (22) | |
| Pre-HCT MRD status (by MFC), n (%) | 0.86 | |||
| MRDneg | 47 (81) | 261 (79) | 308 (80) | |
| MRDpos | 11 (19) | 68 (21) | 79 (20) | |
| Recovered peripheral blood counts before HCT*, n (%) | 38 (66) | 252 (77) | 290 (75) | 0.10 |
| Recovered ANC | 48 (83) | 313 (95) | 361 (93) | 0.002 |
| Recovered platelet count | 38 (66) | 253 (77) | 291 (75) | 0.07 |
| Routine karyotyping before HCT, n (%) | 0.10 | |||
| Normalized karyotype | 31 (53) | 130 (40) | 161 (42) | |
| Abnormal karyotype | 9 (16) | 52 (16) | 61 (16) | |
| Missing/non-informative data | 18 (31) | 147 (45) | 165 (43) | |
| Unrelated donor, n (%) | 25 (43) | 222 (67) | 247 (64) | <0.001 |
| Conditioning regimen, n (%) | ||||
| HD-TBI ± CY or FLU | 36 (62) | --- | 36 (9) | |
| HD-TBI/Tepa/FLU | 22 (38) | --- | 22 (6) | |
| BU/CY | --- | 160 (49) | 160 (41) | |
| BU/FLU | --- | 72 (22) | 72 (19) | |
| Treo/FLU ± LD-TBI | --- | 97 (29) | 97 (25) | |
| Stem cell source, n (%) | 0.56 | |||
| PBSC | 51 (88) | 277 (84) | 328 (85) | |
| BM | 7 (12) | 52 (16) | 59 (15) |
Absolute neutrophil count ≥1,000/µL and platelets ≥100,000/µL.
Abbreviations: BM, bone marrow; BU, busulfan; CY, cyclophosphamide; FLU, fludarabine; HD-TBI, high-dose total body irradiation; HCT, hematopoietic cell transplantation; LD-TBI, low-dose total body irradiation; MFC, multiparameter flow cytometry; PBSC, peripheral blood stem cells; Tepa; thiotepa; Treo, treosulfan; WBC, white blood cell count.
By the day of data cut-off, 113 of the 387 patients (22 with HD-TBI conditioning) relapsed of whom 94 (18 with HD-TBI conditioning) have died. Sixty (4 with HD-TBI conditioning) experienced NRM, for a total of 154 deaths (22 among HD-TBI conditioning) following transplantation (Supplementary Table 1). The median follow-up time after HCT in the 233 patients alive at last contact was 61.8 (range 3.2-145.1) months (for HD-TBI patients [n=36]: 49.4 [3.9-144.5] months; for non-HD-TBI patients [n=197]: 63.5 [3.2-145.1] months; P=0.80). Consistent with our previous analyses,7–11 the 79 patients with MRD before HCT had a significantly higher risk of relapse and shorter RFS as well as shorter OS than the 308 MRDneg patients whereas the risk of NRM was similar at 3-years (Supplementary Table 2). On the other hand, patients who underwent HD-TBI conditioning had post-transplant outcomes that were not statistically significantly different from those who had myeloablative conditioning with non-HD-TBI-based regimens. As depicted in Figure 1 and summarized in Supplementary Table 2, this was true for patients with pre-HCT MRD and those without, indicating that there was not a particular benefit (or disadvantage) of using HD-TBI conditioning in patient subsets stratified by pre-transplant MRD status. Outcomes for patients transplanted in first remission by pre-HCT MRD status and cytogenetic risk are depicted in Supplementary Figure 1.
Figure 1. Association between pre-transplant MRD status and post-transplant outcome for 58 adults who underwent HD-TBI-based and 329 adults who underwent non-HD-TBI-based myeloablative conditioning while in first or second morphologic remission.
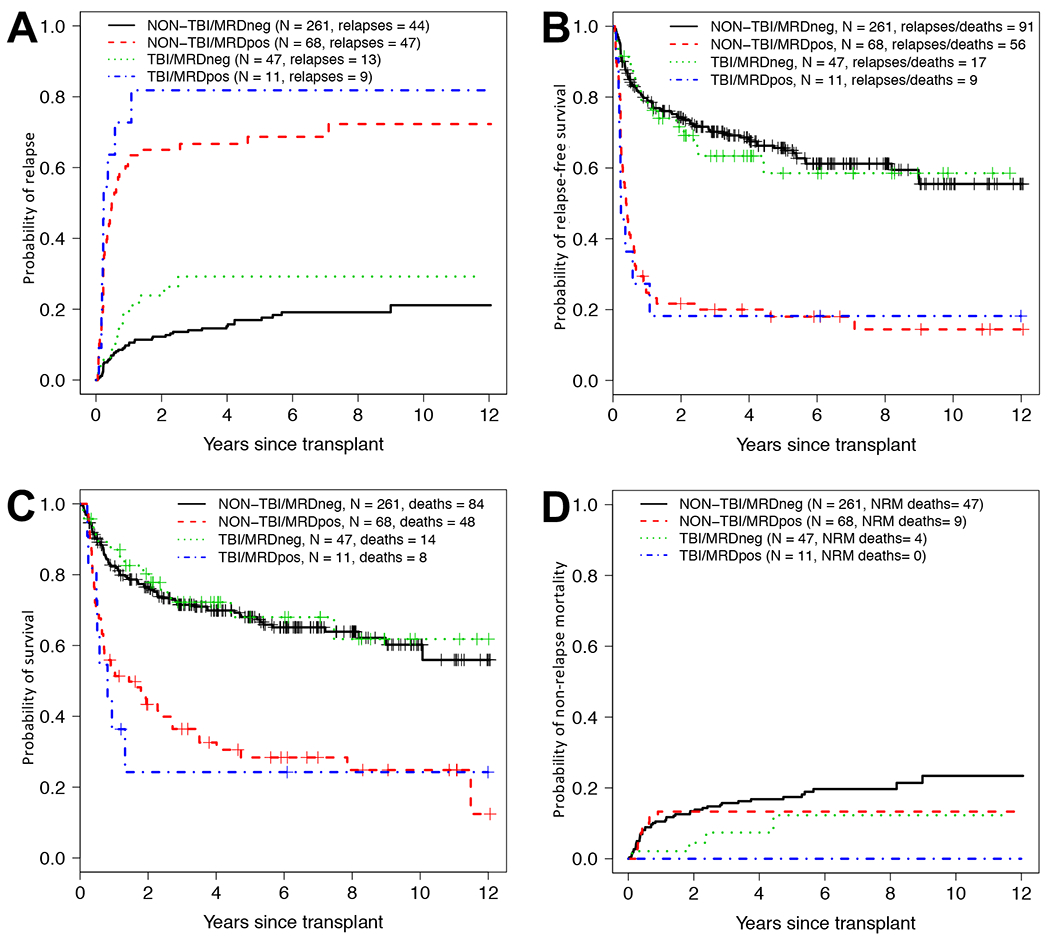
Estimates of (A) cumulative risk of relapse, (B) relapse-free survival, (C) overall survival, and (D) cumulative risk of non-relapse mortality following myeloablative allogeneic HCT. Outcome estimates are shown individually for patients with HD-TBI-based conditioning in MRDneg remission (n=47) or MRDpos remission (n=11) as well as those with HD-TBI-based conditioning in MRDneg remission (n=261) or MRDpos remission (n=68), respectively.
We then developed uni- and multivariable regression models for relapse, RFS, OS, and NRM. In the entire cohort, the unadjusted hazard of HD-TBI conditioning vs. non-HD-TBI conditioning for relapse was 1.49 (0.94-2.37, P=0.089; Supplementary Table 3), the unadjusted hazard for RFS was 1.03 (0.68-1.57, P=0.88), the unadjusted hazard for overall mortality was 0.94 (0.60-1.48, P=0.80), and the unadjusted hazard for NRM was 0.38 (0.14-1.05, P=0.063). For MRDpos vs. MRDneg remission, unadjusted hazard ratios were 6.41 (4.38-9.37; P<0.001) for relapse, 4.37 (3.20-5.98; P<0.001) for RFS, 3.09 (2.22-4.30; P<0.001) for overall mortality, and 0.66 (0.32-1.35, P=0.25) for NRM. As summarized in Supplementary Table 3, statistically significant associations between post-HCT outcomes and cytogenetic risk at diagnosis (for relapse and NRM) and karyotype at the time of HCT (for relapse, RFS, and OS). After adjustment for various covariates as summarized in Supplementary Table 4, HD-TBI conditioning was not independently associated with relapse (hazard ratio [HR]=1.07 [0.60-1.89], P=0.83), RFS (HR=0.82 [0.52-1.30], P=0.41), OS (HR=0.86 [0.53-1.42], P=0.53), or NRM (HR=0.39 [0.14-1.15], P=0.089). On the other hand, being MRDpos before transplantation was associated with increased relapse risk (HR=7.37 [4.88-11.14], P<0.001), shorter RFS (HR=4.67 [3.30-6.60], P<0.001), and shorter OS (HR=3.10 [2.16-4.46], P<0.001) relative to being MRDneg before transplantation, consistent with our previous findings.7–11
Having found no overall difference in post-transplant outcomes between patients who received HD-TBI conditioning and those who received one of three types of non-HD-TBI conditionings, we then performed subset analyses comparing the outcomes of patients receiving non-HD-TBI myeloablative conditioning with BU/CY, BU/FLU, or Treo/FLU (±LD-TBI). Basic characteristics of these 3 patient cohorts are summarized in Supplementary Table 5, whereas estimates of relapse, RFS, OS, and NRM are depicted in Supplementary Figure 2. As detailed in Supplementary Tables 6 and 7, we found very similar hazards for BU/CY vs. BU/FLU or Treo/FLU/±LD-TBI conditioning with regard to relapse (P=0.097 and P=0.17), RFS (P=0.97 and P=0.77), OS (P=0.71 and P=0.72), and NRM (P=0.083 and P=0.43) in univariate regression models as well as after multivariable adjustment (for RFS: P=0.53 and P=0.46; for OS: P=0.83 and P=0.61; and for NRM: P=0.15 and P=0.46) with the exception of relapse for which we found evidence that relapse risk was improved for BU/CY compared to BU/FLU (HR=1.75 [1.02-3.00], P=0.043).
As study strength, MFC-based MRD testing is routinely performed during pre-HCT work-up since 2006 in a largely unchanged fashion, allowing us to include essentially all our patients in this analysis. Over this 13-year period, patients were routinely assigned to myeloablative conditioning unless significant comorbidities were present, or patients were enrolled onto trials comparing conditioning intensities. Results from pre-HCT MRD testing were available to the treating physicians for all patients but while the presence of MRD was perceived as a marker for increased risk of post-HCT relapse, it typically played no major role in the selection of the type of preparative regimen. Important study limitations include its retrospective nature, the substantial differences in patient characteristics between the TBI- and non TBI groups, the fact that conditioning assignments were done in a non-randomized fashion, the small size of some of the patient subsets in our cohort, and the relatively short follow-up time for patients transplanted most recently in our cohort. As another limitation, the majority of patients was referred to our institution for transplantation after receiving induction and consolidation chemotherapy elsewhere. Therefore, molecular testing was not routinely performed and data on mutations could thus not be included in our analyses. Acknowledging these limitations, our data indicate the choice of HD-TBI- versus non-HD-TBI-containing myeloablative preparative regimens for patients with AML should not be influenced by the pre-HCT MRD status of their disease; in both patient subsets, we did not find major benefit for one type of myeloablative conditioning (HD-TBI vs. non-HD-TBI) over the other.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants P01-CA078902, P01-CA018029, and P30-CA015704 from the National Cancer Institute/National Institutes of Health (NCI/NIH). The authors acknowledge the excellent care provided by the physicians and nurses of the HCT teams, the staff in the Long-Term Follow-up office at the Fred Hutchinson Cancer Research Center, the Hematopathology Laboratory at the University of Washington, and the patients for participating in our research protocols.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 2012; 9(10): 579–590. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014; 124(3): 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storb R, Georges GE, Gooley TA. TBI- vs. chemotherapy-based myeloablative conditioning for allogeneic HCT. Biol Blood Marrow Transplant 2019; in press. [DOI] [PubMed] [Google Scholar]
- 5.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127(20): 2391–2405. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010; 116(3): 354–365. [DOI] [PubMed] [Google Scholar]
- 7.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 2011; 29(9): 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122(10): 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia 2015; 29(1): 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: is it time to move toward a minimal residual disease-based definition of complete remission. J Clin Oncol 2016; 34(4): 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016; 30(7): 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


