Abstract
The coronavirus-2019 (COVID-19) infection pandemic has affected the care of patients with heart failure (HF) who have contracted COVID-19 as well as those without COVID-19 who have been impacted by the restructuring of health care delivery. Patients with HF and other cardiovascular comorbidities are at risk for severe disease and complications of infection. Similarly, COVID-19 has been demonstrated to cause myocarditis and may be implicated in new-onset cardiomyopathy. During this pandemic, special considerations are needed for patients with advanced HF, including those supported by durable left ventricular assist devices (LVADs) and heart transplant recipients. The purpose of this review is to summarize emerging data regarding the development of HF secondary to COVID-19 infection in patients with advanced HF and the implications of the pandemic for care of uninfected patients with HF.
Key Words: care delivery, coronavirus, heart failure, heart transplantation, ventricular assist device
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus-2019; HF, heart failure; HT, heart transplant; LVAD, left ventricular assist device; RAAS, renin-angiotensin-aldosterone system; SARS-CoV2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
Highlights
-
•
Patients with HF may be at increased risk for severe disease and complications from COVID-19.
-
•
COVID-19 infection can cause or worsen HF through a variety of mechanisms.
-
•
Delivery of HF care has been significantly restructured during the COVID-19 pandemic.
-
•
Future studies should address the impact of pandemic delays on outcomes in patients with HF.
Since the earliest cases of coronavirus-2019 (COVID-19) infection were reported (1), our care delivery systems have been reorganized and challenged in unprecedented ways. Patients with underlying cardiovascular conditions, including heart failure (HF), are at risk for severe infection and complications (2,3). Special considerations are needed for patients with advanced HF, including those supported by durable left ventricular assist devices (LVADs) and heart transplantation (HT) recipients. This review summarizes emerging data regarding the development of HF secondary to COVID-19 infection in patients with advanced HF and the implications of the pandemic for care delivery in uninfected patients with HF.
Acute Heart Failure In COVID-19
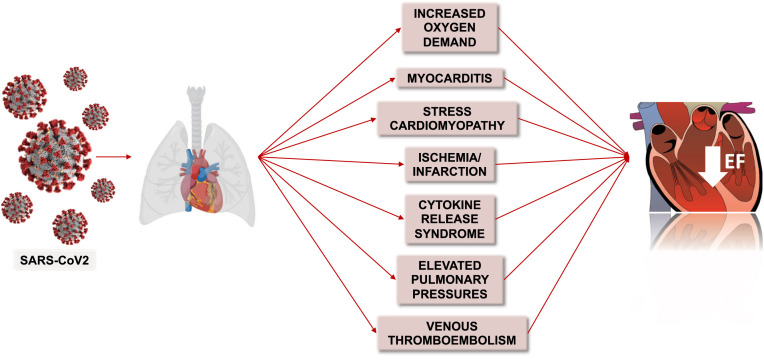
COVID-19 may cause or worsen HF through a variety of mechanisms including myocardial ischemia or infarction, increased oxygen demand, elevations in pulmonary pressures, pulmonary embolism, myocarditis, stress cardiomyopathy, and diffuse cytokine release (4) (Figure 1 ). These mechanisms may concurrently lead to arrhythmia, cardiogenic shock, and sudden cardiac death (2,5).
Figure 1.
Mechanisms of New or Worsening Heart Failure in Patients With COVID-19
Potential contributing factors and mechanisms of worsening heart failure in patients with COVID-19 include increased oxygen demand, myocarditis, stress cardiomyopathy, ischemia or infarction, cytokine release syndrome, elevated pulmonary pressures, and venous thromboembolism.
Patients with COVID-19 infection are at higher risk for thrombosis in the arterial and venous circulations due to endothelial dysfunction, inflammation, oxidative stress, and platelet activation (6). Acute coronary syndromes with plaque rupture leading to type I myocardial infarction may occur in this setting (6). Hypoxemic respiratory failure and increased myocardial oxygen demand may lead to mismatch between oxygen supply and demand and precipitate type 2 myocardial infarction (4); both may trigger decompensation of pre-existing HF or development of de novo acute HF. Right ventricular failure can also develop secondary to elevated pulmonary pressures in the setting of acute respiratory distress syndrome (ARDS) and/or pulmonary embolism (7).
COVID-19-associated myocarditis has been observed as COVID-19 viral particles on endomyocardial biopsy and detection of diffuse myocardial edema on cardiac magnetic resonance (8,9). Attempted myocarditis treatment strategies have included lopinavir/ritonavir, hydroxychloroquine, systemic glucocorticoid therapy, and intravenous immunoglobulin (9,10).
Some data suggest that cardiomyopathy may be common in patients with COVID-19. In a U.S. report of 21 patients with severe COVID-19 disease, 7 (33%) developed cardiomyopathy, which was defined as newly decreased left ventricular systolic function with 1 of the following: 1) increased cardiac biomarkers; 2) decreased central venous oxygen saturation; or 3) clinical signs of shock (11). In another cohort of patients with COVID-19 from China, HF developed in 41 of 83 (49%) of those who died compared to 3 of 94 (3%) of those who recovered (12). Among those who developed HF, approximately 50% did not have a history of hypertension or cardiovascular disease.
The underlying mechanisms for those cardiomyopathies are difficult to elucidate given limitations in inpatient diagnostic testing. For example, the true prevalence of myocarditis secondary to COVID-19 infection may be hard to determine without the use of definitive endomyocardial biopsies and routine cardiac imaging in a wide cohort of infected patients. Accordingly, the myocarditis is often diagnosed by using elevations in biomarkers, echocardiography demonstrating reduction in systolic function, and/or worsening hemodynamics or episodes of arrhythmia.
Large registries such as the American Heart Association COVID-19 Cardiovascular Disease (CVD) registry (13) will help capture the prevalence of systolic dysfunction and incidence of myocardial recovery. Ongoing studies will also explore the pathology of myocarditis and HF more broadly in severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection (14).
Use of temporary mechanical circulatory support
Patients with COVID-19 infection can develop a cytokine release syndrome with hyperinflammation leading to progressive shock and multiorgan failure. In patients with cardiac involvement, important questions remain regarding the use and efficacy of mechanical circulatory support. Device insertion and maintenance require significant equipment, blood products, and personnel. Health systems may have limited resources to deploy these therapies, particularly in older patients with other comorbidities.
The Extracorporeal Life Support Organization has released guidance regarding the use of extracorporeal membrane oxygenation for patients with COVID-19 who develop severe cardiopulmonary failure (15). The organization suggests that, in resource-limited settings, younger patients without comorbidities and/or health care workers should be the highest priority for support. Extracorporeal membrane oxygenation should rarely be used in older patients with significant comorbidities and multiorgan failure (15).
Concerns for patients with chronic heart failure with COVID-19
Use of ACE inhibitors/ARBs
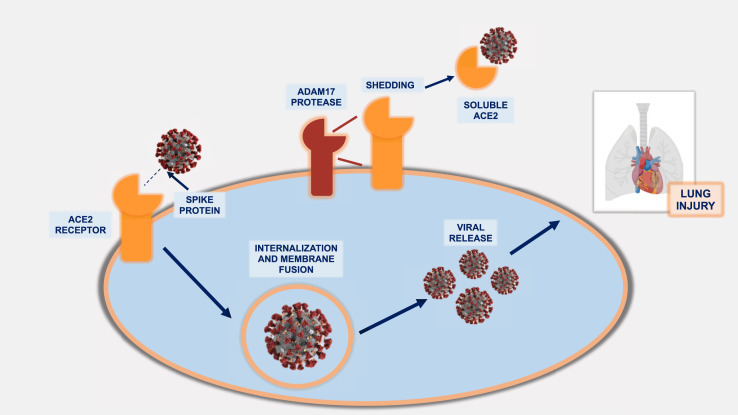
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and angiotensin receptor-neprilysin inhibitors (ARNI) are principal components of guideline-directed medical therapy for patients with chronic systolic HF. SARS-CoV-2 virus, the causative viral agent of COVID-19, uses the ACE2 receptor for cell entry (Figure 2 ), and concerns have been raised regarding this interaction with respect to disease virulence (16). Large retrospective studies have since suggested that the use of ACE inhibitors/ARBs is not associated with increased rates of COVID-19 infection or risk of severe disease (17,18).
Figure 2.
Pathophysiology of the SARS-CoV-2 Virus and the Role of the ACE2 Receptor
The spike proteins of the SARS-CoV-2 virus bind to the ACE2 receptor, leading to viral entry, replication, and SARS-CoV-2 infection. The ADAM17 protease facilitates shedding of ACE2 from the membrane leading to the release of the soluble form of ACE2. Soluble ACE2 may bind the virus and prevent binding to the membrane-anchored ACE2 on cells, preventing cell entry. ACE2 = angiotensin-converting enzyme 2; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
A joint statement from the Heart Failure Society of America and American College of Cardiology/American Heart Association recommends continuation of renin-angiotensin-aldosterone system (RAAS) antagonists in patients who are receiving these medications for HF or other cardiovascular indications (19).
RAAS inhibition may have potential therapeutic benefit. Data from the SARS epidemic demonstrated that injection of SARS-CoV-1 into mice worsened acute lung failure, which was attenuated by RAAS blockade (20). In preclinical studies, sacubitril-valsartan has been shown to reduce levels of proinflammatory cytokines, to increase lymphocyte counts (21), and to inhibit expression of proinflammatory genes including interleukin-6, a potential therapeutic target in COVID-19 (22).
Increased susceptibility to thrombosis
Patients with HF have pre-existing risk factors for venous thromboembolism including possible stasis of blood in the legs, heart, and endothelial injury (23,24). Similarly, patients with ischemic cardiomyopathy and atrial fibrillation are at risk for arterial thrombosis (23). In women with HF, additional thrombotic risks include the use of oral contraceptives, hormone replacement therapy, and breast cancer (25). In patients with durable LVAD support who are already at increased risk for thrombosis, the effects of COVID-19 infection on the risk for stroke or pump thrombosis are currently unknown.
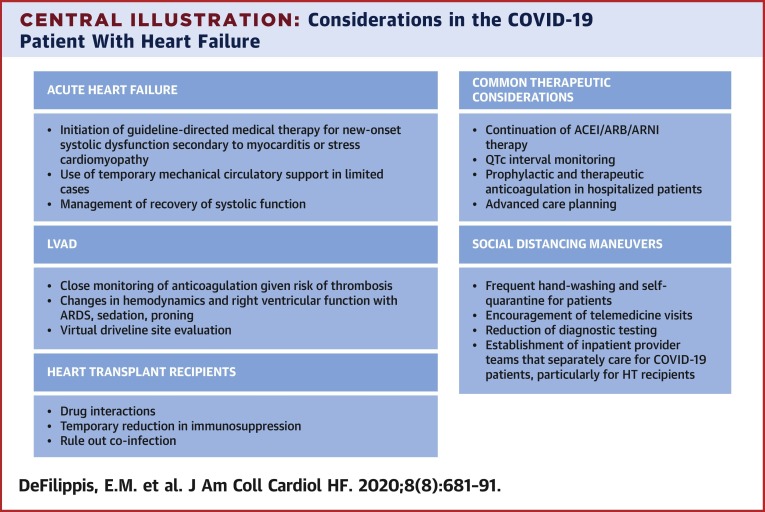
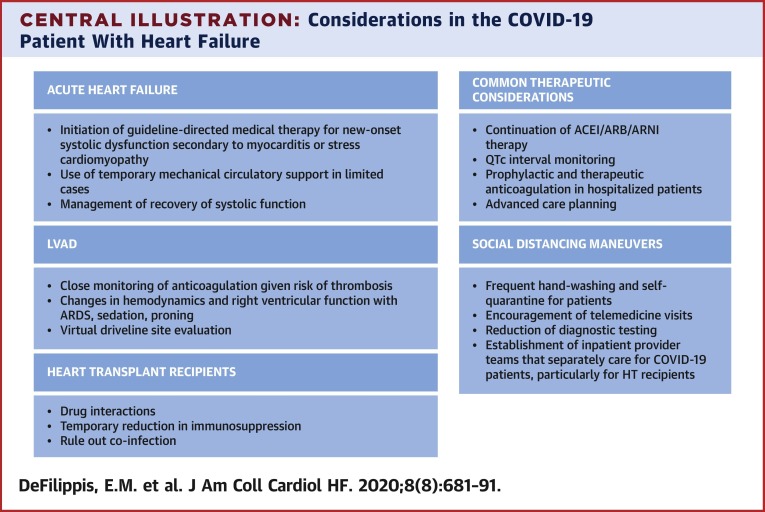
Therapeutic agents in patients with HF who are taking anticoagulation and require admission for COVID-19 should be continued unless a strong contraindication exists. All hospitalized HF patients with COVID-19 infection without a pre-existing indication should receive prophylactic doses of anticoagulation (26). This and other therapeutic considerations are described in the Central Illustration .
Central Illustration.
Considerations in the COVID-19 Patient With Heart Failure
Considerations for patients with coronavirus-2019 (COVID-19) who develop acute heart failure as well as patients with pre-existing heart failure are shown. ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin II receptor blockers; ARDS = acute respiratory distress syndrome; ARNI = angiotensin receptor-neprilysin inhibitors; HT = heart transplant; QTc = QT interval corrected for heart rate.
Decreased cardiopulmonary reserve
Underlying pulmonary disease is common in patients with HF. Approximately 30% of such patients have chronic obstructive lung disease which independently increases their risk for hospitalization and mortality (27). These patients may have pulmonary hypertension as a consequence of parenchymal lung disease in addition to elevated left ventricular filling pressures (28). In patients with COVID-19 infection, hypoxemic respiratory failure and ARDS can exacerbate pulmonary vasoconstriction and interstitial edema, worsening pulmonary hypertension even in patients without pre-existing lung disease (29). In patients with pre-existing biventricular failure, further elevation in pulmonary pressures secondary to ARDS can worsen right ventricular function.
Patients with advanced HF, including those with durable LVAD support, have severely reduced functional capacity (30,31), as measured by peak VO2, and impaired ability to augment cardiac output in response to physiological stressors. These factors collectively decrease their cardiopulmonary reserve.
QT prolongation and monitoring
Despite preliminary reports suggesting that hydroxychloroquine and azithromycin may improve the clinical course in patients with COVID-19, the Centers for Disease Control and Prevention now no longer recommends their use outside of clinical trials. Large observational studies have not demonstrated any mortality benefit or reduction in illness severity (32,33).
Both agents have been associated with QT interval prolongation, increasing the risk for arrhythmia, including Torsades de Pointes, and sudden cardiac death (34,35). Patients with HF often have structural and electric abnormalities leading to delayed ventricular repolarization and QT prolongation on surface electrocardiography (36). Prolonged QTc intervals are independent predictors of adverse outcomes in patients with HF (37). Many patients with HF may be prescribed QT-prolonging drugs as well as loop diuretic agents that can lead to electrolyte abnormalities and increase the risk of serious arrhythmia (38). Additional risk factors for QT interval prolongation in hospitalized patients include female sex, older age, sepsis, and a baseline QTc >450 ms on admission (39).
Underlying inflammatory state
Systemic inflammation underlies both acute and chronic HF (40). The hemodynamic stress of HF stimulates the release of proinflammatory cytokines including tumor necrosis factor alpha, interleukin-6, and interleukin-1 beta (40). Furthermore, coexisting comorbidities such as obesity, type 2 diabetes, and hypertension may perpetuate a persistent inflammatory state leading to multiorgan involvement and endothelial dysfunction (40). Inflammatory markers used to determine COVID-19 severity such as C-reactive protein, lactate dehydrogenase, N-terminal pro–B-type natriuretic peptide, and interleukin-6 may already be elevated in HF, including in patients on LVAD support (40, 41, 42). Therefore, values obtained in the setting of COVID-19 should be compared to prior values, if available.
Advanced care planning
Despite efforts to maintain social distancing, community spread of COVID-19 is increasing. Patients with HF who self-quarantine may still be at risk for acquiring COVID-19 as they commonly have external nursing support. Planning of advanced care is critical for all patients with HF, including all populations on LVAD support and HT recipients, particularly for patients in areas with high prevalence for COVID-19 infection. Ideally, clinicians should initiate these conversations with patients and their caregivers at the onset of their HF diagnosis and not at the time of emergent hospitalization. For patients with HF who develop COVID-19 and require hospital admission, care teams should involve their HF specialists in conversations regarding goals of care, including the deactivation of implantable cardioverter-defibrillators (43). Patients should be encouraged to have these discussions with their families even after admission, using videoconferencing technology, as institutional visitor restrictions frequently limit the bedside presence of family members.
Considerations for special populations with COVID-19
Patients on LVAD support
Patients on LVAD support who contract COVID-19 infection are at risk for severe viral infection due to older age, comorbidities, and immunosuppressed state. They suffer from compromised cellular immunity as shown by aberrant T-cell activation, heightened susceptibility of CD4 T cells to spontaneous apoptosis, and cytokine imbalances (44). This “functionally immunosuppressed state” increases susceptibility to complications from opportunistic infections (45).
Cases of COVID-19 infection have been reported in patients on destination therapy LVAD support who have developed ARDS and multiorgan failure with evidence of cytokine release syndrome (46,47). Both of those cases highlighted the challenges of prone positioning in patients with LVADs, given concerns regarding increased right ventricular pressures and right ventricular failure. Although International Society for Heart and Lung Transplantation guidance suggests that patients with LVADs can be placed in a prone position for the management of hypoxemic respiratory failure (48), more data are needed.
Heart transplantation recipients
Respiratory viruses have been shown to cause rapid progression to pneumonia, greater disease severity, and prolonged viral shedding in solid organ transplantation recipients (49). Post-transplantation patients are more likely to develop bacterial or fungal superinfections due to their immunocompromised state (49). Although the anti-inflammatory effects of immuno-suppressants may mitigate disease severity through reduction in cytokine production (50, 51), experience with SARS and Middle East respiratory syndrome showed that clinical presentations of transplantation patients were similar to those in the general population (2). Notably, mTOR inhibitors have been associated with increased susceptibility to viral infections (52, 53).
Cases of COVID-19 infection have been reported in HT recipients (Table 1 ) (54, 55, 56). Given the immunosuppressed states of these patients, their presentations may be atypical, and high suspicion for COVID-19 illness should be maintained. In the largest report of 28 HT recipients with COVID-19, 79% were hospitalized, and 25% required mechanical ventilation (56). Mycophenolate mofetil was discontinued in 70%, and calcineurin inhibitors were reduced in 26% of patients. Seven patients (25%) died.
Table 1.
Selected Reports of COVID-19 Infection in Heart Transplant Recipients
| First Author (Ref. #) | Number of HT Cases | COVID-Specific Treatment | Changes to HT Immunosuppression | Additional Features |
|---|---|---|---|---|
| Fernandez-Ruiz et al. (54) | 4 | HCQ in 4 (100%) Lopinavir/ritonavir in 2 (50%) Interferon-β in 1 (25%) |
Cyclosporine held in 3 (75%) MMF held in 2 (50%) and continued in 2 (50%) Prednisone continued in 4 (100%) Tacrolimus continued in 1 (25%) |
All presented with fever, cough and abnormal chest imaging 2 patients had severe ARDS requiring intubation and ICU admission, one of whom died 2 other patients with mild courses requiring supplemental oxygen and were discharged home |
| Hsu et al. (67)∗ | 1 | HCQ, remdesivir vs. placebo as part of clinical trial | Continued tacrolimus and prednisone, MMF held | Presented with typical symptoms, found to have lymphopenia and inflammatory markers, discharged home on day 15 |
| Holzhauser et al. (68) | 2 | Patient #1: tocilizumab, IVIG, HCQ, lopinavir/ritonavir Patient #2: HCQ, methylprednisolone, therapeutic anticoagulation |
Patient #1: MPA discontinued, dose reduction of tacrolimus Patient #2: MMF discontinued |
Patient #1 developed hypoxic respiratory failure, multi-organ failure, and death. Patient #2 was discharged home. |
| Latif et. al. (56) | 28 | HCQ in 18 (78%) High-dose corticosteroids in 8 (47%) IL-6 antagonists in 6 (26%) |
MMF discontinued in 16 (70%) Calcineurin inhibitor dose reduced in 6 (26%) |
79% required hospitalization; 25% required mechanical ventilation; 25% in-hospital mortality with 4 patients remaining hospitalized at end of study |
| Li et al. (55) | 2 | Patient #1: ribavirin, IV ganciclovir, high dose corticosteroids, IVIG, oral moxifloxacin and arbidol Patient #2: IV ganciclovir, oral moxifloxacin and arbidol |
Tacrolimus and MMF held in 1 patient; no changes reported in other case | Mild disease courses; did not require ICU admission and both discharged home |
| Mathies et al. (69) | 1 | HCQ | MMF discontinued; sirolimus changed to tacrolimus | Presented with typical symptoms, did not require ICU admission, discharged on hospital day 12, repeat PCR testing negative |
ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus 2019; HCQ = hydroxychloroquine; HT = heart transplantation; ICU = intensive care unit; IV = intravenous; IVIG = intravenous immunoglobulin; MPA = mycophenolic acid; MMF = mycophenolate mofetil; PCR = polymerase chain reaction.
Heart-kidney recipient
For HT patients with COVID-19 illness, transplantation teams should consider reducing doses of calcineurin inhibitors and reducing or holding antimetabolites. Drug interactions between immunosuppressants and COVID-19 therapeutics should be reviewed (57). Leukopenia is common in patients with COVID-19 infection, although it is typically manifest by lymphopenia rather than neutropenia (58). This should be considered in newly transplanted patients who are taking valganciclovir and mycophenolate, which can have similar myelosuppressive effects. In patients who develop allograft dysfunction, distinguishing rejection from viral myocardial involvement may be difficult, as availability of endomyocardial biopsies may be restricted (57).
Healthcare delivery for patients with heart failure
Telemedicine
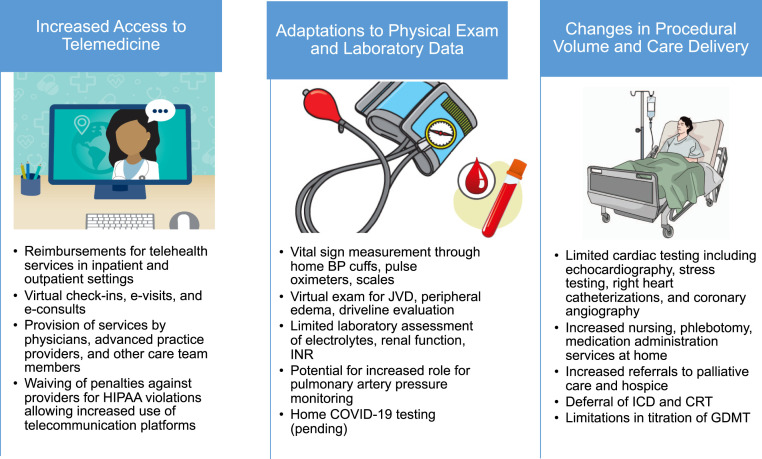
The reorganization of health care structures spurred by the COVID-19 pandemic has significantly affected patients with HF (Figure 3 ). To reduce SARS-CoV-2 transmission and maintain a healthy workforce, health systems have largely transitioned to noncontact care delivery methods for ambulatory care. In response to these challenges, the Centers for Medicare and Medicaid Services broadened access to telehealth services within the Coronavirus Preparedness and Response Supplemental Appropriations Act in early March 2020. Under the 1135 waiver, Medicare will pay for telehealth services provided in inpatient, outpatient, and home settings by a range of providers.
Figure 3.
Reorganization of Care Delivery During the COVID-19 Pandemic
Reorganization of care delivery has resulted in increased access to telemedicine, adaptations to physical examinations, and laboratory data and changes in procedural volume. BP = blood pressure; COVID-19 = coronavirus-2019; CRT = cardiac resynchronization therapy; GDMT = guideline-directed medical therapy; HIPAA = Health Insurance Portability and Accountability Act; ICD = implantable cardioverter defibrillator; INR = international normalized ratio; JVD = jugular venous distension.
Types of services covered include telehealth visits, during which an audiovisual telecommunication system is used; the virtual check-in between patient and provider via telephone or another device, which is conducted to decide whether additional services are needed; and electronic visits (“e-visits”), which are communications between patients and providers through an online portal (59). Commercial payers are also reimbursing for telehealth; however, policies surrounding rates and payments are evolving. Penalties against providers for violations of the Health Insurance Portability and Accountability Act are also being waived, allowing the use of more commonly available telecommunications platforms.
The feasibility and safety of telemedicine for patients with HF is well established; however, its use has not yet been reliably associated with reduction in emergency department visits or hospitalizations (60). Moreover, systematic studies of the benefits and limitations of each component of the telehealth visit should be performed. Considering current limitations, telemedicine offers a convenient and effective substitution for in-person visits. Through virtual visits, HF clinicians can maintain face-to-face interactions with their patients; gain familiarity with patients’ domestic circumstances; obtain vital sign measurement through home blood pressure cuffs, pulse oximeters, and scales; perform limited physical examinations for jugular venous distention, peripheral edema, peripheral catheter and driveline site integrity, and functional capacity; reconcile medications through direct visualization of pill containers; and interact with caregivers. Augmentation of telemonitoring through pulmonary artery pressure monitoring and biosensing devices should be explored. With appropriate precautions, home nursing visits to monitor vital signs, perform phlebotomy, administer intravenous medications, and deliver prescriptions offer a complimentary approach to decentralizing HF care. These visits should be structured in order to limit the number of health care contacts.
Therapeutic inertia in HF care is an ongoing risk during this period. Close monitoring of electrolyte and renal function is critical to safe dose adjustment of guideline-directed therapies for HF with reduced ejection fraction and diuretic agents (61). However, the reduction in available laboratory services during the pandemic and patients’ hesitancy to risk COVID-19 exposure may limit uptitration of RAAS antagonists.
In addition to providing standard HF care, clinicians should regularly inquire about dietary and lifestyle habits related to COVID-19 infection. Due to physical and social isolation and other stresses, changes in nutritional status, food availability, alcohol intake, physical activity, and social support may occur and contribute to worsening HF.
This reliance on remote care has the potential to exacerbate pre-existing health inequities (62). Disadvantaged populations, some underrepresented racial and ethnic groups, and those with limited access to the internet and/or “smart” devices may not derive benefit from the expansion of these innovations. Older adults, who comprise a significant portion of the U.S. population with HF, may have educational, visual, auditory, and cognitive impairments that hinder their participation in remote care. Programs should incorporate social workers and case managers to maximize outreach to these at-risk populations. The option for in-person clinic visits should remain available for patients without access to telemedicine services, high-risk patients (e.g., patients on continuous inotropes) or those for whom physical examination is critical for clinical decision making. Aggressive risk reduction practices should be in place during these visits.
Programs may benefit from establishing triage principles for outpatient virtual versus in-person visits. Virtual visits may be best used for medication titration and optimization for stable patients with ACC/American Heart Association Stage C HF. HT recipients on stable immunosuppression at low risk for allograft rejection and hemodynamically optimized patients with LVAD may be managed remotely. In-person visits should be considered for recently hospitalized patients and patients approaching Stage D HF who are on continuous inotropes, undergoing evaluation for advanced HF therapies, who are newly post-LVAD or HT implantation, and those with new-onset HF. Virtual and in-person ambulatory schedules should be constructed to accommodate both routine and urgent visits.
Given uncertainty surrounding the future course of COVID-19, telemedicine is likely to endure as an important component of advanced HF disease management. Monitoring the safety and efficacy of remote care for HF and establishing evidence-based best practices for virtual visits should become a priority for HF programs.
Additional information regarding existing platforms, workflows, and care models for telemedicine in HF is provided in a recently published statement from the Heart Failure Society of America (60).
Procedural delays
Under guidance from the Centers for Disease Control and Prevention, risk mitigation strategies have included the postponement and cancellation of elective diagnostic and therapeutic procedures (6, 63). For stable patients with advanced HF, these procedures include echocardiograms, stress testing, cardiopulmonary exercise testing, right heart catheterizations, coronary angiography, and implantation or interrogation of cardiac electronic devices, among others. Individualized risk assessment is needed when classifying cases as elective. The rationale for delaying procedures should be reviewed with patients and documented in the medical record (64).
Although delay of these procedures may not immediately affect clinical outcomes, there are important long-term and indirect implications for patients with HF (62). Patients undergoing LVAD/HT evaluation may experience delays in listing and/or surgery leading to worsening nutritional, functional, or hemodynamic status. Completion of the evaluation process can highlight opportunities for optimization of pulmonary vascular resistance, renal function, weight, and adherence.
Centers are encouraged to consider local COVID-19 disease prevalence and resource availability when deliberating the timing of LVAD implantation. Limiting implantation to patients in Interagency Registry for Mechanically Assisted Circulatory Support profiles 1 to 3 is a reasonable approach at this time (48). For HT patients >3 months post-transplantation, programs may defer routine surveillance endomyocardial biopsies in those with stable allograft function with low risk of rejection. For those in the more immediate post-transplant period or at higher risk of rejection, the decision to perform surveillance biopsies should be weighed against the risk of COVID-19 exposure to patient and staff (48).
The Changing Landscape Of Heart Transplantation
The COVID-19 pandemic has had far-reaching implications for donor selection, organ procurement organizations, wait-list candidates, and transplant programs (57). Given the limitations of current testing and risks for asymptomatic transmission and infection, the HT community must be careful to select uninfected donors. Globally, organ procurement organizations in Canada, Italy, Spain and other countries have performed universal screening for all deceased donors (65), recognizing the false negative rates of these tests. In some cases, chest computed tomography may be performed as part of donor assessment to evaluate for radiographic evidence of COVID-19 (48). Only donors who are negative for COVID-19 infection should be considered.
According to International Society for Heart and Lung Transplantation guidance, decisions regarding transplantation should be made at the transplanting center based on COVID-19 community prevalence and potential risks and benefits to the patient (48). In institutions with a high burden of COVID-19 infection, transplants are being reserved for patients in highest urgency statuses whose wait-list mortality risk supersedes the risk of nosocomial infection (57).
Beginning the week of March 15, 2020, transplant programs were able to inactivate waitlisted patients as a COVID-19 precaution (66). Weekly national and regional data is available from the United Network for Organ Sharing (66). Evaluation of changes in transplantation volumes and outcomes over time will be necessary to understand the implications of the COVID-19 pandemic in HT candidates and recipients.
Gaps In Knowledge And Long-Term Impacts
There remains much to learn about the pathophysiology of COVID-19 in patients with HF. Concurrently, robust research is needed in health services focused on elucidating the impacts of telemedicine, elective care deferral, and risk aversion behaviors being practiced by patients and providers during the pandemic. These and other research questions are described in Table 2 . The economic upheaval caused by the COVID-19 pandemic has wide-ranging health consequences, especially for those who already live under conditions of socioeconomic deprivation. The national burdens of HF incidence, prevalence, and undertreatment will likely grow as a result of new COVID-19-related heart disease, delays in the recognition and treatment of ischemic heart disease, rising unemployment, and loss of income and health benefits for large segments of the population (62).
Table 2.
Unanswered Questions Regarding the Care of Patients With Advanced Heart Failure During the COVID-19 Pandemic
| Domain | Question |
|---|---|
| Telemedicine | |
| Safety and efficacy of remote care, home health Safety of visiting nurse, home phlebotomy exposures Capacity for remote routine and urgent/sick visits Impact on patient quality of life Impact on patient, family, caregiver engagement with care team Adjunct diagnostics needed for home care Alteration in GDMT titration schedule Perpetuation or exacerbation of health disparities Applicability of virtual health to palliative care |
|
| Procedural delays | |
| Impacts of delays of ICD, CRT, VAD implantation Safety of noninvasive post-transplant rejection surveillance Alternatives for post-transplantation rejection surveillance and weaning from immunosuppression Impact of transplantation waitlist inactivations |
CRT = cardiac resynchronization therapy; GDMT = guideline-directed medical therapy; ICD = implantable cardioverter-defibrillator; VAD = ventricular assist device.
Despite this devastation, the COVID-19 pandemic has given the HF community opportunities to explore alternative care delivery models, optimize ancillary support structures, and expand the infrastructure for home nursing, palliative care, and hospice services.
Conclusions
The COVID-19 pandemic has significantly impacted our patients with advanced HF, including the LVAD/HT population and our care delivery systems. Over the coming months, anticipated and unforeseen challenges will arise as we manage the ramifications of this pandemic for our patients and the HF community.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Barry Greenberg, MD, was Guest Editor on this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Heart Failureauthor instructions page.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 April 24 doi: 10.1161/CIRCULATIONAHA.120.047430. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 [E-pub ahead of print] doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arentz M., Yim E., Klaff L. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alger H.M., Williams J.H.I.V., Walchok J.G. The role of data registries in the time of COVID-19. Circ Cardiovasc Qual Outcomes. 2020 May;13 doi: 10.1161/CIRCOUTCOMES.120.006766. [DOI] [PubMed] [Google Scholar]
- 14.Heart Failure Society of America Seeking Cardiac Tissue Samples from Myocarditis Patients for Assessing SARS-CoV2 and Myocarditis. 2020. https://hfsa.org/seeking-cardiac-tissue-samples-myocarditis-patients-assessing-sars-cov2-and-myocarditis Available at:
- 15.Bartlett R.H., Ogino M.T., Brodie D. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta N., Kalra A., Nowacki A.S. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020 May 5 doi: 10.1001/jamacardio.2020.1855. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds H.R., Adhikari S., Pulgarin C. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020 May 1 doi: 10.1056/NEJMoa2008975. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26:370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acanfora D., Ciccone M.M., Scicchitano P., Acanfora C., Casucci G. Neprilysin inhibitor–angiotensin II receptor blocker combination (sacubitril/valsartan): rationale for adoption in SARS-CoV-2 patients. Eur Heart J Cardiovasc Pharmacother. 2020;6:135–136. doi: 10.1093/ehjcvp/pvaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Liu G., Zhou W., Zhang W., Wang K., Zhang J. Neprilysin inhibitor-angiotensin II receptor blocker combination therapy (sacubitril/valsartan) suppresses atherosclerotic plaque formation and inhibits inflammation in apolipoprotein e- deficient mice. Sci Rep. 2019;9:6509. doi: 10.1038/s41598-019-42994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhaber S.Z. Venous thromboembolism in heart failure patients: pathophysiology, predictability, prevention. J Am Coll Cardiol. 2020;75:159–162. doi: 10.1016/j.jacc.2019.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Fanola C.L., Norby F.L., Shah A.M. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75:148–158. doi: 10.1016/j.jacc.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speed V., Roberts L.N., Patel J.P., Arya R. Venous thromboembolism and women’s health. Br J Haematol. 2018;183:346–363. doi: 10.1111/bjh.15608. [DOI] [PubMed] [Google Scholar]
- 26.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canepa M., Franssen F.M.E., Olschewski H. Diagnostic and therapeutic gaps in patients with heart failure and chronic obstructive pulmonary disease. J Am Coll Cardiol HF. 2019;7:823–833. doi: 10.1016/j.jchf.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Vachiéry J.-L., Adir Y., Barberà J.A. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Moloney E.D., Evans T.W. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003;21:720–727. doi: 10.1183/09031936.03.00120102. [DOI] [PubMed] [Google Scholar]
- 30.Moss N., Rakita V., Lala A. Hemodynamic response to exercise in patients supported by continuous flow left ventricular assist devices. J Am Coll Cardiol HF. 2020;8:291–301. doi: 10.1016/j.jchf.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Lang C.C., Agostoni P., Mancini D.M. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail. 2007;13:672–679. doi: 10.1016/j.cardfail.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020 May 7 doi: 10.1056/NEJMoa2012410. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang D., Saleh M., Gabriels J. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;S0735-1097 doi: 10.1016/j.jacc.2020.04.032. 35009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 May 1 doi: 10.1001/jamacardio.2020.1834. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link M.G., Yan G.-X., Kowey P.R. Evaluation of toxicity for heart failure therapeutics: studying effects on the QT interval. Circ Heart Fail. 2010;3:547–555. doi: 10.1161/CIRCHEARTFAILURE.109.917781. [DOI] [PubMed] [Google Scholar]
- 37.Vrtovec B., Delgado R., Zewail A., Thomas C.D., Richartz B.M., Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation. 2003;107:1764–1769. doi: 10.1161/01.CIR.0000057980.84624.95. [DOI] [PubMed] [Google Scholar]
- 38.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for drug interactions on QTc in exploratory COVID-19 (Coronavirus Disease 2019) treatment. J Am Coll Cardiol. 2020;75:2623–2624. doi: 10.1016/j.jacc.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tisdale J.E., Jaynes H.A., Kingery J.R. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy S.P., Kakkar R., McCarthy C.P., Januzzi J.L. Inflammation in heart failure. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Radley G., Pieper I.L., Ali S., Bhatti F., Thornton C.A. The inflammatory response to ventricular assist devices. Front Immunol. 2018;9:2651–2657. doi: 10.3389/fimmu.2018.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosman-Rimon L., McDonald M.A., Jacobs I. Markers of inflammation in recipients of continuous-flow left ventricular assist devices. ASAIO J. 1992 2014;60:657–663. doi: 10.1097/MAT.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein N.E., Mather H., McKendrick K. Improving communication in heart failure patient care. J Am Coll Cardiol. 2019;74:1682–1692. doi: 10.1016/j.jacc.2019.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimball P.M., Flattery M., McDougan F., Kasirajan V. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg. 2008;85:1656–1661. doi: 10.1016/j.athoracsur.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 45.Ankersmit H.J., Tugulea S., Spanier T. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–555. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 46.Chau V.Q., Oliveros E., Mahmood K. The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD support. J Am Coll Cardiol Case Rep. 2020 April 8 doi: 10.1016/j.jaccas.2020.04.001. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R., Domenico C., Rao S. Novel coronavirus disease 2019 in a patient on durable left ventricular assist device support. J Card Fail. 2020;26:438–439. doi: 10.1016/j.cardfail.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aslam S., Danziger-Isakov L., Luong M.-L. Guidance from the International Society of Heart and Lung Transplantation Regarding the SARS CoV-2 Pandemic. International Society of Heart and Lung Transplantation; 2020. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_-Guidance-for-Cardiothoracic-Transplant-and-VAD-centers.pdf Available at:
- 49.Fishman J.A., Grossi P.A. Novel coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20:1765–1767. doi: 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romanelli A., Mascolo S. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20:1947–1948. doi: 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abadja F., Atemkeng S., Alamartine E., Berthoux F., Mariat C. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation. 2011;92:396–403. doi: 10.1097/TP.0b013e3182247b5f. [DOI] [PubMed] [Google Scholar]
- 52.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi G., Ozog S., Torbett B.E., Compton A.A. mTOR inhibitors lower an intrinsic barrier to virus infection mediated by IFITM3. Proc Natl Acad Sci U S A. 2018;115:E10069–E10078. doi: 10.1073/pnas.1811892115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Ruiz M., Andrés A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39:496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latif F., Farr M.A., Clerkin K.J. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020 May 13 doi: 10.1001/jamacardio.2020.2159. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeFilippis E.M., Farr M.A., Givertz M.M. Challenges in heart transplantation in the era of COVID-19. Circulation. 2020;141:2048–2051. doi: 10.1161/CIRCULATIONAHA.120.047096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang G., Hu C., Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Medicare and Medicaid Services General Telemedicine Toolkit. https://www.cms.gov/files/document/general-telemedicine-toolkit.pdf Available at:
- 60.Gorodeski E.Z., Goyal P., Cox Z.L. Virtual visits for care of patients with heart failure in the era of COVID-19: a statement from the Heart Failure Society of America. J Card Fail. 2020;S1071-9164:30367-5. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 62.Reza N., DeFilippis E.M., Jessup M. Secondary impact of the COVID-19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007219. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19) Cent Dis Control Prev. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html Available at: [Google Scholar]
- 64.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force, electrophysiology section of the American College of Cardiology, and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;S1547-5271:30289-7. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar D., Manuel O., Natori Y. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.United Network for Organ Sharing COVID-19 and Solid Organ Transplant. https://unos.org/covid/ Available at:
- 67.Hsu J.J., Gaynor P., Kamath M. COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant. 2020;20:1911–1915. doi: 10.1111/ajt.15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holzhauser L., Lourenco L., Sarswat N., Kim G., Chung B., Nguyen A.B. Early Experience of COVID-19 in 2 Heart Transplant Recipients: Case Reports and Review of Treatment Options. Am J Transplant. 2020 Apr 21 doi: 10.1111/ajt.15982. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathies D., Rauschning D., Wagner U. A case of SARS-CoV-2-pneumonia with successful antiviral therapy in a 77-year-old male with heart transplant. Am J Transplant. 2020;20:1925–1929. doi: 10.1111/ajt.15932. [DOI] [PMC free article] [PubMed] [Google Scholar]