Abstract
The persistence of HIV-1 associated neurocognitive disorders (HAND) in the post-cART era, afflicting between 40 and 70% of HIV-1 seropositive individuals, supports a critical need for the development of adjunctive therapeutic treatments. Selective estrogen receptor β agonists, including S-Equol (SE), have been implicated as potential therapeutic targets for the treatment of neurocognitive disorders. In the present study, the therapeutic efficacy of 0.2 mg SE for the treatment of HAND was assessed to address two key questions in the HIV-1 transgenic (Tg) rat. First, does SE exhibit robust therapeutic efficacy when treatment is initiated relatively early (i.e., between 2 and 3 months of age) in the course of viral protein exposure? Second, does the therapeutic utility of SE generalize across multiple neurocognitive domains? Treatment with SE enhanced preattentive processes and stimulus-response learning to the level of controls in all (i.e., 100%) HIV-1 Tg animals. For sustained and selective attention, statistically significant effects were not observed in the overall analyses (Control: Placebo, n = 10, SE, n = 10; HIV-1 Tg: Placebo, n = 10, SE, n = 10). However, given our a priori hypothesis, subsequent analyses were conducted, revealing enhanced sustained and selective attention, approximating controls, in a subset (i.e., 50%, n = 5 and 80%, n = 8, respectively) of HIV-1 Tg animals treated with SE. Thus, the therapeutic efficacy of SE is greater when treatment is initiated relatively early in the course of viral protein exposure and generalizes across neurocognitive domains, supporting an adjunctive therapeutic for HAND in the post-cART era.
Graphical Abstract.
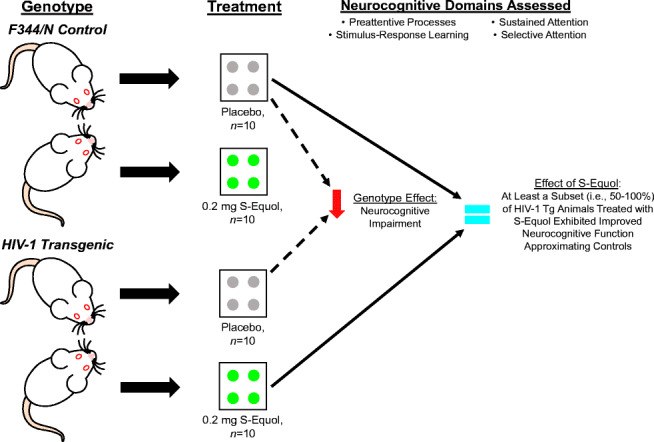
HIV-1 transgenic (Tg) and control animals were treated with either 0.2 mg S-Equol (SE) or placebo between 2 and 3 months of age (Control: Placebo, n = 10, SE, n = 10; HIV-1 Tg: Placebo, n = 10, SE, n = 10). Neurocognitive assessments, tapping preattentive processes, stimulus response learning, sustained attention and selective attention, were conducted to evaluate the utility of SE as a therapeutic for HIV-1 associated neurocognitive disorders (HAND). Planned comparisons between HIV-1 Tg and control animals treated with placebo were utilized to establish a genotype effect, revealing prominent neurocognitive impairments (NCI) in the HIV-1 Tg rat across all domains. Furthermore, to establish the utility of SE, HIV-1 Tg animals treated with SE were compared to control animals treated with placebo. Treatment with 0.2 mg SE ameliorated NCI, to levels that were indistinguishable from controls, in at least a subset (i.e., 50–100%) of HIV-1 Tg animals. Thus, SE supports an efficacious, adjunctive therapeutic for HAND.
Keywords: S-Equol, Preattentive processes, Sustained attention, Selective attention
Introduction
The advent of combination antiretroviral therapy (cART), the primary treatment regimen for individuals with human immunodeficiency virus type 1 (HIV-1), dramatically decreased the severity of neurocognitive deficits associated with HIV-1 (Ances and Ellis 2007). However, HIV-1 associated neurocognitive disorders (HAND) persist, afflicting between 40 and 70% of HIV-1 seropositive individuals (Letendre et al. 2010; McArthur et al. 2010; Heaton et al. 2011). In the post-cART era, HAND has been defined as a progressive, neurodegenerative disease (Cohen et al. 2015; McLaurin et al. 2019a) characterized by alterations in speed of information processing, attention, working memory, and executive function (e.g., Cysique et al. 2004; Garvey et al. 2009; Heaton et al. 2011). Due to the prevalence of HAND in the post-cART era, and its progressive nature (Heaton et al. 2015; Gott et al. 2017; McLaurin et al. 2019a), there is a critical need to develop additional neuroprotective and/or neurorestorative therapeutics.
Estrogen receptors (ER), which belong to the nuclear receptor family of transcription factors, are classified into two primary subtypes, including ERα (Jensen 1962), and ERβ (Kuiper et al. 1996). Although both ERα and ERβ bind to 17β-estradiol with high affinity (Kuiper et al. 1996) and share structural characteristics (e.g., near-identical DNA-binding domain (96%), Kuiper et al. 1996), significant differences in tissue distribution and biological effects have been observed (e.g., Kuiper et al. 1997). Specifically, ERα is predominant in reproductive organs (e.g., uterus, mammary glands), skeletal muscle and bone, playing a critical role in maintaining female reproductive functions (for review, Paterni et al. 2014). ERβ, however, is involved in mediating estradiol signaling in the immune and central nervous systems (for review, Paterni et al. 2014). Within the central nervous system, cells containing ERβ mRNA or immunoactivity are widely dispersed (e.g., Li et al. 1997; Shughrue et al. 1997; Zhang et al. 2002; Gonzalez et al. 2007), and observed in brain regions (e.g., prefrontal cortex, ventral tegmental area, hippocampus) commonly associated with HAND (e.g., Maki et al. 2009; Israel et al. 2019).
Since 2005 (Kendall et al. 2005; Wallace et al. 2006), multiple studies have been conducted to evaluate the utility of estrogenic compounds to protect against the neurotoxic effects of HIV-1 viral proteins. Initial in vitro studies reported that pretreatment with ER agonists blocked neurotoxic effects (Kendall et al. 2005), attenuated oxidative stress (Wallace et al. 2006) and prevented the loss of dopamine transporter function (Wallace et al. 2006) induced by HIV-1 viral proteins (i.e., Tat, gp120). Subsequent investigations were targeted at evaluating whether the neuroprotective effects of estrogen occurred via an ER sensitive or non-receptor mediated mechanism; studies which revealed that the ERβ subtype mediated the 17β-estradiol attenuation of Tat-induced apoptotic signaling in cortical cell cultures (Adams et al. 2010). ERβ, therefore, may support a key target for the development of adjunctive therapeutics for HAND in the post-cART era.
Phytoestrogens, which exhibit a higher affinity for ERβ than ERα (e.g., Kuiper et al. 1998; Mueller et al. 2004), are plant-derived compounds that are structurally similar to 17β-estradiol (Glazier and Bowman 2001). Isoflavones, including genistein, daidzein (DAI), and glycitein, are one class of phytoestrogens commonly found in soy products (Murphy et al. 1982; Setchell 1998). Equol is an active metabolite produced by gut microbiota following the ingestion of the soy derived phytoestrogen DAI (Setchell et al. 1984). S-Equol (SE), the only enantiomer produced by humans (Setchell et al. 2005), exhibits neuroprotective effects via its selective affinity for ERβ (Setchell et al. 2005; Bertrand et al. 2015). Furthermore, when SE crosses the blood-brain-barrier it distributes most significantly to the prefrontal cortex (Lund et al. 2001); a brain region associated with higher-order cognitive functioning. Most critically, however, the translational relevance of SE is evidenced by its progression into clinical trials for Alzheimer’s disease (Ausio Pharmaceuticls; NCT03101085), another progressive, neurodegenerative disorder.
SE has been implicated as a potential adjunctive therapeutic for HAND in both in vitro (Bertrand et al. 2015) and in vivo (Moran et al. 2019) studies. In primary neuronal cell cultures, pretreatment with SE prevented synapse loss induced by the HIV-1 viral protein, Tat (Bertrand et al. 2015). Precursors to SE, including DAI and liquiritigenin (LQ), which also selectively target ERβ (DAI: Casanova et al. 1999; LQ: Mersereau et al. 2008), also prevented Tat induced neuronal apoptosis (Adams et al. 2012) and restored synaptodendritic injury (Bertrand et al. 2014). Given the utility of phytoestrogens to prevent and restore synaptic function in vitro, subsequent in vivo studies were targeted at protecting and/or restoring neurocognitive function following constitutive expression of HIV-1 viral proteins; a therapeutic approach that may be key to effectively treating neurocognitive deficits in HAND. In a dose-response study in the HIV-1 transgenic (Tg) rat, treatment with SE between 6 and 8 months of age enhanced sustained attention, to the level of controls, in a subset (i.e., 40%) of animals (Moran et al. 2019). To date, however, the generalizability of the therapeutic efficacy of SE when treatment occurs at an earlier age and across neurocognitive domains has not yet been assessed.
Neurocognitive functions, including preattentive processes, stimulus-response learning, and attention, are generally componential (Keeler and Robbins 2011). Specifically, sensory input is transformed to motor output via representational knowledge and executive functions (Keeler and Robbins 2011). At the most basic level, HIV-1 seropositive individuals (Minassian et al. 2013) display prominent alterations in preattentive processes (sensorimotor gating); deficits which have been translationally modeled across multiple biological systems used to model HAND (e.g., HIV-1 Tg rat: Moran et al. 2013, McLaurin et al. 2017, 2018; stereotaxic injections of HIV-1 viral proteins: Fitting et al. 2006a, 2006b; gp120 transgenic mice: Henry et al. 2014, Bachis et al. 2016; Tat transgenic mice: Paris et al. 2015). Alterations in the core components of cognitive function, including key components of both representational knowledge (e.g., attention, long-term episodic memory) and executive function (e.g., flexibility, inhibition), have also been reported in clinical (e.g., Heaton et al. 2011; Maki et al. 2015; Kanmogne et al. 2018) and preclinical (e.g., Lashomb et al. 2009; Moran et al. 2014; Repunte-Canonigo et al. 2014; McLaurin et al. 2018, 2019a) studies. Given the componential relationship between neurocognitive functions, examining the effect of SE on the core components of cognitive function (i.e., preattentive processes, stimulus-response learning, sustained attention, and selective attention) will provide one critical test of its therapeutic potential for HAND in the post-cART era.
In light of previous work, the goals of the present study were twofold: 1) Utilizing the HIV-1 transgenic (Tg) rat, developed by Reid et al. (2001), to assess whether SE exhibits greater therapeutic efficacy when treatment is initiated relatively early (i.e., between 2 and 3 months of age) in the course of HIV-1 viral protein exposure; 2) To determine whether the therapeutic utility of SE generalizes across multiple neurocognitive domains, including preattentive processes, stimulus-response learning, sustained attention, and selective attention. It was hypothesized that relatively early initiation of SE would ameliorate neurocognitive impairments across multiple neurocognitive domains in a subset of HIV-1 Tg animals; an effect that would enhance cognitive function to the level of controls. Understanding the generalizability of SE across ages (i.e., 2–3 months of age vs. 6–8 months of age (Moran et al. 2019)) and neurocognitive domains may aid in the development of an efficacious adjunctive therapeutic for HAND in the post-cART era.
Methods
Experimental Design
An experimental timeline for SE treatment and neurocognitive assessments is illustrated in Fig. 1.
Fig. 1.
Schematic of the Experimental Design
Animals
At approximately two months of age, ovariectomized (OVX) female Fischer (F344/N; Harlan Laboratories Inc., Indianapolis, IN) HIV-1 Tg (n = 20) and control (n = 20) rats were delivered to the animal vivarium in two separate batches, one week apart in delivery and age. All animals were pair- or group-housed throughout the duration of experimentation. Rats were handled for one week prior to beginning neurocognitive assessments.
All HIV-1 Tg and control animals were OVX at Harlan Laboratories prior to arrival at the animal vivarium. OVX animals and a minimal phytoestrogen diet (≤20 ppm; Teklad 2020X Global Extruded Rodent Diet (Soy Protein-Free)) were utilized to preclude the potential confounding effect of endogenous hormones. Rodent food and water were available ad libitum throughout the pretest cross-modal prepulse inhibition (PPI) assessment. Animals were placed under food restriction, to maintain 85% body weight, one week prior to beginning operant testing. Rodent food was again provided ad libitum at the conclusion of operant testing and during the posttest cross-modal PPI assessment.
Guidelines established in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH) were utilized for the maintenance of animals in AAALAC-accredited facilities. The animal vivarium was maintained at 21° ± 2°C, 50% ± 10% relative humidity and had a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the project protocol under federal assurance (#D16–00028).
S-Equol
SE was obtained from Cayman Chemical Company (Ann Arbor, MI) and incorporated into 90 mg sucrose pellets by Bio-Serv (Frenchtown, NJ). Each sucrose pellet contained 0.05 mg SE. The placebo group received plain 90 mg sucrose pellets, which were also obtained from Bio-Serv.
At approximately 2–3 months of age and one week prior to beginning operant training, animals began daily treatment with SE or placebo. Animals were randomly assigned to either the SE or placebo group (Control: SE, n = 10, Vehicle, n = 10; HIV-1 Tg: SE, n = 10, Vehicle, n = 10). HIV-1 Tg and control animals treated with SE received four 90 mg sucrose pellets for a daily oral dose of 0.2 mg of SE. A dose-response experimental design previously revealed a linear dose-response with the most effective dose at 0.2 mg SE (Moran et al. 2019). Furthermore, the dose selected yielded a daily amount of 0.25–1.0 mg/kg SE; an amount equivalent to a 2.5–10 mg dose in a 60 kg human (Cf., most elderly Japanese have a daily isoflavone intake of 30–50 mg, Akaza 2012).The placebo group received four 90 mg sucrose pellets. Each rat was administered its treatment at least an hour after neurocognitive assessments and typically consumed their pellets within seconds. Animals were treated after neurocognitive assessments to promote long-term remodeling of neuronal circuitry by preventing synapse loss induced by HIV-1 viral proteins (e.g., Bertrand et al. 2014, 2015). Treatment was continued for 60 days.
Neurocognitive Domain 1: Preattentive Processes (Prepulse Inhibition)
Apparatus
A 10 cm-thick double-walled, 81 × 81 × 116-cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY) enclosed the startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA), providing over 30 dB(A) of sound attenuation relative to the external environment. The ambient sound level in the chamber, in the absence of any stimuli, was 22 dB(A). A high-frequency loudspeaker of the SR-Lab system (Radio Shack model #40-1278B) was mounted inside the isolation cabinet 30-cm above the Plexiglas test cylinder for the presentation of all discrete auditory prepulses and startling stimuli (white noise, frequency range of 5 k–16 k Hz). A microphone was placed inside the Plexiglas cylinder for the measurement and calibration of sound levels (Sound Level Meter: model #2203, Bruël & Kjaer, Norcross, GA). A 22 lx white LED light (Light meter model #840006, Sper Scientific, Ltd., Scottsdale, AZ) was affixed on the isolation cabinet wall in front of the Plexiglas test cylinder for the presentation of discrete visual prepulses. The animal’s whole body startle response to the auditory startle stimulus produced deflection of the Plexiglas test cylinder; a deflection that was converted into analog signals by a piezoelectric accelerometer integral to the bottom of the cylinder. Response signals were digitized (12 bit A to D) and saved to a hard disk. The SR-LAB Startle Calibration System was utilized to calibrate all response sensitivities.
Procedure
Habituation
At approximately 2 months of age, a 36-trial auditory startle test session was conducted to habituate animals to assessment procedures, as well as to the auditory startling stimulus. Habituation was administered beginning with a 5-min acclimation period in the dark with 70 dB(A) background white noise. Subsequently, 36 trials of a 100 dB(A) white noise stimulus (20 msec duration) were presented. The intertrial interval (ITI) was fixed at 10-s. Although HIV-1 Tg animals exhibited an overall decreased startle response relative to controls, no significant differences in the rate of habituation were observed (p > 0.05; data not shown).
Cross-Modal Prepulse Inhibition
HIV-1 Tg and control animals were assessed for PPI of the auditory startle response (ASR) using both auditory and visual prepulse stimuli at 2 months of age (prior to beginning SE treatment) and at 6–7 months of age. The assessment was conducted similar to our prior publication (Moran et al. 2013). In brief, PPI was assessed during a 30-min test session that began with a 5-min acclimation period in the dark with 70 dB(A) background white noise. After the acclimation period, six pulse-only ASR trials with a fixed 10-s ITI were presented. Seventy-two testing trials were subsequently presented, including an equal number of auditory and visual prepulse trials, arranged using an ABBA counterbalanced order of presentation. Testing trials were presented in 6-trial blocks, interdigitated using a Latin-square experimental design, with interstimulus intervals (ISI) of 0, 8, 40, 80, 120, and 4000 msec and a variable ITI (15–25 s). Control trials, including both the 0 and 4000 msec ISI trials, provided a reference ASR within the test session. Mean peak ASR amplitude values were collected for analysis.
Neurocognitive Domains 2 & 3: Stimulus-Response Learning and Sustained Attention (Signal Detection Operant Task)
Apparatus
HIV-1 Tg and control animals were trained and assessed in a signal detection operant task using 22 operant boxes located inside sound-attenuating chambers (Med Associates, Inc., Fairfax, VT). The front wall of the operant chambers included a 45 mg pellet dispenser, two retractable levers, and three panel lights (22 lx). The rear wall of the operant chambers had a house light (5.5 lx). A PC and Med-PC for Windows software (V 4.1.3; Med Associates Inc., Fairfax, VT) controlled the presentation of signals, lever operation, reinforcement delivery, and data collection.
Procedure
Shaping
At approximately 2 months of age, HIV-1 Tg and control animals were trained to lever-press using a standard shaping response protocol (Moran et al. 2014). In brief, a fixed ratio 1 (FR-1) schedule of reinforcement was used to train animals to press both levers for a reinforcer (i.e., 45 mg sucrose pellet). An animal was not reinforced for more than five consecutive presses on a single lever to prevent side bias. All HIV-1 Tg and control animals successfully acquired shaping within 36 days by achieving the criterion of at least 40 reinforcers during the 42-min test session for three consecutive days, with less than 20% variance across days. Following the successful completion of the shaping response protocol, animals were promoted to the signal detection operant task.
Signal Detection Operant Task
The signal detection operant task, tapping sustained attention, employed three vigilance programs, initially described by McGaughy and Sarter (1995), that trained animals to discriminate between signal (i.e., central panel light illumination) and non-signal (i.e., no illumination) trials. Methodology utilized in the present study is similar to our prior publication (McLaurin et al. 2019a) with minor modifications.
In brief, each signal detection operant session began with a 5-min acclimation period in the dark. The presentation of signals (central panel light illumination) and non-signals (no illumination) was randomized across trials throughout the session, with varying ITIs (9 ± 3 s), during which time the levers remained retracted. Two seconds after each trial began, levers were extended until the animal made a response, or 6 s elapsed, whichever occurred first. For half of the animals, lever presses on the left lever during signal trials (Hits) and on the right lever during non-signal trials (Correct Rejections) were rewarded with a 45 mg sucrose pellet. In the same manner, responses on the right lever during signal trials (Misses) and on the left lever during non-signal trials (False Alarms) were not reinforced. The other half of the animals were trained using the reverse set of contingencies.
During the first vigilance program, consisting of 160 trials per session, termination of the central panel light illumination (i.e., the signal) was contingent upon a response. In the second vigilance program, also consisting of 160 trials per session, the central panel light was illuminated for 1 s. Correction trials and force-choice trials, which occurred after an incorrect response, were an integral component of the first two vigilance programs. Specifically, during correction trials, an animal was provided with up to three repetitions of the trial. If an animal failed to respond appropriately to the correction trials, a forced-choice trial occurred during which the same stimulus type (i.e., signal or non-signal) was repeated, but only the correct lever was extended. The lever remained extended until a response was made or 2 min elapsed, whichever occurred first. The third vigilance program consisted of 162 trials per session and manipulated the signal duration (i.e., 1000, 500, 100 msec) across trials using a block randomized experimental design. Correction trials and forced-choice trials were removed in the third vigilance program.
HIV-1 Tg and control animals were trained on each vigilance program, assessing signal detection, until achieving at least 70% accuracy on three consecutive test sessions or until 76 days. Accuracy was calculated as the total number of hits and correct rejections divided by the total number of correct and incorrect responses in a session.
Neurocognitive Domain 4: Selective Attention (Visual Distractor Task)
Apparatus
The assessment of selective attention in HIV-1 Tg and control animals was conducted in the operant chambers described above.
Procedure
For the assessment of selective attention, the signal detection operant task with varying signal durations was divided into three trial blocks, each containing 54 trials. During the second trial block, a 1.5 s visual distractor stimulus was presented at the beginning of each trial. The visual distractor stimulus onset and offset was 1 s prior to and 0.5 s after the signal onset (center panel light), for a 1.5 s total duration. The house light was used as the visual distractor, with an intensity of 5.5 lx measured from the center of the chamber at the level of the animal’s height. The assessment was conducted for three consecutive sessions.
Statistical Analysis
Statistical techniques, including analysis of variance (ANOVA) and regression, were utilized for the analysis of all data (SAS/STAT Software 9.4, SAS Institute, Inc., Cary, NC; SPSS Statistics 26, IBM Corp., Somer NY; GraphPad Software, Inc., La Jolla, CA). Figures were created using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). For all statistical analyses, significance was set at an alpha criterion of p ≤ 0.05. Orthogonal decompositions or the Greenhouse-Geisser df correction factor (Greenhouse and Geisser 1959) were used for variables that classically violate compound symmetry assumptions.
A mixed-factor ANOVA was conducted to examine the effect of the HIV-1 transgene and/or SE treatment on body weight during food restriction (SPSS Statistics 26, IBM Corp., Somer NY). Genotype (HIV-1 Tg vs. control) and treatment (SE vs. placebo) were included as between-subject’s factors, while age served as a within-subject’s factor. Furthermore, linear regression analyses, fit with 95% confidence intervals (CI), were also utilized to directly assess functional form (GraphPad Software, Inc., La Jolla, CA).
Two approaches were utilized to assess the therapeutic efficacy of SE for HAND. First, statistical analyses were conducted on all animals (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 10). Second, when statistically significant effects were not observed in the overall analysis, complementary analyses including a subset of animals were conducted. Based on previous work (Moran et al. 2019), our a priori hypothesis was that SE treatment would only mitigate neurocognitive deficits in a subset of HIV-1 Tg animals. Furthermore, a priori planned comparisons were conducted to evaluate the genotype deficit (i.e., Control Placebo vs. HIV-1 Tg Placebo) and the effect of SE (i.e., amelioration of neurocognitive deficits to approximate control animals treated with placebo; Control Placebo vs. HIV-1 Tg SE).
The temporal process of acquisition (i.e., the number of days to meet criterion), an assessment of stimulus-response learning, was analyzed using a generalized linear mixed effects model with a Poisson distribution (SAS/STAT Software 9.4, SAS Institute, Inc., Cary, NC). Linear regression analyses, fit with 95% CI, were also utilized to directly assess functional form (GraphPad Software, Inc., La Jolla, CA). Genotype (HIV-1 Tg vs. control) and treatment (SE vs. placebo) were included as between-subject’s factors.
A mixed-factor ANOVA with a compound symmetry covariance structure (SAS/STAT Software 9.4, SAS Institute, Inc., Cary, NC) was utilized for the assessment of preattentive processes and sustained attention. Selective attention was assessed by conducting a mixed-factor ANOVA in SPSS (SPSS Statistics 26, IBM Corp., Somer NY). Genotype (HIV-1 Tg vs. control) and treatment (SE vs. placebo) served as between-subject’s factors. ISI (0, 8, 40, 80, 120, 4000), response type (hits, misses), signal duration (1000, 500, 100 msec), and time (Pretest Assessment vs. Posttest Assessment) served as the within-subject’s factors, as appropriate. ISI, trial, signal duration, and time were included as random effects in the analysis, as appropriate. For the analysis of preattentive processes, the dependent variable (i.e., mean peak ASR amplitude) was log transformed. For the analysis of sustained attention, the relative frequency of hits and misses served as the dependent variable. Furthermore, for the analysis of selective attention, the relative frequency of hits and misses during the second trial block (i.e., when the distractor was present), served as the dependent variable.
Results
Body Weight: Somatic Growth
Body weight was assessed as a measurement of somatic growth in HIV-1 Tg and control animals from 8 weeks of age through 28 weeks of age (Fig. 2). At approximately 10 weeks of age, animals were placed on food restriction prior to beginning the signal detection operant task. During the implementation of food restriction (10 Weeks to 28 Weeks of Age), HIV-1 Tg animals, independent of treatment (i.e., SE vs. placebo) weighed significantly less than control animals (Main Effect of Genotype: F(1,36) = 56.6, p ≤ 0.001). Neither a significant main effect of treatment (p > 0.05) nor a genotype x treatment interaction (p > 0.05) were observed.
Fig. 2.

Mean body weight is illustrated as a function of genotype (HIV-1 Tg vs. Control) and age (±95% CI). HIV-1 Tg animals weighed significantly less than the control group across the testing period. Both groups increased significantly in body weight across this period and did not differ in their rates of growth. The x-axis break at 65 days indicates the point at which animals began food restriction, prior to testing
Both HIV-1 Tg and control animals exhibited steady growth across development, evidenced by a linear increase in body weight (Control: R2 = 0.92; HIV-1 Tg: R2 = 0.95). Most notably, however, no significant differences between HIV-1 Tg and control animals were observed in the rate of somatic growth (i.e., β1; F(1,34) = 2.4, p > 0.05). Thus, although HIV-1 Tg animals weighed significantly less than their control counterparts, no significant alterations in the growth trajectory of HIV-1 Tg animals were observed. Furthermore, independent of genotype, treatment with SE did not alter somatic growth.
Neurocognitive Domain 1: Preattentive Processes
HIV-1 Tg and control animals were assessed in PPI, tapping preattentive processes, prior to SE treatment (i.e., Pretest Assessment, 2 months of age; Fig. 3a) and after SE treatment (i.e., Posttest Assessment, 6–7 months of age; Fig. 3b). At both the pretest and posttest assessments, all animals, independent of genotype and/or treatment, displayed maximal inhibition at the 40 msec ISI. Observations of robust inhibition to the visual prepulse at the 40 msec ISI during both test sessions supports the integrity of visual system function. However, significant alterations in the development of perceptual sharpening were observed in HIV-1 Tg animals treated with placebo; deficits that were restored with SE treatment.
Fig. 3.

Mean peak ASR amplitude for prepulse inhibition (PPI), an assessment of preattentive processes, with a visual prepulse is illustrated as a function of genotype (HIV-1 Tg vs. Control), age, and treatment (S-Equol (SE) vs. placebo; ±SEM). At 2 months of age (a), no significant alterations in PPI were observed. However, at 6–7 months of age (b), HIV-1 Tg animals treated with placebo exhibited a prominent deficit in temporal processing relative to control animals; a deficit which was ameliorated in HIV-1 Tg animals treated with SE
Control animals, independent of treatment, exhibited age-related perceptual sharpening evidenced by a sharper ISI function at the posttest assessment relative to the pretest assessment. In sharp contrast, HIV-1 Tg animals treated with placebo failed to develop perceptual sharpening. Specifically, at the posttest assessment, HIV-1 Tg animals treated with placebo displayed a relative insensitivity to the manipulation of ISI, evidenced by a flattening of the ISI function relative to control animals. Treatment with SE, however, ameliorated alterations in the development of perceptual sharpening in the population of HIV-1 Tg animals sampled.
The overall mixed-model ANOVA conducted on mean peak response amplitude (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 10) confirmed our observations, revealing a statistically significant genotype x treatment x ISI interaction [F(5,180) = 3.2, p ≤ 0.008]; an interaction which was subsequently examined by conducting a priori planned comparisons (i.e., Control Placebo vs. HIV-1 Tg Placebo, Control Placebo vs. HIV-1 Tg SE). Comparison of HIV-1 Tg and control animals treated with placebo revealed a statistically significant genotype x ISI interaction [F(5,90) = 5.5, p ≤ 0.001]; an interaction not observed when HIV-1 Tg treated with SE were compared with control animals treated with placebo [p > 0.05]. Thus, treatment with SE ameliorated the marked impairment in the development of perceptual sharpening in the population of HIV-1 Tg animals sampled.
Neurocognitive Domains 2 & 3: Stimulus-Response Learning and Sustained Attention
Stimulus-Response Learning: Temporal Process of Acquisition
Animals were required to meet criterion of 70% accuracy for three consecutive test sessions to successfully acquire each program in the signal detection operant task. The task included a series of three vigilance programs. Independent of treatment, control animals (SE: n = 10; Placebo: n = 10) acquired the signal detection task within 76 test sessions. Furthermore, HIV-1 Tg animals treated with SE successfully acquired the signal detection task within 59 test sessions. In sharp contrast, only 80% (n = 8) of the HIV-1 Tg animals treated with placebo (n = 10) were able to successfully acquire the task. The temporal process of acquisition for all groups, independent of genotype and/or treatment, was well-described by a first-order polynomial (R2s > 0.93; Fig. 4).
Fig. 4.

The number of days required to meet criterion in the signal detection operant task is illustrated as a function of genotype (HIV-1 Tg vs. Control) and treatment (S-Equol (SE) vs. placebo; ±95% Confidence Intervals). (a) HIV-1 Tg animals treated with placebo displayed a pronounced deficit in stimulus-response learning, acquiring the task significantly slower than their control counterparts. (b) In control animals, no significant treatment differences were observed in the temporal process of acquisition. (c) In HIV-1 Tg animals, treatment with SE significantly enhanced the temporal process of acquisition, evidenced by faster acquisition relative to HIV-1 Tg animals treated with placebo
Significant genotype differences were observed between HIV-1 Tg and control animals treated with placebo (Fig. 4A). Specifically, HIV-1 Tg animals acquired the signal detection task significantly slower than their control counterparts, evidenced by significant differences in the parameters of the function [F(2,15) = 6.1, p ≤ 0.01]. HIV-1 Tg animals, therefore, exhibited a marked impairment in stimulus-response learning relative to control animals.
Treatment with SE, however, ameliorated deficits in stimulus-response learning in all HIV-1 Tg animals. Specifically, a generalized linear mixed model with a Poisson distribution (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 8) revealed a significant genotype x treatment interaction [F(1,34) = 10.5, p ≤ 0.003]; an interaction that was further examined via complementary analyses of each genotype. In control animals (Fig. 4B), no significant treatment differences were observed in the temporal process of acquisition [p > 0.05]. In sharp contrast, in HIV-1 Tg animals, treatment with SE significantly enhanced the temporal process of acquisition (Fig. 4C). Specifically, HIV-1 Tg animals treated with SE acquired the signal detection task significantly faster than HIV-1 Tg animals treated with placebo, evidenced by significant differences in the parameters of the first-order polynomial [F(2,14) = 59.1, p ≤ 0.001] and a main effect of treatment [F(1,16) = 7.4, p ≤ 0.015]. Thus, treatment with SE ameliorated the marked impairment in stimulus-response learning in HIV-1 Tg animals.
Sustained Attention: Signal Detection
The effect of the HIV-1 transgene and/or SE treatment was examined by averaging each animal’s performance in the signal detection operant task with varying signal durations (i.e., 1000, 500, 100 msec) across the final three consecutive sessions. To directly assess the temporal components of attention, hits and misses (i.e., response types occurring during signal trials) were the focus of statistical analyses.
The overall mixed-model ANOVA, conducted on the relative frequency of hits and misses at each signal duration, in all animals (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 10) failed to reveal a statistically significant main effect of treatment, genotype x treatment interaction, or higher-order interactions with genotype and treatment (p > 0.05).
Given the a priori hypothesis that SE treatment may only mitigate sustained attention deficits in a subset of HIV-1 Tg animals, additional analyses were conducted. Specifically, analyses were conducted on all control animals (SE: n = 10; Placebo: n = 10), all HIV-1 Tg animals treated with placebo (n = 10), and the top performing 50% of HIV-1 Tg animals treated with SE (n = 5). Performance in HIV-1 Tg animals treated with SE was determined by calculating the area of the signal detection curve where the number of hits was greater than the number of misses (McLaurin et al. 2019b).
SE treatment between 2 and 3 months of age significantly enhanced sustained attention in 50% of the HIV-1 Tg animals (Fig. 5a), evidenced by a statistically significant genotype x treatment x response type interaction [F(1,31) = 16.7, p ≤ 0.001]. Complementary analyses were conducted to determine the locus of the interaction by 1) conducting a priori planned comparisons (i.e., Control Placebo vs. HIV-1 Tg Placebo, Control Placebo vs. HIV-1 Tg SE) and 2) examining each response type (i.e., Hits, Misses). First, comparison of HIV-1 Tg and control animals treated with placebo (Fig. 5b) revealed a statistically significant genotype x response type interaction [F(1,18) = 11.44, p ≤ 0.003]; an interaction not observed when HIV-1 Tg animals treated with SE were compared with control animals treated with placebo (Fig. 5c [p > 0.05]). Second, each response type (i.e., Hits, Misses) was examined individually, revealing a genotype x treatment interaction for both hits [F(1,31) = 4.9, p ≤ 0.04] and misses [F(1,31) = 4.1, p ≤ 0.05]. Independent of signal duration (i.e., mean shift), treatment with SE increased the relative frequency of hits and decreased the relative frequency of misses in HIV-1 Tg animals. Thus, in HIV-1 Tg animals, SE treatment ameliorated sustained attention deficits by improving attention to the stimulus (i.e., hits) and preventing lapses in attention (i.e., misses); an effect that was observed a month after the cessation of treatment.
Fig. 5.

Temporal components of sustained attention were assessed by evaluating hits and misses (i.e., correct and incorrect responses, respectively, during signal trials) (a) The number of hits and misses during the final 3 days in the signal detection operant task are presented independent of signal duration using relative frequencies (mean ± SEM) as a function of genotype (HIV-1 Tg vs. Control) and treatment (S-Equol (SE) vs. Placebo). (b) HIV-1 animals treated with placebo exhibited a prominent deficit in sustained attention, evidenced by a decreased relative frequency of hits and an increased relative frequency of misses, independent of signal duration, relative to control animals treated with placebo. (c) Treatment with SE, however, ameliorated sustained attention deficits in a subset (i.e., 50%) of HIV-1 Tg animals. Independent of signal duration, HIV-1 Tg animals treated with SE exhibited frequencies of hits and misses that approximated control animals treated with placebo
Neurocognitive Domain 4: Selective Attention
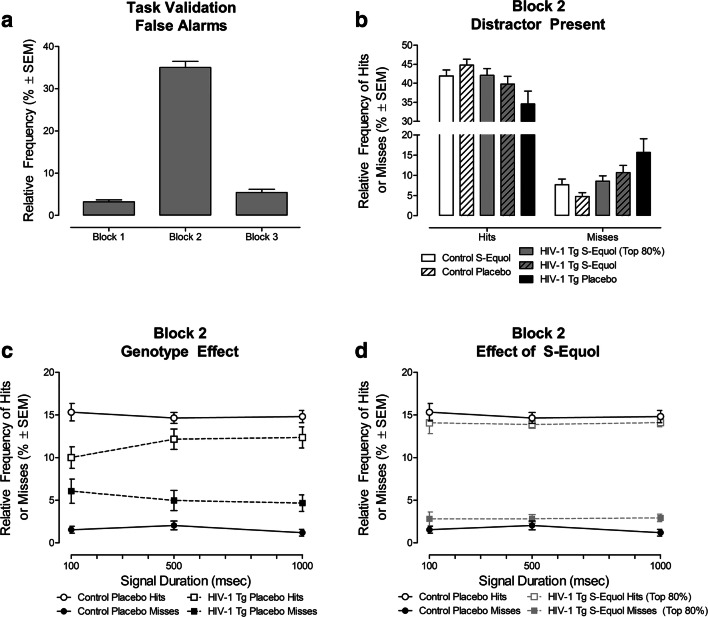
Selective attention was assessed for three consecutive days by presenting a visual distractor at the beginning of each trial in the second trial block (i.e., Trials 54–108). The effect of the HIV-1 transgene and/or SE treatment were examined by averaging each animal’s performance across all three assessments. To directly assess the temporal components of attention, hits and misses (i.e., response types occurring during signal trials) were the focus of statistical analyses. Task validation, however, utilized false alarms, a response type occurring during non-signal trials.
The number of false alarms during each trial block were examined to validate the assessment of selective attention in all animals (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 10; Fig. 6a). A significant main effect of trial block [F(2,72) = 382.2, pGG ≤ 0.001] with a prominent quadratic component [F(1,36) = 458.8, p ≤ 0.001] supports the assessment of selective attention. During the second trial block (i.e., when the distractor was present), a dramatic increase in the relative frequency of false alarms was observed relative to either the first or third trial blocks.
Fig. 6.
(a) The number of false alarms, collapsed across genotype and treatment, is illustrated across 3 trial blocks in the signal detection task. During Block 2, a visual distractor was presented for the assessment of selective attention. Animals exhibited a significantly greater number of false alarms during Block 2 relative to Block 1 or Block 3 validating the assessment of selective attention. (b) The number of hits and misses during the visual distractor task are presented during Block 2, independent of signal duration, using relative frequencies (mean ± SEM) as a function of genotype (HIV-1 Tg vs. Control) and treatment (S-Equol (SE) vs. Placebo). (c) HIV-1 animals treated with placebo exhibited a prominent deficit in selective attention, evidenced by a decreased relative frequency of hits and an increased relative frequency of misses during the second trial block, independent of signal duration, relative to control animals treated with placebo. (d) Treatment with SE, however, ameliorated selective attention deficits in a subset (i.e., 80%) of HIV-1 Tg animals. Independent of signal duration, HIV-1 Tg animals treated with SE exhibited frequencies of hits and misses that approximated control animals treated with placebo
The overall mixed-model ANOVA, conducted on the relative frequency of hits and misses during Block 2, in all animals (Control: SE: n = 10; Placebo: n = 10; HIV-1 Tg: SE: n = 10; Placebo: n = 10) failed to reveal a statistically significant main effect of treatment, genotype x treatment interaction, or higher-order interactions with genotype and treatment (p > 0.05).
Given the a priori hypothesis that SE treatment may only mitigate selective attention deficits in a subset of HIV-1 Tg animals, additional analyses were conducted. Specifically, analyses were conducted on all control animals (SE: n = 10; Placebo: n = 10), all HIV-1 Tg animals treated with placebo (n = 10), and the top performing 80% of HIV-1 Tg animals treated with SE (n = 8). Performance in HIV-1 Tg animals treated with SE was determined by calculating the area of the signal detection curve where the number of hits was greater than the number of misses (McLaurin et al. 2019b).
SE treatment between 2 and 3 months of age significantly enhanced selective attention in 80% of the HIV-1 Tg animals (Fig. 6b), evidenced by a statistically significant genotype x treatment x response type interaction [F(1,34) = 5.7, pGG ≤ 0.023]. Complementary analyses were conducted to determine the locus of the interaction by 1) conducting a priori planned comparisons (i.e., Control Placebo vs. HIV-1 Tg Placebo, Control Placebo vs. HIV-1 Tg SE) and 2) examining each response type (i.e., Hits, Misses). First, comparison of HIV-1 Tg and control animals treated with placebo (Fig. 6c) revealed a statistically significant genotype x response type interaction [F(1,18) = 8.8, pGG ≤ 0.008]; an interaction not observed when HIV-1 Tg animals treated with SE were compared with control animals treated with placebo (Fig. 6d [p > 0.05]). Second, each response type (i.e., Hits, Misses) was examined individually, revealing a statistically significant genotype x treatment interaction for both hits [F(1,34) = 5.3, p ≤ 0.028] and misses [F(1,34) = 5.8, p ≤ 0.021]. Independent of signal duration (i.e., mean shift), treatment with SE increased the relative frequency of hits and decreased the relative frequency of misses in HIV-1 Tg animals. Thus, in HIV-1 Tg animals, SE treatment ameliorated deficits in selective attention by improving attention to the stimulus (i.e., hits) and preventing lapses in attention (i.e., misses); an effect that was observed nearly two months after the cessation of treatment.
Discussion
Two key questions were addressed in the HIV-1 Tg rat to critically test the therapeutic potential of the selective estrogen receptor β agonist (SERBA) SE. First, the therapeutic efficacy of SE was greater when treatment was initiated relatively early (i.e., between 2 and 3 months of age) in the course of viral protein exposure relative to later treatment initiation (i.e., between 6 and 8 months of age; Moran et al. 2019). Second, the therapeutic utility of SE generalizes across multiple neurocognitive domains, including preattentive processes, stimulus-response learning, sustained attention, and selective attention. Dependent upon neurocognitive domain, between 50% (i.e., sustained attention; 80%, selective attention) to 100% (i.e., preattentive processes, stimulus response learning) of HIV-1 Tg animals treated with SE exhibited enhanced neurocognitive function, approximating controls. Results support, therefore, the therapeutic efficacy of selectively targeting ERβ via SE treatment as an adjunctive therapeutic for HAND in the post-cART era. Critically testing novel adjunctive therapeutics is vital to understanding their advantages and opportunities for improving the treatment and diagnosis of HAND.
Neurocognitive functions, including preattentive processes, stimulus-response learning, and attention, are generally componential (Keeler and Robbins 2011). The componential relationship between neurocognitive functions has been examined in the HIV-1 Tg rat via longitudinal mediation; a study that supported preattentive processes as one of the neurobehavioral mechanisms underlying alterations in stimulus-response learning and sustained attention in the HIV-1 Tg rat (McLaurin et al. 2019b). In the present study, treatment with SE enhanced preattentive processes, to the level of controls, in the population (i.e., 100%) of HIV-1 Tg animals sampled. Enhancing lower-order cognitive processes, including preattentive processes may be more straightforward than restoring deficits in more complex cognitive processes (e.g., sustained attention, selective attention); deficits which may require treatment earlier in the course of HIV-1 viral protein exposure (e.g., PD 28) or a longer duration of treatment. Results suggest, therefore, that SE may target one of the neurobehavioral mechanisms underlying HAND, however, future studies utilizing mediation by design are critical to test this hypothesis.
A signal detection operant task, developed by McGaughy and Sarter (1995), was utilized to assess sustained and selective attention. Sustained attention, characterized by the detection of rare, unpredictable, and weak stimuli over long periods of time (Sarter et al. 2001), was examined by requiring animals to attend to a randomly presented stimulus (i.e., central panel illumination), the presence or absence of which indicated which response to make (i.e., which lever to press) to receive a reinforcer (i.e., sucrose pellet). The parametric manipulation of cue characteristics (i.e., presenting a visual distractor during the second trial block) allowed for the assessment of selective attention (Bushnell and Strupp 2009), which requires animals to process the most relevant information, while excluding or inhibiting, irrelevant information (Fuster 2008). The parametric manipulation of signal duration in both the sustained attention task and the selective attention task afforded a critical opportunity to evaluate the temporal aspects of attention. On each trial, independent of task, an animal could emit one of four response choices (i.e., hit, miss, correct rejection, or false alarm), indicative of an animal’s ability to attend to the stimulus (hit, correct rejection), a lapse of attention (miss) or a failure of response inhibition (false alarm).
Treatment with SE enhanced both sustained and selective attention, to the level of controls, in 50% and 80% of HIV-1 Tg animals, respectively, by increasing an animals’ ability to attend to the stimulus (i.e., hits) and decreasing lapses in attention (i.e., misses). In both sustained and selective attention, a subset (i.e., 50% and 80%, respectively) of HIV-1 Tg animals treated with SE exhibited a decreased relative frequency of misses and an increased relative frequency of hits; effects that were independent of signal duration. When HIV-1 Tg animals were treated with SE at a more advanced age (i.e., 6 to 8 months), the enhancement in sustained attention occurred in a smaller subset (i.e., 40%) of animals and was localized to misses at the shortest signal duration (i.e., 100 msec; Moran et al. 2019). Notably, the assessment of two attentional domains in the present study tapped multiple regions of the prefrontal cortex (PFC), with the medial PFC (mPFC) having a primary role in sustained attention (Kim et al. 2016), whereas the lateral PFC plays a more prominent role in selective attention (Kam et al. 2018). SE treatment relatively early in the course of viral protein exposure (i.e., 2 to 3 months), therefore, enhances sustained and selective attention in a global manner, targeting both hits and misses across signal durations.
The perception of time, which has been implicated as an elemental dimension of HAND (e.g., Chao et al. 2004; Matas et al. 2010; Moran et al. 2013; McLaurin et al. 2019b), is a cognitive capacity that is characterized by an organism’s sensitivity to the passage of time (Meck and Benson 2002). Utilization of a series of neurocognitive assessments (i.e., PPI, stimulus-response learning, signal detection, visual distractor task) and time interval manipulations (i.e., from milliseconds to days), as in the present study, provided a critical opportunity to evaluate the generalizability of temporal processing deficits in the HIV-1 Tg rat. Independent of neurocognitive domain, HIV-1 Tg rats treated with placebo exhibited a fundamental deficit in the perception of time relative to control animals, extending previously reported observations (e.g., Moran et al. 2013; McLaurin et al. 2016; McLaurin et al. 2019a; Moran et al. 2019). Treatment with SE, however, ameliorated alterations in the perception of time, evidenced by improvements in preattentive processes, stimulus-response learning, sustained attention and selective attention in 50% to 100% of HIV-1 Tg animals. Most notably, all of the HIV-1 Tg animals treated with SE exhibited an enhancement in preattentive processes and stimulus-response learning. While the improvement in preattentive processes likely reflects the utility of SE to target one of the neurobehavioral mechanisms underlying HAND (McLaurin et al. 2019b), the enhancement of stimulus-response learning may result from the presence of longer temporal intervals (i.e., days relative to msec).
The frontal-subcortical circuit, which includes five parallel segregated circuits linking the basal ganglia and prefrontal cortex (PFC; Alexander et al. 1986; Alexander and Crutcher 1990; Alexander 1994), has been implicated as a potential neuroanatomical substrate for temporal processing (for review, Matell and Meck 2000; Meck and Benson 2002). Specifically, excitatory glutamatergic projections from the prefrontal cortex (PFC) innervate the striatum, which receives dopaminergic projections from the ventral tegmental area and substantia nigra pars compacta (SNpc). The striatum sends GABAergic projections to the globus pallidus, which are subsequently relayed to the thalamus. Excitatory glutamatergic projections from the thalamus then innervate the PFC. Notably, the neuroanatomical regions involved in temporal processing are also involved in the neurocognitive domains assessed in the present study (e.g., preattentive processes: Ellenbroek et al. 1996; stimulus-response learning: Rolls 2004; sustained attention: Kim et al. 2016; selective attention: Kam et al. 2018). Critically, damage to frontal-subcortical circuitry has been observed in HIV-1 seropositive individuals, evidenced by impairments in executive function (e.g., Cysique et al. 2004; Heaton et al. 2011) and neurobehavioral alterations (e.g., apathy: Cole et al. 2007; Kamat et al. 2012; depression: Ciesla and Roberts 2001); results which were translationally modeled in the HIV-1 Tg rat (e.g., executive function impairments: Vigorito et al. 2007; Moran et al. 2014; McLaurin et al. 2019a; motivational dysregulation: Bertrand et al. 2018).
Mechanistically, SE may remodel the frontal-subcortical circuit at the synaptic level by targeting dendritic spines. Dendritic spines, which reflect functionality and capacity for structural change (Lai and Ip 2013), serve as the main postsynaptic compartment of excitatory synapses (Spiga et al. 2014). Long-term modifications (i.e., density, morphology) in dendritic spines may lead to the remodeling of neuronal circuitry and changes in synaptic function targeting a potential neural mechanism (i.e., synaptic dysfunction) underlying HAND (e.g., Gelman and Nguyen 2010; Gelman et al. 2012; Roscoe et al. 2014; Sinharay et al. 2017). Broadly, strong evidence suggests that 17β-estradiol remodels neural circuits by increasing dendritic spine density (e.g., Khan et al. 2013; Hao et al. 2006; Tuscher et al. 2016) and altering dendritic spine morphology (Hao et al. 2006). Specifically, 17β-estradiol promotes the growth and stability of new dendritic spines via the ERβ pathway (Wang et al. 2018) and enhances excitatory glutamatergic synapse formation (Khan et al. 2013).
In vitro studies further support the utility of phytoestrogens, including DAI, LQ, and SE to remodel neural circuits by preventing synapse loss induced by the HIV-1 viral protein, Tat (Bertrand et al. 2014, 2015). Phytoestrogens are one of the few compounds that show potential to restore synaptic connectivity after exposure to HIV-1 Tat (Bertrand et al. 2015) and do so via an ERβ specific mechanism; although other synthetic SERBA compounds have been shown to enhance recovery of neurons following damage (D’Errico et al. 2018). Furthermore, the clinical importance of SERBAs is demonstrated by their progression into clinical trials and experimental studies of other neurodegenerative diseases, including Parkinsons disease (McFarland et al. 2013) and Alzheimer’s disease (Zhao et al. 2013). The development of a therapeutic approach targeting synaptic dysfunction, therefore, may have long-term effects that lead to the remodeling of neural circuitry and enhanced cognitive function.
In conclusion, the present study critically tested the utility of SE as a novel adjunctive therapeutic for the treatment of neurocognitive impairments in HIV-1. When initiated relatively early (i.e., 2 to 3 months of age) in the course of HIV-1 viral protein exposure, SE enhances neurocognitive function in multiple neurocognitive domains. Therefore, selectively targeting ERβ may be an important venue for the development of an efficacious adjunctive therapeutic for HAND.
Acknowledgements
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Disorders and Stroke, NS100624) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program. Dr. Landhing Moran is currently a Health Scientist Administrator at the NIDA Center for Clinical Trials Network.
Funding Information
This study was funded by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Disorders and Stroke, NS100624) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Compliance with Ethical Standards
Conflict of Interest
KA McLaurin declares that she has no conflict of interest. LM Moran declares that she has no conflict of interest. RM Booze declares that she has no conflict of interest. CF Mactutus declares that he has no conflict of interest.
Ethical Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Guidelines established in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH) were utilized for the maintenance of animals in AAALAC-accredited facilities. The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the project protocol under federal assurance (#D16–00028).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER-β mediates 17β-estradiol attenuation of HIV-1 tat-induced apoptotic signaling. Synapse. 2010;64(11):829–838. doi: 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 tat(1-86) exposure. PLoS One. 2012;7(5):e37540. doi: 10.1371/journal.pone.0037540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaza H. Prostate cancer chemoprevention by soy isoflavones: role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012;103(6):969–975. doi: 10.1111/j.1349-7006.2012.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE. Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol. 1994;11:420–431. doi: 10.1097/00004691-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Bachis A, Forcelli P, Masliah E, Campbell L, Mocchetti I. Expression of gp120 in mice evokes anxiety behavior: co-occurrence with increased dendritic spines and brain-derived neurotrophic factor in the amygdala. Brain Behav Immun. 2016;54:170–177. doi: 10.1016/j.bbi.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128(1):140–151. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM. HIV-1 tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front Microbiol. 2015;6:894. doi: 10.3389/fmicb.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Harrod SB, Moran LM, Booze RM. HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Sci Rep. 2018;8(1):7869–7817. doi: 10.1038/s41598-018-25109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Strupp BJ. Assessing attention in rodents. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. 2. Boca Raton, FL: CRC Press/Taylor and Francis; 2009. pp. 130–158. [Google Scholar]
- Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HA. Developmental effects of dietary phytoestorgens in Sprague-dawley rats and interactions of genistein and daidzein with rat estrogen receptors α and β in vitro. Toxicol Sci. 1999;51(2):236–244. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- Chao LL, Lindgren JA, Flenniken DL, Weiner MV. ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol. 2004;115(7):1583–1591. doi: 10.1016/j.clinph.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7:37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MA, Castellon SA, Perkins AC, Ureno OS, Robinet MB, Reinhard MJ, Barclay TR, Hinkin CH. Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. J Int Neuropsychol Soc. 2007;13(3):549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- D’Errico F, Goverse G, Dai Y, Wu W, Stakenborg M, Labeeuw E, De Simone V, Verstockt B, Gomez-Pinilla PJ, Warner M, Di Leo A, Matteoli G, Gustafsson JA. Estrogen receptor β controls proliferation of enteric glia and differentiation of neurons in the myenteric plexus after damage. Proc Natl Acad Sci U S A. 2018;115(22):5798–5803. doi: 10.1073/pnas.1720267115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR. Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience. 1996;75(2):535–542. doi: 10.1016/0306-4522(96)00307-7. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006;318(3):1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006;84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4. London: Academic Press; 2008. [Google Scholar]
- Garvey LJ, Yerrakalva D, Winston A. Correlations between computerized battery testing and a memory questionnaire for identification of neurocognitive impairment in HIV type 1-infected subjects on stable antiretroviral therapy. AIDS Res Hum Retrovir. 2009;25(8):765–769. doi: 10.1089/aid.2008.0292. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J NeuroImmune Pharmacol. 2010;5(1):92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accomodation in HIV-associated neurocognitive disorders and encephalitis. J NeuroImmune Pharmacol. 2012;7:686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Cabrera-Socorro A, Perez Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503(6):790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Gott C, Gates T, Dermody N, Brew BJ, Cysique LA. Cognitive change trajectories in virally suppressed HIV-infected individuals indicate high prevalence of disease activity. PLoS One. 2017;12(3):e0171887. doi: 10.1371/journal.pone.0171887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. doi: 10.1007/BF02289823. [DOI] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26(9):2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group. HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, JA MC, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, CHARTER Group Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell MR, Perry W, Young JW, Minassian A, Translational Methamphetamine AIDS Research Center (TMARC) Group Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav Pharmacol. 2014;25(1):12–22. doi: 10.1097/FBP.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel SM, Hassanzadeh-Behbahani S, Turkeltaub PE, Moore DJ, Ellis RJ, Jiang X. Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum Brain Mapp. 2019;40(10):3010–3026. doi: 10.1002/hbm.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47–54. doi: 10.1353/pbm.1963.0005. [DOI] [PubMed] [Google Scholar]
- Kam JWY, Solbakk AK, Endestad T, Meling TR, Knight RT. Lateral prefrontal cortex lesion impairs regulation of internally and externally directed attention. Neuroimage. 2018;175:91–99. doi: 10.1016/j.neuroimage.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Morgan E, Marcotte TD, Badiee J, Maich I, Cherner M, de Almeida S, de Pereira AP, Ribeiro CE, Barbosa F, Atkinson JH, Ellis R, HNRP Group Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. J Affect Disord. 2012;150(3):1069–1075. doi: 10.1016/j.jad.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Fonsah JY, Tang B, Doh RF, Kengne AM, Umlauf A, Tagny CT, Nchindap E, Kenmogne L, Franklin D, Njamnshi DM, Mbanya D, Njamnshi AK, Heaton RK. Effects of HIV on executive function and verbal fluency in Cameroon. Sci Rep. 2018;8(1):17794. doi: 10.1038/s41598-018-36193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang QG, Brann DW. Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids. 2013;78(6):614–623. doi: 10.1016/j.steroids.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ӓhrlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164(1–2):208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Hӓggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138(3):863–870. 10.1210/endo.138.3.4979 [DOI] [PubMed]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4253. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta. 2013;1832(12):2257–2263. doi: 10.1016/j.bbadis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15(1):14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-beta-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66(2):63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rhees RW, Setchell KD, Lephart ED. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914(1–2):92–99. doi: 10.1016/S0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K. Cognitive function in women with HIV: findings from the Women’s interagency HIV study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas CG, Silva SM, Marcon Bde A, Goncalves IC. Electrophysiological manifestations in adults with HIV/AIDS submitted and not submitted to antiretroviral therapy. Pro Fono. 2010;22(2):107–113. doi: 10.1590/S0104-56872010000200007. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22(1):94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McFarland K, Price DL, Davis CN, Ma JN, Bonhaus DW, Burstein ES, Olsson R. AC-186, a selective nonsteroidal estrogen receptor β agonist, shows gender specific neuroprotection in a Parkinson’s disease rat model. ACS Chem Neurosci. 2013;4(9):1249–1255. doi: 10.1021/cn400132u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep. 2016;6:32831. doi: 10.1038/srep32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Temporal processing demands in the HIV-1 transgenic rat: Amodal gating and implications for diagnostics. Int J Dev Neurosci. 2017;57:12–20. doi: 10.1016/j.ijdevneu.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1 associated neurocognitive disorders. J Neurovirol. 2018;24:229–245. doi: 10.1007/s13365-017-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Mactutus CF. Disruption of timing: NeuroHIV progression in the post-cART era. Sci Rep. 2019;9(1):827. doi: 10.1038/s41598-018-36822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Mactutus CF, Booze RM, Fairchild AJ (2019b) An empirical mediation analysis of mechanisms underlying HIV-1-associated neurocognitive disorders. Brain Res. 1724:146436. 10.1016/j.brainres.2019.146436 [DOI] [PMC free article] [PubMed]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol Cell Endocrinol. 2008;283(1–2):49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W, Translational Methamphetamine AIDS Research Center (TMARC) Group Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J NeuroImmune Pharmacol. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Modeling deficits in attention, inhibition, and flexibility in HAND. J NeuroImmune Pharmacol. 2014;9(4):508–521. doi: 10.1007/s11481-014-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, McLaurin KA, Booze RM, Mactutus CF. Neurorestoration of sustained attention in a model of HIV-1 associated neurocognitive disorders. Front Behav Neurosci. 2019;13:169. doi: 10.3389/fnbeh.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80(1):14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Farmakalidis E, Johnson LD. Isoflavone content of soya-based laboratory animal diets. Food Chem Toxicol. 1982;20(3):315–317. doi: 10.1016/S0278-6915(82)80299-8. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV-1 tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F (2014) Estrogen receptors alpha (ERα) and Beta (ERβ): subtype-selective ligands and clinical potential. Steroids 0:13–29. 10.1016/j.steroids.2014.06.012, 90 [DOI] [PMC free article] [PubMed]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98(16):9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, Califano A, Masliah E, Sanna PP. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:26. doi: 10.1186/1750-1326-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–49. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Roscoe RF, Mactutus CF, Booze RM. HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J NeuroImmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom up. Brain Res Rev. 2001;35:146–160. doi: 10.1016/S0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68(6 Suppl):1333S–13246S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and –beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sinharay S, Lee D, Shah S, Muthusamy S, Papadakis GZ, Zhang X, Maric D, Reid WC, Hammoud DA. Cross-sectional and longitudinal small animal PET shows pre and post-synaptic striatal dopaminergic deficits in an animal model of HIV. Nucl Med Biol. 2017;55:27–33. doi: 10.1016/j.nucmedbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Mulas G, Piras F, Diana M. The “addicted” spine. Front Neuroanat. 2014;8:110. doi: 10.3389/fnana.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36(5):1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J NeuroImmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59(1):51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhu J, Xu T. 17β-estradiol (E2) promotes growth and stability of new dendritic spines via estrogen receptor β pathway in intact mouse cortex. Brain Res Bull. 2018;137:241–248. doi: 10.1016/j.brainresbull.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935(1–2):73–80. doi: 10.1016/S0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Chen S, Schneider LS, Brinton RD. Early intervention with an estrogen receptor β-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013;37(2):403–419. doi: 10.3233/JAD-122341. [DOI] [PMC free article] [PubMed] [Google Scholar]