Abstract
Background
It is difficult to diagnose Bickerstaff’s brainstem encephalitis (BBE) in the acute phase, and emergency physicians could diagnose BBE as an unknown cause of consciousness disturbance.
Case presentation
A 75‐year‐old woman presented with dizziness and weakness in both arms 1 week after an upper respiratory infection. She experienced gradual worsening of consciousness, had dilated pupils and no light reflex. She was suspected of brainstem dysfunction at the upper part of the brainstem; however, there were not significant findings on magnetic resonance imaging, cerebrospinal fluid, or electroencephalography. The auditory brainstem response demonstrated a low voltage, but there was no prolonged latency. At a later date, she was diagnosed with BBE based on serum immunoglobulin G anti‐GQ1b antibody. She was discharged home without any neurological sequelae.
Conclusion
It is necessary to analyze serum immunoglobulin G anti‐GQ1b antibodies to diagnose BBE. Auditory brainstem response would be helpful in detecting lesions and predicting functional recovery.
Keywords: Bickerstaff’s brainstem encephalitis, brain stem, encephalitis, evoked potential, intensive care unit
Auditory brainstem response of patients with Bickerstaff’s brainstem encephalitis demonstrated a low voltage, but there was no prolonged latency.

Introduction
Consciousness disorder is a symptom frequently encountered in the emergency department; however, it is difficult to diagnose this condition in the acute phase. Herein, we describe a case of a patient with progressive consciousness impairment and deep coma who was finally diagnosed with Bickerstaff’s brainstem encephalitis (BBE). We also found that the auditory brainstem response (ABR) is helpful in detecting lesions and predicting functional recovery.
Case Report
A 75‐year‐old woman presented with dizziness and weakness in both arms 1 week after an upper respiratory infection. She was transferred to the emergency department due to difficulty in moving. Her medical history showed that she had breast cancer. Magnetic resonance imaging (MRI) did not reveal any intracranial lesions. Examination of her blood sample and cerebrospinal fluid (CSF) also did not show any abnormalities that caused her symptoms. She was admitted to the hospital for further evaluation. She experienced gradual worsening of consciousness after admission, and on the 6th hospital day, Glasgow Coma Scale E1V1M4, dilated pupils, loss of light reflex, and escape of only both upper limbs were observed; therefore, no reaction was observed in both lower limbs. Tracheal intubation was carried out, followed by ventilator management. A second MRI examination also revealed no significant findings on fluid‐attenuated inversion recovery and diffusion‐weighted imaging. The CSF showed a cell count of 6/µL and a total protein content of 39 mg/dL. Electroencephalogram (EEG) occasionally revealed a slow wave of 2–3 Hz. The ABR demonstrated a low voltage, but there was no prolonged interval of latency between I and V wave (Fig. 1). The somatosensory evoked potential showed bilateral N20. Based on these neurological findings, we supposed the lesion for this neurological deficit was located at the upper part of the brainstem, including the midbrain. On the 10th hospital day, the patient was able to respond to easy verbal commands, and her paralysis was slightly improved. She was considered to be more likely to have Guillain‐Barré syndrome (GBS) or its related disorders, and steroid pulse therapy (1 g/day for 3 days) was initiated. On the 15th hospital day, we noticed left vocal cord paralysis, for which we undertook tracheostomy. The patient’s consciousness recovered, and on the 20th day she was transferred for rehabilitation. At a later date, she showed a positive result for serum immunoglobulin G (IgG)‐type GQ1b antibody; on this basis, we made a diagnosis of BBE. After rehabilitation, the patient was discharged home on the 103rd hospital day without any particular neurological sequelae.
Fig. 1.
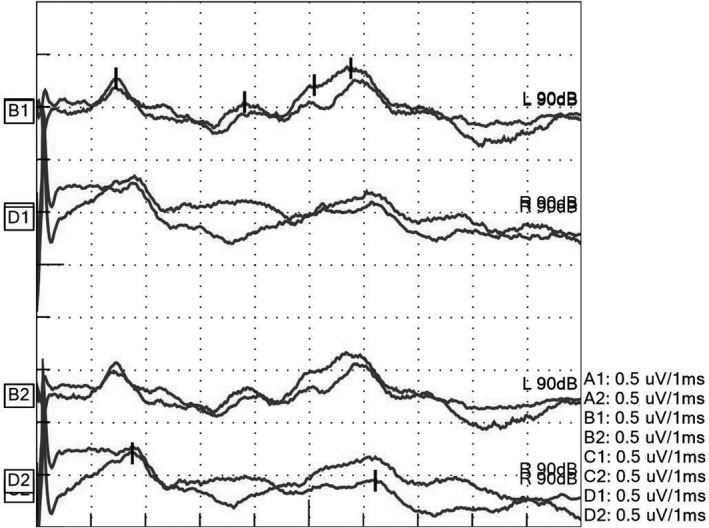
Waveform of the auditory brainstem response on the 7th day of hospitalization of a 75‐year‐old woman with Bickerstaff’s brainstem encephalitis, revealing 2.36 ms in the interval of I–III wave, 1.95 ms in III–V wave, 4.31 ms in I–V wave on the left, and 4.46 ms in I–V wave on the right.
Discussion
We have described a case of a patient with BBE who gradually experienced consciousness disorder but recovered completely after deep coma.
The patient was initially diagnosed with brainstem dysfunction at the upper part of the brainstem, including the midbrain, based on her symptoms of bilateral pupil dilation, loss of light reflex, and other neurological examinations. There were no significant findings in the MRI, EEG, or CSF examinations. Table 1 shows the differential diagnoses of consciousness impairment that physicians find difficult to diagnose in the acute phase. 1 We also analyzed anti‐GQ1b antibody levels for diagnosing BBE in this patient. 2 Finally, we established a diagnosis of BBE in our patient.
Table 1.
Causes of impaired consciousness that can be difficult to diagnose in emergency care
| Pathology | Disease | Diagnosis |
|---|---|---|
| Cerebrovascular disorder | Stroke (cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage) | Brain imaging (CT/MRI) |
| Pituitary stroke | ||
| Autoimmune disease | Myasthenia gravis | Serology |
| Multiple sclerosis, neuromyelitis optica | ||
| Behçet disease, Sweet syndrome | ||
| Acute disseminated encephalomyelitis | Oligoclonal band in CSF | |
| Anti‐GQ1b antibody syndrome (GBS, MFS, BBE) | Serology (GQ1b‐IgG or IgA) | |
| Autoimmune encephalitis (anti‐NMDA receptor encephalitis, Hashimoto encephalopathy) | Serology | |
| Infection | Poliomyelitis | Virology (PCR) |
| Viral encephalitis | Virology (PCR) | |
| Trauma | Diffuse axon injury | Brain imaging (MRI) |
| Tumor | Oncology of the brain | Brain imaging (CT/MRI) |
| Paraneoplastic neurological syndrome | Serology | |
| Dural disease | Hypertrophic pachymeningitis | Enhanced MRI |
| Myelitis | Acute transverse spondylitis | CSF test, spinal MRI |
| Metabolic disease | Trace element deficiency (Wernicke’s encephalopathy, carnitine deficiency) | Serology |
| Others | Vasculitis | Serology |
| Non‐convulsive status epilepticus | EEG | |
| Toxic (e.g. botulinum toxin, drug addiction) | Serology, urinalysis |
BBE, Bickerstaff’s brainstem encephalitis; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalography; GBS, Guillain‐Barré syndrome; Ig, immunoglobulin; MFS, Miller‐Fisher syndrome; MRI, magnetic resonance imaging; NMDA, N‐methyl‐D‐aspartic acid; PCR, polymerase chain reaction.
Bickerstaff’s brainstem encephalitis is an autoimmune disease with the primary lesion in the brainstem. It is believed to be triggered by some type of preinfection, and IgG‐type GQ1b antibodies are detected in the majority of cases. Symptoms are primarily characterized by oculomotor disorders, ataxia, and impaired consciousness. Approximately 20% of cases require ventilator management. 2 , 3 Table 2 shows the diagnostic criteria for BBE. Bickerstaff’s brainstem encephalitis is a rare disease; almost 100 cases are reported every year in Japan, with 120 million habitants. 4 It is considered a variant of GBS and these are related conditions that form a continuous range. Miller‐Fisher syndrome is also a variant of GBS and characterized by acute onset of external ophthalmoplegia, cerebellar ataxia, and the loss of tendon reflexes.
Table 2.
Diagnostic criteria for Bickerstaff’s brainstem encephalitis
| “Defined” Bickerstaff’s brainstem encephalitis must meet the requirements of (1), (2), and (4). |
|---|
| “Probable” Bickerstaff’s brainstem encephalitis must meet the requirements of (1) and (4) or (2), (3), and (4). |
| (1) Acute progressive external ophthalmoplegia, † ataxia, and impaired conscious level by 4 weeks, followed by spontaneous recovery within 12 weeks after onset. |
| (2) Positive for serum IgG anti‐GQ1b antibodies. |
| (3) Incomplete agreement on (1) because of one or more of the following reasons: |
|
|
|
|
| (4) Other conditions are excluded in laboratory and imaging tests. The excluded conditions are Wernicke’s encephalopathy, cerebrovascular disorder, multiple sclerosis, neuromyelitis optica, neuro‐Behçet disease, neuro‐Sweet disease, pituitary apoplexy, viral brainstem encephalitis, myasthenia gravis, brainstem tumor, vasculitis, botulism, Hashimoto encephalopathy. |
Ig, immunoglobulin.
Lateral symmetry is the rule, but mild laterality is also permitted. Features other than the incomplete item(s) must meet (1).
The criteria do not include electrophysiological examinations such as EEG, ABR, or somatosensory evoked potential. Auditory brainstem response is presumed to reflect the activation from the cochlea to the midbrain and can detect small lesions especially in the pons. 5 This patient was suspected to have brainstem lesions based on neurological examinations and was finally diagnosed with BBE. In fact, the ABR did not show prolonged interval of latency between I and V wave. There were differences between ABR findings and neurological examinations. Some studies have described the characteristics of ABR for patients with BBE. Ogawara et al. 6 reported that ABR did not reveal prolonged interval of latency between I and V wave, and the low potential in their study patient with BBE and that of our patient were similar. We built a hypothesis that neural cells with an epitope of anti‐GQ1b antibody were damaged, but neural cells without an epitope of anti‐GQ1b antibody had normal nerve conduction. 7 Bickerstaff’s brainstem encephalitis would not affect the latency of ABR because of several neural cells with normal nerve conduction. However, the potentials of ABR are decreased because the number of normal neural cells decreases. The discrepancy between ABR findings and neurological examinations could be useful for identifying BBE.
Conclusion
It is possible that emergency physicians could diagnose a patient with BBE as an unknown cause of consciousness disturbance. Neurological examinations are important to detect lesions and suspect BBE. It is also necessary to analyze serum IgG anti‐GQ1b antibodies to diagnose BBE. Auditory brainstem response would be helpful in detecting lesions and predicting functional recovery.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Written informed consent was obtained from the patient.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Acknowledgements
We would like to thank the following doctors as well as nurses and other medical staff for patient treatment: Dr. Tetsuro Sekine, Dr. Kentaro Toyama, Dr. Masaaki Inoue, Dr. Megumi Tomita, and Dr. Kazuma Miura (Nippon Medical School Hospital, Tokyo, Japan); Dr. Shizuka Kitajima (Matsue Hospital, Tokyo, Japan); and Professor Susumu Kusunoki, Dr. Motoi Kuwahara, Dr. Keisuke Yoshikawa, Dr. Masaki Yamana, and Dr. Yuta Fukumoto for measurement of serum antibody (Department of Neurology, Kinki University, Osaka, Japan).
Funding Information
No funding information provided.
References
- 1. Cooksley T, Rose S, Holland M. A systematic approach to the unconscious patient. Clin Med (Lond) 2018; 18: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koga M, Kusunoki S, Kaida K et al Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry 2012; 83: 1210–5. [DOI] [PubMed] [Google Scholar]
- 3. Yoshikawa K, Kuwahara M, Morikawa M et al Varied antibody reactivities and clinical relevance in anti‐GQ1b antibody‐related diseases. Neurol Neuroimmunol Neuroinflamm. 2018; 5: e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okda M, Yuki N, Hirata K. Anti‐GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry 2001; 70: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis H. Principles of electric response audiometry. Ann Otol Rhinol Laryngol 1976; 85(SUPPL 28(3 Pt3)): 1–96. [PubMed] [Google Scholar]
- 6. Ogawara K, Kuwabara S, Yuki N. Fisher syndrome or Bickerstaff brainstem encephalitis? Anti‐GQ1b IgG antibody syndrome involving both the peripheral and central nervous systems. Muscle Nerve 2002; 26: 845–9. [DOI] [PubMed] [Google Scholar]
- 7. Chiba A, Kusunoki S, Obata H et al Serum anti‐GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillan‐Barre syndrome: clinical and immunohistochemical studies. Neurology 1993; 43: 1911–7. [DOI] [PubMed] [Google Scholar]


