Abstract
Understanding the environmental justice implications of the mortality impacts of air pollution exposure is a public health priority, as some subpopulations may face a disproportionate health burden. We examined which residential environmental and social factors may affect disparities in the air pollution-mortality relationship in North Carolina, US, using a time-stratified case-crossover design. Results indicate that air pollution poses a higher mortality risk for some persons (e.g., elderly) than others. Our findings have implications for environmental justice regarding protection of those who suffer the most from exposure to air pollution and policies to protect their health.
Keywords: Air pollution, Environmental justice, Health disparities, Mortality
1. Introduction
A large body of literature has demonstrated consistent evidence of the effects of exposure to air pollution on mortality (Achilleos et al., 2019; Di et al., 2017; Qu et al., 2018; Wu et al., 2019; Yu et al., 2019). Such mortality burdens may vary by population and region. However, questions remain on which individual and community factors contribute to differences in the associations between air pollution and health among subpopulations. Understanding these health disparities and their potential determinants is a critical public health concern.
Recent studies on disparities suggest that several factors such as sex, age, pre-existing conditions, race/ethnicity, socioeconomic status (SES), and residential environmental factors such as proximity to green spaces and blue spaces may be associated with higher risk of adverse health outcomes related to exposure to air pollution (Li et al., 2017; Liu et al., 2019; Ou et al., 2008; Qu et al., 2018; Tibuakuu et al., 2018). For example, a study in Hong Kong found that female, the elderly, and people with lower SES had increased risk of death associated with air pollution compared to other populations (Qiu et al., 2015). Another study by Richardson et al. (2013) found that persons in lower-income regions in Europe were more susceptible to the health effects of PM10 than other populations, however the findings varied between Eastern and Western Europe, and by type of mortality. Place or neighborhood factors may play an important role in explaining spatial heterogeneity in air pollution exposure and/or health risk. Living in different residential areas may lead to differential exposure to stressors and access to neighborhood resources (Gee and Payne-Sturges 2004). Although some studies suggested health disparities from the impacts of exposure to air pollution, further work at different locations is needed given the variation in population characteristics across regions and the potential changes in disparities over time given temporal patterns in related variables (e.g., air pollution levels, population structure). Identifying the most important factors related to disparities to air pollution-mortality associations in a given location and the most affected subpopulations is critical to establish appropriate plans and conduct effective interventions to protect public health.
Ozone and PM2.5 are major atmospheric pollutants directly affecting human health. The recent Global Burden of Disease (GBD) estimated that exposure to ambient PM2.5 causes 4.2 million deaths globally, with an additional 254,000 deaths globally caused by ozone exposure (GBD 2015). US burden of disease study lists ambient PM2.5 and O3 pollution as the 8th and 15th leading risk factors in the US in 2010 (Murray and Collaborators US Burden of Disease, 2013). Numerous studies in many parts of the world provided scientific evidence that increased risk of mortality was associated with exposure to these pollutants (Fann et al. 2012; Farhat et al. 2013). The aim of our study was to assess several health disparity factors for major air pollutants with mortality associations. Thus, we chose PM2.5 and O3 as our key exposure of interest.
Recent studies have used predicted air pollutant concentrations, which allow for better spatial and temporal coverage than monitoring data, to estimate the relationship between ambient air pollution and several health outcomes (Bravo et al., 2017; Fann et al., 2018). While these values are estimates, they address the lack of high spatial and temporal resolution in many ambient monitoring networks. Most monitors are located in urban areas, which may not fully reflect exposure in rural regions without monitors. Also, many monitors do not operate continuously throughout the year (e.g., measurements every 3 or 6 days for PM2.5, only for the warm season for O3), which prohibits the investigation of cumulative acute exposures over multiple days. Limited spatial and temporal resolution of some monitoring networks may hinder investigation of exposure and health effects in some regions. Thus, health effect estimates based on monitoring data alone may not fully capture the susceptibility due to differences between communities or subpopulations.
North Carolina (NC) is relatively large and diverse state with underlying geographies that include extensive agricultural regions and forests, coastal areas, and multiple medium-large urban centers. NC has a range of air quality with areas in noncompliance with EPA regulations for criteria pollutants (e.g., O3, PM2.5) and distinct spatial patterns of racial distribution or poverty patterns. This study area allows us to evaluate diverse populations and factors regarding environmental health disparities. Although previous studies in this area explored the relationship between air pollution, race, and SES (Gray et al. 2013) or investigated spatial-temporal association between PM2.5 and daily mortality (Choi et al. 2009), no study evaluated several residential environmental and socioeconomic factors that may affect disparities in air pollution-mortality relationships and assessed multiple disparities, which can contribute to a better understanding of interactions of disparity factors.
This study investigated the health disparities attributable to exposure to air pollutants (PM2.5, O3) in North Carolina, USA. We used Community Multi-scale Air Quality (CMAQ) downscaler output to estimate daily PM2.5 and O3 concentrations for 2002–2013. We evaluated which residential environmental and socio-economic factors affect disparities in air pollution-mortality relationships using a stratified model for each effect modifier. Our study has implications for environmental justice regarding which subpopulations are vulnerable and which factors affect disparities in associations between air pollution and mortality. This work extends current understanding of environmental health disparities.
2. Methods
2.1. Data
We obtained individual-level mortality data for North Carolina from 2002 to 2013 from the North Carolina State Center for Health Statistics, Vital Statistics Department. For each participant, mortality data included date of death, residential location, sex, age at death (<65, ≥65 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, or non-Hispanic other), education (<12 years, high school graduate, 1–4 years of college, ≥5 years of college, or unknown), and marital status (never married, married, widowed, divorced, or unknown). We excluded participants with incomplete data for any variable. We classified mortality data as: total mortality as all causes of death except external causes (International Classification of Diseases, ICD-10, A00-R99), cardiovascular mortality (ICD-10, I00-R99), and respiratory mortality (ICD-10, J00-J99).
Ambient PM2.5 and O3 concentrations for each of North Carolina’s census tracts were obtained for 2002–2013 from the downscaler output from the US Environmental Protection Agency (EPA). The downscaler utilizes air monitoring station data and Community Multiscale Air Quality (CMAQ) output at 12×12km grid cell resolution to estimate daily air pollution concentrations at census tract centroids. Downscaler output includes estimates of daily 24-h average for PM2.5 and daily 8-h maximum for O3. For these estimates we assigned exposure based on the grid cell in which the participant’s residence was located. Additional details for the downscaler modeling approach and evaluation are provided elsewhere (Berrocal et al., 2012). To compare the robustness of effect estimates of downscaler predicted PM2.5 and O3 levels with those generated using monitoring data, we obtained daily 24-h PM2.5 and 8-h maximum O3 measurement values from the EPA’s Air Quality System. We assigned exposures for each participant as the daily measurements from monitors nearest each subject’s residence (based on each subject’s residential location (latitude/longitude)) including monitors outside North Carolina, within 40km of North Carolina’s boundary. We use the downscaler exposure estimates as main analysis and monitor-based values as sensitivity analysis. The total number of cases (i.e., deaths) for downscaler- and monitor-based estimate was 775,338 and 209,669, respectively. There were fewer deaths when using monitor-based estimates as monitor-derived air pollution effect estimates were based on the time and locations for which exposure estimates are available from both methods.
Due to lack of measured daily weather data, we used gridded weather data at the county level. The gridded weather data using Parameter-elevation Regressions on Independent Slopes Model (PRISM) interpolation method are reported on a daily basis and at high spatial resolutions (4×4km grid). PRISM provides data for the continental US. The algorithms and the details have been described elsewhere (Daly et al., 2008; PRISM Climate Group, 2004). A previous study showed good agreement between measured and gridded weather data (Mourtzinis et al., 2017). We used daily levels of temperature and dew point temperature at the county level. County-level values were calculated as the average of all grid cells with centroids within each county.
To assess health disparity factors in the association between exposure to air pollution and mortality, we included several residential environmental and socio-economic factors based on the previous literature review. We considered individual-level factors, residential greenness, proximity to water bodies, median household income, and classification of urbanicity.
As a residential greenness measure, urban vegetation was assessed using the Normalized Difference Vegetation Index (NDVI) derived from the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor aboard the Terra satellite image from NASA’s Earth Observing System. We used the global MODIS product MOD13Q1 version 5, which has been corrected for atmospheric contamination from water, clouds, and aerosols. This product is a 16-day composite at a spatial resolution of 250m. We calculated average NDVI at the ZIP-code level for the study period. We categorized average NDVI as above or below the median (<0.61 or ≥0.61).
We obtained information on water bodies (e.g. river, lake) from the North Carolina Department of Environmental Quality Online GIS to examine effect modification by proximity to water bodies. We calculated the distance from each subject’s residence to water bodies to assess the effect of blue space and categorized proximity to water as above or below the median (<10.2km or ≥10.2km).
To assess community-level effect modification we used 2010 Census data at the census tract level including variables of median household income, as a surrogate for SES, and population size. We classified urbanicity as metropolitan (urban area ≥50,000 people), micropolitan (urban cluster of 10,000–49,999), and rural (urban cluster of <10,000) area. We used median values to define categories of income.
2.2. Statistical analysis
We applied a time-stratified case-crossover design to estimate the association between air pollution and mortality. In this approach, each case acts as his or her own control and thus the method has benefits of controlling for potential confounding from fixed characteristics by design. To avoid selection bias, we applied time-stratified referent selection based on same day of the week of the same year when a death occurred. Each case could be compared to multiple control days.
Some O3 monitors operate only during the warm season (e.g., April-September) when O3 is anticipated to be high. We generated separate effect estimates for the association with mortality for: 1) year-round O3 exposure and 2) warm season O3 exposure (April-September). We examined the lagged effect of air pollutants with single-day lags (lag 0, lag 1, lag 2) and multiday lags (lag 01, lag 02). Lag 0 meant the effect of the air pollution on the same day as the day of mortality (i.e., date of death). Lag 1 refers to the air pollution on the day before the day of death. Lag 02 presented the cumulative effect of the current day and prior 2 days’ air pollution on the current day’s mortality. For monitor-based effect estimates, we investigated the effect of PM2.5 for only single-day lag as most PM2.5 monitors typically record observations every three days.
We conducted additional analyses considering spatial clustering in the model. We accounted for spatial autocorrelation from unmeasured spatially distributed risk factors by including a random intercept for each county where cluster effects are incorporated into the model as independent and identically distributed random variables to account for the within-cluster correlation. We also conducted additional analysis considering NDVI at the county level.
We calculated Population-attributable risks (PARs) based on the calculated effect estimates in this study. The PAR% is the percentage of incidence of a disease within a population (exposed and non-exposed), due to exposure. This statistic describes the percentage incidence of a disease within a population that could be prevented if exposures were eliminated. We estimated Population-attributable risks (PARs) per pollutant using our risk estimates and the following equation: PAR% = 100 × P(R − 1) / [P(R – 1) + 1], for which P is the prevalence of the exposure (i.e., air pollution) in the population and is assumed to be 100% as everyone in the population exposed to air pollution and R is the relative risk (or OR).
To examine the potential effect modifiers, we performed stratified analyses by individual- and community-level factors for total mortality. We then tested statistical significance of differences between effect estimates of strata of a potential effect modifier by calculating the 95% confidence interval as where Q1 and Q2 are the estimates for the two strata of the potential effect modifier (e.g., male and female), and SE1 and SE2 are their respective standard errors. To categorize community-level factors, we tested other cutoff points (e.g., quartile) as well as median value. We also investigated multiple susceptibilities by combinations of potential factors of effect modification (e.g., race/ethnicity and census-tract median income). We fitted conditional logistic regression models to estimate the association between air pollution and mortality. Odds ratios and 95% confidence intervals were calculated on the basis of an increase of 10 μg/m3 in PM2.5 or 10 ppb in O3. All analyses were conducted using SAS (9.4, SAS Institute, Cary, NC, USA) and R (version 3.5.1, R Core Team).
3. Results
During the study period, there were 775,338 cases (i.e., total deaths) with 3,410,015 control days. Table 1 shows characteristics of the study population. The 775,338 total deaths included 261,663 from cardiovascular disease and 86,017 from respiratory disease. The study population had more females than males (52.3% vs. 47.7%). The majority of the deceased were non-Hispanic white (77.7%), and ≥65 years (75.2%). Most subjects had less than a high school level education or were high school graduates (71.2%) and were married or widowed (78.5%). For community-level characteristics, mean census-tract median income was $45,116. Most subjects lived in metropolitan areas (85.0%). For study participants, average NDVI was 0.61 and average distance from residence to water bodies was 12.6km.
Table 1.
Characteristics of study population in NC, 2002–2013
| Characteristics | Total (N=775,338) |
|---|---|
| Cause of death | |
| Total | 775,338 |
| Cardiovascular | 261,663 |
| Respiratory | 86,017 |
| Sex (%) | |
| Male | 369,883 (47.7) |
| Female | 405,441 (52.3) |
| Missing | 14 (0.0) |
| Race/ethnicity | |
| Non-Hispanic White | 602,125 (77.7) |
| Non-Hispanic Black | 158,449 (20.4) |
| Hispanic | 5,307 (0.7) |
| Non-Hispanic Asian | 3,239 (0.4) |
| Non-Hispanic Other | 6,096 (0.8) |
| Missing | 122 (0.0) |
| Age at death | |
| <65 years | 192,631 (24.8) |
| ≥65 years | 582,707 (75.2) |
| Education | |
| <12 years | 303,198 (39.1) |
| High school graduate | 249,042 (32.1) |
| 1–4 years of College | 175,497 (22.6) |
| 5 or more years of college | 36,141 (4.7) |
| Unknown | 11,460 (1.5) |
| Marital status | |
| Never married | 73,592 (9.5) |
| Married | 308,906 (39.8) |
| Widowed | 299,738 (38.7) |
| Divorced | 91,856 (11.9) |
| Unknown | 1,246 (0.2) |
| Community-level factors | |
| Census-tract median income (mean±SD) | $45,116±$18,015 |
| County-level urbanicity | |
| Metropolitan (urban area ≥50,000 people) | 659,332 (85.0) |
| Micropolitan (urban cluster of 10,000–49,999) | 113,692 (14.7) |
| Rural (urban cluster of <10,000) | 2,314 (0.3) |
| Average NDVI | 0.61±0.05 |
| Distance to water bodies (km) | 12.6±10.3 |
| Air Pollution Estimates | |
| Downscaler PM2.5 (μg/m3) | 11.4±5.7 |
| Downscaler O3 (ppb) | 41.7±13.7 |
| Monitor-based PM2.5 (μg/m3) | 11.5±6.1 |
| Monitor-based O3 (ppb) | 31.3±11.1 |
| Weather variables | |
| Temperature (°C) | 14.9±8.7 |
| Dew point temperature (°C) | 8.2±9.8 |
The average PM2.5 concentrations from CMAQ downscaler and EPA monitor were similar, although average O3 concentration for CMAQ downscaler output was higher than EPA monitor concentrations. Spatial variations in air pollution levels are provided in Figure 1. Descriptive statistics for residential environmental factors are provided in Supplementary Table 1 and air pollution levels based on community-level SES are provided in Supplementary Table 2.
Figure 1.
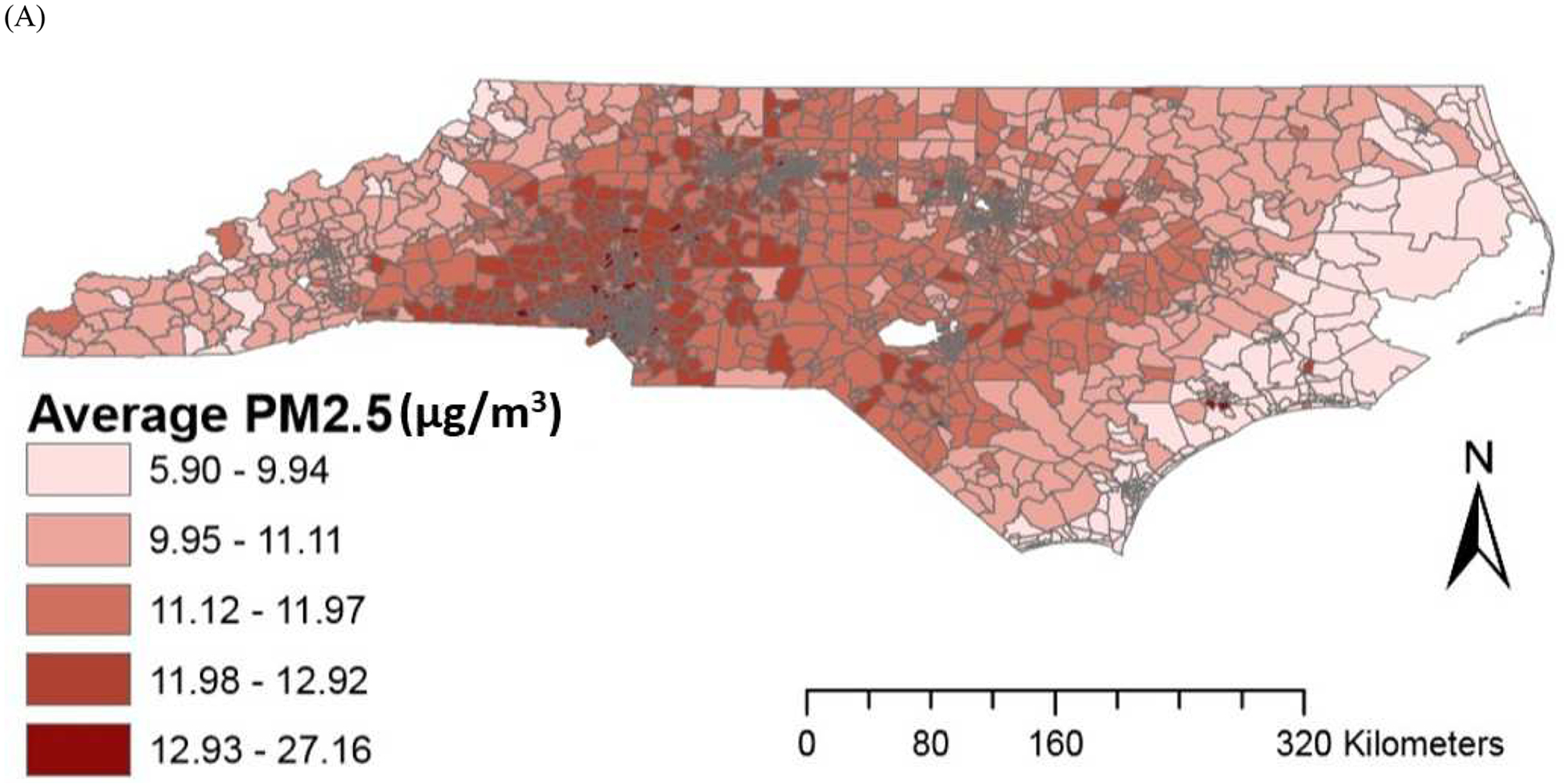
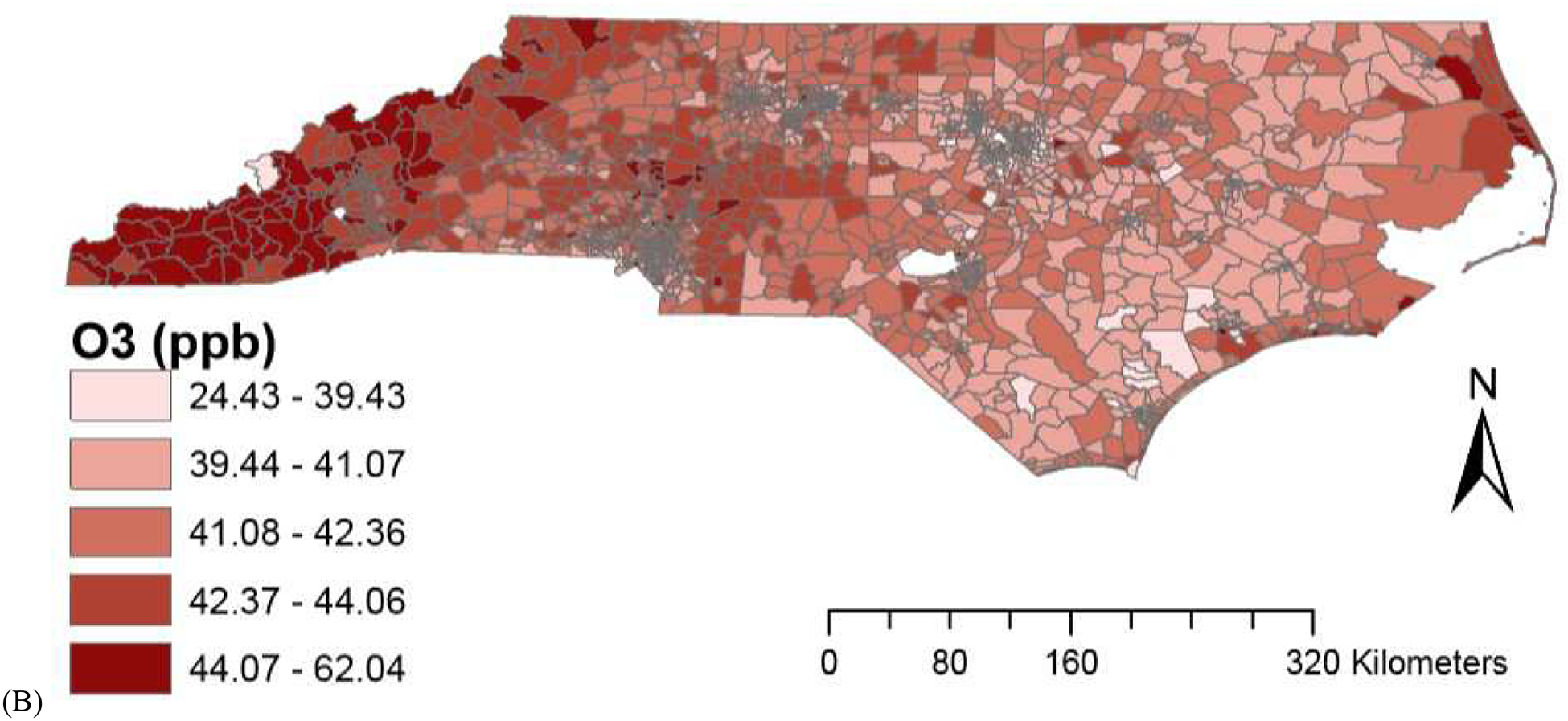
Spatial variations in air pollution levels (A) PM2.5 (B) O3.
Supplementary Table 3 provides correlation coefficients across variables. Strong positive correlations between downscaler- and monitor-derived air pollution concentrations were observed (r=0.96 for PM2.5; r=0.84 for O3). Average NDVI was negatively correlated with county-level total population (r=−0.48).
Table 2 shows odds ratios (OR) and 95% confidence intervals (CI) of the association between exposure using downscaler-derived PM2.5 and O3 for risk of total and cause-specific mortality. All models were adjusted for same day’s temperature and dew point temperature. We observed positive associations between PM2.5 exposure and risk of total and cardiovascular mortality. An 10μg/m3 increase in lag 01 PM2.5 exposure was associated with an OR of 1.019 (95% CI 1.012, 1.025) and 1.017 (95% CI 1.007, 1.028) for total and cardiovascular mortality, respectively. For O3, a 10ppb increase in lag 02 exposure was associated with total mortality (OR 1.006; 95% CI 1.002, 1.010). We did not find statistically significant associations with respiratory mortality.
Table 2.
Odds ratios and 95% confidence intervals of PM2.5 and O3 for total and cause-specific mortality
| Total | Cardiovascular | Respiratory | |
|---|---|---|---|
| Downscaler PM2.5 (per 10μg/m3) | |||
| Lag 0 | 1.015 (1.009, 1.020) | 1.011 (1.002, 1.020) | 1.004 (0.988, 1.020) |
| Lag 1 | 1.015 (1.009, 1.020) | 1.016 (1.007, 1.026) | 1.002 (0.986, 1.019) |
| Lag 2 | 1.004 (0.998, 1.009) | 1.003 (0.993, 1.012) | 0.998 (0.982, 1.015) |
| Lag 01 | 1.019 (1.012, 1.025) | 1.017 (1.007, 1.028) | 1.004 (0.986, 1.023) |
| Lag 02 | 1.018 (1.011, 1.025) | 1.016 (1.004, 1.028) | 1.003 (0.983, 1.025) |
| Downscaler O3 (per 10ppb) | |||
| Lag 0 | 1.004 (1.001, 1.006) | 1.000 (0.995, 1.004) | 1.003 (0.995, 1.012) |
| Lag 1 | 1.004 (1.001, 1.007) | 1.003 (0.997, 1.008) | 0.999 (0.989, 1.009) |
| Lag 2 | 1.003 (1.000, 1.006) | 1.003 (0.998, 1.007) | 1.000 (0.991, 1.008) |
| Lag 01 | 1.005 (1.002, 1.008) | 1.001 (0.996, 1.007) | 1.002 (0.991, 1.012) |
| Lag 02 | 1.006 (1.002, 1.010) | 1.003 (0.996, 1.009) | 1.001 (0.989, 1.012) |
N for downscaler PM2.5 and O3 exposure: 775,338
We performed additional analysis for the warm season (April to September) O3 (Supplementary Table 4). The effect estimates from the warm season were generally similar with those of year-round O3.
To confirm the robustness of our findings, we performed additional analysis comparing the effect estimates based on exposures from the downscaler- and monitor-derived concentrations (Figure 2). Effect estimates from the monitor-derived concentrations were similar to those of the original findings from downscaler-derived concentrations.
Figure 2.
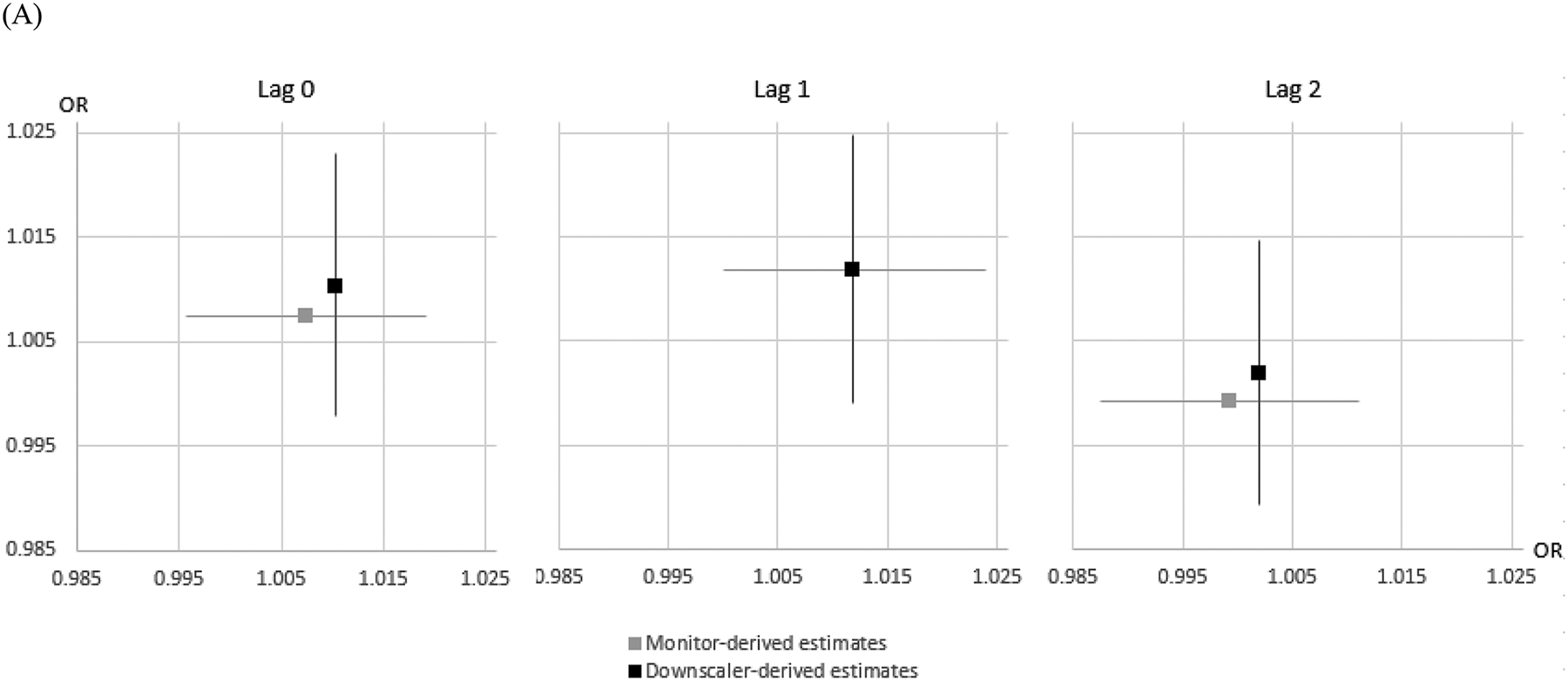
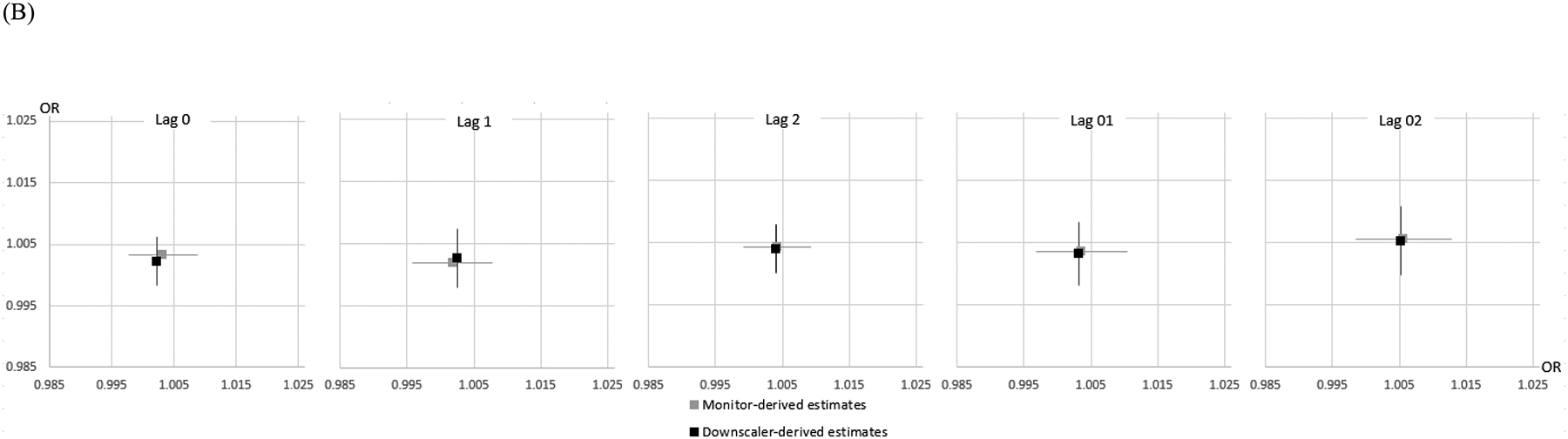
Downscaler- vs. monitor-derived air pollution effect estimates based on the times and locations for which exposure estimates are available from both methods: (A) PM2.5 (B) warm-season O3. Lines reflect 95% intervals, horizontal estimates represent monitor-derived estimates, Vertical estimates represent downscaler-derived estimates.
We observed positive associations between exposure to PM2.5 and O3 and risk of total mortality; for these associations we conducted additional analyses considering spatial autocorrelation in the model. Results were similar to original findings (Supplementary Table 5). We calculated PAR for air pollution and total mortality. The PARs for total mortality due to PM2.5 (lag01) and O3 (lag02) exposure were 1.9% and 0.6% respectively (Supplementary Table 6). Findings from additional analysis considering NDVI at the county level were similar with original findings (Supplementary Table 7).
For the exposure lag and mortality for which we observed the largest and also the most statistically significant associations (i.e., PM2.5 and O3 exposure for total mortality), we evaluated effect modification by community and individual characteristics. Table 3 shows estimated associations between air pollution exposure and total mortality stratified by community-level factors. We investigated the relationship between PM2.5 and O3 exposure and total mortality by residential green space, blue space, urbanicity, and census-tract median income level (Table 3). We did not find any statistically significant differences between groups. However, the association between air pollution and the risk of total mortality was slightly higher, although not statistically different, in areas with less green space, further distance to water bodies, ≥50,000 people, or lower median income level. Estimated associations for O3 showed similar patterns except for urbanicity and census-tract median income level.
Table 3.
Association between air pollution and total mortality, stratified by community-level environmental factors
| PM2.5 | O3 | |
|---|---|---|
| Green space | ||
| Average NDVI <0.61 | 1.020 (1.011, 1.028) | 1.006 (1.000, 1.011) |
| Average NDVI ≥0.61 | 1.018 (1.009, 1.027) | 1.006 (1.001, 1.012) |
| Blue space (proximity to water bodies) | ||
| <10.2 km | 1.017 (1.008, 1.026) | 1.004 (0.999, 1.009) |
| ≥10.2 km | 1.020 (1.011, 1.029) | 1.008 (1.002, 1.013) |
| Urbanicity | ||
| urban area ≥50,000 people | 1.021 (1.015, 1.028) | 1.006 (1.002, 1.010) |
| urban cluster of 10,000–49,999 | 1.001 (0.985, 1.018) | 1.004 (0.994, 1.015) |
| urban cluster of <10,000 | 1.014 (0.890, 1.155) | 1.040 (0.965, 1.121) |
| Census tract median income | ||
| <41,500 USD | 1.021 (1.012, 1.030) | 1.004 (0.999, 1.009) |
| ≥41,500 | 1.016 (1.008, 1.025) | 1.008 (1.002, 1.013) |
PM2.5 lag 01; O3 lag 02
Cutoff for green space, blue space, and median income: 50% median
We also assessed potential effect modification by individual characteristics (Table 4). Stratified analyses showed that associations between PM2.5 exposure and total mortality were higher in males than females although these results were not statistically different. We found higher risk in persons who were non-Hispanic White or non-Hispanic Black, ≥65 years, less educated (<12 years), never married, and widowed. For O3, we observed similar patterns with slightly higher risk in males, persons ≥65 years, and those who were less educated (<12 years).
Table 4.
Association between air pollution and total mortality, stratified by individual-level factors
| Characteristics | PM2.5 | O3 |
|---|---|---|
| Sex | ||
| Male | 1.023 (1.014, 1.032) | 1.008 (1.003, 1.014) |
| Female | 1.015 (1.006, 1.023) | 1.004 (0.999, 1.009) |
| Race/ethnicity | ||
| Non-Hispanic White | 1.020 (1.013, 1.027) | 1.006 (1.001, 1.010) |
| Non-Hispanic Black | 1.017 (1.004, 1.031) | 1.008 (0.999, 1.016) |
| Hispanic | 1.003 (0.931, 1.081) | 0.959 (0.917, 1.003) |
| Non-Hispanic Asian | 0.945 (0.859, 1.040) | 1.024 (0.967, 1.085) |
| Non-Hispanic Other | 1.018 (0.947, 1.094) | 1.017 (0.974, 1.061) |
| Age at death | ||
| <65 years | 1.009 (0.996, 1.021) | 1.005 (0.997, 1.012) |
| ≥65 years | 1.022 (1.015, 1.029) | 1.006 (1.002, 1.011) |
| Education | ||
| <12 years | 1.025 (1.015, 1.035) | 1.007 (1.001, 1.013) |
| High school graduate | 1.016 (1.005, 1.027) | 1.003 (0.997, 1.010) |
| 1–4 years of College | 1.013 (1.000, 1.027) | 1.005 (0.997, 1.013) |
| 5 or more years of college | 1.017 (0.988, 1.046) | 1.013 (0.995, 1.030) |
| Unknown | 1.003 (0.956, 1.052) | 1.013 (0.983, 1.044) |
| Marital status | ||
| Never married | 1.024 (1.004, 1.044) | 1.009 (0.997, 1.021) |
| Married | 1.017 (1.007, 1.027) | 1.006 (1.000, 1.012) |
| Widowed | 1.023 (1.013, 1.033) | 1.007 (1.001, 1.013) |
| Divorced | 1.006 (0.988, 1.024) | 1.000 (0.989, 1.011) |
| Unknown | 1.123 (0.967, 1.303) | 1.077 (0.981, 1.183) |
We conducted additional analysis to assess combined disparities in the associations between air pollution and total mortality by combinations of individual- and community-level characteristics (Table 5). We assessed mortality disparities by combinations of race/ethnicity and census-tract median income level. The highest and most significant association between PM2.5 exposure and total mortality was found in non-Hispanic Black participants living in areas with the lowest community-level SES. Of non-Hispanic Black participants, a significant association between PM2.5 exposure and total mortality was observed only for those living in the lowest census-tract median income level.
Table 5.
Association between PM2.5 exposure and total mortality in urban areas, stratified by combinations of factors
| Census tract median income | Census tract median income | Census tract median income | Census tract median income | |
|---|---|---|---|---|
| < 33,750 (25%) | 33,750–41,500 | 41,500–52,269 | ≥52,269 (75%) | |
| Race/ethnicity | ||||
| Non-Hispanic White | 1.021 (1.003, 1.040) | 1.025 (1.009, 1.041) | 1.021 (1.007, 1.036) | 1.022 (1.008, 1.035) |
| Non-Hispanic Black | 1.035 (1.013, 1.058) | 1.006 (0.976, 1.036) | 1.008 (0.974, 1.043) | 1.020 (0.984, 1.058) |
| Hispanic | 1.005 (0.867, 1.166) | 0.926 (0.777, 1.103) | 1.106 (0.948, 1.286) | 0.971 (0.840, 1.123) |
| Non-Hispanic Asian | 0.978 (0.764, 1.254) | 0.867 (0.687, 1.093) | 0.986 (0.809, 1.202) | 0.906 (0.780, 1.053) |
| Non-Hispanic Other | 1.033 (0.937, 1.139) 1 | 1.171 (0.992, 1.383) | 0.772 (0.587, 1.015) | 0.896 (0.672, 1.195) |
4. Discussion
In this study, we evaluated which subpopulations are vulnerable and which factors affect disparities in associations between exposure to air pollution and risk of mortality. Although the results were not statistically different among groups, some factors such as age, education, and urbanicity were associated with higher risk of total mortality from PM2.5 exposure. For combinations of individual- and community-level factors, the magnitude of health disparities observed was more pronounced for Non-Hispanic Blacks living in lower community-level SES.
Our findings of positive associations between short-term exposure to PM2.5 or O3 and mortality are consistent with those of many studies in the literature, with similar range of effect size (Supplementary Table 8). As an example, a recent study by Wu et al. (2019) reported that increased exposure to particulate matter (PM2.5, PMcoarse, and PM10) in Lanzhou, an industrial city in China, was associated with higher risk of cardiovascular mortality. Other studies observed associations between short-term exposure to PM2.5 and total mortality (Li et al., 2017; Yorifuji et al., 2016). Chen et al. (2017) found strong evidence that short-term exposure to O3 is significantly associated with increased total mortality.
Our findings on the disparities in air pollution–health associations by some individual- and area-level characteristics are consistent with those of previous studies, which find disproportionate health burdens from air pollution. Many studies showed evidence that some factors such as older age, low education, and living in urban areas are associated with higher risk of mortality from air pollution exposure, consistent with our findings (Bravo et al., 2016; Deguen and Zmirou-Navier, 2010; Son et al., 2012). Wong et al. (2008) suggested that people residing in socially deprived communities have higher mortality risk from ambient air pollution. On the other hand, results for effect modification by some factors have varied. Some previous studies found no differences by sex (Ren et al., 2010), while others found higher effect for males (Chen et al., 2010; Son et al., 2012) or females (Kan et al., 2008; Zanobetti and Schwartz, 2000).
In this study, we did not find significant differences by residential environmental factors such as green and blue spaces. Studies on health disparities attributable to air pollution by residential environmental factors such as residential greenness are limited although some research examined the direct effect of greenness on health outcomes. A few recent studies on effect modification of the PM2.5 mortality association found inconsistent results. A recent study by Yitshak-Sade et al. (2019) reported that estimated PM2.5 effects on cardiovascular mortality were attenuated by higher neighborhood greenness in areas with lower socioeconomic status. Another study found positive modification of greenness on the PM2.5 and mortality association (Kioumourtzoglou et al., 2016). Heo and Bell (2019) found that the association between short-term exposure to particulate matter and hospitalization was lower in areas with more green space. Possible mechanisms of how green space might influence health include reduced risks of physical and mental illnesses by increased opportunities for physical activity and other pathways. Moreover, living near green space may benefit health by facilitating social interaction, and can promote recovery from stress (Richardson et al. 2010). Also, proximity to water bodies may reduce exposure to many urban stressors and have beneficial effects on physiological systems that integrate stress response through higher exposure to health promoting factors and behaviors (Crouse et al. 2018).
Previous findings on disparities in mortality risk related to air pollution were inconsistent across different study areas and populations. The patterns of disparities varied depending on the health outcomes and measures of several variables studied. The differences in health disparities we observed may result from several factors such as variation in population characteristics, distribution and/or composition of characteristics and their interactions within groups (e.g., age, education, and racial/ethnic composition in urban/rural population), biological and generic vulnerabilities, access to health care and quality, social and physical environment, and health-related behaviors (Thomson et al., 2006). In the analysis of combined disparities by race/ethnicity and census-tract median income level, we found that Non-Hispanic Blacks living in lower community-level SES (below median) had the highest risk estimate for the association between PM2.5 and total mortality. Our findings indicate that health disparities may relate to socioeconomic differences between/within racial groups; analysis of racial/ethnic differences without consideration of other factors such as socioeconomic status and access to health care may not fully capture the full and complex system. Race/ethnicity and socioeconomic status may be linked through psychosocial pathways such as perceived stress, biological markers of chronic stress (Morello-Frosch et al. 2011; Goodman et al. 2005; Gee et al. 2004). In general, racial minorities tend to have lower socioeconomic status, however, socioeconomic differences do not fully explain racial disparities. Race/ethnicity is highly correlated with residential location. Poorer neighborhoods tend to have higher rates of psychosocial stressors, which may contribute to health disparities.
A previous study conducted within-race analyses, finding that most of the apparent differences in air pollutant effects found across races were explained by socioeconomic and/or health care disparities (Gwynn and Thurston, 2001). Ito and Thurston (1996) found that black females had the highest risk for air pollution impacts for total, respiratory, and cancer mortality in race and sex-specific analysis. This may relate to multiple factors for the subpopulations and their interactions (e.g., correlation between SES and race/ethnicity at the community level, relationship between percentage of racial minorities living in urban areas with higher levels of pollution and/or harmful residential environment, existing health conditions or behaviors) (Martenies et al., 2017). A challenge to the study of disparities in health risk is that many of the characteristics of interest are often correlated. These complexities change the isolation of responsible factors that contribute to health disparities and different impacts of mechanisms on various populations. Evaluation of disparities in health risk relate to multiple relationships among possible disparity factors. Thus, more research at the local scale is needed to consider the complex interactions among factors on health risk from air pollution exposure.
There are several limitations to this work. We used downscaler predictions of air pollution levels that allow us to estimate air pollution concentrations at locations and time periods without monitors. Although we confirmed that the downscaler-derived findings were robust in comparison to results generated using monitoring data for areas and times with monitors, we could not evaluate the effect estimates in areas without monitors (e.g., non-urban areas). Urban and non-urban areas may have different characteristics of exposure (e.g., pollutant mixtures, chemical compositions) and demographics. Thus, further research considering uncertainty on differences in urban and non-urban areas is warranted. We used 2010 Census data to estimate population characteristics for the study period (2002–2013). Using 2010 Census data may not perfectly reflect actual population characteristics for the period 2002–2013. However, assigning more timely data may introduce uncertainty as well due to some issues such as data from different sources, boundary changes over time. For example, ACS and Census data are not equivalent and there are some differences between the ACS and Census data such as residence rules, reference periods, definitions, and methods between the two data sources that can impact comparability. Thus, we used only 2010 Census data for the whole study period for consistency. Also, for our Census variables there is likely to be little relative change across the Census tracts overall across time. Some measures of disparity factors we considered (e.g., census-tract median household income for community-level socioeconomic status) may not fully reflect the actual aspects of each factor, although the correlations between several measures of SES in this study were highly correlated with each other (Supplementary Table 9). Many studies have used several measures such as individual- or community-level measures of income and education to represent SES, however SES has complexities of several correlated factors (e.g., historical income) that may affect the associations and combinations of these variables (Williams et al., 2010).
Strengths of our study include the use of geocoded individual-level mortality data with high spatial and temporal resolution exposure data. Our study was able to estimate the health effect of cumulative short-term exposure to PM2.5 in areas and time periods without daily monitoring data. For some factors, we were able to assess multiple disparities (e.g., race and SES), which contributes to a better understanding of interactions of disparity factors and to environmental justice more broadly.
5. Conclusions
We provide additional evidence confirming previous work indicating that short-term exposure to PM2.5 and O3 are positively associated with increased risk of mortality. Our assessment of combined disparities indicate that the multiple aspects of disparity factors may affect disproportionate mortality burdens from air pollution exposures. The findings from our work have important implications for environmental decision making by identifying priorities for policy intervention on modifiable factors. This work can help focus on more efficient policy actions to mitigate health impacts for vulnerable populations with limited resources. Our findings on environmental health disparities provide valuable evidence for decision makers and help inform future research on environmental justice.
Supplementary Material
Highlights.
Higher PM2.5-mortality effect estimates were associated with age and urbanicity.
Blacks in poor communities had the highest, most certain PM2.5-mortality estimate.
Multiple disparity factors, race and SES, may affect PM2.5-mortality burdens.
Funding Sources
This publication was developed under Assistance Agreement No. RD835871 awarded by the U.S. Environmental Protection Agency to Yale University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD012769. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Achilleos S, Al-Ozairi E, Alahmad B, Garshick E, Neophytou AM, Bouhamra W, et al. 2019. Acute effects of air pollution on mortality: A 17-year analysis in Kuwait. Environ Int 126:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal VJ, Gelfand AE, Holland DM. 2012. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics 68:837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo MA, Ebisu K, Dominici F, Wang Y, Peng RD, Bell ML. 2017. Airborne fine particles and risk of hospital admissions for understudied populations: Effects by urbanicity and short-term cumulative exposures in 708 U.S. Counties. Environ Health Perspect 125(4):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo MA, Son JY, de Freitas CU, Gouveia N, Bell ML. 2016. Air pollution and mortality in São Paulo, Brazil: Effects of multiple pollutants and analysis of susceptible populations. J Expo Sci Environ Epidemiol 26(2):150–61. [DOI] [PubMed] [Google Scholar]
- Chen K, Zhou L, Chen X, Bi J, Kinney PL. 2017. Acute effect of ozone exposure on daily mortality in seven cities of Jiangsu Province, China: No clear evidence for threshold. Environ Res 155:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Pan G, Kan H, Tan J, Song W, Wu Z, et al. 2010. Ambient air pollution and daily mortality in Anshan, China: a time-stratified case-crossover analysis. Sci Total Environ 408(24):6086–91. [DOI] [PubMed] [Google Scholar]
- Choi J, Fuentes M, Reich BJ. 2009. Spatial-temporal association between fine particulate matter and daily mortality. Computational Statistics and Data Analysis 53:2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse DL, Balram A, Hystad P, Pinault L, van den Bosch M, Chen H, et al. 2018. Associations between living near water and risk of mortality among urban Canadians. Environ Health Perspect 126:077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, et al. 2008. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol 28:2031–2064. [Google Scholar]
- Deguen S, Zmirou-Navier D. 2010. Social inequalities resulting from health risks related to ambient air quality-A European review. Eur J Public Health 20(1):27–35. [DOI] [PubMed] [Google Scholar]
- Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. 2017. Association of short-term exposure to air pollution with mortality in older adults. JAMA 318(24):2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N, Coffman E, Timin B, Kelly JT. 2018. The estimated change in the level and distribution of PM2.5-attributable health impacts in the United States: 2005–2014. Environ Res 167:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. 2012. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal 32(1):81–95. [DOI] [PubMed] [Google Scholar]
- Farhat N, Ramsay T, Jerrett M, Krewski D. 2013. Short-term effects of ozone and PM2.5 on mortality in 12 Canadian cities. Journal of Environmental Protection 4:18–32. [Google Scholar]
- Franklin M, Zeka A, Schwartz J. 2007. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of Exposure Science & Environmental Epidemiology 17:279–287. [DOI] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators: Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study. 2015. Lancet 388:1659–1724. 10.1016/S0140-6736(16)31679-8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. 2004. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect 112(17):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SC, Edwards SE, Miranda ML. 2013. Race, socioeconomic status, and air pollution exposure. Environmental Research 126:152–158. [DOI] [PubMed] [Google Scholar]
- Goodman E, McEwen BS, Huang B, Dolan LM, Adler NE. 2005. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosom Med 67(1):9–15. [DOI] [PubMed] [Google Scholar]
- Gwynn RC, Thurston GD. 2001. The burden of air pollution: Impacts among racial minorities. Environ Health Perspect 109(4):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Bell ML. 2019. The influence of green space on the short-term effects of particulate matter on hospitalization in the U.S. for 2000–2013. Environ Res 174:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Thurston GD. 1996. Daily PM10/mortality associations: an investigation of at-risk subpopulations. J Expo Anal Environ Epidemiol 6:79–95. [PubMed] [Google Scholar]
- Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, et al. 2008. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the public health and air pollution in Asia (PAPA) study. Environ Health Perspect 116:1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. 2016. PM2.5 and mortality in 207 US cities: Modification by temperature and city characteristics. Epidemiology 27(2):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Burnett RT, Weichenthal S. 2018. Association of short-term exposure to fine particulate air pollution and mortality: effect modification by oxidant gases. Sci Rep 8(1):16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xue M, Zeng Q, Cai Y, Pan X, Meng Q. 2017. Association between fine ambient particulate matter and daily total mortality: An analysis from 160 communities of China. Sci Total Environ 599–600:108–113. [DOI] [PubMed] [Google Scholar]
- Li Y, Shang Y, Zheng C, Ma Z. 2018. Estimated acute effects of ozone on mortality in a rural district of Beijing, China, 2005–2013: A time-stratified case-crossover study. Int J Environ Res Public Health 15(11):2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xue X, Zhou B, Zhang Y, Sun B, Chen J, et al. 2019. Population susceptibility differences and effects of air pollution on cardiovascular mortality: epidemiological evidence from a time-series study. Environ Sci Pollut Res Int doi: 10.1007/s11356-019-04960-2 [DOI] [PubMed] [Google Scholar]
- Martenies SE, Milando CW, Williams GO, Batterman SA. 2017. Disease and health inequalities attributable to air pollutant exposure in Detroit, Michigan. Int J Environ Res Public Health 14(10). pii: E1243. doi: 10.3390/ijerph14101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. 2011. Understanding the cumulative impacts of inequalities in environmental health: Implications for policy. Health Affairs 30(5):879–887. [DOI] [PubMed] [Google Scholar]
- Mourtzinis S, Edreira JIR, Conley SP, Grassini P. 2017. From grid to field: Assessing quality of gridded weather data for agricultural applications. European Journal of Agronomy 82:163–172. [Google Scholar]
- Murray CJL and US Burden of Disease Collaborators. 2013. The State of US Health, 1990–2010 Burden of Diseases, Injuries, and Risk Factors. JAMA 310:591–606. 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Gascon M, Martinez D, Ponjoan A, Blanch J, Garcia-Gil MDM, et al. 2018. Air pollution, noise, blue space, and green space and premature mortality in Barcelona: A mega cohort. Int J Environ Res Public Health 15(11):2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Tobias A, Querol X, Alastuey A, Amato F, Pey J, Pérez N, Sunyer J. 2011. The effects of particulate matter sources on daily mortality: A case-crossover study of Barcelona, Spain. Environ Health Perspect 119(12):1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou CQ, Hedley AJ, Chung RY, Thach TQ, Chau YK, Chan KP, et al. 2008. Socioeconomic disparities in air pollution-associated mortality. Environ Res 107(2):237–44. [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group, Oregon State University. http://prism.oregonstate.edu
- Qiu H, Tian L, Ho KF, Pun VC, Wang X, Yu IT. 2015. Air pollution and mortality: effect modification by personal characteristics and specific cause of death in a case-only study. Environ Pollut 199:192–7. [DOI] [PubMed] [Google Scholar]
- Qu Y, Pan Y, Niu H, He Y, Li M, Li L, et al. 2018. Short-term effects of fine particulate matter on non-accidental and circulatory diseases mortality: A time series study among the elder in Changchun. PLoS One 13(12):e0209793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Melly S, Schwartz J. 2010. Modifiers of short-term effects of ozone on mortality in eastern Massachusetts-a case-crossover analysis at individual level. Environ Health 9:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E, Pearce J, Mitchell R, Day P, Kingham S. 2010. The association between green space and cause-specific mortality in urban New Zealand: an ecological analysis of green space utility. BMC Public Health 10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson EA, Pearce J, Tunstall H, Mitchell R, Shortt NK. 2013. Particulate air pollution and health inequalities: a Europe-wide ecological analysis. Int J Health Geogr 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JY, Lee JT, Kim H, Yi O, Bell ML. 2012. Susceptibility to air pollution effects on mortality in Seoul, Korea: a case-crossover analysis of individual-level effect modifiers. J Expo Sci Environ Epidemiol 22(3):227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson GE, Mitchell F, Williams MB, editors. 2006. Examining the health disparities research plan of the National Institutes of Health: Unfinished business. Washington DC: National Academies Press (US). [PubMed] [Google Scholar]
- Tibuakuu M, Michos ED, Navas-Acien A, Jones MR. 2018. Air pollution and cardiovascular disease: A focus on vulnerable populations worldwide. Curr Epidemiol Rep 5(4):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, Collins C. 2010. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Ou CQ, Chan KP, Chau YK, Thach TQ, Yang L, et al. 2008. The effects of air pollution on mortality in socially deprived urban areas in Hong Kong, China. Environ Health Perspect 116(9):1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma Y, Wu X, Bai M, Peng Y, Cai W, et al. 2019. Association between particulate matter air pollution and cardiovascular disease mortality in Lanzhou, China. Environ Sci Pollut Res Int doi: 10.1007/s11356-019-04742-w [DOI] [PubMed] [Google Scholar]
- Yitshak-Sade M, James P, Kloog I, Hart JE, Schwartz JD, Laden F, et al. 2019. Neighborhood greenness attenuates the adverse effect of PM2.5 on cardiovascular mortality in neighborhoods of lower socioeconomic status. Int J Environ Res Public Health 16(5):814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Doi H. 2016. Associations of acute exposure to fine and coarse particulate matter and mortality among older people in Tokyo, Japan. Sci Total Environ 542(Pt A):354–9. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yao S, Dong H, Wang L, Wang C, Ji X, et al. 2019. Association between short-term exposure to particulate matter air pollution and cause-specific mortality in Changzhou, China. Environ Res 170:7–15. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2000. Race, gender, and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med 42(5):469–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


