Abstract
Low-molecular-weight heparins (LMWHs) are the mainstay of the prophylaxis and treatment of venous thromboembolism (VTE). Due to their renal elimination, the risk of accumulation with the related bleeding risk may represent a limitation for the use of LMWHs in patients with chronic kidney disease (CKD) as the risk of major bleeding is increased in patients with creatinine clearance (CrCl) < 30 mL/min, especially in patients with cancer. LMWH structure and molecular weight (MW) are heterogeneous among available agents. The elimination of tinzaparin, which has the highest mean MW among LMWHs, is less dependent on renal function as it is also metabolized through the reticuloendothelial system. A subcutaneous therapeutic dose of tinzaparin (175 IU/kg) once daily has been shown to cause no accumulation of anti-factor Xa activity in patients with CrCl ≥ 20 mL/min. Clinical experience from randomized controlled studies has shown no significant impact of CKD on bleeding risk in cancer patients receiving treatment doses of tinzaparin. This suggests that in these patients the use of treatment doses of tinzaparin does not require anticoagulation monitoring or dose adjustment.
Key Points
| Renal elimination of low-molecular-weight heparins (LMWHs) limits their use in patients with venous thromboembolism and renal impairment due to the risk of accumulation and related bleeding. |
| Tinzaparin elimination is less dependent on renal function than other LMWHs, and it is not associated with a significant increase in the risk of bleeding in patients with chronic kidney disease; therefore, treatment doses do not require monitoring or dose adjustment in patients with creatinine clearance ≥ 20 mL/min. |
Introduction
Low-molecular-weight heparins (LMWHs) are viewed as the mainstay for the prevention and treatment of venous thromboembolism (VTE) as they have been shown to be efficient, safe and convenient for short-term and long-term therapy in a large variety of clinical settings. This includes VTE prophylaxis for orthopedic, surgical and medical patients [1], treatment of VTE including cancer-associated thrombosis (CAT), and acute coronary syndromes [2, 3]. Over the past 2 decades, there has been growing evidence that pharmacokinetic (PK) properties of LMWH compounds are not superimposable, especially regarding their elimination by the kidney. Interestingly, tinzaparin elimination is less dependent on renal function than other LMWHs, with potential clinical implications in the management of patients with renal impairment. In the present paper, we focus on this important issue. A search was conducted on Medline for literature published between January 1998 and July 2019 for articles containing the following keywords: “tinzaparin” and “renal insufficiency,” “renal failure,” “renal impairment.” We excluded papers related to patients undergoing hemodialysis or hemofiltration. Only original studies published in English during this period were selected. We also reviewed abstracts from major international meetings.
Tinzaparin Pharmacokinetic and Pharmacodynamic Properties
After subcutaneous injection, LMWHs have an excellent bioavailability of > 85%, with limited inter-individual variability compared with unfractionated heparin (UFH) [4]. Furthermore, LMWHs have linear elimination PK [5], which renders their pharmacodynamic (PD) effect highly predictable and therefore safe in most situations, without the need for monitoring hemostasis to assess efficacy or safety [6].
A variety of pharmaceutical preparations of LMWHs are available. LMWHs have different methods of preparation, resulting in variations in the mean molecular weight (MW) and in the distribution of MW chains, finally leading to different PK and PD profiles. LMWHs are obtained by chemical or enzymatic depolymerization of UFH. This results in the formation of fragments that have a mean MW of approximately one third of the mean MW of UFH. The mean MW of these LMWH compounds ranges from 4300 to 6500 Da (Table 1). Differences in MW are related to the length of oligosaccharide chains resulting from UFH depolymerization. A high degree of depolymerisation results in short chains (< 5400 Da) which are mainly eliminated by the kidney. On the other hand, a low degree of depolymerization results in longer chains (> 5400 Da), which are eliminated by the kidney to a lesser degree, elimination being mainly via the reticuloendothelial system and the liver. A high proportion of short chains are present in nadroparin or enoxaparin, while a substantial proportion of long chains are still present in dalteparin or tinzaparin preparations [7]. Even though LMWHs are obtained from different manufacturing processes, they have a set of common properties which make their antithrombotic activity comparable.
Table 1.
Summary of LMWHs PK and PD characteristics
(adapted from Rey et al. [9])
| Nadroparin | Enoxaparin | Dalteparin | Tinzaparin | |
|---|---|---|---|---|
| Molecular weight (Da) | 4300 | 4500 | 6000 | 6500 |
| t½ (h) | 3.5 | 4.5 | 3–5 | 3–4 |
| Bioavailability (%) | ~ 100 | ~ 100 | 90 | 86.7 |
| Time to peak of anti-Xa activity (h) | 2–3 | 3–5 | 4 | 4–5 |
| Anti-Xa/anti-IIa ratio | 2.5–4.0 | 3.6 | 2.5 | 1.8 |
| Cmax (mean ± SD) | 1.01 ± 0.18 | 1.20 ± 0.17 | 0.59 ± 0.25 | 0.87 ± 0.15 |
Cmax maximal plasma concentration, IIa factor IIa, LMWH low-molecular-weight heparin, PD pharmacodynamic, PK pharmacokinetic, SD standard deviation, t½ elimination half-life, Xa factor Xa
Patients with chronic kidney disease (CKD) are challenging since they are at high risk of both VTE and bleeding [8]. The risk of bleeding may be further increased with the use of LMWHs due to their renal elimination, which may result in their accumulation in renally impaired patients. Elimination is exclusively renal (by glomerular filtration) for LMWHs of low mean MW, such as enoxaparin, and their half-life increases in patients with CKD. The reticuloendothelial system and the liver contribute to the metabolism of LMWHs of higher MW, such as tinzaparin and dalteparin, which are therefore less influenced by renal function [9]. The ratio of renal clearance with respect to total drug clearance is lower for LMWHs with higher mean MW. Compared to enoxaparin, the renal excretion of tinzaparin has been shown to be significantly lower (p < 0.001) in rats with normal renal function [10]. The clearance of LMWHs with larger oligosaccharide chains, such as dalteparin or tinzaparin, has been shown to be less dependent on renal function than it is for LMWHs of lower mean MW, such as enoxaparin or nadroparin [11]. Since tinzaparin has the highest average MW of available LMWHs, it is less likely to accumulate in patients with CKD compared with LMWHs with lower MW.
Low-Molecular-Weight Heparins (LMWHs) in Patients with Chronic Kidney Disease (CKD)
Decreased LMWH clearance has been associated with increased bleeding risks in patients with severe CKD. In a meta-analysis of 18 studies using three preparations of LMWHs, Lim and associates [12] compared the risk of major bleeding and anti-factor Xa (anti-Xa) activity levels in patients receiving LMWH who had severe CKD (creatinine clearance [CrCl] ≤ 30 mL/min) with those in patients without severe renal insufficiency (RI) (CrCl > 30 mL/min). In 12 studies involving 4971 patients, LMWH was associated with a statistically significant increase in the risk for major bleeding in patients with a CrCl of 30 mL/min or less compared with those with a CrCl greater than 30 mL/min (5.0% vs. 2.4%; odds ratio 2.25; 95% confidence interval [CI] 1.19–4.27; p = 0.013) [12]. When analyzed according to LMWH preparation, major bleeding was increased when a standard therapeutic dose of enoxaparin was used (8.3% vs. 2.4%; odds ratio 3.88; 95% CI 1.78–8.45), but may not have been increased when an empirically reduced dose of enoxaparin was used (0.9% vs. 1.9%; odds ratio 0.58; 95% CI 0.09–3.78; p = 0.23 for heterogeneity). There were insufficient data to assess the risk for major bleeding with tinzaparin (two studies), dalteparin (one study), and prophylactic doses of enoxaparin. In a more recent meta-analysis of 20 controlled trials, enoxaparin was associated with a significant increased risk of major bleeding complications in patients with a glomerular filtration rate (GFR) ≤ 60 mL/min compared with other anticoagulants (risk ratio [RR] 1.67; 95% CI 1.12–2.50; p = 0.01), suggesting that only patients with a GFR > 60 mL/min can be safely treated with enoxaparin [13]. In an observational study in a limited number of patients with either VTE or acute coronary ischemia treated with therapeutic doses of enoxaparin (n = 99) or tinzaparin (n = 28), a CrCl < 20 mL/min was associated with an RR of 2.8 (95% CI 1.0–7.8) for bleeding complications with both LMWHs [14].
Tinzaparin Pharmacokinetics in Patients with CKD
PK data on tinzaparin from clinical studies in patients with CKD and the possible consequences on PD were systematically considered and included in this review. When medications were used at full therapeutic doses, nadroparin clearance, but not tinzaparin clearance, was shown to be correlated with CrCl (R = 0.49, p < 0.002) [15]. According to a population PK analysis, the clearance of subcutaneous tinzaparin, based on anti-Xa activity, did not appear to be altered by age, gender or race [16]. However, the analysis predicted a reduction in clearance of 22% in patients with severe CKD (CrCl < 30 mL/min) versus those with normal renal function (CrCl > 80 mL/min) [16]. Despite this, no accumulation (of anti-Xa activity) effect was observed in elderly patients with CKD in several clinical studies [17–19], with no correlation between anti-Xa activity and CrCl in this patient group [19, 20]. Tinzaparin has been safely used for up to 10 days with treatment doses (175 IU/kg once daily) without bioaccumulation in patients with severe CKD (CrCl 20– 29 mL/min, n = 8) even when the CrCl was as low as 20 mL/min [19, 21] (Fig. 1).
Fig. 1.
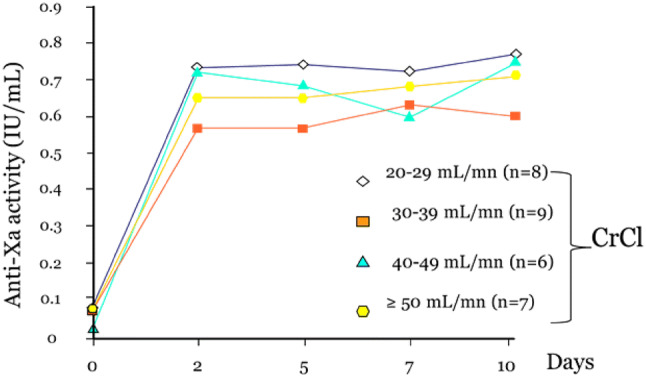
Plasma anti-factor Xa (anti-Xa) activity over a 10-day treatment period according to creatinine clearance (CrCl) value in patients receiving treatment doses of tinzaparin of 175 IU/kg once daily. IU international units
(adapted from Siguret et al. [19])
In a comparative study conducted in 55 elderly patients with CKD (CrCl between 20 and 50 mL/min) receiving prophylactic doses of enoxaparin (4000 IU once daily) or tinzaparin (4500 IU once daily), bioaccumulation of enoxaparin, but not of tinzaparin, was seen after 8 days of treatment, which is likely to expose patients to enoxaparin overdosing [17] (Table 2). In a sub-study of the Innohep in Renal Insufficiency Study (IRIS), peak plasma anti-Xa activity was measured in 87 elderly patients (mean age of 83 ± 5 years) with acute VTE and moderate-to-severe CKD receiving a treatment dose of tinzaparin (175 IU/kg once daily) for a mean treatment duration of 8.4 days [18]. Peak plasma anti-Xa activity was measured on day 2/day 3 and on day 5 or at visit S (VS) (end of tinzaparin treatment). An absence of accumulation was considered if the 90% CI of the (anti-Xa day 5/VS)/(anti-Xa day 2/3) ratio did not exceed the predefined limit of 1.25. No significant accumulation was detected: the mean accumulation ratio was 1.06 (90% CI 1.01–1.11). The accumulation ratios of anti-Xa activities were close in severe and moderate CKD patients (1.05 and 1.07, respectively), suggesting that tinzaparin may not accumulate more in CKD patients with CrCl (Cockcroft formula) between 20 and 30 mL/min (Table 3).
Table 2.
Main anti-Xa PK data in elderly patients with RI receiving prophylactic doses of enoxaparin and tinzaparin
(adapted from Mahé et al. [17])
| Enoxaparin | Tinzaparin | Between-group comparison P value | |||
|---|---|---|---|---|---|
| D1/D8 variation | P valuea | D1/D8 variation | P valuea | ||
| Cmax | 0.55/0.67 | < 0.001 | 0.44/0.46 | 0.3 | < 0.001 |
| AUC IU/mL·min | 354/447 | < 0.001 | 252/273 | 0.11 | < 0.001 |
| Anti-Xa accumulation | 1.22 | < 0.001 | 1.05 | 0.29 | – |
AUC area under the curve, Cmax maximal plasma concentration, IU international units, PK pharmacokinetic, RI renal impairment, Xa factor Xa
aDay 1 vs. day 8
Table 3.
Peak anti-Xa activity in the 87 patients with moderate-to-severe RI receiving a treatment dose of tinzaparin of 175 IU/kg once daily
(adapted from Siguret et al. [18])
| Anti-Xa activity (IU/mL) on day 2/3 Mean ± SD (range) |
Anti-Xa activity (IU/mL) on day 5/VS Mean ± SD (range) |
Anti-Xa activity accumulation ratio Mean ± SD (range) |
|
|---|---|---|---|
| Patients with severe CKD (CrCl ≤ 30 mL/min); n = 21 |
0.97 ± 0.47 (0.32–2.08) |
0.96 ± 0.36 (0.38–1.69) |
1.05 ± 0.25 (0.64–1.49) |
| Patients with moderate RI (30 < CrCl ≤ 60 mL/min); n = 66 |
0.82 ± 0.28 (0.39–1.89) |
0.84 ± 0.29 (0.32–1.81) |
1.07 ± 0.31 (0.37–1.73) |
| Total (n = 87) |
0.86 ± 0.34 (0.32–2.08) |
0.87 ± 0.31 (0.32–1.81) |
1.06 ± 0.30 (0.37–1.73) |
CKD chronic kidney disease, CrCl creatinine clearance, IU international units, RI renal impairment, SD standard deviation, VS visit S (last day of tinzaparin dosing), Xa factor Xa
The potential accumulation of subcutaneous therapeutic doses of tinzaparin (175 IU/kg once daily) in 148 patients with VTE with different degrees of CKD was measured in TRIVET, a prospective multicenter study [22]. Using CrCl > 60 mL/min as the comparison, mean trough anti-Xa levels were significantly higher in patients with CrCl < 30 mL/min and in hemodialysis-dependent patients (p < 0.005). The mean (± standard deviation) anti-Xa levels after up to 7 days of tinzaparin administration in patients with CrCl between 20 and 29 mL/min was 0.29 (0.18) IU/mL, compared with 0.15 (0.12) IU/mL in patients with CrCl > 60 mL/min (Table 4). However, mean trough anti-Xa levels remained below 0.5 IU/mL, considered as the bioaccumulation threshold. Furthermore, there was no accumulation in patients with CrCl < 30 mL/min and < 20 mL/min over time with a stable anti-Xa level: 0.29 (0.19) and 0.30 (0.24), respectively. The authors concluded that therapeutic weight-based doses of tinzaparin can be used in VTE patients with severe renal impairment (CrCl < 30 mL/min) for up to 7 days.
Table 4.
Mean trough anti-Xa levels according to renal function
(adapted from Lim et al. [22])
| No.a | Mean (± standard deviation) trough anti-Xa (IU/mL) | P value | ||
|---|---|---|---|---|
| 1st measurement (day 3–5) | 2nd measurement (day 5–7) | |||
| CrCl (mL/min) | ||||
| > 60 | 55/56 | 0.16 (0.11) | 0.15 (0.12) | 0.40 |
| 30–60 | 34/33 | 0.20 (0.17) | 0.17 (0.09) | 0.28 |
| < 30, non-dialysis dependent | 29/26 | 0.29 (0.23) | 0.29 (0.19) | 0.90 |
| 20–29 | 22/19 | 0.25 (0.19) | 0.29 (0.18) | |
| < 20 | 7/7 | 0.41 (0.32) | 0.30 (0.24) | |
| Dialysis-dependent | 26/21 | 0.38 (0.36) | 0.33 (0.20) | 0.61 |
CrCl creatinine clearance, IU international units, Xa factor Xa
aNumber of patients included for 1st measurement/2nd measurement
Tinzaparin PK was assessed in patients with severe CKD in STRIP, a prospective observational study [23]. Twenty-eight patients with a median estimated glomerular filtration rate (eGFR) (using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula [24]) of 20 mL/min/1.73 m2 received thromboprophylaxis with tinzaparin up to 8 days at the median dosage of 44 IU/kg (interquartile range 42–54) once daily; 19 patients received 3500 IU daily, while nine patients with a body mass index ≥ 30 kg/m2 received 4500 IU daily. A proportion of 54% of patients had an eGFR ≤ 20 mL/min/1.73 m2. The study showed that short-term tinzaparin in patients with severe CKD was not associated with excessive anticoagulation, with peak anti-Xa levels below 0.5 IU/mL and undetectable trough anti-Xa levels.
Tinzaparin Safety in Cancer Patients with CKD
More than half of cancer patients are known to have abnormal renal function with a higher bleeding risk [25]. Patients with CAT are therefore at the highest risk of bleeding when receiving an anticoagulant treatment. The impact of renal impairment on safety in patients with CAT receiving 6-month treatment doses of tinzaparin (175 IU/kg once daily) was assessed from the Comparison of Acute Treatments in Cancer Haemostasis (CATCH) trial, a randomized control study [26, 27]. Patients with CKD (GFR < 60 mL/min/1.73 m2) on tinzaparin showed no difference in recurrent VTE, clinically relevant bleeding (CRB), major bleeding or mortality rates versus those on warfarin. Major bleeding did not significantly differ between tinzaparin patients with or without CKD (4.3% vs. 2.5%, respectively; RR 1.72; 95% CI 0.48–6.17) as well as CRB (14.5% vs. 12.7%, respectively; RR 1.14; 95% CI 0.61–2.16). These findings are consistent with results from a post-hoc analysis of the CLOT study in which dalteparin, an LMWH compound with long chains, compared with vitamin K antagonist, significantly reduced the risk of VTE recurrence in patients with cancer and renal impairment (hazard ratio 0.15; 95% CI 0.03–0.65; p = 0.01), while bleeding rates were similar with both treatments (p = 0.47) [28].
Clinical Practice Implications
The use of LMWHs requires prior assessment of renal function since CKD tends to be underdiagnosed [29]. Since some LMWHs are mainly eliminated by the kidney, any renal dysfunction is likely to cause their accumulation, resulting in an increased bleeding risk. The assessment of anti-Xa activity at peak level has been proposed as a biomarker to detect an accumulation and/or an overdosage in specific situations including CKD. Given the various PD profiles of LMWH compounds, physicians should be aware of specific overdosage values that have been shown to be associated with an increased risk of bleeding, especially in phase 2/phase 3 studies; for instance, 1.5 IU/mL as the therapeutic dose of tinzaparin and 1.8 IU/mL as the therapeutic dose of nadroparin.
Although there is no specific CrCl threshold at which the risk for LMWH accumulation becomes clinically significant, an estimated CrCl of about 30 mL/min is a reasonable cut-off value based on the available literature; however, this depends on the LMWH. If an LMWH with low mean MW (i.e., nadroparin or enoxaparin) is used in patients with an estimated CrCl of < 30 mL/min, anti-Xa activity monitoring and/or dose reduction should be considered to ensure that there is no accumulation. The recommended treatment dosage of enoxaparin for patients with a CrCl < 30 mL/min who have acute coronary syndromes or VTE is 50% of the usual dosage (i.e., 1 mg/kg once daily) [30].
The LMWHs have different metabolic pathways, and tinzaparin, despite its renal elimination, does not cause any accumulation of anti-Xa activity in patients with CrCl ≥ 20 mL/min and therefore does not require dosage adjustment.
The apparent difference in tinzaparin clearance in patients with severe CKD may reflect metabolism by hepatic mechanisms, possibly due to the higher mean MW of tinzaparin compared with other LMWHs. Anti-Xa activity monitoring in patients treated with tinzaparin is not generally necessary, but some authorities suggest that monitoring be done in obese patients and in those with CKD. The Cancer and the Kidney International Network (C-KIN) states that, “Among LMWHs, tinzaparin presents with the clearest data on its use in CKD patients, demonstrating no accumulation at the usual dosage, and thus there is no need for dosage adjustment.” The same network recommended that among LMWHs, tinzaparin should be the treatment of choice in cancer patients with CKD [31]. This is consistent with the absence of significant impact of CKD on the bleeding risk in cancer patients treated with tinzaparin based on the clinical experience from randomized controlled trials.
Conclusions
The risk of bleeding is an essential component of the surveillance of patients treated with LMWHs. Patients with CKD may have an increased bleeding risk due to LMWH accumulation, which may be detected by the increase in anti-Xa activity. LMWHs with relatively high mean MW are associated with a low risk of accumulation of anti-Xa activity given the prominent participation of the reticuloendothelial system in their metabolism.
Based on available PK data, there is consistent evidence that standard therapeutic doses of tinzaparin (175 IU/kg once daily) cause no clinically significant accumulation of anti-Xa activity in patients with a CrCl ≥ 20 mL/min. This suggests that there is no need for systematic anti-Xa activity monitoring or dosage adjustment of tinzaparin in these patients.
Compliance with Ethical Standards
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Hélène Helfer, Virginie Siguret, and Isabelle Mahé declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
References
- 1.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JR, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, ACC/AHA Task Force Members, Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 3.Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA, Pabinger I, Solymoss S, Douketis J, Kakkar A. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17(10):e452–e466. doi: 10.1016/S1470-2045(16)30369-2. [DOI] [PubMed] [Google Scholar]
- 4.Handeland GF, Abildgaard U, Holm HA, Arnesen KE. Dose adjusted heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. Eur J Clin Pharmacol. 1990;39(2):107–112. doi: 10.1007/BF00280041. [DOI] [PubMed] [Google Scholar]
- 5.Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 6.Boneu B, de Moerloose P. How and when to monitor a patient treated with low molecular weight heparin. Semin Thromb Hemost. 2001;27(5):519–522. doi: 10.1055/s-2001-17961. [DOI] [PubMed] [Google Scholar]
- 7.Bisio A, Vecchietti D, Citterio L, Guerrini M, Raman R, Bertini S, Eisele G, Naggi A, Sasisekharan R, Torri G. Structural features of low-molecular-weight heparins affecting their affinity to antithrombin. Thromb Haemost. 2009;102(5):865–873. doi: 10.1160/TH09-02-0081. [DOI] [PubMed] [Google Scholar]
- 8.Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the Atherosclerosis Risk in Communities (ARIC) study. Clin J Am Soc Nephrol. 2016;11(10):1735–1743. doi: 10.2215/CJN.02170216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rey JB, Launay-Vacher V. Utilisation des héparines de bas poids moléculaire (HBPM) chez le patient insuffisant rénal en milieu hospitalier. J Pharm Clin. 2011;30(2):61–74. [Google Scholar]
- 10.Johansen KB, Schroeder M, Lundtorp L, Mousa SA. XXIst congress of the international society on thrombosis and haemostasis. Genève 2007, (2007).
- 11.Johansen KB, Balchen T. Tinzaparin and other low-molecular-weight heparins: what is the evidence for differential dependence on renal clearance? Exp Hematol Oncol. 2013;2:21. doi: 10.1186/2162-3619-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144(9):673–684. doi: 10.7326/0003-4819-144-9-200605020-00011. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann P, Keller F. Increased major bleeding risk in patients with kidney dysfunction receiving enoxaparin: a meta-analysis. Eur J Clin Pharmacol. 2012;68(5):757–765. doi: 10.1007/s00228-011-1149-6. [DOI] [PubMed] [Google Scholar]
- 14.Cestac P, Bagheri H, Lapeyre-Mestre M, Sie P, Fouladi A, Maupas E, Leger P, Fontan B, Massip P, Montastruc JL. Utilisation and safety of low molecular weight heparins: prospective observational study in medical inpatients. Drug Saf. 2003;26(3):197–207. doi: 10.2165/00002018-200326030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Mismetti P, Laporte-Simitsidis S, Navarro C, Sie P, d’Azemar P, Necciari J, Duret JP, Gaud C, Decousus H, Boneu B. Aging and venous thromboembolism influence the pharmacodynamics of the anti-factor Xa and anti-thrombin activities of a low molecular weight heparin (nadroparin) Thromb Haemost. 1998;79(6):1162–1165. doi: 10.1055/s-0037-1615034. [DOI] [PubMed] [Google Scholar]
- 16.Barrett JS, Gibiansky E, Hull RD, Planes A, Pentikis H, Hainer JW, Hua TA, Gastonguay M. Population pharmacodynamics in patients receiving tinzaparin for the prevention and treatment of deep vein thrombosis. Int J Clin Pharmacol Ther. 2001;39(10):431–446. [PubMed] [Google Scholar]
- 17.Mahé I, Aghassarian M, Drouet L, Bal Dit-Sollier C, Lacut K, Heilmann JJ, Mottier D, Bergmann JF. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586. doi: 10.1160/TH06-09-0513. [DOI] [PubMed] [Google Scholar]
- 18.Siguret V, Gouin-Thibault I, Pautas E, Leizorovicz A. No accumulation of the peak anti-factor Xa activity of tinzaparin in elderly patients with moderate-to-severe renal impairment: the IRIS substudy. J Thromb Haemost. 2011;9(10):1966–1972. doi: 10.1111/j.1538-7836.2011.04458.x. [DOI] [PubMed] [Google Scholar]
- 19.Siguret V, Pautas E, Fevrier M, Wipff C, Durand-Gasselin B, Laurent M, Andreux JP, d’Urso M, Gaussem P. Elderly patients treated with tinzaparin (Innohep) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days. Thromb Haemost. 2000;84(5):800–804. [PubMed] [Google Scholar]
- 20.Pautas E, Gouin I, Bellot O, Andreux JP, Siguret V. Safety profile of tinzaparin administered once daily at a standard curative dose in two hundred very elderly patients. Drug Saf. 2002;25(10):725–733. doi: 10.2165/00002018-200225100-00005. [DOI] [PubMed] [Google Scholar]
- 21.Pautas E, Siguret V, d’Urso M, Laurent M, Gaussem P, Fevrier M, Durand-Gasselin B. Monitoring of tinzaparin in a ten day treatment dose in elderly patients. Rev Med Interne. 2001;22(2):120–126. doi: 10.1016/S0248-8663(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim W, Crowther M, Wang L, Douketis J, Schnurr T, Moreau C, Clase C, et al. Assessment of low-molecular-weight heparin accumulation in patients with chronic kidney disease: results from the TRIVET study. J Thromb Haemost. 2016;14(Suppl. 1):1–168. [Google Scholar]
- 23.Projean D, Lalonde S, Morin J, Nogues E, Seguin A, Vincent A, Lafrance JP, Masson V, Kassis J, Fafard J, Lordkipanidze M. Study of the bioaccumulation of tinzaparin in renally impaired patients when given at prophylactic doses—the STRIP study. Thromb Res. 2019;174:48–50. doi: 10.1016/j.thromres.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monreal M, Falga C, Valle R, Barba R, Bosco J, Beato JL, Maestre A, RIETE Investigators Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med. 2006;119(12):1073–1079. doi: 10.1016/j.amjmed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA, CATCH Investigators Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 27.Bauersachs R, Lee AYY, Kamphuisen PW, Meyer G, Janas MS, Jarner MF, Khorana AA. Renal impairment, recurrent venous thromboembolism and bleeding in cancer patients with acute venous thromboembolism-analysis of the CATCH study. Thromb Haemost. 2018;118(5):914–921. doi: 10.1055/s-0038-1641150. [DOI] [PubMed] [Google Scholar]
- 28.Woodruff S, Feugere G, Abreu P, Heissler J, Ruiz MT, Jen F. A post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis. 2016;42(4):494–504. doi: 10.1007/s11239-016-1386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janus N, Mahe I, Launay-Vacher V, Laroche JP, Deray G. Renal function and venous thromboembolic diseases. J Mal Vasc. 2016;41(6):389–395. doi: 10.1016/j.jmv.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launay-Vacher V, Scotté F, Riess H, Ashman N, McFarlane P, et al. Thrombosis and kidney disease in cancer: comorbidities defining a very high risk patient: a position paper from the Cancer & the Kidney International Network. J Onco Nephrol. 2018;2(2–3):37–49. doi: 10.1177/2399369318809102. [DOI] [Google Scholar]


