Abstract
Tea plants (Camellia sinensis O. Kuntze) can hyperaccumulate fluoride (F) in leaves. Although, aluminum (Al) can alleviate F toxicity in C. sinensis, the mechanisms driving this process remain unclear. Here, we measured root length, root activity, soluble proteins content, and levels of peroxidase, superoxide dismutase, catalase, malondialdehyde (MDA), and chlorophyll in tea leaves after treatment with different F concentrations. In addition, we focused on the content of organic acids, the gene transcription of malate dehydrogenase (MDH), glycolate oxidase (GO) and citrate synthase (CS) and the relative enzyme activity involved in the tolerance to F in C. sinensis. We also examined the role of Al in this process by analyzing the content of these physiological indicators in tea leaves treated with F and Al. Our results demonstrate that increased MDA content, together with decreased chlorophyll content and soluble proteins are responsible for oxidative damage and metabolism inhibition at high F concentration. Moreover, increased antioxidant enzymes activity regulates ROS damage to protect tea leaves during F stress. Furthermore, exogenous Al alleviated F stress in tea leaves through the regulation of MDA content and antioxidant enzymes activity. In addition, organic acids in exudate stimulated root growth in tea plants exposed to low F concentrations are regulated by MDH, GO, and CS. In addition, Al can stimulate the exudation of organic acids, and may participate in regulating rhizosphere pH of the roots through the interaction with F, eventually leading to the response to F stress in C. sinensis.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00813-2) contains supplementary material, which is available to authorized users.
Keywords: Camellia sinensis, Fluoride, Aluminum, Root growth, Organic acid
Introduction
Tea, processed from the leaves of tea plant (Camellia sinensis (L.) O. Kuntze), is one of the most economically important non-alcoholic beverages worldwide. Fluoride (F), the 13th most abundant element in the earth crust, often is recognized for its inhibition of plant growth because many plants species are sensitive to F (Singh et al. 2018; Weinstein and Halscher-Herman 1982). However, the contents of F range from 871 to 1337 mg/kg in mature leaves and higher than 2000 mg/kg in old leaves in C. sinensis (Shu et al. 2003), confirming the fact that tea plant is a hyper-accumulator of F. Tea-based beverages contain approximately 40–90% of the F of the tea leaves, that in turn accumulates in the body of tea drinkers (Smith 2001). Uptake of appropriate amounts of F through tea contributes to the health of bones and teeth. However, over-intake of F through excessive tea consumption has negative effects, such as skeletal and dental fluorosis (Lu et al. 2004; Cao et al. 2003). Therefore, it is meaningful to understand the mechanism of F accumulation in C. sinensis in order to control F content in tea products.
Previous studies investigated the accumulation of F and its influences in C. sinensis. Zhang et al. (2013a) revealed that absorption of F in tea roots is likely an active and energy dependent process, and that F accumulate eventually in tea leaves. Although C. sinensis can hyperaccumulate a large amount of F without any poisoning symptom, excessive F accumulation also can cause a series of physiological changes, for example, the number of epidermal hairs is increased and stomatal apertures are decreased on tea leaves exposed to high F. Moreover, increasing F content reduces photosynthesis rate and chlorophyll fluorescence. Meanwhile the content of antioxidant enzymes manganese superoxide dismutase (Mn-SOD), ascorbate peroxidase (APX), and catalase (CAT) decreases at high F concentration. In contrast, low or moderate F concentrations induce antioxidant enzymes expression to scavenge ROS and to resist oxidative stress. Furthermore, betaine and proline are also involved in osmotic regulation of tea plant tolerance to F stress. All these changes together regulate tea plant growth in response to F-induced stress (Cai et al. 2016). Additionally, there is a tight relationship with aluminum (Al) during tea plant accumulates F. Previous reports showed that Al3+ pretreatment causes a significant increase in F content in tea plants (Ruan et al. 2003). In the xylem, F is transported as an Al-F complex and disassociates in leaves (Nagata et al. 1993). Liang et al. (1996) found that the AlF2+, AlF2+ and AlF4− are mainly accumulate in roots and translocate from roots to leaves in tea plants. The ratio between Al and F influences the accumulation of F in tea plants grown in hydroponics solution (Zhang et al. 2013b). These findings indicate that tea plants probably absorb both F and Al combined in an Al-F complex.
Al toxicity is responsible for reducing root elongation, interfering nutrient uptake, and decreasing crop production in acidic soils. Therefore, studying the regulatory mechanism of plants responding to Al toxicity is of great significance for promoting plant growth. Previous studies have shown that organic acids are involved in response to Al toxicity in plant growth. The release of organic acids from roots protects plant root cells in response to Al (Kinraide et al. 2005). Among these, citrate, malate, and oxalate acid are the main metabolites released from the root apex in response to Al3+ (Kochian et al. 2005). For example, Malate dehydrogenase (MDH) in Chinese cabbage (Brassica campestris ssp. pekinensis), a gene involved in malate synthesis, induces stronger Al resistance in Arabidopsis under treated with Al (Li et al. 2016). Proteome analysis of wheat roots revealed that Al stress upregulated oxalate oxidase (OxO) 2 precursor and MDH proteins (Oh et al. 2014). In addition, enhancing the citrate synthase (CS) gene expression confers tolerance to Al stress in Nicotiana tabacum, Carica papaya (della Fuente et al. 1997), and Citrus junos (Deng et al. 2009). Klug and Horst (2010) also found that the oxalate exudation enhances Al tolerance and allows Al accumulation in buckwheat. Moreover, tea plants, as Al hyperaccumulators, do not show Al toxicity symptoms compared to other plants under relatively high Al concentration (Mukhopadyay et al. 2012).
A large number of studiers on the role of tea plants organic acids in the absorption of Al have been reported recently. The roots of C. sinensis can secrete oxalate (Morita et al. 2011) and increase citrate synthesis (Xu et al. 2017) in response to Al exposure. A previous RNA-Seq transcriptome study revealed that high Al concentration significantly increases the expression of genes related to organic acids such as malate, oxalate and citrate acids (Li et al. 2017b). Recent study has shown that Al can influence the organic acids synthases activity by regulating the expression of CS, MDH and glycolate oxidase (GO) genes, and then altering the contents and secretions of organic acids in tea roots (Li et al. 2017a). However, the activity of both MDH and CS decreases after F treatments in tea rhizosphere (Wang et al. 2013). Although Al can alleviate F toxicity in tea by forming Al-F complexes, the precise molecular mechanisms behind the Al-depended regulation of F tolerance and of Al accumulating in tea plants have not been deciphered yet.
Here, to investigate the response of C. sinensis to F treatment, we measured root length, root growth, soluble proteins, POD, SOD, CAT, MDA and chlorophyll content in tea leaves under different F concentration. In addition, we focused on the content of organic acids, MDH, GO, and CS enzymes involved in C. sinensis tolerance to F stress. We monitored the expression of MDH, GO, and CS genes and the activity of the respective enzymes in C. sinensis in response to F treatment. Furthermore, we examined the role of Al in rescuing F stress observing it effect on these physiological indicators. Our findings undoubtedly provide a better understanding on how Al alleviates F toxicity in tea plants.
Method and material
Plant materials
Two-year-old root cuttings tea plant (Camellia sinensis (L.) O. Kuntze cv. Longjingchangye) were collected from the Nanjing Agricultural University (Nanjing, China). The tea plants were hydroponically grown and pre-cultured in a standardized nutrient solution and in a climate chamber for 2 weeks according to Wan et al. (2012). Pre-incubated tea plants were then cultured in standardized nutrient solutions containing a gradient of different concentrations of F− (NaF) (0, 4, 8, 16 mg/L). In addition, other pre-incubated tea plants were transferred to medium (8 or 16 mg/L F−) at different concentration of Al3+ (0, 0.4 or 2 mM/L). All treatments were carried out in triplicates. During the hydroponic culture, using 0.5 M NaOH or HCl adjusted the pH of the solution every day. The tea root activity of each treatment was measured every week using 2, 3, 5-triphenyltetrazolium chloride (TTC) method (Khan et al. 2014; Ruf and Brunner 2003). During the 5-week treatment, the third and fourth leaves of every treatment were collected for identifying chlorophyll soluble sugar, POD, SOD, CAT, MDA content. The root and root exudates were collected for identification and quantification of organic acids, MDH, GO and CS enzymes and genes.
Chlorophyll, soluble proteins, and MDA content, and Antioxidant enzymes activity analysis
The chlorophyll of 0.2 g fresh tea leaves for each sample was extracted by 95% ethanol and then the extract was measured spectrophotometrically at 645 nm and 663 nm for chlorophyll contents as previously described (Arnon 1949). Total soluble protein was extracted with 50 mM Na2HPO4–KH2PO4 (pH 7.0) and 5% (w/v) insoluble polyvinylpolypyrrolidone (PVPP) and quantified with the Bradford method using bovine serum albumin (BSA) as standard (Bradford 1976). The content of MDA in the tea leaves was measured by thiobarbituric acid (TBA) according to previous report (Dhindsa et al. 1981).
For assay of antioxidant enzyme activities, the fresh tea leaves samples at different F treatments were immediately ground with a pre-cooled mortar and pestle in 2 mL ice-cold extraction buffer containing 50 mM K2PO4–KOH (pH 7.8) and 4% (w/v) insoluble PVPP. Total SOD activity was determined using monoformazan formation as previous described (Hajiboland et al. 2013). The POD activity was assayed using guaiacol (Merck, Darmstadt, Germany) as substrate (Hajiboland et al. 2013). The CAT activity was measured using iodometric titration according to Hajiboland et al. (2013).
Identification and quantification of organic acids in root exudates
Cutting 0.2 g roots from 5-week-treated plants and transferring into 0.5 mM CaCl2 solution (pH 4.5) for 24 h, then the roots were gathered, purified, eluted and dried according to the method of Yamada et al. (2014). Then each sample was taken to extract organic acids (Ding et al. 2014). Organic acids were identified and quantified using a Shimadzu LC-20A series of high-performance liquid chromatography (HPLC) equipped with the C18 analytical column (250 mm × 4.6 mm i.d., 5 μm nominal particle size) (Zhang et al. 2014).
MDH, GO and CS enzyme activities detection
The fresh roots from each sample were harvested and washed after 5-week treatment. The roots (1.0 g) were immersed in liquid N2 and homogenized with 2 mL ice-cold Tris–HCl extraction buffer. The clarified supernatant was used for MDH, GO and CS activity tests after centrifuged using 15,000g at 4 °C for 10 min. MDH activity was detected by the vanish of NADH at 340 nm for 1 min on the basic of the previous report (Johnson et al. 1994). GO activity was measured by detecting the rate of H2O2 formation at 30 °C according to Yamada et al. (2014). CS activity was monitored by detecting the decrease of acetly CoA to CoA with DTNB at 412 nm for 3 min (Mugai et al. 2000).
QRT-PCR analysis of MDH, GO and CS genes
The expression profiles of MDH, GO and CS genes in tea roots were measured by qRT-PCR during different concentration F and Al. These genes were identified from C. sinensis transcriptome data (Pan et al. 2016). Roots from each treatment were washed and collected after 5-week treatment. Total RNA was extracted from each sample using the EASYspin plus plant RNA mini kit (Aidlab Biotechnologies Co., Ltd) on the basis of the manufacturer’s instructions. In this study, specific primers for qRT-PCR were designed using Primer Premier 5.0. The β-actin gene of C. sinensis was selected as an internal reference. The primers of selected MDH, GO and CS genes are listed in Additional file 1: table S1. Each amplification reaction contained 10 μL SYBR® Premix Ex Taq™ II (Takara), 5 μL cDNA, 0.4 μL each primer and 4.2 μL H2O. The PCR cycling regime of thermocycling were listed as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 10 s, 55 °C for 15 s, and 72 °C for 20 s. The experiments were performed with three independent biological replicates and technical replicates. Relative expression level was calculated by the 2−ΔΔCt method by Livak and Schmittgen (2001).
All of the presented data are mean values of at three independent measurements and shown as the mean ± SE. All statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA), and significance of difference were carried out by Ducan’s test and ANOVA.
Results
Effect of F treatments on tea root and leaf
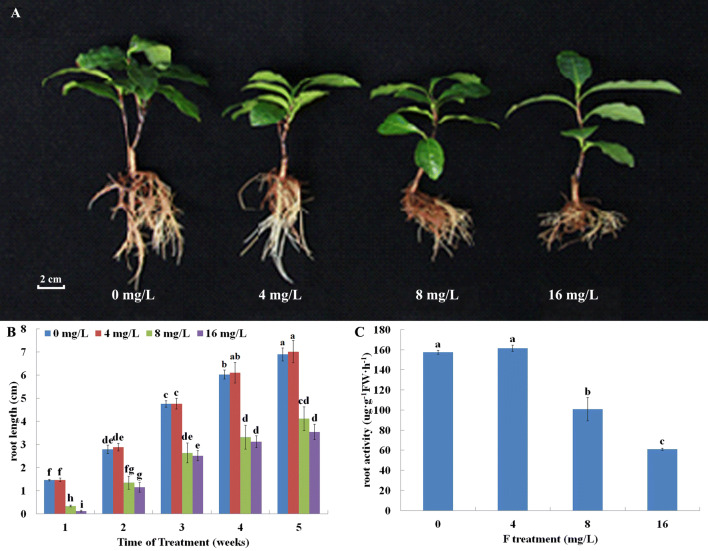
We used root length and activity to measure F resistance in tea plants (Fig. 1). After 5 weeks of F treatment exposure to 8 or 16 mg/L F inhibited root activity, while 4 mg/L F slightly promoted root activity (Fig. 1a). Root length and activity was both markedly affected in the presence of F when compared to those grown in the absence of F. Although there was no significant difference, relatively low F treatment (4 mg/L) increased the root length and activity. In contrast, root length and activity decreased under high F concentrations (> 8 mg/L) (Fig. 1b, c). In addition, to verify the influence of chlorophyll in tea leaves under F treatment, we measured chlorophyll a and b content. The results showed that the contents of chlorophyll a, b, and a + b were reduced by F treatments (Fig. 2).
Fig. 1.
Effects of F treatments on tea root growth and activity. a Appearance of tea roots exposed to different F− concentrations (0, 4, 8 or 16 mg/L) for 5 weeks. b Lengths of tea root exposed to different concentrations of F (0, 4, 8 or 16 mg/L) for 5 weeks. c Tea root activity expose to different concentrations of F (0, 4, 8 or 16 mg/L) after 5 weeks
Fig. 2.
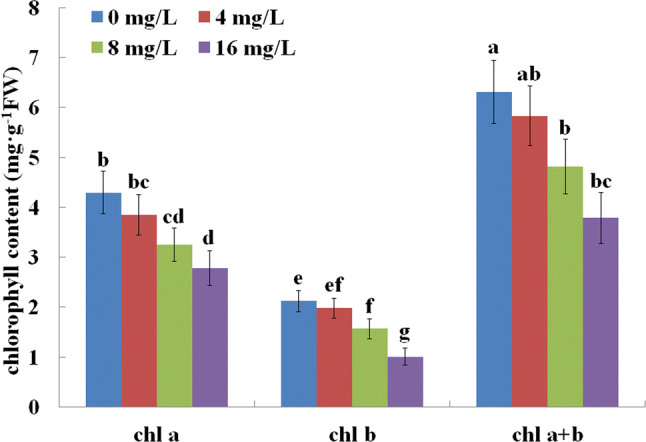
Effects of F treatments on chlorophyll content in C. sinensis leaves
Effect of F on MDA content, antioxidant enzymes activity, and soluble proteins in tea leaves
MDA content, antioxidant enzymes activity, and soluble proteins are major indicators of the extent to which plant growth is affected under biotic or abiotic stress. Here, we measured MDA content, POD, SOD and CAT activity, and soluble proteins in plants exposed to different F concentrations (Fig. 3). After 5 week of F treatment, the MDA content show not significant difference in tea leaves treated with 4 mg/L F when compared to those grown in the absence of F (Fig. 3a). However, high F concentration (> 8 mg/L) increased MDA content in tea plants. Similarly, POD, SOD, and CAT activity did not change significantly at low concentrations of F, while they significantly increased in tea leaves exposed to high concentrations of F (Fig. 3b–d). Soluble proteins decreased gradually with the increase in concentration of F (Fig. 3).
Fig. 3.
Effects of F treatments on MDA content, antioxidant enzymes activity, and soluble protein levels in C. sinensis leaves. MDA content, antioxidant enzymes activity and soluble proteins were detected after treatment by 0, 4, 8 or 16 mg/L F−. a MDA; b SOD; c CAT; d POD; e soluble proteins
Effects on MDA content and antioxidant enzymes activity under F and Al regulation in tea leaves
In order to observe whether Al was involved in the growth of tea leaves under F stress, we measured MDA content and antioxidant enzymes activity in tea leaves exposed to high F concentration (> 8 mg/L) and to different Al treatment leaves. MDA content decreased gradually after treatment with high F concentrations (> 8 mg/L) and Al (> 0.4 mM/L) (Fig. 4A). In addition, POD, SOD, and CAT activity showed different responses to Al treatment (Fig. 4b–d). POD activity was not affected in plants treated with high F concentrations (> 8 mg/L) and different Al concentration. SOD activity increased under 8 mg/L F treatment with the increase in Al concentration. In contrast, SOD activity did not change in tea leaves treated with 16 mg/L F treatment and different Al concentrations. Moreover, treatments with moderate (0.4 mM/L) Al concentration caused a significant decrease in CAT activity in tea leaves under treated with high F concentration (> 8 mg/L). These results indicated that adding Al regulated MDA, SOD, and CAT in tea leaves treated with high F concentrations. However, Al did not affect POD activity in the same conditions.
Fig. 4.
Effects of different Al3+ concentrations (0, 0.4 or 2 mM) on MDA content and antioxidant enzymes activity in C. sinensis leaves exposed to high F concentrations. The MDA content and antioxidant enzymes activity were detected after treatment with different Al3+ (0, 0.4 or 2 mM) concentrations in tea leaves exposed to high F concentrations (> 8 mg/L). a MDA; b SOD; c CAT; d POD
Organic acids metabolism, and related enzymes activity and gene expression in tea roots after F treatment
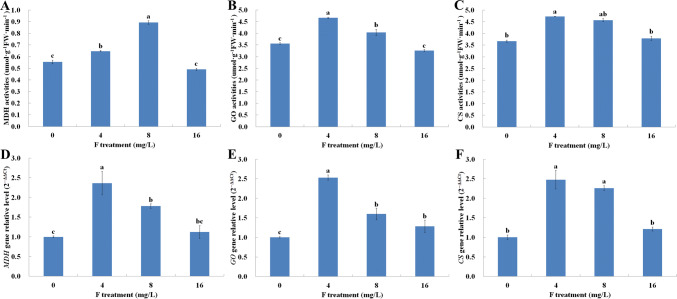
We quantified malate, oxalate and citrate enzymes in roots and root exudates in tea plant under different F concentration treatments. Malate and oxalate content in exudates and roots, as well as citrate content increased and then decreased gradually, peaking in tea roots treated with 4 mg/L F. However, these organic acids content in exudates showed no significant changes in tea roots treated with 16 mg/L F when compared with those grown in the absence of F. Interesting, the citrate exudate in tea root increased significantly only after a treatment with 8 mg/L F (Fig. 5). Furthermore, we also measured the expression of genes coding enzymes related to organic acids and their relative activity in tea roots at different F concentrations. The results showed a similar trend in enzyme activity and gene expression. In particular, MDH, GO and CS activity and the expression of their genes in roots increased after treated with 4 mg/L F, and dropped gradually with the increasing in F concentration. However, these indicators showed no significant changes at 16 mg/L F concentration (Fig. 6). These data suggested that low concentration F increased the concentration of malate, oxalate, and citrate in exudates and content in tea roots, and that MDH, GO and CS activity and gene expression regulate this process. In addition, high F concentrations reduced the concentration of organic acids in exudates and in roots, but did not result in significant differences compared to the control treatment.
Fig. 5.
Effects of F treatments on the content of organic acids in exudates and roots of C. sinensis. Organic acids were identified after treatment with 0, 4, 8 or 16 mg/L F− for 5 weeks. a–c Malate, oxalate, and citrate exudate; d–f malate, oxalate, and citrate content, respectively
Fig. 6.
Effects of F treatments on activity and genes expression of enzymes related to organic acids in C. sinensis roots. Values were measured after treatment with 0, 4, 8 or 16 mg/L F− for 5 weeks. a–c MDH, GO and CS enzymes; d–f expression of MDH, GO, and CS genes
Effects of F on organic acids content in exudates and roots in tea plants exposed to Al treatment
We further measured the malate, oxalate and citrate content in exudate and roots of tea leaves exposed to high F concentrations (> 8 mg/L) and different Al treatments. We detected very low levels of the malate, oxalate, and citrate in exudates and roots in different F concentration without Al treatment. Malate, oxalate, and citrate content in exudates and roots increased dramatically with the increase in Al concentration (Fig. 7). These results confirm that Al enhanced the organic acids content in both exudates and root in tea roots exposed to high F concentration. In addition, 0.4 mM Al significantly increased malate, oxalate, and citrate content in exudates and roots of plants exposed to 8 mg/L F compared to those exposed to 16 mg/L F.
Fig. 7.
Effects of different Al3+ concentrations (0, 0.4, 2 mM) on organic acid content in exudates and roots of C. sinensis exposed to high F concentrations. Values were measured after treatment with different Al3+ concentrations (0, 0.4 or 2 mM) in tea roots exposed to high F concentrations (> 8 mg/L). a–c Malate, oxalate, and citrate content in exudates; d–f malate, oxalate, and citrate content in roots
Discussion
Tea plants often do not present F toxicity symptoms even when they accumulate a large amount of F because tea plant is a hyperaccumulator of F (Ruan and Wong 2001). Here we present that low F concentration (4 mg/L) slightly enhanced tea roots growth, while high F concentration significantly reduced root length (Fig. 1). Moreover, we observed that increasing F concentration induced a decrease in photosynthetic pigments (chlorophyll a and b and a + b) in the tea plants (Fig. 2), which confirm the previous report that high F concentration treatments have a negative effect on photosynthesis in tea leaves (Cai et al. 2016). Abiotic stress adversely influences photosynthesis reducing the level of photosynthetic pigments (Mishra et al. 2006). This might explain the reduction of photosynthesis levels in tea leaves exposed to high F levels (Cai et al. 2016). In addition, our results are consistent with those of Cai et al. (2016), showing an elevated MDA content in tea leaves after F treatment. Increasing the MDA content is responsible for oxidative damage and it induces lipid peroxidation (Weber et al. 2004). Interesting, our findings showed that increasing F concentrations enhanced the activity of the antioxidant enzymes SOD, CAT, and POD, which usually prevent ROS damage to plants (Arora et al. 2002). However, previous study showed that Mn-SOD and CAT activity are reduced in tea plants exposed to 5 mg/L F or higher (Cai et al. 2016). This is likely due to differences in the resistance against F between different tea cultivars. Furthermore, the reduction in soluble protein level in response to F treatment might lead a change in the metabolism of tea leaves, eventually leading to growth inhibition.
Exudation of organic acid in plant roots influences ion solubility and uptake, indirectly affecting the physical properties of the rhizosphere and the dynamics of root growth (Blagoveshchensky and Kologrivova 1945). In the present study, the content of oxalic, citric, and malic acid in exudates and roots increased in tea roots treated with 4 mg/L F and decreased under high F concentration. Previous study has demonstrated that organic acids can promote root growth (Nardi et al. 2002), suggesting that organic acids might be responsible for slight increase in tea roots growth after treatment with low F concentrations (4 mg/L) However, Wang et al. (2013) found that F induces a decrease in the secretion of malic and citric acid, as well as in their accumulation in tea roots. This might account for the higher resistance against F in tea root of the variety ‘longjingchaye’. Furthermore, the GO, CS and MDH activity and their related genes expression profiles were consistent with the content of oxalic, citric and malic acid (Fig. 5). Previous studies show that the GO, CS, and MDH synthases in tea plant are involved in the activity of oxalic, citric, and malic acid (Li et al. 2017a), respectively. GO has been considered as a key component mediating oxalate biosynthesis and enhance oxalate accumulation in some plants (Li and Franceschi 1990; Xu et al. 2006). The MDH can catalyze the reversible reactions of malate and oxaloacetic acid and using NAD+ or NADP+ as coenzymes for generating malate (Schulze et al. 2002). CS, the first enzyme involved in tricarboxylic acid (TCA) cycle, is involved in the combination of oxaloacetate and acetyl CoA to produce citrate (Unger et al. 1989). Our results indicated that low F concentration stimulated the expression of GO, CS, and MDH genes, which regulate the activity of organic acid synthases and ultimately affect the contents of organic acids in tea roots. Moreover, Baunthiyal and Ranghar (2014) reported that F affected plant growth and development by interfering with respiration. Citrate and malate are widely involved in respiration thorough the tricarboxylic acid cycle (TCA). We therefore speculate that the modulation of genes expression and of enzyme activity of factors related to citrate and malate synthesis can influence TCA-dependent respiration in response to F in C. sinensis.
Tea plant is highly tolerant to Al and is an Al accumulator species. Al can alleviate F toxicity by forming Al-F complexes, reducing the amount of F in tea leaves (Yang et al. 2016). However, the exact mechanism by which Al alleviates F induced stress is complicated and has not been fully understood. Here, we detected the content of MDA and antioxidant enzymes in tea leaves treated with different F concentrations in the presence of Al. We found that MDA content of tea leaves decreased with the increase in treatment concentrations of F and Al. Previous study showed that the content of MDA in tea leaves is reduced by 0–0.32 mM Al, but increased significantly at 0.53 mM Al (Li et al. 2011). Our results indicated that low Al concentrations reduced MDA content in tea leaves under F treatment. In contrast, F still reduced the content of MDA in tea leaves under high concentration Al. We speculate that both of F and Al influence the content of MDA, which in turns alleviates oxidative damage and prevent the product of lipid peroxidation in tea leaves (Weber et al. 2004). In addition, SOD activity increased and CAT activity decreased under F treatment by adding extra Al. These results are consistent with a previous report showing that Al increase SOD activity and decreases CAT activity in tea leaves (Li et al. 2011). This suggests that exogenous Al regulates SOD and CAT activity in tea leaves during F stress. These above results confirm that Al can modulate the content of MDA and SOD to protect cells from free radical injury in F-stressed tea leaves.
Organic acids act in tolerance mechanism associated with Al exclusion, and it is believed that their presence in exudates of Nicotiana tabacum chelates Al (della Fuente et al. 1997). Previous studies indicate that the root apex releases the citrate, malate, and oxalate as the main metabolites in response to Al stress (Kochian et al. 2005). Treatment with Al (0–2 mM) enhances the content of citrate, malate, and oxalate in exudates and roots in C. sinensis (Li et al. 2017a). In the present study, the contents of citrate, malate, and oxalate acids in exudates and root under F stress were significantly enhanced by Al treatment. It is noteworthy that citrate, malate, and oxalate acids are present in large concentrations in root cells and involved in the buffering of cytosolic pH (Wang et al. 2010). Moreover, there is a close link between rhizosphere pH and exudation of organic acids in response to Al stress (Wang et al. 2006, 2010). Al alleviates F toxicity by forming Al-F complexes, suppressing the amount of free F− in tea plants (Yang et al. 2016). Therefore, it is reasonable to speculate that adding Al can form Al-F complexes and alleviates rhizosphere pH through the stimulation of exudation of organic acids, ultimately leading to the response to F stress in C. sinensis.
Conclusion
In conclusion, our results demonstrate that F treatment influences physiological indicators in tea leaves. Increased MDA content is responsible for oxidative damage in tea leaves exposed to high F concentration. In turn, increased antioxidant enzymes activity regulates ROS damage to protect tea leaves during F stress. In addition, the growth of tea roots might be regulated by organic acids exudate under F treatment, and this process is regulated by MDH, GO and CS enzymes activities and related genes expression. Furthermore, exogenous Al alleviates F stress in tea leaves through the regulation of MDA content and antioxidant enzymes activity, and stimulates the synthesis and exudation of organic acids in tea roots responding to F stress. These results help us to understand the Al-mediated mechanism to alleviate F toxicity in tea plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Primers used for qRT-PCR of MDH, GO, and CS genes in tea plant (DOCX 14 kb)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31972458, 31770733), the Earmarked Fund for China Agriculture Research System (no. CARS-19), the Earmarked Fund for Jiangsu Agriculture Research System (no. JATS[2019]423).
Author contributions
YW, JP and DL conceived the study and wrote the manuscript. DL, ZS and JZ are responsible for the determination of F and Al content and measurement of soluble proteins, POD, SOD, CAT, MDA and chlorophyll content. ZS, DL and JP are responsible for the analyses of organic acids contents and organic acids enzyme activities. JP, XY, JZ and AX were responsible for hydroponic culture and root elongation measurement. XY, JZ and AX were responsible for qRT-PCR experiment and analysis. YW, WF, XZ, BW and YM critically reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junting Pan and Dongqin Li contributed equally.
Contributor Information
Junting Pan, Email: 2014104092@njau.edu.cn.
Dongqin Li, Email: ldq123asd@163.com.
Jiaojiao Zhu, Email: 2016104092@njau.edu.cn.
Zaifa Shu, Email: shuzaifa@163.com.
Xiaoli Ye, Email: 2015104090@njau.edu.cn.
Anqi Xing, Email: 2017104087@njau.edu.cn.
Bo Wen, Email: njauwb@njau.edu.cn.
Yuanchun Ma, Email: spring1980@163.com.
Xujun Zhu, Email: zhuxujun@njau.edu.cn.
Wanping Fang, Email: fangwp@njau.edu.cn.
Yuhua Wang, Email: wangyuhua@njau.edu.cn.
References
- Arnon DI. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–1238. [Google Scholar]
- Baunthiyal M, Ranghar S. Physiological and biochemical responses of plants under fluoride stress: an overview. Fluoride. 2014;47:287–293. [Google Scholar]
- Blagoveshchensky AV, Kologrivova AJ. Growth of roots as stimulated by certain organic acids. Doklady Akademii Nauk Soiuza Sovetskikh Sotsialisticheskikh Respublik. 1945;48:440. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai HM, Dong YY, Li YY, Li DX, Peng CY, Zhang ZZ, Wan XC. Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol Plant. 2016;38:144. [Google Scholar]
- Cao J, Zhao Y, Liu JW, Xirao RD, Danzeng SB, Daji DW, Yan Y. Brick tea fluoride as a main source of adult fluorosis. Food Chem Toxicol. 2003;41:535–542. doi: 10.1016/s0278-6915(02)00285-5. [DOI] [PubMed] [Google Scholar]
- della Fuente JM, Ramirez Rodriguez V, Cabrera Ponce JL, Herrera Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Deng W, Luo KM, Li ZG, Yang YW, Hu N, Wu Y. Overexpression of Citrus junos mitochondrial citrate synthase gene in Nicotiana benthamiana confers aluminum tolerance. Planta. 2009;230:355–365. doi: 10.1007/s00425-009-0945-z. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumbdhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101. [Google Scholar]
- Ding HY, Wen DN, Fu ZW, Qian HF. The secretion of organic acids is also regulated by factors other than aluminum. Environ Monit Assess. 2014;186:1123–1131. doi: 10.1007/s10661-013-3443-5. [DOI] [PubMed] [Google Scholar]
- Hajiboland R, Rad SB, Barcelo J, Poschenrieder C. Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis) J Plant Nutr Soil Sci. 2013;176:616–625. [Google Scholar]
- Johnson JF, Allan DL, Vance CP. Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus-Albus. Plant Physiol. 1994;104:657–665. doi: 10.1104/pp.104.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, Cheng Z, Ghazanfar B, Khan MA, Zhu YX. Acetyl salicylic acid and 24-epibrassinolide enhance root activity and improve root morphological features in tomato plants under heat stress. Acta Agric Scand. 2014;64:304–311. [Google Scholar]
- Kinraide TB, Parker DR, Zobel RW. Organic acid secretion as a mechanism of aluminium resistance: a model incorporating the root cortex epidermis, and the external unstirred layer. J Exp Bot. 2005;56:1853–1865. doi: 10.1093/jxb/eri175. [DOI] [PubMed] [Google Scholar]
- Klug B, Horst WJ. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum) New Phytol. 2010;187:380–391. doi: 10.1111/j.1469-8137.2010.03288.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Pineros MA, Hoekenga OA. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195. [Google Scholar]
- Li XX, Franceschi VR. Distribution of peroxisomes and glycolate metabolism in relation to calcium oxalate formation in Lemna minor L. Eur J Cell Biol. 1990;51:9–16. [PubMed] [Google Scholar]
- Li CL, Xu HM, Xu J, Chun XY, Ni DJ. Effects of aluminium on ultrastructure and antioxidant activity in leaves of tea plant. Acta Physiol Plant. 2011;33:973–978. [Google Scholar]
- Li QF, Zhao J, Zhang J, Dai ZH, Zhang LG. Ectopic expression of the chinese cabbage malate dehydrogenase gene promotes growth and aluminum resistance in Arabidopsis. Front Plant Sci. 2016;7:1180. doi: 10.3389/fpls.2016.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shu Z, Ye X, Zhu J, Pan J, Wang W, Chang P, Cui C, Shen J, Fang W, Zhu X, Wang Y. Cell wall pectin methyl-esterification and organic acids of root tips involve in aluminum tolerance in Camellia sinensis. Plant Physiol Biochem. 2017;119:265–274. doi: 10.1016/j.plaphy.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang J, Song XW, Zhang ZW, Jiang Y, Zhu YL, Zhao H, Ni DJ. An RNA-Seq transcriptome analysis revealing novel insights into aluminum tolerance and accumulation in tea plant. Planta. 2017;246:91–103. doi: 10.1007/s00425-017-2688-6. [DOI] [PubMed] [Google Scholar]
- Liang J, Shu T, Lin H. The aluminium complexes in the xylem sap of tea plant. J Chin Agric Soc. 1996;34:695–702. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Y, Guo WF, Yang XQ. Fluoride content in tea and its relationship with tea quality. J Agric Food Chem. 2004;52:4472–4476. doi: 10.1021/jf0308354. [DOI] [PubMed] [Google Scholar]
- Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere. 2006;65:1027–1039. doi: 10.1016/j.chemosphere.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Morita A, Yanagisawa O, Maeda S, Takatsu S, Ikka T. Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminum. Soil Sci Plant Nutr. 2011;57:796–802. [Google Scholar]
- Mugai EN, Agong SG, Matsumoto H. Aluminium tolerance mechanisms in Phaseolus vulgaris L.: citrate synthase activity and TTC reduction are well correlated with citrate secretion. Soil Sci Plant Nutr. 2000;46:939–950. [Google Scholar]
- Mukhopadyay M, Bantawa P, Das A, Sarkar B, Bera B, Ghosh P, Mondal TK. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. Biometals. 2012;25:1141–1154. doi: 10.1007/s10534-012-9576-0. [DOI] [PubMed] [Google Scholar]
- Nagata T, Hayatsu M, Kosuge N. Aluminum kinetics in the tea plant using 27Al and 19F NMR. Phytochemistry. 1993;32:771–775. [Google Scholar]
- Nardi S, Sessi E, Pizzeghello D, Sturaro A, Rella R, Parvoli G. Biological activity of soil organic matter mobilized by root exudates. Chemosphere. 2002;46:1075–1081. doi: 10.1016/s0045-6535(01)00160-6. [DOI] [PubMed] [Google Scholar]
- Oh MW, Roy SK, Kamal AM, Cho K, Cho SW, Park CS, Choi JS, Komatsu S, Woo SH. Proteome analysis of roots of wheat seedlings under aluminum stress. Mol Biol Rep. 2014;41:671–681. doi: 10.1007/s11033-013-2905-8. [DOI] [PubMed] [Google Scholar]
- Pan JT, Wang WD, Li DQ, Shu ZF, Ye XL, Chang PP, Wang YH. Gene expression profile indicates involvement of NO in Camellia sinensis pollen tube growth at low temperature. BMC Genom. 2016;17:809. doi: 10.1186/s12864-016-3158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan JY, Wong MH. Accumulation of fluoride and aluminium related to different varieties of tea plant. Environ Geochem Health. 2001;23:53–63. [Google Scholar]
- Ruan JY, Ma LF, Shi YZ, Han WY. Uptake of fluoride by tea plant (Camellia sinensis L) and the impact of aluminium. J Sci Food Agric. 2003;83:1342–1348. [Google Scholar]
- Ruf M, Brunner I. Vitality of tree fine roots: reevaluation of the tetrazolium test. Tree Physiol. 2003;23:257–263. doi: 10.1093/treephys/23.4.257. [DOI] [PubMed] [Google Scholar]
- Schulze J, Tesfaye M, Litjens RHMG, Bucciarelli B, TreppG Miller S, Samac D, Allan D, Vance CP. Malate plays a central role in plant nutrition. Plant Soil. 2002;247:133–139. [Google Scholar]
- Shu WS, Zhang ZQ, Lan CY, Wong MH. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere. 2003;52:1475–1482. doi: 10.1016/S0045-6535(03)00485-5. [DOI] [PubMed] [Google Scholar]
- Singh G, Kumari B, Sinam G, Kriti Kumar N, Mallick S. Fluoride distribution and contamination in water, soil and plants continuum and its remedial technologies, an Indian perspective: a review. Environ Pollut. 2018;239:95–108. doi: 10.1016/j.envpol.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Smith AJ. The bio-availability of fluoride from black tea. J Dent. 2001;29:15–21. doi: 10.1016/s0300-5712(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Unger EA, Hand JM, Cashmore AR, Vasconcelos AC. Isolation of a cDNA encoding mitochondrial citrate synthase from Arabidopsis thaliana. Plant Mol Biol. 1989;13:411–418. doi: 10.1007/BF00015553. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xu RK, Li XH. Proton release by tea plant (Camellia sinensis L.) roots as affected by nutrient solution concentration and pH. Plant Soil Environ. 2012;58:429–434. [Google Scholar]
- Wang P, Bi SP, Ma LP, Han WY. Aluminum tolerance of two wheat cultivars (Brevor and Atlas 66) in relation to their rhizosphere pH and organic acids exuded from roots. J Agric Food Chem. 2006;54:10033–10039. doi: 10.1021/jf0611769. [DOI] [PubMed] [Google Scholar]
- Wang SL, Wang P, Wang CY. Changes in rhizosphere pH and exudation of organic acids of masson pine (Pinus massoniana) seedlings under aluminum stress. J Ecol Rural Environ. 2010;26:87–91. [Google Scholar]
- Wang LX, Tang JH, Xiao B, Yang YJ, Yu YB. Enhanced release of fluoride from rhizosphere soil of tea plants by organic acids and reduced secretion of organic acids by fluoride supply. Acta Agric Scand Sect B Soil Plant Sci. 2013;63:426–432. [Google Scholar]
- Weber H, Chetelat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004;37:877–888. doi: 10.1111/j.1365-313x.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- Weinstein LH, Halscher-Herman R (1982) Physiological responses of plants to fluorine. In: Effects of gaseous air pollutants in agriculture and horticulture, pp 139–167
- Xu HW, Ji XM, He ZH, Shi WP, Zhu GH, Niu JK, Li BS, Peng XX. Oxalate accumulation and regulation is independent of glycolate oxidase in rice leaves. J Exp Bot. 2006;9:9. doi: 10.1093/jxb/erj131. [DOI] [PubMed] [Google Scholar]
- Xu QS, Wang Y, Ding ZT, Fan K, Ma DX, Zhang YL, Yin Q. Aluminum induced physiological and proteomic responses in tea (Camellia sinensis) roots and leaves. Plant Physiol Biochem. 2017;115:141–151. doi: 10.1016/j.plaphy.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Yamada M, Higashiyama T, Kishino S, Kataoka M, Ogawa J, Shirnizu S, Isobe K. Novel alcohol oxidase with glycolate oxidase activity from Ochrobactrum sp. AIU 033. J Mol Catal B Enzymat. 2014;105:41–48. [Google Scholar]
- Yang Y, Liu Y, Huang CF, Silva JD, Zhao FJ. Aluminium alleviates fluoride toxicity in tea (Camellia sinensis) Plant Soil. 2016;402:179–190. [Google Scholar]
- Zhang L, Li Q, Ma LF, Ruan JY. Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze) Plant Soil. 2013;366:659–669. [Google Scholar]
- Zhang XC, Gao HJ, Zhang ZZ, Wan XC. Influence of aluminum on absorption and distribution of fluoride in tea plants (Camellia sinesis L.) Food Science. 2013;34:147–150. [Google Scholar]
- Zhang FG, Meng XH, Yang XM, Ran W, Shen QR. Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol Biochem. 2014;83:250–257. doi: 10.1016/j.plaphy.2014.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for qRT-PCR of MDH, GO, and CS genes in tea plant (DOCX 14 kb)