Abstract
Drought stress is one of the most prevalent environmental factors limiting faba bean (Vicia faba L.) crop productivity. β-aminobutyric acid (BABA) is a non-protein amino acid that may be involved in the regulation of plant adaptation to drought stress. The effect of exogenous BABA application on physiological, biochemical and molecular responses of faba bean plants grown under 18% PEG-induced drought stress were investigated. The results showed that the application of 1 mM of BABA improved the drought tolerance of faba bean. The application of BABA increased the leaf relative water content, leaf photosynthesis rate (A), transpiration rate (E), and stomatal conductance (gs), thereby decreased the water use efficiency. Furthermore, exogenous application of BABA decreased production of hydrogen peroxide (H2O2), malondialdehyde and electrolyte leakage levels, leading to less cell membrane damage due to oxidative stress. Regarding osmoprotectants, BABA application enhanced the accumulation of proline, and soluble sugars, which could improve the osmotic adjustment ability of faba bean under drought challenge. Interestingly, mended antioxidant enzyme activities like catalase, guaiacol peroxidase, ascorbate peroxidase and superoxide dismutase and their transcript levels may lead to counteract the damaging effects of oxidative stress and reducing the accumulation of harmful substances in BABA-treated faba bean plants. In addition, exogenous BABA significantly induced the accumulation of drought tolerance-related genes like VfMYB, VfDHN, VfLEA, VfERF, VfNCED, VfWRKY, VfHSP and VfNAC in leaves and roots, suggesting that BABA might act as a signal molecule to regulate the expression of drought tolerance-related genes.
Keywords: β-Aminobutyric acid (BABA), Drought stress, Gene expression, Vicia faba, qRT-PCR
Introduction
Water deficit is one of the most prevalent environmental stress factors that greatly influence plant growth and development, and cause substantial decline in crop yields worldwide. Faba bean (Vicia faba L.) is used for human consumption, animal feed and green-manure legume, as well as in the wheat based cropping systems due to its ability to fix atmospheric nitrogen in the northern favorable regions of the country. According to Amri et al. (2016), faba bean is the most important grain legume cultivated in Tunisia occupying more than 75% (55,000 ha) of the total food legume planted area in the country. However, the Tunisian dry grain yield average (about 1 t/ha) is below the worldwide average yield (1.7 t/ha). The low harvest is mainly due to the lack of adapted cultivars, the sensitivity of local material to environmental stresses (mainly water deficit, especially in semi-arid regions), diseases and pests. It is worth to note that the genome complexity (13,000 Mb) compared to other legume species (450 Mb and 1200 Mb in Medicago sativa and Glycine max, respectively) made it difficult to breed elite cultivars suitable for local and regional ecosystem and enhancing the role of faba bean for conservation agriculture in arid and semi-arid regions in Tunisia. Ouji et al. (2017) showed that drought affects the development, growth and yield components in that plant, leading to a significant loss in productivity. Drought stress induces several physiological, biochemical and molecular disruptions on faba bean plant (Abid et al. 2017; Siddiqui et al. 2015). Moreover, Kabbadj et al. (2017) reported that drought disturbed various enzymatic pathways and caused impaired photosynthesis. In addition to traditional breeding methods and molecular tools, new various recent strategies have been proposed to improve plant performance against environmental stresses. Among these strategies, the stimulation of natural defense in plants by inducing resistance could be considered one of the best. These methods include the use of antioxidants, vitamins and osmoprotectant compounds as seed priming or as foliar spray. In this regard, previous studies indicated that exogenous application of various plant growth regulators (such as abscisic acid, brassinolides, cytokinins, salicylic acid and gibberellins) and osmoprotectants (like proline, trehalose and glycinebetaine) can also enhance drought tolerance of crops as sunflower (Hussain et al. 2014), lucerne (An et al. 2014), maize (Akter et al. 2014) and soybean (EL Sabagh et al. 2018). According to the above cited authors, the application of these compounds promotes compatible solutes and antioxidants accumulation in order to maintain osmotic balance and scavenging of the reactive oxygen species (ROS) and consequently ensure stability of membrane structures, enzymes, and other macromolecules under drought stress. Such priming treatment is a very promising strategy in modern crop production management as it allows the plants to respond faster and stronger to abiotic or biotic stress (Xiao et al. 2017). Several studies revealed that polyamines (like putrescine, spermidine and spermine) and non-protein amino acids such as γ-aminobutyric acid (GABA) accumulated in response to abiotic stresses could play key role in the response of plants to environmental factors (Li et al. 2016a, b; Yuan et al. 2016).
It is well documented that inducing priming by exogenous application of natural compound GABA or β-aminobutyric acid (BABA), its isomer synthetic form, could promote osmotic balance via synthesis of osmolytes and increase antioxidant enzymes activity and their transcript levels hence contributing to the reduction of oxidative damage (Vijayakumari et al. 2016). It has been reported that BABA-treated plants showed enhanced accumulation of abscisic acid (ABA), a phytohormone playing a crucial role in drought-stress response and tolerance in plants. Suggesting that BABA priming led to an ABA-dependent mechanisms (Jakab et al. 2005), later on Tworkoski et al. (2011) demonstrated that both ABA-dependent and independent mechanisms act through BABA priming. These two protection mechanisms through the exogenous application of BABA have been reported in several plant species. Ton et al. (2005) revealed that BABA usage improved the response of Arabidopsis to drought stress by enhancing the transcript accumulation of ABA-dependent RAB18 and RD29 genes. Shaw et al. (2016) indicated that BABA-induced the up-regulation of antioxidant enzymes (APX, SOD, GR and GSH) in maize enhancing the plant defense and detoxification processes against drought. Moreover, an ABA accumulation, inducing a partial stomatal closure and leading to reduced water use under drought conditions have been reported in BABA treated maize (Shaw et al. 2016), wheat (Du et al. 2012) and Arabidopsis (Jakab et al. 2005). However, no studies of priming effects of BABA on drought stress tolerance in faba bean were carried out so far. The objective of this study was to determine the effect of exogenous application of BABA on faba bean response and tolerance to water deficit stress.
Materials and methods
Plant material, growth condition and treatments
Faba bean seeds (cv. Badii cultivated in sub-humid areas of Tunisia) were surface-sterilized for 5 min in 5% sodium hypochlorite (NaClO) solution and then rinsed 5 times with sterile distilled water to remove all sterilizing agents and followed by germination at 23 °C. One week after germination, uniform seedlings were transplanted in plastic pots filled with perlite and were irrigated with half-strength Hoagland nutritive solution (Hoagland and Arnon 1950) when required. Three-week-old seedlings (corresponding to four fully expanded leaves) were uprooted, washed with distilled water and transferred to aerated hydroponic system (height 14 cm, width 17 cm, length 40 cm). 15 seedlings where considered for each hydroponic system containing 5 l of Hoagland solution. At interval of 48 h, the nutrient medium was replaced and the seedlings’ root were exposed to BABA pretreatment for 2 days by adding 0.5, 1 and 1.5 mM of BABA to the Hoagland solution (pH 6.5). After BABA pretreatment, seedlings were subject to drought stress by adding 18% of polyethylene glycol (PEG-6000; − 0.58 MPa) in the nutritive solution (Michel and Kaufmann 1973). Untreated seedlings in nutrient solution were taken as control. In total, five treatments were created: (1) control; (2) PEG treatment (18% PEG-6000); (3) BABA (0.5 mM) + PEG (18% PEG-6000); (4) BABA (1 mM) + PEG (18% PEG-6000) and (5) BABA (1.5 mM) + PEG (18% PEG-6000). Drought stress was induced for 2 days. The experiment was conducted in a completely randomized design with three replications in a growth chamber under controlled conditions (temperature of 23 ± 2 °C, relative humidity 55–65%, light 270 μmol of photons m−2 s−1 photosynthetic active radiations and a 16/8 h day/night photoperiod). The experimental boxes were covered with black plastic to reduce the exposure of roots to light, and continuously aerated in order to avoid the algal growth and prevent the roots from developing hypoxia. Two days after treatments, seedlings were harvested and physiological and biochemical parameters were measured. For molecular analyses, leaf and root samples were snap frozen in liquid N2, and stored at − 80 °C for further analysis.
Measurement of photosynthesis
Gas exchange measurements such as photosynthetic rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) were made at 10 a.m. on the youngest fully expanded attached leaves and uniform in terms of age using a Portable Photosynthesis System (LCpro +, Inc., UK). The photosynthetically active radiation in the leaf chamber was set at 980 µmol m−2 s−1 during the monitoring. Five seedlings were used as replicates for each treatment.
Determination of relative water content (RWC)
RWC was estimated in the fully-expanded leaf (from the top) according to the method of González and González-Vilar (2001). Leaf samples were weighed for fresh weights (FW) and then saturated in deionized distilled water at 4 °C in the dark for 24 h and their turgid weights were recorded (TW). Then, they were oven-dried at 65 °C for 72 h and their dry weights (DW) were recorded. The RWC was finally calculated using the following formula: RWC (%) = [(FW − DW)/(TW − DW)] × 100.
Determination of proline, soluble sugars, hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents and leaf electrolyte leakage (EL) level
Proline content was determined by ninhydrine reaction according to Bates et al. (1973) with slight modifications. Leaf dry samples (100 mg) were homogenized with 3 ml of a 3% (w/v) sulfosalicylic acid solution and centrifuged at 13,800 rpm for 10 min. Then, 2 ml of supernatant was added with 2 ml of glacial acetic acid and acid ninhydrin reagent. The mixture was incubated at 100 °C in water bath for 1 h and then cooled on ice. The homogenate was extracted with 4 ml of toluene at room temperature and the upper phase was separated and absorbance was measured at 520 nm using spectrophotometer (Spectro UV–Vis Dual Beam PC, UV-S-2007; LABOMED, INC.). l-proline was used for standard curve construction.
Soluble sugars content was determined spectrophotometrically according to Dubois et al. (1951) based on the method of phenolsulfuric acid. About 100 mg dry weight of leaves was homogenized with 5 ml of 80% ethanol, extract was filtered and 1 ml of the extract was mixed with 0.5 ml of 5% phenol solution and 2.5 ml of 98% sulphuric acid. The mixture was incubated for 1 h and absorbance was measured at 490 nm. The level of soluble sugars was expressed in µg g−1 DW.
Content of H2O2 was measured according to the procedure of Velikova et al. (2000). About 0.5 g fresh weights of leaves was homogenized with 5 ml 0.1% (w/v) trichloroacetic acid (TCA) in ice bath and the mixture was centrifuged at 13,800 rpm for 15 min. Then 1 ml of 10 mM potassium phosphate buffer and 2 ml of 1 M KI were added to 1 ml of the supernatant. The absorbance of supernatant was measured at 390 nm and H2O2 content was quantified using a standard curve.
Lipid peroxidation was estimated by the level of malondialdehyde (MDA) production as described by Dhindsa et al. (1981). Fresh leaf samples (1 g) were homogenized in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA) solution and centrifuged at 13,800 rpm for 15 min. To 1 ml of supernatant, 4 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA was added and heated at 100 °C for 30 min before cooled at room temperature. The absorbance of the supernatant was recorded at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. The concentration of MDA was expressed in nmol g−1 FW, using a molar extinction coefficient equal to 155 × 105 mM−1 cm−1.
Electrolyte leakage (EL) level was determined according to the method of Murray et al. (1989) with slight modifications. Leaf discs were cut from fully expanded leaves and placed in 5 ml deionized water for 3 h at 25 °C and initial conductivity (Ci) of the solutions were determined. Then, leaf samples were boiled at 100 °C for 30 min and cooled at room temperature to measure the final conductivity (Cf). EL was estimated as a percentage (%) of initial to final conductivity by the following formula: EL (%) = (Ci/Cf) × 100%.
Assays of antioxidant enzymes
Enzyme extract was prepared by homogenizing 1 g of plant leaves from each treatment in an ice cold mortar with 10% (w/w) polyvinylpyrrolidone (PVP) and 1 ml of 50 mM phosphate buffer (pH 7.8) containing 0.1% (v/v) triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 2 mM EDTA. The homogenate was centrifuged at 13,800 rpm for 15 min at 4 °C and the obtained supernatant was collected for determining the activities of antioxidant enzymes. Protein content was determined according to the method of Bradford (1976).
Superoxide dismutase (SOD) activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm following Del Longo et al. (1993). One unit (U) of SOD activity was defined as the quantity of enzyme necessary to inhibit the NBT reduction by 50% at 25 °C. The reaction mixture contained 50 mM K phosphate (pH 7.8), 0.1 mM EDTA, 13 mM l-methionine, 2 µM riboflavin and 75 µM NBT. Catalase (CAT) activity was assayed according to Cakmak and Marschner (1992) by measuring the decline of absorbance at 240 nm caused by the catabolization of H2O2 (10 mM) for 1 min calculated using extinction coefficient (ε = 39.4 mM−1 cm−1).
Ascorbate peroxidase (APX) activity was assayed according to Nakano and Asada (1981) by monitoring the decrease of ascorbate at 290 nm during 1 min using extinction coefficient (ε = 2.8 mM−1 cm−1). The reaction mixture contained 1.5 mM H2O2 in 50 mM phosphate buffer (pH 7.8).
Guaiacol peroxidase (GPX) activity was assayed according to Polle et al. (1994) by following the increase in absorbance at 470 nm due to guaiacol oxidation in a reaction solution for 1 min and calculated using extinction coefficient (ε = 25.5 mM−1 cm−1). The reaction solution contained 100 mM potassium phosphate buffer (pH 7.8), 20 mM guaiacol, 10 mM H2O2 and 50 µl enzymes extract.
Total RNA extraction and cDNA synthesis
Total RNA was isolated from frozen root and leaf samples following the protocol described by Chang et al. (1993). Total RNA was quantified using micro-spectrophotometry (NanoDrop Technologies, Inc.). Then, RNA integrity and possible DNA contamination were checked by agarose gel electrophoresis (1.5%). Before cDNA synthesis, the total RNA samples were treated with 5 U of RNase-free DNase I (Thermo Fisher Scientific) for 30 min at 37 °C in order to eliminate the DNA contamination. cDNA was synthesized from 5 µg of total RNA using 200 U of RevertAid M-MuLV reverse transcriptase (Biomatik; Wilmington, Delaware, USA) as described by the manufacturer.
Real-time reverse transcription-PCR (qRT-PCR)
qRT-PCR was carried out in a 7300 Real-Time PCR Detection System (Applied Biosystems, Foster City, USA). VfELFA-1 gene was used as endogenous control for all reactions, and primer pairs were designed using the Primer3 Input (version 0.4.0) software (Rozen and Skaletsky 2000) (http://frodo.wi.mit.edu/primer3/). For qRT-PCR, Maxima SYBR Green/ROX qPCR Master Mix (2 ×) kit (Biomatik; Wilmington, Delaware, USA) was used according to manufacturer’s instruction. The reactions were performed in 30 µl volume containing 2 µl of first strand cDNA, 200 µM each of gene specific primers (Table 1), 15 μl Maxima SYBR Green/ROX qPCR Master Mix (2 ×) and 12 μl H2O. The thermal protocol consisted of 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 1 min. All reactions were performed in triplicate. Melting curves were obtained by slow heating from 65 °C to 95 °C at 0.5 °C/s and continuous monitoring of the fluorescence signal. The relative expression levels were calculated using the 2−ΔΔCt method (Schmittgen and Livak 2008). The heat maps are generated using R package (http://www.r-project.org/) to compare the expression profiling of the transcriptome in different stress treatments.
Table 1.
Primers used for quantitative real time PCR assays
| Transcript | GenBank accession ID | Forward and reverse primer 5′–3′ | Tm (°C) |
|---|---|---|---|
| VfCAT | JQ043348 | Forward: 5′-TGCATTTTGTCCTGCCATTA-3′ | 58 |
| Reverse: 5′-TCCAAGTCTGTGCCTCTGTG-3′ | |||
| VfAPX | FL507355 | Forward: 5′-CATTGAAAAGGCCAAGAGGA-3′ | 59 |
| Reverse: 5′-TGCTTAATGGTTCCGAAAGGT-3′ | |||
| VfSOD | JQ043347 | Forward: 5′-CAGGGCTTCATGGTTTTCAT-3′ | 60 |
| Reverse: 5′-GACGGGTTTCATCTTCAGGAG-3′ | |||
| VfGST | JR964463 | Forward: 5′-CTTACCGCACCCAAAAACTT-3′ | 59 |
| Reverse: 5′-CCAATCTTGCCTTCAGATCC-3′ | |||
| VfMYB | FL505090 | Forward: 5′-TCCGTTCGACCAGGTAACTT-3′ | 60 |
| Reverse: 5′-ATCCTGGTCTCAAACGTGGT-3′ | |||
| VfDHN | FL506814 | Forward: 5′-CAGATGAAACAAACTACTCAAAC-3′ | 58 |
| Reverse: 5′-AAGCTTCCTGGTACTGGAGGA-3′ | |||
| VfLEA | FL507910 | Forward: 5′-TGACCAGAAGCCAGTGTGAG-3′ | 59 |
| Reverse: 5′-CGGGAGTACCAACGGATATG-3′ | |||
| VfERF | EU543659 | Forward: 5′-TGCTGCTTTTCATTTTCGTG-3′ | 59 |
| Reverse: 5′-AGGCGCTGTAAGAGGCATAG-3′ | |||
| VfNCED | FL505829 | Forward: 5′-ACAATGTCAGCAGATCCCGT-3′ | 60 |
| Reverse: 5′-GCAATGGTTGTCTGCCTGTT-3′ | |||
| VfWRKY | KO324181 | Forward: 5′-CCGCTGTTTGCAGTTATTGA-3′ | 60 |
| Reverse: 5′-TCATTCATTTCGGTCCACAA-3′ | |||
| VfHSP | FL504801 | Forward: 5′-TCTCAAGCTGGTGGGTCTTT-3′ | 59 |
| Reverse: 5′-AAATCCTTCAATCGGCGCTC-3′ | |||
| VfNAC | JR969093 | Forward: 5′-ATGCTGCATCGTTCTCAGTG-3′ | 59 |
| Reverse: 5′-TGATTGGGTTCTGTGTCGAA-3′ | |||
| VfELF1A | AJ222579 | Forward: 5′-GTGAAGCCCGGTATGCTTGT-3′ | 58 |
| Reverse: 5′-CTTGAGATCCTTGACTGCAACATT-3′ |
Statistical analysis
All data obtained was subjected to one-way analysis of variance (ANOVA) with three replications per treatment and means were separated by Tukey’s post hoc test (P ≤ 0.05) using SPSS program version 16.
Results
Photosynthesis and gas exchange parameters
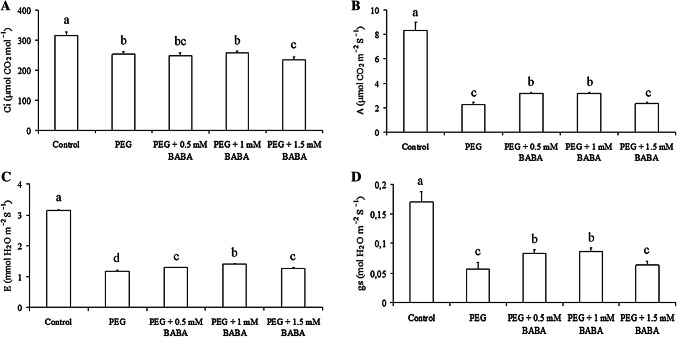
In order to determine the effects of drought stress and BABA treatment on the gas-exchange parameters of Badii plants, A, E, gs and Ci were measured. Water deficit considerably affected A, gs, Ci, and E (Fig. 1). ANOVA analysis of gas exchange parameters showed significant differences between treatments for all parameters. Treated plants showed significant decrease in Ci by 19.31% for PEG (18%) in comparison with the control plants (Fig. 1a) and by 21.66%, 18.50% and 25.55% under the combined effect of PEG and BABA (0.5, 1 and 1.5 mM, respectively). Variation amongst treatments was significant for A (Fig. 1b). Indeed, A decreased more for treatments PEG (72.95%) and PEG + 1.5 mM BABA (71.87%) than for PEG + 0.5 mM BABA and PEG + 1 mM BABA (61.77 and 61.89, respectively). Interestingly, gs showed similar trends to A for all treatments with a decrease of 67.05% and 62.94% for PEG and PEG + 1.5 mM BABA, respectively and 51.17% and 49.41% for PEG + 0.5 mM BABA and PEG + 1 mM BABA, respectively (Fig. 1d). E decreased by 62.42%, 58.28%, 55.73% and 59.87% in PEG, PEG + 0.5 mM BABA, PEG + 1 mM BABA and PEG + 1.5 mM BABA, respectively, compared with that in controls (Fig. 1c). As compared to treated BABA plants, PEG-stressed plants without BABA application exhibited a significant reduction in A, gs and E. Furthermore, 1 mM BABA-treated plants showed significantly higher E than 0.5 and 1.5 mM BABA-treated plants under water deficit whilst gs and A were significantly higher in 0.5 and 1 mM BABA-treated plants than that in 1.5 mM BABA-treated plants.
Fig. 1.
Effects of PEG (18%) and BABA treatments (0.5 mM, 1 mM and 1.5 mM) on intercellular CO2 concentration (a), photosynthetic rate (b), transpiration rate (c) and stomatal conductance (d). Vertical bars indicate ± SE of mean (n = 3). Different letters denote significant differences (Tukey’s HSD, P < 0.05)
RWC and electrolyte leakage (EL) level, soluble sugars, proline, H2O2 and MDA content
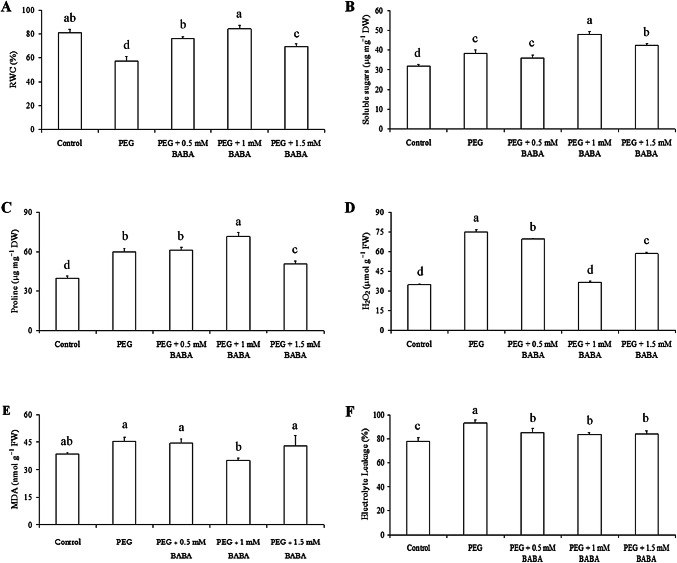
Drought stress resulted in a significant decrease in RWC in BABA untreated plants, but exogenous application of BABA alleviated drought-induced decline in RWC (Fig. 2a). When treated with 18% PEG solution for 2 days, leaf RWC dropped from almost 81% to only 57.5% (decreased 0.29 fold). It was shown that BABA-treated plants maintained significantly higher RWC than untreated plants under water deficit. Indeed, 0.5, 1 and 1.5 mM BABA-treated plants had 32.17%, 46.52% and 20.86% higher RWC than BABA-untreated plants. Interestingly, 1 mM BABA-treated plants showed significantly high RWC than plant treated with PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA.
Fig. 2.
Effects of PEG (18%) and BABA treatments (0.5 mM, 1 mM and 1.5 mM) on relative water content (a), soluble sugars (b), Proline (c), hydrogen peroxide (d), malondialdehyde (e) content and electrolyte leakage level (f). Vertical bars indicate ± SE of mean (n = 3). Different letters denote significant differences (Tukey’s HSD, P < 0.05)
As shown in Fig. 2b, drought stress had strongly affected the accumulation of soluble sugars in Badii leaves in different treatments to reach its maximum after 2 days of drought stress with application of 1 mM BABA. Thus, compared to control treatment, plants treated with PEG, PEG + 0.5 mM BABA, PEG + 1 mM BABA and PEG + 1.5 mM BABA, had 19.53%, 12.50%, 50% and 31.25% higher soluble sugars content, respectively. However, when comparing the soluble sugars accumulation in leaves of BABA-untreated plants under drought stress with PEG + BABA treatments, we noted an increase percentage of 33% and 11% under PEG + 1 mM BABA and PEG + 1.5 mM BABA treatments, respectively, and almost no effect of 0.5 mM BABA.
Regarding proline content, an obvious increase in the leaves of Badii after 2 days of drought was recorded. Moreover, proline content on BABA-treated plants was significantly higher compared to the non-treated plants under drought stress (Fig. 2c). Thus, proline content was 50.31%, 54.08%, 79.24% and 27.04% higher in plants treated with PEG, PEG + 0.5 mM BABA, PEG + 1 mM BABA and PEG + 1.5 mM BABA than that in control plants. As a result, PEG + 1 mM BABA-treated plants showed significantly higher (increased by 19%) proline content throughout the drought stress as compared to non-treated BABA plants. On the other hand, exogenous 0.5 mM BABA-treatment had no effect on proline accumulation in leaves of Badii under drought stress even more this content decreased by 15.50% in PEG + 1.5 mM BABA treated plants comparing with only PEG treated plants.
With application of drought challenge, no significant change of H2O2 content in leaves of Badii treated with 1 mM BABA compared to controls was observed. Although, PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA-treated plants exhibited 117%, 100% and 70%, respectively, higher H2O2 content than controls (Fig. 2d).
Lipid peroxidation, measured as MDA content in leaves, did differ between treatments. MDA content showed an increase by 18%, 15.50% and 11.50% after PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA treatment, respectively in leaves of Badii, while decreased by 10% in plants treated with PEG + 1 mM BABA compared to controls (Fig. 2e). Interestingly, only exogenous application of 1 mM BABA effectively reduced the increase trend of MDA in drought stressed plants. PEG + 1 mM BABA-treated plants showed significant lower MDA content (almost 23%) compared to PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA treated plants.
At 2 days of drought challenge, PEG, PEG + 0.5 mM BABA, PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants showed 19%, 9%, 7% and 8%, respectively, higher EL than controls with a measurable significant difference (Fig. 2f). Drought stress caused a considerable increase of EL level in leaves of Badii, but exogenous BABA effectively reduced the increase trend of EL. However, BABA concentration almost had no effect on EL level in leaves of Badii under drought stress.
Antioxidant enzymes activities
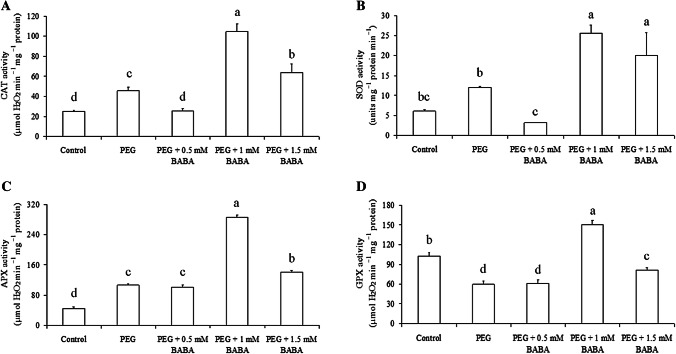
Activities of CAT, SOD, APX and GPX antioxidant enzymes were measured (Fig. 3). In general, drought stress and exogenous application of BABA induced changes in antioxidant enzymes actions. At 2 days of drought stress, significant differences of CAT activity between PEG + BABA-treated plants and non-treated plants were observed (Fig. 3a). Except PEG + 0.5 mM BABA treated plants that did not show any significant difference compared to controls. CAT activity increased by 82%, 318% and 156% in PEG, PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants, respectively, as compared to controls. Accordingly, the degree of increase in CAT activity was the highest in 1 mM BABA-treated plants. SOD activity was relatively unchanged or slightly increased in untreated-BABA plants during drought application. Nonetheless, SOD activity slightly decreased in PEG + 0.5 mM BABA treated plants but significantly increased by 318% and 227% in PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants, respectively (Fig. 3b). With the imposition of drought stress for 2 days, the activity of APX increased with 140%, 130%, 550% and 221% in PEG, PEG + 0.5 mM BABA, PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants compared to controls (Fig. 3c). All the BABA treatments increased the activity of APX in leaves of Badii under drought, but this increase was much more prominent in 1 mM BABA-treated plants. Except in PEG + 1 mM BABA treated plants, the GPX activity was lowered in all drought-stressed compared with well-watered plants at 2 days of treatment (Fig. 3d). A 41.46%, 40.19% and 20.09% reduction in GPX activity was observed in PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA-treated plants. However, the application of 1 mM BABA resulted in a 47% higher GPX activity in drought-stressed than control plants. When compared with drought stress alone, exogenous BABA application significantly increased GPX activity in 1 mM BABA and 1.5 mM BABA-treated plants, while exogenous application of 0.5 mM BABA almost have no effect on GPX activity. Moreover, the increase in GPX activity following BABA application was more pronounced in 1 mM BABA than in 1.5 mM BABA-treated plants.
Fig. 3.
Effects of PEG (18%) and BABA treatments (0.5 mM, 1 mM and 1.5 mM) on the activities of catalase (a), superoxide dismutase (b), ascorbate peroxidase (c) and guaiacol peroxidase (d) in leaves of Badii. Vertical bars indicate ± SE of mean (n = 3). Different letters denote significant differences (Tukey’s HSD, P < 0.05)
Effect of exogenous application of BABA on drought stress-related genes expression
To reveal the effect of exogenous BABA on drought stress-related genes expression, qRT-PCR experiment was performed and data were visualized using heat maps. Heat map of the changes in the expression patterns of 12 drought stress-related genes (VfCAT, VfAPX, VfSOD, VfGST, VfMYB, VfDHN, VfLEA, VfERF, VfNCED, VfWRKY, VfHSP and VfNAC) in response to exogenous application of BABA and drought in Badii leaves (Fig. 4) and in roots (Fig. 5) were depicted. Data analysis showed that all genes were found to be expressed under drought and control conditions. Furthermore, as compared to control plants, higher level of expression was measured for all genes including genes encoding antioxidant enzymes (CAT, SOD, APX and GST) in treated and untreated-BABA under drought stress condition in leaves and roots of Badii. All studied genes were expressed diversely under drought stress in treated and untreated-BABA plants. In leaves, the genes expression could be divided into four clusters (Fig. 4). Cluster 1 included 4 members (VfAPX, VfNCED, VfWRKY and VfNAC, 33.50%) of 12 studied drought related genes, which were widely upregulated in PEG, PEG + 0.5 mM BABA and PEG + 1 mM BABA-treated plants compared to control plants. However, under PEG + 1.5 mM BABA treatment VfWRKY and VfNAC were weakly upregulated but VfAPX and VfNCED were weakly downregulated. The 4 members (VfDHN, VfLEA, VfHSP and VfMYB, 33.50%) of cluster 2 were mainly upregulated after drought treatment. Indeed, VfDHN was upregulated under PEG and BABA treatments. However, VfLEA and VfHSP were upregulated in PEG, PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants compared to controls. And VfMYB was upregulated in PEG and PEG + 1 mM BABA-treated plants and nearly unchanged under PEG + 0.5 mM BABA and PEG + 1.5 mM BABA treatment. All members (VfCAT, VfSOD, VfGST, 25%) of cluster 3 were mainly upregulated under PEG and BABA treatments. The VfERF of cluster 4 (8%) was upregulated under PEG and PEG + 0.5 mM BABA treatments but nearly unchanged under PEG + 1 mM BABA and PEG + 1.5 mM BABA treatments. For roots, the heat map also clustered the analyzed genes in four groups (Fig. 5). Cluster 1 contains 5 members (42%). Overall, drought stress up-regulated the expression of VfCAT, VfLEA, VfWRKY, VfHSP and VfAP in faba bean treated BABA (both 0.5 mM and 1 mM) but showed downregulated in drought-stressed plants treated with 1.5 mM BABA. Cluster 2 mainly consists of 5 genes (42%) and was divided into 3 sub-clusters. Sub-cluster I had 2 genes (VfSOD and VfDHN), which were widely upregulated in PEG and PEG + 0.5 mM BABA treated plants. Sub-cluster II had also 2 genes (VfNCED and VfNAC). VfNCED was upregulated under PEG + 1 mM BABA-treated plants while VfNAC was upregulated under both PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants. Sub-cluster III, had only 1 member (VfGST), this gene was expressed at high level only under PEG treatment but BABA treatments did not affect VfGST expression compared to control plants. Cluster 3 had only 1 gene (VfERF, 8%), which showed a significantly upregulation under PEG and PEG + 0.5 mM BABA treatments but downregulated in PEG + 1.5 mM BABA-treated plants. Cluster 4 contained VfWRKY gene (8%), which was mainly upregulated in PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA-treated plants and in particular was highly induced in PEG + 1 mM BABA-treated plants compared to controls.
Fig. 4.
Heat map representation of the effects of PEG (18%) and BABA treatments (0.5 mM, 1 mM and 1.5 mM) on the genes expression in the leaves of Badii. White and red indicate higher and lower expression values, respectively. Intensity of the colors is proportional to the absolute value of log2 of the fold difference in expression (color figure online)
Fig. 5.
Heat map representation of the effects of PEG (18%) and BABA treatments (0.5 mM, 1 mM and 1.5 mM) on the genes expression in the roots of Badii. White and red indicate higher and lower expression values, respectively. Intensity of the colors is proportional to the absolute value of log2 of the fold difference in expression (color figure online)
Discussion
Drought stress is one of the major environmental stresses that can severely limit faba bean plant growth and productivity. Several studies reported that exogenous application of plant growth regulators (PGRs) including γ-aminobutyric acid (GABA) and β-aminobutyric acid (BABA) might play an important role in regulating growth and development as well as in enhancing abiotic and biotic stress tolerance in plants (Ton et al. 2009; Yong et al. 2017). BABA induces drought tolerance and constitute an effective strategy to mitigate drought damage in plants like Arabidopsis thaliana (Jakab et al. 2005), Brassica napus (Mohamadi et al. 2017), Vigna radiata (Jisha and Puthur 2016a, b), Linum usitatissimum (Quero et al. 2015), Solanum tuberosum (Sos-Hegedus et al. 2014) and Zea mays (Shaw et al. 2016). All these reports support the hypothesis that BABA exhibits a protective function against drought and other abiotic stresses. A significant reduction in A, gs, Ci, and E under drought stress had been reported earlier in a number of crops including faba bean (Abid et al. 2017). Exogenous GABA application improved maize seedlings growth by improving net photosynthetic rate and gas exchange capacities (Li et al. 2016a, b). More recently, Wang et al. (2017a, b) suggested that exogenous GABA application alleviated salt damage and improved maize seedlings growth by improving photosynthesis and chlorophyll fluorescence parameters. In the present investigation, drought stress caused a marked reduction in A, gs, Ci, and E in Badii. However, exogenous application of BABA mitigated the adverse effects of drought on photosynthesis especially at 1 mM. The PEG + 1 mM BABA-treated plants were superior to the PEG, PEG + 0.5 mM BABA and PEG + 1.5 mM BABA-treated plants with respect to these gas exchange attributes. The improvement of gas exchange parameters in Badii plants under 1 mM BABA treatment could be due to the maintenance of cell turgor and the regulation of various physio-biochemical processes. Previous research showed that after 7 days of dehydration, BABA-treated apple trees demonstrated higher E, gs and A compared to untreated trees (Workoski et al. 2011). However, according to Jakab et al. (2005) application of BABA may induce drought and salt stress tolerance in Arabidopsis thaliana but not directly affect gas exchange parameters. The result obtained in the present investigation agrees with the previous report of Du et al. (2012) which suggested that exogenous application of BABA through the rooting medium was more effective compared to the other modes of application in alleviating the adverse effects of drought on different gas exchange attributes. A significant decline in RWC was clearly observed in plants grown only under water stress while BABA treated plants maintained significantly higher RWC under drought stress than non-treated plants. The present study revealed that exogenous application of BABA at 1 mM via rooting medium helped Vicia faba plants to maintain the RWC and hence to mitigate the adverse effects of drought stress. These data indicates that BABA has influence on the osmotic potential suggesting that the observed increase in RWC may be due to a net accumulation of solute content. These findings are in line with an earlier report on apple (Tworkoski et al. 2011).
The accumulation of soluble sugars and proline as osmoprotectants in response to drought is well documented (Pomortsev et al. 2018). In other crop species, osmotic adjustment of leaf is strongly correlated with drought resistance. Both analyzed osmoprotectants increased compared to the controls. Moreover, BABA application resulted in a significant increase in these osmoprotectants contents in PEG + 1 mM BABA and PEG + 1.5 mM BABA-treated plants relative to untreated plants. Interestingly, PEG + 1 mM BABA-treated plants exhibited higher osmoprotectants accumulation. The present findings indicated that BABA promoted soluble sugars and proline accumulations in faba bean plants under drought stress. The increase of both osmoprotectants in the BABA-treated plants could facilitate the maintenance of favorable turgor pressure resulting in a better osmotic adjustment which ultimately promotes the expansion of cells and thus promoting the growth. This effect of BABA corroborated with the results of previous studies in Vigna radiata where exogenous BABA increased soluble sugars and proline contents under NaCl/PEG-stressed conditions (Jisha and Puthur 2016a, b). The authors proposed the positive effects of exogenous BABA on alleviating the oxidative stress associate with osmolytes accumulation.
Du et al. (2012) found that BABA reduced oxidative damage in drought-stressed wheat. They reported that under drought pressure ROS (O2– and H2O2) accumulation in BABA-treated wheat was lower than that without BABA application. The results in the present study disclosed that H2O2 contents were significantly affected by PEG and BABA concentrations. Actually, the leaf H2O2 content increased significantly under drought stress relative to the controls. However, BABA-treated plants showed significant reduction in H2O2 content as compared to non-treated under drought stress, indicating that BABA reduced the level of ROS in faba bean (cv. Badii) under drought. This result is similar to the one previously observed in rapeseed cultivar Madonna (Mohamadi et al. 2017). Interestingly, PEG + 1 mM BABA-treated plants exhibited the lowest H2O2 level, almost similar to the level measured in control plants.
The MDA level, index of lipid peroxidation was higher in PEG, PEG + 0.5 mM BABA-treated plants and PEG + 1.5 mM BABA-treated compared to the controls. Only PEG + 1 mM BABA-treated plants showed reduction of MDA content in comparison to the control plants. Thus, 1 mM BABA treatment could reduce MDA content in drought-stressed faba bean, thereby reducing the membranes oxidative damage. The reduction in MDA content in leaves of BABA-treated plants under abiotic challenge was already reported in V. radiata (Jisha and Puthur 2016a, b), Brassica napus (Mohamadi et al. 2017) and wheat (Du et al. 2012).
PEG-induced drought stress caused steep rise of EL level in leaves of Badii, but exogenous BABA effectively reduced the increase trend of EL. This result is similar to that observed previously by Mohamadi et al. (2017), which reported that progressive drought stress induced significant increase of EL level in leaves of Brassica napus L. (cv. Madonna) but BABA application observably inhibited the rising trend. The data suggest that BABA lead to maintaining the integrity of cellular membrane under drought pressure considered as a part of drought tolerance mechanisms.
Previous studies demonstrated that the application of BABA decreased MDA, O2– and H2O2 contents in drought-stressed plants which exhibited elevated enzymatic activities in antioxidant related enzymes such as glutathione reductase (GR), peroxidase (POX), APX, SOD and CAT compared to untreated controls. Thus BABA application may mitigate the adverse effects of ROS, thereby effectively alleviated drought-caused oxidative damage and improved plant response to drought stress (Du et al. 2012; Jisha and Puthur 2016a, b). In the current study, ROS accumulation was lower in treated plants with BABA (specifically by 1 mM) compared to untreated, which might be attributed to the enhancement of antioxidant enzymes activities (APX, GPX, CAT and SOD) responsible to alleviate the negative effect of ROS and reduce the oxidative damage, which was consistent with the results of Du et al. (2012) in wheat. Those BABA-activated antioxidant enzymes could play key roles in scavenging ROS like H2O2 and reducing membrane lipid peroxidation under drought stress in faba bean. Moreover, exogenous application of BABA significantly improved SOD, APX and POD activities in B. napus seedlings exposed to drought stress (Rajaei and Mohamadi 2013). Also, it has been shown that BABA treatment influenced the nitrogen metabolism of Vigna radiata drought-stressed seedlings through enhancing the activity of nitrate reductase (NR) enzyme (Jisha and Puthur 2016a, b). Moreover, Shaw et al. (2016) found that exogenous BABA can improve drought stress effects in Zea mays by the activation of antioxidant defense systems through modulating glutathione metabolism or increasing the activity of O2– and H2O2-scavenging enzymes such as APX, SOD, GR, dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDAR).
Our findings also suggested that BABA mediated antioxidant defense in Vicia faba at the molecular level through up-regulating multiple genes (VfSOD, VfCAT, VfAPX and VfGST) encoding antioxidant enzymes in response to drought. Interestingly, RT-qPCR analysis showed that the expression pattern of VfSOD, VfCAT and VfAPX involved in antioxidant defense mechanism largely corroborated with biochemical analysis. The study of Shaw et al. (2016) showed that BABA promote the accumulation of genes encoding antioxidant enzymes in maize under drought stress, thereby mediated antioxidant defense in this species. Moreover, these authors revealed the accumulation of various signaling proteins such as transcription factors (including NAC, ERF, WRKY and MYB) playing fundamental role in plant response to abiotic stress. In the current study exogenous BABA significantly increased the accumulation of some selected genes (VfMYB, VfDHN, VfLEA, VfERF, VfNCED, VfWRKY, VfHSP and VfNAC) encoding abscisic acid (ABA)-dependent and ABA-independent signaling indicating that BABA could significantly promote the accumulation of selected genes in leaves and roots of faba bean under drought stress, thereby improving drought tolerance of faba bean. Previous research showed that BABA-treated wheat (Du et al. 2012) displayed higher ABA accumulation compared to non-treated plants under drought, suggesting that BABA-induced tolerance in plants by increasing ABA under drought stressed conditions. In addition, Jakab et al. (2005) demonstrated that BABA-induced water stress tolerance in Arabidopsis is based on elevated ABA accumulation resulting in higher expression of the ABA-dependent RAB-18 and RD-29A genes. In the current study, almost all the genes tested were up-regulated in leaves and roots of BABA-treated plants, suggesting that BABA might act as a signal molecule to regulate the ABA-dependent and independent genes expressions in response to drought stress in Vicia faba. VfNCED, VfLEA, VfDHN, VfNAC, VfMYB and VfWRKY which are ABA-dependent (Agarwal and Jha 2010; Yang et al. 2012; Zhang et al. 2016) were up-regulated; suggesting that ABA may play a crucial role in the BABA enhancement of drought stress tolerance in that crop. Interestingly, exogenous BABA significantly increased the accumulation of genes encoding dehydrins (VfLEA and VfDHN) in leaves and roots of faba bean under drought stress, indicating that BABA-enhanced expression of VfLEA and VfDHN could contribute to improve drought tolerance associated with the maintenance of better membranes stability and water status in the plant (Li et al. 2018). Previous research showed a positive correlation of dehydrins expression with crops tolerance to drought like shown in rice (Kumar et al. 2014). According to these authors, over-expression of OsDhn1 gene improved drought and salt stress tolerance through scavenging reactive oxygen species. Heat shock proteins (HSPs) can play an essential role in protecting plants against stress by reestablishing normal protein conformation and thus cellular homeostasis (Wang et al. 2004). Previous studies demonstrated that overexpression of certain HSPs improved rice drought tolerance (Xiang et al. 2018). Current results showed that VfHSP, known as molecular chaperones, was up-regulated by BABA under drought stress, indicating regulatory roles of BABA for activation of HSPs. The data of current study demonstrated that exogenously applied BABA up-regulated four transcription factors (VfERF, VfNAC, VfWRKY and VfMYB) under drought stress, indicating their positive association with drought tolerance in faba bean. The results of Jin et al. (2018) confirmed that the overexpression of an OsERF101 factor could significantly enhance drought tolerance of Oryza sativa. Gao et al. (2018) also found that transgenic wheat overexpressing TaWRKY2 significantly increased its drought tolerance. In addition, transgenic Arabidopsis lines transformed with maize MYB48 gene displayed drought tolerance (Wang et al. 2017a, b). In rice, transgenic seedlings overexpressing OsNAC14 exhibited significantly improved drought tolerance (Shim et al. 2018). Exogenous application of GABA in creeping bentgrass (Agrostis stolonifera) up-regulated some drought stress-related genes including HSP90, DHN3, MYB13 and WRKY75 and genes encoding antioxidant enzymes (SOD, CAT, POD, APX, MDHAR, DHAR and GR) under drought and heat stresses (Li et al. 2018). In the current study, the exogenous application of BABA promoted the up-regulation of selected drought stress-related gene transcripts suggesting that these genes may be involved in BABA-improved drought tolerance and could be used as key candidate genes for improving drought tolerance in this species.
The results from this study demonstrate that exogenous application of BABA was effective in mitigating physiological response of drought stress damage and the effects were more pronounced at the application rate of 1 mM than 0.5 mM and 1.5 mM. Indeed, a significant up-regulation of genes related to the abscisic acid (ABA) pathway such as VfNCED (a key gene involved in ABA biosynthesis) and VfMYB (involved in both stomatal and non-stomatal limitations of photosynthesis) has been shown in faba bean plants treated with PEG + 1 mM BABA which could lead to decrease stomatal conductance in order to minimize water loss. Therefore, stomatal closure is followed by reduced photosynthesis, the rate of transpiration under water deficit conditions which reduces the inflow of CO2 into the leaves results in the accumulation of ROS (H2O2) which is the lower in treated plants with PEG + 1 mM BABA compared to other treatments due to the enhancement of antioxidant enzymes activities (SOD, CAT, APX and GPX) responsible to mitigate the negative effect of ROS and reducing the oxidative. Osmotic adjustment in plants treated with PEG + 1 mM BABA is also could be provided by accumulation of osmoprotectants such as proline and soluble sugars and overexpression of VfLEA and VfDHN proteins which help the treated plants to maintain a state of osmotic balance and turgor in the cell, cell respiration and contributes to membrane stabilization, which ensures the increase in required energy.
Conclusion
In summary, we can conclude that exogenous application of the appropriate concentration of BABA (1 mM) could be an effective technique to alleviate adverse effects of drought stress on faba bean through the prevention of ROS accumulation by activating the plant antioxidant system and improving osmotic adjustment. Indeed, exogenous BABA increased RWC and improved photosynthetic capacity through the improvement of A, gs and E in leaves of this legume. In addition, exogenous application of BABA improved proline and soluble sugars accumulation, but reduced MDA and H2O2 synthesis. BABA could also increase antioxidant enzyme activities (CAT, SOD, APX and GPX) and genes transcript levels associated with better maintenance of cell membrane stability in faba bean plants under drought stress. Moreover, in the present study, leaves and roots of BABA-treated plants exhibited significantly higher VfGST, VfMYB, VfDHN, VfLEA, VfERF, VfNCED, VfWRKY, VfHSP and VfNAC transcript levels compared to the non-treated during drought stress, suggesting that these selected genes may be involved in BABA-improved drought tolerance in Vicia faba.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abid G, Hessini K, Aouida M, Aroua I, Baudoin JP, Muhovski Y, Mergeai G, Sassi K, Machraoui M, Souissi F, Jebara M. Agro-physiological and biochemical responses of faba bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress. Biotechnol Agron Soc Environ. 2017;21:146–159. [Google Scholar]
- Agarwal PK, Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signaling. Biol Plant. 2010;54:201–212. [Google Scholar]
- Akter N, Rafiqul Islam M, Abdul Karim M, Hossain T. Alleviation of drought stress in maize by exogenous application of gibberellic acid and cytokinin. J Crop Sci Biotechnol. 2014;17:41–48. [Google Scholar]
- Amri M, Abbes Z, Trabelsi I, Omri N, Allagui MB, Najar A, Kumari S, Selmi H, Mediouni J, Ben Saleh H, Maalouf F, Halila MH, Kharrat M (2016) Achievements of the national faba bean (Vicia faba L.) breeding program in Tunisia. In: International conference on pulses, Marrakesh, Morocco, 18–20 April, p 96
- An Y, Zhou P, Liang J. Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 2014;65:274–286. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992;98:1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Del Longo OT, Gonzalez CA, Pastori GM, Trippi VS. Antioxidant defenses under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol. 1993;34:1023–1028. [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlation with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:96–101. [Google Scholar]
- Du YL, Wang ZY, Fan JW, Turner NC, Wang T, Li FM. β-Aminobutyric acid increases abscisic acid accumulation and desiccation tolerance and decreases water use but fails to improve grain yield in two spring wheat cultivars under soil drying. J Exp Bot. 2012;63:4849–4860. doi: 10.1093/jxb/ers164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167–168. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- EL Sabagh A, Hossain A, Islam MS, Barutcular C, Fahad S, Ratnasekera D, Kumar N, Meena RS, Vera P, Saneoka H. Role of osmoprotectants and soil amendments for sustainable soybean (Glycine max L.) production under drought condition: a review. J Exp Biol Agric Sci. 2018;6:32–41. [Google Scholar]
- Gao H, Wang Y, Xu P, Zhang Z. Overexpression of a WRKY transcription factor TaWRKY2 enhances drought stress tolerance in transgenic wheat. Front Plant Sci. 2018;9:997. doi: 10.3389/fpls.2018.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González L, González-Vilar M. Determination of relative water content. In: Reigosa Roger MJ, editor. Handbook of plant ecophysiology techniques. Dordrecht: Springer; 2001. pp. 207–212. [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plant without soil. Calif Agric Exp Stn Circ. 1950;342:32. [Google Scholar]
- Hussain S, Saleem MF, Iqbal J, Ibrahim M, Atta S, Ahmed T, Rehmani MIA. Exogenous application of abscisic acid may improve the growth and yield of sunflower hybrids under drought stress. Pak J Agric Sci. 2014;51:49–58. [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Métraux JP, Mauch-Mani B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol. 2005;139:267–274. doi: 10.1104/pp.105.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Pan Y, Zheng X, Cheng X, Liu M, Ma H, Ge X. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol Biol. 2018;98:51–65. doi: 10.1007/s11103-018-0762-5. [DOI] [PubMed] [Google Scholar]
- Jisha KC, Puthur JT. Seed priming with beta-amino butyric acid improves abiotic stress tolerance in rice seedlings. Rice Sci. 2016;23:242–254. [Google Scholar]
- Jisha KC, Puthur JT. Seed priming with BABA (β-aminobutyric acid): a cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma. 2016;253:227–289. doi: 10.1007/s00709-015-0804-7. [DOI] [PubMed] [Google Scholar]
- Kabbadj A, Makoudi B, Mouradi M, Pauly N, Frendo P, Ghoulam C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE. 2017 doi: 10.1371/journal.pone.0190284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Lee SC, Kim JY, Kim SJ, Aye SS, Kim SR. Over-expression of dehydrin gene, OsDhn1, improves drought and salt stress tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.) J Plant Biol. 2014;57:383–393. [Google Scholar]
- Li W, Liu J, Ashraf U, Li G, Li Y, Lu W, Gao L, Han F, Hu J. Exogenous γ-aminobutyric Acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front Plant Sci. 2016;7:919. doi: 10.3389/fpls.2016.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yu J, Peng Y, Huang B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera) Sci Rep. 2016;6:30338. doi: 10.1038/srep30338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Peng Y, Huang B. Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera) Int J Mol Sci. 2018;19:1623. doi: 10.3390/ijms19061623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadi N, Baghizadeh A, Saadatmand S, Asrar Z. Alleviation of oxidative stress induced by drought stress through priming by β-aminobutyric acid (BABA) in rapeseed (Brassica napus L.) plants. Iran J Plant Physiol. 2017;7:2203–2210. [Google Scholar]
- Murray MB, Cape JN, Flower D. Quantification of frost damage in plant tissues by rates of electrolyte leakage. New Phytol. 1989;113:307–311. doi: 10.1111/j.1469-8137.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Ouji A, Naouari M, Mouelhi M, Ben Younes M. Yield and yield components of faba bean (Vicia faba L.) as influenced by supplemental irrigation under semi-arid region of Tunisia. World J Agric Res. 2017;5:52–57. [Google Scholar]
- Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of norway spruce Picea abies L. Plant Physiol. 1994;106:53–60. doi: 10.1104/pp.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomortsev A, Dorofeev N, Sokolova L, Zorina S, Katysheva N. Physiological and biochemical response of winter triticale crowns at different soil moisture levels. Pak J Biol Sci. 2018;21:387–393. doi: 10.3923/pjbs.2018.387.393. [DOI] [PubMed] [Google Scholar]
- Quero A, Fliniaux O, Elboutachfaiti R, Petit E, Guillot X, Hawkins S, Courtois J, Mesnard F. β-aminobutyric acid increases drought tolerance and reorganizes solute content and water homeostasis in flax (Linum usitatissimum) Metabolomics. 2015;11:1363–1375. [Google Scholar]
- Rajaei P, Mohamadi N. Effect of beta-aminobutyric acid (BABA) on enzymatic and non-enzymatic antioxidants of Brassica napus L. under drought. Int J Biosci. 2013;3:41–47. [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shaw AK, Bhardwaj PK, Ghosh S, Roy S, Saha S, Sherpa AR, Saha SK, Hossain Z. β-aminobutyric acid mediated drought stress alleviation in maize (Zea mays L.) Environ Sci Pollut Res. 2016;23:2437–2453. doi: 10.1007/s11356-015-5445-z. [DOI] [PubMed] [Google Scholar]
- Shim JS, Oh N, Chung PJ, Kim YS, Choi YD, Kim JK. Overexpression of OsNAC14 improves drought tolerance in rice. Front Plant Sci. 2018;9:310. doi: 10.3389/fpls.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS, Bukhari NA. Response of different genotypes of faba bean plant to drought stress. Int J Mol Sci. 2015;16:10214–10227. doi: 10.3390/ijms160510214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos-Hegedus A, Juhasz Z, Poor P, Kondrak M, Antal F, Tari I, Mauch-Mani B, Banfalvi Z. Soil drench treatment with ß-aminobutyric acid increases drought tolerance of potato. PLoS ONE. 2014 doi: 10.1371/journal.pone.0114297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux JP, Mauch-Mani B. Dissecting the β-aminobutyric acid induced priming phenomenon in Arabidopsis. Plant Cell. 2005;17:987–999. doi: 10.1105/tpc.104.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, van der Ent S, Van Hulten M, Pozo M, van Oosten V, van Loon LC, Mauch-Mani B, Turlings TCJ, Pieterse CMJ. Priming as a mechanism behind induced resistance against pathogens, insects and abiotic stress. IOBC-WPRS Bull. 2009;44:3–13. [Google Scholar]
- Tworkoski T, Wisniewski M, Artlip T. Application of BABA and s-ABA for drought resistance in apple. J Appl Hortic. 2011;13:85–90. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Vijayakumari K, Jisha KC, Puthur JT. GABA/BABA priming: a means for enhancing abiotic stress tolerance potential of plants with less energy investments on defence cache. Acta Physiol Plant. 2016;38:230–244. [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:245–251. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S. γ-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep. 2017;7:43609. doi: 10.1038/srep43609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Liu M, Bo C, Wang X, Ma Q, Cheng B, Cai R. Overexpression of a maize MYB48 gene confers drought tolerance in transgenic Arabidopsis plants. J Plant Biol. 2017;60:612–621. [Google Scholar]
- Xiang J, Chen X, Hu W, Xiang Y, Yan M, Wang J. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 2018;37:1585–1595. doi: 10.1007/s00299-018-2331-4. [DOI] [PubMed] [Google Scholar]
- Xiao W, Fu-lai L, Dong J. Priming: a promising strategy for crop production in response to future climate. J Integr Agric. 2017;16:60345–60352. [Google Scholar]
- Yang ZB, Eticha D, Albacete A, Rao IM, Roitsch T, Horst WJ. Physiological and molecular analysis of the interaction between aluminium toxicity and drought stress in common bean (Phaseolus vulgaris) J Exp Bot. 2012;63:3109–3125. doi: 10.1093/jxb/ers038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong B, Xie H, Li Z, Li YP, Zhang Y, Nie G, Zhang XQ, Ma X, Huang LK, Yan YH, Peng Y. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol. 2017;8:1107. doi: 10.3389/fphys.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhong M, Shu S, Du N, Sun J, Guo S. Proteomic and physiological analyses reveal putrescine responses in roots of cucumber stressed by NaCl. Front Plant Sci. 2016;7:1035. doi: 10.3389/fpls.2016.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu H, Yang X, Li Q, Ling J, Wang H, Gu X, Huang S, Jiang W. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol Biochem. 2016;108:478–487. doi: 10.1016/j.plaphy.2016.08.013. [DOI] [PubMed] [Google Scholar]