Abstract
Heat stress is a major constraint of yield in grain legumes including peas. Increasing global warming and human population now urge to develop climate resilient varieties. The present experiment was conducted over 2 years to evaluate the heat tolerance of 211 pea genotypes. In the present study, the field pea genotypes showed a wide variation for reproductive stage heat stress (RSHS) quantitative traits. Significant positive correlations were found between no. of seeds per plant and no. of pods per plant; seed diameter (mm) and 25-seed weight (g) in heat tolerant as well as heat susceptible genotypes. Principal component analysis revealed two major principal components contributed approximately 91% of total variations and heat tolerant and susceptible genotypes separately formed two major clusters. Stepwise multiple regression analysis revealed that no. of seeds per plant was the best predictor for no. of pods per plant. On the basis of four RSHS traits, the most prominent heat tolerant pea genotypes identified in the present study JP-625, IARI-2877, PMR-38 II, EC-318760, EC-328758 and IARI-2904 would better combat RSHS and provide yield stability under changing climatic conditions.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00803-4) contains supplementary material, which is available to authorized users.
Keywords: Seed diameter, 25-Seed weight, Plant type, Correlation, Climate resilience
Introduction
By the end of the twenty-first century, the earth’s climate is predicted to warm by an average of 2–4 °C (IPCC 2013). It has also been predicted that yield of most of the crop would decline at temperature much above 30 °C (Schlenker and Roberts 2009). Adoption of agronomic practices can buffer the crops against warmer and dry environment (Hobbs et al. 2008) but it is always challenging to produce more food for an ever-increasing global population. Heat stress affect many physiological processes of the plant cell including increased membrane permeability, inactivation of enzymes in chloroplast and mitochondria, inhibition of protein synthesis, protein degradation and loss of membrane integrity (Ashraf and Harris 2005). Such detrimental effects may eventually lead to starvation, inhibition of growth, reduced ion flux, production of toxic compounds and reactive oxygen species (ROS) (Seginer 1997; Dias et al. 2011).
Heat stress during germination, vegetative growth and reproductive growth have been studied in various crops such as wheat (Sharma et al. 2005), rice (Weerakoon et al. 2008), chickpea (Devasirvatham and Tan 2018), faba bean (Bishop et al. 2016) and cotton (Cottee et al. 2010), while only limited research has been conducted in pea (Wang et al. 2006). In general, the cool season food legumes (peas, lentil, chickpea and faba bean) are more sensitive to heat than warm season legumes (cowpea, soybean, groundnut, pigeonpea, and mung bean). Among cool season legumes, chickpea have best heat tolerance followed by faba bean, lentil and pea (Wery et al. 1993). Further, heat stress during reproductive stage is more detrimental than at vegetative stage in cool season legumes (Farooq et al. 2017). Reproductive stage heat stress (RSHS) in food legumes showed reduced yield, pod and seed set when exposed to day temperatures of a certain limit i.e., 32 °C during floral development in common bean (Porch and Jahn 2001), 33 °C in cowpea (Ahmed et al. 1992), 28 °C in faba bean (Bishop et al. 2016) and 30 °C in pea (McDonald and Paulsen 1997). So, RSHS is now becoming an important limiting factor in the production of a number of food legumes including peas in West Asia, North Africa and Indian subcontinent.
Pea (Pisum sativum L., 2n = 14) belonging to family Fabaceae is an important food legume crop with a rich history of genetic research. Pea is one of the six major pulse cultivated globally and is the second highest yielding legume in the world after common bean (Phaseolus vulgaris L.). In India, pea as food legume is grown in about 0.83 m ha area with annual production of 0.73 m tones (Siebert and Ewert 2014). RSHS is increasingly becoming a serious constraint to pea production in India because of late sowing due to late harvest of rice in rice-pea/mung/vegetables cropping system, shortening of cool winter periods and sudden increase in temperature at the time of flowering and pod setting in late February-April. Pea cultivation with improved heat tolerance is now more realized to cut down the yield losses in those areas where the crop is exposed to high temperatures at reproductive stage. Heat tolerant pea cultivars will provide a better adaptability for different sowing dates and enhance opportunities for expending the pea cultivation in new areas where rice crop is often harvested late. Little research has been published on the cool season food legume crops, specifically the mechanisms by which high temperature is tolerated or avoided. For successful breeding for heat tolerance, it is necessary not only to identify sources of tolerance but also to have a simple, rapid, and efficient selection scheme for consistently detecting differences in heat tolerance among a large number of genotypes (Adams 1967). Keeping the aforesaid in view, the present work has been undertaken to screen and to characterize pea genotypes with enhanced RSHS tolerance.
Materials and methods
Planting material
A set of two hundred eleven pea genotypes were identified for the experiment which are being made available by the Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India. The genotypes were maintained under AICRP (All India Coordinated Research Project) on MULLaRP (Mung, Urd, Lentil, Lathyrus, Rajmash and Peas), ICAR, New Delhi, India. The test genotypes consisted of adopted exotic collections, advanced breeding lines and known varieties of fieldpeas.
Experimental conditions and heat screening
A set of 211 pea genotypes were heat screened during crop seasons 2014–15 and 2015–16 at Polyhouse facility, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi. The experimental site is situated in South-Eastern part of Varanasi at 25° 15′ North latitude and 83° 03′ East longitude at an elevation of 129.23 m above the mean sea level. The soil of this region i.e., middle Indo-Gangetic region is sandy loam with pH of ~ 7.7 and electrical conductivity of ~ 0.21 dS/m. Every genotype was grown in four earthen pots, filled with local soil, each pot containing five plants. Two pots representing one replication thus, two replications were maintained. The maximum and minimum temperatures ranged 15 to 38 °C respectively in the polyhouse and 75% relative humidity were maintained throughout the experiment. The polyhouse facility was used in particular to conduct the present investigation as it mimics the greenhouse effect with a control on temperature and moisture during the crop growth and development.
Five plants were randomly selected from each replication for observation before bud initiation. Data were recorded on four quantitative traits viz., number of pods plant, number of seeds per pod, 25-seeds weight (g) and seed diameter (mm) which are most severely affected by the heat stress during reproductive stage of the pea crop designated as RSHS traits. Seed diameter was recorded in millimeter (mm) using Vernier calipers.
Data analysis
Quantitative data were analyzed using the SPSS16.0 software to perform univariate analysis (mean, standard error and coefficient of variation %). Correlation coefficients among different parameters were calculated using PROC CORR function of SAS (version 9.2; SAS Institute Inc. Cary NC 2010). Principal component analysis was performed by PROC PRIN COMP of SAS. In addition, stepwise multiple regression analysis was conducted with number of pods per plant as response variable, and the other three heat screening quantitative traits, i.e. number of seeds per plant, 25-seed weight (g) and seed diameter (mm) as predictor variables.
Results
Evaluation of heat tolerance
In the present study, a wide range of variation was found in four RSHS quantitative traits. Pea genotypes yielding 15 pods per plant were initially identified as heat tolerant. Out of 211 genotypes, 67 genotypes showed no pod setting whereas, 108 genotypes ranged 1–14 pods per plant and grouped as heat susceptible genotypes. Only 36 genotypes were found to have 15 or more pods per plant and categorized as heat tolerant genotypes (Table 1). At time of flowering and pod initiation, bud drop, premature pod drops and wilting of leaflets were observed in most of the genotypes (Fig. 1). Number of pods per plant ranged from 1–45 with an average of 10.29 pods and no. of seeds per plant ranged from 1 to 197 with an average of 32 seeds among all the 144 pea genotypes (Table 1). Coefficient of variation % was comparatively higher among 108 heat susceptible and 144 overall genotypes than 36 heat tolerant pea genotypes for four RSHS traits studied. However, average 25-seed weight (g) and seed diameter (mm) were comparable among three categories of pea genotypes (Table 1).
Table 1.
Mean values of four RSHS quantitative traits in pea screened under polyhouse conditions (excluding 67 pea genotypes showed no pod setting)
| Category of genotype | Parameter | RSHS quantitative trait | |||
|---|---|---|---|---|---|
| No. of pods per plant | No. of seeds per plant | 25-Seed weight (g) | Seed diameter (mm) | ||
| Heat susceptible (108)* | Range | 1–14 | 1–58 | 1.5–7.0 | 45.30–84.60 |
| Mean | 6.15 | 19.31 | 4.13 | 66.17 | |
| SE | 0.34 | 1.17 | 0.09 | 0.70 | |
| CV (%) | 56.99 | 63.08 | 23.21 | 10.99 | |
| Heat tolerant (36) | Range | 15–45 | 35–197 | 3.5–6.7 | 53.10–80.70 |
| Mean | 22.61 | 69.89 | 4.6 | 67.38 | |
| SE | 1.29 | 5.44 | 0.13 | 0.92 | |
| CV (%) | 34.16 | 46.69 | 16.30 | 8.18 | |
| Overall (144) | Range | 1–45 | 1–197 | 1.5–7.0 | 45.30–84.60 |
| Mean | 10.29 | 32.08 | 4.26 | 66.43 | |
| SE | 0.73 | 2.47 | 0.08 | 0.58 | |
| CV (%) | 84.63 | 91.57 | 22.03 | 10.39 | |
*Value within parentheses indicated the number of genotypes in each category
Fig. 1.

Screening for heat tolerance in pea under polyhouse conditions. a Pea plants encircled showed the effect of heat stress b Effect of heat stress on leaflets, flowers and pods of pea
Estimates of correlation coefficients among four heat tolerance quantitative traits are presented in Table 2. Number of seeds per plant was significantly associated with number of pods per plant for all the three categories of genotypes i.e., heat susceptible, heat tolerant and overall (r = 0.831–0.936, p < 0.0001). 25-seed weight (g) showed non-significant but positive association with no. of pods per plant and no. of seeds per plant among heat susceptible pea genotypes whereas, heat tolerant pea genotypes showed highly significant negative association. It implies that, as the seed weight (g) increases in heat tolerant pea genotypes, no. of pods as well as no. of seeds per plant decreases. Association of seed diameter (mm) with 25-seed weight (g) was significant and positive among all the three categories of genotypes.
Table 2.
Estimates of correlation coefficients among four RSHS quantitative traits among 144 pea genotypes (excluding 67 pea genotypes showed no pod setting)
| RSHS quantitative trait | Category of genotypes | No. of pods per plant | No. of seeds per plant | 25-Seed weight (g) |
|---|---|---|---|---|
| No. of seeds per plant | Heat susceptible | 0.894*** | ||
| Heat tolerant | 0.831*** | |||
| Overall | 0.936*** | |||
| 25-Seed weight (g) | Heat susceptible | 0.077 | 0.092 | |
| Heat tolerant | − 0.375*** | − 0.257** | ||
| Overall | 0.140*** | 0.138** | ||
| Seed diameter (mm) | Heat susceptible | 0.107 | 0.085 | 0.725*** |
| Heat tolerant | − 0.054 | − 0.086 | 0.647*** | |
| Overall | 0.087 | 0.066 | 0.714*** |
**, ***Significant at p ≤ 0.01 and 0.001 level of significance, respectively
Regression and principal component analysis of heat tolerance
Stepwise multiple regression analysis (MRA) using no. of pods per plant (as dependent variable) has been carried out with three other RSHS quantitative traits as independent variables among three categories of pea genotypes. MRA results revealed statistically highly significant and positive association of no. of seeds per plant (independent variable) with no. of pods per plant (dependent variable) among three categories of pea genotypes (Table 3). Proportion of phenotypic variation (R2) explained by no. of seeds per plant was also very high to estimate no. of pods per plant among three categories of pea genotypes (R2 ranged from 69.0 to 87.7%). The significance of this association was also revealed by low standard errors and high β coefficients and t values (p < 0.0001) (Table 3).
Table 3.
Association of dependent variable with independent variable (s) and coefficients as revealed by stepwise multiple regression analysis (MRA)
| Independent variable | Category of genotypes | r | R2 | R2 change | F change | Standard error | Standardized beta (β) coefficient | t value | p value |
|---|---|---|---|---|---|---|---|---|---|
| No. of seeds per plant | Heat susceptible | 0.894 | 0.799 | 0.798 | 422.61 | 0.013 | 0.894 | 20.56 | 0.0001 |
| Heat tolerant | 0.831 | 0.691 | 0.681 | 75.86 | 0.023 | 0.831 | 8.71 | 0.0001 | |
| Overall | 0.936 | 0.877 | 0.876 | 995.62 | 0.009 | 0.936 | 31.55 | 0.0001 |
Dependent variable = No. of pods per plant. Two independent variables viz., 25-seed weight (g) and Seed diameter (mm) are excluded by stepwise MRA (Criteria: Probability-of-F-to-enter ≤ 0.050, Probability-of-F-to-remove ≥ 0.100)
Relationships between different quantitative traits were revealed by Principal Component Analysis (PCA). For each trait, factor loading of more than 0.92 was considered as significant (p < 0.001). In the present study, the two principal components (PCs) exhibited more than 1.0 Eigen value and explained about 91.31% of total variation among the 144 pea genotypes for four RSHS quantitative traits (Table 4). First principal component (PC1) was the most important and explained about 51.72% of total variance. Important loading factors for PC1 were no. of pods and no. of seeds per plant with loading value of 0.982 and 0.983, respectively. The second PC contributed 39.59% of the variation among the 144 pea genotypes. PC2 was positively defined by 25-seed weight (g) and seed diameter (mm). It was important to note that all the four RSHS traits positively influenced the PCs variation (Table 4).
Table 4.
Eigen value, cumulative variance and scores of the two major factors obtained from the PCA of four RSHS quantitative traits among 144 pea genotypes (excluding 67 pea genotypes showed no pod setting)
| Variable | PC 1 | PC 2 |
|---|---|---|
| Eigen value | 2.069 | 1.584 |
| Cumulative variance (%) | 51.72 | 91.31 |
| No. of pods per plant | 0.982*** | 0.065 |
| No. of seeds per plant | 0.983*** | 0.051 |
| 25-Seed weight (g) | 0.093 | 0.921*** |
| Seed diameter (mm) | 0.017 | 0.927*** |
Rotation method: Varimax with Kaiser normalization
***Significant at p < 0.001 (Significant factor loading was observed above 0.92)
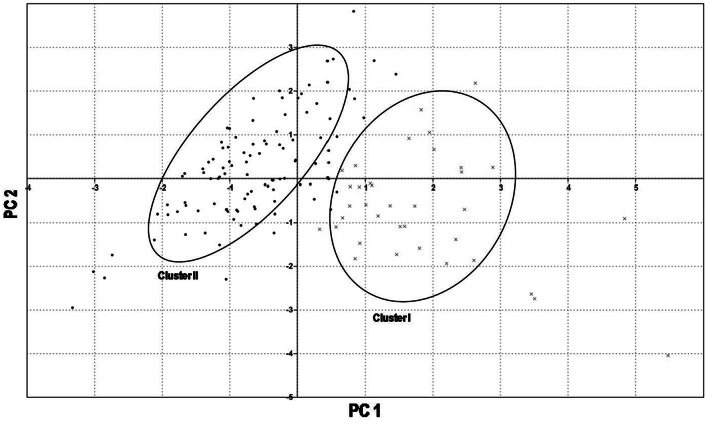
On the basis of projection of the principle components (PCs), 144 pea genotypes were grouped into two major clusters (Fig. 2). These two major clusters i.e., Cluster I and Cluster II were belonging to heat tolerant (≥ 15 pods per plant) and heat susceptible (≤ 14 pods per plant) pea genotypes, respectively. There is a marked difference in the no. of pods and no. of seeds per plant in heat tolerant and susceptible genotypes. Mean no. of pods per plant and no. of seeds per plant (22.61 and 69.89) of heat tolerant genotypes was more than three times higher than heat susceptible genotypes (6.15 and 19.31). But, mean 25-seed weight (g) and seed diameter (mm) are almost comparable among two clusters.
Fig. 2.
Clustering of the 144 pea genotypes on the basis of four RSHS quantitative traits in principal component analysis (PCA). Black dot (•) denotes heat susceptible genotypes (≤ 14 pods per plant) and cross (×) mark denotes heat tolerant genotypes (≥ 15 pods per plant)
Discussion
Under increasing global temperature, grain legumes will play a major role as an important component of crop rotation in different agricultural systems (Farooq et al. 2017). Heat stress during the reproductive stage affects pollen viability, fertilization, pod set and seed development leading to flowers and pods drop and substantial losses in grain yield. Heat stress often leads to soil moisture deficit during reproductive growth stages of grain legumes thus predisposes them to necrotrophic pathogens such as Rhizoctonia bataticola causing dry root rot disease (Gaur et al. 2015). In legumes, selection for heat tolerance can be made effective either by planting the crop at high-temperature hot spot or under late sown conditions and selecting the tolerant plants on the basis of number of filled pods per plant and seed yield (Gaur et al. 2015). Thus, the present study screened 211 pea genotypes for reproductive stage heat stress under polyhouse conditions. Out of 211, 67 genotypes have shown no pod setting and rest 144 genotypes were grouped as 36 heat tolerant and 108 heat susceptible genotypes depending upon the number of pods per plant. Comparative study of heat susceptible and tolerant genotypes (on the basis of four RSHS quantitative traits), showed that there is ample scope for the selection and subsequent improvement for heat tolerance in pea. A drastic reduction in seed size (mm) was observed among heat susceptible genotypes as compared to heat tolerant genotypes. Kumar et al. (2016) have also reported a severe reduction in lentil seed size (ranged from 2.4 to 67.2%) under heat stress conditions.
The estimates of correlation coefficients showed ambiguity among the association of different RSHS quantitative traits within three categories of pea genotypes e.g., 25-seed weight (g) is negatively associated with no. of seeds and no. of pods per plant however, these two traits were positively associated in heat susceptible and overall genotypes. The results obtained from heat tolerant pea genotypes are more understandable and will be considered for conclusive association among different RSHS traits in the present study. The discrepancy in the correlation estimates may be subjected to large variation found in heat susceptible and overall genotypes as compared to heat tolerant pea genotypes. Compensation of yield contributing traits has already being reported in a number of crop species e.g., Faba bean (Bishop et al. 2016), Arabidopsis thaliana (Creissen et al. 2013), Chickpea (Gan et al. 2003), Corn (Haag et al. 2017) etc. In the present study, allocation of photosynthetic products to increase seed weight resulted in decreasing the no. of pods and seeds per plant among heat tolerant pea genotypes. However, significant positive correlation between 100-seed weight (g) and no. of pods per plant is reported in peas (Kujur et al. 2014; Mohapatra et al. 2017).
It is worth to mention that, plant types have no significant effect on heat stress tolerance in the present study, as most of the pea genotypes are belonging to normal foliaged plant type (data not shown). Some of the semi-leafless plant type genotypes (e.g., VL-40, KPMR-615, DDR-61, KPMR-557 etc.) are grouped under heat susceptible category while some are belonging to heat tolerant category (e.g., HUDP-25, IPF-400, HFP-4, DDR-56 etc.) (Supplementary Table 1). However, semi-leafless plant type has been considered as ideal plant type for better yield and lodging resistance in pea (Singh and Srivastava 2015). So, more elaborate experiments need to be conducted to envisage the effect of plant type on heat stress reaction.
In the present study, the field pea genotypes showed a wide variation for RSHS quantitative traits. Significant positive correlations were found between no. of seeds per plant and no. of pods per plant in heat tolerant as well as heat susceptible genotypes. However, significant negative correlation of 25-seed weight (g) was found with no. of pods and no. of seeds per plant in heat tolerant genotypes. The anomalies in interrelationships between different RSHS traits among heat tolerant and susceptible groups may be attributed to the selectable compensation between different yield traits under stress conditions. Principal Component Analysis revealed two major principal components contributed approximately 91% of total variations among 144 pea genotypes. Stepwise multiple regression analysis revealed that no. of seeds per plant was the best predictor for no. of pods per plant. There is a drastic yield loss under heat stress conditions and it need to improve the heat stress resilience of peas. On the basis of four RSHS traits, the most prominent heat tolerant pea genotypes identified in the present study are JP-625, IARI-2877, PMR-38 II, EC-318760, EC-328758 and IARI-2904 (Supplementary Table 1). These heat tolerant pea genotypes would serve as parental breeding material for pea improvement programs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Authors are thankful to Prof. C. P. Srivastava, Pea Breeder, Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India for providing the pea genotypes for the present work.
Abbreviations
- AICRP
All India Coordinated Research Project
- g
Gram
- mm
Millimeter
- MULLaRP
Mung, Urd, Lentil, Lathyrus, Rajmash and Peas
- MRA
Multiple regression analysis
- PCA
Principal component analysis
- PC
Principal component
- RSHS
Reproductive stage heat stress
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams MW. Basis of yield component compensation in crop plants with special reference to the field bean, Phaseolus vulgaris. Crop Sci. 1967;7:505–510. doi: 10.2135/cropsci1967.0011183X000700050030x. [DOI] [Google Scholar]
- Ahmed FE, Hall AE, DeMason DA. Heat Injury during floral development in Cowpea (Vigna unguiculata, Fabaceae) Am J Bot. 1992;79:784–791. doi: 10.1002/j.1537-2197.1992.tb13655.x. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Abiotic stresses: plant resistance through breeding and molecular approaches. New York: Howarth Press Inc.; 2005. pp. 277–300. [Google Scholar]
- Bishop J, Potts SG, Jones HE. Susceptibility of faba bean (Vicia faba L.) to heat stress during floral development and anthesis. J Agron Crop Sci. 2016;202:508–517. doi: 10.1111/jac.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottee NS, Tan DKY, Bange MP, Cothren JT, Campbell LC. Multilevel determination of heat tolerance in cotton (Gossypium hirsutum L.) under field conditions. Crop Sci. 2010;50:2553–2564. doi: 10.2135/cropsci2010.03.0182. [DOI] [Google Scholar]
- Creissen HE, Jorgensen TH, Brown JKM. Stabilization of yield in plant genotype mixtures through compensation rather than complementation. Ann Bot. 2013;112:1439–1447. doi: 10.1093/aob/mct209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasirvatham V, Tan DKY. Impact of high temperature and drought stresses on chickpea production. Agronomy. 2018;8:145–151. doi: 10.3390/agronomy8080145. [DOI] [Google Scholar]
- Dias CV, Mendes JS, dos Santos AC, Pirovani CP, da Silva GA, Micheli F, Gramacho KP, Hammerstone J, Mazzafera P, de Mattos Cascardo JC. Hydrogen peroxide formation in cacao tissues infected by the hemibiotrophic fungus Moniliophthora perniciosa. Plant Physiol Biochem. 2011;49:917–922. doi: 10.1016/j.plaphy.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Farooq M, Nadeem F, Gogoi N, Ullah A, Alghamdi SS, Nayyar H, Siddique KHM. Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pastor Sci. 2017;68:985–1005. doi: 10.1071/CP17012. [DOI] [Google Scholar]
- FAO Database (2017) Food agriculture organization database. http://www.faostat.fao.org
- Gan YT, Liu PH, Stevenson FC, McDonald CL. Interrelationships among yield components of chickpea in semiarid environments. Can J Plant Sci. 2003;83:759–767. doi: 10.4141/P02-145. [DOI] [Google Scholar]
- Gaur PM, Samineni S, Krishnamurthy L, Kumar S, Ghanem ME, Beebe S, Rao I, Chaturvedi SK, Basu PS, Nayyar H, Jayalakshmi V, Babbar A, Varshney RK. High temperature tolerance in grain legumes. Legume Perspect. 2015;7:23–24. [Google Scholar]
- Haag LA, Holman JD, Ransom J, Roberts T, Maxwell S, Zarnstorff ME, Murray L. Compensation of corn yield components to late-season stand reductions in the central and northern great plains. Agron J. 2017;109:524–531. doi: 10.2134/agronj2015.0523. [DOI] [Google Scholar]
- Hobbs PR, Sayre K, Gupta R. The role of conservation agriculture in sustainable agriculture. Phil Trans R Soc B. 2008;363:543–555. doi: 10.1098/rstb.2007.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . Intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013. pp. 1–27. [Google Scholar]
- Kujur S, Singh AK, Srivastava CP. Multivariate analysis of yield and lodging traits in a diverse collection of pea (Pisum sativum L.) J Food Leg. 2014;27:293–296. [Google Scholar]
- Kumar J, Kant R, Kumar S, Basu PS, Sarker A, Singh NP. Heat tolerance in lentil under field conditions. Leg Genom Genet. 2016;7:1–11. [Google Scholar]
- McDonald GK, Paulsen GM. High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil. 1997;196:47–58. doi: 10.1023/A:1004249200050. [DOI] [Google Scholar]
- Mohapatra C, Chand R, Singh AK, Dixit GP. Principal component analysis for quantitative traits and powdery mildew resistance in pea (Pisum sativum L.) J Food Leg. 2017;30:43–47. [Google Scholar]
- Porch T, Jahn M. Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ. 2001;24:723–731. doi: 10.1046/j.1365-3040.2001.00716.x. [DOI] [Google Scholar]
- Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. PNAS. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seginer I. Transpirational cooling of a greenhouse crop with partial ground cover. Agric For Meteorol. 1997;41:265–281. [Google Scholar]
- Sharma KD, Pannu RK, Behl RK (2005) Effect of early and terminal heat stress on biomass portioning, chlorophyll stability and yield of different wheat genotypes. In: Singh KB (ed) Proceedings of the international conference on sustainable crop production in stress environments: management and genetic options, 9–12 February, pp 87–194
- Siebert S, Ewert F. Future crop production threatened by extreme heat. Environ Res Lett. 2014;9:41–51. [Google Scholar]
- Singh AK, Srivastava CP. Effect of plant types on grain yield and lodging resistance in pea. Ind J Genet. 2015;75:69–74. [Google Scholar]
- Wang J, Gan YT, Clarke F, McDonald CL. Response of chickpea yield to high temperature stress during reproductive development. Crop Sci. 2006;46:2171–2178. doi: 10.2135/cropsci2006.02.0092. [DOI] [Google Scholar]
- Weerakoon WMW, Maruyama A, Ohba K. Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L) J Agron Crop Sci. 2008;194:135–140. doi: 10.1111/j.1439-037X.2008.00293.x. [DOI] [Google Scholar]
- Wery J, Turc O, Lecoeur J. Mechanism of resistance to cold, heat and drought in cool-season legumes, with special reference to chickpea and pea. In: Singh KB, Saxena MC, editors. Food legumes. Chichester: Wiley; 1993. pp. 271–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.