Abstract
Ethnicity, geography, and dietary habits are known to have dominant roles in modulating the gut microbiota. Two major ethnic groups Ahom and Bodo in the north-east of India consume traditionally prepared rice beer which contains various microbes and substances that promote the growth of such microbes, known as prebiotics. This study aimed to understand the effect of traditionally prepared rice beer on gut microbiota. A total of 134 (67 from each group) volunteers including non-drinkers and drinkers from three locations were recruited. Fecal and blood samples were collected to study fecal bacterial and metabolite profiles and biochemical markers, respectively. Amplicon 16S rRNA gene sequencing (region V3–V4) by next-generation sequencing showed similar alpha and beta diversities in both the ethnic groups. However, with rice beer consumption the abundance of Firmicutes, Bacteroidetes, Fusobacteria phyla was higher in the drinkers (p < 0.05) of Ahom whereas only Firmicutes were higher in Bodo ethnic group. At the genus level, the bacterial abundance of Faecalibacterium and Roseburia were lower in the drinkers (p < 0.05) of both communities. Gas chromatography–mass spectrometry for the detection of fecal metabolites also revealed lower butyric acid in the feces of drinkers (p < 0.05). This study showed the effects of traditionally prepared rice beer on human gut microbiota and fecal metabolites. Further research is required to understand their effect on health.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02280-8) contains supplementary material, which is available to authorized users.
Keywords: Diet, Gut microbiota, Health, Metabolites, Rice beer
Introduction
Gut microbiota has a profound influence on human physiology and health. Trillions of bacteria in the gut belonging to diverse taxonomic lineages determine the well-being of an individual (Imhann et al. 2018; Qin et al. 2012; Zhao 2013). These bacteria impact host composition in immunological, metabolic, and neurological landscapes (Adak and Khan 2018). Recent studies have indicated that the composition of bacterial species is influenced by various exogenous (diet, pollutant, environment, microbes etc.) and endogenous (genotype, lifestyle, health and diseases, blood groups etc.) factors (Blaser 2008; Turnbaugh et al. 2007). Among these factors, diet plays a crucial role in shaping the gut microbiota (Wu et al. 2011). Understanding the effects of various dietary components and habits on the modulation of gut microbiota have been a research focus (Tao et al. 2019; Wijayabahu et al. 2019). There is recent evidence and consumer perception of the health benefits of fermented foods and beverages.
The importance of tribal food practices has been instrumental in the shaping of gut microbiota and its associated factors (Borah et al. 2019). Also variations ranging from foraging to hunting and finally to western diet have shown a potential role in gut microbial dynamics (De Filippis et al. 2016; Schnorr et al. 2014). Fermented foods and beverages are useful because they help provide a spectrum of probiotics to foster a vigorous microbiome (Annunziata and Mariani 2018). The two main effects of the daily consumption of fermented foods are upon the immune system and upon metabolic function (Rezac et al. 2018).
The identification and use of microbes for improved food properties have been used and studied extensively for various fermented food (Yadav et al. 2019). These fermented foods may often contain beneficial microbes having properties such as probiotics or preservative functions (Kaur et al. 2019; Schoina et al. 2020; Yadav et al. 2019). Traditionally prepared rice beer is a fermented beverage that has been consumed since ancient times as part of socio-cultural activities of many ethnic groups in the north-eastern state of Assam (India). It is prepared by mixing cooked rice with a starter cake containing Lactic Acid Bacteria (LAB) and Saccharomyces as dominant microbes. Locally named as Xaaj and Joubishi, prepared by the Ahom and Bodo ethnic groups are the two prominent rice beer variants. This rice beer contains an average of 9.41–19.33% alcohol along with antiradical activities ranging from 2.479–22.31% (Bhuyan et al. 2014). Metagenomic studies also reported the 18 core bacterial genera correlating with 66 metabolites in their functional pathway. Traditionally prepared rice beer harbors diverse bacteria having probiotic efficacy and various compounds, such as amino acids, oligosaccharides etc. which may influence the gut ecosystem (Das et al. 2019). This study aimed to understand the effects of traditionally prepared rice beer (Xaaj and Joubishi) on gut bacteria in two major ethnic groups of Assam, Ahom, and Bodo. A comparative analysis of fecal bacteria and metabolites and serum biochemical parameters were carried out between non-drinkers and drinkers.
Methods
Recruitment of volunteers
Written informed consent was taken from the volunteers along with a standard questionnaire. A total of 134 healthy volunteers of Ahom (n = 67) and Bodo (n = 67) ethnic groups were recruited from three locations for each in Assam (India). Healthy individuals (both male and female) of (i) 20–40 years of age, (ii) who had not taken any antibiotic three months prior to sample collection and (iii) without any health issues were included for the study. The volunteers were divided into non-drinkers (ND) and drinkers (D). Details about the volunteers including medical history and dietary habits were recorded in a standard questionnaire. The height and bodyweight of the volunteers were recorded for body mass index (BMI) determination (Health 1998).
Sample collection and preservation
Fecal samples from the volunteers were collected in RNA later® (Cat. No. R0901, Sigma-Aldrich, USA) in sterile stool collection tubes and transported in chilled conditions to the laboratory for metagenomic DNA extraction. For metabolite analysis, fresh fecal samples were collected in sterile containers and transported in chilled conditions to the laboratory. Blood samples were drawn from the volunteers in K3 EDTA (ethylenediaminetetraacetic acid) vials and plasma was separated immediately by centrifugation at 3000 rpm for 5 min at room temperature. All the samples were maintained at − 80 °C until further analysis.
Biochemical analyses of plasma
The plasma samples were analyzed using standard biochemical assay kits from CCS® [Coral Clinical Systems, Tulip Diagnostics (P) Ltd., Goa, India] following the manufacturer’s instructions. Serum glucose, cholesterol, albumin, globulin, total protein, liver parameter tests including serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT) and alkaline phosphatase (ALP) along with direct and total bilirubin contents were analyzed following standard protocols and standard reference values (Davies and Morris 1993; Sandler 2001; Simon-Manso et al. 2013).
DNA extraction and next-generation sequencing (NGS)
Metagenomic DNA was extracted from the fecal samples using QIAAmp® DNA stool Mini kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions. The amount of double-stranded DNA was quantified by a fluorometer (Quantas™, Promega, Madison, USA). Fecal metagenomic DNA samples were subjected to Next Generation Sequencing (NGS) analysis with Macrogen Inc. (Seoul, South Korea). PCR amplification was performed for the V3–V4 region of 16S rRNA using 341F-805R primer pairs. Metagenomic libraries were prepared using Nextera XT kit following Illumina MiSeq protocol (Bronner et al. 2013). Sequencing was carried out in an Illumina MiSeq machine (MiSEq 2500) following 2 × 300 bp paired-end chemistry on the multiplexed pooled samples. The trimmed sequences in FASTQ files were then uploaded to the Metagenomic RAST server (MG-RAST) (Project ID: mgp85400 and mgp85176). Sequences with an average Phred score lower than 25, containing ambiguous bases, homopolymer; run exceeding 6, having mismatches in primers, or sequence length shorter than 100 bp were removed. The details of the samples with MG-RAST ids have been presented in Online Resource 1. The taxonomic assignment was carried out with 97% homology within the Greengenes database. Bacterial abundance data at phylum, class, order, family, and genus levels were downloaded from the MG-RAST server.
Functional prediction
Raw NGS sequences were simultaneously analyzed within Quantitative Insights into Microbial Ecology (QIIME) package. The paired-end sequences were joined prior to closed reference OTU picking within the Greengenes 13_5 as reference database (97% homology). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (Langille et al. 2013) program was used to predict functionalities of the fecal bacteria of the samples and the data was analyzed in the context of Kyoto encyclopedia of genes and genomes (KEGG) modules. The predictions were then collapsed into functional categories under a given subsystem hierarchy at level II and level III derived from KEGG modules (Ogata et al. 1999). Statistical Analysis of Metagenomic Profiles (STAMP) is a graphical software package used for analyzing taxonomic and functional profiles using statistical hypothesis tests and exploratory plots (Parks et al. 2014). It was used for visualization in which Welch’s t test was performed for differentiating functional predictions between non-drinker and drinkers.
Non-targeted fecal metabolite profiling
Extraction
Extraction and derivatization of the metabolites were carried out using methods as described earlier with some modifications (Dehingia et al. 2017). Briefly, 40 mg of lyophilized fecal sample was extracted with 1 ml of HPLC grade methanol (Merck, Mumbai, India) and kept in a shaker overnight at 1200 rpm. The samples were centrifuged at 10,000 rpm for 10 min at 10 °C. The extract was then dried at 60 °C using a vacuum evaporator and resuspended in a 40 µl of pyridine containing 20 mg/ml of methoxyamine hydrochloride and incubated at 30 °C for 90 min and derivatized with 20 µl of N-methyl-N-trimethylsilyl trifluoroacetamide with 1% trimethylchlorosilane (Merck, USA) at 37 °C for 30 min. The samples were then centrifuged at 3000 rpm for 5 min and a 20 µl of the supernatants of 5 samples from each group (non-drinkers and drinkers) were pooled to make 1 composite sample. Further, a 50 µl of supernatant from the composite sample was transferred to a glass insert in autosampler vials for analysis.
Gas chromatography mass spectrometry (GC–MS) analysis
GC–MS analysis was performed for each of the derivatized samples using Shimadzu GC 2010 Plus-triple quadrupole (TP-8030) GC–MS/MS system fitted with EB-5MS column (length: 30 m, thickness: 0.25 μm, ID: 0.25 mm). A 1 µl of the sample was injected in splitless mode at 300 °C using helium as carrier gas at 1 ml/min flow rate. The oven program was set at 70 °C initially, ramped at 1 °C/min for 5 min up to 75 °C. Subsequently, it was increased at 10 °C/min upto 150 °C, held for 5 min followed by increasing at the same rate upto 300 °C and held for 5 min. The mass spectrometer was operated at a continuous scan from 45 to 600 m/z in the electron ionization (EI) mode at 70 ev with 200 °C as source temperature. Peak identification was performed using National Institute of Standards and Technology library, USA by matching the mass spectra. Metabolites enlisted in the online human metabolite database (HMDB) which are of microbial origins were selected for analysis.
Statistical analysis
All statistical analyses of plasma biochemical parameters were performed within the IBM SPSS Statistics 20 (SPSS Inc, Chicago, IL). The gut bacterial diversity among non-drinkers and drinkers of both the groups were ordinated using UniFrac distance metrics (Lozupone and Knight 2005). The bacterial abundance data across the two groups were subjected to Mann Whitney U test using IBM SPSS Statistics 20. Metabolite analysis was performed within MetaboAnalyst 4.0 package customized for metabolomics study (Xia et al. 2015). Data transformation and scaling were performed as described earlier (Dehingia et al. 2015). Normalized data were subjected to heatmap analysis to assess the differences in the metabolite profiles. To study the relationships among the core gut bacteria and the metabolites of bacterial origins, Spearman’s rank correlation was performed in R environment using the Hmisc package (Harrell Jr and Harrell Jr 2018). Metabolites having significant correlations with the core genera were plotted in Cytoscape (v 3.6.1) using the Metscape to visualize the network of co-occurrence (Shannon et al. 2003). Networks were visualized using prefuse force-directed layout where the nodes represent the bacterial genera and the edges represent the correlations with the metabolites. All statistical significance was set at p < 0.05.
Results
BMI and serum biochemical analyses
Based on the information collected during sample collection, anthropometric values were used for the calculation of BMI (body mass index) between the groups. In the Ahom ethnic group, BMI for non-drinkers and drinkers were 20.43 ± 0.67 and 22.07 ± 0.44, respectively. On the other hand, BMI for the Bodo ethnic group was 22.25 ± 0.58 and 21.80 ± 0.50 for non-drinkers and drinkers, respectively. There was no significant difference in BMI between non-drinkers and drinkers in none of the ethnic groups. All the serum biochemical parameters data were within the normal ranges (Online Resource 2).
The fecal bacterial diversity based on the NGS data
On an average of 147,748 high-quality reads of the average length of 301 nucleotides were obtained from 16S amplicon sequencing on Illumina Miseq platform covering the V3–V4 region of 16S rRNA region. Operational taxonomic units (OTUs) clustering at 97% cutoff yielded a total of 1444 OTUs for the entire data set. Sufficient coverage of bacterial diversity was indicated by the slopes of the rarefaction curves (Online Resource 3). The microbial diversity within and between samples were estimated based on alpha and beta-diversity indices, respectively. To measure the species richness within an ethnic group, observed OTU, ACE (abundance-based coverage estimators), and Chao1 indices were determined and for diversity estimates, Shannon and Simpson indices were considered. The various alpha diversity indices are represented in the Online Resource 4. Tukey’s HSD test for multiple comparisons indicated no significant differences in alpha-diversity indices between non-drinkers and drinkers. Beta-diversity analysis using UniFrac principal coordinate analysis (PCoA) of OTUs (grouped at 97% sequence identity) did not indicate differences in bacterial diversity between non-drinkers and drinkers of rice beer (Online Resource 5).
Taxonomic assignment using the Greengenes database in the MG-RAST server yielded a table assigning classification at phylum, class, order, family and genus levels. The major phyla identified were Bacteroidetes and Firmicutes followed by Actinobacteria, Spirochates, and Proteobacteria. Firmicutes to Bacteroidetes (F:B) ratio for non-drinkers and drinkers of the Ahom group were 0.69 and 0.72, whereas of the Bodo ethnic group were 0.59 and 0.54, respectively (Online Resource 6). A total of 199 bacterial families were detected in both the ethnic groups, where Prevotellaceae, Ruminococcaceae, Lachnospiraceae, Veillonellaceae, Clostridiaceae, Eubacteriaceae, Bacteroidaceae and Bifidobacteriaceae were dominant (> 1%). At genus level, the core bacteria present in at least 80% of the volunteers with at least 0.1% relative abundance in both the ethnic groups were computed. In both the ethnic groups, Acetivibrio, Anaeroplasma, Bacteroides, Bifidobacterium, Blautia, Clostridium, Collinsella, Dialister, Ethanoligenens, Eubacterium, Faecalibacterium, Hespellia, Lactobacillus, Megasphaera, Parabacteroides, Paraprevotella, Peptoniphilus, Prevotella, Roseburia, Ruminococcus, Sarcina, Selenomonas and Veillonella were the core bacterial genera.
Bacteria affected by rice beer consumption
Relative abundance of the bacterial taxa between non-drinkers and drinkers were compared (Table 1). In case of Ahom ethnic group, the phyla Firmicutes, Bacteroidetes, and Fusobacteria and the classes Bacteroidia, Erysipelotrichi and Fusobacteria were higher in the drinkers (p < 0.05). However, the class Flavobacteria was lower in the drinkers (p < 0.05). Bacteria of the orders Bacteriodales, Erysipelotrichales and Fusobacteriales were higher in the drinkers in contrast to Flavobacteriales which were lower in the drinkers (p < 0.05). At the family level, the abundance of Desulfohalobiaceae, Flavobacteriaceae, Peptococcaceae, Rikenellaceae, Thermoactinomycetaea were lower whereas Erysipelotrichaceae, Fusobacteriaceae, Prevotellaceae were higher in the drinkers (p < 0.05).
Table 1.
Bacterial taxa affected by consumption of rice beer in the non-drinkers (ND) and drinkers (D) in the Ahom and Bodo ethnic groups
| Taxonomic levels | Ahom (% abundance) | p value | Bodo (% abundance) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| ND | D | ND | D | |||||
| Phylum | Firmicutes | 33.50 | 35.08 | 0.046 | Firmicutes | 31.64 | 27.93 | 0.045 |
| Bacteroidetes | 47.92 | 48.36 | 0.041 | |||||
| Fusobacteria | 0.05 | 0.32 | 0.016 | |||||
| Class | Bacteroidia | 47.91 | 48.36 | 0.041 | Clostridia | 26.77 | 22.23 | 0.037 |
| Erysipelotrichi | 0.70 | 1.46 | 0.011 | |||||
| Flavobacteria | 0.02 | 0.01 | 0.017 | |||||
| Fusobacteria | 0.05 | 0.32 | 0.016 | |||||
| Order | Bacteroidales | 47.91 | 48.36 | 0.040 | Clostridiales | 26.76 | 22.23 | 0.039 |
| Erysipelotrichales | 0.70 | 1.46 | 0.010 | Lactobacillales | 0.51 | 0.88 | 0.039 | |
| Flavobacteriales | 0.02 | 0.01 | 0.020 | |||||
| Fusobacteriales | 0.05 | 0.32 | 0.020 | |||||
| Family | Desulfohalobiaceae | 0.04 | 0.02 | 0.030 | Lachnospiraceae | 7.65 | 5.11 | 0.010 |
| Erysipelotrichaceae | 0.70 | 1.46 | 0.010 | |||||
| Flavobacteriaceae | 0.02 | 0.01 | 0.020 | |||||
| Fusobacteriaceae | 0.05 | 0.32 | 0.020 | |||||
| Peptococcaceae | 0.19 | 0.04 | 0.000 | |||||
| Prevotellaceae | 43.87 | 44.24 | 0.050 | |||||
| Rikenellaceae | 0.32 | 0.18 | 0.010 | |||||
| Thermoactinomycetaceae | 0.01 | 0.00 | 0.050 | |||||
In the Bodo ethnic group, the phyla Firmicutes (phyla), Clostridia (class), Clostridiales (order), and Lachnospiraceae (family) were lower with the consumption of rice beer, whereas Lactobacillales (order) was higher in the drinkers (p < 0.05).
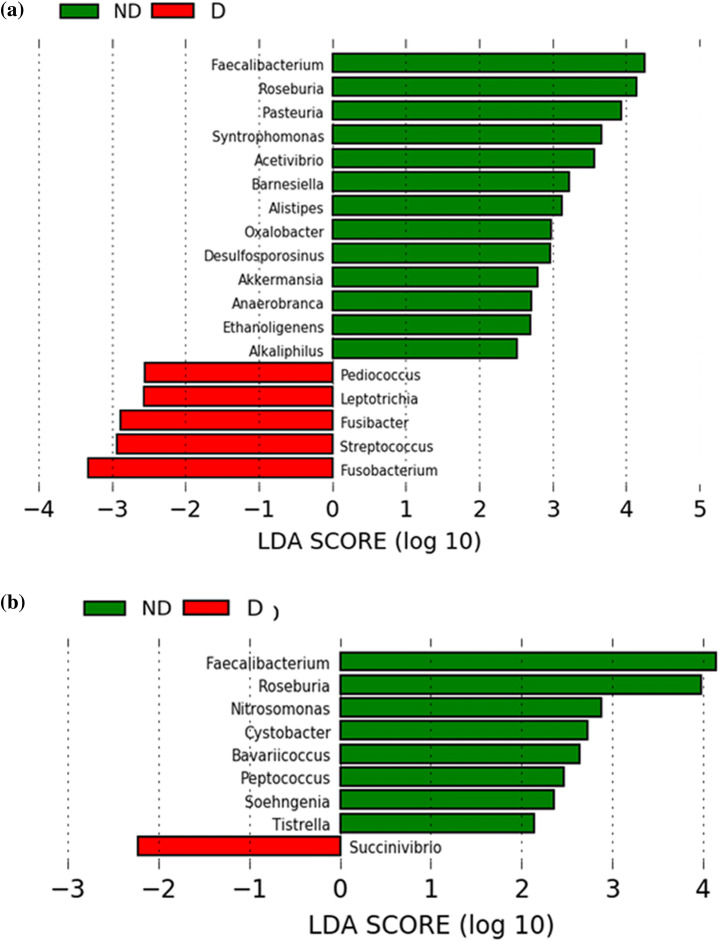
Differential abundance at the genus level was detected by LefSe {linear discriminant analysis (LDA) effect size} for minimizing the number of differences. In addition to discriminating features by statistical analysis, LefSe also performs additional tests to assess the consistency of difference with respect to expected biological behavior. LEfse analysis with effect size (LDA score > 2) detected 18 bacterial genera in the Ahom and 10 genera in the Bodo ethnic groups, respectively, showing marked differences between non-drinkers and drinkers (Fig. 1). In the Ahom ethnic group, the bacterial genera Faecalibacterium, Roseburia, Pasteuria, Syntrophomonas, Acetivibrio, Barnesiella, Alistipes, Oxalobacter, Desulfosporosinus, Akkermensia, Anaerobranca, Ethanoligenens, and Alkaliphilus were more abundant in the non-drinkers (p < 0.05) and Pediococcus, Leptotrichia, Fusibacter, Streptococcus, and Fusobacterium were more abundant in the drinkers (p < 0.05). Similarly, in the Bodo ethnic group, Faecalibacterium, Roseburia, Nitrosomonas, Cystobacter, Bavaricoccus, Peptococcus, Soehngenia and Tistrella were more abundant in the non-drinkers and in the drinkers Succinivibrio was more abundant (p < 0.05). A bar plot showing the differences in the abundance of these genera is presented in Fig. 2. In both the ethnic groups, Faecalibacterium and Roseburia decreased in the drinkers. The abundance of Faecalibacterium reduced by 29.59 and 25.15% in the drinkers of the Ahom and Bodo ethnic groups, respectively (p < 0.05). Similarly, the abundance of Roseburia in the drinkers decreased by 49.94 and 53.05% in the Ahom and Bodo ethnic groups, respectively (p < 0.05).
Fig. 1.
Linear discriminant analysis (LDA) effect size (LEfSe) results showing bacterial genera differentially abundant in the non-drinkers (ND) and drinkers (D) of aAhom and bBodo ethnic groups
Fig. 2.
Bar plot showing the decrease in the genera Faecalibacterium and Roseburia in the non-drinkers (ND) and drinkers (D) of aAhom (ND = 16; D = 51) and bBodo (ND = 21; D = 46) ethnic groups. Mann Whitney U test was performed between ND and D at p < 0.05
Functional predictions of the gut microbiota
Differences were observed in the KEGG orthologues (KO) composition based on fecal bacterial diversity between the non-drinkers and drinkers. STAMP profiles of the predicted functions are presented in Fig. 3 for both the ethnic groups. A total of 328 pathways (expressed in both the ethnic groups) representing cellular processes, signaling, environmental information processing, genetic information processing, human diseases, metabolism and organismal system categories involved in microbial metabolisms were detected (p < 0.05). Pathways involved in the biosynthesis of type II polyketide products, ether lipid metabolism, selenocompound metabolism, nitrogen metabolism, and calcium signaling pathway were over-represented in the drinkers compared to the non-drinkers, where lipid biosynthesis proteins were higher in the Ahom ethnic group (p < 0.05). Notably, in the Bodo ethnic group, in the drinkers a significant increase in taurine and hypotaurine metabolism and bile secretion pathways was observed (p < 0.05).
Fig. 3.
Extended error bar plot showing the normalized relative abundance of KEGG metabolic pathways (at level II) based on the gut bacteria of non-drinkers (ND) and drinkers (D) of aAhom and bBodo ethnic groups
Fecal metabolite profile
A total of 250 metabolites were detected in the GC–MS analysis of the fecal samples. However, only 40 metabolites categorized as of microbial origins were taken into consideration for further analysis. The compounds identified by GC–MS along with their peak area has been listed in Online Resource 7. The metabolite profiles included 3 short-chain fatty acids (SCFA), 9 amino acids, 4 saccharides, 4 sugar alcohols, 6 bile acids, 13 organic acids and compounds such as indole, methane, and piperidine. The differences in the metabolites (peak area percentage) among non-drinkers and drinkers in both the ethnic groups are presented in heatmaps (Fig. 4). The metabolites clustered based on the ward algorithm considering Pearsons distance measure. Based on the similarity, separate clusters were observed for non-drinkers and drinkers in both the ethnic groups.
Fig. 4.
Heatmap plots based on the metabolites profile of non-drinkers (ND) and drinkers (D) of aAhom and bBodo ethnic groups identified by GC–MS analysis
In the case of Ahom non-drinkers, propanoic, butyric, and cis-vaccenic acids were found to be higher in abundance (p < 0.05) as compared to drinkers. Likewise, in the Bodo non-drinkers, butyric acid, rhamnose, arabinose, glycine, hydroxycinnamic acid, indole, formic acid, ursodeoxycholic acid, acetic acid, and benzoic acid were higher in abundance (p < 0.05).
Correlation of fecal metabolites to the core bacterial genera
The correlation networks created using Cytoscape for both the ethnic groups have been presented in Fig. 5. In the Ahom ethnic group, most of the core bacteria had positive correlations with the SCFAs except for Bifidobacterium which had a negative correlation with valeric acid (r = − 0.33, p = 0.01). Roseburia, which was a dominant bacteria in the non-drinkers was positively correlated with valeric acid (r = 0.68, p = 0.03) and cholestanol (r = 0.47, p = 0.03). Acetivibrio had positive correlations with acetic acid (r = 0.34, p < 0.01), butanoic acid (r = 0.00, p < 0.00) and amino acids (r > 0.28, p < 0.02). Ethanoligenens had positive correlations with acetic acid, butanoic acid, proline, valine, and alanine (r > 0.26, p < 0.05) and negative correlations with arabitol, rhamnose, lactic acid, butanedioic acid, deoxycholic acid and butanol (r < − 0.29, p < 0.05). Prevotella which was higher in the drinkers, had negative correlations with carboxylic acid (r = − 0.30, p = 0.02) and propionic acid (r = − 0.29, p = 0.03).
Fig. 5.
Network of correlating genera with fecal metabolites of aAhom and bBodo ethnic groups. The nodes in diamond represent bacterial genera and the edges represent the type of correlation (green: positive; red: negative) to the spherical nodes representing fecal metabolites
In the case of the Bodo ethnic group, Faecalibacterium which was more abundant in the non-drinkers had positive correlations with arabitol, propanoic acid, acetic acid, trehalose and deoxycholic acid (r > 0.29, p < 0.05). However, Faecalibacterium was negatively correlated to pentanoic acid, oleic acid, and butanol (r > − 0.29, p < 0.05). On the other hand, Roseburia, which was also in higher abundance in the non-drinkers had a positive correlation with cholestanol (r = 0.47, p = 0.00) and negative correlation with rhamnose (r = − 0.30, p < 0.04).
Discussion
The human gut microbiota is influenced by factors such as ethnicity, diet, lifestyle etc. Diet has a dominant role in modulating the microbiota in the gut ecosystem and is the easiest route for therapeutic intervention (Wu et al. 2011). Long-term dietary habits and those associated with ethnicity have been a recent focus in gut microbiome research (Cuevas-Sierra et al. 2019). The gut microbiota of an individual has been shown to be affected by long-term diet in the American and European populations (De Filippo et al. 2010; Wu et al. 2011). Although gut microbiota of some of the Indian population has been studied (Bhute et al. 2016; Dehingia et al. 2015; Ghosh et al. 2014), the role of ethnic dietary habits on gut microbiota has not been explored. Traditionally prepared rice beer is an alcoholic fermented beverage that is rich in carbohydrates, amino acids, sugar alcohol, and organic acids. Moreover it contains a consortium of microflora, prebiotics, and metabolites of nutritional values (Bora et al. 2016; Das et al. 2014). The rice beer consumed by the ethnic communities of Assam is rich in microbial and metabolite content. Ahom and Bodo communities of Assam has been reported to consume such beverages which are rich in lactic acid bacteria. Culture-independent study of rice beer showed the presence of 18 core bacteria in which the LAB Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, and Weissella were dominant. Also the presence of potent prebiotics such as cellobiose and mannobiose in their metabolite profile enhances its nutritional value (Das et al. 2019). Few studies had reported the microbial composition of the starter cake and the biochemical composition of the rice beer. The crude protein content has been reported to be 1.5–2.51% along with the presence of calcium, sodium, potassium, iron, and phosphorus as mineral compounds (Bhuyan et al. 2014; Das et al. 2014; Ghosh et al. 2014; Sha et al. 2017). Therefore, this study emphasized the effect of rice beer consumption, on the gut bacterial profiles of two major ethnic groups of Assam, Ahom and Bodo.
To evaluate the effect of rice beer consumption on health, BMI, and plasma biochemical parameters such as SGOT, SGPT, ALP, bilirubin along with glucose and cholesterol levels were tested and found to be in the normal range. BMI of the population was within a normal range indicating the volunteers recruited in our study were apparently healthy. Moreover, the normal BMI range in the drinkers indicates that long term rice beer consumption did not lead to alcohol-induced obesity. Although the rice beer consumed by the Ahom and Bodo ethnic groups are alcoholic beverages containing 4.1–4.3% (v/v) ethanol,(Das et al. 2014) there was no significant difference in the biochemical parameter indicating no apparent detrimental effect on health.
To gather further insight into the effect of rice beer consumption on the gut bacterial profile, the bacterial diversity was studied by culture-independent technique. Alpha and beta diversity indices reflecting the richness and evenness of gut bacteria did not vary significantly between non-drinkers and drinkers of rice beer. However, there were significant differences in various bacterial taxa with respect to rice beer consumption, though this variation was distinctive for either ethnic groups.
Detection of biomarkers for the study groups through LEfSe revealed Roseburia and Faecalibacterium as two bacterial genera over-represented in the non-drinker groups. These gut commensals are known to produce butyrate from non-digestible dietary fibers (Louis and Flint 2009). Butyrate is made from carbohydrates via glycolysis by the combination of two molecules of acetyl-CoA to form acetoacetyl-CoA. It is then followed by stepwise reduction to butyryl-CoA (Cummings and Englyst 1987). The final step in butyrate formation from butyryl-CoA is carried out in two different pathways. In the first pathway, butyryl-CoA is phosphorylated to form butyryl-phosphate and subsequently transformed to butyrate via butyrate kinase. In the second pathway, the CoA moiety of butyryl-CoA is transformed to acetate via butyryl-CoA: acetate CoA-transferase leading to the formation of butyrate and acetyl-CoA (Cummings and Englyst 1987). In concordance to the LEfSe analysis, metabolomic data also indicated a lower level of butyrate in the drinkers which may render them prone to gastrointestinal disorders, and its depletion was earlier observed in cases of alcohol dependence syndrome, ulcerative colitis and Inflammatory Bowel Disease (Dubinkina et al. 2017). The bacterial genera Porphyromoas, Fusobacterium, Leptotrichia, and Streptococcus which were earlier reported as oral microflora, were found to be abundant in the drinkers of the Ahom ethnic group. Increase abundance of Porphyromonas was previously reported in patients with nonalcoholic fatty liver disease (NAFLD). Fusobacterium can activate the E-cadherin/β-catenin signaling pathway and was previously shown to be associated with oncogenic and inflammatory genes (Rubinstein et al. 2013) and also its strong adhesive and invasive abilities results in malignant transformation of epithelial cells which has direct association with colorectal cancer (McCoy et al. 2013). Leptotrichia was reported to be enriched in the oral microbiome in high alcoholic subjects (Fan et al. 2018) was also observed in this study in drinkers, which could be due to flushing of oral microbiota by rice beer. On the other hand, Succinivibrio which was the only bacterial genus increased in the Bodo drinkers was earlier reported in the cases of NAFLD (Li et al. 2018). Functional predictions of the gut microbiota in the Bodo drinkers indicated high taurine and hypotaurine metabolism along with bile acid secretion which can be correlated to the higher cholestanol levels in fecal metabolites. An increase in cholestanol or coprostanol (5-dihydro derivative of cholesterol) induces a pathological condition named cerebrotendinous xanthomatosis (CTX) which leads to higher bile acid secretion and metabolism (Salen and Grundy 1973). Higher expression of bile acid secretion in the Bodo drinkers can be related to the higher abundance of cholestanol in their fecal metabolite profiles.
The metabolomic study revealed that in the Ahom non-drinkers, SCFAs were higher, whereas saccharides were lower. Since fiber utilizing bacteria such as Faecalibacterium and Roseburia were higher in the non-drinkers, they might have converted the available saccharides into SCFAs which is an indicator of good gut health (Louis and Flint 2009). A similar trend was also observed in the Bodo group suggesting that rice beer-drinking lowers the bacterial population responsible for maintaining gut health. Acetivibrio is known to produce acetate (SCFA) from fibres (Khan et al. 1984). Acetivibrio was positively correlated to acetic acid in the Ahom ethnic group, its abundance was also higher in the non-drinkers. In the Bodo non-drinkers, acetic acid was also higher, however, though there was an increase in abundance of Acetivibrio in the non-drinkers, it was not significant.
This is the first report on the effect of traditional rice beer on gut microbiota. Further research should focus on understanding the effects of such altered gut microbiota and reduced SCFAs on human health. Also rice beer being a traditional beverage can be improved by using fermentation technology and microbes having enhanced values.
In conclusion, this study reports the effects of traditionally prepared rice beer on gut microbiota. Although the two groups are different (ethnicity and geography), their gut microbiota were similar. However, the consumption of traditional rice beer prepared by the ethnic groups had a differential effect on the gut microbiota. Butyric acid-producing bacterial genera Faecalibacterium and Roseburia were reduced in the gut microbiota of the drinkers along with a lower abundance of butyric acid in the fecal samples indicating a probable detrimental effect of rice beer consumption on gut health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Institutional level Biotech Hub (DBT, Govt. of India) of IASST for providing the facilities. We are thankful to the volunteers for providing the samples.
Author contributions
DD, SD and AA performed the experiments and data analysis. DD wrote the manuscript. MRK designed the study.
Funding
This research was funded by Department of Biotechnology (DBT), Ministry of Science and Technology under Unit of Excellence Project (BT/550/NE/U-Excel/2014, Sl. No. 449).
Availability of data and material
The datasets generated during and/or analysed during the current study are available in the MG-RAST repository (https://www.mg-rast.org) Project ID: mgp85400 and mgp85176.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethics approval
This study was approved by the Ethics Committee (Human studies), Institute of Advanced Study in Science and Technology (IASST), Guwahati, India (NO IEC(HS)/IASST/1082/2014-15/8) and was conducted following relevant guidelines and regulations.
Consent to participate
Written informed consents were taken from the volunteers along with a standard questionnaire.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- Adak A, Khan MR (2018) An insight into gut microbiota and its functionalities Cell Mol Life Sci 1–21 10.1007/s00018-018-2943-4 [DOI] [PMC free article] [PubMed]
- Annunziata A, Mariani A (2018) Consumer perception of sustainability attributes in organic and local food. Recent Patents Food Nutr Agric 9:87–96 10.2174/2212798410666171215112058 [DOI] [PubMed]
- Bhute S, et al. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of Prevotella and Megasphaera in Indian subjects. Front Microbiol. 2016;7:660. doi: 10.3389/fmicb.2016.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan DJ, Barooah MS, Bora SS, Singaravadivel K (2014) Biochemical and nutritional analysis of rice beer of North East India
- Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prevent Res. 2008;1:308–311. doi: 10.1158/1940-6207.CAPR-08-0170. [DOI] [PubMed] [Google Scholar]
- Bora SS, Keot J, Das S, Sarma K, Barooah M (2016) Metagenomics analysis of microbial communities associated with a traditional rice wine starter culture (Xaj-pitha) of Assam, India. 3 Biotech 6:153. 10.1007/s13205-016-0471-1 [DOI] [PMC free article] [PubMed]
- Borah T, Gogoi B, Khataniar A, Gogoi M, Das A, Borah D. Probiotic characterization of indigenous Bacillus velezensis strain DU14 isolated from Apong, a traditionally fermented rice beer of Assam. Biocatal Agric Biotechnol. 2019;18:101008. doi: 10.1016/j.bcab.2019.01.046. [DOI] [Google Scholar]
- Bronner IF, Quail MA, Turner DJ, Swerdlow H. Improved protocols for illumina sequencing. Curr Protoc Hum Genet. 2013;79:18.12.11–18.12.42. doi: 10.1002/0471142905.hg1802s79. [DOI] [PubMed] [Google Scholar]
- Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10:S17–S30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Englyst HN. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45:1243–1255. doi: 10.1093/ajcn/45.5.1243. [DOI] [PubMed] [Google Scholar]
- Das AJ, Khawas P, Miyaji T, Deka SC. HPLC and GC-MS analyses of organic acids, carbohydrates, amino acids and volatile aromatic compounds in some varieties of rice beer from northeast India. J Inst Brew. 2014;120:244–252. doi: 10.1002/jib.134. [DOI] [Google Scholar]
- Das S, Deb D, Adak A, Khan MR. Exploring the microbiota and metabolites of traditional rice beer varieties of Assam and their functionalities. 3 Biotech. 2019;9:174. doi: 10.1007/s13205-019-1702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- De Filippis F, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehingia M, Sen S, Bhaskar B, Joishy TK, Deka M, Talukdar NC, Khan MR. Ethnicity influences gut metabolites and microbiota of the tribes of Assam India. Metabolomics. 2017;13:69. doi: 10.1007/s11306-017-1206-y. [DOI] [Google Scholar]
- Dehingia M, Talukdar NC, Talukdar R, Reddy N, Mande SS, Deka M, Khan MR. Gut bacterial diversity of the tribes of India and comparison with the worldwide data. Sci Rep. 2015;5:18563. doi: 10.1038/srep18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinkina VB, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6:59. doi: 10.1186/s40168-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TS, et al. Gut microbiomes of Indian children of varying nutritional status. PLoS ONE. 2014;9:e95547. doi: 10.1371/journal.pone.0095547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell Jr FE, Harrell Jr MFE (2018) Package ‘Hmisc’ R foundation for statistical computing
- Health NIo (1998) Clinical guidelines for the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes Res 6:51S–209S [PubMed]
- Imhann F, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Ghoshal G, Banerjee UC (2019) Traditional bio-preservation in beverages: fermented beverages. In: Preservatives and preservation approaches in beverages. Elsevier, New York, pp 69–113
- Khan A, Meek E, Sowden L, Colvin JR. Emendation of the genus Acetivibrio and description of Acetivibrio cellulosolvens sp. nov., a nonmotile cellulolytic mesophile. Int J Syst Evol Microbiol. 1984;34:419–422. doi: 10.1099/00207713-34-4-419. [DOI] [Google Scholar]
- Langille MG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, et al. Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci China Life Sci. 2018;61:770–778. doi: 10.1007/s11427-017-9303-9. [DOI] [PubMed] [Google Scholar]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS ONE. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Rezac S, Kok CR, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1. doi: 10.3389/fmicb.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G, Grundy SM. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J Clin Invest. 1973;52:2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler SG. Primary hematology: Ayalew Tefferi, MD, ed. Totowa, NJ: Humana Press, 2001. 472 pages. $125. Hardcover Transfus. 2001;41:850–850. doi: 10.1046/j.1537-2995.2001.41060850.x. [DOI] [Google Scholar]
- Schnorr SL, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoina V, Terpou A, Papadaki A, Bosnea L, Kopsahelis N, Kanellaki M. Enhanced aromatic profile and functionality of cheese whey beverages by incorporation of probiotic cells immobilized on Pistacia terebinthus. Resin Foods. 2020;9:13. doi: 10.3390/foods9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SP, Jani K, Sharma A, Anupma A, Pradhan P, Shouche Y, Tamang JP. Analysis of bacterial and fungal communities in Marcha and Thiat, traditionally prepared amylolytic starters of India. Sci Rep. 2017;7:10967. doi: 10.1038/s41598-017-11609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Manso Y, et al. Metabolite profiling of a NIST standard reference material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal Chem. 2013;85:11725–11731. doi: 10.1021/ac402503m. [DOI] [PubMed] [Google Scholar]
- Tao J, Li S, Gan R-Y, Zhao C-N, Meng X, Li H-B (2019) Targeting gut microbiota with dietary components on cancer: effects and potential mechanisms of action. Crit Rev Food Sci Nutr 1–13. 10.1080/10408398.2018.1555789 [DOI] [PubMed]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayabahu AT, Waugh SG, Ukhanova M, Mai V. Dietary raisin intake has limited effect on gut microbiota composition in adult volunteers. Nutr J. 2019;18:14. doi: 10.1186/s12937-019-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Mandeep, Shukla P (2019) Probiotics of diverse origin and their therapeutic applications: a review. J Am Coll Nutr 1–11 [DOI] [PubMed]
- Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the MG-RAST repository (https://www.mg-rast.org) Project ID: mgp85400 and mgp85176.