Abstract
Flag leaf and shoot growth at heading stage as well as ultimate yield capacity of ten wheat cultivars were assessed in a pot experiment under normal and drought conditions. Drought was imposed by withholding 25% of field capacity from the 45- day old plants for 21 days followed by normal irrigation until maturity. Leaf succulence degree and stomatal opening area as well as shoot biomass, density and distribution decreased in all cultivars in response to drought but to different degrees. On contrary, leaf sclerophylly degree and water saturation deficit increased in all cultivars as a result of drought. At the same time, drought caused marked alterations in leaf transpiration rate, hair features, abscisic acid content, osmotic adjustment and fatty acid profile of the concerned cultivars; with ultimate variable capacity for yield. The drought- induced changes in the estimated traits were graphically represented in a single map then they were correlated with each other. The considered cultivars could be eventually clustered based on their drought response; with Sids cultivars being the most drought tolerant whereas Shandaweel 1 and Giza 168 being the most sensitive.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00807-0) contains supplementary material, which is available to authorized users.
Keywords: Drought, Fatty acids, Leaf water status, Shoot, Wheat, Yield
Introduction
Wheat (Triticum aestivum L.) is one of the most economically important crops involved worldwide in daily human diet as a basic source of carbohydrates and proteins (Marček et al. 2019). However, wheat is mainly cultivated in arid and semi- arid regions where limited water availability can cause marked yield loss reaching up to 29% of its productivity (Daryanto et al. 2016). Therefore, exploring the response of different wheat cultivars to drought is of great significance especially with the accelerated climate change that caused drought incidence to become more severe (Fang and Xiong 2015). In this regard, numerous studies indicated that plants could sense drought when water availability around their root system became limited. In consequence, plants usually close stomata in a trial to minimize water loss via transpiration as a result of a hormonal signal activated under drought in the form of abscisic acid (ABA) (Saradadevi et al. 2017). Such response is often reflected on leaf succulence, sclerophylly and water saturation deficit (Mickky et al. 2018).
Under drought, some organic osmolytes such as soluble sugars and soluble nitrogen compounds could be accumulated in the stressed plants to reduce cellular dehydration and cope with stress (Jogawat 2019). In addition, some studies, though being limited, have pointed out to the involvement of some non- polar metabolites, such as fatty acids, in plant drought tolerance (Sánchez-Martín et al. 2018). For wheat, the impact of drought on ultimate yield usually correlates not only with flag leaf growth but also with the whole shoot growth that considerably varies with the duration and severity of stress in addition to the plant cultivar and its growth stage (Mickky et al. 2019). Thus, the main objective of the present study was to compare the response of ten wheat cultivars to drought stress by assessing their vegetative growth, mainly flag leaf water status, fatty acid profile and the whole shoot vigor, to be linked with their productivity at maturation. In such a way, drought- tolerant cultivars are expected to be identified and differentiated from the drought- sensitive ones; with adopting appropriate statistical procedures to discuss the results and reach an overall conclusion.
Materials and methods
Experimental design
Pure strains of wheat (Triticum aestivum L.), cultivar Misr 1, Misr 2, Gemmeiza 9, Gemmeiza 11, Sids 12, Sids 13, Sakha 93, Sakha 94, Shandaweel 1 and Giza 168, were obtained from Sakha Agricultural Research Center. Healthy grains were cultivated in clay/sand soil (2/1, w/w) within plastic pots. While kept under natural conditions (minimum/maximum light intensity, temperature and relative humidity of 185/1406 µmol m−2 s−1, 15/27 °C and 40/77%; respectively at midday time), plants were irrigated to field capacity. When the plants became 45- day old, 25% of irrigation water was held for 21 days then the plants were normally irrigated until maturation. Another set of plants were irrigated to field capacity during the whole experimental period serving as control. Sampling was performed twice; flag leaf and shoot at heading (65 ± 3 days after sowing) in addition to yield at maturity (115 ± 5 days after sowing).
Estimation of vegetative traits at heading and yield traits at maturity
Leaf succulence degree was computed as the difference between leaf fresh and dry mass divided by leaf area, while leaf sclerophylly degree was computed as leaf dry mass divided by leaf area (Witkowski and Lamont 1991). Leaf dehydration was gravimetrically determined as a percentage of leaf water saturation deficit (Gietler et al. 2016), while transpiration rate was determined using gas exchange system (LCi, ADC BioScientific Ltd., UK). Stomatal and hair features were studied in leaf micrographs obtained from scanning electron microscopy. Micrographs of abaxial epidermis were analyzed using ImageJ 1.34S software for stomatal opening area, hair length as well as stomatal and hair frequency (their number in the unit mm2).
Leaf ABA content was determined by HPLC (Ali et al. 2011). Regarding osmotic adjustment, partial osmotic pressure of leaf water extract was expressed in terms of its electric conductivity, while total soluble sugar and total soluble nitrogen were quantified in the water extracts using anthrone and Kjeldahl method; respectively (AOAC 2000). Leaf fatty acid profile was assessed by GC–MS (Kitson et al. 1996). Also, shoot biomass and length were determined; with shoot density determined as its dry mass to length ratio and shoot distribution as its fresh mass to length ratio (Arduini et al. 1994). At maturity, biomass yield (grained shoot mass), straw yield, grain yield and crop yield (grained spike mass) were determined.
Statistical analysis
Replicates of the pooled data (ten for agronomic traits and three for biochemical analyses) were analyzed by CoHort/CoStat 6.311 software for analysis of variance at p ≤ 0.05. For each cultivar, the percent change in its traits in response to drought was recorded; and the computed sets of percent change (320 data points) were summarized as bar map. Spearman correlation coefficients among the drought- induced percent changes in the estimated traits were determined by Past 3.20 software; and the significant correlations at p ≤ 0.05 were displayed as bubble map. In addition, cluster analysis of the considered cultivars was performed by Minitab 18 software with Euclidean distance of single linkage.
Results and discussion
Changes in leaf water status at heading
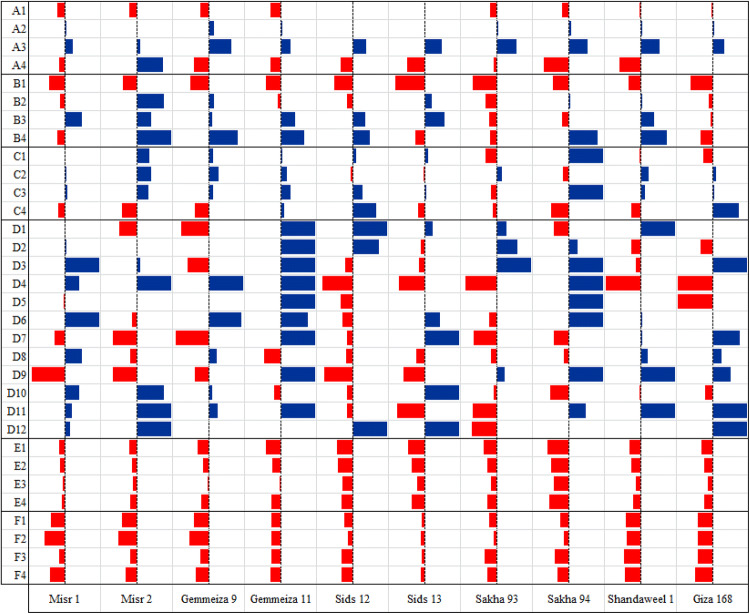
The results obtained indicated that drought significantly decreased leaf succulence degree of the studied wheat cultivars except in the two Sids cultivars, Shandaweel 1 and Giza 168 where such decrease was non- significant at p ≤ 0.05. The maximum drought- induced decrease in leaf succulence degree was recorded in the two Gemmeiza cultivars, while the minimum decrease was recorded in the two Sids cultivars (Fig. 1 and Table S1). Leaf succulence was assumed to indicate adaptation to drought; where more succulent organs were supposed to have more capacity for water storage (Jones 2011). On contrary, leaf sclerophylly degree non- significantly increased in response to drought in almost all wheat cultivars; with the cultivar Gemmeiza 9 showing the maximum drought- induced increase as well as the two Sids cultivars and Misr 2 showing the minimum increase (Fig. 1 and Table S1). In this regard, Edwards et al. (2000) pointed out to leaf sclerophylly as (1) an adaptive response to drought, (2) a consequence of little nutrient supply and (3) a strategy to improve leaf longevity by increasing its carbon gain. Matching the results obtained herein, Mickky et al. (2018) recorded that drought decreased leaf succulence degree of alfalfa plants with corresponding increase in their leaf sclerophylly degree.
Fig. 1.
Bar map of drought- induced percent change (positive blue or negative red) in the considered traits of ten wheat cultivars (A1 = leaf succulence degree, A2 = leaf sclerophylly degree, A3 = leaf water saturation deficit, A4 = leaf transpiration rate, B1 = stomatal opening area, B2 = stomatal frequency, B3 = hair length, B4 = hair frequency, C1 = leaf ABA content, C2 = leaf partial osmotic pressure, C3 = leaf total soluble sugar content, C4 = leaf total soluble nitrogen content, D1 = leaf palmitoleic acid proportion, D2 = oleic acid proportion, D3 = linoleic acid proportion, D4 = lauric acid proportion, D5 = tridecanoic acid proportion, D6 = myristic acid proportion, D7 = pentadecanoic acid proportion, D8 = palmitic acid proportion, D9 = heptadecanoic acid proportion, D10 = stearic acid proportion, D11 = arachidic acid proportion, D12 = behenic acid proportion, E1 = shoot fresh mass, E2 = shoot dry mass, E3 = shoot density, E4 = shoot distribution, F1 = biomass yield, F2 = straw yield, F3 = grain yield, F4 = crop yield)
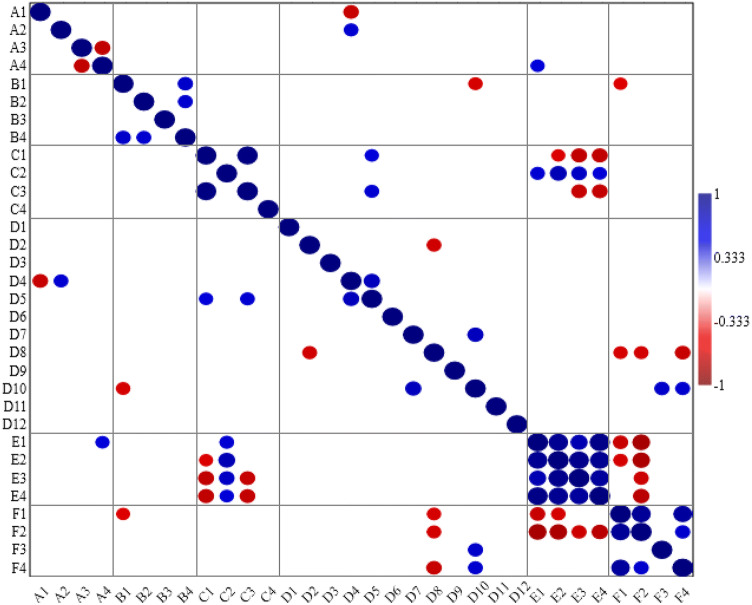
Also, drought significantly increased leaf water saturation deficit in all the concerned wheat cultivars except in Misr 2 where such increase was non- significant (Fig. 1 and Table S1). In this context, leaf dehydration could be indicated from its water saturation deficit as one of the first symptoms of plant response to drought (Gietler et al. 2016). Data obtained also revealed that drought decreased leaf transpiration rate in all the concerned cultivars except in Misr 2 where drought significantly increased it (Fig. 1 and Table S1). The recorded drought- induced decrease in transpiration rate matches the results obtained by Novick et al. (2016). The drop in transpiration rate under drought could be ascribed to stomatal closure induced by ABA overproduction (Saradadevi et al. 2017). However, the drought- induced change in leaf transpiration rate of the concerned cultivars negatively correlated with that in their leaf water saturation deficit (Fig. 2) indicating that the recorded reduction in transpiration rate may be insufficient to recover leaf dehydration.
Fig. 2.
Bubble map of correlation coefficient (significant at p ≤ 0.05) among the drought- induced percent change in the considered traits of ten wheat cultivars (A1 = leaf succulence degree, A2 = leaf sclerophylly degree, A3 = leaf water saturation deficit, A4 = leaf transpiration rate, B1 = stomatal opening area, B2 = stomatal frequency, B3 = hair length, B4 = hair frequency, C1 = leaf ABA content, C2 = leaf partial osmotic pressure, C3 = leaf total soluble sugar content, C4 = leaf total soluble nitrogen content, D1 = leaf palmitoleic acid proportion, D2 = oleic acid proportion, D3 = linoleic acid proportion, D4 = lauric acid proportion, D5 = tridecanoic acid proportion, D6 = myristic acid proportion, D7 = pentadecanoic acid proportion, D8 = palmitic acid proportion, D9 = heptadecanoic acid proportion, D10 = stearic acid proportion, D11 = arachidic acid proportion, D12 = behenic acid proportion, E1 = shoot fresh mass, E2 = shoot dry mass, E3 = shoot density, E4 = shoot distribution, F1 = biomass yield, F2 = straw yield, F3 = grain yield, F4 = crop yield)
The results of the current study also revealed that drought generally decreased stomatal opening area of the concerned cultivars; but non- significantly affected their stomatal frequency except in Misr 2 where drought significantly increased it (Fig. 1 and Plate S1). Such alterations in stomatal behavior come in parallelism with the drought- induced change in transpiration rate. Supporting this finding, it was recorded that transpiration rate under drought is regulated by stomatal activity, size and density (Nir et al. 2014). Regarding leaf epidermal hairs, the results obtained herein revealed that drought increased hair length in all cultivars except in the two Sakha cultivars and Giza 168 where drought decreased it. For hair frequency, it increased under drought in all cultivars except in Misr 1, Sids 13, Sakha 93 and Giza 168 where the reverse was noticed (Fig. 1 and Plate S1). Moreover, the drought- induced change in hair frequency positively correlated with that in stomatal opening area and stomatal frequency (Fig. 2). In this context, leaf epidermal hairs were supposed to mediate drought tolerance by (1) minimizing water loss via transpiration, (2) maximizing radiation reflection thereby protecting the underlying tissues from radiation damage and (3) improving leaf capacity to take advantage of water condensation or light rain (Karabourniotis and Bornman 1999).
Data also indicated that drought decreased leaf ABA content in Sakha 93, Shandaweel 1 and Giza 168; but increased it in the remaining cultivars except in Misr 1 where it remained unchanged (Fig. 1 and Table S1). Among the strategies developed by plants to withstand drought, stomatal conductance of the plant leaves was found to decrease in response to a signal originating in root in the form of ABA which moves up to leaves where it elevates cytosolic concentration of calcium ions in guard cells causing stomatal closure (Blatt 2000). Another strategy to cope with drought is the osmotic adjustment, where drought caused marked increase in leaf osmotic pressure of the studied wheat cultivars except in the two Sids cultivars and Sakha 94 where the reverse was recorded. Leaf total soluble sugar content increased in response to drought in all cultivars except Sakha 93, while total soluble nitrogen content increased by drought in Gemmeiza 11, Sids 12 and Giza 168 but decreased in the remaining cultivars (Fig. 1 and Table S1). Matching these results, Moinuddin and Khannu-Chopra (2004) noticed marked variation in the potency of different chickpea cultivars for osmotic adjustment under drought. The role of osmotic adjustment under drought is thought to be accomplished by (1) lowering cellular osmotic potential with subsequent continuation of cell expansion, (2) mitigating the reverse effects of stress on enzymatic activity and/or (3) scavenging free radicals and stabilizing cellular membranes (Jogawat 2019).
Changes in leaf fatty acid profile at heading
Data in Fig. 1 and Table S1 revealed that drought decreased leaf palmitoleic acid proportion (in Misr 2, Gemmeiza 9 and Sakha 94), oleic acid proportion (in Misr 2, Gemmeiza 9, Sids 13, Shandaweel 1 and Giza 168), linoleic acid proportion (in Gemmeiza 9, Sids 12, Sids 13 and Shandaweel 1), lauric acid proportion (in Sids 12, Sids 13, Sakha 93, Shandaweel 1 and Giza 168), tridecanoic acid proportion (in Misr 1, Sids 12 and Giza 168), myristic acid proportion (in Misr 2, Sids 12 and Sakha 93), pentadecanoic acid proportion (in Misr 1, Misr 2, Gemmeiza 9, Sids 12, Sakha 93 and Sakha 94) and palmitic acid proportion (in Misr 2, Gemmeiza 11, Sids 12, Sids 13, Sakha 93 and Sakha 94). Drought also decreased heptadecanoic acid proportion (in Misr 1, Misr 2, Gemmeiza 9, Sids 12 and Sids 13), stearic acid proportion (in Gemmeiza 11, Sids 12, Sakha 93, Sakha 94, Shandaweel 1 and Giza 168), arachidic acid proportion (in Sids 12, Sids 13 and Sakha 93) and behenic acid proportion (in Sakha 93). For the remaining cultivars, fatty acids proportions either increased or remained unchanged in response to drought.
In response to drought, marked increase in polyunsaturated fatty acids proportions was recorded in Arabidopsis leaves (Gigon et al. 2004). Also, earlier studies indicated lower proportions of polyunsaturated fatty acids in drought- sensitive cultivars; with an opposite higher or unchanged proportions in their tolerant relatives in response to drought (Repellin et al. 1997). Thus, strong relation was previously suggested between the plant capacity to maintain or upgrade its polyunsaturated fatty acid content and its drought tolerance. In the same context, Sánchez-Martín et al. (2018) reported that the drought- tolerant oat cultivar had higher content of polyunsaturated linolenic and linoleic acid when droughted. On contrary, the sensitive cultivar had higher content of the saturated arachidic, stearic and palmitic acids when droughted. According to Upchurch (2008), polyunsaturated fatty acids play a fundamental role in the maintenance of cellular membranes and stability of proteins. Supporting the assumption that leaf fatty acid profile may control plant response to drought, significant positive correlation was recorded herein for the concerned wheat cultivars between the drought- induced change in their (1) leaf lauric acid proportion and its sclerophylly degree, (2) leaf tridecanoic acid proportion and its ABA content as well as (3) leaf tridecanoic acid proportion and its total soluble sugar content. Also, significant negative correlation was recorded between the drought- induced change in (1) leaf lauric acid proportion and its succulence degree as well as (2) leaf stearic acid proportion and its stomatal opening area (Fig. 2).
Changes in shoot growth at heading
Data in Fig. 1 and Table S1 revealed that drought decreased shoot biomass, density and distribution of the concerned cultivars; but such decrease was non- significant in the two Misr cultivars (for shoot fresh mass), the two Misr cultivars, the two Gemmeiza cultivars and Giza 168 (for shoot dry mass), all cultivars except the two Sids cultivars and Sakha 94 (for shoot density) as well as the two Misr cultivars, Gemmeiza 11, Shandaweel 1 and Giza 168 (for shoot distribution). The drought- induced decrease in shoot biomass was previously noticed in other studies; but when different cultivars were studied, the degree of such reduction varied greatly among cultivars (Tahir and Mehid 2001). In this context, a common impact of drought on crop plants is marked reduction in shoot biomass and/or length as a result of (1) the drop in photosynthetic output, (2) difficulty in mineral uptake, (3) disturbance in carbohydrate and protein metabolism and/or (4) inhibition of transpiration (Farooq et al. 2009). For that, significant positive correlation was recorded herein between the drought- induced change in shoot fresh mass and leaf transpiration rate; along with negative correlation between the drought- induced change in in shoot growth criteria and leaf stomatal opening area (Fig. 2).
Changes in yield capacity at maturity
Data in Fig. 1 and Table S1 also revealed that drought decreased biomass, straw, grain and crop yield of all wheat cultivars; with the maximum percent of decrease in Shandaweel 1 or Giza 168, while the minimum percent of decrease was recorded in Sids 13. Such drop in yield under drought can be attributed to the stress- induced accumulation of ABA (Farooq et al. 2009). In this regard, significant negative correlation was recorded herein between the drought- induced change in biomass yield and that in leaf stomatal opening area at heading (Fig. 2). Moreover, the reduction in yield capacity of the droughted plants can be also linked with the ill impact of stress on flag leaf growth and the translocation of photo- assimilates to the developed grains (Farooq et al. 2009). So, significant positive correlation was recorded herein between the drought- induced change in each of grain and crop yield with leaf stearic acid proportion at heading (Fig. 2).
Cluster analysis of the concerned cultivars
Based on the drought- induced change in their traits, the studied wheat cultivars could be sequestered into three groups. The two Sids cultivars, Sakha 93 and Misr 1 were recognized as drought tolerant; with the minimum drought- induced decrease in their shoot growth traits at heading as well as the minimum decrease in their yield at maturity. On contrary, Shandaweel 1, Giza 168 and Misr 2 were grouped together as the most drought sensitive; with almost the maximum drought- induced decrease in their shoot growth traits and yield capacity. The remaining cultivars were clustered as drought moderate (Fig. 3). Similar clustering order was identified for the concerned cultivars when evaluating other vegetative and yield parameters (Mickky et al. 2019).
Fig. 3.

Cluster analysis of ten wheat cultivars based on the drought- induced percent change in their traits
Conclusion
According to the results obtained from the present study, the two Sids cultivars seemed to be the most tolerant to drought; so they can be recommended for cultivation with little water supply, while Shandaweel 1, Giza 168 and Misr 2 proved to be the most drought sensitive. In addition, such drought- tolerant cultivars can be employed in breeding programs in a trial to obtain more efficient cultivars requiring the minimum amount of irrigation water.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the members of Sakha Agricultural Research Center for their help in obtaining pure strains of the wheat cultivars used in the current study.
Funding
This study was funded by the Scientific Research Unit of Mansoura University (Grant Number Competitive Project 8.12.2014).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
5. References
- Ali K, Gujjar R, Niwas R, Gopal M. A rapid method for estimation of abscisic acid and characterization of ABA regulated gene in response to water deficit stress from rice. Am J Plant Physiol. 2011;6:144–156. doi: 10.3923/ajpp.2011.144.156. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of AOAC (Association of Official Analytical Chemists) 17. Gaithersburg: AOAC; 2000. [Google Scholar]
- Arduini I, Godbold DG, Onnis A. Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pinaster seedlings. Physiol Plant. 1994;92:675–680. doi: 10.1111/j.1399-3054.1994.tb03039.x. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Ca2+ signaling and control of guard-cell volume in stomatal movements. Curr Opin Plant Biol. 2000;3:196–204. doi: 10.1016/S1369-5266(00)00064-9. [DOI] [PubMed] [Google Scholar]
- Daryanto S, Wang L, Jacinthe PA. Global synthesis of drought effects on maize and wheat production. PLoS ONE. 2016;11:e0156362. doi: 10.1371/journal.pone.0156362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C, Read J, Sanson G. Characterising sclerophylly: some mechanical properties of leaves from heath and forest. Oecologia. 2000;123:158–167. doi: 10.1007/s004420051001. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SM. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Gietler M, Nykiel M, Zagdańska BM. Changes in the reduction state of ascorbate and glutathione, protein oxidation and hydrolysis leading to the development of dehydration intolerance in Triticum aestivum L. seedlings. Plant Growth Regul. 2016;79:287–297. doi: 10.1007/s10725-015-0133-z. [DOI] [Google Scholar]
- Gigon A, Matos A, Laffray D, Zuily-fodil Y, Pham-Thi A. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia) Ann Bot. 2004;94:345–351. doi: 10.1093/aob/mch150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat A. Osmolytes and their role in abiotic stress tolerance in plants. In: Roychoudhury A, Tripathi D, editors. Molecular plant abiotic stress: biology and biotechnology. Hoboken: Wiley; 2019. [Google Scholar]
- Jones LA. Anatomical adaptations of four Crassula species to water availability. Biosci Horizons. 2011;4:13–22. doi: 10.1093/biohorizons/hzr002. [DOI] [Google Scholar]
- Karabourniotis G, Bornman J. Penetration of UV-A, UV-B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiol Plant. 1999;105:655–661. doi: 10.1034/j.1399-3054.1999.105409.x. [DOI] [Google Scholar]
- Kitson GF, Larsen SB, McEwen NC. Gas chromatography and mass spectrometry: a practical guide. New York: Academic Press; 1996. [Google Scholar]
- Marček T, Hamow KA, Végh B, Janda T, Darko E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE. 2019;14:e0212411. doi: 10.1371/journal.pone.0212411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickky BM, Abbas MA, El-Shhaby OA. Alterations in photosynthetic capacity and morpho-histological features of leaf in alfalfa plants subjected to water deficit-stress in different soil types. Indian J Plant Physiol. 2018;23:426–443. doi: 10.1007/s40502-018-0383-7. [DOI] [Google Scholar]
- Mickky BM, Aldesuquy HS, Elnajar MI. Drought-induced change in yield capacity of ten wheat cultivars in relation to their vegetative characteristics at heading stage. Physiol Mol Biol Plants. 2019;25:1137–1148. doi: 10.1007/s12298-019-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moinuddin K, Khannu-Chopra R. Osmotic adjustment in chickpea in relation to seed yield and yield parameters. Crop Sci. 2004;44:449–455. [Google Scholar]
- Nir I, Moshelion M, Weiss D. The Arabidopsis gibberellin methyl transferase 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014;37:113–123. doi: 10.1111/pce.12135. [DOI] [PubMed] [Google Scholar]
- Novick KA, Miniat CF, Vose JM. Drought limitations to leaf-level gas exchange: results from a model linking stomatal optimization and cohesion-tension theory. Plant Cell Environ. 2016;39:583–596. doi: 10.1111/pce.12657. [DOI] [PubMed] [Google Scholar]
- Repellin A, Pham-Thi AT, Tashakorie A, Sahsah Y, Daniel C, Zuily-Fodil Y. Leaf membrane lipids and drought tolerance in young coconut palms (Cocos nucifera L.) Eur J Agron. 1997;6:25–33. doi: 10.1016/S1161-0301(96)02034-5. [DOI] [Google Scholar]
- Sánchez-Martín J, Canales FJ, Tweed JK, Lee MR, Rubiales D, Gómez-Cadenas A, Arbona V, Mur LA, Prats E. Fatty acid profile changes during gradual soil water depletion in oats suggests a role for jasmonates in coping with drought. Front Plant Sci. 2018;9:1077. doi: 10.3389/fpls.2018.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradadevi R, Palta JA, Siddique KHM. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front Plant Sci. 2017;8:1251. doi: 10.3389/fpls.2017.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir MHN, Mehid SS. Evaluation of open pollinated sunflower (Helianthus annuus L.) populations under water stress and normal conditions. Int J Agric Biol. 2001;3:236–238. [Google Scholar]
- Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- Witkowski E, Lamont B. Leaf specific mass confounds leaf density and thickness. Oecologia. 1991;88:486–493. doi: 10.1007/BF00317710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.