Abstract
The spike traits of wheat can directly affect yield. F2 and F2:3 lines derived from the cross of the multi-spikelet female 10-A and the uni-spikelet male BE89 were used to detect QTLs for spike length (SL), total spikelet number per spike (TSS), kernel number per spike (KNS) and thousand-kernel weight (TKW) in four different environments. A total of 1098 SNP and 5 SSR were used to construct genetic map of 2398.1 cM with the average distance of 2.2 cM between markers. A total of 11 QTLs were identified for spike traits, including three QTLs for SL, five QTLs for TSS, two QTLs for KNS and one QTL for TKW. The QTLs mapped to chromosomes 2D, 4A, 6A, 7A and 7B explained 8.2–37.8% of the phenotypic variation in single environment. The major QTL confidence interval with distance of 0.5 cM was located on chromosome 4A and detected in multiple environments, which can explain more than 30% of the phenotypic variation for SL, TSS and KNS. Combining IWGSC RefSeq v1.0 and RNA-seq data for 10-A and BE89, we identified 16 genes expressed on spike or grain in four QTL regions. These findings provide insights into improving wheat yield through increasing spikletes in wheat, particularly through the use of the multi-spikelet female 10-A for breeding.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00823-0) contains supplementary material, which is available to authorized users.
Keywords: Wheat, Multi-spikelet, Spike-related trait, QTL, RNA-seq
Introduction
Common wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD subgenomes) is one of the most important food crops in the world (Gupta et al. 2008). Spike traits, including total spikelet number per spike (TSS), kernel number per spike (KNS) and thousand-kernel weight (TKW), are important determinants for wheat yield (Cui et al. 2013). Given the importance of spikes on yield, wheat varieties are divided into large-spike, intermediate and multi-spike types.
In recent years, many QTLs related to spike traits have been mapped. The numbers of QTLs controlling spike-related traits depend on population and genotype of the cultivar. Kumar et al. (2007) detected QTLs for spike length and kernel number per spike on eight chromosomes and nine chromosomes, respectively. QTLs controlling kernel number per spike and total spikelet per spike were detected on chromosomes 6B, 1D, 4D and 1B, 6B, 3D in different populations, and contributed to 4.94–10.97% of the phenotypic variation (Mcintyre et al. 2010; Cui et al. 2012). Ma et al (2007) reported a major QTL for spike length at chromosome 7D using recombinant inbred lines and an immortalized F2 population, and this QTL explained 29.7–36.3% of the phenotypic variation. Major QTLs for thousand kernel weight were detected at chromosomes 3A and 6A (Deng et al. 2017), 2A and 2D (Li et al. 2015), 7A (Su et al. 2016), and these QTLs explained more than 15% of the phenotypic variation.
Multi-spikelet wheat has higher spikelet number per spike and kernel number per spike. Therefore, wheat cultivars can be improved for higher yield by using multi-spikelet wheat. The wheat germplasm 10-A is a multi-spikelet line with more than 26 spikelets per spike. It is a valuable parental resource for breeding high-yield wheat. Through chromosome locating method, the loci that control the yield of wheat in 10-A were located at chromosomes 5A, 7A, 1B, 2B, 6B, 2D and 7D (Zheng et al. 1994). In addition, the 1RS chromosome fragment was detected in 10-A by analyzing rye prolamines (Wei and Zhou 1999).
In this study, F2 and F2:3 lines derived from the cross 10-A × BE89 were used to construct a genetic map with 1098 SNP and length of 2398.11 cM using wheat GBS 1.0 platform. QTLs associated with SL, TSS, KNS and TKW were detected in four environments. Candidate genes were observed in confidence intervals by Analysis for IWSGC Refseq v1.0 and RNA-seq data of 10-A and BE89, and were verified by qRT-PCR. These results will provide insight for improving wheat yield through increasing spikelets, particularly the utility of the multi-spikelet germplasm 10-A.
Materials and methods
Plant materials
One F2 population and three F2:3 lines from a cross between 10-A and BE89 were used for this study. Female 10-A is a wheat line with unbranching multi- spikelet, large spike and low grain weight. Male BE89 is a wheat line with low spikelet number, more total tiller and short spike that resulted from the cross ‘Batavia × Erine’ (Fig. 1). The F2 population consisted of 186 plants, and was grown at Sichuan Agricultural University, Wenjiang District, Sichuan Province, China in 2016, and used for genetic map construction and phenotype evaluation. Seeds from the F2 population were randomly divided into three parts to generate three F2:3 lines to evaluate the phenotype of the population.
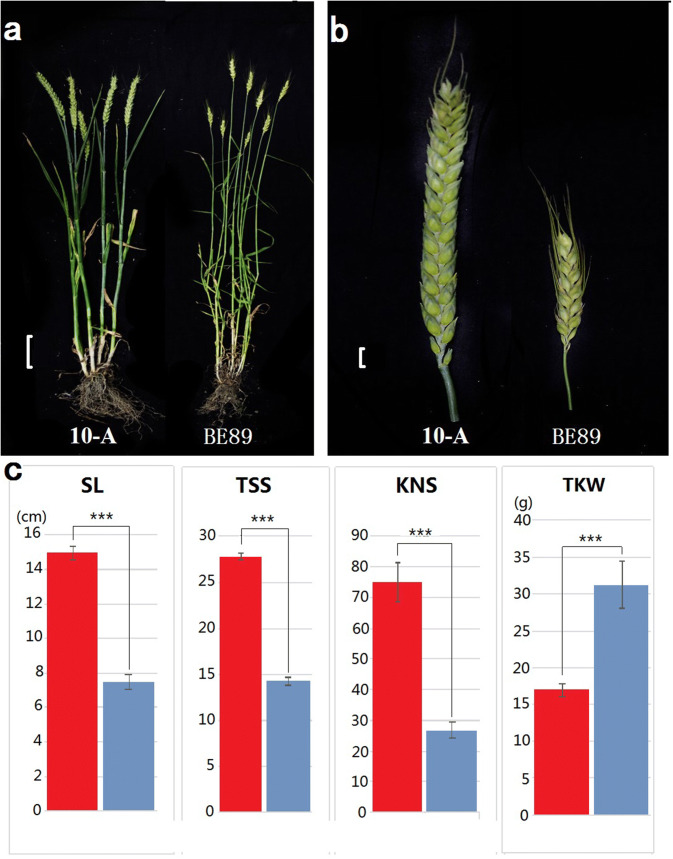
Fig. 1.
The differences in spike traits between female 10-A and male BE89. a The differences of whole plant phenotype between parents. Scale bar represents 10 cm. b The differences in spike morphology between parents. Scale bar represents 1 cm. c The differences of four spike traits between parental lines. The red column represents 10-A and the blue column represents BE89. Abbreviations: SL spike length, TSS total spikelet per spike, KNS kernel number per spike, and TKW thousand-kernel weight
Phenotypic evaluation and data analysis
The F2 population was evaluated at Wenjiang (103°51′ E, 30°43′ N, E1) in 2016. The F2:3 lines were evaluated in three different environments: Wenjiang (E2), Chongzhou (103° 38′ E, 30° 32′ N, E3) and Ya’ an (103° 01′ E, 29° 54′ N, E4) in 2017. Each line consisted of single seeds planted in one row that was 2 m in length with 10 cm between each plant, and the interval between every row was 30 cm. The field was managed following the common practices for wheat production. Spike traits, including spike length (SL, cm), total spikelet per spike (TSS), kernel number per spike (KNS) and thousand-kernel weight (TKW, g) were measured by randomly choosing five plants in each row. Phenotypic data was counted using Microsoft Office Excel 2013. Statistical analyses were performed using IBM SPSS Statistics 20.0 and the broad-sense heritability (H2) for each trait was estimated using SAS 9.2.
Genotyping
Genomic DNA was extracted from 2 g leaves at the four-leaf stage of the F2 seedling, and the DNA was sent to Diversity Arrays Technology Pty Ltd (Canberra, Australia) for genotyping using the Wheat GBS 1.0 platform. Of the 4011 SNP markers, the ones with no difference in both parental lines and did not conform to the Chi-square test of Mendel's law (1:2:1) were removed. This resulted in 1219 SNP markers remained and were used to construct the genetic linkage map.
Polymorphism between 10-A and BE89 was tested using 108 SSR markers based on GrainGene 2.0 (https://wheat.pw.usda.gov/GG2/). Regions of interest were amplified by PCR with the following conditions (20 µL total volume): 2 µL of 10 × Taq Plus Buffer, 1.6 µL of dNTP Mixture (10 mM), 10 pmol of each forward and reverse primer, 1.5 ng of template DNA, 0.3 µL of Taq Plus DNase (2.5 U/µL), and double distilled H2O to make up the final volume. 10 × Taq Plus Buffer, dNTP Mixture and Taq Plus DNase were obtained from TIANGEN biochemical technology (Beijing, China). The PCR program was as follows: pre-denaturation at 94 °C for 5 min; amplification for 38 cycle denaturing at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min; and extension at 72 °C for 7 min. PCR products were mixed with 5 µL of Loading buffer and denatured at 95 °C for 5 min, then the mixture was detected by electrophoresis using 6% polyacrylamide gel and 1 × TBE Buffer for 1.5 h–2.0 h at constant power 80 W. Silver staining is used to visualize fragments, size of which are used to determine genotypes. There were 21 SSR markers polymorphic between the parental populations, of which 5 were included in the genetic map (Table S1).
Map construction and QTL analysis
The unanchored markers were sorted into groups using the MAP function in IciMapping 4.0 with a logarithm of odds (LOD) threshold of 3 according to anchored markers. The linkage map was constructed using JoinMap 4.0, and markers were sorted using recombination frequency of 0.35 and LOD threshold of 4 using the Regression Mapping Algorithm and Kosambi mapping function.
The QTL for spike traits were detected through inclusive composite interval mapping (ICIM) using IciMapping 4.0, and a test of 1000 permutations was used to identify the LOD threshold that corresponded to a genome-wide false discovery rate of 5% (p < 0.05).
Prediction of candidate gene
We used Triticeae Multi-omics Center (https://202.194.139.32/blast/viroblast.php) to search for SNP flanking sequences of QTL confidence interval in order to obtain their physical location on chromosomes. The candidate genes in QTL confidence interval were obtained on Annotation browser (https://wheat-urgi.versailles.inra.fr/Tools/Annotation-browser) according to their above physical location by referring to IWGSC RefSeq v1.0, and were made analysis of expression patterns on expVIP (https://www.wheat-expression.com).
RNA-seq analysis
Leaves, young spike (W3) and seeds (W10, Waddington et al. 1983) were collected from both parental genotypes. 2 g of leaves were collected from one plant at the floral differentiation stage as one sample. Young spike was collected from 15 plants at the floral differentiation stage to mix as one sample. Seeds were collected from one plant at the seed filling stage as one replication. A total of 18 samples were obtained (two genotypes, three tissues and three biological replications). Total RNA was extracted using TRIzol reagent (Invitrogen). Digital gene expression libraries were generated using the NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biosystems), following the manufacturer’s instructions and sequencing was performed on a HiSEquation 2000 platform (Illumina).
Reference genome sequences and annotations were downloaded from the CS genome website (ftp://ftp.ensemblgenomes.org/pub/plants/release-42/). Reads were aligned to the genome using TopHat (v.2.09; Trapnell et al. 2009; Kim et al. 2013) with the following parameters: read-mismatches of 2; segment mismatches of 1; max-multihits of 20; r of 0. Reads were mapped to the gene models using HTSeq v.0.6.1 (https://www-huber.embl.de/users/anders/HTSeq/). For all comparisons, read counts were normalized to the aligned RPKM (Mortazavi et al. 2008) to obtain relative levels of expression. Differential gene expression analysis between stages, each with two biological replicates, was implemented using DESeq (Anders and Huber 2010), and the number of reads in each sample assigned to each gene was modeled using a negative binomial distribution.
The mean and variance were estimated from the read counts and differential expression between different conditions under the null hypothesis, which posited that read counts should be similar across all the conditions. Therefore one test was performed between two conditions to identify differentially expressed genes. For each gene, an adjusted p value was computed by DESeq, and those with an adjusted p value of 0.05 were considered as differentially expressed. The Pearson correlations between biological replicates were calculated using the R packages COR function using the RPKM values. A Venn diagram was made using a gene list for each stage derived by combining the genes from all genotypes, and this was mapped using the vennDiagram function in R version 3.5.2. For differentially expressed genes (DEGs), Gene Ontology (GO) functional enrichment analysis was performed using the Gene ontology database (https://geneontology.org/) to annotate the gene function of differentially expressed genes. Functional categories of putative unigenes were grouped using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/kegg/).
DEGs identified in the RNA-seq data between 10-A and BE89 that belonged to target regions were located using EnsemblePlants (https://plants.ensembl.org/Triticum_aestivum/Info/Index). We used Triticeae Multi-omics Center (https://202.194.139.32/getfasta/index.html) to obtain the sequences of the DEGs by their location on the chromosome and those sequences were subjected to a BLAST search in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
qRT-PCR analysis
Combining the information on candidate genes and DEGs in QTL confidence interval, a total of ten candidate genes were selected and verified by qRT-PCR. Total RNA was isolated from young spike at bi-ridged stage using Plant RNA Purification Kit V1.5 (BIOFIT, China) according to the manufacturer’s instruction. Using PrimScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Japan), 1ug of total RNA was reverse transcribed into the complementary DNA (cDNA) according to the manufacturer’s instruction. The primers of candidate genes and reference gene were designed by Primer Premier 5 software.
qRT-PCR was conducted on the CFX96 real-time PCR system (Bio-Rad, USA). All reactions were performed using ChamQTM Universal SYBR® qPCR Master Mix Q1711-02/03 (Vazyme, China) in triplicate of each sample. Total of 10 μL reaction volume contained 5 μL 2 × ChamQ Universal SYBR qPCR Master Mix, 0.5 μL each primer (10 μM), 1 μL cDNA template and 3 μL DNase/RNase free water. Reaction protocol was set with three-step cycling conditions: 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and melting curve collected in 60 °C for 60 s, 95 °C for 15 s. Relative expression of candidate genes were calculated by 2−ΔΔCt method.
Result and analysis
Phenotypic analysis
Among the four spike traits that were measured in this study, TSS of female 10-A was always over 28 in the four environments that were tested. This was twice as much as TSS in male BE89, which was an average of 14 across all of the environments. SL and KNS of female 10-A were 14.97 cm and 55 kernels, respectively, and they were significantly higher than that of male BE89, which had 7.5 cm SL and 26.8 kernels in E1 (Wenjiang in 2016). TKW was 31.19 g for BE89 and 16.93 g for 10-A. In the F2 population and F2:3 line, the average of SL ranged from 10.18 cm (E3, Chongzhou in 2017) to 11.08 cm (E2, Wenjiang in 2017) in the four environments, where the coefficient of variation (CV) was highest as 25.69% in E2 and the lowest as 12.45% in E4 (Ya’an in 2017). The means of TSS were 22.1 in E2 and E4, and above 20 in E1 and E3. The maximum TSS was 29 in E2 and E4, and the CV of TSS was highest in E1 (14.86%). The average KNS ranged from 23.8 to 35.6 in different environments, and the CV of KNS was more than 30% in all of the environments. The mean value of TKW was low at E3 (27.77 g), moderate at E1 and E4 (34.09 g and 35.36 g, respectively), and high at E2 (41.82 g). The mean of CV for the four environments was 27.54%, where E3 had the highest variation at 34.95% and E2 had the lowest variation at 19.97%. The skewness and kurtosis for SL, TSS, KNS and TKW indicated that they had a normal distribution and showed the genetic characteristics of typical quantitative traits. The broad-sense heritability (H2) was 0.60 for SL, 0.84 for TSS, 0.71 for KNS and 0.58 for TKW, suggesting that genetic factors played a major role in the variation of these four spike traits (Table 1).
Table 1.
Phenotypic analysis of spike traits in 10-A and BE89 and their offspring
| Trait name | H2 | Environment | Parents | Population | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-A | BE89 | Mean ± SD | Variance | Min | Max | Kurtosis | Skewness | C.V. (%) | |||
| SL | 0.84 | E1 | 14.97 | 7.50 | 10.70 ± 2.15 | 4.64 | 5.5 | 16.70 | −0.69 | 0.09 | 20.13 |
| E2 | 16.00 | 8.33 | 11.08 ± 2.85 | 8.10 | 7.63 | 14.90 | 0.63 | −0.24 | 25.69 | ||
| E3 | 14.83 | 8.50 | 10.18 ± 1.28 | 1.65 | 6.80 | 13.77 | 0 | −0.13 | 12.61 | ||
| E4 | 15.76 | 8.20 | 10.95 ± 1.36 | 1.86 | 7.53 | 15.13 | 0.06 | 0.16 | 12.45 | ||
| TSS | 0.60 | E1 | 28.80 | 14.30 | 20.80 ± 3.10 | 9.50 | 14.00 | 28.00 | −0.82 | 14.00 | 14.86 |
| E2 | 28.30 | 14.00 | 22.10 ± 2.70 | 7.61 | 14.70 | 29.00 | −0.48 | 14.70 | 12.50 | ||
| E3 | 28.20 | 13.30 | 20.20 ± 2.70 | 7.12 | 13.70 | 28.30 | −0.02 | 13.70 | 13.23 | ||
| E4 | 29.50 | 14.50 | 22.10 ± 2.60 | 6.52 | 15.70 | 29.00 | −0.26 | 15.70 | 11.55 | ||
| KNS | 0.71 | E1 | 55.00 | 26.80 | 26.10 ± 11.60 | 133.30 | 5.00 | 55.00 | −0.77 | 5.00 | 44.31 |
| E2 | 48.30 | 18.30 | 35.60 ± 11.4 | 130.40 | 9.70 | 63.70 | −0.55 | 9.70 | 32.10 | ||
| E3 | 43.30 | 20.30 | 23.80 ± 8.60 | 74.40 | 4.00 | 48.00 | −0.30 | 4.00 | 36.21 | ||
| E4 | 52.00 | 20.70 | 33.10 ± 11.16 | 124.59 | 3.00 | 59.70 | 0.20 | 3.00 | 33.81 | ||
| TKW | 0.58 | E1 | 17.50 | 31.40 | 34.09 ± 8.64 | 74.59 | 10.00 | 53.60 | 0.11 | 10.00 | 25.33 |
| E2 | 17.10 | 29.15 | 41.82 ± 8.35 | 69.77 | 12.00 | 58.43 | 1.54 | 12.00 | 19.97 | ||
| E3 | 16.50 | 33.30 | 27.77 ± 9.70 | 94.17 | 10.00 | 53.52 | 5.99 | 10.00 | 34.95 | ||
| E4 | 16.60 | 30.89 | 35.36 ± 10.58 | 111.9 | 10.00 | 63.66 | 0.98 | 10.00 | 29.92 | ||
Abbreviations: SL spike length; TSS total spikelet per spike; KNS kernel number per spike; TKW thousand-kernel weight; H2 broad-sense heritability; C.V. coefficient of variation; E1 Wenjiang where F2 lines were planted in 2016; E2 Wenjiang where F2:3 lines were planted in 2017; E3 Chongzhou where F2:3 lines were planted in 2017; E4 Ya’an where F2:3 lines were planted in 2017
Genetic map construction
A total of 1103 markers (1098 SNP and 5SSR) were used to construct a genetic map across 21 chromosomes. This map consisted of 31 linkage groups, with total length of 2398.1 cM and an average distance of 2.7 cM between each marker. The five SSR markers were mapped at chromosomes 5A and 7A, which was consistent with the location of known chromosomal markers. Chromosome 5B had the most markers, with 118 markers and a length of 162.3 cM, and chromosome 6D had the fewest markers, with 9 markers and a length of 30.9 cM. The longest chromosome was 7A, which was 239.1 cM with 85 markers. The shortest chromosome was 3D, which was 8.0 cM with 10 markers. The length of the genetic map for subgenomic A, B and D were 1100.1 cM, 892.4 cM and 405.6 cM, respectively, with 463, 515, 125 markers and a density of 2.4 cM, 1.73 cM and 3.3 cM, respectively (Table S1).
QTL analysis
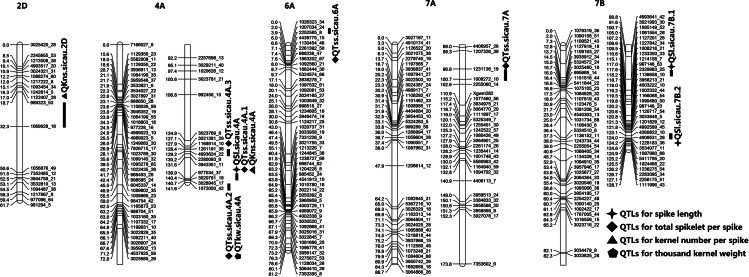
The wheat population was evaluated in four different environments to identify QTLs for spike traits, and criteria for QTL was LOD value higher than 3 and phenotypic variance explanation (PVE) higher than 8%. A total of 11 significant QTLs were detected, which were located on chromosomes 2D, 4A, 6A, 7A and 7B (Table 2, Fig. 2). The QTLs explained between 8.2% and 37.8% of the phenotypic variation. Two QTL clusters controlling two or three traits were observed on chromosome 4A, with interval lengths of 0.54 cM and 0.86 cM. Interval 977534_37-3020781_18 controlled SL, TSS and KNS, and explained more than 30% of the phenotypic variation. The interval 3028945_17-1072050_42 explained 30% of TSS and 18% of TKW phenotypic variation.
Table 2.
QTLs for spike traits detected in four environments of the 10-A × BE89 mapping population
| Trait Name | QTL | Environment | Interval | LOD | PVE (%) | Add | Dom | Distance (cM) |
|---|---|---|---|---|---|---|---|---|
| SL | QSl.sicau-4A | E1 | 977534_37-3020781_18 | 18.6 | 36.6 | 1.6 | 0.4 | 0.54 |
| E2 | 4.8 | 11.5 | 1.1 | 1.2 | ||||
| QSl.sicau-7B.1 | E3 | 2329983_25-1136658_10 | 10.7 | 8.8 | −0.7 | -0.1 | 0.72 | |
| QSl.sicau-7B.2 | E3 | 4260822_10-4909531_26 | 17.7 | 15.9 | 1 | 0.1 | 1 | |
| TSS | QTss.sicau-4A.1 | E2 | 977534_37-3020781_18 | 26.2 | 37.8 | 2.3 | 1.4 | 0.54 |
| QTss.sicau-4A.2 | E1 | 3028945_17-1072050_42 | 24.6 | 30.1 | 2.5 | 0.3 | 0.86 | |
| E4 | 19.6 | 30.2 | 2.1 | 0.7 | ||||
| QTss.sicau-4A.3 | E3 | 1261191_36-4993624_22 | 5.4 | 13.8 | 1.2 | 0.5 | 0.78 | |
| QTss.sicau-6A | E1 | 2252585_8-1007034_24 | 12.8 | 13.3 | −1.7 | 0.2 | 2.06 | |
| QTss.sicau-7A | E2 | 1231136_19-1008272_10 | 7 | 8.2 | 1.1 | 0.1 | 5.06 | |
| KNS | QKns.sicau-2D | E3 | 989323_53-1069928_18 | 4.7 | 9.1 | 4.1 | −0.8 | 13.6 |
| QKns.sicau-4A | E1 | 977534_37-3020781_18 | 12.6 | 28.2 | 7.9 | 2.5 | 0.54 | |
| E2 | 14.5 | 32.2 | 7.1 | 6.3 | ||||
| E4 | 6.6 | 14.6 | 5.5 | 2.9 | ||||
| TKW | QTkw.sicau-4A | E2 | 3028945_17-1072050_42 | 7.2 | 18.1 | 4.6 | 2.7 | 0.86 |
Abbreviations: SL spike length; TSS total spikelet per spike; KNS kernel number per spike; TKW thousand-kernel weight; LOD logarithm of odds; PVE phenotypic variation estimated from marker regression against the phenotype; Add additive effect; Dom dominant effect; cM centimorgan; E1 Wenjiang where F2 population were planted in 2016; E2 Wenjiang where F2:3 lines were planted in 2017; E3 Chongzhou where F2:3 lines were planted in 2017; E4 Ya’an where F2:3 lines were planted in 2017
Fig. 2.
The genetic maps and QTL for spike traits of chromosomes 2D, 4A, 6A, 7A and 7B. Abbreviations: QSl QTLs for spike length; QTss QTLs for total spikelet per spike; QKns QTLs for kernel number per spike; QTkw QTLs for thousand-kernel weight
For SL, we detected three QTLs in three environments (E1, E2 and E3), which were located on chromosomes 4A and 7B. The phenotypic variance was explained by a single QTL ranging from 8.8% (QSl.sicau-7B.1) to 36.6% (QSl.sicau-4A). Additive effects of QSl.sicau-4A and QSl.sicau-7B.2 were positive, indicating that the allele with increased length of spike was from female 10-A. QSl.sicau-4A had the highest LOD and PVE, with 18.6 and 36.6% in E1, respectively.
For TSS, five major QTLs were mapped on chromosomes 4A, 6A and 7A. QTss.sicau-6A and QTss.sicau-7A were detected in E1 and E2, and these QTLs explained 13.3% and 8.2% of the phenotypic variation with LOD score of 12.8 and 7, respectively. QTss.sicau-4A.2 explained about 30% of the phenotypic variation in E1 and E4, and the QTL had a confidence interval of 0.86 cM. QTss.sicau-4A.1 (E2) and QTss.sicau-4A.3 (E3) on chromosome 4A explained 37.8% and 13.8% of the phenotypic variation, respectively. Their LOD score was 26.2 and 5.4, respectively. The additive effects of QTss.sicau-4A.1, QTss.sicau-4A.2, QTss.sicau-4A.3 and QTss.sicau-7A, were positive, indicating that female 10-A as allele donor on total spikelet per spike and this was consistent with the multiple spikelet of 10-A.
Two QTLs contributing to KNS were detected on chromosomes 2D and 4A in the four environments. QKns.sicau-4A explained 28.2% (E1), 32.2% (E2) and 14.6% (E4) of the phenotypic variation. QKns.sicau-2D explained 9.1% of the phenotypic variation in E3. We only identified QTkw.sicau-4A controlling TKW in E2, and it had LOD score of 7.2 and explained 18.1% of the phenotypic variation.
Candidate gene prediction
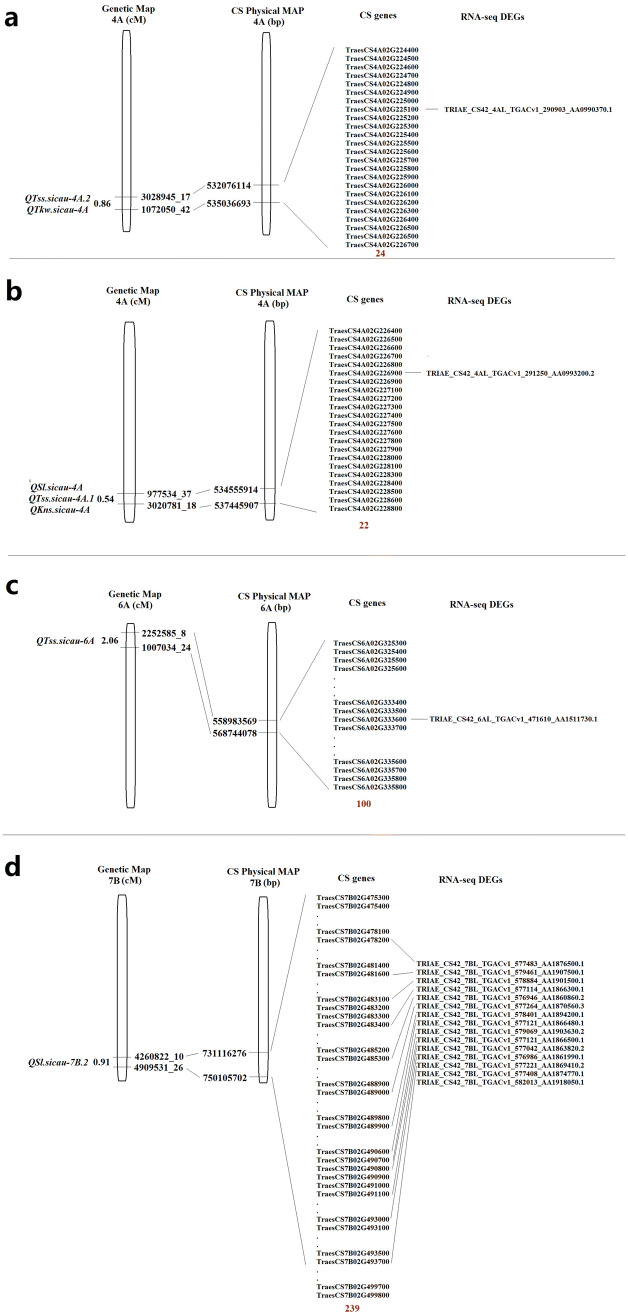
We selected confidence intervals of four major QTL regions (C1, C2, C3 and C4) and identified their physical size on the wheat reference genome sequence. The regions contained QTss.sicau-4A.2 and QTkw.sicau-4A(C1), QSl.sicau-4A, QTss.sicau-4A.1 and QKns.sicau-4A (C2), QTSS.sicau-6A(C3), QSl.sicau-7B.2(C4). The physical length of the four intervals were 2.96 Mb (C1), 8.89 Mb (C2), 9.76 Mb (C3), and 18.99 Mb (C4), respectively. Referring to IWGSC RefSeq v1.0, 24 genes in C1, 22 genes in C2, 100 genes in C3, and 239 genes in C4 were mapped to the target regions (Table S2, Supplementary 1).
We selected five tissues of seven stages of development time-course of Chinese Spring for analyzing candidate genes’ expression pattern. A total of 12 genes in C1, 15 genes in C2, 67 genes in C3, and 65 genes in C4 were expressed in spike or grain (Fig. S1).
RNA sequencing analysis
A total of 3739, 2575 and 2531 DEGs between 10-A and BE89 were observed in the young spike (W3), leaves (W3) and seeds (W10), respectively. These genes were found on 21 chromosomes, and most of the genes were on chromosome 4A (Table S3). Of the DEGs, 1306 (in young spike), 859 (in leaves) and 1185 (in seeds) were up-regulated, and 2433 (in young spikes), 1716 (in leaves), and 1446 (in seeds) genes were down-regulated in BE89 compared to 10-A. A total of 430 differentially expressed genes were found in all three tissues. A total of 415 DEGs were shared in the spike primordium and leaves, and 443 DEGs were shared in young spike and seeds, and 161 DEGs were shared in leaves and seeds (Fig. S2). DEGs (Supplementary 2) in the young spike, seeds and leaves with p value of 0.01 were then subjected to GO and KEGG analyses. A total of 1159 genes (373 DEGs in young spike, 391 DEGs in seeds and 395 DEGs in leaves) were annotated in the GO database. These genes belonged to cellular component, metabolic process, single-organism process, binding, catalytic activity, transporter activity, cell part, membrane and organelle categories. In the KEGG analysis, 258 genes (79 DEGs in young spike, 87 DEGs in seeds and 92 DEGs in leaves) were categorized as belonging to protein metabolic process, nucleosome assembly, microtubule-based movement, ribosome biogenesis, pentose-phosphate RNA processing, iron-sulfur cluster assembly by, carbohydrate transport, pentose-phosphate shunt, carbohydrate metabolic process, photosynthsis, protein-chromophore linkage and single transduction pathways (Table S4, Fig. S3).
Based on the DEGs that were obtained between 10-A and BE89, 18 DEGs were mapped in the four candidate regions C1, C2, C3 and C4. One of the DEGs between seeds and leaves was located in the C1 region, one DEG that was up-regulated in the leaf was in the C2 region, and one DEG in the leaves was located in the C3 region. In contrast, the C4 region contained 15 DEGs, of which 11 were differentially expressed in the leaves, two in the young spike and leaves, one in the young spike and seeds, and one in the young spike (Table S5). Of 18 DEGs, 16 were overlapped with candidate genes in four confidence intervals (Fig. 3).
Fig. 3.
The candidate genes and their comparison with DEGs of RNA-seq of four confidence intervals.a–d The candidate genes and their comparison with DEGs of RNA-seq in the a C1, b C2, c C3, and d C4. Abbreviations: DEGs differential expression genes
qPCR analysis of candidate genes
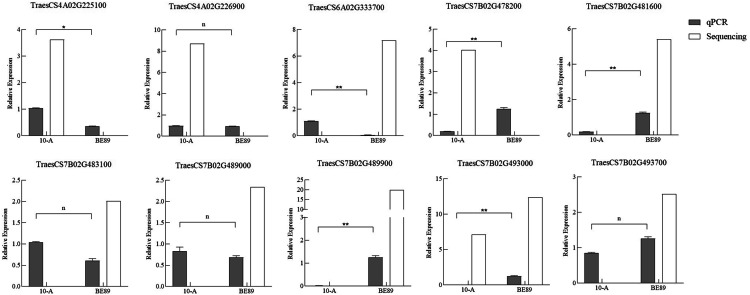
Ten candidate genes were selected for qRT-PCR analysis (Fig. S1). The result showed that a total of six genes expressed differently between young spikes of 10-A and BE89. Among those genes, the gene TraesCS4A02G225100 expressed higher in 10-A than in BE89 and showed significant difference (p < 0.05). Five genes (TraesCS6A02G333700, TraesCS7B02G478200, TraesCS7B02G481600, TraesCS7B02G489900 and TraesCS7B02G493000) were significantly differentially expressed between 10-A and BE89 (p < 0.01). Among them, the gene TraesCS6A02G333700 was expressed higher in 10-A than in BE89, while other four genes were expressed higher in BE89. The expression patterns of six genes were consistent partially with results of RNA-seq, which were TraesCS4A02G225100, TraesCS7B02G481600, TraesCS7B02G489900, TraesCS7B02G493000 (Fig. 4).
Fig. 4.
Comparison between sequencing and qRT-PCR analysis of ten candidate genes. White representes RNA-seq data, and gray representes qRT-PCR. The bars represent standard error of the mean (SEM). *, ** and n represent p < 0.05, p < 0.01 and no significant difference
Discussion
Wheat yield characterizations include productive spike per unit area, kernel number per spike and kernel weight (Ma et al. 2007). Increasing kernel number per spike is an important approach to improve wheat yield (Ortiz-Monasterio et al. 1997). Total kernel number is affected strongly by total spikelet number per spike (TSS) and number of viable florets per spikelet, and TSS is positively correlated with spike length (SL) (Ma et al. 2007). We found that female 10-A and male BE89 showed divergent spike traits. WE constructed a genetic map using their hybrid F2 and F2:3 populations to identify QTLs that control spike traits. These QTLs can be further used to identify genes or markers of interest to improve breeding of wheat. Eleven major QTLs for spike traits were detected, where each QTL explained 8.2% to 37.8% of the phenotypic variation. The PVE of four major QTLs, QSl.sicau-4A, QTss.sicau-4A.1, QTss.sicau-4A.2 and QKns.sicau-4A, were over 30%. The RNA sequencing analysis showed that one DEG between the parental cultivars was located in C1, C2 and C3, respectively, and 16 DEGs were located in C4. These QTLs and candidate genes can contribute to further understanding of target traits, fine mapping and developing molecular markers.
Other researchers used their own F2 population to locate QTLs for spike traits. Wang et al. (2011) found major QTLs for SL that mapped to chromosome 2D, 4A and 6B with 10% of PVE or higher. Major QTLs for TKW and KNS mapped on chromosome 4B, and these QTLs explained 16% and 18% of the phenotypic variation, respectively. Thanh and Vladutu (2013) detected major QTLs that regulated SL, TSS and KNS on chromosome 2A, which explained 70%, 33% and 7% of the phenotypic variation, respectively. In this study, we detected QTLs for spike traits on chromosomes 4A and 7B (for SL), 4A, 6A and 7A (for TSS), 2D and 4A (for KNS), 4A (for TKW), respectively. Chen et al. (2017) and Millet (1986) have reported loci controlling TSS on chromosomes 1A, 1D and 5B. Our analysis mapped QTLs for TSS to chromosomes 4A, 6A and 7A, and this likely resulted from the differences in the cultivars used to investigate these traits. QTss.sicau-4A.2 was detected on E1 and E4, and explained 36.6% and 11.5% of the phenotypic variation; however, this region has not been reported in other studies before. For kernel number per spike, the QTLs were reported on 1A, 1D, 2A, 2B, 4A, 4B, 4D, 5A and 6B (Blanco et al. 2012). We also detected QKns.sicau-4A (E1, E2 and E4) on 4A, but QKns.sicau-2D may be a new locus in our results.
In recent years, RNA sequencing (RNA-seq) has been used for QTL mapping in wheat. Ramirez-Gonzalez et al. (2015) developed 22 markers linked to stripe rust resistance gene Yr15 using RNA-Seq of bulked pool wheat F2 populations. Also using RNA-seq technology, multiple differential genes were found in the wheat QTLs for Fusarium head blight Fhb1 and Qfhs.ifa-5A using four near isogenic lines (Kugler et al. 2013). Here, we mapped QTLs for spike traits using F2 and F2:3 populations by combining Wheat GBS 1.0 platform and RNA-seq, and this allowed for rapid detection of candidate genes in target regions.
In this study, there were 24, 22, 100 and 239 (total of 385) candidate genes in region C1, C2, C3 and C4 according to IWSGC RefSeq v1.0, respectively. Of 385 candidate genes, 18 genes showed different expression between the parents. By expression pattern analysis for 18 candidate genes, a total of ten genes were expressed at spike or/and seed. We selected six genes to verify the expression by qRT-PCR, one from C1, one from C3 and four from C4. A gene TRIAE_CS42_4AL_TGACv1_290903_AA0990370.1 (C1), named TaSPS, was located from 533,000,096 to 533,006,978 bp (total length of 6883 bp) on chromosome 4AL. This gene has 12 exons with transcript length of 4044 bp, and translation length of 1347aa. It is homologous to sucrose-phosphate synthase (SPS) in Aegilops taushii (XM_020321656.1), Hordeum vulgare (AK356780.1), Brachypodium (XM_003577628.4), Zea mays (XM_008679801.1), Oryza sativa (XM_015762188.2) and Triticum aestivum (AF310160.1) with sequence similarity of 83% or higher. SPS (EC 2.4.1.14) is a key rate-limiting enzyme that controls sucrose synthesis in crops (Winter and Huber 2000), and the synthesis of sucrose affects growth, development and yield (Huber and Huber 1996). SPS was first detected on wheat germ, and was subsequently found in germinated seed of wheat and rice (Hawker 1971). SPS genes have five families in gramineous crops (A, B, C, DIII and DIV) in moncotyledons, and three families (A, B and C) in dicotyledons (Castleden et al. 2004). Rocher et al. (1989) found that SPS activity in Zea mays was positively correlated with growth at the seedling stage. Seneweera et al. (1995) reported that SPS activity was positively correlated with blade elongation rate in rice. These results suggest that SPS genes affect crop growth. Overexpression of SPS genes in rice and tobacco results in taller plants compared to the control (Ishimaru et al. 2004; Park et al. 2008). Micallef et al. (1995) and Baxter et al. (2003) revealed that SPS transgenic tomatoes and tobacco had more total inflorescence compared to wild-type plants. Therefore high SPS activity promotes crop growth and development. In addition, SPS overexpression increases fruit crop yield and results in higher dry weight of seeds in tomato (Micallef et al. 1995). In transgenic potato overexpressing SPS, stem and leaf weight, tuber yield and root weight were higher than the control group (Tobias et al. 1999). There was only one differentially expressed gene, TaSPS, in the target interval C1 that controlled total spikelet per spike (TSS) and thousand-kernel weight (TKW). We speculate that this gene may affect TSS and TKW of wheat based on our results and previous studies on the role of SPS in crop growth and yield. For other five candidate genes in C3 and C4, three genes had no obvious homologs in other species based on BLAST search. One gene TRIAE_CS42_7BL_TGACv1_578401_AA1894200.1 was in chromosome 7B ranged from 743649262 to 743660024 bp with 9 exons. Its homolog was the disease resistance protein RGA3 (LOC109782965) in Aegilops tauschii. Another gene TRIAE_CS42_7BL_TGACv1_577483_AA1876500.1 was in chromosome 7B ranged from 733527185 to 733530566 bp with 7 exons. Its homolog of cDNA was TaMYB59 with query cover of 63% in Triticum aestivum L.
Future studies to verify and focus on the effect of TaSPS on the spike phenotype of wheat would be important to validate our results. For the candidate genes in the confidence intervals, we would further analyze their annotated functions and verify them. The genes affect target traits would be subjected to further functional studies.
Conclusion
In this study, F2 and F2:3 lines are derived from the multi-spikelet wheat germplasm 10-A. The QTLs for spike length (SL), total spikelet number per spike (TSS), kernel number per spike (KNS) and thousand-kernel weight (TKW) are mapped on five chromosomes, QSl.sicau-4A, QTss.sicau-4A.1 and QKns.sicau-4A can explain more than 30% of the phenotypic variation. Referring to Chinses Spring genome and analysis of expression pattern, 158 candidate genes were expressed on spike or grain in four QTL regions. And combined with RNA-seq results of 10-A and BE89, among 158 candidate genes, a total of ten genes show differences in expression between 10-A and BE89.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2017YFD0100900, 2016YFD0100102-13) and The Applied Basic Research Programs of Science and Technology Department of Sichuan Province (2018JY0020).
Author contributions
C-HK, X-F Z, KY, Z-PZ, LD carried out experiments, and analyzed the data; Z-EP, JM, Q-TJ, G-YC, J-RW analyzed the data and contributed to writing; C-HK, Y-MW, Y-LZ, and WL conceived and designed the experiments, and wrote the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cheng-Hao Kuang and Xiao-Fang Zhao have contributed equally to this paper.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter CJ, Foyer CJ, Rolfe SA, Quick WP. Elevated sucrose-phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. J Exp Bot. 2003;54(389):1813–1820. doi: 10.1093/jxb/erg196. [DOI] [PubMed] [Google Scholar]
- Blanco A, Mangini G, Giancaspro A, Giove S, Colasuonno P, Simeone R, et al. Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol Breed. 2012;30(1):79–92. doi: 10.1007/s11032-011-9600-z. [DOI] [Google Scholar]
- Castleden CK, Aoki N, Gillespie VJ, Macrae EA, Quick WP, Buchner P, et al. Evolution and function of the sucrose-phosphate synthase gene families in wheat and other grasses. Plant Physiol. 2004;135(3):1753–1764. doi: 10.1104/pp.104.042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wu XY, Wu K, Zhang JP, Liu WH, Yang XM, et al. Novel and favorable genomic regions for spike related traits in a wheat germplasm pubing 3504 with high grain number per spike under varying environments. J Integr Agric. 2017;16(11):2386–2401. doi: 10.1016/S2095-3119(17)61711-8. [DOI] [Google Scholar]
- Cui F, Ding A, Li J, Zhao C, Wang L, Wang X, et al. QTL detection of seven spike-related traits and their genetic correlations in wheat using two related RIL populations. Euphytica. 2012;186(1):177–192. doi: 10.1007/s10681-011-0550-7. [DOI] [Google Scholar]
- Cui F, Zhao C, Li J, Ding A, Li X, Bao Y, et al. Kernel weight per spike: what contributes to it at the individual QTL level? Mol Breed. 2013;31(2):265–278. doi: 10.1007/s11032-012-9786-8. [DOI] [Google Scholar]
- Deng ZY, Cui Y, Han QD, Fang WQ, Li JF, Tian JC. Discovery of consistent QTLs of wheat spike-related traits under nitrogen treatment at different development stages. Front Plant Sci. 2017;8:2120. doi: 10.3389/fpls.2017.02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Mir RR, Mohan A, Kumar J. Wheat genomics: present status and future prospects. Int J Plant Genomics. 2008;2008:1–36. doi: 10.1155/2008/896451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker JS. Enzymes concerned with sucrose synthesis and transformations in seeds of maize, broad bean and castor bean. Phytochemistry. 1971;10(10):2313–2322. doi: 10.1016/S0031-9422(00)89872-6. [DOI] [Google Scholar]
- Huber SC, Huber JL. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47(2):431. doi: 10.1146/annurev.arplant.47.1.431. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Ono K, Kashiwagi T. Identification of a new gene controlling plant height in rice using the candidate-gene strategy. Planta (Berlin) 2004;218(3):388–395. doi: 10.1007/s00425-003-1119-z. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. Tophat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler KG, Siegwart G, Nussbaumer T, Ametz C, Spannagl M, Steiner B, et al. Quantitative trait loci-dependent analysis of a gene co-expression network associated with fusarium head blight resistance in bread wheat (Triticum aestivum L.) BMC Genomics. 2013;14(1):728. doi: 10.1186/1471-2164-14-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gill BS, Faris JD. Identification and characterization of segregation distortion loci along chromosome 5B in tetraploid wheat. Mol Genet Genomics. 2007;278(2):187–196. doi: 10.1007/s00438-007-0248-7. [DOI] [PubMed] [Google Scholar]
- Li CL, Bai GH, Carver BF, Chao SM, Wang ZH. Single nucleotide polymorphism markers linked to QTL for wheat yield traits. Euphytica. 2015;206(1):89–101. doi: 10.1007/s10681-015-1475-3. [DOI] [Google Scholar]
- Ma ZQ, Zhao DM, Zhang CQ, Zhang ZZ, Xue SL, Lin F, et al. Molecular genetic analysis of five spike-related traits in wheat using RIL and immortalized F2 populations. Mol Genet Genomics. 2007;277(1):31–42. doi: 10.1007/s00438-006-0166-0. [DOI] [PubMed] [Google Scholar]
- Mcintyre CL, Mathews KL, Rattey A, Chapman SC, Drenth J, Ghaderi M, et al. Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions. Theor Appl Genet. 2010;120(3):527–541. doi: 10.1007/s00122-009-1173-4. [DOI] [PubMed] [Google Scholar]
- Micallef BJ, Haskins KA, Vanderveer PJ, Roh KS, Sharkey STD. Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an increased capacity for sucrose synthesis. Planta. 1995;196(2):327–334. doi: 10.1007/BF00201392. [DOI] [Google Scholar]
- Millet E. Genetic control of heading date and spikelet number in common wheat (T. aestivum L.) line ‘NOA’. Tag Theor Appl Genet. 1986;72(1):105. doi: 10.1007/BF00261463. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Ortiz-Monasterio RJI, Sayre KD, Rajaram S, Mcmahon M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997;37(3):898–904. doi: 10.2135/cropsci1997.0011183X003700030033x. [DOI] [Google Scholar]
- Park JY, Canam T, Kang KY, Ellis DD, Mansfield SD. Over-expression of an arabidopsis family a sucrose phosphate synthase (SPS) gene alters plant growth and fibre development. Transgenic Res. 2008;17(2):181–192. doi: 10.1007/s11248-007-9090-2. [DOI] [PubMed] [Google Scholar]
- Ramirez-Gonzalez RH, Segovia V, Bird N, Fenwick P, Holdgate S, Berry S, et al. RNA-seq bulked segregant analysis enables the identification of high-resolution genetic markers for breeding in hexaploid wheat. Plant Biotechnol J. 2015;13(5):613–624. doi: 10.1111/pbi.12281. [DOI] [PubMed] [Google Scholar]
- Rocher JP, Prioul JL, Lecharny A, Reyss A, Joussaume M. Genetic variability in carbon fixation, sucrose-p-synthase and ADP glucose pyrophosphorylase in maize plants of differing growth rate. Plant Physiol. 1989;89(2):416–420. doi: 10.1104/pp.89.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneweera SP, Basra AS, Barlow EW, Conroy JP. Diurnal regulation of leaf blade elongation in rice by CO2 (Is it related to sucrose-phosphate synthase activity?) Plant Physiol. 1995;108(4):1471. doi: 10.1104/pp.108.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZQ, Jin SJ, Lu Y, Zhang GR, Chao SM, Bai GH. Single nucleotide polymorphism tightly linked to a major QTL on chromosome 7A for both kernel length and kernel weight in wheat. Mol Breed. 2016;36(2):15. doi: 10.1007/s11032-016-0436-4. [DOI] [Google Scholar]
- Thanh PT, Vladutu CI. Molecular genetic analysis of domestication traits in emmer wheat. I: map construction and QTL analysis using an F2 papulation. Biotechnol Biotec Eq. 2013;27(2):3627–3637. doi: 10.5504/BBEQ.2013.0008. [DOI] [Google Scholar]
- Tobias DJ, Hirose T, Ishimaru K, Ishige T, Ohkawa Y, Kano-Murakami Y, et al. Elevated sucrose-phosphate synthase activity in source leaves of potato plants transformed with the maize Sps gene. Plant Prod Sci. 1999;2(2):92–99. doi: 10.1626/pps.2.92. [DOI] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. Tophat: discovering splice junctions with RNA-seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. A quantitative scale of spike initial and pistil development in barley and wheat. Annual of Botany. 1983;51:119–130. doi: 10.1093/oxfordjournals.aob.a086434. [DOI] [Google Scholar]
- Wang JS, Liu WH, Wang H, Li LH, Wu J, Yang XM, et al. QTL mapping of yield-related traits in the wheat germplasm 3228. Euphytica. 2011;177(2):277–292. doi: 10.1007/s10681-010-0267-z. [DOI] [Google Scholar]
- Wei YM, Zhou YH. Detection of the rye chromatin in multispikelet wheat germplasm 10-A background using fluorescence in situ hybridization (FISH) and RFLP markers. Acta Bot Sin. 1999;41(7):722–725. [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem. 2000;35(4):37. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Zheng YL, Yan J, Yang JL. Effect of chromosomes on grain yield per plant in the common wheat multispikelet line 10-A. Hereditas. 1994;16(4):27–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.