Abstract
WRKY transcription factors (TFs) are a large plant-specific family of TFs that govern development and biotic/abiotic stress responses in plants. We have identified SlWRKY23 as a gene primarily expressed in roots. SlWRKY23 encodes a protein of 320 amino acids that functions as a transcriptional activator. It is transcriptionally up-regulated by ethylene, BAP and salicylic acid treatment but suppressed by IAA. Expression of SlWRKY23 in transgenic Arabidopsis affects sensitivity of roots to ethylene, JA and auxin with transgenic plants showing hypersensitivity to ethylene, JA and auxin-mediated primary root growth inhibition. This hypersensitivity is correlated with higher expression of ERF1 and ARF5 that mediate responses to these hormones. SlWRKY23 expression also affects aerial growth with transgenic plants showing greater number of leaves but smaller rosettes. Flowering time is reduced in transgenic lines and these plants also show a greater number of inflorescence branches, siliques and seeds. The siliques are longer and compactly packed with seeds but seeds are smaller in size. Root biomass shows a 25% decrease in transgenic SlWRKY23 Arabidopsis plants at harvest compared with controls. The studies show that SlWRKY23 regulates plant growth possibly through modulation of genes controlling hormone responses.
Keywords: WRKY, IAA, Salicylic acid, Primary root, Seed, Lateral root
Introduction
Plant growth and root/shoot architecture are controlled by developmental cues and change with age and in response to environmental and edaphic conditions. Dynamic changes in hormone levels and responses during development, adaptation and defense, guide plant growth. Hormone responses, in turn, are tightly controlled at the cellular and tissue level by several factors including by regulation of transcription factors (TFs). A large number of TFs that are adapted for plant-specific functions, have been identified in Arabidopsis and plants of different families (Reichmann and Ratcliffe 2000).
The WRKY TF family is a large plant-specific family with members having at least one conserved DNA-binding region (WRKY domain) of about 60 amino acids, consisting of a highly conserved WRKYGQK peptide sequence and a zinc finger motif (Rushton et al. 1996; Eulgem et al. 2000). The WRKY family consists of 74 members in Arabidopsis and 109 members in rice while 81 members have been identified in tomato (Huang et al. 2012). WRKYs can be classified into three main groups based on the number of WRKY domains and the type of zinc-finger motifs (Eulgem et al. 2000). Group I WRKYs contain two WRKY domains with a C2H2 zinc-finger motif. Group II WRKYs have a single WRKY domain and a C2H2 zinc-finger motif and can be further divided into five subgroups (II-a, b, c, d, and e). Group III WRKYs have a single domain with a C2HC zinc-finger motif.
The WRKY TF family has a multi-faceted role primarily in biotic and abiotic stress responses. A large number of WRKYs are known to function in defense against both biotrophic and necrotrophic organisms. These include AtWRKY33 that acts as a positive regulator of resistance against the necrotrophic fungi, Alternaria brassicicola and Botrytis cinerea (Zheng et al. 2006) and AtWRKY38 and AtWRKY62 that function negatively in basal resistance against P. syringae (Kim et al. 2008). In rice, OsWRKY13 contributes to resistance to Xanthomonas oryzae pv oryzae and Magnaportha grisea that cause bacterial blight and fungal blast respectively, by activating SA-biosynthesis and responsive genes (Qiu et al. 2007, 2008). In tomato, expression of SlWRKY31 and SlWRKY33 (homologues of AtWRKY33) compromise tolerance to B. cinerea. Similarly, expression of SlWRKY45 (a homologue of AtWRKY40) enhances susceptibility to the root-knot nematode Meloidogyne javanica by decreasing the expression of JA- and SA marker genes (Chinnapandi et al. 2017). WRKY family members also regulate abiotic stress responses. For instance, AtWRKY46 from Arabidopsis functions in stress tolerance by modulating ABA signaling and auxin homeostasis (Ding et al. 2015). In addition, WRKY18, WRKY40 and WRKY60 function in a complex that represses ABA responses (Liu et al. 2012). In tomato, SlWRKY45 was induced by cold treatment (Chen et al. 2015) while SlWRKY39, an orthologue of AtWRKY40, was induced by salt, drought, ABA, SA and JA (Huang et al. 2012; Sun et al. 2015). Two other genes, SlWRKY32 and SlWRKY74, were induced by drought stress (Huang et al. 2012). AtWRKY70, associated with an increase in SA responses during defense, negatively regulates water stress responses (Li et al. 2004; Chen et al. 2017).
Apart from their roles in stress responses, a few WRKY genes are also known to be involved in developmental processes although these are less explored. A WRKY protein, MINISEED3/AtWRKY10, is expressed in the endosperm and controls seed size (Luo et al. 2005). AtWRKY23 controls lateral root development as well as embryonal development by governing auxin responses (Grunewald et al. 2008, 2012, 2013). AtWRKY75 and AtWRKY12 promote flowering while AtWRKY13 negatively regulates it (Li et al. 2016; Zhang et al. 2018). AtWRKY53 is a key regulator of leaf senescence and functions in combination with AtWRKY18 and other proteins (Zentgraf et al. 2010; Potschin et al. 2014).
We were interested in root architecture and factors governing it. In this paper, we have identified SlWRKY23 as a gene that is primarily expressed in roots. We show that SlWRKY23 expression is governed strongly by different hormones and that its expression affects not only root responses in Arabidopsis but also aerial architecture and yield, indicating that it may be an important developmental regulator of tomato growth.
Materials and methods
Plant materials and conditions for growth and phytohormone treatment
Tomato (Solanum lycopersicum cv. Ailsa Craig) was used for isolation of SlWRKY23. Plants were grown in a glasshouse maintained at 23–25 °C. Various tissues like roots and leaves were collected from 15-day-, 1-month and 2-month-old plants. Other tissues such as flowers, and fruits were also collected. Fruit samples included pulp and seeds from mature ripened fruits. All samples were immediately frozen in liquid nitrogen and stored at − 70 °C until further use.
To study the expression of SlWRKY23 in response to different hormones, plants were grown on ½ MS agar in petriplates. After 15 days, these were transferred to test tubes containing ½ MS in hydroponic conditions and treated with hormones such as 1-aminocyclopropane-1-carboxylic acid (ACC, 10 µM), abscisic acid (ABA, 50 µM), indole acetic acid (IAA, 1 µM), 6-benzyl aminopurine (BAP, 1 µM), jasmonic acid (JA, 10 µM) and salicylic acid (SA, 10 µM). Treatments of roots were carried out for 0 h, 0.5 h, 1 h, 2 h and 4 h. For studying the effects of these hormones on root development, seeds of transgenic plants over-expressing SlWRKY23 were grown on ½ MS agar plates for 4 days and then transferred to plates containing either ACC (5 µM), IAA (0.5 µM), JA (10 µM) or SA (10 µM). The JA and SA treatments were carried out for 5 days post-transfer when measurable differences in root growth were observed while for auxin and ethylene these were conducted for 11 days post-transfer to observe the differences since these were not clear in 5 days.
Arabidopsis thaliana, ecotype Columbia (Col-0) was used for ectopic expression studies of SlWRKY23. Plants were grown under 16 h-light/8 h-dark, in soilrite in a culture room maintained at 22 ± 2 °C, with an optimized light intensity of 100 μE m−2 s−1 (Philips, India) and a relative humidity of 78 ± 4% at 25 °C.
RNA extraction and preparation of cDNA
Total RNA was extracted from various tissues and samples described above using Spectrum Plant Total RNA kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions. cDNA was prepared using the MuMLV Revertaid reverse transcriptase (Fermentas) and used for the expression analysis by real-time polymerase chain reaction (RT-PCR).
Real time PCR reaction and amplification conditions
RT-PCR was performed to quantify the expression of SlWRKY23 using the primers SlWRKY23-Fq (CATCTCTCTTCTCACTTCCTTCAT) and SlWRKY23-Rq (TCCAAAACCG TTGTTGTCAATG). Reactions were performed in three biological replicates and three technical replicates on an ABI StepOnePlus machine (Applied Biosystems Inc, USA). The analyzed real time reaction data was the mean of biological and technical triplicates. SYBR Select PCR Master Mix (ABI, USA) was used to run the reactions at the following cycle conditions: step 1, 50 °C, 2 min; step 2, 95 °C 10 min; step 3 (95 °C 15 s, 60 °C 1 min), × 40 cycles. Relative gene expression was calculated using 2−ΔCT method with SlCAC (SlCAC-Fq CCTCCGTTGTGATGTAACTGG and SlCAC-Rq (ATTGGTGGAAAGTAACA TCATCG) as an internal control in tomato (Expósito-Rodríguez et al. 2008). For studies on expression of different hormone-responsive genes, primers specific for ERF1 (AtERF1-Fq CGA CAGCGAGTTCGGTTACT and AtERF1-Fq GCTCTCGGTGAAGCA AGGAT), ERF2 (AtERF2-Fq GTTTGAGACGGCGGAAGATG and AtERF2-Rq CCTCAACGGAAAATTCAA TAAAGCG), ARF5 (AtARF5-Fq ATGGTGGAGGAACTATAACAAC and AtARF5-Rq GCTTTCTTGTACCTGACTGGTC), ARF7 (AtARF7-Fq TATGGCACGCTTGTGCTG and AtARF7-Rq TCAGTCTGCTTCTGCATTGAAGCC) and ARF19 (AtARF19-Fq ACAGCGA GCAAGTTGCAGCA and AtARF19-Rq GGTATCAGCATGTAA TGTAACACTG) were used for real time analysis.
Isolation of SlWRKY23 from tomato and transformation of Arabidopsis
SlWRKY23 was identified as a gene primarily expressed in roots from a tissue related transcriptome. The complete SlWRKY23 (963 bp) ORF was isolated from 2-month-old tomato root cDNA using the primer pair SlWRKY23-F0 (GGATCCATGGAAAGCTACAAAGAC) and SlWRKY23-R0 (GGATCCTCATCTGTTGTAGTCATG) as follows: denaturation at 94 °C for 3 min, 35 cycles of 10 s denaturation at 94 °C, 10 s annealing at 55 °C and extension at 72 °C for 60 s and final extension at 72 °C for 5 min. The PCR-amplified fragment was cloned in pTZ57R (Thermo scientific), confirmed by sequencing and then cloned in the plant expression vector pBI121 at BamHI in sense orientation for expression driven by CaMV35S promoter. This construct was introduced into A. thaliana (ecotype Columbia) by the floral dip method (Clough and Bent 1998). Transgenic seeds were screened on kanamycin (50 mg/L) and integration checked using the primers CaMV35S-F (GTAAGGGATGACGCACAATCC) and SlWRKY23Rq. These plants were grown subsequently to the homozygous T3 generation. Three independent lines L-3-4-2, L-4-4-1 and L-5-2-2 were chosen for all analyses that included germination, rosette growth, number of branches flowering time, silique and seed size and total yield. All phenotypic studies were carried out using 15 plants per line.
Phylogenetic analysis
CLUSTALW programme was used for multiple sequence alignment. Phylogenetic analysis was carried out using homologues of SlWRKY23 identified from a BLAST analysis. Protein sequences were fetched from NCBI with the following accession numbers: NP_001352739.1 (Solanum lycopersicum), XP_004239067.1 (Solanum lycopersicum), XP_004251825.2 (Solanum lycopersicum), XP_004233585.1 (Solanum lycopersicum), NP_182248.1 (Arabidopsis thaliana), NP_193551.1 (Arabidopsis thaliana), NP_001321561.1 (Arabidopsis thaliana), XP_025875631.1 (Oryza sativa), XP_015637917.1 (Oryza sativa),XP_015645717.1(Oryza sativa), NP_001288832.1 (Brassica rapa), XP_016676103.1 (Gossypium hirsutum), XP_016718752.1 (Gossypium hirsutum), NP_001313893.1 (Gossypium hirsutum), NP_001275158.1 (Solanum tuberosum), NP_001311512.1 (Capsicum annuum), XP_003554557.1 (Glycine max) WP_143240623.1 (Bifidobacterium adolescentis). Phylogenetic analysis was carried out using MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 (Tamura et al. 2013) and a UPGMA dendrogram was constructed by the bootstrap method with a Bootstrap value of 500.
Trans-activation assay
To check the nature of SlWRKY23 as an activator or repressor, it was first cloned in the yeast vector pGBKT7. A yeast transcriptional assay was carried out using the fusion of SlWRKY23-GAL4DNA BD in pGBKT7 to activate the MEL1 alpha galactosidase gene. Transformed Y187 cells were grown on SD/-Trp plate. Diploid cells having pGBKT7-lam and pGADT7-T vector were used as a negative control while diploid cells having pGBKT7 in fusion with p53 and pGADT7-T vector were used as a positive control. A blue colour after colony lift filter assay in presence of X-α gal indicated transcriptional activation.
Germination assays
For germination analysis, 100 sterilized seeds from each transgenic Arabidopsis line were sown on ½ MS medium. Seeds were stratified at 4 °C for 48 h and then transferred to 22 °C and the germination percentage was calculated at 24 h. For root length measurements, the plates were positioned vertically for the evaluation of root growth.
Statistical analysis
Duncan’s multiple range tests (P < 0.05) and one-way ANOVA test (significance level of P < 0.05) were used to analyze real time PCR and plant phenotypic analysis data respectively using SPSS software.
Results
SlWRKY23 shows similarity to other WRKY23-like proteins and functions as a transcriptional activator
SlWRKY23 (Solyc01g 079260.2.1) was identified as a 963 nt ORF encoding a 320 amino acid protein. The protein has a single WRKY domain that encompasses the region between 169 and 228 amino acids. It also possesses a single C2H2 type Zn finger motif from 195 to 226 amino acids. Based on its sequence characteristics it belongs to the group IIc type WRKY proteins (Huang et al. 2012). An amino acid alignment with WRKY23-like members from other plants revealed conservation only within the WRKY domain and the immediate flanking portions. Considerable differences were observed at the N and C-terminal ends of the protein (Fig. 1a). A phylogenetic analysis revealed closest similarity of SlWRKY23 with the potato StWRKY4 (Fig. 1b). Its closest homologue in Arabidopsis was AtWRKY23, known to be involved in auxin responses in embryonic development, lateral root formation, venation and defence (Grunewald et al. 2008, 2012, 2013; Prat et al. 2018). Similarity of WRKY23 to WRK57 homologues was also observed.
Fig. 1.
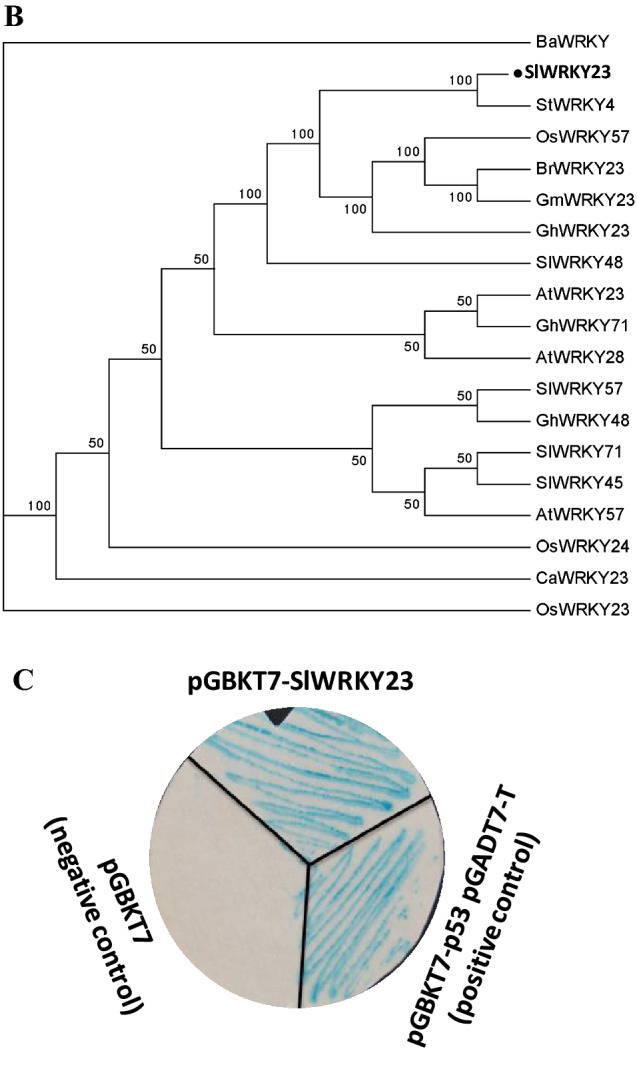
Sequence analysis, phylogeny and nature of SlWRKY23. a Alignment of the amino acid sequence of SlWRKY23 with its closest homologues in other plants. Sequences were aligned using the CLUSTAL W programme and presented using BOX shade programme. Black boxes indicate identical amino acids and grey boxes show the conserved residues. b Phylogenetic analysis of the full-length SlWRKY23 amino acid sequence with closest homologues in different plants. Analysis was carried out using the MEGA5 software with a bootstrap value of 500. c SlWRKY23 functions as a transcriptional activator. A yeast transcriptional assay was carried out using SlWRKY23 (in a fusion with GAL4DNA BD) in pGBKT7 to activate the MEL1 alpha galactosidase gene. Transformed Y187 cells were grown on SD/Trp plate. pGBKT7 in fusion with p53 and pGADT7-T vector was used as a positive control while the pGBKT7 empty vector was used as a negative control. Colony-lift filter assay was performed to indicate positive colonies which show blue color in the presence of X-α gal
Functionally, WRKY proteins are known to regulate processes by either activating or suppressing the expression of target genes (Robatzek and Somssich 2002; Xie et al. 2005). In order to investigate the nature of SlWRKY23 function, we first carried out a yeast transcriptional activation assay. The gene was cloned in pGBKT7 and expression of the downstream MEL1 α-galactosidase marker gene was studied using X-alpha-Gal solution as substrate. SlWRKY23 clearly functioned as an activator of transcription as evident from the blue coloured yeast colonies using the SlWRKY23-GAL4DNA BD fusion in pGBKT7 (Fig. 1c).
SlWRKY23 is transcribed primarily in roots and differentially regulated by various hormones
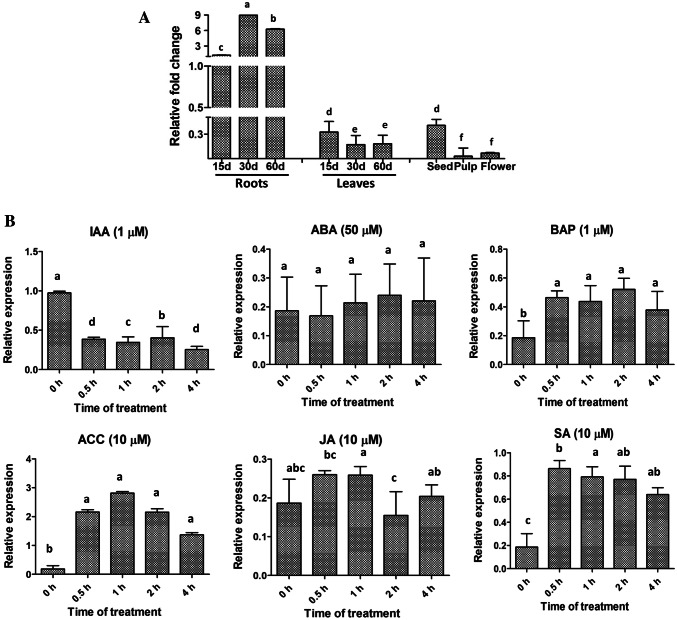
To obtain an insight into SlWRKY23 expression during plant development, steady state transcript levels of SlWRKY23 were estimated in different organs like roots, leaves, flowers, fruit and seeds and in different stages of plant development. SlWRKY23 was expressed primarily in roots (Fig. 2a). Within roots, it showed an age-dependent increase with expression increasing almost eightfold between roots of 15- and 30-day-old plants. It remained at least fivefold higher in roots of 2-month-old plants. Compared to roots, steady state transcript levels in leaves of 15-day-old plants were just ~ 25% of 15-day roots and these reduced further to less than half at 30 and 60 days. Expression in seeds was similar to that of 15-day leaves while expression in flowers and fruit pulp was just 10–25% of that observed in 15-day leaves.
Fig. 2.
Real-time PCR analysis of SlWRKY23 transcript accumulation in different tissues and in response to different hormones. aSlWRKY23 expression in different plant tissues at different stages of development. RNA was isolated from roots and leaves of 15-day, 1-month and 2-month-old plants, flowers and seeds/pulp from red mature fruits. Reactions were run in triplicates with SlCAC as the internal control for normalization. Error bars represent ± SE of three biological replicates. Expression values were analyzed by one-way ANOVA and compared using Duncan’s multiple range test (DMRT). Values on the bar carrying different letters are significantly different. bSlWRKY23 transcript accumulation in roots of 15-day-old plants in response to different hormones. Total RNA was isolated from roots of control plants and plants treated with IAA (1 µM), BAP (1 µM), ABA (50 µM) or ACC (10 µM) at various time intervals after treatment. Reactions were run in triplicates with SlCAC as the internal control for normalization. Error bars represent ± SE of three biological replicates. Analysis was carried out as described in a
We next studied the regulation of SlWRKY23 in roots by different hormones (Fig. 2b). SlWRKY23 transcription in roots was strongly and rapidly activated by ethylene, SA and BAP but suppressed by auxin. ACC (the precursor of ethylene) caused a more than tenfold increase in transcription within 30 min of treatment to roots, with peak levels at 1 h. Thereafter, there was a steady decline up to 4 h although transcripts levels still remained ~ sevenfold higher than control. SlWRKY23 also responded rapidly to BAP with an ~ twofold increase in transcript levels within 30 min that stayed high up to 4 h. SA too caused a 4-4.5-fold increase in transcript levels of SlWRKY23. Surprisingly, auxin treatment rapidly reduced transcript levels of the gene to just about 40% within 30 min and these remained low throughout the period of treatment. ABA and JA did not alter the transcript levels of SlWRKY23 much.
Constitutive expression of SlWRKY23 in Arabidopsis increases sensitivity of roots to growth inhibition by ethylene, auxin and JA
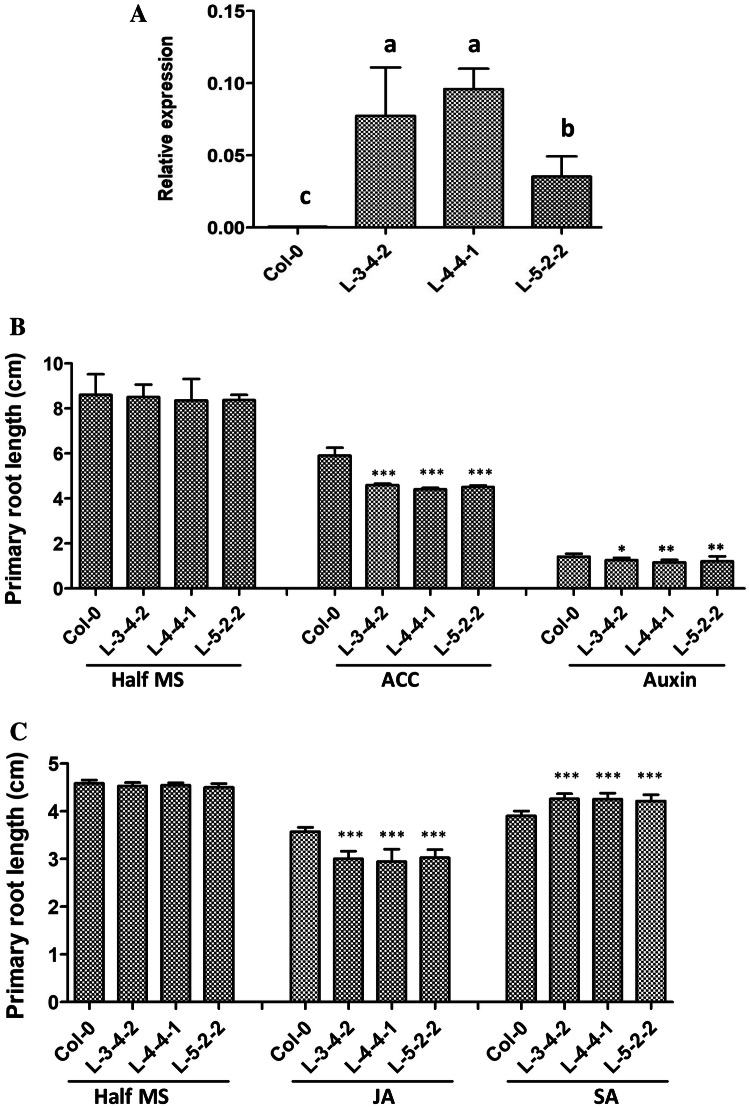
To obtain an idea about the possible function of SlWRKY23, transgenic Arabidopsis lines expressing SlWRKY23 under the constitutive CaMV35S promoter were generated. Analysis of growth was carried out in three independent homozygous T3 generation lines, L-3-4-2, L-4-4-1 and L-5-2-2 that showed high transcript levels of the SlWRKY23 transgene (Fig. 3a). The transgenic lines did not show changes in root growth when observed during the early stages of development at 9 days in absence of any hormone (Fig. 3b). However, since the genes were strongly responsive to hormones like ethylene, auxin and SA and likely to regulate their signalling, we compared root growth of control and transgenic SlWRKY23 over-expressing lines in presence of these hormones. Treatment with 5 µM ACC inhibited root growth in control by about ~ 30% with root length decreasing from 8.6 ± 0.54 cm to 5.9 ± 0.35 cm (Fig. 3b). Interestingly, a greater inhibition of root growth was observed in all transgenic lines with root growth reducing from 8.40 ± 0.06 to 4.5 ± 0.08 cm, representing an inhibition of ~ 46%. Treatment with auxin (which strongly suppressed SlWRKY23 transcription at 1 µM) was inhibitory at 0.5 µM and suppressed root growth in control and transgenic lines by ~ 83–85% (Fig. 3b). Nevertheless, suppression was slightly but significantly higher in transgenic lines although not as prominent as with ACC. Treatment with JA (10 µM) also reduced root growth by approximately 22% in controls compared to untreated roots (Fig. 3c). As in the case of ACC, the extent of primary root growth inhibition was much higher in transgenic lines with ~ 35% inhibition. Treatment with 10 µM SA led to only a marginal suppression of approximately 13% in root growth in controls. Transgenic plants, however, showed a lesser but nevertheless significant inhibition of 6% in root growth (Fig. 3c). The results showed that expression of SlWRKY23 rendered root growth in transgenic plants hypersensitive to inhibition by ethylene and JA and to a lesser extent with auxin.
Fig. 3.
Alteration in primary root length of transgenic Arabidopsis over-expressing SlWRKY23 in response to different hormones. a Real time expression analysis of SlWRKY23 in roots of homozygous lines of transgenic Arabidopsis over-expressing SlWRKY23. UBIQUITIN10 was used as an internal control. b Effect of ACC and IAA on primary root growth. Seeds of control and transgenic SlWRKY23 lines were germinated and allowed to grow for 4 days on ½ MS medium and then transferred to medium containing either 5 µM ACC or 0.5 µM IAA and allowed to grow for another 11 days and primary root length measured. Experiments were carried in three separate sets. Data represent mean values ± SE. Significant differences are shown as *P < 0.05; **P < 0.01 and ***P < 0.001. c Effect of JA and SA on primary root growth. Seeds of control and transgenic SlWRKY23 lines were germinated and allowed to grow for 4 days on ½ MS medium and then transferred to medium containing either 10 µM JA or 10 µM SA and allowed to grow for another 5 days and primary root length measured. Experiments were carried in three separate sets. Data represent mean values ± SE. Significant differences are shown as *P < 0.05; **P < 0.01 and ***P < 0.001
Constitutive expression of SlWRKY23 in Arabidopsis is associated with higher transcript levels of ERF1 and ARF5 but reduced transcript levels of ARF7
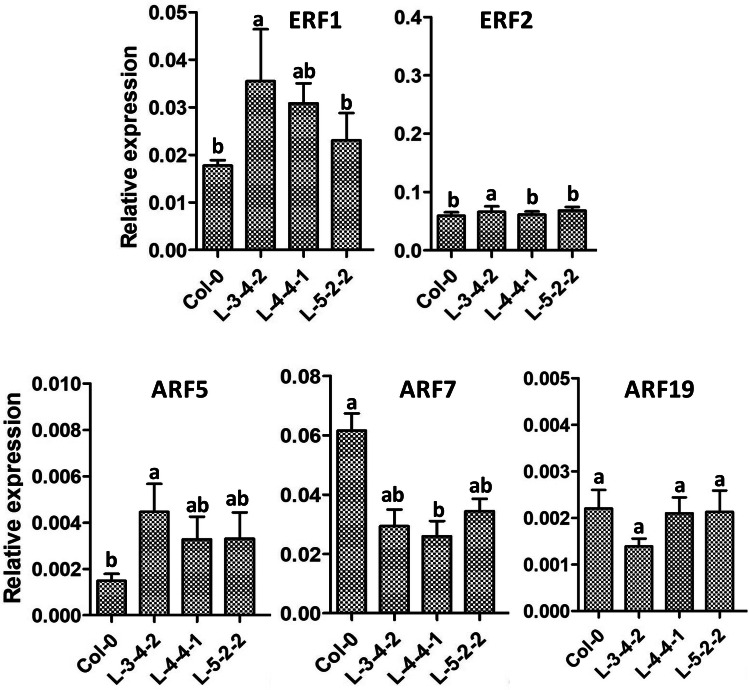
Since responses of root to inhibition by ethylene, JA and auxin were increased, we next studied the expression of a few genes known to govern downstream responses of hormones like ethylene, JA and auxin. Transcript levels of ERF1 and ERF2, both of which mediate ethylene and JA signalling, were monitored along with those of ARF5, ARF7 and ARF19 which regulate auxin responses during development and primary and lateral root formation (Przemeck et al. 1996; Hardtke and Berleth 1998; Mao et al. 2016; Lorenzo et al. 2003; Okushima et al. 2007). As shown in Fig. 4, basal levels of ERF1 transcripts were significantly higher in transgenic lines compared to control in at least two of the lines while expression of ERF2 did not undergo a change. Transcript levels of ARF5 were also higher in all three transgenic lines by almost two fold while those of ARF7 were reduced to just 50% in the transgenic lines (Fig. 4). No change was observed in expression of ARF19, a paralogue of ARF7. The results suggested that SlWRKY23 could alter expression levels of downstream signalling components of different hormones and thereby modulate the hormone responses.
Fig. 4.
Real-time PCR analysis of transcript accumulation of ERF1, ERF2, ARF5, ARF7 and ARF19 in roots of transgenic Arabidopsis lines over-expressing SlWRKY23. Seeds of control and transgenic SlWRKY23 lines were germinated and allowed to grow for 4 days on ½ MS medium. RNA was isolated from roots. Reactions were run in triplicates with UBIQUITIN10 as the internal control for normalization. Error bars represent ± SE of three biological replicates. Expression values were analyzed by one-way ANOVA and compared using Duncan’s multiple range test (DMRT). Values on the bar carrying different letters are significantly different
SlWRKY23 over-expression in Arabidopsis affects vegetative growth, flowering time, aerial architecture and silique size/seed number and root growth at later stages
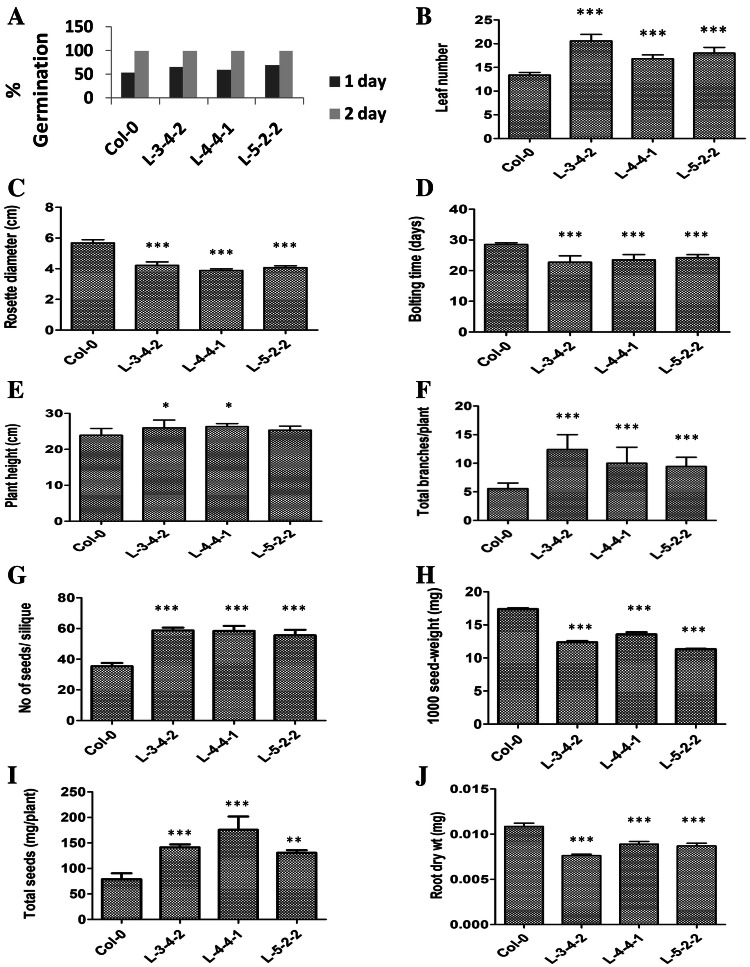
Besides root sensitivity to ethylene and auxin, over-expression of SlWRKY23 in the transgenic Arabidopsis lines also altered various aspects of aerial plant growth. Germination in transgenic lines was normal with no differences compared to control (Fig. 5a). However, leaf size and leaf number was considerably altered. All transgenic lines had a greater leaf number with 17–21 leaves, compared with control, which had about 13–14 leaves (Fig. 5b). The leaves were, however, smaller in size with rosettes that were 25% smaller in diameter in transgenic lines than in controls (Fig. 5c).
Fig. 5.
Alterations in aerial phenotypes of transgenic SlWRKY23 over-expressing Arabidopsis lines. Transgenic SlWRKY23 over-expressing lines were monitored at different stages throughout the course of their life cycle. Phenotypic analysis was carried out on 15 plants per line. Data represent mean values ± SE. Significant differences are shown as *P < 0.05; **P < 0.01 and ***P < 0.001. a Germination (n = 100), b leaf number, c Rosette diameter, d flowering time, e inflorescence height, f number of inflorescence branches, g number of seeds per silique, h 1000 seed weight, i seed yield, j root dry weight at harvest
Flowering time was advanced in transgenic SlWRKY23 over-expression lines with respect to time despite the increase in number of leaves. Inflorescence bolts were initiated at around 22–24 days with 17–20 leaves in transgenic lines compared to 27–28 days and 12–13 leaves in control (Fig. 5d). As the plants went into the reproductive phase, clear differences were seen in the aerial architecture. The inflorescence shoots were slightly but significantly taller in lines L-3-4-2 and L-4-4-1 (Fig. 5e). All transgenic lines showed a substantial increase in the number of flowering branches (Fig. 5f). Compared to an average of five branches in control, the transgenic line L-3-4-2 showed 10–15 branches, while L-4-4-1 and L-5-2-2 showed 7–13 branches. These branches contained siliques that were longer in size and more densely packed with a higher number of seeds/silique in all transgenic lines compared to control (Fig. 5g). Seed size was however reduced, as also apparent from the 1000-seed weight where a decrease of almost 29% in the weight was observed (Fig. 5h). Nevertheless, the increase in flowering branches and siliques in all the three transgenic lines led to a substantial 1.7–2-fold increase in total seed weight per plant (Fig. 5i). Interestingly, root growth showed a reduction at harvest in all transgenic lines compared to control (Fig. 5j). Root dry weight decreased by about 20% in all the lines with the greatest reduction observed in line L-3-4-2. The above results showed that SlWRKY23 expression could influence various aspects of plant growth through its effects on downstream targets.
Discussion
Regulation of root growth is under the control of several hormones and the expression of various transcriptional regulators. Collectively these respond to developmental needs and edaphic conditions and alter the root architecture to optimize plant growth. In this study, we have identified SlWRKY23 as a predominantly root-expressed gene in tomato, the expression of which increases considerably at later stages of plant growth. It is one of 83 WRKY genes known in tomato, a large number of which are regulated by biotic and abiotic stresses (Bai et al. 2018). Its transcription is under strong hormonal control, being induced in roots by hormones like ethylene (~ tenfold), SA (~ fivefold) and BAP (~ 2.5-fold) within 1 h of their treatment but strongly reduced by auxin to less than 50% within 30 min of treatment. All these hormones are known to affect root growth in different ways (Marchant et al. 2002; Casimiro et al. 2003; Laplaze et al. 2007; Street et al. 2015; Pasternak et al. 2019). Our studies show that manipulation of SlWRKY23 affects downstream responses of some of these hormones and affects root and shoot architecture.
Ethylene reduces primary root growth in Arabidopsis in a concentration-dependent manner through the auxin pathway (Swarup et al. 2007; Ruzika et al. 2007) by transcriptionally activating the auxin biosynthesis gene ASA1 (Mao et al. 2016). Both ethylene and cytokinin also suppress lateral root growth while auxin promotes it. Thus, transcriptional activation of SlWRKY23 by ethylene and BAP and its suppression by auxin in tomato roots would suggest a function in suppression of root growth. Indeed, transgenic Arabidopsis over-expressing SlWRKY23 show an increase in sensitivity of roots to ethylene and auxin and a greater reduction in primary root growth in response to these hormones compared to controls but not in their absence (Fig. 3b). The increase in sensitivity of roots to ethylene may be explained partly due to higher expression of ERF1. ERF1 is a key transcription factor in the ethylene pathway and an increase in its levels would increase ethylene responses (Solano et al. 1998). ERF1 is also a downstream positive regulator of JA responses (Lorenzo et al. 2003) and thus an increase in ERF1 levels could also increase JA responses and inhibit root growth as seen in SlWRKY23 over-expression lines (Fig. 3c). Further, ERF1 transcriptionally activates the auxin biosynthesis gene ASA1 (Mao et al. 2016). An increase in auxin levels would increase auxin signalling as evident from increased expression of the auxin response factor gene, ARF5. This, in turn, would increase sensitivity to auxin which inhibits root growth. The reduced differences between control and transgenic SlWRKY23 lines upon treatment with auxin is most likely due to higher concentrations of auxin used in the experiment which suppress root growth by more than 80%. The inhibition of roots by an increase in sensitivity to ethylene, JA and auxin and the simultaneous 50% reduction in transcript levels of the key mediator of lateral root development, ARF7 (Figs. 3b, c, 4, Okushima et al. 2007) may explain the reduction in root biomass by ~ 25% at later stages. While some of the changes in transgenic SlWRKY23 root in response to ethylene and auxin are described by expression of ERF1, ARF5 and ARF7, these are not sole regulators of the root phenotypes. It is likely that these as well as other targets of SlWRKY23, not studied by us, may differentially influence tissue phenotypes in SlWRKY23 over-expression lines with tissue-specific post-translational modifications of the TFs in response to ethylene or auxin adding further complexities.
Besides root development, expression of SlWRKY23 affects several aspects of aerial architecture such as leaf size, number of branches, silique, seed size and number. The reduction in leaf size may be a manifestation of the reduction in expression of ARF7 which is known to promote leaf expansion (Wilmoth et al. 2005). In addition, the increase in ethylene sensitivity from increased ERF1 expression may also explain the reduction in rosette size since ethylene is known to inhibit cell expansion (Kieber et al. 1993; Vaseva et al. 2018). One of the most prominent effects of SlWRKY23 expression is on the number of inflorescence branches which are considerably higher compared to controls (Fig. 5f). This increase was associated with an increase in number of siliques and total seed weight although the seeds were smaller in size than controls. The increase in branches could be an effect associated with higher cytokinin responses or a change in balance between auxin and cytokinin responses. Considering that auxin suppresses SlWRKY23 but BAP increases its transcription, it is likely that SlWRKY23 expression may activate cytokinin responses but suppress auxin responses through its action on some downstream targets that may also be common to Arabidopsis. This might manifest itself in the increased branching observed in the transgenic lines. Interestingly, another WRKY gene, AtWRKY71/EXB1, was shown to positively regulate branching by activating transcription of RAX1-3 (REGULATOR OF AXILLARY MERISTEMS 1-3) genes which initiated axillary meristems (Guo et al. 2015). The AtWRKY71 protein functions by reducing auxin responses and incidentally belongs to the same clade as WRKY23 and WRKY57 which are close homologues of SlWRKY23. Thus, SlWRKY23 and its homologues may have a conserved function in controlling branching. It should be noted however, that although the closest homologue of SlWRKY23 in Arabidopsis is AtWRKY23, similarity with AtWRKY23 is only shared within the WRKY domain and its immediate flanking portions (~ 85% amino acid identity) while the regions other than the WRKY domain show just ~ 25% identity. AtWRKY23, unlike SlWRKY23, is transcriptionally up-regulated by auxin and regulates auxin action in development of lateral roots, embryonic stem cell specification and nematode-plant interactions (Grunewald et al. 2012, 2013; Prat et al. 2018). It plays a key role in PIN polarization by regulating PIN2 lateralization and thereby mediating auxin responses required for lateral root development and vascular tissue formation during leaf venation. It also mediates auxin action in root stem cell specification through ARF5/MP and BODENLOS (Grunewald et al. 2013). In many respects, the effects of SlWRKY23 are in contrast to what is known about AtWRKY23 in Arabidopsis. These include reduction in transcription by auxin and the suppression of root growth by SlWRKY23 over-expression. These effects are probably associated with differences in the portion flanking the WRKY domain. These differences may affect binding and interactions of SlWRKY23 with other proteins and may impart it with regulatory properties that are different from AtWRKY23. Considering that Arabidopsis and tomato have different growth habits and a different architecture, these changes may cause the two putative orthologues to be regulated differently to suit the needs of each plant. Further studies through suppression of SlWKY23 in tomato will provide a better idea about its function in tomato.
In conclusion, we show that SlWRKY23 functions in development and controls root and shoot architecture partly through its effects on key regulators of ethylene, auxin and JA.
Acknowledgements
We are thankful to the Council of Scientific and Industrial Research (CSIR), India for financial support to Deepika Singh and Pratima Debnath. The work was supported by CSIR, India under the project BSC0204 and MLP26. Integral Manuscript Communication No: IU/R&D/2019-MCN000678 and NBRI Publication No. CSIR-NBRI_MS/2020/02/06.
Author’s contributions
VAS and APs conceptualized and designed the experiments, DS and PD carried out the experiments. VAS, APS, Roohi analysed the data. APS and VAS wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded by CSIR, India under the Projects BSC0204 and MLP26. NBRI Publication No. CSIR-NBRI_MS/2020/02/06.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai Y, Sunarti S, Kissoudis C, Visser RG, van Der Linden G. The role of tomato WRKY genes in plant responses to combined abiotic and biotic stresses. Front Plant Sci. 2018;9:801. doi: 10.3389/fpls.2018.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003;8:165–171. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang Y, Liu C, Zheng Y, Xu M, Wu N. Characterization of WRKY transcription factors in Solanum lycopersicum reveals collinearity and their expression patterns under cold treatment. Biochem Biophys Res Commun. 2015;464:962–968. doi: 10.1016/j.bbrc.2015.07.085. [DOI] [PubMed] [Google Scholar]
- Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell. 2017;29:1425–1439. doi: 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnapandi B, Bucki P, Braun MS. SlWRKY45 nematode-responsive tomato WRKY gene enhances susceptibility to the root knot nematode M. javanica infection. Plant Signal Behav. 2017;12:e1356530. doi: 10.1080/15592324.2017.1356530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Ding ZJ, Yan JY, Li CX, Li GX, Wu YR, Zheng SJ. Transcription factor WRKY 46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. Plant J. 2015;84:56–69. doi: 10.1111/tpj.12958. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler F, Inze D, Beeckman T, Gheysen G. A role for AtWRKY23 in feeding site establishment of plant parasitic nematodes. Plant Physiol. 2008;148:358–368. doi: 10.1104/pp.108.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, De Smet I, Lewis DR, Lofke C, Jansen L, Goeminne G, Bossche RV, Karimi M, De Rybel B, Vanholme B, Teichmann T. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc Natl Acad Sci. 2012;109:1554–1559. doi: 10.1073/pnas.1121134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, De Smet I, De Rybel B, Robert HS, Van De Cotte B, Willemsen V, Gheysen G, Weijers D, Friml J, Beeckman T. Tightly controlled WRKY23 expression mediates Arabidopsis embryo development. EMBO Rep. 2013;14:1136–1142. doi: 10.1038/embor.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Zhang J, Wang X, Han X, Wei B, Wang J, Li B, Yu H, Huang Q, Gu H, Qu LJ. The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis. Plant Cell. 2015;27:3112–3127. doi: 10.1105/tpc.15.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gao Y, Liu J, Peng X, Niu X, Fei Z. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol Genet Genomics. 2012;287:495–513. doi: 10.1007/s00438-012-0696-6. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1 a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lai Z, Fan B, Chen Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20:2357–2371. doi: 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, Offringa R. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET. The WRKY70 transcription factor a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang H, Yu D. Arabidopsis WRKY transcription factors WRKY12 and WRKY13 oppositely regulate flowering under short day conditions. Mol Plant. 2016;9:1492–1503. doi: 10.1016/j.molp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Yan L, Wu Z, Mei C, Lu K, Yu YT, Liang S, Zhang XF, Wang XF, Zhang DP. Cooperation of three WRKY-domain transcription factors WRKY18 WRKY40 and WRKY60 in repressing two ABA-responsive genes ABI4 and ABI5 in Arabidopsis. J Exp Bot. 2012;63:6371–6392. doi: 10.1093/jxb/ers293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A. MINISEED3 (MINI3) a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci. 2005;102:17531–17536. doi: 10.1073/pnas.0508418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JL, Miao ZQ, Wang Z, Yu LH, Cai XT, Xiang CB. Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet. 2016;12:e1005760. doi: 10.1371/journal.pgen.1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Groot EP, Kazantsev FV, Teale W, Omelyanchuk N, Kovrizhnykh V, Palme K, Mironova VV. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019;180:1725–1739. doi: 10.1104/pp.19.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschin M, Schlienger S, Bieker S, Zentgraf U. Senescence networking: WRKY18 is an upstream regulator a downstream target gene, and a protein interaction partner of WRKY53. J Plant Growth Regul. 2014;33:106–118. [Google Scholar]
- Prat T, Hajny J, Grunewald W, Vasileva M, Molnar G, Tejos R, Schmid M, Sauer M, Friml J. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLoS Genet. 2018;14:e1007177. doi: 10.1371/journal.pgen.1007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S. OsWRKY13 mediates rice disease resistance by regulating defense related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Xie W, Liu H, Li X, Xiong L, Wang S. Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13 a positive regulator of disease resistance. Mol Plant. 2008;1:538–551. doi: 10.1093/mp/ssn012. [DOI] [PubMed] [Google Scholar]
- Reichmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3:423–434. doi: 10.1016/s1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002;16:1139–1149. doi: 10.1101/gad.222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Gene Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IH, Aman S, Zubo Y, Ramzan A, Wang X, Shakeel SN, Kieber JJ, Schaller GE. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol. 2015;169:338–350. doi: 10.1104/pp.15.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XC, Gao YF, Li HR, Yang SZ, Liu YS. Over-expression of SlWRKY39 leads to enhanced resistance to multiple stress factors in tomato. J Plant Biol. 2015;58:52–60. [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseva II, Qudeimat E, Potuschak T, Du Y, Genschik P, Vandenbussche F, Van Der Straeten D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc Natl Acad Sci. 2018;17:E4130–E4139. doi: 10.1073/pnas.1717649115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005;43:118–130. doi: 10.1111/j.1365-313X.2005.02432.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Zhang ZL, Zou X, Huang J, Ruas P, Thompson D, Shen QJ. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005;137:176–189. doi: 10.1104/pp.104.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentgraf U, Thomas L, Ying M. The complex regulation of WRKY53 during leaf senescence of Arabidopsis thaliana. Eur J Cell Biol. 2010;89:133–137. doi: 10.1016/j.ejcb.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen L, Yu D. Transcription factor WRKY75 interacts with DELLA proteins to affect flowering. Plant Physiol. 2018;176:790–803. doi: 10.1104/pp.17.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]