Abstract
The role of ethylene (through application of ethephon) in the regulation of nickel (Ni) stress tolerance was investigated in this study. Ethephon at concentration of 200 µl l−1 was applied to mustard (Brassica juncea) plants grown without and with 200 mg kg−1 soil Ni to study the increased growth traits, biochemical attributes, photosynthetic efficiency, nutrients content, activities of antioxidants such as superoxide dismutase, ascorbate peroxidase, glutathione reductase, and glutathione peroxidase, glyoxalase systems and enhanced the proline metabolism. In the absence of ethephon, Ni increased oxidative stress with a concomitant decrease in photosynthesis, growth and nutrients content. However, application of ethephon positively increased growth traits, photosynthetic parameters, nutrients content and also elevated the generation of antioxidants enzymes and glyoxalase systems, proline production to combat oxidative stress. Plants water relations and cellular homeostasis were maintained through increased photosynthetic efficiency and proline production. This signifies the role of ethylene in mediating Ni tolerance via regulating proline production and photosynthetic capacity. Ethephon can be used as an exogenous supplement on plants to confer Ni tolerance. The results can be exploited to develop tolerance in plants via gene editing technology encoding enzymes responsible for proline synthesis, antioxidant defence, glyoxalase systems and photosynthetic effectiveness.
Keywords: Antioxidant enzymes, Ethylene, Glyoxalase systems, Nickel, Proline
Introduction
Natural resources have been polluted with rapid industrial developments in the last few decades. The addition of heavy metals (HMs) to agricultural soil due to the rapid industrialization is a potential threat to agricultural land (Nabi et al. 2019). Heavy metals are classified as essential and non-essential element for plants in physiological conditions (Asati et al. 2016). Nickel (Ni) has been proposed as essential elements whose requirement was constructing to be 0.01–5 µg g−1 dry weight by plants (Rizwan et al. 2017). Nickel is concerned in activation of enzymes such as urease, glyoxalase-I system and methyl-CoM reductase, superoxide dismutases (SOD) peptide deformylases and hydrogenase metabolism (Fabiano et al. 2015; Harish Sundaramoorthy et al. 2008; Küpper and Kroneck 2007; Polacco et al. 2013; Shahzad et al. 2018). Nickel also plays a vital role in the maintaining the cellular redox state, stress tolerance/defence and optimizing nutrient use efficiency (Vatansever et al. 2017). However, its deficiency in plants leads to chlorosis, reduces iron uptake, alters nitrogen metabolism, retards plant growth and senescence and necrotic lesions in leaves (Sirko and Brodzik 2000; Brown 2007; Chen et al. 2009; Polacco et al. 2013). In contrast, high concentration of Ni is reported to have toxic effects on plant growth and development and causes phyto-toxicity in plants and reduces photosynthesis and water relations, induces mineral nutrients deficiency and growth inhibition (Foy et al. 1978; Seregin and Kozhevnikova 2006; Matraszek and Hawrylak-Nowak 2010; Georgiado et al. 2018). Studies deal with Ni toxicity in plants has shown nutrients imbalances (Saad et al. 2016), low nutrient uptake (Charles and Issac 2014), decreased chlorophyll content (Sirhindi et al. 2016) and reduction in stomatal conductance (Velikova et al. 2011). Besides this, reduced PSII activity, Rubisco activity and damage of photosynthetic apparatus has also reported under high level of Ni presence (Chen et al. 2009; Hussain et al. 2013).
Higher concentration of Ni in plant cells is also responsible for inducing oxidative stress, redox imbalance, replacement of essential functional groups and metabolic arrest (Pandey and Sharma 2002; Sharma et al. 2011; Khan and Khan 2017; Rizwan et al. 2017). Additionally, it is also a potential risk to the ecological system and human health via food chain (N'guessan et al. 2009; He et al. 2012; Naveedullah et al. 2013). Plants adopt various strategies to avoid higher level of HMs in agricultural soil through regulating various defense mechanisms. Among these defense mechanisms, a well-regulated antioxidant systems which is composed of ROS scavenging enzymes such as SOD, ascorbate peroxidase (APX), glutathione reductase (GR) and glutathione peroxidase (GPX) has been reported (Rasool et al. 2013; Khan and Khan 2017; Asgher et al. 2018). Recent studies in plants have demonstrated that, in response to abiotic stress, a highly reactive and cytosolic α,β-dicarbonyl aldehyde compounds called methylglyoxal (MG) formed in excessive amounts (Hossain et al. 2009; Hasanuzzaman and Fujita 2013; Kaur et al. 2014). In addition to ROS, MG is highly toxic and cause ultra-structural damage, mutations, proteins inactivation and oxidative damage to cellular machinery (Kaur et al. 2014). Conversely, at the same time in plants, MG detoxified by thiol-dependent enzymes namely glyoxalase I (Gly I) and glyoxalase II (Gly II), which can regulate MG homeostasis in plants (Kaur et al. 2016; Hasanuzzaman et al. 2017). When both antioxidant and glyoxalase systems function coordinately, plants can illustrate considerable resistance against oxidative damage induced by toxicity of stresses (Upadhyaya et al. 2011; Álvarez Viveros et al. 2013; Mahmud et al. 2018). Another defence mechanism is proline metabolism which includes its metabolizing enzymes; pyrroline-5-carboxylate synthetase (P5CS), glutamyl kinase (GK) and proline oxidase (PROX) has been reported as potential mechanism for abiotic stress tolerance. It has been established that proline detoxifies excess ROS, maintains the cellular osmotic environment, protects biological membranes, photosynthetic activity and stabilizes enzymes/proteins under stress conditions (Rejeb et al. 2014; Per et al. 2017; Jiang et al. 2019).
Ethylene is a gaseous phytohormone that potentially triggers developmental processes ranging from seed germination to senescence and is also involved in abiotic stress tolerance (Pahwa et al. 2017; Khan et al. 2017; Dubios et al. 2018). The action of ethylene is linked to the concentration of ethylene in plants which is responsible for increase in tolerance under stress conditions (Dubios et al. 2018). Additionally, ethephon (2-chloroethyl phosphonic acid; an ethylene releasing compound) has great agricultural importance and converted to ethylene in plant cells, after conversion it becomes available for regulating physiological functions and may induce wide range of physiological mechanisms (Yang 1969; Khan and Khan 2014a, b). This property makes ethephon an important technology in applied crop physiology research. However, the involvement of ethylene in regulation of plant tolerance to elevated Ni levels through modulation of major defense systems (proline metabolism, glyoxalase system and antioxidant system) has not been demonstrated during plant growth transition from vegetative to maturity. Considering the imperative role of ethylene in plant stress responses at two stages, the present study, was designed to assess the influence of exogenous ethylene in mitigating high Ni-induced responses on growth and physiological attributes of Indian mustard plants.
Material and methods
Plant material and growth conditions
Indian mustard (Brassica juncea L. var. Type 59) seeds were surface sterilized with 0.01 g l−1 mercuric chloride solution and were sown in earthen pots filled with soil [peat and compost, 4:1 (v/v); mixed with sand, 3:1 (v/v)]. Four healthy plants of nearly equal size in each pot were maintained. The pots were kept in the green house of the Botany Department, Aligarh Muslim University, Aligarh (India). The experiment was designed to study the role of ethylene in Ni stress alleviation in ethylene-more responsive mustard var. Type 59 (Khan and Khan 2014a). The plants grown with 200 mg Ni kg−1 soil were treated with 200 μl l−1 ethephon at 20 days after sowing (DAS). Each of the treatment was given in 50 ml together with 0.5% surfactant teepol. In addition, a control group of plants and plants treated with 200 μl l−1 ethephon alone were also maintained. The treatments were arranged in a completely randomized block design and number of replicates were four (n = 4). All parameters were studied at 30 DAS (vegetative stage) and 60 DAS (maturity stage) respectively.
Determination of lipid peroxidation, electrolyte leakage and methylglyoxal content
Lipid peroxidation in leaves was determined by estimating the content of thiobarbituric acid reactive substances (TBARS) as described by Dhindsa et al. (1981). The details have been described earlier (Khan et al. 2015). For measuring electrolyte leakage earlier method of Mobin and Khan (2007) was adopted. MG content was adopted the method of Wild et al. (2012). Leaf samples were homogenized, using perchloric acid (5%) followed by centrifugation (11,000 × g). To neutralize the supernatant a saturated solution of sodium carbonate was used. The neutralized extract was further mixed with Na-P and N-acetyl-L-cysteine and the product N-α-acetyl-S-(1-hydroxy-2-oxoprop-1-yl) cysteine formation was observed spectrophotometrically at 288 nm. A standard curve of MG was engaged to calculate the MG content and expressed in μmol g−1 FW.
Determination of antioxidant enzymes
Fresh leaf tissue (200 mg) were homogenized with an extraction buffer containing 0.05% (v/v) Triton X-100 and 1% (w/v) PVP in potassium-phosphate buffer (100 mM, pH 7.0) using chilled mortar and pestle. At 4 °C, the homogenate was centrifuged at 15,000 × g for 20 min. The supernatant obtained after centrifugation was used for assay superoxide dismutase (SOD; EC, 1.15.1.1) GSH reductase (GR; EC, 1.6.4.2) and GSH peroxidase (GPX; EC 1.11.1.9) enzymes and for the assay of ascorbate peroxidase (APX; EC 1.11.1.11). 2.0 mM ascorbate was supplemented with extraction buffer.
Activity of SOD was determined by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT), according to the methods of Beyer and Fridovich (1987) and Giannopolitis and Ries (1977). A 5-ml reaction mixture containing 5 mM HEPES (pH 7.6), 0.1 mM EDTA, 50 mM Na2CO3 (pH 10.0), 13 mM methionine, 0.025% (v/v) Triton X-100, 63 mmol NBT, 1.3 mmol riboflavin, and the enzyme extract was illuminated for 15 min and a control set was not illuminated to correct for background absorbance. A unit of SOD was defined as the amount of enzyme required to use 50% inhibition of the reaction of NBT at 560 nm.
Activity of APX was determined by the method of Nakano and Asada (1981) by recording the decrease in absorbance of ascorbate at 290 nm. The assay mixture contained phosphate buffer (50 mM, pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2, and the enzyme extract. APX activity was calculated by using the extinction coefficient 2.8 mM−1 cm−1. One unit of the enzyme is the amount necessary to decompose 1 μmol of substrate per min at 25 °C.
Activity of GR was determined adopting the method of Foyer and Halliwell (1976) by monitoring the GSH-dependent oxidation of NADPH. The assay mixture contained phosphate buffer (25 mM, pH 7.8), 0.5 mM oxidized GSH, 0.2 mM NADPH, and the enzyme extract. GR activity was calculated by using extinction coefficient at 6.2 mM−1 cm−1. One Unit of the enzyme was amount necessary to decompose 1 µmol of NADPH min−1 at 25 °C.
Activity of GPX was determined by adopting the method of Hasanuzzaman et al. (2012). One ml reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7), 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 U ml−1 GR, 1 mM GSH and 0.25 mM H2O2.Oxidation of NADPH was recorded at 340 nm and activity of the enzymes was calculated using extinction coefficient at 6.2 mM−1 cm−1. One unit of the enzyme was amount necessary to decompose 1 µmol of NADPH min−1 at 25 °C. Protein was estimated according to Bradford (1976) using bovine serum albumin as standard.
Measurement of glyoxalase systems activity
Glyoxalase I (EC: 4.4.1.5) assay was determined by the method of Hasanuzzaman et al. (2018). The assay mixture contained 100 mM K-phosphate buffer (pH 7.0), 15 mM MgSO4, 1.7 mM GSH and 3.5 mM MG in a final volume of 700 µl. The reaction was started by the addition of MG and the increase in absorbance was recorded at 240 nm for 1 min. The activity was calculated using the extinction coefficient of 3.37 mM−1 cm−1.
Glyoxalase II (EC: 3.1.2.6) activity were measured determined the method of Principato et al. (1987) by monitoring the formation of GSH at 412 nm for 1 min. The reaction mixture contained 100 mM Tris–HCl buffer (pH 7.2), 0.2 mM DTNB and 1 mM S-D-lactoylglutathione (SLG) in a final volume of 1 ml. The reaction was started by the addition of SLG and the activity was calculated using the extinction co-efficient of 13.6 mM−1 cm−1.
Determination of nutrients content
The determination of mineral nutrients (nitrogen; N, phosphorous; P, potassium; K, and calcium; Ca) was done in acid-peroxide digested oven-dried leaf sample. K, and Ca were measured using flame photometer (Khera-391: Khera Instruments, New Delhi), whereas N and P were determined by using the methods of Lindner (1944) and Fiske and Subba Row (1925), respectively.
Determination of proline content and activity of proline metabolizing enzymes
Proline content was determined spectrophotometrically by adopting the ninhydrin method of Bates et al. (1973). The method was modified as described by Khan et al. (2013). Activities of pyrroline-5-carboxylate synthetase (P5CS; EC; 2.7.2.11/1.2.2.41), γ-glutamyl kinase (GK; EC; 2.7.2.11) and proline oxidase (PROX; EC; 1.5.99.8), enzyme extract was prepared as used by Khan et al. (2013). Pyrroline-5-carboxylate reductase was assayed according to Rena and Splittstoesser (1975) with a slight modification. The details of determination have been described recently (Asgher et al. 2018). Activity of GK was assayed by the method of Hayzer and Leisinger (1980) with slight modification. The details of determination have been described earlier (Khan et al. 2013). Proline oxidase activity was determined adopting the method of Huang and Cavalieri (1979) with slight modification. The details of determination have been described earlier (Khan et al. 2013).
Water potential and osmotic potential
Leaf water potential was measured using the plant second leaf from the top by using the water potential system (Psypro, WESCOR, USA). The leaf was frozen in liquid N in a sealed polythene bag and cell sap was extracted with the help of a disposable syringe. The extracted sap was used for the determination of osmotic potential using a vapour pressure osmometer (5520, WESCOR, USA).
Measurements of photosynthetic attributes, relative growth rate and plant dry mass
Gas exchange parameters (stomatal conductance, intercellular CO2 concentration and net photosynthesis) were measured in fully expanded uppermost leaves of plants in each treatment using CID-340, Photosynthesis System, Bio-Science, USA. The measurements were done between 11.00 and 12.00 h at light saturating intensity and at 430 ± 5 μmol mol−1 atmospheric CO2 concentrations.
Chlorophyll content was measured with the help of a SPAD chlorophyll meter (SPAD 502 DL PLUS, Spectrum Technologies).
Activity of ribulose-1,5-bisphosphate carboxylase (Rubisco; EC; 4.1.1.39) was determined spectrophotometrically by the adopting the method of Usuda (1985) by monitoring nicotinamide adenine dinucleotide (NADH) oxidation at 30 °C at 340 nm during the conversion of 3-phosphoglycerate to glycerol 3-phosphate after the addition of enzyme extract to the assay medium. The other detail has been given as Khan and Khan (2014a, b).
Dry mass of plants was recorded after drying the sample in a hot air oven at 80 °C till constant weight. Relative growth rate was calculated using the following formula given by Radford (1967).
Statistical analysis
Data were analyzed statistically, and standard error was calculated. Analysis of variance was performed on the data using SPSS (ver. 17.0 Inc., USA) to determine the significance at p < 0.05. Least significant difference (LSD) was calculated for the significant data to identify difference in the mean of the treatment and data are presented as mean ± SE (n = 4).
Results
Application of ethephon reduces nickel-induced oxidative stress and MG content
For the assessment of the influence of ethylene in reducing Ni-induced oxidative stress, TBARS content and electrolyte leakage were analyzed after the application of ethephon. Plants treated with Ni showed higher oxidative stress in comparison to control plants. Nickel treatment to plants enhanced TBARS content and electrolyte leakage by about 1.3-times and 3.4-times at 30 DAS and 86% and 2.9-times at 60 DAS, respectively compared to the control. Supplementation of ethephon proved better in lowering oxidative stress under Ni stress at both measurement stages. Ethephon application reduced TBARS content and electrolyte leakage by 49% and 62% in Ni-treated plants at 30 DAS, and by 1.42 and 1.25 times at 60 DAS (Table 1). Plants treated with Ni enhanced MG production in compared to control plants and equally increased MG content by about 63% at 30 DAS and 60 DAS. Application of ethephon under Ni stress decreased MG content by about 16% at 30 DAS and 12% at 60 DAS compared to Ni-treated plants (Table 1).
Table 1.
Electrolyte leakage, TBARS content, methylglyoxal content, chlorophyll content, net photosynthesis, stomatal conductance, intercellular CO2 concentration, Rubisco activity, relative growth rate and plant dry mass in the leaf of mustard (Brassica juncea L.) cv. Type 59 at 30 DAS and 60 DAS. Plants were grown with/without nickel stress (200 mg kg−1 soil) and treated with 200 µl l−1 ethephon at 20 DAS
| 30 DAS | 60 DAS | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Ethephon | Nickel | Ethephon + nickel | Control | Ethephon | Nickel | Ethephon + nickel | |
| Electrolyte leakage (%) | 1.98 ± 0.09e | 1.20 ± 0.06g | 6.90 ± 0.34a | 4.12 ± 0.2b | 1.24 ± 0.06f | 0.81 ± 0.04h | 3.59 ± 0.2c | 2.82 ± 0.2d |
| TBARS content (nmol g−1 FW) | 17.61 ± 0.8e | 12.74 ± 0.6g | 41.58 ± 2.0a | 26.32 ± 1.3b | 13.24 ± 0.6f | 8.23 ± 0.4h | 24.12 ± 1.2c | 21.47 ± 1.0d |
| Methylglyoxal content (mol g−1 FW) | 45.8 ± 1.2e | 50 ± 1.7de | 74 ± 1.4b | 62 ± 1.8c | 48.8 ± 1.5e | 54.6 ± 1.3d | 81 ± 2.0a | 71 ± 2.3b |
| Chlorophyll content (SPAD value) | 36.7 ± 1.3c | 50.8 ± 1.2b | 23.1 ± 1.31e | 30.2 ± 1.27d | 47.2 ± 1.23b | 69.2 ± 1.31a | 26.7 ± 1.25de | 34.5 ± 1.24c |
| Net photosynthesis (µmol CO2 m−2 s−1) | 17.15 ± 0.8C | 22.09 ± 1.1b | 11.67 ± 0.5f | 14.60 ± 0.7d | 21.78 ± 1.0b | 30.54 ± 1.5a | 13.95 ± 0.6e | 16.42 ± 0.8e |
| Intracellular CO2 concentration (µmol CO2 mol−1) | 234 ± 8.1c | 296 ± 8.7b | 162 ± 7.5e | 197 ± 8.3d | 287 ± 9.0b | 399 ± 8.6a | 176 ± 8.8de | 226 ± 7.8c |
| Stomatal conductance (mmol CO2 m−2 s−1) | 302 ± 11.2c | 372 ± 13.4b | 227 ± 8.9e | 265 ± 10.4d | 364 ± 12.7b | 464 ± 14.7a | 259 ± 10.5de | 293 ± 10.7cd |
| Rubisco activity (µmol CO2 m−2 s−1) | 32.2 ± 1.3c | 44.2 ± 2.0b | 19.2 ± 1.1e | 27.9 ± 1.3cd | 42.2 ± 2.0b | 62.8 ± 2.4a | 23.8 ± 1.4de | 30.3 ± 1.24c |
| Relative growth rate (mg g−1 d−1) | 15.42 ± 0.8c | 18.93 ± 0.9b | 9.57 ± 0.5 h | 13.87 ± 0.7e | 17.23 ± 0.7b | 24.23 ± 1.2a | 13.67 ± 0.7e | 15.83 ± 0.8c |
| Plant dry mass (g plant−1) | 4.35 ± 0.2d | 6.52 ± 0.3b | 2.23 ± 0.1i | 3.81 ± 0.2g | 4.82 ± 0.2c | 7.25 ± 0.4a | 3.11 ± 0.16e | 4.12 ± 0.20e |
Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
Application of ethephon enhanced antioxidant systems under Ni stress
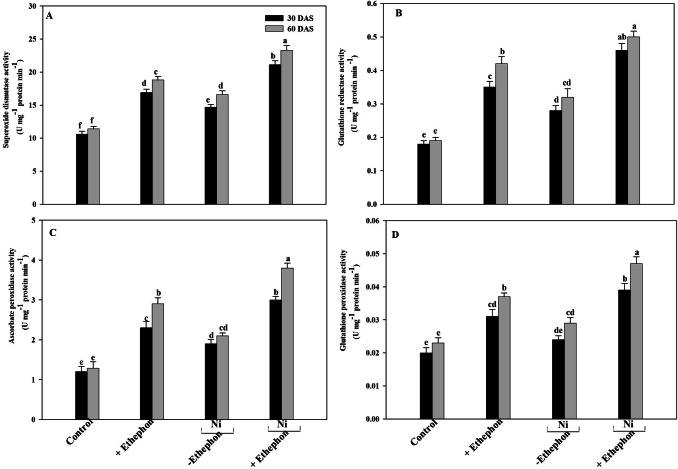
Nickel treatment resulted in increased SOD, APX, GR and GPX activity by 39%, 58%, 56% and 20% at 30 DAS and 46%, 63%, 68% and 61% at 60 DAS compared to control. In non-stressed control plants, application of ethephon increased activity of SOD APX, GR and GPX 60%,92%,95% and 55% at 30 DAS and 65%,125%,121% and 61% at 60 DAS over the control. Application of ethephon to Ni-treated plants resulted in significantly increased the activity of SOD, APX, GR, and GPX 99%, 150%, 156% and 95% at 30 DAS and 105%, 195%, 163% and 104% at 60 DAS plants compared to the control (Fig. 1).
Fig. 1.
SOD activity (a) GR activity (b) APX activity (c) GPX activity (d) of mustard leaf (Brassica juncea L.) cv. Type 59 at 30 and 60 DAS. Plants were grown with/without Ni and treated with 200 µl l−1 ethephon at 20 DAS. Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
Ethephon application enhances glyoxalase system enzymes
Gly I was improved with Ni stress by 61% at 30 DAS and 66% at 60 DAS over control plants (Fig. 2). Supplementation of ethephon to Ni treated plant significantly enhanced the activity Gly I by 85% and 88% was observed at 30 DAS and 60 DAS respectively, compared to control plants, Gly II increased with Ni stress was recorded by 88% at 30 DAS and 111% at 60 DAS in comparison to the control plants. However, external application of ethephon with Ni treated plants enhanced the activity of Gly II by 150% and 178% at 30 DAS and 60 DAS respectively, compared to control plants (Fig. 2).
Fig. 2.
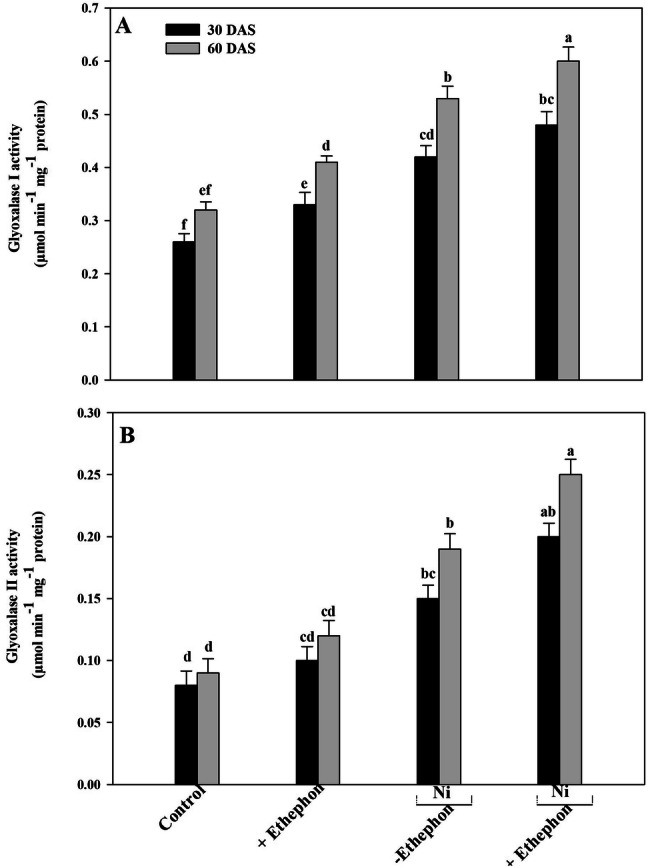
Activities of GI (a) GII (b) in mustard plants (Brassica juncea L.) cv. Type 59 at 30 and 60 DAS. Plants were grown with/without Ni and treated with 200 µl l−1 ethephon at 20 DAS. Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
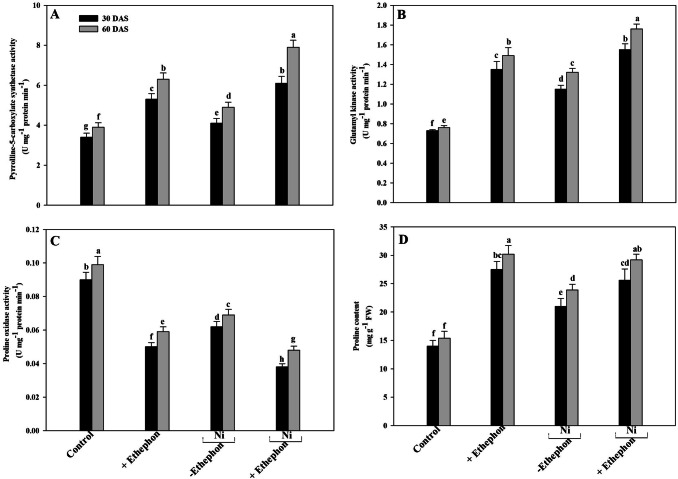
Application of ethephon increases proline metabolism under Ni stress
To study the involement of proline metabolism in Ni stress tolerance under the influence of ethylene, assessment of proline accumulation and activity of proline metabolizing enzymes, P5CS, GK and PROX was done. The activity of P5CS and GK increased in Ni-stressed plants and with ethephon plus Ni treatments. Application of ethephon increased P5CS activity by 20.55% at 30 DAS and 25.6% at 60 DAS and GK activity by 57.5% at 30 DAS and 73.6% at 60 DAS in Ni-stressed plants compared to the control. Contrarily, the activity of PROX reduced under no-stress and Ni-stress with ethephon treatment at 30 DAS. At 60 DAS activity of P5CS and GK increased in Ni-treated plants and with ethylene plus Ni. Application of ethylene increased P5CS and GK activity by 2 times under Ni stress in comparison to the control plants. However, PROX activity reduced under no-stress and metals-stressed plants with ethephon treatment (Fig. 3).
Fig. 3.
Activities of pyrroline-5-carboxylate synthetase (a) glutamyl kinase (b) proline oxidase (c) and proline content (d) in the leaf of mustard (Brassica juncea L.) cv. Type 59 at 30 and 60 DAS. Plants were grown with/without Ni and treated with 200 µl l−1 ethephon at 20 DAS. Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
Accumulation of proline increased on ethephon supplementation as well as with Ni treatment. Nickel caused an increase of 42.8% in proline at 30 DAS while the higher increase of 53.2% occurred at 60 DAS in comparison to the control. Ethephon further enhanced proline accumulation under Ni stress and maximum proline accumulation occurred with the combined Ni plus ethephon treatment at 60 DAS compared with the control (Fig. 3).
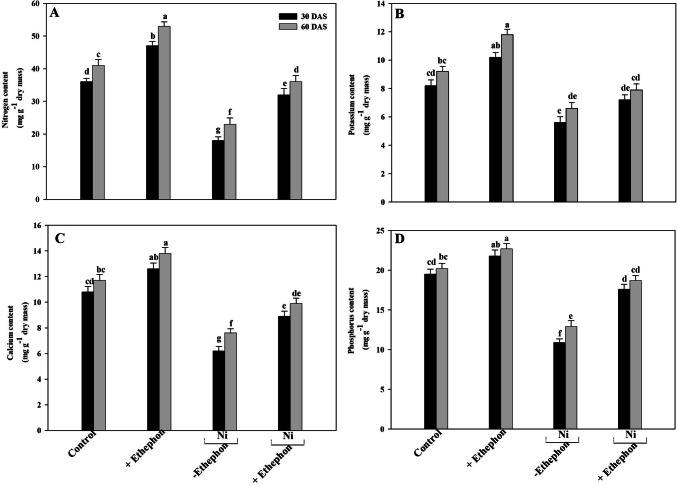
Ethylene supplementation maintained nutrients content
Treatment of Ni stress decreased N, P, K, and Ca content in plants in comparison to control plants. Ni treatment decreased N by 50.0 and 44%, P by 44.1 and 36.1%, K by 69% and 50.6%, and Ca by 42.5 and 35% at 30 DAS and 60 DAS, respectively, compared to control. Application of ethephon on Ni grown plants completely alleviated the Ni effects and increased the nutrients content significantly at 60 DAS then at 30 DAS compared to control (Fig. 4).
Fig. 4.
Content of nitrogen (a) potassium (b) calcium (c) and phosphorous (d) of mustard (Brassica juncea L.) cv. Type 59 at 30 and 60 DAS. Plants were grown with/without Ni and treated with 200 µl l−1 ethephon at 20 DAS. Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
Influence of ethephon application on water relations under Ni stress
Application of ethephon alone resulted in the increase of water potential by 31.08%, 28.5% and osmotic potential by 31.3% and 29.4% at 30 DAS and 60 DAS, respectively compared to the control. The plants subjected to Ni treatment showed reduced water potential at 30 DAS by 1.8 times and by 2.1 times at 60 DAS compared to the control. Application of ethylene to Ni-stressed plants increased water potential by 28.5% and 33.7% and osmotic potential by 37.8% and 43.4% compared to the control (Fig. 5).
Fig. 5.

Leaf water potential (a) and osmotic potential (b) of mustard (Brassica juncea L.) cv. Type 59 at 30 and 60 DAS. Plants were grown with/without Ni and treated with 200 µl l−1 ethephon at 20 DAS. Data are presented as treatments mean ± SE (n = 4). Data followed by same letter are not significantly different by LSD test at p < 0.05
Application of ethephon reverses effects of Ni stress on relative growth rate and plant dry mass
Growth of plants reduced with Ni compared to the control plants. Ethephon exposure to plants without metals treatment enhanced relative growth rate (RGR) and plant dry mass compared to the control plants. The decrease in plant dry mass was limited to 48.7% and 35.4% in Ni treated plants at 30 and 60 DAS, respectively. Ni reduced RGR by 37.9% and 20.6% at 30 and 60 DAS compared to control (Table 1). The decrease in plant dry mass was limited in Ni by 25%, RGR in Ni is 23.5% compared to the control (Table 1).
Application of ethephon reverses effects of Ni stress on photosynthetic attributes
Photosynthetic attributes were reduced in plants treated with Ni in comparison to the control plants, but the ethephon application reversed the adverse effect of Ni. Ethephon supplementation to plants without Ni treatment enhanced net photosynthesis by about 28.8% and 40.2%, chlorophyll content by 38.4% and 46.6%, Rubisco activity 37.3% and 48.9%, stomatal conductance by 23.1% and 27.5%, intracellular CO2 concentration by 26.4% and 39% at 30 and 60 DAS, respectively compared to the control plants. Ethephon application to Ni treated plants controlled the adverse effects of Ni on photosynthetic attributes (Table 1).
Discussion
The unrestricted anthropogenic activities resulted in addition of HMs in agricultural soils led to increase the risk for plant growth and development (Khan and Khan 2017). The essentiality of Ni for plants at lower concentration and its adverse effects at higher concentration on the life cycle of the plants have been reported (Ghori et al. 2019; Georgiado et al. 2018). However, Ni toxicity and their adverse effects and how plants can cope Ni toxicity has not been reporetd much.
Phytohormones are essential for normal growth and development of plants and for striving in stress conditions. The involvement of ethylene in response to abiotic stresses is available in the literature but little has been reported on HMs and the information is scanty on Ni stress. Exogenous application of ethylene to improve photosynthetic efficiency and growth has been reported (Khan et al. 2019; Dubios et al. 2018; Van Oosten and Maggio 2015). Additionally, it has been shown that ethephon supplemention affects antioxidant metabolism and photosynthetic characteristics significantly (Thao et al. 2015; Wakeel et al. 2018; Khan et al. 2017). However, studies on the ethylene regulated proline metabolism, antioxidant machinery and glyoxalase systems under Ni stress tolerance in mustard plants are scanty.. In this present study, it was found that ethylene released from ethephon treatment played significant role in reducing the detrimental effect of Ni through enhanced antioxidant, glyoxalase systems and proline metabolism to improve water relations, photosynthetic and growth traits.
The findings strongly imply that higher Ni level produce oxidative stress and MG content in mustard plant and causes membrane damage. However, application of ethephon significantly increased the activity of antioxidant enzymes (SOD, APX, GR, and GPX) along with reduced oxidative stress markers and may also be associated with protecting photosynthetic and growth traits in mustard plants. It has been suggested that high activity of Gly I and II are essential for excess production of MG and plant stress tolerance (Hasanuzzaman et al. 2011). It was observed that the activity of Gly I and Gly II decreased under Ni stress, however upon ethephon application glyoxalase system activity increased with cumulative effect on growth traits. Photosynthesis in higher plants is sensitive to metals inhibiting accessory pigments and chlorophyll biosynthesis (Zhang et al. 2003; Ahmad et al. 2009). Under metal stress, the decrease in photosynthesis is due to stomata closure which resulted in decreased leaf transpiration rate and reduced leaf CO2 concentration (Parveen and Ashraf 2010). In Arachis hypogaea an inhibition in the net photosynthetic rate due to decreased stomatal conductance and photosynthetic pigment content (Shi and Cai 2008) due to the increased stomatal closure (Gupta et al. 2017) is reported. It was recorded that, chlorophyll content and photosynthetic apparatus decreased collectively with plant dry mass and RGR under Ni-stressed condition. It may be suggested that reduced growth and photosynthesis is due to the limited distribution of mineral nutrients under stressed conditions. Moreover, Ni toxicity reduced chlorophyll content might be due to inhibition of enzymes for chlorophyll biosynthesis (Kaveriammal and Subramani 2013). The photosynthetic machinery can be improved or restored by exogenous application of ethephon under Ni stress. When we applied ethephon to Ni containing grown plants exogenouly, the position altered remarkably. Such enhancing effects of exogenous application of ethephon in terms of increased photosynthetic efficiency can be considered as lead findings for enhancing productivity of the plants.
Proline under stress in plants is accountable for stabilization of protein, membranes integrity and the protection of cellular structures (Salinas et al. 2013; Kishor et al. 2015; Per et al. 2017). Proline also functions as a compatible osmolytes by acting as a enzyme protectant, ROS scavenger, cell redox balancer, and efficiently protect photosystem structures (Kishor et al. 2005; Verbruggen and Hermans 2008; Naliwajski and Skłodowska 2014). These roles of proline might play a key function in the survival of plants under Ni stress. In the present research, effort was made to investigate the role of proline production in facilitating Ni detoxification and ameliorating stress in mustard plants. Application of ethephon resulted in higher proline content by modulating metabolizing enzymes with minimizing oxidative stress effects induced by Ni stress. The enhanced of proline accumulation after ethephon supplementation resulted from induced GK activity and inhibited PROX activity. It has been reported that activity of P5CS and GK plays an imperative role in controlling the level of proline and environmental stress in plants (Szabados and Savoure 2010; Naliwajski and Skłodowska 2014; Iqbal et al. 2015). Khan et al. (2008) have shown that ethylene plays a key role in N metabolism and increases NR activity and N content in Brassica juncea. Additionally, Iqbal et al. (2014, 2015) reported that increased proline metabolism had ethylene effects on plants under salt stress. In the present study, it is shown that ethylene-induced increase in proline metabolism contributed to tolerance to Ni stress and increased photosynthetic and growth attributes. It was also observed that ethephon-induced accumulation of proline protected photosynthetic apparatus from Ni-induced oxidative stress by maintaining water relations and by decreasing oxidative stress parameters in Ni-stressed plants. Proline synthesis may provide shelter against photo-inhibition under stress environment by restoring the pool of the terminal electron acceptor of the photosynthetic electron transport chain by increasing the antioxidants metabolism and prevent the oxidative damages (Szabados and Savoure 2010). Moreover, in the present study the enhancement in water potential by ethephon treatment in Ni-stressed plants helped in increased retaining photosynthesis and growth of Ni-stressed plants.
It has been shown that HMs have toxic effects on plant cellular machinery by disturbing the uptake of major nutrients such as N, P, K and Ca and impairment in many physiological processes such photosynthesis, transport of photosynthates and growth traits of plants (Munns and Tester 2008; Khan et al. 2009; Siddiqui et al. 2013; Sirhindi et al. 2016). Maintaining proper plant nutritional status under stressful environments can be one of the most desirable strategies to protect the damaging effects of metals and which ultimately can avoid HMs entry into the food chain (Arif et al. 2016). In this research we also focused on modulation of essential nutrients through application of ethephon under Ni stress condition and special attention was made on nutrients content (N, P, K, and Ca) and significant decrease under Ni stress was recorded (Fig. 4).
Conclusion and future prospects
The work reported that plants grown with Ni exhibited reduced photosynthesis and growth due to Ni-induced oxidative stress. Additionally, it showed that Ni stress-mediated responses occur via ethylene and could be manipulated to augment photosynthesis and growth. Application of ethephon to Ni-stressed plants showed higher antioxidant machinery, glyoxalase system and proline metabolism and helped in improvement of osmotic adjustments, photosynthesis and growth traits. It may be said that ethylene works centrally in the regulation of photosynthesis and growth of plants. The use of ethephon to plants grown under Ni stress may be adopted as the strategy for alleviation of stress because of increased photosynthetic efficiency along with reduced Ni toxicity in mustard plants. Also there is an urgent need to investigate genetic architecture and complex metabolic networks interaction to develop Ni-tolerant genotypes under higher Ni levels.
Acknowledgements
MIRK gratefully acknowledges the award of MANF [F.40-3 (M/S)/2009 (SA-III/MANF)] by the University Grants Commission, New Delhi, India. MFA and MTR acknowledge the generous support from the Researcher Supporting Project number (RSP-2019-122) King Saud University, Riyadh, Saudi Arabia.
Author contributions
MIRK and NAK conceptualized the study and wrote the manuscript; MIRK and BJ conducted the experiments and collected the data, MIRK, BJ, MFA, MTR analyses the data and helped in preparing the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad I, Naeem M, Khan NA. Effects of cadmium stress upon activities of antioxidative enzymes, photosynthetic rate, and production of phytochelatins in leaves and chloroplasts of wheat cultivars differing in yield potential. Photosynthetica. 2009;47:146–151. [Google Scholar]
- Álvarez Viveros MF, Inostroza-Blancheteau C, Timmermann T, González M, Arce-Johnson P. Overexpression of Gly I and Gly II genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol Biol Rep. 2013;40:3281–3290. doi: 10.1007/s11033-012-2403-4. [DOI] [PubMed] [Google Scholar]
- Arif N, Vaishali Y, Shweta S, Swati S, Parvaiz A, Rohit KM, Shivesh S, Durgesh K, Tripathi NKD, Devendra KC. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci. 2016;4:69. [Google Scholar]
- Asati A, Pichhode M, Nikhil K. Effect of heavy metals on plants: an overview. Int J Appl Innov Eng Manag. 2016;5:56–66. [Google Scholar]
- Asgher M, Per TS, Verma S, Pandith SA, Masood A, Khan NA. Ethylene supplementation increases PS II efficiency and alleviates chromium-inhibited photosynthesis through increased nitrogen and sulfur assimilation in mustard. J Plant Growth Regul. 2018;37:1300–1317. [Google Scholar]
- Bates LE, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the Quantitation of micro-gram quantities of proteins utilising the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown PH. Nickel. In: Barker AV, Pilbean DJ, editors. Handbook of plant nutrition. New York: Taylor and Francis; 2007. pp. 395–402. [Google Scholar]
- Charles IO, Issac UI. Effect of nickel concentrations on Amaranthus spinosus uptake of nutrients and heavy metals in soil. J Appl Phytotechnol Environ Sanit. 2014;3:87–91. [Google Scholar]
- Chen C, Huang D, Liu J. Functions and toxicity of nickel in plants: recent 10 advances and future prospects. Clean Soil Air Water. 2009;37:304–313. [Google Scholar]
- Dhindsa RH, Plumb-Dhindsa P, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101. [Google Scholar]
- Dubios M, Broeck LVD, Inze D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:311–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano CC, Tezotto T, Favarin JL, Polacco JC, Mazzafera P. Essentiality of nickel in plants: a role in plant stresses. Front Plant Sci. 2015;6:754. doi: 10.3389/fpls.2015.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CH, Subba Row Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Foy CD, Chaney RL, White MC. The physiology of metal toxicity in plants. Annu Rev Plant Physiol. 1978;29:511–566. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Georgiado EC, Kowalska E, Patla K, Kulbat K, Smolinska B, Leszczynska J, Fotopoulos V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.) plants. Front Plant Sci. 2018;9:862. doi: 10.3389/fpls.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M. Heavy metal stress and responses in plants. Int J Environ Sci Technol. 2019;16:1807–1828. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Jatav PK, Verma R, Kothari SL, Kachhwaha S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ Sci Pollut Res. 2017;24:23915–23925. doi: 10.1007/s11356-017-0057-4. [DOI] [PubMed] [Google Scholar]
- Harish Sundaramoorthy S, Kumar D, Vaijapurkar SG. A new chlorophycean nickel hyperaccumulator. Bioresour Technol. 2008;99:3930–3934. doi: 10.1016/j.biortech.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Fujita M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology. 2013;22:584–596. doi: 10.1007/s10646-013-1050-4. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353. doi: 10.1007/s12011-011-8958-4. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res. 2012;149:248–261. doi: 10.1007/s12011-012-9419-4. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci. 2017;18:200. doi: 10.3390/ijms18010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Khan MIR, Fujita M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. South Afr J Bot. 2018;115:50–57. [Google Scholar]
- Hayzer DJ, Leisinger TH. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 1980;118:287–293. doi: 10.1099/00221287-118-2-287. [DOI] [PubMed] [Google Scholar]
- He S, He Z, Yand X, Baligar VC. Mechanism of nickel uptake and hyperaccumulation by plants and implication for soil remediation. Adv Agron. 2012;117:117–189. [Google Scholar]
- Hossain MA, Hossain MZ, Fujita M. Stress induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci. 2009;3:53–64. [Google Scholar]
- Huang AHC, Cavalieri AJ. Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol. 1979;63:531–535. doi: 10.1104/pp.63.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MB, Ali S, Azam A, Hina S, Farooq MA, Ali B, Bharwana SA, Gill MB. Morphological, physiological and biochemical responses of plants to nickel stress: a review. Afr J Agric Res. 2013;8:1596–1602. [Google Scholar]
- Iqbal N, Umar S, Khan NA, Khan MIR. A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot. 2014;100:34–42. [Google Scholar]
- Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jiang K, Wu B, Wang C, Ran Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat. Ecotoxicol Environ Saf. 2019;167:345–353. doi: 10.1016/j.ecoenv.2018.10.048. [DOI] [PubMed] [Google Scholar]
- Kaur C, Singla-Pareek SL, Sopory SK. Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit Rev Plant Sci. 2014;33:429–456. [Google Scholar]
- Kaur C, Sharma S, Singla-Pareek SL, Sopory SK. Methylglyoxal detoxification in plants: role of glyoxalase pathway. Indian J Plant Physiol. 2016;21:377–390. [Google Scholar]
- Kaveriammal S, Subramami A. Toxic effect of nickel chloride (NiCl2) on the growth behavoiur and biochemical constituent of groundnut seedlings (Arachis hypogeae L.) Int J Res Bot. 2013;3:48–52. [Google Scholar]
- Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, et al. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88:424–438. [Google Scholar]
- Kishor PB, Hima Kumari P, Sunita MSL, Sreenivasulu N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front Plant Sci. 2015;6:544. doi: 10.3389/fpls.2015.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MIR, Khan NA. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma. 2014;251:1007–1019. doi: 10.1007/s00709-014-0610-7. [DOI] [PubMed] [Google Scholar]
- Khan NA, Khan MIR. The ethylene: from senescence hormone to key player in plant metabolism. J Plant Biochem Physiol. 2014;2:124. [Google Scholar]
- Khan MIR, Khan N. Reactive oxygen species and antioxidant system in plants: role and regulation under abiotic stress. Berlin: Springer; 2017. [Google Scholar]
- Khan NA, Mir MR, Nazar R, Singh S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and N accumulation in mustard (Brassica juncea L.) Plant Biol. 2008;10:534–538. doi: 10.1111/j.1438-8677.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- Khan NA, Nazar R, Anjum NA. Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hortic. 2009;122:455–460. [Google Scholar]
- Khan MIR, Iqbal N, Masood A, Per TS, Khan NA. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav. 2013;8:263–274. doi: 10.4161/psb.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MIR, Nazir F, Asgher M, Per TS, Khan NA. Selenium and sulfur influence ethylene formation and alleviate cadmium induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol. 2015;173:9–18. doi: 10.1016/j.jplph.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Khan NA, Khan MIR, Ferrante A, Peter P. Ethylene: a key regulatory molecule in plants. Front Plant Sci. 2017;8:1782. doi: 10.3389/fpls.2017.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MIR, Jahan B, AlAjmi MF, Rehman MT, Khan NA. Exogenously-sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.) Plants (Basel) 2019;8(12):pii: E540. doi: 10.3390/plants8120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Kroneck PM. Nickel in the environment and its role in the metabolism of plants and cyanobacteria. Metal Ions Life Sci. 2007;2:31–62. [Google Scholar]
- Lindner RC. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1944;19:70–89. doi: 10.1104/pp.19.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf. 2018;147:990–1001. doi: 10.1016/j.ecoenv.2017.09.045. [DOI] [PubMed] [Google Scholar]
- Matraszek R, Hawrylak-Nowak B. Growth and mineral composition of nickel-stressed plant under conditions of supplementation with excessive amounts of calcium and iron. J Toxicol Environ Health. 2010;73:1260–1273. doi: 10.1080/15287394.2010.492015. [DOI] [PubMed] [Google Scholar]
- Mobin M, Khan NA. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol. 2007;164:601–610. doi: 10.1016/j.jplph.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanism of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- N’guessan M, Probst JL, Bur T, Probst A. Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France): where do they come from? Sci Total Environ. 2009;407:2939–2952. doi: 10.1016/j.scitotenv.2008.12.047. [DOI] [PubMed] [Google Scholar]
- Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot. 2019;161:120–133. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Naliwajski MR, Skłodowska M. Proline and its metabolism enzymes in cucumber cell cultures during acclimation to salinity. Protoplasma. 2014;251:201–209. doi: 10.1007/s00709-013-0538-3. [DOI] [PubMed] [Google Scholar]
- Naveedullah, Hashmi MZ, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y. Risk assessment of heavy metals pollution in agricultural soils of siling reservoir watershed in Zhejiang province, China. Biomed Res Intern. 2013;2013:590306. doi: 10.1155/2013/590306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa K, Ghai N, Kaur J, Singh I, Singh S, Dhingra M. Influence of ethylene and cobalt chloride on photosynthetic parameters and pedicel anatomy of pigeonpea (Cajanus cajan L.) genotypes. J Environ Biol. 2017;38:367–374. [Google Scholar]
- Pandey N, Sharma CP. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002;163:753–758. [Google Scholar]
- Parveen N, Ashraf M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak J Bot. 2010;42:1675–1684. [Google Scholar]
- Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MI, Anjum NA. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Polacco JC, Mazzafera P, Tezotto T. Opinion–nickel and urease in plants: still many knowledge gaps. Plant Sci. 2013;199:79–90. doi: 10.1016/j.plantsci.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Principato GB, Rosi G, Talesa V, Giovanni E, Uotila L. Purification and characterization of two forms of glyoxalase II from the liver and brain of wistar rats. Biochim Biophys Acta. 1987;911:349–355. doi: 10.1016/0167-4838(87)90076-8. [DOI] [PubMed] [Google Scholar]
- Radford PJ. Growth analysis formulae-their use and abuse. Crop Sci. 1967;7:171–175. [Google Scholar]
- Rasool S, Ahmad A, Siddiqi TO, Ahmad P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant. 2013;35:1039–1050. [Google Scholar]
- Rejeb KB, Abdelly C, Savouré A. How reactive oxygen species and proline face stress together. Plant Physiol Biochem. 2014;80:278–284. doi: 10.1016/j.plaphy.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Rena AB, Splittstoesser WE. Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons. Phytochemistry. 1975;14:657–661. [Google Scholar]
- Rizwan M, Imtiaz M, Dai Z, Mehmood S, Adeel M, Liu J, Tu S. Nickel stressed responses of rice in Ni subcellular distribution, antioxidant production, and osmolyte accumulation. Environ Sci Pollut Res. 2017;24:20587–20598. doi: 10.1007/s11356-017-9665-2. [DOI] [PubMed] [Google Scholar]
- Saad R, Kobaissi A, Robin C, Echevarria G, Benizri E. Nitrogen fixation and growth of Lens culinaris as affected by nickel availability:a pre-requisite for optimization of agromining. Environ Exp Bot. 2016;131:1–9. [Google Scholar]
- Salinas R, Sánchez E, Ruíz JM, Lao MT, Romero L. Proline, betaine, and choline responses to different phosphorus levels in green bean. Commun Soil Sci Plant Anal. 2013;44:465–472. [Google Scholar]
- Seregin IV, Kozhevnikova AD. Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol. 2006;53:257–277. [Google Scholar]
- Shahzad B, Tanveer M, Rehman A, Cheema SA, Fahad S, Rehman S, Anket Sharma A. Nickel; whether toxic or essential for plants and environment—a review. Plant Physiol Biochem. 2018;132:641–651. doi: 10.1016/j.plaphy.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Sharma I, Pati PK, Bhardwaj R. Effect of 24-epibrassinolide on oxidative stress markers induced by nickel-ion in Raphanus sativus L. Acta Physiol Plant. 2011;33:1723–1735. [Google Scholar]
- Shi GR, Cai QS. Photosynthetic and anatomic responses of peanut leaves to cadmium stress. Photosynthetica. 2008;46:627–630. [Google Scholar]
- Siddiqui MH, Al-Whaibi MH, Ali HM, Sakran AM, Basalah MO, AlKhaishany MY. Mitigation of nickel stress by the exogenous application of salicylic acid and nitric oxide in wheat. Aust J Crop Sci. 2013;7:1780. [Google Scholar]
- Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci. 2016;7:591. doi: 10.3389/fpls.2016.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirko A, Brodzik R. Plant ureases: roles and regulation. Acta Biochim Pol. 2000;47:1189–1195. [PubMed] [Google Scholar]
- Szabados L, Savoure A. Proline: multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Thao NP, Khan MIR, Thu NBA, Hoang XLT, Asgher M, Khan NA, Tran LP. Role of ethylene and its crosstalk with other signaling molecules in plant responses to heavy metals. Plant Physiol. 2015;169:73–84. doi: 10.1104/pp.15.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW. Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett. 2011;33:2297–2307. doi: 10.1007/s10529-011-0684-7. [DOI] [PubMed] [Google Scholar]
- Usuda H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985;26:1455–1463. [Google Scholar]
- Van Oosten MJ, Maggio A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ Exp Bot. 2015;111:135–146. [Google Scholar]
- Vatansever R, Ozyigit II, Filiz E. Essential and beneficial trace elements in plants, and their transport in roots: a review. Appl Biochem Biotechnol. 2017;181:464–482. doi: 10.1007/s12010-016-2224-3. [DOI] [PubMed] [Google Scholar]
- Velikova V, Tsonev T, Loreto F, Centritto M. Changesin photosynthesis, mesophyll conductance to CO2, and isoprenoide missions in Populus nigra plants exposed to excess nickel. Environ Pollut. 2011;159:1058–1066. doi: 10.1016/j.envpol.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Wakeel A, Ali I, Wu M, Raza Khan A, Jan M, Ali A, Gan Y. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ Exp Bot. 2018;161:166–179. [Google Scholar]
- Wild R, Ooi L, Srikanth V, Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal Bioanal Chem. 2012;403:2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- Yang SF. Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol. 1969;44:1203–1204. doi: 10.1104/pp.44.8.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Shi W, Jin Z, Shen Z. Response of oxidative enzymes in cucumber chloroplasts to cadmium toxicity. J Plant Nutr. 2003;26:1779–1788. [Google Scholar]