Abstract
Purpose of Review
The purposes of the review are as follows: (1) to define acute rhinosinusitis (ARS) and their phenotypes, (2) to highlight the ARS management according to international guidelines, (3) to compare the physicians’ management with the ARS guideline recommendations, and (4) to report ARS socioeconomic burden.
Recent Findings
Bacterial and non-bacterial ARS have similar symptoms, although they can be discriminated by using a combination of specific signs and symptoms. The prescription of antibiotics should be limited to clearly suspected bacterial ARS. There is an overuse of diagnosis tools and treatment prescriptions. The total cost per ARS episode in Europe is over €1000.
Summary
ARS is mainly an inflammatory disease triggered by viral infection, and few cases end up developing bacterial infection. In most of the cases, it is a self-resolving disease which diagnosis is mainly clinical and the treatment symptomatic. The incidence of complications is low and independent of antibiotic use. There is a high socioeconomic burden associated to ARS.
Keywords: Acute rhinosinusitis, Common cold, Respiratory tract infections, Bacterial acute rhinosinusitis, EPOS, Costs
Introduction
Acute rhinosinusitis (ARS) is an inflammatory disease affecting the nose and paranasal sinuses with duration up to 12 weeks. The main trigger cause is a viral infection (common cold) that can be prolonged on time (post-viral) and, in a small number of patients, may develop a bacterial infection. It is important to discriminate the different phenotypes of ARS to understand the diagnostic and therapeutic requirements in every individual case [1••].
ARS has a significant impact on quality of life [2••], although it usually is a self-resolving disease and the incidence of chronicity or complications is very low. Despite this, both primary care physicians and ENT specialists abuse diagnostic tools and overuse drug prescriptions [3•].
The aim of the present article is to review the incidence of ARS, discuss its etiology (inflammation versus infection), describe ARS different phenotypes, and analyze the recommendations of international guidelines for its management. Furthermore, we will highlight the use and abuse of diagnostic tools and prescribed medications, while exploring the similarities and differences between children and adult disease.
Definition
According to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) [1••], ARS should be suspected when there are two or more nasal symptoms, one of which should be either nasal congestion/blockage/obstruction or rhinorrhea (anterior or post-nasal drip), while the others could be either facial pain/pressure or reduction/loss of smell, lasting up to 12 weeks. In children, ARS should be considered when there are two or more of the following symptoms: nasal blockage/congestion, discolored nasal discharge, and cough.
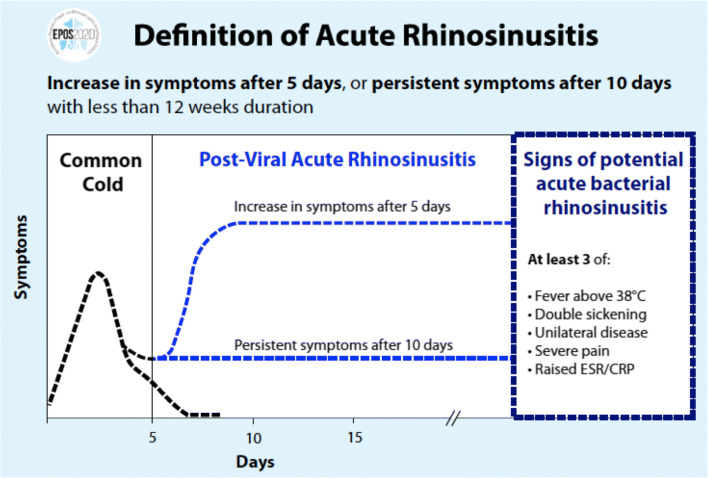
Although it is important to note that there are other infectious etiologies (bacteria, fungi) of this illness, the most common is caused by viruses. The disease may present in three main clinical phenotypes: viral ARS or common cold when the episode lasts up to 10 days and post-viral ARS when symptoms persist longer than 10 days or worsens after 5 days. Bacterial ARS is defined by the presence of three or more of the following clinical findings: fever (≥ 38 °C), severe local pain, double sickening, unilateral disease (with discolored mucus), or elevation of C-reactive protein (CRP)/erythrocyte sedimentation ratio (ESR) in blood test (Fig. 1) [1••].
Fig. 1.
Definition of ARS phenotype bases on EPOS 2020 Consensus. The duration of symptoms is used to differentiate viral ARS (common cold) from post-viral ARS, which is considered when the symptoms persist longer than 10 days or worsen after 5 days. Bacterial ARS should be suspected at any time when the presence of three or more of the sings or symptoms related to bacterial ARS are found
American guidelines (ICAR) note similar definitions and symptoms but stratify to ARS, when symptoms last up to 4 weeks, and sub-acute rhinosinusitis when the duration is between 4 and 12 weeks. Like the European guidelines, they consider viral ARS when the disease duration is less than 10 days [4••].
Epidemiology/Incidence of Diseases
The prevalence of ARS in the general population is variable depending on different studies, noted to be between 6% and 15% [5, 6]. Viral ARS or common cold has a very high incidence, presenting two to five episodes per person a year [7]. In children, this incidence could be up to four times higher [8], with URTIs being one of the main causes of primary care consultations [9•]. Post-viral ARS is less common, with an incidence of about 3 episodes per 100 inhabitants a year [10] in adults (Iceland) with a lower frequency in pediatric populations and differences noted among different age groups (2 cases per 100,000 in ≤ 4 years old, 4–7 cases per 100,000 in 5–14 years old, and 18 cases per 100,000 in 15–17 years old) [11]. In a recent study in Germany, the incidence was found to be 18.8 episodes per 1000 inhabitants per year [12••]. Classically, the incidence of bacterial ARS is estimated to be 0.5–2% of all ARS viral infections, although recent studies have suggested it to be higher. The rate of positive cultures is about 50% in patients with clinical suspicion of bacterial ARS [13•].
Many predisposing factors for ARS have been described: environmental dampness, anatomical factors (particularly in recurrent ARS episodes [14]), mucocilliary impairment, smoking, as well as anxiety and depression [1••]. There is also a higher incidence of episodes during the cold months (especially in patients with CRS at baseline [15•]). On the other hand, laryngo-pharyngeal reflux was not found to be a clear underlying factor [1••].
One of the most interesting and controversial predisposing factors to develop ARS is allergic rhinitis (AR). The incidence of ARS in patients with AR was reported to be 4.4 times higher than in non-allergic rhinitis [16], but other authors have concluded that the presence of allergy in ARS patients might be incidental [17]. A recent study supports however the role of an atopic phenotype as a risk factor to develop ARS in children [18•] while other studies have demonstrated that AR is an insignificant risk factor [19•]. Since there is a clear difficulty in discriminating an AR exacerbation and ARS, exploring symptoms, such as sneezing, itching, and specific triggers worsening symptoms, which would be indicative of AR, can be quite helpful.
Inflammation Versus Infection
ARS is mainly an inflammatory disease of the nose and paranasal sinuses. Usually, a viral infection triggers the inflammatory cascade in the context of common cold. In few cases, this inflammatory condition of the mucosa may facilitate a bacterial infection [1••]. As a result, three different ARS phenotypes are described: viral, post-viral, and bacterial ARS. But it should be noted that these entities often overlap, and their symptoms are very similar. Regarding viral ARS, the rhinovirus has been found to be the cause of 50% of the common cold episodes [20] although other viruses such as adenovirus, coronavirus, influenza virus, and even SARS-CoV-2 virus (responsible for the recent COVID-19 pandemics) could also be involved [21]. Typically, the common cold has a duration of up to 7 to 10 days. When the symptoms persist after the viral disease (over 10 days), the clinical process is called post-viral ARS [1••]. In a few number of cases (0.5–2% of all common colds), this inflammatory condition may lead into a bacterial infection [22].
Children vs Adults
Although ARS seems to have similar pathophysiology in children and adults, viral ARS incidence is however greater in children, while post-viral ARS incidence is more common in adults [1••]. The symptomatology may also differ. While in adults cough is considered a secondary symptom (except for SARS-CoV-2 virus), in children, it is one of the main common symptoms [23] being strongly considered in the diagnostic protocols [1••, 4••]. On the other hand, posterior rhinorrhea and hyposmia, which are cardinal symptoms in adult, are not in children, likely because it is not easy for the young child to describe or acknowledge them. As in adults, no complementary tests are needed to diagnose ARS and its treatment, if there is no suspicion of bacterial origin or complication, should be strictly symptomatic [1••]. A detailed description of medical therapy in children and adults is provided later in the treatment section.
Diagnosis
According to EPOS and American guidelines [1••, 4••], ARS diagnosis is strictly based on the sudden onset of ≥ 2 nasal symptoms (nasal congestion/obstruction, rhinorrhea, facial pressure, or loss of smell). This diagnosis may be supported by endoscopic findings (mucosal edema or rhinorrhea), but they are not necessary in a primary care consultation. Although the use of imaging tests is not recommended, except in complicated cases [24••, 25], a clear overuse of diagnostic tools has been found, where physicians recommended plain X-ray or CT scan in 70% and 22% of post-viral ARS episodes and even in 55% and 12% of viral (common cold) episodes, respectively [3•].
The most difficult issue is to correctly diagnose bacterial ARS (ABRS). Although a rather invasive technique, the gold standard to diagnose ABRS is the antral puncture and culture [26]. The culture of middle meatus secretions obtained under endoscopic visualization has been demonstrated to have similar specificity and sensibility [27•]. However, a culture result takes a few days, and is not useful in the acute situation. Some authors have considered the presence of opacification in the sinuses in X-ray or CT can predict a bacterial origin; however, it has been clearly demonstrated that this is not specific for ABRS. As a matter of fact, the majority of patients with common cold present with opacification due to mucous in the paranasal sinuses [28••]. For this reason, recent studies have been trying to find biochemical markers or specific symptoms that could help to differentiate bacterial from non-bacterial ARS. Regarding symptoms, unilateral facial or dental pain has been identified as a good predictor of bacterial ARS [29], but with limited evidence. Dental pain in the superior jaw has been recently identified to be the symptom more strongly related to bacterial ARS [30••]. Classically, the purulence of nasal discharge has also been considered a sign of bacterial infection, but a recent work by Ebell et al. [30••] has showed however that discolored discharge may be also present in post-viral and even in viral cases, thus invalidating the previous correlation with ABRS. A body temperature ≥ 38 °C has also been associated with a high risk of bacterial infection [31]. Concerning inflammatory biomarkers, Hansen et al. [31] and Autio et al. [32••], in a recent systematic review, noted that an elevation of C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR) support the diagnosis of ABRS, but with poor sensitivity and without being considered a diagnostic marker of the disease. With that said, it does seem clear that the presence of low rates of CRP provides evidence against the use of antibiotics [25].
The EPOS consensus [1••] recommends the use of a combination of signs and symptoms to determine the probability of bacterial origin and defines ABRS when 3 or more of the following five criteria are present: discolored discharge with unilateral predominance, severe local pain, fever ≥ 38 °C, double sickening, or elevation of CRP/ESR. These EPOS criteria have been demonstrated to have better specificity than IDSA (Infectious Diseases Society of America) criteria for diagnosing bacterial ARS [33•]. The IDSA guideline considers bacterial ARS when there are ≥ 1 of the following criteria: symptoms lasting for more than 10 days with no improvement, severe symptoms from the onset (fever ≥ 39 °C or discolored discharge from the beginning and during 3–4 days), or double sickening after 5–6 days [34].
To summarize, in normal situations (apart from virus epidemics or pandemics), there is no need for complementary diagnostic tools to diagnose viral or post-viral ARS, while a blood test to determine CRP/ESR may be helpful when ABRS is suspected.
Treatment
The first step in common cold and ARS management is the prevention of viral infection mainly reinforcing the use of hygiene rules such as hand washing. In special epidemic situations, such as the 2020 COVID-19 pandemics, more strict recommendations such as social distancing, facemask and eye guards, as well as home confinement may be required [35].
The updated management of viral (common cold) and post-viral ARS, and ABRS according to EPOS2020, is summarized in Table 1. The highlights for medical therapy, according to phenotypes and different guidelines [1••, 4••], include the following:
- Common cold:
- Recommended therapy (mainly symptomatic): paracetamol; non-steroidal anti-inflammatory drugs (NSAIDs); second-generation antihistamines with short-term benefit in reducing symptoms the first 2 days [36•]; nasal decongestants with small effect in nasal congestion in adults [37]; combination of analgesics and nasal decongestants [38]; ipratropium bromide for reducing rhinorrhea [39]; probiotics; zinc when administered the first 24 h after the onset of symptoms [40, 41•]; nasal saline irrigations [42]; vitamin C, in selected patients with suspected deficit or with high levels of physical activity [43]; and some herbal medicines (BNO 1016, cineole, and Andrographis paniculata SHA-10) [1••].
Postviral ARS:
- Recommended therapy: symptomatic treatment; INCS, although the beneficial effect in symptoms is clear, as ARS is a self-limited disease, consider the need of their use depending on the severity of symptoms [1••]; sinfrontal, a homeopathy product with slight benefit but low evidence [51]; and some herbal compounds such as Cyclamen europaeum, which improves some symptoms but with low evidence [52], Pelagorium sidoides [53], and BNO 1016, mainly for nasal congestion [54].
-
3.Bacterial ARS:
- Recommended therapy: symptomatic treatment and antibiotics, especially amoxicillin/penicillin (beta-lactams) are effective in adult patients with signs and symptoms of ABRS; data is very limited in children, demonstrating lack of efficacy compared with placebo, but with more adverse events [1••]. Sodium hyaluronate plus saline solution may have and additive effect to antibiotics [55•]. Oral corticosteroids added to antibiotics have shown a moderate effect reducing facial pain. Currently, there is a need for quality research in ABRS on the full range of medications and in particular, topical and oral corticosteroids, antihistamines, decongestants, and saline and steam inhalation [1••].
Table 1.
Acute rhinosinusitis treatment and recommendations for both adults and children based slightly modified from EPOS2020 (Fokkens 2020)
| Acute viral rhinosinusitis (common cold) | Acute post-viral rhinosinusitis | Acute bacterial rhinosinusitis | |
|---|---|---|---|
| Antibiotics |
Recommendation against (1a -) in children and adults |
Recommendation against (1a -) in children and adults |
Careful patient selection to avoid unnecessary use. Recommendation in adults (1a)a No recommendation in children (1a -) |
| Nasal corticosteroids | Recommendation against (1a -) |
Are effective reducing the symptoms, but as a self-limiting disease they are optional in adults (1a) No advise can be made in children (low quality of evidence) |
No studies |
| Systemic corticosteroids | No studies | Recommendation against (1a -) in adults | Insufficient datab |
| Antihistamines | Short-term beneficial effect the overall symptoms in adults (1a) | Low quality of evidence studies, no additive beneficial effect in studies in adults and children | Low quality of evidence studies, no additive beneficial effect in studies in adults and children |
| Nasal decongestants | Multiple doses may have a small positive effect on nasal congestion in adults (1a) without increase the risk of adverse events | May be effective in improving mucociliary clearance in the acute phase. Absence of clinically relevant data | Insufficient data |
| Antihistamine + nasal decongestant + analgesic | Some general benefit in adults and older children with common cold (1a). No evidence in young children | Insufficient data | No studies |
| Ipratropium bromide | Improves rhinorrea but has no effect on nasal obstruction (1a) | Insufficient data | Insufficient data |
| Saline irrigation | Slight benefits decreasing the symptoms of URTIs | Very low quality of evidence, but it may be beneficial in adults (1b) | Insufficient data. No advice can be given about the use of nasal saline irrigation |
| Zinc | Acetate or gluconate ≥75 mg/day when taken within 24 h of onset of symptoms reduces the duration of common cold (1a) | No studies | No studies |
| Herbal medicines |
BNO1016, cineole and Andrographis paniculata SHA-10 extract have significant impact on symptoms of common cold without important adverse events (1b). Echinacea is not reccomended (1a -) |
In adults, BNO1016 tablets and Pelargonium sidoides drops and Myrtol (and other essential oil) capsules have significant impact on symptoms (1b) | Insufficient data |
BNO1016 (Sinupret) is an extract of five herbal drugs (gentian root, prímula flower, sorrel herb, elder flower, and verbena herb). 1a: Systematic review (with homogeneity) of RCTs. 1b: Individual RCT (with narrow confidence intervals)
URTI upper respiratory tract infection
aFrom the limited data available, it seems that especially beta-lactams (amoxicillin/penicillin) are effective and moxifloxacin (fluoroquinone) is not. The efficacy of beta-lactams is evident at day 3 where patients already experience better symptom improvement and continue with a higher number of cures at completion of treatmen
bIn ABRS, a short curse of oral corticosteroids (3–5 days) can be prescribed if severe unilateral pain is present
cSecond-generation antihistamines could be prescribed for the treatment of concomitant allergic rhinitis
The challenge in discriminating bacterial from non-bacterial ARS often leads to an over-diagnosis of ABRS, which result in an overuse of diagnostic testing and early unnecessary prescription of antibiotics. In a study from the UK, 88% of the consultations for rhinosinusitis resulted in antibiotic prescription, while only 11% were deemed appropriate [56••]. The same was true in the Netherlands where 34% of the interviewed primary care physicians chose an antibiotic as treatment for a patient with moderate severe acute rhinosinusitis [57]. In a study from Spain, even when ABRS patients were excluded, the use of antibiotics was found to be around 60% in patients with common cold and 70% in those with post-viral ARS [3•]. The American Rhinosinusitis guidelines highlight the fact that although effective in adults, the actual benefit of antibiotics is small, needing to treat between 11 and 15 patients to get 1 individual to improve [4••]. The overuse of antibiotics has also been associated with an increment of antibiotic resistance, which is directly related to increased morbidity and mortality due to resistant bacterial infections [58, 59•]. So, once again, in spite of the clinical suspicion of ABRS, the decision to treat a patient with antibiotics should be made on an individual basis. In order to help to decrease the inappropriate use of antibiotics for ARS, published studies emphasize the importance of physician communication skills on the use of antibiotics, responsible justification, and peer comparison and training of physicians, to help patients understand the downside to inappropriate prescriptions [1••].
Besides antibiotics, other medications such as antihistamines and mucolytic have not shown any benefit in treating post-viral. Despite this, many physicians continue to prescribe these agents regularly (~ 50%) as reported in several studies from Spain [3•], France [60], or Asia [6].
Complications
The incidence of ABRS complications has been shown to be approximately 3:1,000,000 per year despite the different utilization of antibiotics in the various countries [1••]. Specifically, it has been demonstrated that the use of antibiotics does not prevent complications [61•]. Complications of ABRS are typically classified as orbital (60–80%), intracranial (15–20%), and rarely osseous (5%) [1••]. Orbital complications, the most commonly related to ABRS, are a consequence (in decreasing frequency) of ethmoid, maxillary, frontal, and rarely the sphenoid sinusitis [62]. Orbital complications commonly affect children [63•, 64•], a population that is known to express fewer clinical signs and symptoms, and thus, it is important to have a high level of clinical suspicion.
According to EPOS recommendations, one should rule out a complication when a patient presents with one or more of the following signs and/or symptoms: periorbital edema/erythema, displaced globe, double vision, ophtalmoplegia, reduced visual acuity, severe headache, frontal swelling, signs of sepsis, or other neurological signs [1••].
Regarding diagnosis of complications, the accuracy of a clinical diagnosis is estimated to be around 82% and the accuracy of CT 91% [65]. MRI is, however, considered the “gold standard,” as it is more sensitive than CT scan. When available, MRI should be the imaging modality of choice, having the additional diagnostic value to exclude or confirm cavernous sinus thrombosis and soft tissue involvement [66, 67].
According to EPOS guidelines, the main indications for surgical intervention in orbital complications of ABRS are evidence of subperiosteal or intraorbital abscess in CT scan or MRI (exception for small volume abscesses). Subperiosteal abscess in children is not an absolute indication for immediate surgical intervention. Conservative measures can be safe and effective if appropriately used. A reduced visual acuity, loss of color vision, affected afferent pupillary reflex, or inability to assess vision, however, are indications for urgent surgery. When conservative treatment is chosen, progression or no improvement in orbital signs (diplopia, ophthalmoplegia, proptosis, swelling, chemosis) or in the general condition (fever, infection parameters), after 48 h of intravenous antibiotic treatment is also an indicator of the need for emergency surgery [1••].
Endocranial complications of ABRS are usually associated with fronto-ethmoidal or sphenoid rhinosinusitis [68] and include epidural or subdural empyema, brain abscess, meningitis, cerebritis, and superior sagittal and cavernous sinus thrombosis. They may present with specific central nervous system signs, such as nausea and/or vomiting, neck stiffness, and altered mental stat, or non-specific symptoms and signs (high fever, headache, reduced consciousness), or can even be silent [69]. The pathogens most commonly isolated are Streptococcus and Staphylococcus species including methicillin-resistant (MRSA) and anaerobes [70]. The recommended treatment involves neurosurgical drainage procedures and endoscopic drainage of the paranasal sinuses (most often the frontal sinus) [71•].
Societal Burden and Socioeconomic Costs
Despite the fact that ARS is usually a self-limited, with low risk of further morbidity, it presents a considerable burden to public health [4••], being an important cause of work absenteeism [72]. As reported above, a significant overuse of diagnostic tests and medications has been reported in multiple countries [3•, 6, 57], with very few studies addressing the economic impact of ARS. In the 1990s, the cost of ARS reached US$3390 million per year in the USA [73]. In Europe, a total cost of ~ €1100 per ARS episode was recently examined, with the major cost (75%) attributable to indirect costs [2••]. Recent data from Spain demonstrated that direct costs of ARS where greater in postviral (~ €440) than viral (~ €320) ARS episodes, and not surprisingly, severe cases resulted in greater direct cost [74], with the main driver of direct cost attributable to medical visits [72, 74]. As the economic costs are quite large, there is a clear unmet need with further research needed to optimize appropriate testing and therapy. Concerning medical visits, health education should be improved and encouraged, teaching the public that ARS is a self-limited and non-complicated disease, which usually only requires symptomatic treatment, while medical consultations should be restricted to severe or complicated cases. On the other hand, decreasing the costs related to diagnosis and treatment are directly linked to medical management. Svensson et al. showed that the cost of treating ARS with topical corticosteroid was much lower compared with the use of amoxicillin [75]. Regarding the costs related to antibiotic use, Cramer et al. reported a dramatic decrease in costs when t guideline recommendations were followed, compared with when they were not (US$352 vs. US$166 million per year) [76••]. Therefore, knowledge of up-to-date guidelines and scientific recommendations is strongly recommended for both primary care physicians and specialists in order to avoid the overuse of diagnostic tools and prescription of unnecessary medications, especially antibiotics, in the management of ARS [77•].
Conclusions
The first and most important rule for common cold and ARS management is the prevention of viral infection through hygiene behavior such as hand washing. In special epidemic situations such as the 2020 COVID-19 pandemics, more strict recommendations such as social distance and home confinement may be required.
Post-viral ARS is mainly an inflammatory disease, which usually begins as a viral infection (common cold, URTI, or viral ARS), but may persist longer than 7–10 days or worsen after 5 days. Some rare cases (less than 2%) may develop bacterial ARS.
The incidence of viral ARS is very high (2–5 episodes/person/year) while post-viral ARS has an incidence about 3 episodes per 100 people a year.
The most common ARS symptoms are nasal blockage or congestion and anterior or posterior rhinorrhea. In adults, facial pain or pressure and loss of smell are also cardinal symptoms, while in children, cough is more relevant.
The diagnosis is clinical, based on the sudden onset of nasal symptoms (nasal blockage/obstruction, nasal discharge/rhinorrhea, hyposmia, and facial pain/pressure), and there is no need for complementary tests.
The distinction between bacterial and non-bacterial ARS remains a diagnostic challenge. The presence of a fever, unilateral focality, local pain, and elevation of CPR/ERS seems to be the best way to predict bacterial ARS.
In special situations, such as the 2020 COVID-19 pandemics, a sudden severe loss of smell (anosmia), even with the absence of other nasal or general symptoms (dry cough, fever), should be considered a symptom of suspicion while the definitive diagnosis should be specific by using a PCR test for the SARS-Cov-2 virus.
Viral ARS treatment should be symptomatic (analgesic, NSAIDs). Some herbal compounds or minerals like zinc may also help.
Intranasal corticoids have proven to be useful in post-viral ARS, but, being a self-resolving disease, its use should be individualized. Antibiotics, mucolytic, and antihistamines have not demonstrated any benefit in patients with post-viral ARS.
Antibiotics have only shown some effect in bacterial ARS, although there is a high rate of resolution even without their use. Therefore, individual considerations, taking into account the adverse effects and increased drug resistances, have to be made before prescribing antibiotics.
Complications are very uncommon, and their incidence is not dependent on the antibiotic use. Orbital complications are common in children while intracranial complications are less frequent. The presence of ophthalmological or neurological symptoms should raise the suspicion of a complication and imaging tests should be obtained. Therapeutic management of ARS complications includes hospital admission and intravenous antibiotics and often requires surgery (ORL and/or neurosurgery).
The economic burden of ARS is incredibly high due to the large number of medical visits, the misuse of diagnostic testing, and the overuse of medications, as well as for the high indirect costs. Disseminating the concept of ARS being a mild and self-resolving disease among patients and physicians remains an unmet need that is required to reduce the high costs of this illness.
Footnotes
This article is part of the Topical Collection on Rhinosinusitis
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francesca Jaume, Email: fjaumemonroig@hotmail.com.
Joaquim Mullol, Email: jmullol@clinic.cat.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Fokkens W, Lund V, Hopkins HP, Kern R, Reitsma S, et al. EPOS2020: European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 2.Stjärne P, Odebäck P, Ställberg B, Lundberg J, Olsson P. High costs and burden of illness in acute rhinosinusitis: real-life treatment patterns and outcomes in Swedish primary care. Primary Care Resp Journal. 2012;21:174–179. doi: 10.4104/pcrj.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaume F, Quintó L, Alobid I, Mullol J. Overuse of diagnostic tools and medications in acute rhinosinusitis in Spain: a population-based study (the PROSINUS study) BMJ Open. 2018;8(1):e018788. doi: 10.1136/bmjopen-2017-018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 5.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 6.Wang DY, Wardani RS, Singh K, Thanaviratananich S, Vicente G, Xu G, Zia MR, Gulati A, Fang SY, Shi L, Chan YH, Price D, Lund VJ, Mullol J, Fokkens WJ. A survey on the management of acute rhinosinusitis among Asian physicians. Rhinology. 2011;49(3):264–271. doi: 10.4193/Rhino10.169. [DOI] [PubMed] [Google Scholar]
- 7.Turner RB. Epidemiology, pathogenesis and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78:531–540. doi: 10.1016/S1081-1206(10)63213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachert C, Hormann K, Mosges R, Rasp G, Riechelmann H, Müller R, et al. An update on the diagnosis and treatment of sinusitis and nasal polyposis. Allergy. 2003;58:176–191. doi: 10.1034/j.1398-9995.2003.02172.x. [DOI] [PubMed] [Google Scholar]
- 9.Finley CR, Chan DS, Garrison S, Korownyk C, Kolber MR, Campbell S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018;64:832–840. [PMC free article] [PubMed] [Google Scholar]
- 10.Oskarsson JP, Halldorsson S. An evaluation of diagnosis and treatment of acute sinusitis at three healthcare centers. Laeknabladid. 2010;96:531–535. doi: 10.17992/lbl.2010.09.313. [DOI] [PubMed] [Google Scholar]
- 11.Uijen JH, Bindels PJ, Schellevis FG, van der Wouden JC. ENT problems in Dutch children: trends in incidence rates, antibiotic prescribing and referrals 2002-2008. Scand J Prim Health Care. 2011;29:75–79. doi: 10.3109/02813432.2011.569140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmans R, Wagemakers A, van Drunen C, Hellings P, Fokkens W. Acute and chronic rhinosinusitis and allergic rhinitis in relation to comorbidity, ethnicity and environment. PloS one. 2018;13:e0192330. doi: 10.1371/journal.pone.0192330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SS, Ference EH, Evans CT, Tan BK, Kern RC, Chandra RK. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and metaanalysis. Laryngoscope. 2015;125:57–69. doi: 10.1002/lary.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftus PA, Lin J, Tabaee A. Anatomic variants of the paranasal sinuses in patients with recurrent acute rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:328–333. doi: 10.1002/alr.21658. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper JR, Hirsch AG, Bandeen-Roche K, Tan BK, Schleimer RP, Kern RC, et al. Prevalence, severity, and risk factors for acute exacerbations of nasal and sinus symptoms by chronic rhinosinusitis status. Allergy. 2018;73:1244–1253. doi: 10.1111/all.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatz M, Zeiger RS, Chen W, Yang S-J, Corrao MA, Quinn VP. The burden of rhinitis in a managed care organization. Ann Allergy Asthma Immunol. 2008;101:240–247. doi: 10.1016/S1081-1206(10)60488-7. [DOI] [PubMed] [Google Scholar]
- 17.Pant H, Ferguson BJ, Macardle PJ. The role of allergy in rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:232–238. doi: 10.1097/moo.0b013e32832ad3c0. [DOI] [PubMed] [Google Scholar]
- 18.Lin SW, Wang SK, Lu MC, Wang CL, Koo M. Acute rhinosinusitis among pediatric patients with allergic rhinitis: a nationwide, population-based cohort study. PLoS One. 2019;14(2):e0211547. doi: 10.1371/journal.pone.0211547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leo G, Incorvaia C, Cazzavillan A, Consonni D, Zuccotti GV. Could seasonal allergy be a risk factor for acute rhinosinusitis in children? J Laryngol Otol. 2018;132(2):150–153. doi: 10.1017/S0022215118000038. [DOI] [PubMed] [Google Scholar]
- 20.Heikkinen T, Järvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benninger MS, Senior BA. The development of the rhinosinusitis disability index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh N, Hoberman A, Kearney DH, Colborn DK, Kurs-Lasky M, Jeong JH, Haralam MA, Bowen A’D, Flom LL, Wald ER. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J. 2013;32:1061–1065. doi: 10.1097/INF.0b013e31829bb2c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scadding G, Hellings P, Alobid I, Bachert C, Fokkens W, van Wijk RG, et al. Diagnostic tools in rhinology EAACI position paper. Clin Transl Allergy. 2011;1(1):2. doi: 10.1186/2045-7022-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebell MH, McKay B, Guilbault R, Ermias Y. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612–e632. doi: 10.3399/bjgp16X686581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benninger MS, Appelbaum PC, Denneny JC, Osguthorpe DJ, Stankiewicz JA. Maxillary sinus puncture and culture in the diagnosis of acute rhinosinusitis: the case for pursuing alternative culture methods. Otolaryngol Head Neck Surg. 2002;127:7–12. doi: 10.1067/mhn.2002.124847. [DOI] [PubMed] [Google Scholar]
- 27.Benninger MS, Payne SC, Ferguson BJ, Hadley JA, Ahmad N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:3–9. doi: 10.1016/j.otohns.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Gwaltney JM, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 29.Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol. 1988;105:343–349. doi: 10.3109/00016488809097017. [DOI] [PubMed] [Google Scholar]
- 30.Ebell MH, McKay B, Dale A, Guilbault R, Ermias Y. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164–172. doi: 10.1370/afm.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen JG, Hojbjerg T, Rosborg J. Symptoms and signs in culture-proven acute maxillary sinusitis in a general practice population. APMIS. 2009;117:724–729. doi: 10.1111/j.1600-0463.2009.02526.x. [DOI] [PubMed] [Google Scholar]
- 32.Autio TJ, Koskenkorva T, Leino TK, Koivunen P, Alho OP. Longitudinal analysis of inflammatory biomarkers during acute rhinosinusitis. Laryngoscope. 2017;127(2):E55–E61. doi: 10.1002/lary.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seresirikachorn K, Snidvongs K, Chitsuthipakorn W, et al. EPOS2012 has better specificity com-pared to IDSA2012 for diagnosing acute bacterial rhinosinusitis. Rhinology. 2018;56:241–244. doi: 10.4193/Rhin17.261. [DOI] [PubMed] [Google Scholar]
- 34.Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM, Jr, Infectious Diseases Society of America IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72–e112. doi: 10.1093/cid/cir1043. [DOI] [PubMed] [Google Scholar]
- 35.Lotfinejad N, Peters A, Pittet D. Hand hygiene and the novel coronavirus pandemic: the role of healthcare workers. J Hosp Infect. 2020. [DOI] [PMC free article] [PubMed]
- 36.• De Sutter AI, Saraswat A, van Driel ML. Antihistamines for the common cold. Cochrane Database Syst Rev. 2015:CD009345 This systematic review stated that antihistamines have a limited but significant short-term (days one and two of treatment), but not in the mid to long-term, beneficial effect on the severity of overall symptoms for common cold in adults. [DOI] [PMC free article] [PubMed]
- 37.Deckx L, De Sutter AI, Guo L, Mir NA, van Driel ML. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst Rev. 2016;10:CD009612. doi: 10.1002/14651858.CD009612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Sutter AI, van Driel ML, Kumar AA, Lesslar O, Skrt A. Oral antihistamine-decongestant-analgesic combinations for the common cold. Cochrane Database Syst Rev. 2012:CD004976. [DOI] [PubMed]
- 39.AlBalawi ZH, Othman SS, Alfaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database Syst Rev. 2013;6:CD008231. doi: 10.1002/14651858.CD008231.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev. 2011;2013:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 41.Hemila H, Fitzgerald JT, Petrus EJ, Prasad A. Zinc acetate lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis. Open Forum Infect Dis. 2017;4:ofx059. doi: 10.1093/ofid/ofx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King D, Mitchell B, Williams CP, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015:CD006821. [DOI] [PMC free article] [PubMed]
- 43.Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013:CD000980. [DOI] [PMC free article] [PubMed]
- 44.Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013:CD000247. [DOI] [PubMed]
- 45.Hayward G, Thompson MJ, Perera R, Del Mar CB, Glasziou PP, Heneghan CJ. Corticosteroids for the common cold. Cochrane Database Syst Rev. 2015;10:CD008116. doi: 10.1002/14651858.CD008116.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh M, Singh M, Jaiswal N, Chauhan A. Heated, humidified air for the common cold. Cochrane Database Syst Rev. 2017;8(8):CD001728. doi: 10.1002/14651858.CD001728.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karsch-Volk M, Barrett B, Kiefer D, Bauer R, Ardjomand-Woelkart K, Linde K. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2014:CD000530. [DOI] [PMC free article] [PubMed]
- 48.Hawke K, van Driel ML, Buffington BJ, McGuire TM, King D. Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children. Cochrane Database Syst Rev. 2018;4:CD005974. doi: 10.1002/14651858.CD005974.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quick M. Cochrane commentary: probiotics for prevention of acute upper respiratory infection. Explore (NY) 2015;11(5):418–420. doi: 10.1016/j.explore.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Lee HK, Hwang IH, Kim SY, Pyo SY. The effect of exercise on prevention of the common cold: a meta-analysis of randomized controlled trial studies. Kor J Fam Med. 2014;35:119–126. doi: 10.4082/kjfm.2014.35.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zabolotnyi DI, Kneis KC, Richardson A, Rettenberger R, Heger M, Kaszkin-Bettag M, Heger PW. Efficacy of a complex homeopathic medication (Sinfrontal) in patients with acute maxillary sinusitis: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial. Explore (NY) 2007;3(2):98–109. doi: 10.1016/j.explore.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Pfaar O, Mullol J, Anders C, Hormann K, Klimek L. Cyclamen europaeum nasal spray, a novel phytotherapeutic product for the management of acute rhinosinusitis: a randomized double-blind, placebo-controlled trial. Rhinology. 2012;50:37–44. doi: 10.4193/Rhino11.141. [DOI] [PubMed] [Google Scholar]
- 53.Timmer A, Gunther J, Rucker G, Motschall E, Antes G, Kern WV. Pelargonium sidoides extract for acute respiratory tract infections. Cochrane Database Syst Rev. 2008:CD006323. [DOI] [PubMed]
- 54.Neubauer N, März RW. Placebo-controlled, randomized double-blind clinical trial with Sinupret sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. Phytomedicine. 1994;1:177–181. doi: 10.1016/S0944-7113(11)80061-9. [DOI] [PubMed] [Google Scholar]
- 55.Ciofalo A, de Vincentiis M, Zambetti G, et al. Olfactory dysfunction in acute rhinosinusitis: intranasal sodium hyaluronate as adjuvant treatment. Eur Arch Otorhinolaryngol. 2017;274:803–808. doi: 10.1007/s00405-016-4277-x. [DOI] [PubMed] [Google Scholar]
- 56.Pouwels KB, FCK D, DRM S, Robotham JV, Smieszek T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018;73:19–26. doi: 10.1093/jac/dkx502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmans R, Schermer T, van Weel C, Fokkens W. Management of rhinosinusitis in Dutch general practice. Prim Care Respir J. 2011;20:64–70. doi: 10.4104/pcrj.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Kraker ME, Davey PG, Grundmann H, BURDEN study group Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter D, Charlett A, Conti S, Robotham JV, Johnson AP, Livermore DM, et al. A risk assessment of antibiotic pan-drug-resistance in the UK: Bayesian analysis of an expert elicitation study. Antibiotics. 2017;6:9. doi: 10.3390/antibiotics6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klossek JM, Mesbah K. Presentation and treatment of acute maxillary sinusitis in general practice: a French observational study. Rhinology. 2011;49(1):84–89. doi: 10.4193/Rhino09.126. [DOI] [PubMed] [Google Scholar]
- 61.Hansen FS, Hoffmans R, Georgalas C, Fokkens WJ. Complications of acute rhinosinusitis in The Netherlands. Fam Pract. 2012;29(2):147–153. doi: 10.1093/fampra/cmr062. [DOI] [PubMed] [Google Scholar]
- 62.Chandler JR, Langenbrunner DJ, Stevens ER. The pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;80:1414–1428. doi: 10.1288/00005537-197009000-00007. [DOI] [PubMed] [Google Scholar]
- 63.El Mograbi A, Ritter A, Najjar E, Soudry E. Orbital complications of rhinosinusitis in the adult population: analysis of cases presenting to a tertiary medical center over a 13-year period. Ann Otol Rhinol Laryngol. 2019;128:563–568. doi: 10.1177/0003489419832624. [DOI] [PubMed] [Google Scholar]
- 64.Schollin Ask L, Hultman Dennison S, Stjarne P, et al. Most preschool children hospitalized for acute rhinosinusitis had orbital complications, more common in the youngest and among boys. Acta Paediatr. 2017;106:268–273. doi: 10.1111/apa.13650. [DOI] [PubMed] [Google Scholar]
- 65.Younis RT, Anand VK, Davidson B. The role of computed tomography and magnetic resonance imaging in patients with sinusitis with complications. Laryngoscope. 2002;112:224–229. doi: 10.1097/00005537-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Dankbaar JW, van Bemmel AJ, Pameijer FA. Imaging findings of the orbital and intracranial complications of acute bacterial rhinosinusitis. Insights Imaging. 2015;6(5):509–518. doi: 10.1007/s13244-015-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Germiller J, Monin D, Sparano A, Tom L. Intracranial complications of sinusitis in children and adolescents and their outcomes. Arch Otolaryngol Head Neck Surg. 2006;132(9):969–976. doi: 10.1001/archotol.132.9.969. [DOI] [PubMed] [Google Scholar]
- 68.Garin A, Thierry B, Leboulanger N, Blauwblomme T, Grevent D, Blanot S, Garabedian N, Couloigner V. Pediatric sinogenic epidural and subdural empyema: the role of endoscopic sinus surgery. Int J Pediatr Otorhinolaryngol. 2015;79:1752–1760. doi: 10.1016/j.ijporl.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Chaiyasate S, Fooanant S, Navacharoen N, Roongrotwattanasiri K, Tantilipikorn P, Patumanond J. The complications of sinusitis in a tertiary care hospital: types, patient characteristics, and outcomes. Int J Otolaryngol. 2015;2015:709302. doi: 10.1155/2015/709302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deutschmann MW, Livingstone D, Cho JJ, Vanderkooi OG, Brookes JT. The significance of Streptococcus anginosus group in intracranial complications of pediatric rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2013;139:157–160. doi: 10.1001/jamaoto.2013.1369. [DOI] [PubMed] [Google Scholar]
- 71.Kou YF, Killeen D, Whittemore B, et al. Intracranial complications of acute sinusitis in children: the role of endoscopic sinus surgery. Int J Pediatr Otorhinolaryngol. 2018;110:147–151. doi: 10.1016/j.ijporl.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharyya N. Contemporary assessment of the disease burden of sinusitis. The economic burden and symptom manifestations of chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23:392–395. doi: 10.2500/ajra.2009.23.3355. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy DW. First line management of sinusitis: a national problem? Overview. Otolaryngol Head Neck Surg. 1990;103:847–854. doi: 10.1177/01945998901030S502. [DOI] [PubMed] [Google Scholar]
- 74.Jaume F, Quintó L, Alobid I, Mullol J. Direct costs of acute rhinosinusitis in spain—a prospective and Observational study (Prosinus). J Investig Allergol Clin Immunol (Under revision). [DOI] [PubMed]
- 75.Svensson J, Lundberg J, Olsson P, Stjärne P, Tennval GR. Cost-effectiveness of mometasone furoate nasal spray in the treatment of acute rhinosinusitis. Prim Care Respir J. 2012;21(4):412–418. doi: 10.4104/pcrj.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cramer JD, Kern RC, Tan BK, Peters AT, Evans CT, Smith SS. Potential national savings from prescribing guideline-recommended antibiotics for acute rhinosinusitis. Laryngoscope. 2016;26(3):579–581. doi: 10.1002/lary.25719. [DOI] [PubMed] [Google Scholar]
- 77.Piltcher OB, Kosugi EM, Sakano E, Mion O, Testa JRG, Romano FR, et al. How to avoid the inappropriate use of antibiotics in upper respiratory tract infections? A position statement from an expert panel. Braz J Otorhinolaryngol. 2018;84(3):265–279. doi: 10.1016/j.bjorl.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]