Abstract
Development of abiotic stress tolerant rice cultivars is necessary for sustainable rice production under the scenario of global climate change, dwindling fresh water resources and increase in salt affected areas. Several genes from rice have been functionally validated by using EMS mutants and transgenics. Often, many of these desirable alleles are not available indica rice which is mainly cultivated, and where available, introgression of these alleles into elite cultivars is a time and labour intensive process, in addition to the potential introgression of non-desirable genes due to linkage. CRISPR-Cas technology helps development of elite cultivars with desirable alleles by precision gene editing. Hence, this study was carried out to create mutant alleles of drought and salt tolerance (DST) gene by using CRISPR-Cas9 gene editing in indica rice cv. MTU1010. We used two different gRNAs to target regions of DST protein that might be involved in protein–protein interaction and successfully generated different mutant alleles of DST gene. We selected homozygous dst mutant with 366 bp deletion between the two gRNAs for phenotypic analysis. This 366 bp deletion led to the deletion of amino acid residues from 184 to 305 in frame, and hence the mutant was named as dst∆184–305. The dst∆184–305 mutation induced by CRISPR-Cas9 method in DST gene in indica rice cv. MTU1010 phenocopied EMS-induced dst (N69D) mutation reported earlier in japonica cultivar. The dst∆184–305 mutant produced leaves with broader width and reduced stomatal density, and thus enhanced leaf water retention under dehydration stress. Our study showed that the reduction in stomatal density in loss of function mutants of dst is, at least, in part due to downregulation of stomatal developmental genes SPCH1, MUTE and ICE1. The Cas9-free dst∆184–305 mutant exhibited moderate level tolerance to osmotic stress and high level of salt stress in seedling stage. Thus, dst mutant alleles generated in this study will be useful for improving drought and salt tolerance and grain yield in indica rice cultivars.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00819-w) contains supplementary material, which is available to authorized users.
Keywords: CRISPR-Cas9, Drought and salt tolerance, Inducer of CBF expression1/scream, Mute, Speechless, Stomata, Salt tolerance
Introduction
Rice (Oryza sativa L.) is an important food crop of the world. Rice grain makes up to 20% of the world’s dietary energy supply, and more than three billion people across the globe eat rice daily as major staple food (Birla et al. 2017). We need to produce 40% more rice for food security by 2050 (Ray et al. 2013). To achieve this goal, breaking the yield barriers, improving climate resilience, biotic and abiotic stress tolerance and resource use efficiency such as water and nitrogen use efficiency, are necessary. Hence, in addition to molecular breeding approaches, use of genome editing technologies is necessary to sustain rice production.
Genome editing is a precision mutagenesis tool for functional genomics and crop improvement. Recently, rice is being used to model crop system for functional genomics by using genome editing technologies viz., Transcriptional Activator-Like Effector nucleases (TALEN) (Li et al. 2012) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-associated endonuclease Cas9 (CRISPR/Cas9) (Feng et al. 2013; Xu et al. 2014; Wang et al. 2016; Meng et al. 2017; Miao et al. 2018). Of the various genome editing technologies, the RNA-guided CRISPR-Cas9 technology is easy to use. In this technology, guide-RNA makes Watson–Crick base pairing with complimentary genomic DNA strand, and Cas9 endonuclease make double strand DNA cleavage in the genome DNA at 3 bases upstream to PAM (protospacer adjacent motif) site. The double stand break (DSB) caused by Cas9 is repaired by non-homologous end joining (NHEJ) or homologous recombination (HR) by cell’s native machinery. The NHEJ repair mechanism is error prone and thus leads to deletions/insertion mutation at double strand break site. In HR mechanism, exogenous template is used to create more precise mutations (Chen et al. 2019). In addition, DSB dependent CRISPR-Cas9 technology, DBS-independent CRISPR-dCas9/nCas9 base-editing technologies have been standardized for rice and other crops. Adenine base editors (ABEs, adenosine deaminase fused with dCas9/nCas9) and cytosine base editors (CBEs, cytidine deaminase fused with dCas9/nCas9) are used to create precise A → G and C → T mutation respectively (Kim 2018; Chen et al. 2019; Hua et al. 2019). In this study, we selected CRISPR-Cas9 based NHEJ mutation method for gene editing.
Several alleles of genes associated with economic traits have been characterized mainly in japonica genotype (Li et al. 2018; Yao et al. 2018). Many of these genes for tolerance to biotic and abiotic stresses, nutritional quality improvement, flowering, grain quality and yield traits have been edited by using TALENs, CRISPR-Cas9, CRISPR-Cpf1 and base editing methods (reviewed in Mishra et al. 2018; Jun et al. 2019). CRISPR-Cas9 approach can be used to create these economically important mutants in indica varieties, which occupy about 80% of rice cultivated area in the world. Standardization of efficient genome editing methods for indica rice cultivars is necessary to harness the advantages of cutting-edge biotechnology (Hiei et al. 2015). MTU1010 (Cotton Dora Sannalu) is a popular mega rice variety cultivated in India (Singh et al. 2016). Hence, in this study MTU1010 was selected for genome editing using CRISPR-Cas9 method.
Considering the demand for enhanced productivity of rice in regions with fresh water scarcity and salt affected areas, we selected the drought and salt tolerance (OsDST) gene as a candidate gene. Large-scale screening of EMS mutants of japonica rice cv. Zhonghua11 (ZH11) led to the identification of dst (drought and salt tolerance) mutant of rice. OsDST gene encodes a zinc finger transcription factor. The dst mutant had two substitutions which resulted in N69D and A162T changes in the amino acid sequence of the dst protein. Transgenic complementation analysis revealed that N69D mutation is the cause for dst mutant phenotype. The N69 is a conserved amino acid residue in the zinc finger motif which is necessary for transcriptional activity of the protein. This mutation enhanced the leaf width, reduced stomatal density and enhanced stomatal closure through modulation of H2O2 homeostasis. The loss of function of DST protein due to N69D mutation enhanced the drought and salt tolerance of rice (Huang et al. 2009). In another study, a single nucleotide insertion mutant (A in between 214th and 215th nucleotide) resulting in frame shift mutation (DSTreg1) which destroyed the C-terminal EAR motif was identified in a cultivated indica rice cv. Zhefu 802 (ZF802). The DSTreg1 mutation impaired the transcriptional activation of Gn1a/OsCKX2 (Grain number 1a/Cytokinin Oxidase 2) gene, resulting in increased cytokinin level in the reproductive meristem, and thus increased grain number (Li et al. 2013). However, no deletion mutants were developed and analyzed to ascertain the function of DST gene in japonica or indica rice. Therefore in this study, we selected DST gene for CRISPR-Cas9 mediated genome editing in indica rice cv. MTU1010.
Materials and methods
MTU1010 seeds were obtained from Division of Genetics, ICAR-IARI, New Delhi. Matured seeds of MTU1010 were used as explants for Agrobacterium mediated transformation and generation of mutants using CRISPR-Cas9.
Construction of CRISPR/Cas9 vector for OsDST gene
The DST gene was sequenced from indica rice cv. MTU1010, and this sequence was used for gRNA design. Two gRNAs 5'-GGTGGGCTGCAGTACTACGC-3' (gRNA1) and 5'-GGAGCTAGAGGCTCAAGTTG-3' (3'-CAACTTGAGCCTCTAGCTCC-5') (gRNA2) were designed to target two different regions of OsDST (Online Resource 1) using CRISPR-PLANT (https://www.genome.arizona.edu/crispr2/). These gRNAs were cloned separately in p2XSgR-SpCas9-Os vector (kindly provided by Prof. JK Zhu, Shanghai Center for Plant Stress Biology, Shanghai 201,602, PR China) following the protocol described by Mao et al. (2013). The clones with individual gRNA were confirmed by PCR and sequencing. The sgRNA1 and Cas9 expression cassettes from the recombinant p2XSgR-SpCas9-Os vector was cloned into pCAMBIA1300 at HindIII and KpnI sites to construct pC1300-POsU3::sgRNA1-PUQ::SpCas9 vector. The sgRNA2 expression cassette from recombinant p2XSgR-SpCas9-Os vector was PCR amplified using OsU3-KpnI-F and OsU3-EcoRI-R primers, and cloned into pC1300-POsU3::sgRNA1-PUQ::SpCas9 vector at KpnI and EcoRI sites. The resultant recombinant binary vector pC1300-POsU3::sgRNA1-PUQ::SpCas9n-POsU3::sgRNA2, was confirmed by restriction enzyme digestion and DNA sequencing. This construct was introduced into Agrobacterium tumefaciens strain EHA105 and used for transformation of rice cv. MTU1010. The sequences of primers used in this study are given in Online Resource 2.
Agrobacterium mediated genetic transformation of rice cv. MTU1010
For callus induction, healthy MTU1010 seeds were manually dehusked and then washed 2–3 times with autoclaved sterile double distilled water. Seeds were surface sterilized with 70% ethanol (v/v) for 90 s followed by 4–5 times with sterile double distilled water. Seeds were further surface sterilized with 50% (v/v) commercial bleach with gentle shaking for 20 min followed by 5–6 times wash with sterile double distilled water. Seeds were dried on autoclaved Whatman paper (3 mm) for an hour. 15–20 seeds were inoculated per plate (100 mm × 90 mm) on callus induction media (CIM) and incubated at 26 ± 2 °C in dark conditions. The compositions of media used are given in Online Resource 3. In this experiment, two types of media were tested to optimize the callus induction in MTU1010. Previous studies used CIM with 2,4-D (2.5 mg/l) alone or combination of 2,4-D (2.5 mg/l) and 6-BAP (0.25 mg/l) for different indica varieties (Sahoo et al. 2011). For MTU1010, combination of 2,4-D (2.5 mg/l) and 6-BAP (0.25 mg/l) were used. Further, maltose can influence the degree of differentiation and thus the efficiency of regeneration in different plants (Strickland et al. 1987; Chu et al. 1990; Jain et al. 1995; Kumar et al. 2005). Hence, we used maltose as a carbon source in the CIM in this study.
Agrobacterium mediated transformation was carried out following method of Sahoo and Tuteja (2012) with some modifications. Media compositions used in this study are given in Online Resource 3. A single colony of Agrobacterium tumefaciens strain EHA105 harbouring pC1300-POsU3::sgRNA1-PUQ::SpCas9n-POsU3::sgRNA2 constructs were inoculated in 5 ml of liquid of YEM (yeast extract mannitol) medium and incubated at 28 °C for 24 h in a rotary shaker. Depending on the growth of the culture, 0.5-1 ml of the primary culture was inoculated in 100 ml of YEM liquid medium with Rifampicin (Duchefa) 10 mg/l and Kanamycin (Duchefa) 50 mg/l, and incubated at 28 °C over-night in a rotary shaker at 200 rpm. The next day, Agrobacterium culture with an absorbance 0.6–1.0 was centrifuged for 20 min at 4000 rpm at 20 °C to pellet the cells. The pellet was resuspended in 10–20 ml of resuspension medium containing 150 µM acetosyringone (Himedia). The microcalli were immersed in resuspension medium with gentle shaking in an incubator shaker for 20 min. After infection, the media was discarded, the calli were blotted on a filter paper and air-dried for 5–10 min in a laminar flow hood. The microcalli were transferred on to co-cultivation media and incubated in dark at 25 °C for 48 h.
Excess Agrobacterium was removed by washing the calli for 4–5 times with sterile double distilled water until no turbidity observed in water, followed by sterile double distilled water containing 300 mg/l cefotaxime. Then, the calli were blotted to dry on sterile Whatman no. 1 paper and transferred to selection medium (SM1) containing 50 mg/l hygromycin, 300 mg/l cefotaxime and 200 mg/l Timenitin. Calli were maintained in the SM1 medium in growth incubator at 26 ± 2 °C dark for 15 days. After 15 days of selection, the resistant calli were selected and transferred to selection medium (SM2) containing 300 mg/l cefotaxime and 50 mg/l hygromycin, and cultured in growth incubator at 26 ± 2 °C dark for 15 days. After two rounds of selection, white portions of proliferated calli were isolated and transferred to fresh selection medium (SM2) and maintained in growth incubator at 26 ± 2 °C dark for 10–15 days. The resistant proliferated calli were isolated and transferred to regeneration medium containing 35 mg/l hygromycin and kept in dark for 1 week and then transferred to 16 h light/8 h dark photoperiod for 10–15 days.
The regenerated healthy shoots were separated and transferred on rooting media and kept in light for 10–15 days. The rooted plants were transferred to full strength Yoshida solution to enhance the root growth for 1 week and then transferred to soilrite mixture (Keltech Banglore, India) in pots for hardening. Different step of rice transformation are given in Online Resource 4. The hardened rooted plants were grown to maturity at transgenic greenhouse (ICAR-IARI, New Delhi, India). Putative transgenic plants were subjected to molecular analysis.
Molecular characterization of genome edited DST plants
Genomic DNA was isolated from putative T0 plants and used as template for confirmation by PCR using U3 promoter and sgRNA gene specific primers, and SpCas9 specific primers. To identify the mutation in OsDST gene in Cas9 PCR confirmed T0 plants, the OsDST genomic region covering the gRNA target regions was amplified by PCR using LATaq polymerase (TAKARA Japan). The DST PCR amplicons from T0 plants were sequenced by Sanger sequencing method. The DNA sequences of the DST PCR amplicons from T0 plants were analyzed using DSDecodeM (http://dsdecode.scgene.com/) (Liu et al. 2015; Ma et al. 2015) and CRISPR-ID (Dehairs et al. 2016) tools. Seeds from these sequence confirmed mutants were used to raise T1 plants. The DST PCR amplicons from T1 plants were again sequenced by Sanger sequencing method and mutation in DST gene was confirmed. T1 plants with confirmed mutation in DST gene were further analysed by PCR to identify Cas9 free dst mutants. Since in T0 generation, mutation is induced, T0 is considered as M1 generation. The transgene free mutant obtained from T1 generation is considered as M2. M2 plants were selfed to produce M3 generation.
Morphological traits of gene-edited dst∆184–305 mutant
Homozygous deletion mutant dst∆184–305 produced by genome editing was analysed for phenotypic variations in M3 generation. Flag leaf width was measured in the wild type MTU1010 plants and GEd M3 dst∆184–305 mutant plants. Stomatal density was measured following the previously described protocol (Kusumi et al. 2012). To measure stomatal density, fully expanded leaves were selected and imprints using instant glue were taken at abaxial side and collected on cover slip. Stomata were observed on 40× magnification in light microscope (EVOS XL Core, life technologies, USA).
qRT-PCR expression analysis of stomatal developmental genes in dst∆184–305 mutant
Total RNA was isolated from developing leaves of wild type MTU1010 plants and dst∆184–305 mutant (M3) plants using pure link RNA isolation kit (Invitrogen) according to manufacture’s instructions. DNA contamination was removed by using DNaseI treatment (Ambion). 2 µg of total RNA was used for synthesis of first-strand cDNA using Superscript cDNA synthesis kit (Invitrogen) following manufacturer’s instructions. qRT-PCR was performed with the KAPA superfast syber mix (KAPA biosystems, USA) in a total volume of 10 μl on the step one Real-Time PCR system (Applied Biosystems) following the manufacturer’s manual. All the reactions were run in three biological replicates with two technical replicates for each sample. Data were normalized to rice UBIQUITIN5 gene (Jain et al. 2006) and the comparative Ct relative quantification method (2−ΔΔCt) was used for data analysis (Livak and Schmittgen 2001). The primer sequences used for qRT-PCR analysis are given on Online resource 2.
Excised leaf water loss assay
Water loss rate was measured according to methods described by Verslues et al. (2006) with some modifications. To measure water loss under dehydration conditions, fully expanded penultimate leaves (n = 15) from WT and dst∆184–305 mutant (M3) plants at vegetative stage were detached and immediately weighed to record fresh weight, and then kept it in water for 2 h to make it turgid. After weighing turgid weight, leaves were exposed to air at a culture room at 28 °C and constant light for dehydration. The leaves were weighed an interval of 15 min up to 150 min. Leaves were finally oven dried for 48 h at 80˚C to a constant dry weight (DW). Excised Leaf Water loss (ELWL) was measured using the following formula ELWL (%) = [(FW–desiccated weight)/FW] × 100 for each 15 min. The relative water content was measured by following formula RWC (%) = [(FW-DW)/(TW-DW)] X100 for each 15 min interval to calculate change in leaf water status.
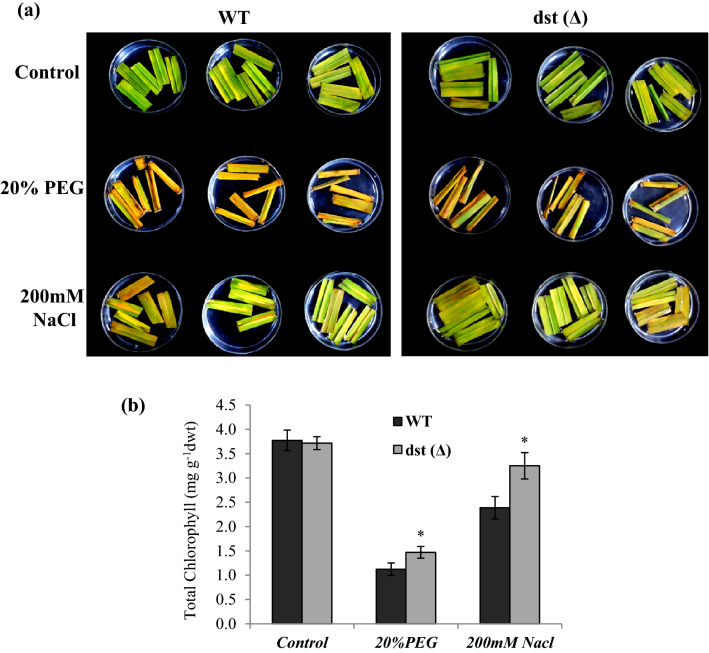
Chlorophyll retention assay
To measure the chlorophyll retention under stress conditions, fully expanded penultimate leaves from WT and dst∆184–305 mutant (M3) plants at vegetative stage were used. Leaves were cut into small pieces and floated on water (Control), 20% PEG6000 (Osmotic stress) and 200 mM NaCl (salt stress) in a petriplate, and incubated in a culture room under 28 °C and 16 h/8 h light/dark cycle for 4 days. At the end of the experiment, photographs were taken and chlorophyll was extracted by using Dimethylsulphoxide (DMSO) and estimated as described by Hiscox and Israelstam 1979. 50 mg of fresh leaf sample was weighed and added into 10 ml of DMSO in a glass vial. The vials were covered with aluminium foil to avoid photo oxidation of pigments and kept at 65 °C for 5 h. The vials were taken out and cooled down to room temperature. The absorbance of the extract was measured at 663 and 645 nm, with DMSO as blank. Total chlorophyll was calculated by using the formula [(8.02 × A663) + (20.2 × A645)].
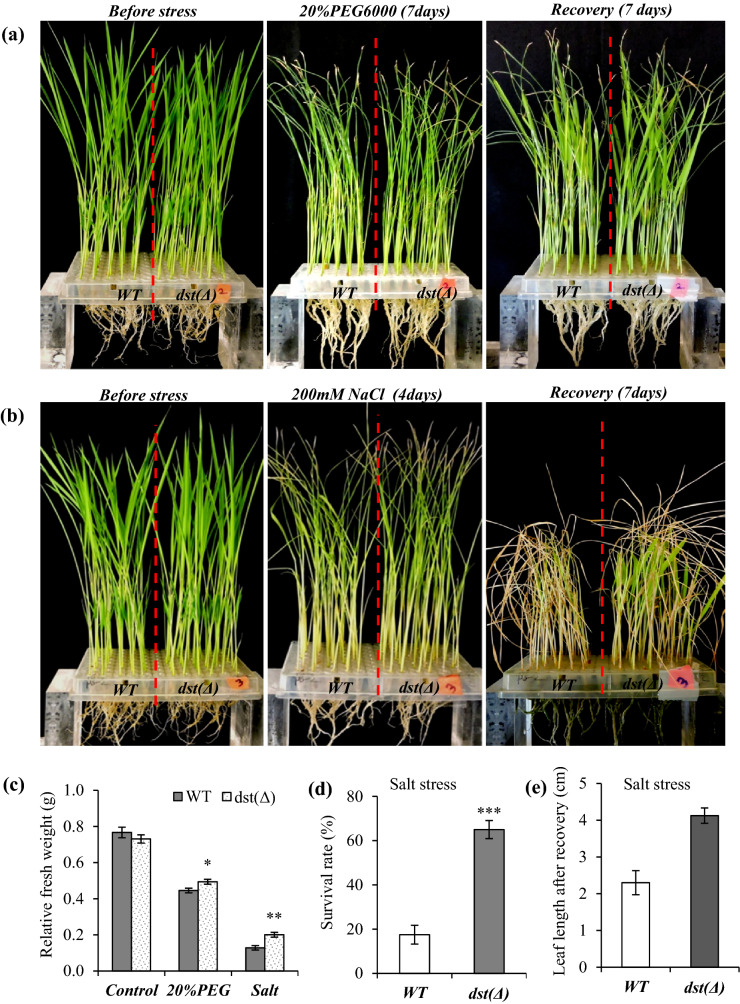
Abiotic stress tolerance assay
For drought and salt tolerance assay, uniformly germinated seeds of WT and homozygous Cas9 free dst∆184–305 mutant (M4) plants were transferred into 96 well PCR plates with the bottom of the tubes cut opened to permit the root growth. These seedlings were grown in half strength Yosida solution at a culture room with 16 h light/8 h dark at 28 °C. Uniformly grown 2 weeks old seedlings were subjected to 20% PEG6000 (− 0.49 MPa) Osmotic stress and 200 mM NaCl (− 1.01 MPa; 20 dS/m) salt stress. Stress period was 4 days for salt stress and 7 days for PEG-induced osmotic stress. After the stress treatments, pictures were taken and treated plates were recovered by transferring them to Yosida solution. Relative fresh weight, leaf elongation rate and survival rate of seedlings were taken after 7 days.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0.2 for Windows (La Jolla, CA, USA). Student’s t-tests were used in comparisons between means of genotypes or means of treatments of a genotype.
Results and discussion
Molecular analysis of genome-edited lines
The putative T0 transgenic plants were confirmed by SpCas9 specific primers (Online Resource 5A-B). PCR amplicon of DST gene from 30 different T0 lines were sequenced by Sanger sequencing to identify CRISPR-Cas9 mediated mutations at gRNA1 and gRNA2 sites. DST PCR amplicon from mutants yielded a chromatogram with superimposed signal peaks beginning around Cas9 cleavage site in the sequence (Online Resource 5C). Analysis of these superimposed sequence chromatograms using DSDecodeM and CRISPR-ID tool led to the identification of mutation and classification into biallelic and heterozygous mutations (Online Resource 5D). In total, 19 mutant plants were identified from 30 T0 transgenic plants. The frequency of mutation at gRNA1 and gRNA2 sites was 33.3 and 30.0%, respectively (Online Resource 6). This is within in the range of mutation frequency reported for Oryza sativa ssp. japonica cultivars which varied from 20 to 100% for different genes in different studies (Feng et al. 2013; Zhou et al. 2014; Li et al. 2016; Xu et al. 2017).
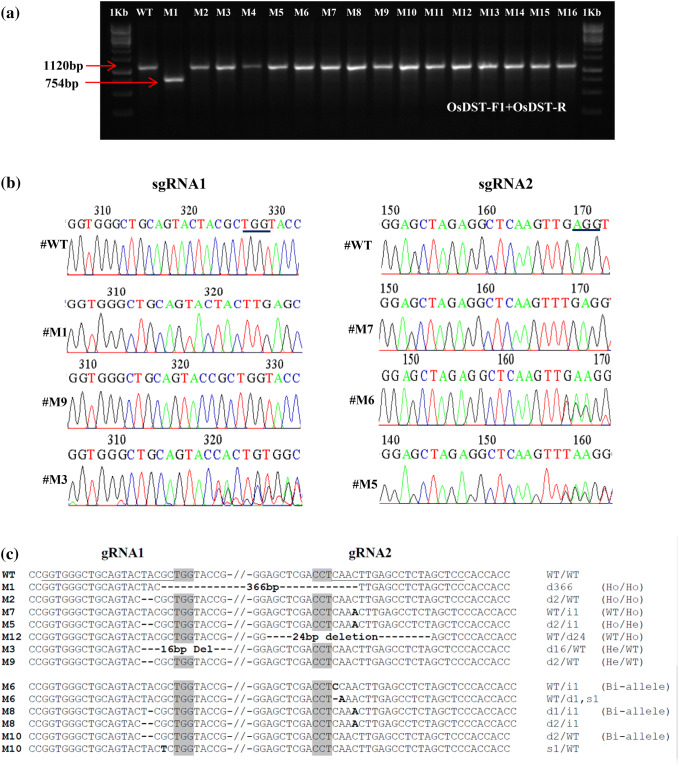
In T1 generation, DNA sequencing of DST PCR amplicon from 17 selected dst mutant lines (Fig. 1a and b) led to the identification of homozygous, heterozygous and biallelic mutants (Fig. 1c and d). At gRNA1 site, 6 homozygous, 3 heterozygous and 2 biallelic mutations were found, while at gRNA2 site, 5 homozygous, 3 heterozygous and 1 biallelic mutations were found. A homozygous mutant with 366 bp deletion between the PAM sites of two gRNAs was selected for further study. This deletion resulted in an in-frame deletion of amino acid residues from 184 to 305 in the DST protein, and thus the mutant was named as dst∆184–305 (Online Resource 7). In the mutant dst∆184–305 protein, N-terminal EAR motif and zinc finger domain are intact, while rest of the protein is truncated including the C-terminal EAR motif. DST protein contains two EAR motifs, one each at N- and C-terminal, respectively, and a DNA binding ZF domain (Huang et al. 2009; Li et al. 2013). The EAR motifs are necessary for transcriptional repression (Hiratsu et al. 2004; Tan et al. 2008; Tiwari et al. 2004; Pauwels et al. 2010) or activation (Huang et al. 2009; Li et al. 2013). DST with functional EAR motifs has been shown to function as transcriptional activator (Huang et al. 2009; Li et al. 2013) and loss of C-terminal EAR motif resulted in loss of transcriptional activity as in case of DSTreg1 mutant (Li et al. 2013). Since earlier studies showed that loss of the function of DST due to N69D mutation (dst) in zinc finger DNA binding domain that abolishes DNA binding and enhanced stress tolerance (Huang et al. 2009), and loss of C-terminal EAR motif enhanced grain number (Li et al. 2013), we selected the loss of function dst∆184–305 mutant for further studies.
Fig. 1.
Molecular confirmation of dst mutants (T1). a PCR amplification of (T1) OsDST transgenic plants by using OsDST-F1 and OsDST-R primers. b Chromatograms of selected mutations induced by CRISPR-Cas gene editing at gRNA1 and gRNA2 target sites of OsDST gene. c Representative dst Indel mutations of alleles identified from sequence analysis of PCR amplicons from dst mutants shown. The wild type sequence was shown at the top. The underlined sequence is gRNA target sites. PAM site nucleotides are shaded. Bold dash in and around gRNA region in different mutants indicates deletion, while Capital nucleotides are insertion or substitution; d, deletion; s, substitution; i, insertion; Ho, Homozugous; WT, Wild Type
Seeds from homozygous dst∆184–305 mutant (T1) were harvested at maturity, and T2 seedlings were raised. All the 17 T2 progenies of 366 bp deletion mutant genotyped showed expected amplicon of 754 bp confirming homozygous 366 bp deletion mutation. Of these 17 homozygous mutants, 3 plants were Cas9 free (Online resource 8). These three M3 plants were advanced to M4 generation.
Phenotypic analysis of dst mutants
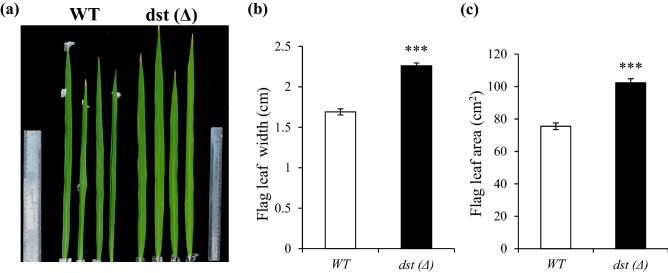
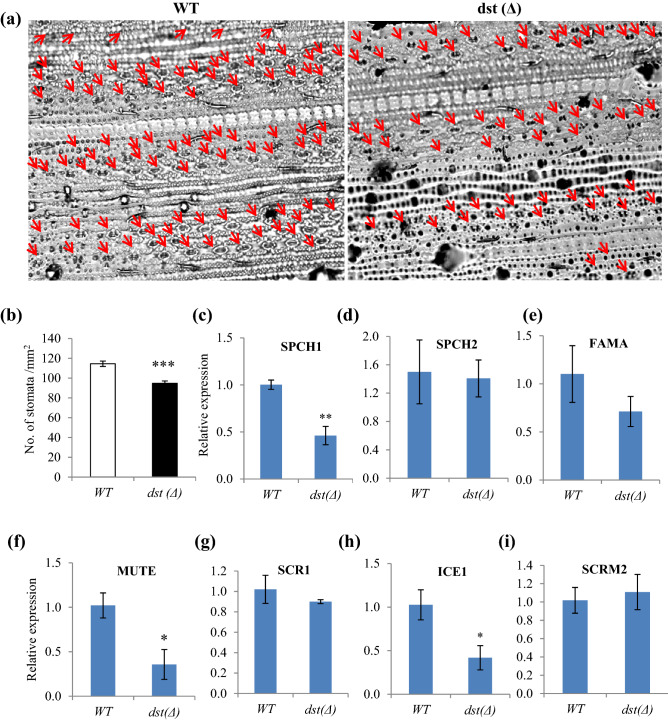
The dst∆184–305 mutant showed significantly broader leaf width and enhanced leaf area. The average flag leaf width was about 1.6 cm in WT MTU1010, while it was about 2.0 cm in genome edited dst∆184–305 mutant plants (Fig. 2a–c). Microscopic examination of stomata in MTU1010 and dst∆184–305 mutant plants revealed that stomatal density decreased significantly in dst∆184–305 mutant plants (Fig. 3 a and b). Thus, the dst∆184–305 mutant in indica cv. MTU1010 created by genome editing phenocopies the EMS-induced dstN69D mutant in japonica cv. Zhonghua11 (Huang et al. 2009).
Fig. 2.
The dst∆184–305 mutation confers enhanced flag leaf area. a and b Flag Leaf width. c Flag leaf area of WT and dst∆184–305 mutant. Data was recorded as a mean ± SEM for at least (n = 15) leaves. Asterisks indicate statistically significant differences (***P < 0.001)
Fig. 3.
The dst∆184–305 mutation reduces stomatal density. a Stomatal density of WT and dst∆184–305 mutant (M3 generation). Stomata in abaxial side of flag leaf was examined in light microscope (40×). b Stomatal density was calculated for at least five plants as a mean ± SEM (n = 5). The asterisk represents the significant differences between the two treatments (***P ≤ 0.001). c–i qRT-PCR expression analysis of genes involved in stomatal development. Data represents mean of 3 biological replicates (n = 3, ± SD). The asterisks represents the significant differences between the two treatments (*P ≤ 0.05 and **P ≤ 0.01)
Although reduction in stomatal density in loss of function mutant of DST was reported (Huang et al. 2009), the mechanism has not been examined earlier. Hence, we examined the expression of genes involved in stomatal development such as SPEECHLESS1 (SPCH1), SPCH2, FAMA, MUTE, SCARECROW1 (SCR1), INDUCER OF CBF EXPRESSION 1 (ICE1)/SCREAM (SCRM) and SCRM2 in the developing leaf of WT and dst∆184–305 mutant. The SMF basic helix-loop-helix (bHLH) transcription factor proteins SPEECHLESS (SPCH), MUTE and FAMA regulate initiation, meristemoid differentiation and guard cell morphogenesis (Pillitteri et al. 2007), and loss of function of any of these proteins leads to impairment in stomatal development (Zoulias et al. 2018). SCREAM1/ICE1 and SCREAM2 bHLH TFs regulate SPCH and MUTE, and positively regulate stomatal development. Loss of function of ice1/scrm1 and scrm2 resulted in total loss of stomata (Kanaoka et al. 2008). Our results revealed that expression levels of SPCH1, MUTE and ICE1 were significantly lower in dst∆184–305 mutant than that of WT MTU1010 plant (Fig. 3c–i). This is consistent with the reduced stomatal density in dst∆184–305 mutant (Fig. 3a and b). A recent study showed loss of function mutants of ice1 and spch1, developed by CRISPR-Cas mediated editing, with drastically reduced stomatal number in rice (Wu et al. 2019).
In order to understand the possible direct transcriptional role of DST protein on the expression of in SPCH1, MUTE and ICE1 genes in rice, we searched for the presence of DST binding cis-element (DBS) TGNTANN(A/T)T (Huang et al. 2009; Li et al. 2013) in the promoters of SPCH1, MUTE and ICE1 genes. We found one DBS TGaTAtgTT at 716–708 upstream to initiation codon in the SPCH1 promoter, and two DBS viz., TGaTAtaAT, TGcTAccTT at 1064–1056 and 237–229 upstream to initiation codon, respectively, in ICE1 promoter (Online resource 9). This suggests that DST protein may directly bind to the promoter and positively regulate the stomatal developmental genes SPCH1 and ICE1. Thus, the reduction in stomatal number in the loss of function dst∆184–305 mutant could be attributed, at least in part due to, reduction in the expression of SPCH1, MUTE and ICE1 genes.
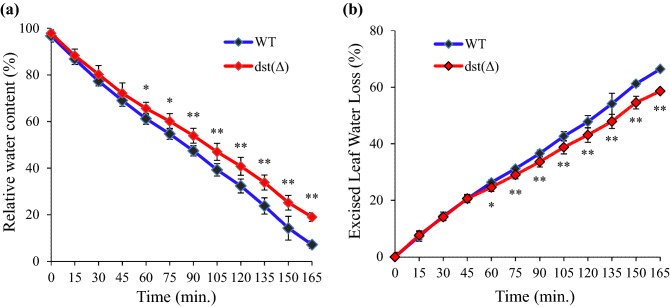
Since dst∆184–305 mutant showed reduced stomatal density, we analysed the excised leaf water loss (ELWL) to confirm the minimization of whole leaf transpiration. The dst∆184–305 mutant showed significantly less water loss (Fig. 4). This may be due to reduced stomatal density (Fig. 3) as well as faster stomatal closure (Huang et al. 2009).
Fig. 4.
Excised leaf water loss of dst∆184–305 mutant (M3 generation). a Change in RWC of excised leaf. b Excised leaf water loss. The asterisks represents the significant differences between the two treatments. Error bars ± SD (n = 15). Asterisks indicate statistically significant differences (**P ≤ 0.01 and *P ≤ 0.05)
We further examined chlorophyll retention of the excised leaf under osmotic and salt stress. Chlorophyll retention was significantly higher in the excised leaves of dst∆184–305 mutant under osmotic and salt stresses (Fig. 5). Analysis of WT and dst∆184–305 mutant (M4) seedlings for tolerance to osmotic stress and NaCl stress showed that seedling biomass was significantly higher in the dst∆184–305 mutant than that of WT at the end of the recovery from these stresses (Fig. 6a–c). Further, only ~ 20% of WT seedlings survived the 200 mM NaCl stress, while > 65% of dst∆184–305 mutant seedlings survived the salt stress (Fig. 6d). In addition, during the recovery period, the rate of leaf elongation in dst∆184–305 mutant was significantly higher than that of WT (Fig. 6e). Huang et al. (2009) showed that loss of function mutant of dstN169D is tolerant to drought and salt stresses. DST Co-activator 1 (DCA1) was found to interact with N-terminal domain of DST and act as co-activator. TOS17 mutant of dca1 showed enhanced tolerance to drought and salt stresses, while overexpression of DCA1 conferred susceptibility of transgenic rice (Cui et al. 2015). Thus, the stress tolerance phenotype found in loss-of-function mutant dst∆184–305 in this study is consistent with earlier findings. This study further showed that different allele generated by CRISPR-Cas9 genome editing in indica cultivar can phenocopy EMS mutant dstN69D of japonica cultivar. In addition, our study showed that the reduction in stomatal density in loss of function mutants of DST is due to downregulation of stomatal developmental genes SPCH1, MUTE and ICE1 in dst mutants.
Fig. 5.
Chlorophyll retention assay of dst∆184–305 mutant (M3 generation). a Fully expanded leaves of WT and dst∆184–305 mutant were cut into small pieces and floated on water (control), 20% PEG6000 and 200 mM NaCl salt stress for 4 days. Photographs were taken and chlorophyll was estimated. b Total leaf chlorophyll content in control, 20% PEG6000, 200 mM NaCl treated leaves. Data was recorded in three biological replicates (n = 3). Asterisks indicate statistically significant differences (*P < 0.05)
Fig. 6.
Drought and Salt tolerance of dst∆184–305 mutant (M4 generation). a Drought tolerance of WT and dst∆184–305 mutant. Fourteen days old seedlings were treated with 20% PEG6000 (7 days) and then recovered for a week. b Salt stress tolerance of WT and dst∆184–305 mutant. Fourteen days old seedlings were treated with 200 mM NaCl salt stress (4 days) and then recovered for a week. c Relative fresh weight after recovery of WT and dst∆184–305 mutant treated with 20% PEG6000 and 200 mM NaCl stress. d and e Survival rate and expanded leaf length after recovery in 200 mM salt stress. Data was recorded for at least 20 seedling per replicate. Error bars ± SD (n = 3). Asterisks represents the significant differences between the two treatments (*P ≤ 0.05 and **P ≤ 0.01 and ***P ≤ 0.001)
Conclusion
In the present study, we have standardized the protocol for CRISPR-Cas9 mediated genome editing in indica mega rice cv. MTU1010. Earlier mutation in dst gene was developed in rice by using genome editing approach. We developed a loss-of-function deletion mutant dst∆184–305 in indica cultivar which phenocopies EMS-induced N69D amino acid substitution mutant previously identified in japonica cultivar. The dst∆184–305 mutant showed reduced stomatal density accompanied by an increase in leaf water retention under dehydration stress. Further, at seedling stage stress tolerance assay, dst∆184–305 mutant exhibited moderate level of tolerance to osmotic stress and high level of tolerance to NaCl stress. We also showed that reduction in stomatal density is associated with reduction in stomatal development genes SPCH1, MUTE and ICE1 in dst mutant. Since more than 2800 genes controlling important agronomic traits have been cloned and characterized in rice, mostly in japonica cultivars, CRISPR-Cas9 genome editing approach can be used to generate these alleles in indica genotypes for improvement of yield and stress tolerance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Indian Council of Agricultural Research (ICAR) -National Agricultural Science Fund, New Delhi, Grant No. NASF/CRISPR-Cas-7003/2018-19, and ICAR-Indian Agricultural Research Institute, New Delhi, Grant No. CRSCIARISIL20144047279.
Authors contribution
SKVV prepared gene constructs, optimized callus induction, transformation and developed GEd plants; PY and AW developed GE plants. RKV and SKV did molecular analysis of transgenic plants. SKVV and MVR wrote the manuscript. VC designed the experiments, helped in data analysis and edited the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Birla DS, Malik K, Sainger M, Chaudhary D, Jaiwal R, Jaiwal PK. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.) Crit Rev Food Sci Nutr. 2017;57:2455–2481. doi: 10.1080/10408398.2015.1084992. [DOI] [PubMed] [Google Scholar]
- Chen K, Wang Y, Zhang R, Zhang H, Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol. 2019;70:667–697. doi: 10.1146/annurev-arplant-050718-100049. [DOI] [PubMed] [Google Scholar]
- Chu CC, Hill RD, Brule-Babel AI. High frequency of pollen embryoid formation and plant regeneration in Triticum aestivum L. on monosaccharide containing media. Plant Sci. 1990;66:255–262. doi: 10.1016/0168-9452(90)90211-6. [DOI] [Google Scholar]
- Cui LG, Shan JX, Shi M, Gao JP, Lin HX. DCA1 acts as a transcriptional co-activator of DST and contributes to drought and salt tolerance in rice. PLoS Genet. 2015;11(10):e1005617. doi: 10.1371/journal.pgen.1005617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehairs J, Talebi A, Cherifi Y, Swinnen JV. CRISP-ID: decoding CRISPR mediated indels by Sanger sequencing. Sci Rep. 2016;6:28973. doi: 10.1038/srep28973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Zhang B, Ding W, Liu X, Yang D, Wei P, Cao F, Zhu S, Zhang F, Mao Y, Zhu JK. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23:1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ishida Y, Komari T (2015) Rice, Indica (Oryza sativa L.). In: Wang K. (eds). Agrobacterium Protocols. Methods Mol Biol 1223. Springer, New York, NY [DOI] [PubMed]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 2004;34(5):733–739. doi: 10.1046/j.1365-313X.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- Hiscox JD, Israelstam GF. A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Hua K, Tao X, Zhu JK. Expanding the base editing scope in rice by using Cas9 variants. Plant Biotechnol J. 2019;17:499–504. doi: 10.1111/pbi.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009;23:1805–1817. doi: 10.1101/gad.1812409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Khehra GS, Lee S-H, Blackhall NW, Marchant R, Davey MR, Power JB, Cocking EC, Gosal SS. An improved procedure for plant regeneration from indica and japonica rice protoplasts. Plant Cell Rep. 1995;14:515–519. doi: 10.1007/BF00232786. [DOI] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Jun R, Xixun H, Keijan W, Chun W. Development and application of CRISPR/Cas system in rice. Rice Sci. 2019;26(2):69–76. doi: 10.1016/j.rsci.2019.01.001. [DOI] [Google Scholar]
- Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu JK, Torii KU. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell. 2008;20(7):1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Precision genome engineering through adenine and cytosine base editing. Nat Plants. 2018;4:148–151. doi: 10.1038/s41477-018-0115-z. [DOI] [PubMed] [Google Scholar]
- Kumar KK, Maruthasalam S, Loganathan M, Sudhakar D, Balasubramanian P. An improved Agrobacterium mediated transformation protocol for recalcitrant elite indica rice cultivars. Plant Mol Biol Rep. 2005;23:67–73. doi: 10.1007/BF02772648. [DOI] [Google Scholar]
- Kusumi K, Hirotsuka S, Kumamaru T, Iba K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot. 2012;63:5635–5644. doi: 10.1093/jxb/ers216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Liu B, Spalding MH, Weeks DP, Yang B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol. 2012;30:390–392. doi: 10.1038/nbt.2199. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao B, Yuan D, Duan M, Qian Q, Tang L, Wang B, Liu X, Zhang J, Wang J, Sun J, Liu Z, Feng YQ, Yuan L, Li C. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc Natl Acad Sci USA. 2013;110:3167–3172. doi: 10.1073/pnas.1300359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li X, Zhou Z, Wu P, Fang M, Pan X, Lin Q, Luo W, Wu G, Li H. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front Plant Sci. 2016;7:377. doi: 10.3389/fpls.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiao J, Chen L, Huang X, Cheng Z, Han B, Zhang Q, Wu C. Rice functional genomics research: past decade and future. Mol Plant. 2018;11:359–380. doi: 10.1016/j.molp.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Liu W, Xie X, Ma X, Li J, Chen J, Liu YG. DSDecode: a web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol Plant. 2015;8:1431–1433. doi: 10.1016/j.molp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma X, Chen L, Zhu Q, Chen Y, Liu YG. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol Plant. 2015;8:1285–1287. doi: 10.1016/j.molp.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Mao Y, Zhang H, Xu N, Zhang B, Gou F, Zhu JK. Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant. 2013;6:2008–2011. doi: 10.1093/mp/sst121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Yu H, Zhang Y, Zhuang F, Song X, Gao S, Gao C, Li J. Construction of a genome-wide mutant library in rice using CRISPR/Cas9. Mol Plant. 2017;10:1238–1241. doi: 10.1016/j.molp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, Zhu JK. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc Natl Acad Sci USA. 2018;115:6058–6063. doi: 10.1073/pnas.1804774115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Joshi RK, Zhao K. Genome editing in rice: recent advances, challenges, and future implications. Front Plant Sci. 2018;9:1361. doi: 10.3389/fpls.2018.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, García-Casado G, Witters E, Inzé D, Long JA, De Jaeger G, Solano R, Goossens A. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464(7289):788–791. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445(7127):501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. Yield trends are insufficient to double global crop production by 2050. PLoS ONE. 2013;8(6):e66428. doi: 10.1371/journal.pone.0066428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo RK, Tuteja N. Development of agrobacterium-mediated transformation technology for mature seed-derived callus tissues of indica rice cultivar IR64. GM Crops Food. 2012;3:123–128. doi: 10.4161/gmcr.20032. [DOI] [PubMed] [Google Scholar]
- Sahoo KK, Tripathi AK, Pareek A, Sopory SK, Singla-Pareek SL. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods. 2011;7:49. doi: 10.1186/1746-4811-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Singh Y, Xalaxo S, Verulkar S, Yadav N, Singh S, Singh N, Prasad KSN, Kondayya K, Rao PVR, Rani MG, Anuradha T, Suraynarayana Y, Sharma PC, Krishnamurthy SL, Sharma SK, Dwivedi JL, Singh AK, Singh PK, Nilanjay Singh NK, Kumar R, Chetia SK, Ahmad T, Rai M, Perraju P, Pande A, Singh DN, Mandal NP, Reddy JN, Singh ON, Katara JL, Marandi B, Swain P, Sarkar RK, Singh DP, Mohapatra T, Padmawathi G, Ram T, Kathiresan RM, Paramsivam K, Nadarajan S, Thirumeni S, Nagarajan M, Singh AK, Vikram P, Kumar A, Septiningshih E, Singh US, Ismail AM, Mackill D, Singh NK. From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016;242:278–287. doi: 10.1016/j.plantsci.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Strickland SG, Nichol JW, McCaU CM, Stuart DA. Effect of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci. 1987;48:113–121. doi: 10.1016/0168-9452(87)90138-5. [DOI] [Google Scholar]
- Tan L, Li X, Liu F, Sun X, Li C, Zhu Z, Fu Y, Cai H, Wang X, Xie D, Sun C. Control of a key transition from prostrate to erect growth in rice domestication. Nat Genet. 2008;40(11):1360–1364. doi: 10.1038/ng.197. [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16(2):533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang C, Liu P, Lei P, Hao W, Gao Y, Liu YG, Zhao K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE. 2016;11(4):e0154027. doi: 10.1371/journal.pone.0154027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Chen L, Yu Q, Zhou W, Gou X, Li J, Hou S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019;223(1):220–232. doi: 10.1111/nph.15766. [DOI] [PubMed] [Google Scholar]
- Xu R, Li H, Qin R, Wang L, Li L, Wei P, Yang J. Gene targeting using the Agrobacterium tumefaciens mediated CRISPR-Cas system in rice. Rice. 2014;7(1):5. doi: 10.1186/s12284-014-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Qin R, Li H, Li D, Li L, Wei P, Yang J. Generation of targeted mutant rice using a CRISPR-Cpf1 system. Plant Biotechnol J. 2017;15:713–717. doi: 10.1111/pbi.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Li G, Yu Y, Ouyang Y. funRiceGenes dataset for comprehensive understanding and application of rice functional genes. Gigascience. 2018;7(1):1–9. doi: 10.1093/gigascience/gix119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liu B, Weeks DP, Spalding MH, Yang B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acid Res. 2014;42:10903–10914. doi: 10.1093/nar/gku806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulias N, Harrison EL, Casson SA, Gray JE. Molecular control of stomatal development. Biochem J. 2018;475(2):441–454. doi: 10.1042/BCJ20170413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.