Abstract
Algal supplements can improve crop productivity and afford protection against abiotic stress by virtue of their rich content of plant nutrients and bioactive compounds. The present work investigates the relative efficiency of the biomass and extract of the brown alga Dictyota dichotoma in protection of rice against salinity and water stress. Rice (Oryza sativa L.) cv. Sakha 101 was grown on a silty clay soil amended with the aqueous extract and powder of D. dichotoma under NaCl and PEG 6000 stress at water potential of − 0.492 MPa. Abiotic stress, particularly water stress, reduced rice growth and concentrations of K+ and protein but increased soluble sugars, starch, proline and Na+ concentrations of plant tissues, with counterbalancing effect of algal amendment. The benefit of algal amendment was greater for algal extract than algal powder and under water stress than salt stress. Algal amendment and abiotic stress promoted catalase and peroxidase activities in rice leaves with variable effect on polyphenol oxidase. The benefit of D. dichotoma to rice can be related to macro- and micro-nutrients, growth hormones, phenolics, flavonoids, sterols, vitamins and fucoidan. The production of toxic intermediates as a result of fermentation of the algal biomass in the paddy soil might reduce the benefit of algal amendment. Although rice is salt-sensitive, it is more resistant to salt stress than to drought stress. The ability of rice to retain Na+ in the root is pivotal for stress resistance, but the role of K+ partitioning is less evident.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00805-2) contains supplementary material, which is available to authorized users.
Keywords: Algal amendment, Antioxidant enzymes, Minerals, Rice, Salinity, Water stress
Introduction
The challenge of meeting the requirements of the growing world population from agricultural and horticultural crops can be met by following different approaches. The traditional approach is to fix the soil problems, via reclamation of the barren (salt-affected) lands, to match plant requirements. Another sophisticated approach is to modify the plant potentiality to match the problem soil using genetic engineering for the production of transgenic plants, which should meet the criteria of high yield, good quality, tolerance to harsh environments and resistance to pests and pathogens. Between these costly options, there is a simple and safe practice, exemplified by amendment of crop species with beneficial additives that can improve overall plant productivity and aid in alleviating the impact of abiotic stress. Several additives have been manipulated to improve plant performance, including growth-stimulating hormones, polyamines, and antioxidants. Application of polyamines, for example, ameliorated the toxic effect of abiotic stress on growth and performance of plants (Gupta et al. 2013).
In addition to the above-mentioned chemical additives, association of the plant with beneficial organisms or addition of their extracts to the soil can improve plant productivity and confer tolerance against abiotic stress. For example, inoculation with the plant growth promoting rhizobacteria Bacillus amyloliquefaciens can confer tolerance to rice against various abiotic stresses such as salt, drought and extreme temperatures via positively modulating gene expression (Tiwari et al. 2017). Likewise, algal extracts can provide promising amendments to alleviate the impact of biotic and abiotic stresses on the performance of higher plants (El-Eslamboly et al. 2019; Zou et al. 2019). Seaweeds are well-known valuable sources of plant macronutrients and micronutrients. Extracts of different algal species can enhance plant growth and protect plants from biotic and abiotic stresses through activation of the anti-oxidative systems and providing hormones and micronutrients (Nabti et al. 2016). Uniquely, the beneficial role of seaweed extract on wheat growth under salt stress has been attributed to algal polysaccharides (Zou et al. 2019).
The toxic effect of salinity on plant performance includes an osmotic effect (tissue dehydration due to the very negative water potential) and a specific ion effect. The latter effect can be manifested either as induction of nutritional imbalance or oxidative damage to biomolecules. The specific ion effect of salinity, which arises at relatively low salt concentrations, might be due to Na+ such as in rice (Lin and Kao 2001) and faba bean (Slabu et al. 2009), Cl− such as in citrus (Moya et al. 2003) or both the two ions such as in Medicago truncatula (Aydi et al. 2008).
Rice (Oryza sativa L.) is a well-domesticated cereal crop with numerous cultivars of divergent yield potentiality and attributes and represents the staple food for a large sector of the world population (Tiwari et al. 2017). In brief, rice has been be categorized as C3 (Zhu et al. 2010), ammonium-tolerant, calcifuge or acid-tolerant and relatively natrophobic species (Broadley et al. 2012), with ample water requirements. Hence, rice is susceptible to drought and salinity stresses at both the vegetative and reproductive stages; with increasing salt sensitivity as the plant development progresses from germination up to maturity (Alam et al. 2004). Unfortunately, the growing demand for rice is synchronized with an aggravated deterioration of soil and water quality and a severe shortage of water supplies all over the world, particularly in the arid regions. Therefore, efforts must be directed towards breeding of new lines of rice, with better tolerance to salt and drought stress. Also, trials must be done to improve performance and to maintain yield of the existing rice cultivars under abiotic stress via manipulation of safe and economic regimes such as amendment with algal extracts.
The present work aims to assess the relative sensitivity of a popular Egyptian cultivar of rice (Sakha 101) to salt stress and water stress, and also to assess the relative potentiality of the aqueous extract and powder of the seaweed D. dichotoma to ameliorate the impact of abiotic stress on rice growth and performance. D. dichotoma is one of the most promising seaweeds, with appreciable nutritive and therapeutic potentialities (Ward et al. 2017). This species is of widespread occurrence and grows along the Red Sea coast of Egypt throughout the year, but flourishes enormously in autumn. Among the several Egyptian rice cultivars, cv. Sakha 101 has been proven to be the most productive one (Fazaa et al. 2016). The underlying mechanisms of the injury of salt and water stresses on rice and the probable mechanisms of alleviation of abiotic stress by algal amendments have been suggested.
Materials and methods
Plant material
Seeds of rice (Oryza sativa L. cv. Sakha 101) were obtained from the Experimental Station of Agricultural Research at Giza, Egypt. Fronds of the brown alga Dictyota dichotoma (Hudson) J.V. Lamouroux were collected from semi-exposed shores at Hurghada, Red Sea coast of Egypt (27° 13′ N, 33° 45′ E). The experimental alga belongs to order Dictyotales, family Dictyotaceae. Fronds were washed with distilled water to remove adhered particles and salts from the surface, shade-dried on blotting paper and ground to a fine powder.
Preparation of treatment solutions
A stock aqueous extract of D. dichotoma was prepared by soaking 100 g of the powdered algal fronds in 1 L of boiling distilled water (100 °C) for 6 h. The slurry was passed through nylon cloth, followed by filtration in Whatman No. 1 filter paper, and the volume of supernatant was made up to 1 L with distilled water. The composition of algal samples from the same site was estimated by Ward et al. (2017). The concentrations of macronutrients (% DW) were: N 3.1, P 0.17, K 3.8, Ca 2.5 and Mg 0.06, and those of micronutrients (µg g−1 DW) were: Fe 23, Mn 85, Cu 22, Ni 24, Zn 6 and Co 7. The concentrations of growth hormones (µg g−1 DW) were: cytokinins 260, auxins 45, gibberellins 12 and abscisic acid 24. The concentrations of some other bioactive compounds (mg g−1 DW) were: phenolics 1.5, flavonoids 1.4, terpenes 23.7, sterols 40.3, vitamin E 0.49, vitamin C 0.63 and fucoidan 57.
Rice plants were supplied with a complete nutrient solution containing the following macronutrients (mM): N 16 (12 mM NH4+ and 4 mM NO3−), K 6, P 1, Ca 2, Mg 1 and S 9.5, and the micronutrients (µM): Fe 100, Mn 10, Cu 1, Zn 1, B 50 and Mo 0.5. Macronutrients were provided in the form of the following salt solutions (mM): (NH4)2SO4 6, Ca(NO3)2 2, KH2PO4 1, K2SO4 2.5, MgSO4 1. The micronutrient solution was prepared according to Smith et al. (1983) and contained the following salts (μM): FeEDTA 0.1, MnSO4·4H2O 10, CuSO4·5H2O 1, ZnSO4·7H2O 1, H3BO3 50, Na2MoO4·2H2O 0.5. Salt stress and water stress were induced by using NaCl and PEG 6000, respectively. To prepare isosmotic solutions of NaCl and PEG, the following formulae of Money (1989) were used:
where Π is the water potential (MPa) and C is the molarity of the solution. According to these equations, 120 mM NaCl and 29.4 mM PEG 6000 (176.5 g L−1) are isosmotic solutions with a water potential of − 0.492 MPa.
Growth conditions
Rice plants, either amended with the powder or the equivalent dose of the aqueous extract of D. dichotoma, were grown under the impact of water stress, induced by PEG 6000 or salt stress, induced by NaCl. Twenty-seven pots were first divided into two groups in a ratio of 1:2. One-third of the pots (9 pots) were devoted to algal powder treatment, where the soil was thoroughly mixed with the fine algal powder at a rate of 20 g/pot before planting. The other two thirds (18 pots) was left native to receive either no amendment (9 pots) or an aqueous algal extract equivalent to 20 g (9 pots) at equal split doses throughout the experimental period. A preliminary germination experiment revealed that the boiled aqueous extract of D. dichotoma at 20 g L−1 maximized germination of rice seeds.
Uniform seeds of rice were surface-sterilized in 10% (v/v) Chlorox for 20 min, washed thoroughly with tap water and germinated in 12 cm Petri dishes lined with soft tissue saturated with 0.5 mM CaSO4 for 2 days. By that time seeds sprouted and produced about 3 mm-long radicles. Sprouts, three per pot, were planted in sealed plastic pots of 20 cm diameter and 25 cm height, full of soil which was either previously mixed with the algal powder or left native to receive either the equivalent of the aqueous algal extract or no amendment. Before planting, the soil was adequately watered with tap water up to field capacity. The soil was silty clay (3.5% sand, 42.2% silt and 54.3% clay), with a pH of 7.86 ± 0.11, water holding capacity of 49.1 ± 3.52%, EC of 1.32 ± 0.05 dS m−1 and organic carbon content of 0.464 ± 0.02%. Seven days after planting, seedlings received the nutrient solution in excess to form a one cm layer above the surface. Seedlings were thinned successively to one per pot within the first 5 days from application of the nutrient solution. Each of the three algal amendment treatments (no amendment, algal extract and algal powder) was then subdivided into three groups (three pots each) according to the stress treatment. The stress treatments included the control (receiving only the full nutrient solution), water stress induced by irrigation with PEG 6000 at a water potential of − 0.492 MPa and salt stress by irrigation with 120 mM NaCl (isosmotic to the PEG solution), both superimposed on the nutrient solution. In the group receiving aqueous algal extract, the extract was superimposed on the nutrient solution and applied to plants at 10 equal successive doses so as to provide the equivalent of the 20 g algal powder throughout the time course of the experiment. Solutions were added to the soil so as to form a layer of 1 cm above the soil surface, and this level was maintained throughout the experimental period. Samples of the standing water above the soil surface were frequently analyzed for Na+ to maintain salinity and water potential at the prescribed level by the addition of either water or treatment solutions.
Plants were grown in a greenhouse at the Faculty of Science, Damietta University. The environmental conditions were: irradiance of about 2000 µmol m−2 s−1 from natural sunlight in a 14/10 h light/dark period, with day/night temperature of 35/25 °C and relative humidity of about 80% on the average.
Harvest and measurements
One harvest was made at the mid-vegetative stage (58 days from imposing abiotic stress). Plants were extracted from soil, cleaned thoroughly by washing with tap water and distilled water, plotted gently and separated into roots and shoots. Number of tillers, dimensions of the second youngest leaf and fresh weights of shoot and root were recorded. The second youngest leaf was immediately dipped in liquid nitrogen and kept at − 80 °C and used for assay of photosynthetic pigments, proline, protein and the activities of catalase (CAT), peroxidase (POX) and polyphenol oxidase (PPO). Dry weights of shoot and root were recorded after drying in an air-forced oven at 80 °C for 48 h, and shoot dry weight was corrected for the frozen leaf. Dry plant matter was ground into a fine powder prior to estimation of soluble sugars and starch in shoot and of minerals in both shoot and root.
Plant analysis
Estimation of photosynthetic pigments
Leaf photosynthetic pigments were determined by extracting a frozen leaf segment in 80% acetone according to the procedure and formulae of Wellburn and Lichtenthaler (1984).
Estimation of proline content
Proline content was assayed in the frozen leaves according to the procedure of Bates et al. (1973). Proline concentration was calculated with reference to standard proline solutions in the range of 0–50 µg mL−1 prepared in 3% sulfosalicylic acid.
Estimation of carbohydrate fractions
The carbohydrate fractions were assayed in the powdered leaves according to Brányiková et al. (2011). Soluble sugars were extracted in boiling 70% ethanol and assayed by using 8.6 mM anthrone in 80% v/v H2SO4. For assay of starch, the debris left after extraction of soluble sugars was re-suspended in 1.6 M perchloric acid in a water bath at 70 °C for 2 h and the released sugars were determined using the anthrone method. Sugar concentration was calculated with reference to a glucose calibration curve in the range of 0–100 µg mL−1.
Estimation of protein content and enzyme activity
Frozen leaf samples were ground in liquid nitrogen and homogenized in 50 mM sodium phosphate buffer (pH 7.0) containing 2 mM EDTA, 5 mM β-mercaptoethanol and 4% (w/v) polyvinylpyrrolidine-40. The homogenate was centrifuged at 30,000 × g for 30 min at 4 °C. The supernatant was used for the assay of protein content as well as catalase, peroxidase, and polyphenol oxidase activities.
Protein content was determined according to the method of Bradford (1976) with reference to a standard curve of bovine serum albumin in the range of 0–100 μg mL−1 prepared in 0.15 N NaCl. Catalase (CAT, EC 1.11.1.6) activity was assayed according to the procedure described by Luck (1974). Peroxidase (POX, EC 1.11.1.7) activity was assayed according to the procedure described by Reddy et al. (1995). Polyphenol oxidase (PPO, EC 1.14.18.1) activity was assayed according to the procedure described by Luh and Phithakpol (1972). Enzyme activity was expressed as enzyme unit mg−1 protein min−1.
Determination of the plant mineral content
Potassium and sodium were extracted by boiling the powdered plant material in distilled water according to the method of Hansen and Munns (1988) and assayed in the clear extract by using a Jenway PFP7 flame photometer. The partitioning of K+ and Na+ within the plant was estimated in terms of the root K+ ratio (RKR) and root Na+ ratio (RNaR), which denote the element content in the root relative to the total plant content.
Statistical analysis
The experiment was factorial with two factors and three replications, in a completely randomized design. The main factors were (1) algal amendment with three levels: no amendment, aqueous algal extract and algal powder; and (2) abiotic stress with three levels: control, salt stress and water stress. Data processing was performed using SPSS version 22. Two-way ANOVA assessed the effect of the main factors (algal amendment and abiotic stress) and their interaction on rice growth and performance. Mean separation was performed according to the Duncan’s multiple range test at P ≤ 0.05. Correlation analysis was estimated as the Pearson correlation coefficient (R) at different levels of significance. Principal component analysis (PCA) to outline the relationships among the different growth and biochemical parameters of rice under the different treatments was carried out using Canoco for windows version 4.5.
Results
Algal amendment × stress interaction on rice growth
Two-way ANOVA shows very highly significant effects (P < 0.001) of algal amendment and abiotic stress and their interaction on rice growth (data not shown). The beneficial effect of algal amendment on rice growth was more evident for the aqueous algal extract than the algal powder, under stress conditions than in non-stressed plants and under drought stress than salt stress (Figs. 1, 2). For example, in control plants the increase in shoot dry weight due to algal extract and algal powder amounted to 51% and 7%, respectively above the non-amended plants (NAP). The respective increases were 300% and 238%, under salt stress and up to 340% and 275% under water stress. Root dry weight followed a pattern similar to that of shoot dry weight. The retardation in plant growth due to abiotic stress was most severe due to water stress of NAP. Shoot dry weight of NAP was reduced by 75% and 83% under the impact of salt stress and water stress, respectively. But, the reduction was cut down to 35% and 50% due to salt stress and water stress, respectively in extract-amended plants (EAP) and further to 22% and 39%, respectively in powder-amended plants (PAP). By contrast, the reduction in root dry weight was comparable under salt stress and water stress, and averaged around 86% in NAP, 46% in EAP and 57% in PAP (Fig. 1). The effect of algal amendment on R/Sh ratio of rice was, in the overall, marginal, except with the 52% increase due to algal extract in the water-stressed plants. By contrast, salt stress and water stress led to an average reduction of 24% in the R/Sh ratio, irrespective of the amendment regime, with a relatively mild effect in EAP.
Fig. 1.

Dry weight of shoot (a) and root (b) and the root/shoot dry weight ratio (c) of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended with either the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Fig. 2.
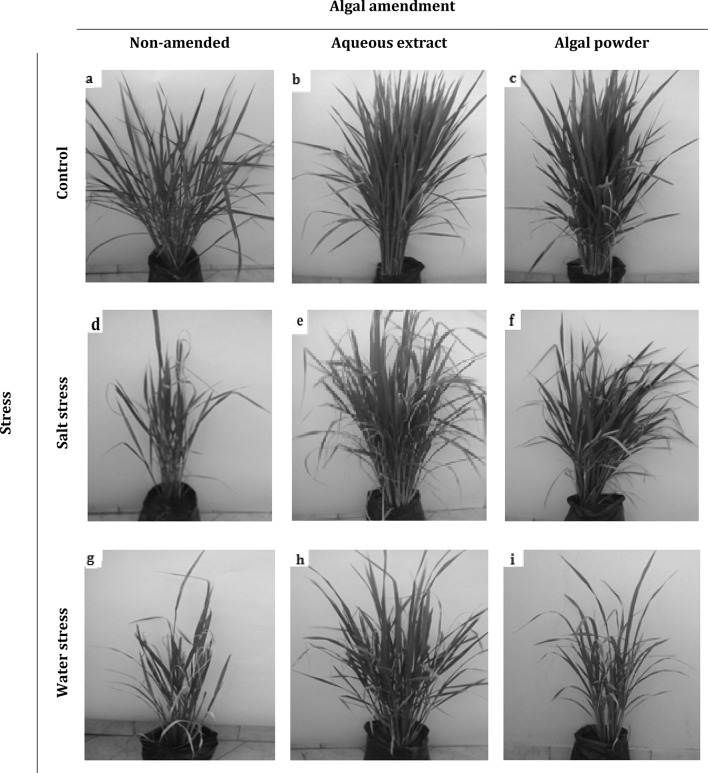
Vigor of rice plants under the different algal amendment × stress combinations. a Non-amended control, b extract-amended control, c powder-amended control, d non-amended salt-stressed, e extract-amended salt-stressed, f powder-amended salt-stressed, g non-amended water-stressed, h extract-amended water-stressed and i powder-amended water-stressed
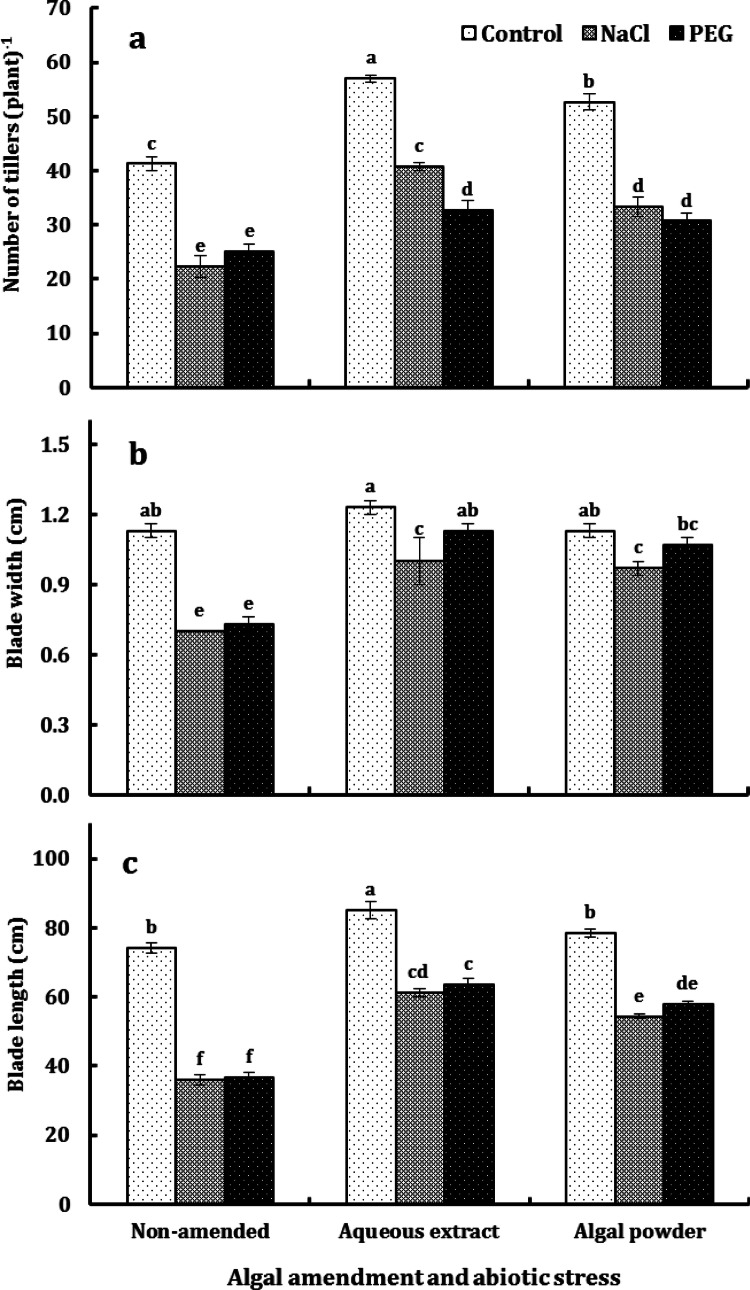
In control and water-stressed plants, both aqueous extract and algal powder increased the number of tillers by an average of 30%; but under salt stress, the increase amounted to 28% and 49% due to aqueous extract and algal powder, respectively. By contrast, water stress and salt stress reduced the number of tillers by an average of 39%, with a relatively mild effect due to salt stress in EAP (Fig. 3). The increase in leaf dimensions due to the algal amendment was marginal in control plants; but under abiotic stress, the increase in leaf width averaged around 50% under salt stress and 40% under water stress. By contrast, the increase in leaf length was comparable in salt-stressed and water-stressed plants and averaged around 72% due to algal extract and 54% due to algal powder. The reduction in leaf dimensions due to abiotic stress was more severe in NAP (37% in leaf width and 51% in leaf length) than in amended plants (12% in leaf width and 27% in leaf length), irrespective of type of stress and mode amendment application (Fig. 3).
Fig. 3.
Number of tillers per plant (a), blade width (b) and blade length (c) of the second youngest leaf of O. sativa L. cv. Sakha 101 grown on a silty clay soil amended with either the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Changes in photosynthetic pigments
The contents of photosynthetic pigments in rice leaves were very highly significantly affected (P < 0.001) by algal amendment and abiotic stress and their interaction (data not shown). Algal extract increased Chl a concentration by an average of 33%, irrespective of the stress regime; but the effect of algal powder was 27% increase in the control plants, marginal increase in water-stressed plants and 30% decrease in salt-stressed plants. The increase in Chl b concentration due to algal extract varied from 19% in the control plants to 34% in water-stressed plants and up to 87% in salt-stressed plants; this in contrast to a relatively moderate and variable effect of algal powder which ranged from 13 and 43% increases in the control and water-stressed plants, respectively to 20% decrease in salt-stressed plants. The beneficial effect of algal extract on carotenoids concentration was most evident (52% increase) in water-stressed plants, moderate (33% increase) in the control plants and almost non-existent in water-stressed plants; but the effect of algal powder varied from 29% increase in the control plants to an average 20% decrease in both water-and salt-stressed plants. The adverse effect of abiotic stress on Chl a concentration was more pronounced in PAP (with 59% and 39% reductions due to salt stress and water stress, respectively) than in EAP and NAP, in which the reduction due to both stresses averaged around 28%. The reduction in Chl b concentration due to salt stress was most severe (60%) in PAP, moderate (43%) in NAP and mild (only 11%) in EAP; but the impact of water stress was stronger in NAP and EAP with an average 37% reduction than in PAP with 25% reduction. In NAP and PAP, the reduction in carotenoids concentration due to salt stress and water stress averaged around 28% and 56%, respectively; but, in EAP, salt stress induced 49% reduction versus only 15% reduction due to water stress (Fig. 4).
Fig. 4.
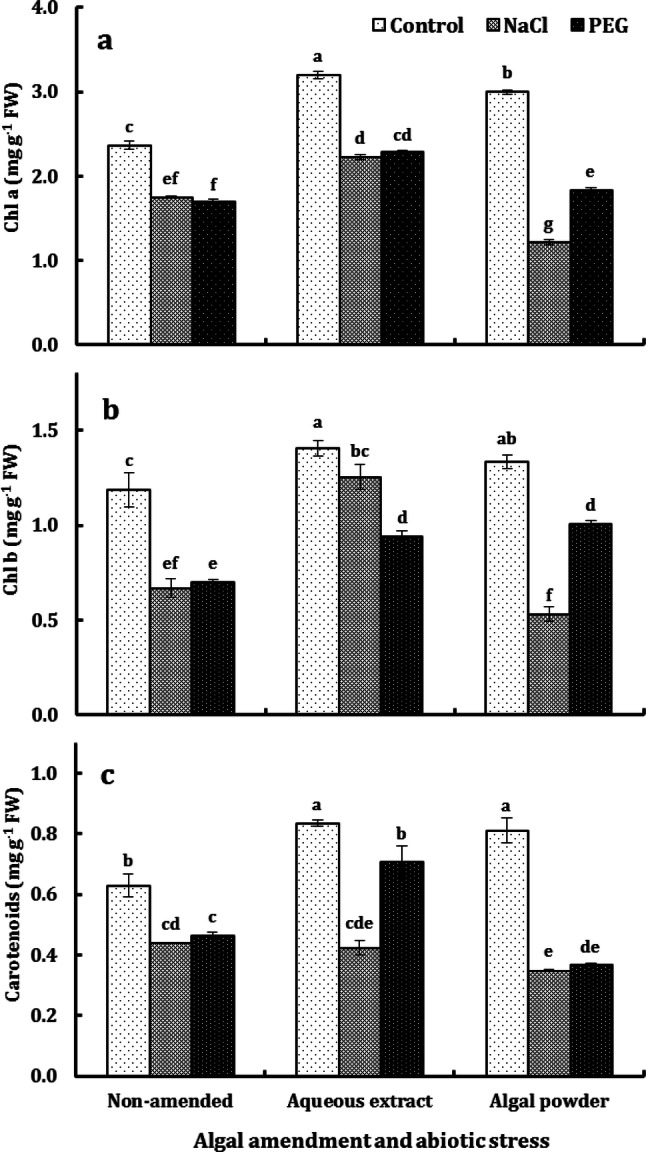
Concentrations of chlorophyll a (a), chlorophyll b (b) and carotenoids (c) in the second youngest leaf of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended with either the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Response of leaf metabolites
The concentrations of soluble sugars, starch and proline in rice leaves were very highly significantly affected (P < 0.001) by algal amendment and abiotic stress and their interaction (data not shown). Algal amendment had no effect on soluble sugars and starch contents of control plants but led to mild reductions under stress conditions. By contrast, both salt stress and water stress led to moderate increases in soluble sugars and starch contents, which were most pronounced in NAP but least expressed in EAP (Table 1). Both algal amendment (mildly) and abiotic stress (substantially) increased proline concentration of rice leaves. The limited effect of algal amendment was most pronounced due to algal powder in water-stressed plants but least evident due to the algal extract in salt-stress plants. The increase in proline concentration due to salt stress and water stress averaged around 100% in PAP and 76% in both NAP and EAP (Table 1).
Table 1.
Concentrations of total soluble sugars (TSS), starch and proline in the second youngest leaf of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended with either the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa
| Algal amendment and abiotic stress | TSS (mg g−1 DW) | Starch (mg g−1 DW) | Proline (µg g−1 FW) |
|---|---|---|---|
| No amendment | |||
| Control | 110.8 ± 0.67a | 122.3 ± 2.21a | 58.70 ± 0.74a |
| NaCl | 135.7 ± 0.66g | 141.1 ± 0.73d | 99.70 ± 1.03c |
| PEG | 139.8 ± 0.94h | 167.5 ± 2.50e | 110.2 ± 1.38d |
| Aqueous algal extract | |||
| Control | 113.3 ± 0.87b | 122.9 ± 2.06a | 68.70 ± 0.94b |
| NaCl | 119.9 ± 0.36c | 129.5 ± 1.07b | 120.2 ± 4.09e |
| PEG | 124.9 ± 0.72d | 137.3 ± 0.42cd | 119.4 ± 1.64e |
| Algal powder | |||
| Control | 113.1 ± 0.74b | 123.2 ± 1.39a | 66.60 ± 1.43b |
| NaCl | 127.1 ± 0.69e | 124.5 ± 0.49a | 139.0 ± 3.88g |
| PEG | 129.5 ± 0.52f | 135.0 ± 0.92c | 131.2 ± 1.94f |
Each value is the mean of three replicates ± SE
Means with common letters are non-significantly different at P ≤ 0.05
Protein content and antioxidant enzyme activity
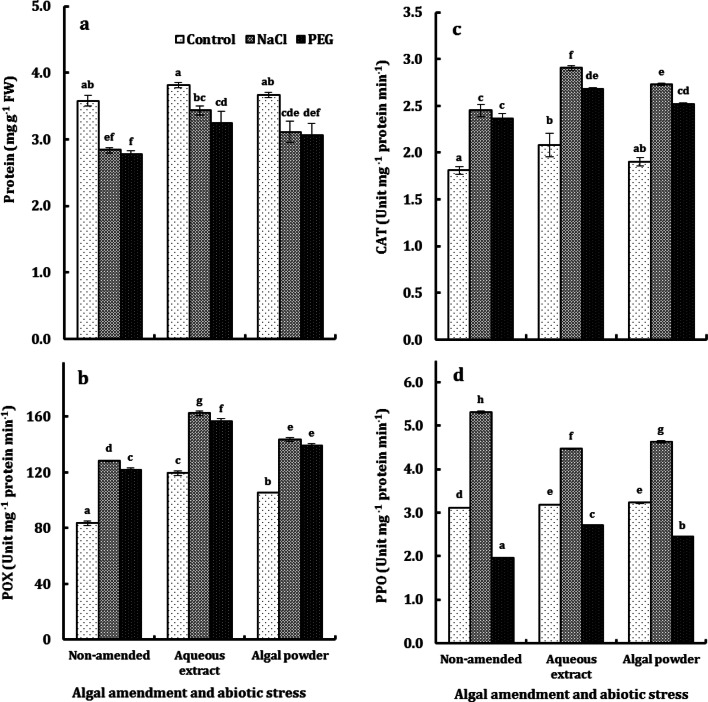
Protein concentration and the activities of CAT and POX in rice leaves were very highly significantly (P < 0.001) affected by the main factors but with non-significant interaction. By contrast, PPO activity was non-significantly affected by algal amendment but very highly significantly affected by abiotic stress and the amendment × stress interaction (data not shown). The increase in protein concentration of rice leaves due to the algal amendment was marginal, being either non-significant in the control or mild in stressed plants. The reduction in protein concentration was comparable under the impact of salt stress and water stress and averaged around 22% in NAP and 14% in both EAP and PAP (Fig. 5). Treatments, in general, and abiotic stress in particular, promoted POX and CAT activities in rice leaves, with a stronger effect of algal extract compared with algal powder. The promotion in POX activity, due to abiotic stress, was greater for salt stress than water stress and in the NAP than in EAP and PAP. Likewise, salt stress promoted CAT activity to a greater extent than did water stress, independent of the amendment regime. Both algal extract and algal powder affected PPO activity comparably, and the effect varied from 14% inhibition in salt-stressed plants to 31% promotion in water-stressed plants, with almost no effect in the control plants. Salt stress induced a promotion in PPO activity versus an inhibitory effect of water stress, and the differential effect of both types of stress was more pronounced in the NAP (with 71% promotion due to salt stress versus 37% reduction due to water stress) than in EAP and PAP, with an average 42% promotion due to salt stress versus 20% inhibition due to water stress (Fig. 5).
Fig. 5.
Protein content (a) and the activity of POX (b), CAT (c) and PPO (d) in the second youngest leaf of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended with either the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Mineral content of shoot and root
Two-way ANOVA shows very highly significant effect (P < 0.001) of algal amendment and abiotic stress and their interaction on concentrations and partitioning of K+ and Na+ in the shoot and root of rice. However, the effect of algal amendment on plant K+ concentration was stronger (greater F ratio) than that of abiotic stress but the reverse was true for Na+ concentration and the K/Na ratio (data not shown).
Both algal extract and algal powder increased shoot K+ concentration by an average of 48% in the control plants; but the increase was greater and differential in stressed plants, in favor of the algal extract under water stress and of algal powder under salt stress. The beneficial effect of algal amendment on root K+ was limited compared to that on shoot K+ and was most pronounced in salt-stressed plant but least in control plants. By contrast, abiotic stress reduced K+ concentration of rice tissues, with greater impact of salt stress than that of water stress and in NAP than in both EAP and PAP. The reduction in shoot K+ concentration due to salt stress amounted to 44% in NAP and averaged around 28% in both EAP and PAP; but that due to water stress averaged around 27% in both NAP and PAP and 11% in EAP. The reduction in root K+ concentration under the impact of salt stress varied from 57% in NAP to 42% in PAP and 26% in EAP; while that due to water stress amounted to 26% in NAP and only 6% in both EAP and PAP (Fig. 6a, b).
Fig. 6.
Concentrations of K+ and Na+ in the shoot (a, c) and root (b, d) of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended either with the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Algal amendment decreased Na+ concentration of rice tissues, and the effect was comparable for algal extract and algal powder and was most pronounced under salt stress but least in control plants. The reduction in shoot Na+ concentration-due to algal extract and algal powder -averaged around 9% in the control plants, 59% in water-stressed plants and 74% in salt-stressed plants. Likewise, the reduction in root Na+ concentration averaged around 18% in both the control and water-stressed plants and 67% in salt-stressed plants. By contrast, abiotic stress, particularly salt stress, increased tissue Na+ concentration, with greater magnitude in the shoot than in root and in NAP than in amended plants. The increase in shoot Na+ concentration amounted to 10 and 1.7 folds under salt stress and water stress, respectively in NAP but averaged around 220% and 23%, respectively in both EAP and PAP. Likewise, the increase in root Na+ concentration amounted to 580% and 72% under salt stress and water stress, respectively in NAP but averaged around 166% and 64%, respectively in both EAP and PAP (Fig. 6c, d).
The effect of algal amendment on the K/Na ratio of plant tissues was limited and variable in the control plants (17% increase in the shoot and 25% reduction in the root) versus a substantial increase under the impact of abiotic stress, which averaged around 10 and 6 folds in the shoot and root, respectively under salt stress and 300% and 63%, respectively under water stress. By contrast, abiotic stress decreased K/Na ratio, and the reduction was more severe in the shoot than the root; under salt stress than water stress and in NAP than in amended plants. In NAP, the K/Na ratio of both shoot and root was reduced by an average of 95% due to salt stress and 66% due to water stress. In both EAP and PAP, the reduction in the K/Na ratio of shoot and root averaged around 50% due to salt stress and 7% due to water stress (Fig. 7a, b).
Fig. 7.
K/Na ratio in the shoot (a) and root (b) and the RKR (c) and the RNaR (d) of O. sativa L. cv. Sakha 101 grown on a silty clay soil, amended either with the aqueous extract or powder of the brown alga D. dichotoma, under the impact of NaCl and PEG 6000 at ψw of − 0.492 MPa. Each column represents the mean of three replicates ± SE. Columns with common letters are non-significantly different at P ≤ 0.05
Both algal amendment and abiotic stress reduced the RKR of rice, with a greater effect of the aqueous algal extract than algal powder and of salt stress than water stress. The reduction in RKR due to algal extract amounted to 45% in control plants, 21% in salt-stressed plants and 14% in water-stressed plants but that due to algal powder amounted to 13%, 26% and 3%, respectively. The reduction due to salt stress amounted to 39% in NAP, 13% in EAP and 48% in PAP; but the effect of water stress varied from 27 and 18% reductions in NAP and PAP, respectively to 15% increase in the EAP. Whereas algal amendment increased RNaR, abiotic stress decreased it, with a pronounced increasing effect due to aqueous algal extract and decreasing effect due to salt stress. The change in RNaR due to algal extract varied from marginal reduction in control plants to 36% and 130% increases in salt-stressed and water-stressed plants, respectively; versus negligible increases due to algal powder in control and salt-stressed plants and 83% increase in water-stressed plants. The reduction in RNaR due to salt stress averaged around 47% in NAP and PAP to 17% in EAP, but the effect of drought stress varied from 51 and 12% reductions in NAP and PAP, respectively to 31% increase in EAP (Fig. 7c, d).
Discussion
The present work suggests that the beneficial effect of algal amendment on rice growth emerges quite efficiently under stress conditions to restore plant growth, with greater efficiency of the aqueous algal extract above algal powder and under the impact of the severe water stress compared with salinity stress. In agreement with our findings, foliar application of putrescine (Gupta et al. 2013) and Zn (Ashraf et al. 2014) had limited effect on the growth of control plants but ameliorated the toxic effect of salt stress on growth and performance of rice. The beneficial role of algal extracts in alleviating the impact of abiotic stress on Triticum aestivum was attributed partially to providing growth hormones and micronutrients (Nabti et al. 2016) or to the algal polysaccharides which contributed in the reduction of lipid peroxidation and enhancement of antioxidant activities and ion compartmentalization (Zou et al. 2019). Fronds of the investigated alga (D. dichotoma) contains considerable contents of macro- and micro-nutrients, growth promoters such as cytokinins, auxins, and gibberellins, secondary metabolites such as flavonoids, phenolics and the unique algal product fucoidan (Ward et al. 2017).
Among the plant macronutrients, K+ seems to play a prominent role in the maintenance of enzyme activity and osmotic adjustment under stress conditions, which justifies the intimate positive correlation between rice growth in one hand and K+ concentration and the K/Na ratio of the plant in the other hand (Table 2 and Fig. 8). In addition, the present work suggests that the beneficial role of algal amendment, particularly algal extract, in improving K+ uptake was associated with preferential allocation of K+ to the shoot; i.e. lowered RKR. In support to this conclusion, the drought resistance of wheat was positively correlated with leaf K+ concentration (Wang et al. 2013). Nevertheless, the inferior beneficial effect of the algal powder relative to algal extract can be attributed to the complex organic fractions of the algal biomass such as carbohydrates, alginates, proteins and lipids, which are amenable to fermentation in the paddy rice environment, with the production of toxic intermediates such as alcohols, ketones, aldehydes, and short chain fatty acids. The lower effect of algal powder was assigned to the production of sulfides as a consequence of anaerobic decomposition of sulfated organic substances of algae (Nabti et al. 2016). The restricted seed germination and plant growth of cotton under anaerobic microhabitats amended with wheat straw have been related to excessive levels of phenolics and short chain fatty acids rather than to oxygen limitation or elevated ethylene levels (Narwal 2012).
Table 2.
Correlation analysis of the performance parameters of O. sativa L. cv. Sakha 101 amended with either the aqueous extract or powder of the brown alga D. dichotoma and grown under the impact of salt stress and water stress
+ + + and − − −denote very highly significant (P < 0.001) positive and negative correlation, respectively, + + and − − highly significant (P < 0.01) correlation, + and − significant (P < 0.05) correlation and ns non-significant (P > 0.05) correlation
The embedded table illustrates the alternate negative correlation between rice growth in one hand and POX and PPO activities in the other hand, versus the consistent correlation with CAT
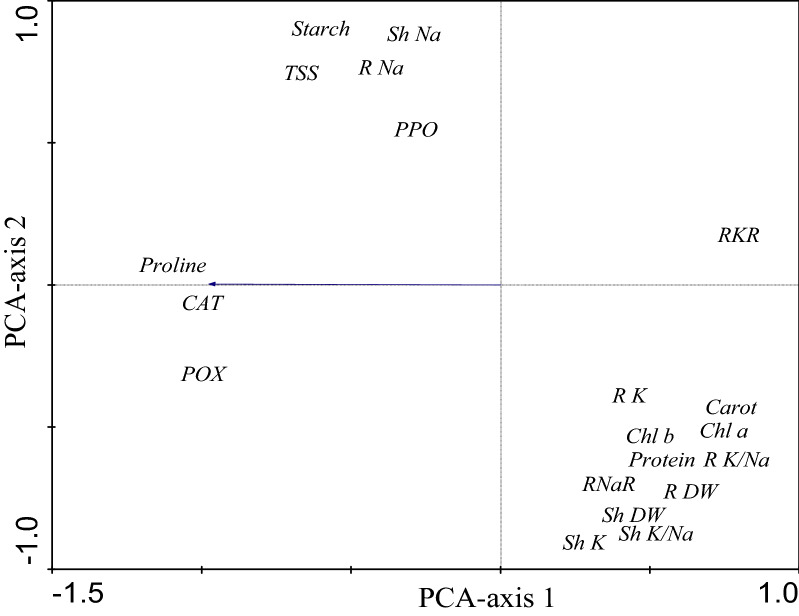
Fig. 8.
Principle component analysis (PCA) of the different growth and biochemical variables of rice at the nine treatment combinations to grasp the relationship between the variables
The severe reduction in rice growth by the moderate salinity of 120 mM NaCl suggests that rice is a salt sensitive species, which coincides with the classification of rice as a natrophobic and salt-sensitive species (Broadley et al. 2012). Alam et al. (2004) reported a considerable reduction in rice growth even at 4.5 dS m−1 (about 45 mM NaCl) with complete growth cessation at 12.5 dS m−1. Nevertheless, the present findings suggest that rice, particularly cv. Sakha 101, is more resistant to salt stress than to drought stress, which seems reasonable in view of the paddy habitat of rice plantations. Consequently, the beneficial effect of the algal amendment in restoration of plant performance was more evident under the more severe water stress than under salt stress. Furthermore, salt sensitivity of rice is expected to aggravate as the plant development progresses from germination to the vegetative and reproductive stages (Razzaq et al. 2020). In agreement with this conclusion, we found that whereas 120 mM NaCl reduced shoot biomass of rice by 73% below the control, it has been estimated to reduce seed germination and early embryo growth by only 57% (Ward et al. 2017). The reduced rice growth under the impact of abiotic stress might be associated with differential impact on the growth of root and shoot; that is abiotic stress (except water stress on EAP) might target root growth specifically. However, Munns (2002) claimed that leaf growth is usually more affected by salinity than root growth; and that although roots are the part of the plant in direct contact with salt or drought stress, they are surprisingly robust.
The impact of salt stress on plant growth is expected to surpass that of water stress; since the former involves an extra specific ion effect in addition to the osmotic effect shared by the two types of stress. Nevertheless, several hypotheses can be forwarded to resolve the observed stronger impact of water stress on rice growth. First, the absorbed salt ions might serve as cheap osmotica to alleviate the osmotic component of salinity stress. This hypothesis is, however, not likely in light of the significant negative correlation between plant growth and tissue Na+ concentrations (Table 2 and Fig. 8) and in view of the fact that rice is a natrophobic and salt-sensitive species (Broadley et al. 2012). Alternatively, it seems that inducing water stress by manipulating PEG 6000 might yield a different response than the mere physical drought induced by water withholding from the soil. Although PEG 6000 is assumed not to cross cellular membranes because of its high molecular weight; yet, the aggressive impact of water stress on rice growth suggests a specific toxic effect of PEG. It is probable that either the polymer molecules themselves or some associated contaminants such as traces of the monomers or the chemicals used in the manufacture of the polymer can cross the cellular membranes and exert a toxic effect in the symplast. The greater inhibitory effect of PEG on Sesuvium portulacastrum growth compared with NaCl has been ascribed either to the presence of toxic contaminants, reduced oxygen availability or inhibition of water movement to root by virtue of the great viscosity of PEG solutions (Slama et al. 2007). However, the contribution of reduced oxygen availability is not likely for rice since paddy rice is well-adapted to anaerobic conditions.
The reduced rice growth under the impact of abiotic stress was intimately associated with increased levels of leaf carbohydrates (soluble sugars and starch) and also proline. It has been suggested that the first effect of salinity on plant performance is the induction of water deficit; which necessitates lowering of the plant water potential. This can be accomplished via accumulation of osmolytes, including mineral ions and compatible organic solutes such as soluble sugars and proline. Proline accumulation in plants is correlated with enhanced salt resistance (Kibria et al. 2017). In addition to its role as a compatible solute, proline can afford protection for biomolecules and membranes against the oxidative damage induced by abiotic stress and hence can aid in the maintenance of membrane integrity and K+ homeostasis of the cell (Ashraf and Foolad 2007). However, the increased concentrations of starch (osmotically inactive) along with soluble sugars in rice leaves under the impact of abiotic stress can be attributed to either a more severe reduction in shoot growth (leaf area) than that in photosynthesis or to impeded export of assimilates from the source leaves. Accumulation of starch under the impact of salt stress has been observed in certain cultivars of rice (Pattanagul and Thitisaksakul 2008) and wheat (Sadak 2019). Starch accumulation has been proposed by Balibrea et al. (2000) to result from increased alkaline invertase activity which hydrolyzes sucrose and converts it into simpler sugars, from which starch may be synthesized. According to this hypothesis, starch accumulation should be fulfilled at the expense of soluble sugars, which is not the case in our study. Therefore, the rise in soluble sugars and starch levels might be a symptom or consequence of stress rather than an adaptive mechanism. To verify if the accumulation of soluble sugars and starch is a consequence of or an adaptive mechanism to abiotic stress, different rice cultivars of divergent salt-tolerance should be manipulated.
The strong positive correlation of pigment content of rice leaves with K+ concentration, along with a less negative, but significant, correlation with Na+ concentration (Table 2 and Fig. 8) suggests that abiotic stress can adversely affect pigment synthesis through retarded K+ uptake to a greater extent than through enhanced Na+ uptake. The adverse effect of salt stress on chlorophyll synthesis in rice has been attributed to accumulation of Na+ in the leaves (Kibria et al. 2017). The reduced protein content, concomitant with proline accumulation in rice leaves under the impact of abiotic stress, suggests that proline accumulation occurs as a consequence of protein degradation. Degradation of protein in favor of the accumulation of a number of amino acids such as glycine betaine and proline can aid in osmotic adjustment, protection of enzymes and membranes from salt injury and detoxification of reactive oxygen species (Banu et al. 2010). The effect of NaCl salinity on protein synthesis may be due to Cl− toxicity in sensitive species (e.g., soybean), whereas in the more salt-tolerant barley Na/K imbalance in the leaves is probably the responsible factor (George et al. 2012). It has also been suggested that Na+ in the cytoplasm impairs ribosomal attachment to rRNA by competing with K+ for binding sites (Tester and Davenport 2003).
In addition to the specific ion effect and disturbance of ionic homeostasis, abiotic stress accelerates the generation of reactive oxygen species (ROS) including superoxide radicals, hydrogen peroxide, and hydroxyl radicals in plant cells which cause oxidative damage to membranes, proteins, and nucleic acids (Banu et al. 2010). Therefore, plants manipulate an array of enzymatic and non-enzymatic antioxidant defense systems to protect cells against the damaging effects of ROS (Moriwaki et al. 2008). The consistent negative correlation of rice growth and leaf photosynthetic pigments in one hand with CAT activity in the other hand versus a less frequent correlation with POX and almost no correlation with PPO suggests that enhanced CAT activity can be considered a universal indicator of abiotic stress in rice, irrespective of stress severity which can be aided partially by POX (under severe stress in NAP and PAP) and marginally by PPO under mild stress (in EAP) (Table 2). The apparent weak negative correlation of POX or the no correlation of PPO with rice growth and leaf photosynthetic pigments was resolved into strong negative correlations in alternating pattern between the two enzymes; where correlation with POX was evident under the severe stress in NAP and PAP while that with PPO emerged under the mild stress in EAP. In partial agreement with this conclusion, Gondim et al. (2012) demonstrated that among the antioxidant enzymes catalase, guaiacole peroxidase and ascorbate peroxidase, catalase was the most responsive to H2O2 application to maize foliage, with earlier activity relative to the other two enzymes and lower lipid peroxidation of leaf cell membranes, and this was linked to gene expression regulation.
Enhanced synthesis of secondary metabolites such as phenolics and anthocyanins under stressful conditions aids in the protection of the cellular structures from oxidative damage (Keutgen and Pawelizik 2008). However, the positive association between rice growth and PPO activity under water stress versus a negative association under salt stress, might signify that phenolics, at low levels, as a result of the promoted PPO activity under salt stress, play a protective role in plants but become themselves toxic when accumulated to high levels as a result of the inhibited PPO activity under drought stress. This might partially explain the less severe impact of salt stress on rice growth relative to water stress and the overall better performance of plants amended with the algal extract over those amended with algal powder. In support to this hypothesis El-Sheekh et al. (2016), claimed that phenolics are beneficial to fenugreek at low concentrations but turn toxic at higher levels.
In salt-sensitive plants, the specific ion effect of salinity rather than the osmotic effect seems the likely cause of plant injury; for in these species plant growth is retarded by too low salt levels to induce water deficit. For graminaceous crops, Na+ is the primary cause of the specific ion damage (Nemati et al. 2011). Sodium toxicity arises mainly from its competition with K+; making the cytosolic K/Na ratio of greater importance than the Na+ concentrations per se. Furthermore, toxicity of Na+ is more likely in the paddy rice, since the combination of waterlogging and salinity can lead to increased Na+ concentration in the plant tissues. The Na+ exclusion mechanisms rely upon high metabolic activity in the roots and thus are dependent on soil aeration. The present work revealed that in addition to the strong positive correlation of rice growth with K+ concentration, along with the negative correlation with Na+ concentration of plant tissues, there was also a strong positive correlation with the RNaR (Table 2 and Fig. 8). This might mean that the stress-relieving effect of the algal amendment on rice can be partially accomplished via enhanced retention of the plant Na+ content by the root to avoid buildup of Na+ in the shoot. This will result in maintenance of high K/Na ratio in the shoot under abiotic stress. In this regard salt stress of some cultivars of rice was found to be positively correlated with the K/Na ratio in the shoot (Aslam et al. 2003; Gerona et al. 2019). The cytosolic K/Na ratio may be critical for NaCl tolerance rather than the absolute Na+ concentration per se (Carden et al. 2003). Nemati et al. (2011) demonstrated that salt tolerance in rice is achieved via exclusion mechanisms to prevent Na+ and Cl– accumulation in the leaf which allows the plant to avoid ion toxicity under saline conditions. In addition, the significant negative correlation of CAT and PPO with RNaR and RKR means that the enhanced activities of these two enzymes are dependent on partitioning of high proportion of the plant ionic content to the shoot irrespective of the type of ion. Meanwhile, the highly significant negative correlation of CAT, PPO and POX with RKR suggests that activities of the three enzymes seem to depend on partitioning of K+ in particular.
Conclusion
The beneficial effect of D. dichotoma aqueous extract on rice can be related to a variety of bioactive components including growth hormones, phenolics, flavonoids, sterols, vitamins and fucoidan in addition to macro-and micro-nutrients. The inferiority of the algal powder relative to the aqueous extract in alleviation of abiotic stress on rice might be related to the production of toxic intermediates as a result of fermentation of the algal biomass in the paddy soil, which might counteract the beneficial effect of algal amendment. Although rice is a natrophobic, salt-sensitive species; yet it is more resistant to salt stress than to drought stress, suggesting a more toxic effect of PEG than that of salt ions. The ability of the plant to retain Na+ in the root and to control its translocation to the shoot is pivotal for stress resistance. Accumulation of proline and soluble sugars can be considered as signs of the impact of abiotic stress on rice. Growth retardation under the impact of abiotic stress was invariably associated with promotion of CAT but was related to POX under severe stress and PPO under moderate stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Experimental Station of Agricultural Research at Giza, Egypt for providing rice seeds. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Taha Mohamed El-Katony, Email: tmsoliman2000@yahoo.co.uk.
Mohamed Ali Deyab, Email: Modeyab@yahoo.com.
Magda Faiz El-Adl, Email: magdaeladl@yahoo.com.
Fatma Mohamed El-Nabway Ward, Email: fatma2028@yahoo.com.
References
- Alam MZ, Stuchbury T, Naylor REL, Rashid MA. Effect of salinity on growth of some modern rice cultivars. J Agron. 2004;3(1):1–10. doi: 10.3923/ja.2004.1.10. [DOI] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ Exp Bot. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Ashraf MY, Iqbal N, Ashraf M, Akhter J. Modulation of physiological and biochemical metabolites in salt stressed rice by foliar application of zinc. J Plant Nutr. 2014;37(3):447–457. doi: 10.1080/01904167.2013.864309. [DOI] [Google Scholar]
- Aslam M, Muhammad N, Qureshi RN, Ahmad Z, Nawaz S, Javaid A. Calcium and salt-tolerance of rice. Commun Soil Sci Plant Anal. 2003;34(19–20):3013–3031. doi: 10.1081/CSS-120025222. [DOI] [Google Scholar]
- Aydi S, Sassi S, Abdelly C. Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant Soil. 2008;312(1–2):59–67. doi: 10.1007/s11104-008-9656-7. [DOI] [Google Scholar]
- Balibrea ME, Della'Amico J, Bolarín MC, Pérez-Alfocea F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol Plant. 2000;110(4):503–511. doi: 10.1111/j.1399-3054.2000.1100412.x. [DOI] [Google Scholar]
- Banu MN, Hoque MA, Watanable-Sugimoto M, Islam MM, Uraji M, Matsuoka K, Nakamura Y, Murata Y. Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci Biotechnol Biochem. 2010;74(10):2043–2049. doi: 10.1271/bbb.100334. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline of water stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M. Microalgae—novel highly efficient starch producers. Biotechnol Bioeng. 2011;108(4):766–776. doi: 10.1002/bit.23016. [DOI] [PubMed] [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. Function of nutrients: micronutrients. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. London: Academic Press; 2012. pp. 191–248. [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ. Signal cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol. 2003;131(2):676–683. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Eslamboly AASA, Abd El-Wanis MM, Amin AW. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt J Biol Pest Control. 2019;29(18):1–9. doi: 10.1186/s41938-019-0122-z. [DOI] [Google Scholar]
- El-Sheekh MM, Ismail MM, Hamouda MM. Influence of some brown seaweed extracts on germination and cytological responses of Trigonella foenum-graecum L. Biotechnol Indian J. 2016;12(9):104. [Google Scholar]
- Fazaa M, El-Sabagh A, Anis G, El-Rewainy I, Barutçular C, Hatipoğlu R, Islam MS. The agronomical performances of doubled haploid lines of rice (Oryza sativa L.) derived from another culture. J Agric Sci. 2016;8(5):177–183. doi: 10.5539/jas.v8n5p177. [DOI] [Google Scholar]
- George E, Horst WJ, Neumann E. Adaptation of plants to adverse chemical soil conditions. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. London: Academic Press; 2012. pp. 409–472. [Google Scholar]
- Gerona MEB, Deocampo MP, Egdane JA, Ismail AM, Dionisio-Sese ML. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 2019;26(4):207–219. doi: 10.1016/j.rsci.2019.05.001. [DOI] [Google Scholar]
- Gondim FA, Gomes-Filho E, Costa JH, Alencar NLM, Prisco JT. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol Biochem. 2012;56:62–71. doi: 10.1016/j.plaphy.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Gupta K, Dey A, Gupta B. Plant polyamines in abiotic stress responses. Acta Physiol Plant. 2013;35(7):2015–2036. doi: 10.1007/s11738-013-1239-4. [DOI] [Google Scholar]
- Hansen EM, Munns DN. Effect of CaSO4 and NaCl on mineral content of Leucaena leucocephala. Plant Soil. 1988;107:101–105. doi: 10.1007/BF02371550. [DOI] [Google Scholar]
- Keutgen AJ, Pawelizik E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008;107(4):1413–1420. doi: 10.1016/j.foodchem.2007.09.071. [DOI] [Google Scholar]
- Kibria MG, Hossain M, Murata Y, Hoque A. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017;24(3):155–162. doi: 10.1016/j.rsci.2017.05.001. [DOI] [Google Scholar]
- Lin CC, Kao CH. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil. 2001;230(1):135–143. doi: 10.1023/A:1004876712476. [DOI] [PubMed] [Google Scholar]
- Luck H. Catalase. In: Gergmeyer H-U, editor. Methods in enzymatic analysis. 2. New York: Academic Press; 1974. pp. 885–894. [Google Scholar]
- Luh BS, Phithakpol B. Characteristics of polyphenol oxidase related to browning in cling peaches. J Food Sci. 1972;37(2):264–268. doi: 10.1111/j.1365-2621.1972.tb05832.x. [DOI] [Google Scholar]
- Money NP. Osmotic pressure of aqueous polyethylene glycols: Relationship between molecular weight and vapor pressure deficit. Plant Physiol. 1989;91(2):766–769. doi: 10.1104/pp.91.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki T, Yamamoto Y, Aida T, Funahashi T, Shishido T, Asada M, Prodhan SH, Komamine A, Motohashi T. Overexpression of the Escherichia coli catalase gene, katE, enhances tolerance to salinity stress in the transgenic indica rice cultivar, BR5. Plant Biotechnol Rep. 2008;2(1):41–46. doi: 10.1007/s11816-008-0046-7. [DOI] [Google Scholar]
- Moya JL, Gomez-Cadenas A, Primo-Millo E, Talon M. Chloride absorption in salt-sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. J Exp Bot. 2003;54(383):825–833. doi: 10.1093/jxb/erg064. [DOI] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Nabti E, Jha B, Hartmann A. Impact of seaweeds on agricultural crop production as biofertilizer. Int J Environ Sci Technol. 2016;14(5):1119–1134. doi: 10.1007/s13762-016-1202-1. [DOI] [Google Scholar]
- Narwal SS. Allelopathy in crop production. Jodhpur: Scientific Publishers; 2012. [Google Scholar]
- Nemati I, Moradi F, Gholizadeh S, Esmaeili MA, Bihamta MR. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 2011;57(1):26–33. doi: 10.17221/71/2010-PSE. [DOI] [Google Scholar]
- Pattanagul W, Thitisaksakul M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Biol. 2008;46(10):736–742. [PubMed] [Google Scholar]
- Razzaq A, Ali A, Safdar LB, Zafar MM, Rui Y, Shakeel A, Shaukat A, Ashraf M, Gong W, Yuan Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J Food Sci. 2020;85(1):14–20. doi: 10.1111/1750-3841.14983. [DOI] [PubMed] [Google Scholar]
- Reddy KP, Subhani SM, Khan PA, Kumar KB. Effect of light and benzyladenine on dark-treated graving rice (Oryza sativa) leaves—changes in peroxidase activity. Plant Cell Physiol. 1995;26(1):987–994. [Google Scholar]
- Sadak MS. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent. 2019;43:53. doi: 10.1186/s42269-019-0098-6. [DOI] [Google Scholar]
- Slabu C, Zörb C, Steffens D, Schubert S. Is salt stress of faba bean (Vicia faba) caused by Na+ or Cl– toxicity? J Plant Nutr Soil Sci. 2009;172(5):644–650. doi: 10.1002/jpln.200900052. [DOI] [Google Scholar]
- Slama I, Ghnaya T, Hessini K, Messedi D, Savouré A, Abdelly C. Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ Exp Bot. 2007;61(1):10–17. doi: 10.1016/j.envexpbot.2007.02.004. [DOI] [Google Scholar]
- Smith GS, Johnston CM, Cornforth IS. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983;94(4):537–548. doi: 10.1111/j.1469-8137.1983.tb04863.x. [DOI] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91(5):503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Prasad V, Chauhan PS, Lata C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front Plant Sci. 2017;8:1510. doi: 10.3389/fpls.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zheng Q, Shen Q, Guo S. The critical role of potassium in plant stress response. Int J Mol Sci. 2013;14(4):7370–7390. doi: 10.3390/ijms14047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward FM, Deyab MA, El-Katony TM. Biochemical composition and bioactivity of Dictyota from Egypt. Saarbrückeny: Lambert Academic Publishing; 2017. [Google Scholar]
- Wellburn AR, Lichtenthaler K (1984) Formulae and programme to determine total carotenoids and chlorophyll a and b of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research, vol 2. Martinus Nijjhoff, Dordrecht, The Netherlands, pp 9–12
- Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Ann Rev Plant Biol. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]
- Zou P, Lu X, Zhao H, Yuan Y, Meng L, Zhang C, Li Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front Plant Sci. 2019;10:48. doi: 10.3389/fpls.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.