Abstract
Various cutaneous manifestations have been observed in patients with COVID‐19 infection. Herpes zoster is a viral skin disease caused by varicella zoster that remains dormant in the dorsal root ganglia of cutaneous nerves following a primary chicken pox infection. In this report, we describe two cases COVID infection who first presented with herpes zoster. We are here by suggesting that the clinical presentation of HZ at the time of the current pandemic even in patients giving mild or no suggestive history of upper respiratory symptoms should be considered as an alarming sign for a recent subclinical SARS CoV2 infection.
Keywords: COVID19, Herpes zoster, immune suppression, SARS CoV2, skin rash
1. INTRODUCTION
Severe acute respiratory syndrome corona virus 2 (SARS CoV2) is the most recently identified member of the zoonotic pathogens of coronaviruses, it started by causing an outbreak of pneumonia in December 2019 in Whuan City of China. 1
Although SARS‐CoV and Middle East respiratory syndrome corona virus (MERS‐CoV) are both closely related to SARS‐CoV‐2 and have bat reservoirs, SARS‐CoV‐2 is markedly more infectious with a high potential for human transmission. The novel virus was officially named SARS‐CoV‐2, with the disease termed COVID‐19. 2
Most patients with COVID‐19 exhibit mild‐to‐moderate symptoms, but approximately 15% of the cases progress to severe pneumonia and about 5% eventually develop acute respiratory distress syndrome (ARDS), septic shock, and/or multiple organ failure. 3 , 4 Finding tools for recognition of asymptomatic carriers would be very helpful in the management of this outbreak.
A wide array of skin manifestations in COVID 19 infection were reported including macuulopapular eruptions, morbilliform rashes, urticaria, chickenpox‐like lesions, livedo reticularis, covid toe, erythema multiforme and pityriasis rosea, and several other patterns. 5
We present two cases of clinically diagnosed Herpes zoster infection attending two different clinics, both patients' showed no or mild symptoms of COVID 19 infection and denied any history of contact with known or suspected COVID19 cases.
1.1. Case 1
A 68‐year‐old man presented with painful blisters on the right side of his right loin. The pain was severe, continuous and stabbing in nature. He gave a history of initial stabbing pain followed by onset of blisters. The blisters were initially small and few in number; they later increased in number covering the entire right half of the loin with a watery discharge that turned hemorrhagic Figure 1. No relevant medical history was given except for rare bouts of untreated hypertension and hypercholesterolemia. The area surrounding the vesicular eruption was flared and tender to touch. The patient was discharged and prescribed valaciclovir 1 g twice daily for 1 week, acyclovir cream, and paracetamol for his fever.
FIGURE 1.
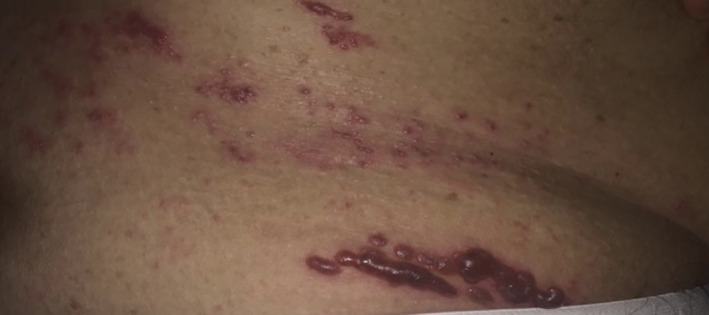
Male patient presenting with multiple blisters on his right loin
Two days following the presentation, the patient developed a heavy cough, sore throat, dyspnea, and fever that required hospitalization. A chest CT showed patchy ground glass infiltration at the peripheral and base of the both lungs consistent with the COVID‐19 infection. A nasopharyngeal smear test revealed a COVID‐19 infection.
1.2. Case 2
A 60‐year‐old female patient attended the dermatology clinic with low grade fever and a vesicular painful rash. On examination, she presented with painful, itchy, vesicular rash affecting the left side of her chest and nape of the neck. The pain was severe enough to freeze her neck. Three days prior to presentation, she experience stabbing pain aggravated by slashing water on the neck or chest. This was followed by the development of the rash. Her oral and ocular mucosae were free of any presentations. Concomitantly she had headache and myalgia but no digestive or urinary symptoms Figures 2 and 3. She had no relevant past history except for controlled hypertension but was gasping and dyspneic for which her attendants believed it was a seasonal allergy bout. She was prescribed acyclovir 800 mg five times daily for 5 days, prednisolone 5 mg twice daily for 5 days as well besides topical application of calamly lotion. She was advised to consult a pulmonologist for her chest symptoms. Chest x‐ray and a positive nasopharyngeal smear test were consistent with COVID‐19 infection.
FIGURE 2.
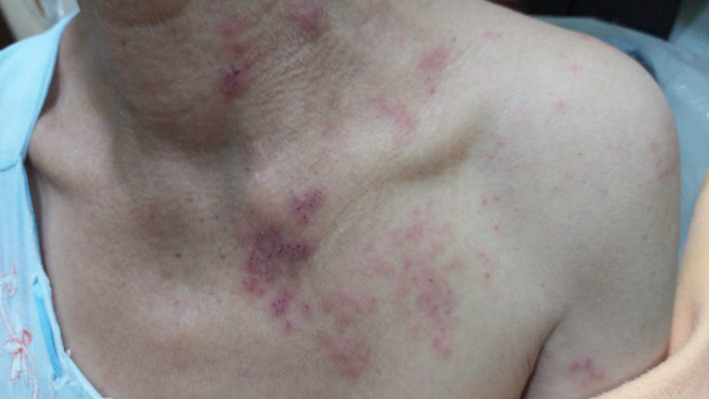
Female patient with blisters on her chest
FIGURE 3.
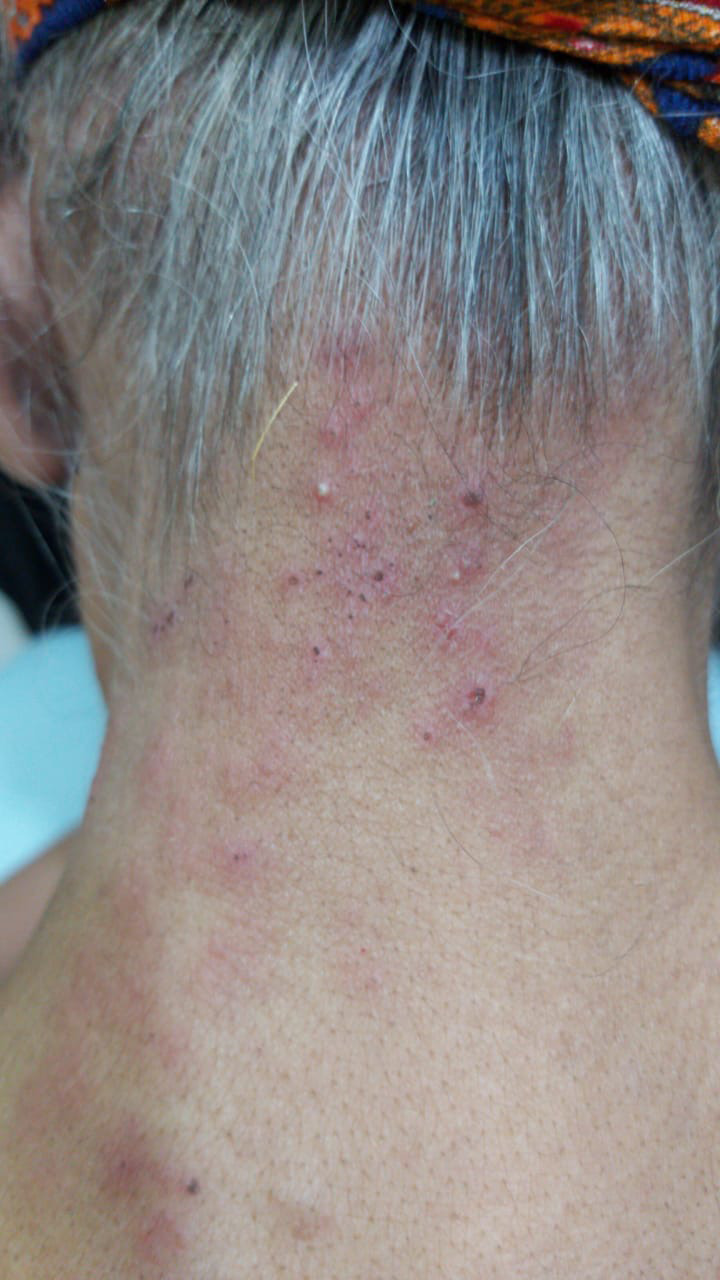
Female patient with zosteriform rash on her nape of the neck
Incubation time of COVID‐19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea were speculated to be clinical symptoms; however, many cases might be asymptomatic. Besides medical and travel history, diagnosis can be confirmed by detection of viral RNA by reverse‐transcriptase polymerase chain reaction (RT‐PCR) for nasopharyngeal swabs or bronchoalveolar fluid. Immunocompromised, old patients, of male gender and suffering from cardiovascular conditions or debilitating chronic conditions are at an increased risk of severe disease and poor outcome. 2
Herpes zoster represents a cutaneous viral condition caused by reactivation of varicella zoster dormant at the dorsal root ganglia of cutaneous nerve endings following a primary early episode of chicken pox. The condition is characterized by occurrence of multiple, painful, unilateral vesicles, and ulceration and shows a typical single dermatome innervated by single dorsal root or cranial sensory ganglion. Involvement of three or more dermatomes is known as disseminated zoster and seen in immunocompromised individuals. 6
Several studies agreed that COVID 19 infection is associated mostly with reduction in lymphocytes, monocytes, and eosinophils 7 , 8 , 9 , 10 with drastically reduced numbers of CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells. 11
In addition, the follow‐up of the dynamic changes of the lymphocyte counts along the course of the disease showed that the nonsurvivors developed more severe lymphopenia over time. White blood cell counts and neutrophil counts were higher in nonsurvivors than found in survivors and also the lymphocyte counts continued to decrease until death occurred in non‐surviving patients. 12
Lower percentages of T‐cell functional markers is also observed with in the T lymphocytes population as COVID‐19 patients showed decreased percentages of CD107a + CD8+, IFN‐γ + CD8+, and IL‐2 + CD8+ T cells and mean fluorescence intensity of granzyme B + CD8+ T cells when compared to normal controls. 13
It is reasonable to hypothesize that, in addition to the activation‐induced cell death, SARS‐CoV‐2 could directly infect lymphocytes, particularly T cells, and initiate or promote the cell death of lymphocytes, which eventually lead to lymphopenia and impaired antiviral responses. 14 It is also postulated that the functional damage of CD4+ T cells may have predisposed COVID‐19 patients to severe disease. 15
We are here by suggesting that the clinical presentation of HZ at the time of the current pandemic even in patients giving mild or no suggestive history of upper respiratory symptoms should be considered as an alarming sign for a recent subclinical SARS CoV2 infection. Thorough follow‐up of such patients is important and advice for COVID exclusion is suggested. These patients can unknowingly infect others and contribute to the spread of the COVID‐19 infection.
Also our observation would recommend further studies before supporting the use of broad immunosuppression in patients with overwhelming viral illness as beneficial anti‐inflammatory effects should be always weighed up against delay of virus clearance. 16
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatologic Therapy. 2020;33:e13666. 10.1111/dth.13666
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars‐cov‐2. Cell. 2020;181(2):223‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020. 10.1111/dth.13549.. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dayan RR, Peleg R. Herpes Zoster‐typical and atypical presentations. Postgrad Med. 2017;129(6):567‐571. [DOI] [PubMed] [Google Scholar]
- 7. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0369.. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar A, Anil A, Praveen S, et al. Clinical features of COVID‐19 and factors associated with severe clinical course: a systematic review and meta‐analysis. SSRN Electron J. 2020. [Google Scholar]
- 10. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020. 10.1111/all.14309. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;4:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):541‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID‐19: a double‐edged sword? Lancet. 2020;395(10230):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]


