Abstract
Coronavirus disease 2019 (COVID‐19) has been a pandemic worldwide. The data about COVID‐19 in renal transplant recipient are deficiency. Herein, we report two COVID‐19 cases in renal transplant recipients. Both cases were discharged following a treatment regimen including discontinued immunosuppressant and low‐dose methylprednisolone‐based therapy. There were no signs of rejection during the treatment. These successfully treated cases can provide helpful information about the management of COVID‐19 in renal transplant recipients.
Keywords: COVID‐19, immunosuppressant, methylprednisolone, renal transplant recipient, SARS‐CoV‐2
SARS‐CoV‐2 is a new recognized highly contagious coronavirus which has caused a global pandemic named coronavirus disease 2019 (COVID‐19). 1 , 2 Previous studies have reported the clinical characters and treatment strategies of COVID‐19. 3 , 4 , 5 However, the data about COVID‐19 in renal transplant recipients are deficiency. Renal transplant recipients are immunocompromised due to the use of immunosuppressant. Herein, we report the clinical feature and management of two COVID‐19 cases in renal transplant recipients.
1. CASE1
A 48‐year‐old man was admitted on February 10 with the chief complaint of intermittent fever for 13 days with a peak temperature of 37.5°C, accompany by chest tightness asthenia without coughing. On February 6, a nasopharyngeal swabs specimen was obtained and sent for detection of SARS‐CoV‐2 nucleic acid; this was reported back within 24 hours as positive. At the same day, a pulmonary computed tomography (CT) scan showed pulmonary inflammation. Local protocol for COVID‐19 was activated, and the patient was diagnosed with COVID‐19 and isolated immediately.
This patient received a renal transplantation in 2009 and was taking oral immunosuppressants consisting of tacrolimus, mycophenolate mofetil, and prednisone with stable allograft function. From February 6, he had discontinued the immunosuppressant use, taking his doctor's advice.
The physical examination on admission revealed a body peak temperature of 39°C, pulse of 95 beats per minute, respiratory rate of 20 breaths per minute, blood pressure of 140/86 mm Hg, and oxygen saturation below 93%. Laboratory results demonstrated a normal leukocyte count (4.5 × 109/L), a normal percentage and count of lymphocyte (24.8% 1.12 × 109/L), C‐reactive protein (CRP) elevation (49.68 mg/L), and a slightly increased level of procalcitonin (PCT) (0.07 ug/L). Renal function was stable (creatinine 1.27 mg/dL cystatin C 1.48 mg/L), and pulmonary CT on February 13 reported the two lungs showed flocculent density increasing shadow and ground glass‐like change, with two lower lungs as the focus (Figure 1).
FIGURE 1.
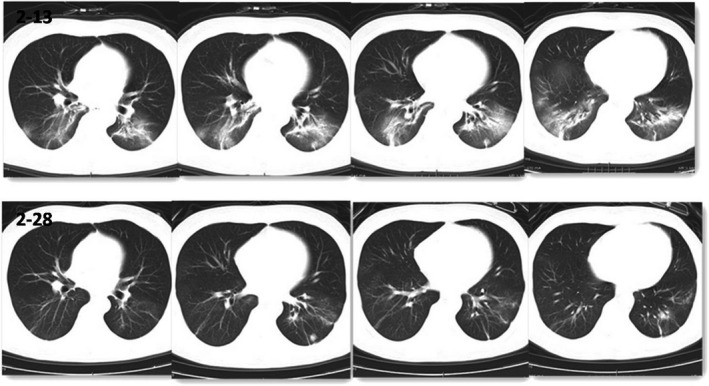
Comparison of CT of case 1 on February 13 and 28
The patient had a peak fever of T39°C on admission with dyspnea. Low‐dose methylprednisolone (40 mg qd) was given with symptomatic supportive treatment. The patient's symptoms were improved gradually. SARS‐CoV‐2 nucleic acid test was negative for consecutive three times, and CT scan on February 28 showed that bilateral lung lesions were absorbed more obviously than before (Figure 1). The patient took the resumption of mycophenolate mofetil tacrolimus and discharged on March 3.
2. CASE2
A 65‐year‐old woman was admitted on February 8 with the chief complaint of intermittent fever for 9 days with a peak temperature of 39°C, accompany by cough expectoration and chest distress muscle ache weakness for 4 days. A SARS‐CoV‐2 nucleic acids test was performed and confirmed positive 5 days prior to hospitalization. She was diagnosed with COVID‐19 and isolated immediately with the administration of arbibol and moxifloxacin, but it was not effective.
She received renal transplantation on 2011 and was taking oral immunosuppressants consisting of tacrolimus, prednisone, and mycophenolate mofetil after surgery. The allograft function was stable. She stopped the immunosuppressants and only reserved prednisone in the guidance of the doctor on the second day onset of illness.
Physical evaluation showed that body temperature was 37.3°C, respiratory rate was 22 breaths per minute, oxygen saturation was only 83% in ambient air and could reach above 93% with oxygen therapy supplied at 3‐5 L/min. Laboratory results on hospital day 2 revealed a normal leukocyte count (4.7 × 109/L), a reduced lymphocyte count (0.72 × 109/L), a low percentage of lymphocytes (15.3%), and a normal thrombocyte and hemoglobin. The level of CRP elevated significantly (40.14 mg/L). Serum creatinine was 0.63 mg/dL, and cystatin C was 1.05 mg/L. The level of plasma albumin slightly decreased (32.9 g/L) with negative proteinuria.
The patient was given high‐flow nasal oxygen therapy (HFNT) at 40 L/min immediately after admission. On second day in hospital, the patient's condition was deteriorating accompanied by chest tightness more obvious after exercise. Non‐invasive ventilator (NIV) was administered at 50% oxygen concentration and oxygen flow 40 L/min. Low‐dose methylprednisolone (40 mg qd) and human immunoglobulin for intravenous injection (IVIG) (5 g qd) were given with symptomatic supportive treatment. The symptoms of the patient were alleviated gradually. NIV was switched to HFNT and at last to nasal cannula oxygen therapy till discharge. IgG (164.91) and IgM (294.78) of SARS‐CoV‐2 were positive on March 09. The nucleic acids tests had been intermittent positive until March 14. From March 16, the tests were consecutive twice negative. The patient was cured (Figure 2) and discharged on March 20 without signs of rejection during the hospital period.
FIGURE 2.
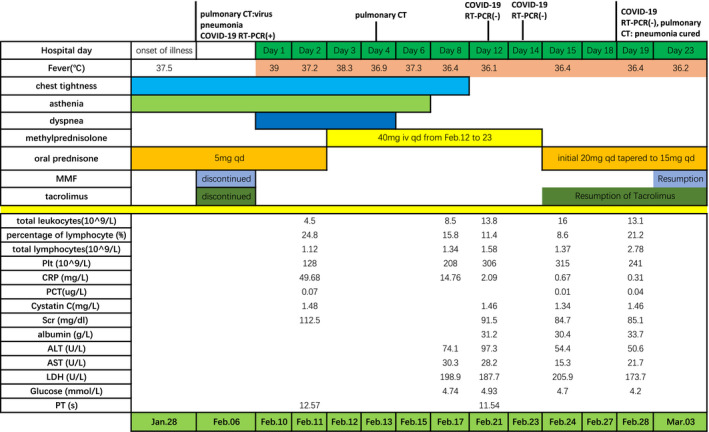
Symptoms, laboratory results and treatments of case 1 according to day of hospitalization
3. DISCUSSION
Coronavirus disease 201 has been a global pandemic with nearly two million population infected. Not only anti‐infection therapy but also adjustment of immunosuppressant should be the key point in deal with allograft recipients with COVID‐19. But there is little experience in coronavirus infection. In severe pneumonia caused by CMV or Pneumocystis carinii as an opportunistic infection, discontinuing the immunosuppressants timely is the key role in renal transplant recipients. Cases of coronavirus infection in renal transplant recipients were rarely reported before. A recent research reveals that in liver transplant recipients, COVID‐19 does not seem to cause more severe conditions. 6 However, both cases we reported had dyspnea and high fever. We observed the rapid deterioration of illness in the second case. Discontinuation of the immunosuppressants as soon as the diagnosis with COVID‐19 was taken in both cases. The duration of discontinuing immunosuppressants was respective 18 days and 22 days. We did not observe the sign of rejection during the long duration. Zhong et al 7 reported the emergence of rejection following the discontinuation of immunosuppressant in a liver transplant recipient who was diagnosed with COVID‐19 several days after liver transplantation. Nevertheless, the time was the highest risk of rejection period. In the same report, maintenance of the immunosuppressants not only delayed the elimination of virus but also leaded to severe bone marrow suppression in another renal transplant recipient. In consideration of high mortality of COVID‐19 once it develops into critical ill, 8 timely discontinuation of immunosuppressant to reduce the risk of critical ill should be considered especially in recipients with stable allograft function and low risk of rejection.
Even though corticosteroid use in COVID‐19 has not a consensus conclusion, 9 , 10 in our cases, daily methylprednisolone was administrated. The use of methylprednisolone played an important role in suppressing inflammatory storms promoting lung inflammation absorption and preventing pulmonary fibrosis. It also could prevent rejection during the free of immunosuppressant period. It is very important to have the proper dose of methylprednisolone to avoid the side effect. During 2003, SARS coronavirus (SARS‐CoV) pandemic period, some researchers administrated methylprednisolone to cases of SARS. 11 , 12 , 13 The dose of methylprednisolone generally exceeded 2‐3 mg/kg/d even 500‐1000 mg per day. The controversy about the dose and effect lasted for a long time. Considering the inclusive evidence and urgent clinical demand, physicians from the Chinese Thoracic Society have developed an expert consensus statement on the use of corticosteroids in COVID‐19. 14 The dosage of methylprednisolone was suggested to be low to moderate (≤0.5‐1 mg/kg per day methylprednisolone or equivalent). It was much lower than before to balance the effect and side effect.
We notice that the count of lymphocyte on discharge was 2.5‐4 times that at admission (Figure 3, Figure 4). It reflected the importance of the immunity in the improvement of illness. It took the second case nearly 50 days to eliminate virus. The count of lymphocyte of the second case was under the normal level at admission. In renal transplant recipients, immunocompromise condition would delay eliminating virus.
FIGURE 3.
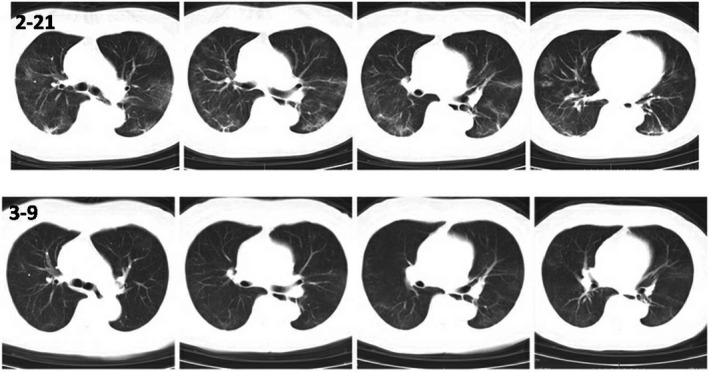
Comparison of CT of case 2 on February 21 and March 9
FIGURE 4.
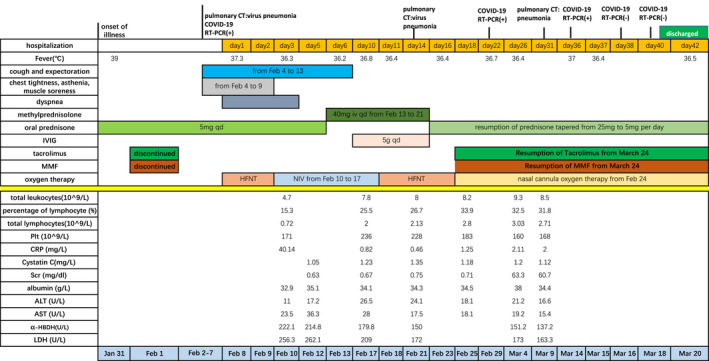
Symptoms, laboratory results and treatments of case 2 according to day of hospitalization
4. SUMMARY
In conclusion, although only two COVID‐19 cases in renal transplant recipients were showed, it may give some experience for therapeutic management. For renal transplant recipients with stable function and low risk of rejection, it is beneficial to discontinue immunosuppressant onset of COVID‐19. Low dose of methylprednisolone through the process has a good balance between suppression of inflammation storm and side effect.
AUTHORS CONTRIBUTIONS
Cheng DR, JQ Wen, ZZ Liu, TF Lv and JS Chen contributed to the design and implementation of this report as well as the analysis of the results and the writing of the manuscript. PLT: platelets, Scr: serum creatinine LDH: lactate dehydrogenase, α‐HBDH: α ‐ hydroxybutyrate dehydrogenase, CRP: C‐reactive protein, PCT: procalcitonin, ALT: alanine aminotransferase, AST: aspartate aminotransferase, PT: prothrombin Time, HFNT: high‐flow nasal oxygen therapy, NIV: non‐invasive ventilator, MMF: mycophenolate mofetil, IVIG: intravenous injection immunoglobulin.
Cheng D, Wen J, Liu Z, Lv T, Chen J‐S. Coronavirus disease 2019 in renal transplant recipients: Report of two cases. Transpl Infect Dis. 2020;22:e13329. 10.1111/tid.13329
REFERENCES
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. [DOI] [PubMed] [Google Scholar]
- 7. Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;395(10225):683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao Z, Zhang F, Xu M, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52(8):715‐720. [DOI] [PubMed] [Google Scholar]
- 12. Sung JJ, Wu A, Joynt GM, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho JC, Ooi GC, Mok TY, et al . High‐dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168(12):1449–1456. [DOI] [PubMed] [Google Scholar]
- 14. Zhao JP, Hu Y, Du RH, et al. Expert consensus on the use of corticosteroid in patients with 2019‐nCoV pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E007. [DOI] [PubMed] [Google Scholar]


